Behavioral and Pharmacokinetics Studies of N-Methyl-2-Aminoindane (NM2AI) in Mice: An Aminoindane Briefly Used in the Illicit Drug Market
Abstract
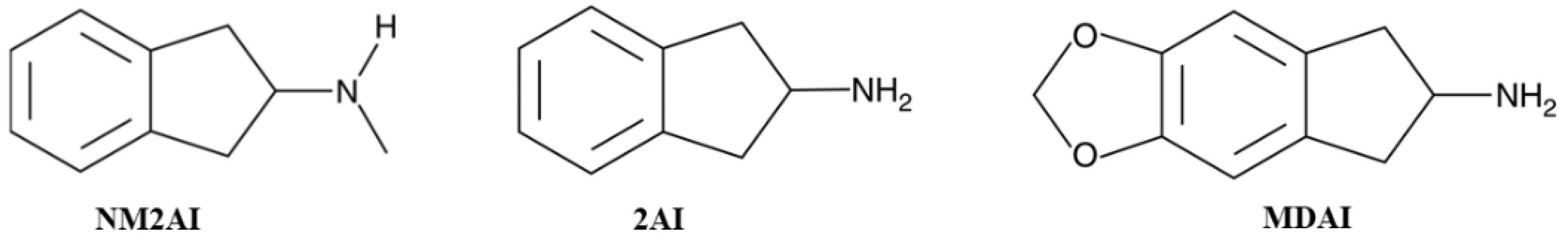
1. Introduction
2. Results
2.1. Behavioral Studies
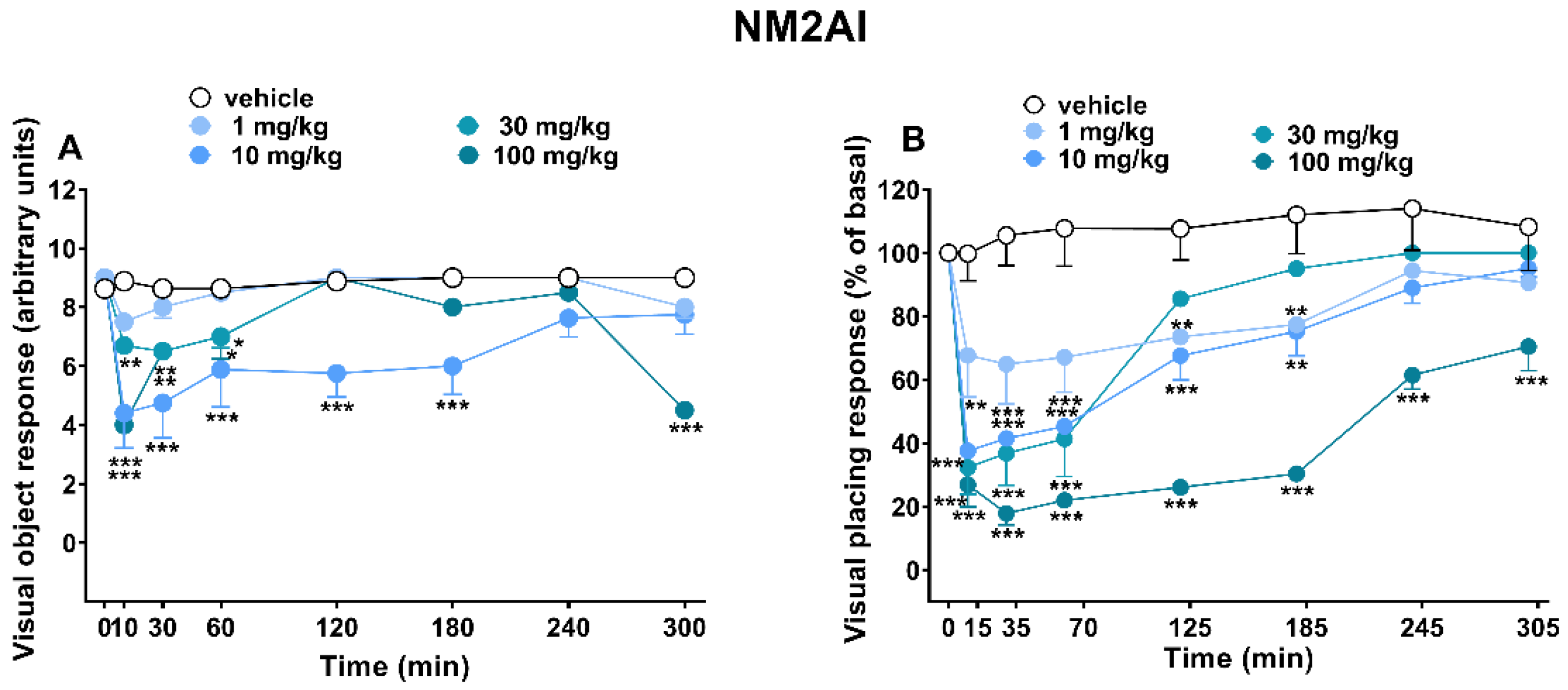
2.1.1. Evaluation of the Visual Response
2.1.2. Evaluation of Acoustic Response
2.1.3. Evaluation of Tactile Response
2.1.4. Evaluation of Body Temperature
2.1.5. Evaluation of Pain Induced by Two Stimuli: Mechanical (Tail Pinch Test) and Thermal (Tail Withdrawal)
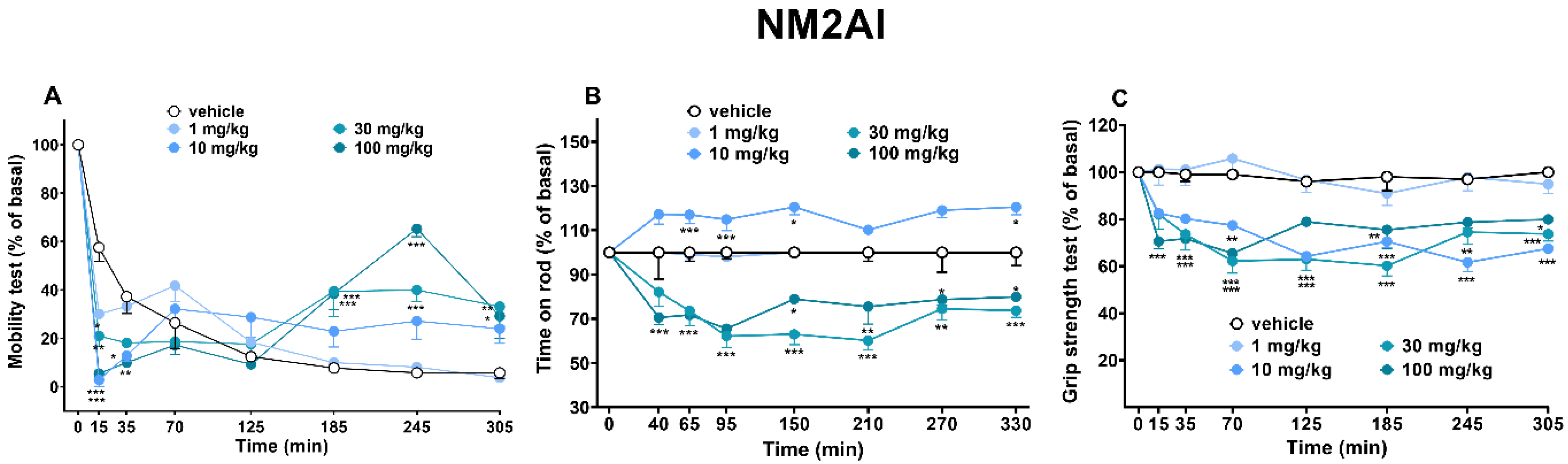
2.1.6. Sensorimotor Activity Assessment (Accelerod and Immobility Time Test)
2.1.7. Evaluation of Skeletal Muscle Strength (Grip Strength Test)
2.2. Startle/Prepulse Inhibition Analysis
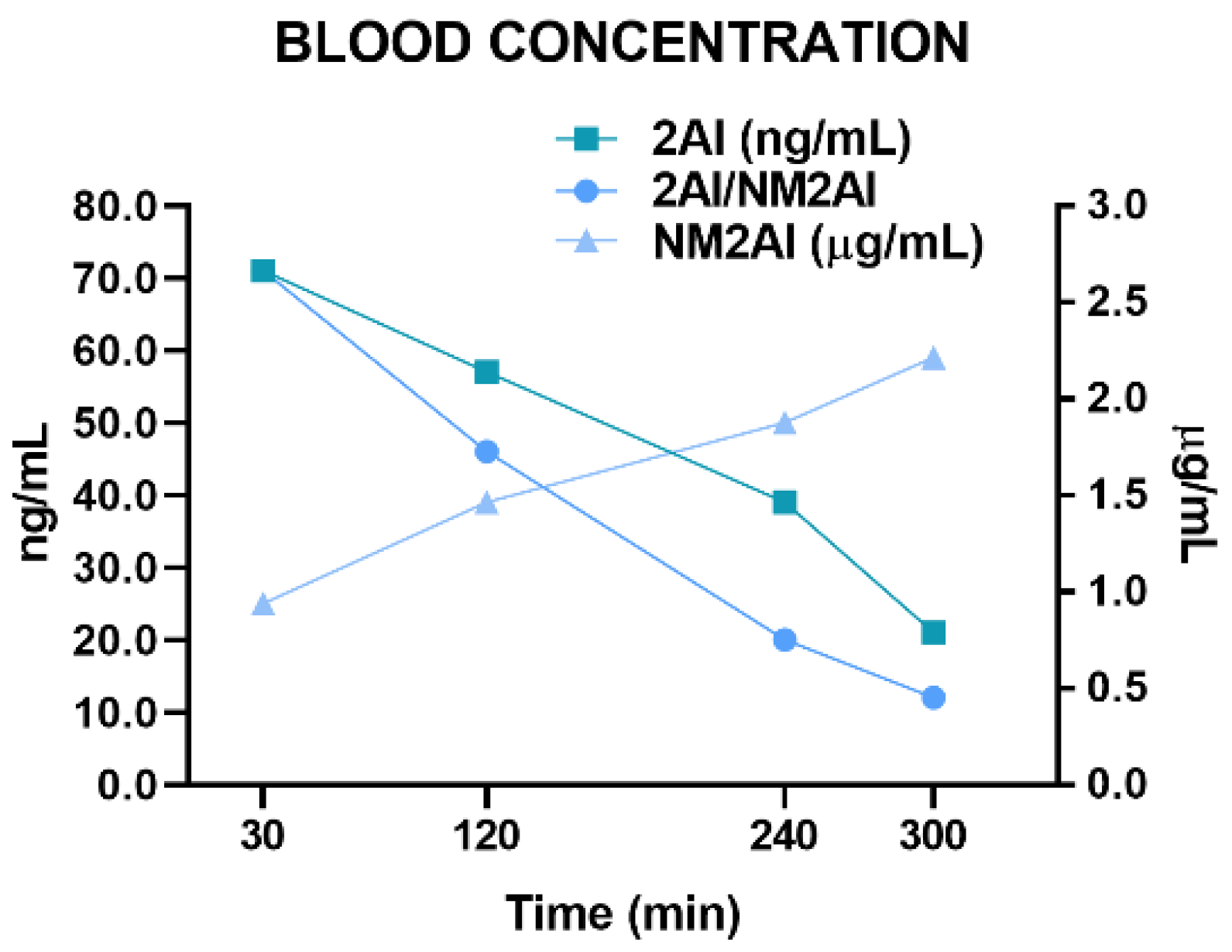
2.3. Pharmacokinetic Study
3. Discussion
3.1. Visual Responses
3.2. Core Body Temperature
3.3. Analgesic Profile Responses
3.4. Motor Activity and Grip Strength
3.5. PPI Effect
4. Materials and Methods
4.1. Animals
4.2. Drug Preparation and Dose Selection
4.3. Experimental Protocol of Behavioral Studies
4.3.1. Evaluation of the Visual Responses (Visual Object and Placing Test)
4.3.2. Evaluation of the Acoustic Response
4.3.3. Evaluation of Tactile Response
4.3.4. Evaluation of Body Temperature (Core and Surface)
4.3.5. Evaluation of Pain Induced by Two Different Stimuli: Mechanical (Tail Pinch Test) and Thermal (Tail Withdrawal)
4.3.6. Sensorimotor Activity Assessment (Accelerod and Immobility Time Test)
4.3.7. Evaluation of Skeletal Muscle Strength (Grip Strength Test)
4.4. Startle/Prepulse Inhibition Analysis
4.5. Pharmacokinetic Study
4.6. Statistical Analysis
4.7. Blood Sample Analysis
4.7.1. Blood Preparation
4.7.2. LC–HRMS Apparatus
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NPS | New Psychoactive Substances |
| NM2AI | N-methyl-2-aminoindane |
| 2AI | 2-aminoindane |
| MDAI | 5,6-methylenedioxy-2-aminoindane |
| MDMA | 3,4-methylenedioxymethamphetamine |
References
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). European Drug Report 2021: Trends and Developments; Publications Office of the European Union: Luxembourg, 2021. [Google Scholar]
- United Nations Office on Drugs and Crime (UNODC). Current NPS Threats. 2020. Available online: https://www.unodc.org/documents/scientific/Current_NPS_Threats_Vol.3.pdf. (accessed on 2 December 2022).
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). European Drug Report 2022: Trends and Developments; Publications Office of the European Union: Luxembourg, 2022. [Google Scholar]
- Odoardi, S.; Romolo, F.S.; Strano-Rossi, S. A snapshot on NPS in Italy: Distribution of drugs in seized materials analysed in an Italian forensic laboratory in the period 2013–2015. Forensic Sci. Int. 2016, 265, 116–120. [Google Scholar] [CrossRef]
- Rhumorbarbe, D.; Morelato, M.; Staehli, L.; Roux, C.; Jaquet-Chiffelle, D.O.; Rossy, Q.; Esseiva, P. Monitoring new psychoactive substances: Exploring the contribution of an online discussion forum. Int. J. Drug Policy. 2019, 73, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Drug Enforcement Administration Toxicology (DEA TOX). Testing Program. 2021. Available online: https://www.deadiversion.usdoj.gov/dea_tox/index.html (accessed on 2 December 2022).
- Mestria, S.; Odoardi, S.; Federici, S.; Bilel, S.; Tirri, M.; Marti, M.; Strano Rossi, S. Metabolism Study of N-Methyl 2-Aminoindane (NM2AI) and Determination of Metabolites in Biological Samples by LC-HRMS. J. Anal. Toxicol. 2021, 45, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Corkery, J.M.; Elliott, S.; Schifano, F.; Corazza, O.; Ghodse, A.H. MDAI (5,6-methylenedioxy-2-aminoindane; 6,7-dihydro-5H-cyclopenta[f][1,3]benzodioxol-6-amine; “sparkle”; “mindy”) toxicity: A brief overview and update. Hum. Psychopharmacol. Clin. Exp. 2013, 28, 345–355. [Google Scholar] [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Annual Report 2012: The State of the Drugs Problem in Europe; Publications Office of the European Union: Luxembourg, 2012. [Google Scholar]
- Sumnall, H.R.; Cole, J.C.; Jerome, L. The varieties of ecstatic experience: An exploration of the subjective experiences of ecstasy. J. Psychopharmacol. 2006, 20, 670–682. [Google Scholar] [CrossRef]
- Gallagher, C.T.; Assi, S.; Stair, J.L.; Fergus, S.; Corazza, O.; Corkery, J.M.; Schifano, F. 5,6-methylenedioxy-2-aminoindane: From laboratory curiosity to ‘legal high’. Humanist. Psychol. 2012, 27, 106–112. [Google Scholar] [CrossRef]
- Nichols, D.E.; Brewster, W.K.; Johnson, M.P.; Oberlender, R.; Riggs, R.M. Nonneurotoxic tetralin and indan analogues of 3,4-(methylenedioxy)amphetamine (MDA). J. Med. Chem. 1990, 33, 703–710. [Google Scholar] [CrossRef]
- Kovar, K.A. Chemistry and pharmacology of hallucinogens, entactogens and stimulants. Pharmacopsychiatry 1998, 31 (Suppl. 2), 69–72. [Google Scholar] [CrossRef]
- Gatch, M.B.; Dolan, S.B.; Forster, M.J. Locomotor, discriminative stimulus, and place conditioning effects of MDAI in rodents. Behav. Pharmacol. 2016, 27, 497–505. [Google Scholar] [CrossRef]
- Marti, M.; Neri, M.; Bilel, S.; Di Paolo, M.; La Russa, R.; Ossato, A.; Turillazzi, E. MDMA alone affects sensorimotor and prepulse inhibition responses in mice and rats: Tips in the debate on potential MDMA unsafety in human activity. Forensic Toxicol. 2019, 37, 132–144. [Google Scholar] [CrossRef]
- European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). Annual Report 2008: Evolution of the Phenomenon in Europe; Publications Office of the European Union: Luxembourg, 2008. [Google Scholar]
- Psychonautwiki. 2019. Available online: https://psychonautwiki.org/wiki/NM-2-AI (accessed on 2 December 2022).
- United Nations Office on Drugs and Crime (UNODC). World Drug Report 2015; United Nations Publication; Sales No. E.15.XI.6; UNODC: Washington, DC, USA, 2015. [Google Scholar]
- Bluelight. 2013. Available online: https://bluelight.org/xf/threads/nm2ai-n-methyl-2-aminoindane.682634/ (accessed on 2 December 2022).
- Páleníček, T.; Lhotková, E.; Žídková, M.; Balíková, M.; Kuchař, M.; Himl, M.; Mikšátková, P.; Čegan, M.; Valeš, K.; Tylš, F.; et al. Emerging toxicity of 5,6-methylenedioxy-2-aminoindane (MDAI): Pharmacokinetics, behaviour, thermoregulation and LD50 in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 69, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Iversen, L.; Gibbons, S.; Treble, R.; Setola, V.; Huang, X.P.; Roth, B.L. Neurochemical profiles of some novel psychoactive substances. Eur. J. Pharmacol. 2013, 700, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Simmler, L.D.; Rickli, A.; Schramm, Y.; Hoener, M.C.; Liechti, M.E. Monoamine transporter and receptor interaction profiles of a new series of designer cathinones. Neuropharmacology 2014, 79, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Luethi, D.; Kolaczynska, K.E.; Docci, L.; Krähenbühl, S.; Hoener, M.C.; Liechti, M.E. Pharmacological profile of mephedrone analogs and related psychoactive substances. Neuropharmacology 2018, 134 Pt A, 4–12. [Google Scholar] [CrossRef]
- Luethi, D.; Liechti, M.E. Designer drugs: Mechanism of action and adverse effects. Arch. Toxicol. 2020, 94, 1085–1133. [Google Scholar] [CrossRef]
- Fuller, R.W.; Baker, J.C.; Molloy, B.B. Biological Disposition of Rigid Analogs of Amphetamine. J. Pharm. Sci. 1977, 66, 271–272. [Google Scholar] [CrossRef]
- Pinterova, N.; Horsley, R.R.; Palenicek, T. Synthetic Aminoindanes: A Summary of Existing Knowledge. Front. Psychiatry 2017, 17, 236. [Google Scholar] [CrossRef]
- McCorvy, J.D.; Roth, B.L. Structure and function of serotonin G protein-coupled receptors. Pharmacol. Ther. 2015, 150, 129–142. [Google Scholar] [CrossRef]
- Beleslin, D.; Varagic, V. The effect of cooling and of 5-hydroxytryptamine on the peristaltic reflex of the isolated guinea-pig ileum. Br. J. Pharmacol. Chemother. 1958, 13, 266–270. [Google Scholar] [CrossRef]
- Masson, J. Serotonin in retina. Biochimie 2019, 161, 51–55. [Google Scholar] [CrossRef]
- Trakhtenberg, E.F.; Pita-Thomas, W.; Fernandez, S.G.; Patel, K.H.; Venugopalan, P.; Shechter, J.M.; Morkin, M.I.; Galvao, J.; Liu, X.; Dombrowski, S.M.; et al. Serotonin receptor 2C regulates neurite growth and is necessary for normal retinal processing of visual information. Dev. Neurobiol. 2017, 77, 419–437. [Google Scholar] [CrossRef] [PubMed]
- De-Giorgio, F.; Bilel, S.; Ossato, A.; Tirri, M.; Arfè, R.; Foti, F.; Serpelloni, G.; Frisoni, P.; Neri, M.; Marti, M. Acute and repeated administration of MDPV increases aggressive behavior in mice: Forensic implications. Int. J. Leg. Med. 2019, 133, 1797–1808. [Google Scholar] [CrossRef] [PubMed]
- Canazza, I.; Ossato, A.; Trapella, C.; Seri, C.; Rimondo, C.; Serpelloni, G.; Marti, M. 2C-B and 25I-NBOMe impair sensorimotor functions in mice. In Proceedings of the Italian Society of Pharmacology “Addictive Disorders: From Neurobiology to Novel Therapeutic Strategies”, Palermo, Italy, 27–28 March 2015. [Google Scholar]
- Tirri, M.; Ponzoni, L.; Bilel, S.; Arfè, R.; Braida, D.; Sala, M.; Marti, M. Acute DOB and PMA Administration Impairs Motor and Sensorimotor Responses in Mice and Causes Hallucinogenic Effects in Adult Zebrafish. Brain Sci. 2020, 10, 586. [Google Scholar] [CrossRef] [PubMed]
- Tirri, M.; Bilel, S.; Arfè, R.; Corli, G.; Marchetti, B.; Bernardi, T.; Boccuto, F.; Serpelloni, G.; Botrè, F.; De-Giorgio, F.; et al. Effect of -NBOMe Compounds on Sensorimotor, Motor, and Prepulse Inhibition Responses in Mice in Comparison with the 2C Analogs and Lysergic Acid Diethylamide: From Preclinical Evidence to Forensic Implication in Driving Under the Influence of Drugs. Front. Psychiatry 2022, 13, 875722. [Google Scholar] [CrossRef] [PubMed]
- Ossato, A.; Bilel, S.; Gregori, A.; Talarico, A.; Trapella, C.; Gaudio, R.M.; De-Giorgio, F.; Tagliaro, F.; Neri, M.; Fattore, L.; et al. Neurological, sensorimotor and cardiorespiratory alterations induced by methoxetamine, ketamine and phencyclidine in mice. Neuropharmacology 2018, 141, 167–180. [Google Scholar] [CrossRef]
- Canal, C.E.; Morgan, D. Head-twitch response in rodents induced by the hallucinogen 2,5-dimethoxy-4-iodoamphetamine: A comprehensive history, a re-evaluation of mechanisms, and its utility as a model. Drug Test. Anal. 2012, 4, 556–576. [Google Scholar] [CrossRef]
- Gonzalez-Maeso, J.; Sealfon, S.C. Agonist-trafficking and hallucinogens. Curr. Med. Chem. 2009, 16, 1017–1027. [Google Scholar] [CrossRef]
- Gonzalez-Maeso, J.; Weisstaub, N.V.; Zhou, M.; Chan, P.; Ivic, L.; Ang, R.; Lira, A.; Bradley-Moore, M.; Ge, Y.; Zhou, Q.; et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron 2007, 53, 439–452. [Google Scholar] [CrossRef]
- Lambert, F.M.; Bras, H.; Cardoit, L.; Vinay, L.; Coulon, P.; Glover, J.C. Early postnatal maturation in vestibulospinal pathways involved in neck and forelimb motor control. Dev. Neurobiol. 2016, 76, 1061–1077. [Google Scholar] [CrossRef]
- Manier, S.K.; Felske, C.; Eckstein, N.; Meyer, M.R. The metabolic fate of two new psychoactive substances—2-aminoindane and N-methyl-2-aminoindane—studied In Vitro and In Vivo to support drug testing. Drug Test. Anal. 2020, 12, 145–151. [Google Scholar] [CrossRef]
- Wakita, R.; Tanabe, S.; Tabei, K.; Funaki, A.; Inoshita, T.; Hirano, T. Differential regulations of vestibulo-ocular reflex and optokinetic response by β- and α2-adrenergic receptors in the cerebellar flocculus. Int. J. Leg. Med. 2017, 134, 1695–1711. [Google Scholar] [CrossRef] [PubMed]
- Liechti, M.E. “Ecstasy” (MDMA): Pharmakologie, Toxikologie und Behandlung der akuten Intoxikation [“Ecstasy” (MDMA): Pharmacology, toxicology, and treatment of acute intoxication]. Dtsch. Med. Wochenschr. 2003, 128, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Liechti, M.E.; Kunz, I.; Kupferschmidt, H. Acute medical problems due to Ecstasy use. Case-series of emergency department visits. Swiss Med. Wkly. 2005, 135, 652–657. [Google Scholar] [PubMed]
- Simmler, L.D.; Hysek, C.M.; Liechti, M.E. Sex differences in the effects of MDMA (ecstasy) on plasma copeptin in healthy subjects. J. Clin. Endocrinol. Metab. 2011, 96, 2844–2850. [Google Scholar] [CrossRef]
- Simmler, L.D.; Rickli, A.; Schramm, Y.; Hoener, M.C.; Liechti, M.E. Pharmacological profiles of aminoindanes, piperazines, and pipradrol derivatives. Biochem. Pharmacol. 2014, 88, 237–244. [Google Scholar] [CrossRef]
- Dafters, R.I. Hyperthermia following MDMA administration in rats: Effects of ambient temperature, water consumption, and chronic dosing. Physiol. Behav. 1995, 58, 877–882. [Google Scholar] [CrossRef]
- Green, A.R.; O’Shea, E.; Saadat, K.S.; Elliott, J.M.; Colado, M.I. Studies on the effect of MDMA (‘ecstasy’) on the body temperature of rats housed at different ambient room temperatures. Br. J. Pharmacol. 2005, 146, 306–312. [Google Scholar] [CrossRef]
- Docherty, J.R.; Green, A.R. The role of monoamines in the changes in body temperature induced by 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) and its derivatives. Br. J. Pharmacol. 2010, 160, 1029–1044. [Google Scholar] [CrossRef]
- Elmore, J.S.; Baumann, M.H. Repeated Exposure to the “Spice” Cannabinoid JWH-018 Induces Tolerance and Enhances Responsiveness to 5-HT1A Receptor Stimulation in Male Rats. Front. Psychiatry 2018, 9, 55. [Google Scholar] [CrossRef]
- Yano, H.; Adhikari, P.; Naing, S.; Hoffman, A.F.; Baumann, M.H.; Lupica, C.R.; Shi, L. Positive Allosteric Modulation of the 5-HT1A Receptor by Indole-Based Synthetic Cannabinoids Abused by Humans. ACS Chem. Neurosci. 2020, 11, 1400–1405. [Google Scholar] [CrossRef]
- Clark, W.G. Changes in body temperature after administration of amino acids, peptides, dopamine, neuroleptics and related agents. Neurosci. Biobehav. Rev. 1979, 3, 179–231. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.G.; Lipton, J.M. Changes in body temperature after administration of amino acids, peptides, dopamine, neuroleptics and related agents: II. Neurosci. Biobehav. Rev. 1985, 9, 299–371. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.G.; Lipton, J.M. Changes in body temperature after administration of adrenergic and serotonergic agents and related drugs including antidepressant: II. Neurosci. Biobehav. Rev. 1986, 10, 153–220. [Google Scholar] [CrossRef] [PubMed]
- Levin, N.; Graham, B.E.; Kolloff, H.G. Physiologically Active Indanamines1. J. Org. Chem. 1944, 09, 380–391. [Google Scholar] [CrossRef]
- Solomons, E.; Sam, J. 2-Aminoindans of pharmacological interest. J. Med. Chem. 1973, 16, 1330–1333. [Google Scholar] [CrossRef] [PubMed]
- Erowid. 2020. Available online: https://www.erowid.org/experiences/exp_pdf.php?ID=49250&format=pdf (accessed on 2 December 2022).
- Marks, D.M.; Shah, M.J.; Patkar, A.A.; Masand, P.S.; Park, G.Y.; Pae, C.U. Serotonin-norepinephrine reuptake inhibitors for pain control: Premise and promise. Curr. Neuropharmacol. 2009, 7, 331–336. [Google Scholar] [CrossRef]
- Schifano, F.; Albanese, A.; Fergus, S.; Stair, J.L.; Deluca, P.; Corazza, O.; Davey, Z.; Corkery, J.; Siemann, H.; Scherbaum, N.; et al. Mephedrone (4-methylmethcathinone; ‘meow meow’): Chemical, pharmacological and clinical issues. Psychopharmacology 2011, 214, 593–602. [Google Scholar] [CrossRef]
- Baumann, M.H.; Ayestas, M.A., Jr.; Partilla, J.S.; Sink, J.R.; Shulgin, A.T.; Daley, P.F.; Brandt, S.D.; Rothman, R.B.; Ruoho, A.E.; Cozzi, N.V. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 2012, 37, 1192–1203. [Google Scholar] [CrossRef]
- Giannotti, G.; Canazza, I.; Caffino, L.; Bilel, S.; Ossato, A.; Fumagalli, F.; Marti, M. The Cathinones MDPV and α-PVP Elicit Different Behavioral and Molecular Effects Following Acute Exposure. Neurotox. Res. 2017, 32, 594–602. [Google Scholar] [CrossRef]
- Nevins, M.E.; Nash, S.A.; Beardsley, P.M. Quantitative grip strength assessment as a means of evaluating muscle relaxation in mice. Psychopharmacology 1993, 110, 92–96. [Google Scholar] [CrossRef]
- Elliott, S.; Evans, J. A 3-year review of new psychoactive substances in casework. Forensic Sci. Int. 2014, 243, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.G.; Perez, J.A.; Pease, J.P.; Long, J.P.; Flynn, J.R.; Rusterholz, D.B.; Dryer, S.E. Comparison of biological effects of N-alkylated congeners of beta-phenethylamine derived from 2-aminotetralin, 2-aminoindan, and 6-aminobenzocycloheptene. J. Med. Chem. 1980, 23, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef]
- Ossato, A.; Canazza, I.; Trapella, C.; Vincenzi, F.; De Luca, M.A.; Rimondo, C.; Varani, K.; Borea, P.A.; Serpelloni, G.; Marti, M. Effect of JWH-250, JWH-073 and their interaction on “tetrad”, sensorimotor, neurological and neurochemical responses in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 67, 31–50. [Google Scholar] [CrossRef] [PubMed]
- Ossato, A.; Vigolo, A.; Trapella, C.; Seri, C.; Rimondo, C.; Serpelloni, G.; Marti, M. JWH-018 impairs sensorimotor functions in mice. Neuroscience 2015, 300, 174–188. [Google Scholar] [CrossRef]
- Vigolo, A.; Ossato, A.; Trapella, C.; Vincenzi, F.; Rimondo, C.; Seri, C.; Varani, K.; Serpelloni, G.; Marti, M. Novel halogenated derivates of JWH-018: Behavioral and binding studies in mice. Neuropharmacology 2015, 95, 68–82. [Google Scholar] [CrossRef]
- Arfè, R.; Bilel, S.; Tirri, M.; Frisoni, P.; Serpelloni, G.; Neri, M.; Boccuto, F.; Bernardi, T.; Foti, F.; De-Giorgio, F.; et al. Comparison of N-methyl-2-pyrrolidone (NMP) and the “date rape” drug GHB: Behavioral toxicology in the mouse model. Psychopharmacology 2021, 238, 2275–2295. [Google Scholar] [CrossRef]
- Fantinati, A.; Ossato, A.; Bianco, S.; Canazza, I.; De-Giorgio, F.; Trapella, C.; Marti, M. 1-cyclohexyl-x-methoxybenzene derivatives, novel psychoactive substances seized on the internet market. Synthesis and in vivo pharmacological studies in mice. Hum. Psychopharmacol. 2017, 32, e2560. [Google Scholar] [CrossRef]
- Canazza, I.; Ossato, A.; Trapella, C.; Fantinati, A.; De Luca, M.A.; Margiani, G.; Vincenzi, F.; Rimondo, C.; Di Rosa, F.; Gregori, A.; et al. Effect of the novel synthetic cannabinoids AKB48 and 5F-AKB48 on “tetrad”, sensorimotor, neurological and neurochemical responses in mice. In Vitro and In Vivo pharmacological studies. Psychopharmacology 2016, 233, 3685–3709. [Google Scholar] [CrossRef]
- Bilel, S.; Azevedo, N.J.; Arfè, R.; Tirri, M.; Gregori, A.; Serpelloni, G.; DeGiorgio, F.; Frisoni, P.; Neri, M.; Calò, G.; et al. In Vitro and In vivo pharmacological characterization of the synthetic opioid MT-45. Neuropharmacology 2020, 171, 108110. [Google Scholar] [CrossRef]
- Tirri, M.; Arfè, R.; Bilel, S.; Corli, G.; Marchetti, B.; Fantinati, A.; Vincenzi, F.; De-Giorgio, F.; Camuto, C.; Mazzarino, M.; et al. In Vivo Bio-Activation of JWH-175 to JWH-018: Pharmacodynamic and Pharmacokinetic Studies in Mice. Int. J. Mol. Sci. 2022, 23, 8030. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tirri, M.; Corli, G.; Arfè, R.; Marchetti, B.; Bilel, S.; Bernardi, T.; Boccuto, F.; Odoardi, S.; Mestria, S.; Strano-Rossi, S.; et al. Behavioral and Pharmacokinetics Studies of N-Methyl-2-Aminoindane (NM2AI) in Mice: An Aminoindane Briefly Used in the Illicit Drug Market. Int. J. Mol. Sci. 2023, 24, 1882. https://doi.org/10.3390/ijms24031882
Tirri M, Corli G, Arfè R, Marchetti B, Bilel S, Bernardi T, Boccuto F, Odoardi S, Mestria S, Strano-Rossi S, et al. Behavioral and Pharmacokinetics Studies of N-Methyl-2-Aminoindane (NM2AI) in Mice: An Aminoindane Briefly Used in the Illicit Drug Market. International Journal of Molecular Sciences. 2023; 24(3):1882. https://doi.org/10.3390/ijms24031882
Chicago/Turabian StyleTirri, Micaela, Giorgia Corli, Raffaella Arfè, Beatrice Marchetti, Sabrine Bilel, Tatiana Bernardi, Federica Boccuto, Sara Odoardi, Serena Mestria, Sabina Strano-Rossi, and et al. 2023. "Behavioral and Pharmacokinetics Studies of N-Methyl-2-Aminoindane (NM2AI) in Mice: An Aminoindane Briefly Used in the Illicit Drug Market" International Journal of Molecular Sciences 24, no. 3: 1882. https://doi.org/10.3390/ijms24031882
APA StyleTirri, M., Corli, G., Arfè, R., Marchetti, B., Bilel, S., Bernardi, T., Boccuto, F., Odoardi, S., Mestria, S., Strano-Rossi, S., & Marti, M. (2023). Behavioral and Pharmacokinetics Studies of N-Methyl-2-Aminoindane (NM2AI) in Mice: An Aminoindane Briefly Used in the Illicit Drug Market. International Journal of Molecular Sciences, 24(3), 1882. https://doi.org/10.3390/ijms24031882







