Roles of Estrogen, Estrogen Receptors, and Estrogen-Related Receptors in Skeletal Muscle: Regulation of Mitochondrial Function
Abstract
1. Introduction
2. Estrogen Actions in Muscle
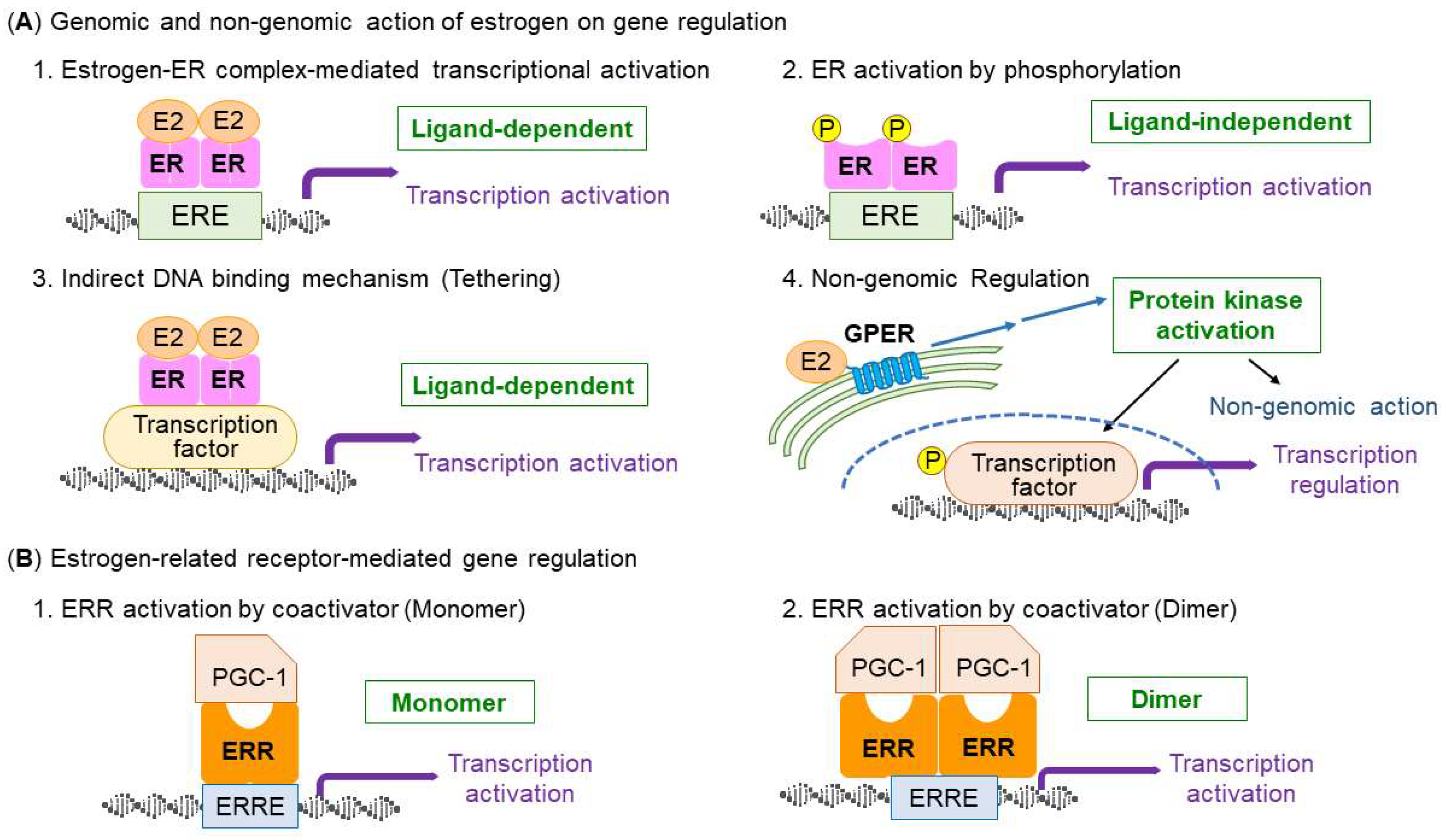
3. Types and Structure of Estrogen Receptors
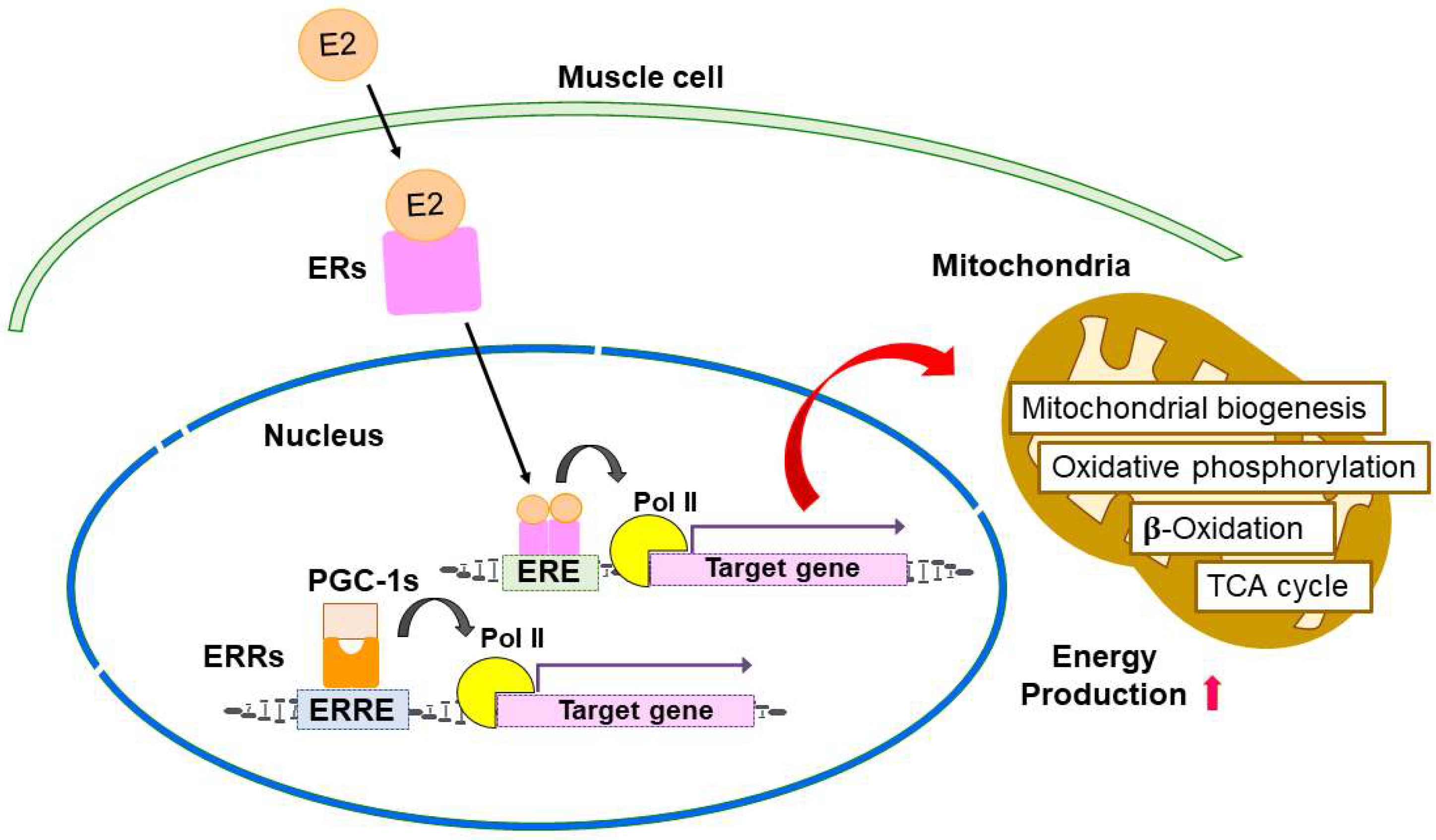
4. Estrogen-ER Signaling in the Regulation of Skeletal Muscle Function and Mitochondria
5. Similarities of Estrogen-Related Receptors with ERs
6. ERRs Signaling in the Regulation of Skeletal Muscle Function and Mitochondria
7. Potential Clinical Implications of ER- and ERR-Dependent Muscle Disease Models and Tissue Engineering
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.-L.; Huang, Z.-Y.; Yu, K.; Li, J.; Fu, X.-W.; Deng, S.-L. Estrogen Biosynthesis and Signal Transduction in Ovarian Disease. Front. Endocrinol. (Lausanne) 2022, 13, 827032. [Google Scholar] [CrossRef]
- Chou, C.-H.; Chen, M.-J. The Effect of Steroid Hormones on Ovarian Follicle Development. Vitam. Horm. 2018, 107, 155–175. [Google Scholar] [CrossRef]
- Noyola-Martínez, N.; Halhali, A.; Barrera, D. Steroid Hormones and Pregnancy. Gynecol. Endocrinol. 2019, 35, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Hilton, H.N.; Clarke, C.L.; Graham, J.D. Estrogen and Progesterone Signalling in the Normal Breast and Its Implications for Cancer Development. Mol. Cell Endocrinol. 2018, 466, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.; Herrera, G.; Anamthathmakula, P.; Rock, J.; Willie, A.; Harris, E.; Takemaru, K.-I.; Winuthayanon, W. Roles of Steroid Hormones in Oviductal Function. Reproduction 2020, 159, R125–R137. [Google Scholar] [CrossRef] [PubMed]
- de Paoli, M.; Zakharia, A.; Werstuck, G.H. The Role of Estrogen in Insulin Resistance: A Review of Clinical and Preclinical Data. Am. J. Pathol. 2021, 191, 1490–1498. [Google Scholar] [CrossRef]
- Meng, Q.; Li, Y.; Ji, T.; Chao, Y.; Li, J.; Fu, Y.; Wang, S.; Chen, Q.; Chen, W.; Huang, F.; et al. Estrogen Prevent Atherosclerosis by Attenuating Endothelial Cell Pyroptosis via Activation of Estrogen Receptor α-Mediated Autophagy. J. Adv. Res. 2021, 28, 149–164. [Google Scholar] [CrossRef]
- Geraci, A.; Calvani, R.; Ferri, E.; Marzetti, E.; Arosio, B.; Cesari, M. Sarcopenia and Menopause: The Role of Estradiol. Front. Endocrinol. (Lausanne) 2021, 12, 682012. [Google Scholar] [CrossRef]
- Sipilä, S.; Törmäkangas, T.; Sillanpää, E.; Aukee, P.; Kujala, U.M.; Kovanen, V.; Laakkonen, E.K. Muscle and Bone Mass in Middle-Aged Women: Role of Menopausal Status and Physical Activity. J. Cachexia Sarcopenia Muscle 2020, 11, 698–709. [Google Scholar] [CrossRef]
- Pellegrino, A.; Tiidus, P.M.; Vandenboom, R. Mechanisms of Estrogen Influence on Skeletal Muscle: Mass, Regeneration, and Mitochondrial Function. Sports Med. 2022, 52, 2853–2869. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef] [PubMed]
- Rathnayake, N.; Alwis, G.; Lenora, J.; Lekamwasam, S. Factors Associated with Measures of Sarcopenia in Pre and Postmenopausal Women. BMC Womens Health 2021, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Kiel, D.P.; Cooper, C.; et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J. Am. Geriatr. Soc. 2020, 68, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.K.; Rook, K.M.; Siddle, N.C.; Bruce, S.A.; Woledge, R.C. Muscle Weakness in Women Occurs at an Earlier Age than in Men, but Strength Is Preserved by Hormone Replacement Therapy. Clin. Sci. (Lond.) 1993, 84, 95–98. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.L.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Santilli, V.; Bernetti, A.; Mangone, M.; Paoloni, M. Clinical Definition of Sarcopenia. Clin. Cases Miner. Bone Metab. 2014, 11, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Dam, T.V.; Dalgaard, L.B.; Ringgaard, S.; Johansen, F.T.; Bisgaard Bengtsen, M.; Mose, M.; Lauritsen, K.M.; Ørtenblad, N.; Gravholt, C.H.; Hansen, M. Transdermal Estrogen Therapy Improves Gains in Skeletal Muscle Mass After 12 Weeks of Resistance Training in Early Postmenopausal Women. Front. Physiol. 2021, 11, 596130. [Google Scholar] [CrossRef]
- Javed, A.A.; Mayhew, A.J.; Shea, A.K.; Raina, P. Association Between Hormone Therapy and Muscle Mass in Postmenopausal Women: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2019, 2, e1910154. [Google Scholar] [CrossRef]
- Onambélé-Pearson, G.L.; Tomlinson, D.J.; Morse, C.I.; Degens, H. A Prolonged Hiatus in Postmenopausal HRT, Does Not Nullify the Therapy’s Positive Impact on Ageing Related Sarcopenia. PLoS ONE 2021, 16, e0250813. [Google Scholar] [CrossRef]
- Greising, S.M.; Baltgalvis, K.A.; Lowe, D.A.; Warren, G.L. Hormone Therapy and Skeletal Muscle Strength: A Meta-Analysis. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64A, 1071–1081. [Google Scholar] [CrossRef]
- Armeni, E.; Paschou, S.A.; Goulis, D.G.; Lambrinoudaki, I. Hormone Therapy Regimens for Managing the Menopause and Premature Ovarian Insufficiency. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101561. [Google Scholar] [CrossRef] [PubMed]
- Oxfeldt, M.; Dalgaard, L.B.; Jørgensen, A.A.; Hansen, M. Hormonal Contraceptive Use, Menstrual Dysfunctions, and Self-Reported Side Effects in Elite Athletes in Denmark. Int. J. Sports Physiol. Perform. 2020, 15, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Ekenros, L.; von Rosen, P.; Solli, G.S.; Sandbakk, Ø.; Holmberg, H.-C.; Hirschberg, A.L.; Fridén, C. Perceived Impact of the Menstrual Cycle and Hormonal Contraceptives on Physical Exercise and Performance in 1,086 Athletes from 57 Sports. Front. Physiol. 2022, 13, 954760. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, Y.M.; Nandu, T.S.; Kelleher, A.M.; Ramos, E.I.; Gadad, S.S.; Kraus, W.L. Genome-Wide Analysis and Functional Prediction of the Estrogen-Regulated Transcriptional Response in the Mouse Uterus. Biol. Reprod. 2020, 102, 327–338. [Google Scholar] [CrossRef]
- Greising, S.M.; Carey, R.S.; Blackford, J.E.; Dalton, L.E.; Kosir, A.M.; Lowe, D.A. Estradiol. Treatment, Physical Activity, and Muscle Function in Ovarian-Senescent Mice. Exp. Gerontol. 2011, 46, 685–693. [Google Scholar] [CrossRef]
- Nagai, S.; Ikeda, K.; Horie-Inoue, K.; Shiba, S.; Nagasawa, S.; Takeda, S.; Inoue, S. Estrogen Modulates Exercise Endurance along with Mitochondrial Uncoupling Protein 3 Downregulation in Skeletal Muscle of Female Mice. Biochem. Biophys. Res. Commun. 2016, 480, 758–764. [Google Scholar] [CrossRef]
- Kitajima, Y.; Ono, Y. Estrogens Maintain Skeletal Muscle and Satellite Cell Functions. J. Endocrinol. 2016, 229, 267–275. [Google Scholar] [CrossRef]
- Oydanich, M.; Babici, D.; Zhang, J.; Rynecki, N.; Vatner, D.E.; Vatner, S.F. Mechanisms of Sex Differences in Exercise Capacity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 316, R832–R838. [Google Scholar] [CrossRef]
- Chaiyasing, R.; Ishikawa, T.; Warita, K.; Hosaka, Y.Z. Absence of Estrogen Receptors Delays Myoregeneration and Leads to Intermuscular Adipogenesis in a Low Estrogen Status: Morphological Comparisons in Estrogen Receptor Alpha and Beta Knock out Mice. J. Vet. Med. Sci. 2021, 83, 1022–1030. [Google Scholar] [CrossRef]
- Christianto, A.; Baba, T.; Takahashi, F.; Inui, K.; Inoue, M.; Suyama, M.; Ono, Y.; Ohkawa, Y.; Morohashi, K.-I. Sex Differences in Metabolic Pathways Are Regulated by Pfkfb3 and Pdk4 Expression in Rodent Muscle. Commun. Biol. 2021, 4, 1264. [Google Scholar] [CrossRef]
- Green, H.J.; Fraser, I.G.; Ranney, D.A. Male and Female Differences in Enzyme Activities of Energy Metabolism in Vastus Lateralis Muscle. J. Neurol. Sci. 1984, 65, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Azuma, K.; Ikeda, K.; Inoue, S. Mechanisms Underlying the Regulation of Mitochondrial Respiratory Chain Complexes by Nuclear Steroid Receptors. Int. J. Mol. Sci. 2020, 21, 6683. [Google Scholar] [CrossRef]
- Tang, Z.-R.; Zhang, R.; Lian, Z.-X.; Deng, S.-L.; Yu, K. Estrogen-Receptor Expression and Function in Female Reproductive Disease. Cells 2019, 8, 1123. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, N.; Silveyra, P. Estrogen Receptor Signaling Mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef]
- Arao, Y.; Hamilton, K.J.; Lierz, S.L.; Korach, K.S. N-Terminal Transactivation Function, AF-1, of Estrogen Receptor Alpha Controls Obesity through Enhancement of Energy Expenditure. Mol. Metab. 2018, 18, 68–78. [Google Scholar] [CrossRef]
- Hewitt, S.C.; Korach, K.S. Estrogen Receptors: New Directions in the New Millennium. Endocr. Rev. 2018, 39, 664–675. [Google Scholar] [CrossRef]
- Weikum, E.R.; Liu, X.; Ortlund, E.A. The Nuclear Receptor Superfamily: A Structural Perspective. Protein Sci. 2018, 27, 1876–1892. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Junaid, M.; Li, C.-D.; Saleem, S.; Humayun, F.; Shamas, S.; Ali, S.S.; Babar, Z.; Wei, D.-Q. Dynamics Insights Into the Gain of Flexibility by Helix-12 in ESR1 as a Mechanism of Resistance to Drugs in Breast Cancer Cell Lines. Front. Mol. BioSci. 2019, 6, 159. [Google Scholar] [CrossRef] [PubMed]
- Arao, Y.; Korach, K.S. The Physiological Role of Estrogen Receptor Functional Domains. Essays Biochem. 2021, 65, 867–875. [Google Scholar] [CrossRef]
- Cagnet, S.; Ataca, D.; Sflomos, G.; Aouad, P.; Schuepbach-Mallepell, S.; Hugues, H.; Krust, A.; Ayyanan, A.; Scabia, V.; Brisken, C. Oestrogen Receptor α AF-1 and AF-2 Domains Have Cell Population-Specific Functions in the Mammary Epithelium. Nat. Commun. 2018, 9, 4723. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogenic Control of Mitochondrial Function. Redox Biol. 2020, 31, 101435. [Google Scholar] [CrossRef] [PubMed]
- Arao, Y.; Korach, K.S. Transactivation Function-1-Mediated Partial Agonist Activity of Selective Estrogen Receptor Modulator Requires Homo-Dimerization of the Estrogen Receptor α Ligand Binding Domain. Int. J. Mol. Sci. 2019, 20, 3718. [Google Scholar] [CrossRef] [PubMed]
- Prossnitz, E.R.; Barton, M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat. Rev. Endocrinol. 2011, 7, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Aryan, L.; Younessi, D.; Zargari, M.; Banerjee, S.; Agopian, J.; Rahman, S.; Borna, R.; Ruffenach, G.; Umar, S.; Eghbali, M. The Role of Estrogen Receptors in Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 4314. [Google Scholar] [CrossRef] [PubMed]
- Tutzauer, J.; Sjöström, M.; Bendahl, P.-O.; Rydén, L.; Fernö, M.; Leeb-Lundberg, L.M.F.; Alkner, S. Plasma Membrane Expression of G Protein-Coupled Estrogen Receptor (GPER)/G Protein-Coupled Receptor 30 (GPR30) Is Associated with Worse Outcome in Metachronous Contralateral Breast Cancer. PLoS ONE 2020, 15, e0231786. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Mauvais-Jarvis, F.; Prossnitz, E.R. Roles of G Protein-Coupled Estrogen Receptor GPER in Metabolic Regulation. J. Steroid Biochem. Mol. Biol. 2018, 176, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Paciuc, J. Hormone Therapy in Menopause. Adv. Exp. Med. Biol. 2020, 1242, 89–120. [Google Scholar] [CrossRef]
- Levin, V.A.; Jiang, X.; Kagan, R. Estrogen Therapy for Osteoporosis in the Modern Era. Osteoporos Int. 2018, 29, 1049–1055. [Google Scholar] [CrossRef]
- Zhou, Z.; Ribas, V.; Rajbhandari, P.; Drew, B.G.; Moore, T.M.; Fluitt, A.H.; Reddish, B.R.; Whitney, K.A.; Georgia, S.; Vergnes, L.; et al. Estrogen Receptor α Protects Pancreatic β-Cells from Apoptosis by Preserving Mitochondrial Function and Suppressing Endoplasmic Reticulum Stress. J. Biol. Chem. 2018, 293, 4735–4751. [Google Scholar] [CrossRef]
- Capllonch-Amer, G.; Lladó, I.; Proenza, A.M.; García-Palmer, F.J.; Gianotti, M. Opposite Effects of 17-β Estradiol. and Testosterone on Mitochondrial Biogenesis and Adiponectin Synthesis in White Adipocytes. J. Mol. Endocrinol. 2014, 52, 203–214. [Google Scholar] [CrossRef]
- Farhat, F.; Amérand, A.; Simon, B.; Guegueniat, N.; Moisan, C. Gender-Dependent Differences of Mitochondrial Function and Oxidative Stress in Rat Skeletal Muscle at Rest and after Exercise Training. Redox Rep. 2017, 22, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Ignacio, D.L.; Fortunato, R.S.; Silvestre, D.; Matta, L.; de Vansconcelos, A.L.; Carvalho, D.P.; Galina, A.; Werneck-de-Castro, J.P.; Cavalcanti-de-Albuquerque, J.P. Physical Exercise Improves Mitochondrial Function in Ovariectomized Rats. J. Endocrinol. 2022, 254, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.J.; Ryan, T.E.; Lin, C.-T.; Zeczycki, T.N.; Neufer, P.D. Impact of 17β-Estradiol. on Complex I Kinetics and H2O2 Production in Liver and Skeletal Muscle Mitochondria. J. Biol. Chem. 2018, 293, 16889–16898. [Google Scholar] [CrossRef]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [CrossRef]
- Ikeda, K.; Horie-Inoue, K.; Inoue, S. Functions of Estrogen and Estrogen Receptor Signaling on Skeletal Muscle. J. Steroid Biochem. Mol. Biol. 2019, 191, 105375. [Google Scholar] [CrossRef]
- Lin, C.; Yang, Q.; Guo, D.; Xie, J.; Yang, Y.-S.; Chaugule, S.; DeSouza, N.; Oh, W.-T.; Li, R.; Chen, Z.; et al. Impaired Mitochondrial Oxidative Metabolism in Skeletal Progenitor Cells Leads to Musculoskeletal Disintegration. Nat. Commun. 2022, 13, 6869. [Google Scholar] [CrossRef]
- Ribas, V.; Drew, B.G.; Zhou, Z.; Phun, J.; Kalajian, N.Y.; Soleymani, T.; Daraei, P.; Widjaja, K.; Wanagat, J.; de Aguiar Vallim, T.Q.; et al. Skeletal Muscle Action of Estrogen Receptor α Is Critical for the Maintenance of Mitochondrial Function and Metabolic Homeostasis in Females. Sci. Transl. Med. 2016, 8, 334ra54. [Google Scholar] [CrossRef] [PubMed]
- Ribas, V.; Nguyen, M.T.A.; Henstridge, D.C.; Nguyen, A.-K.; Beaven, S.W.; Watt, M.J.; Hevener, A.L. Impaired Oxidative Metabolism and Inflammation Are Associated with Insulin Resistance in ERalpha-Deficient Mice. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E304–E319. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.C.; Mader, T.L.; Cabelka, C.A.; Iñigo, M.R.; Spangenburg, E.E.; Lowe, D.A. Deletion of Estrogen Receptor α in Skeletal Muscle Results in Impaired Contractility in Female Mice. J. Appl. Physiol. (1985) 2018, 124, 980–992. [Google Scholar] [CrossRef]
- Cabelka, C.A.; Baumann, C.W.; Collins, B.C.; Nash, N.; Le, G.; Lindsay, A.; Spangenburg, E.E.; Lowe, D.A. Effects of Ovarian Hormones and Estrogen Receptor α on Physical Activity and Skeletal Muscle Fatigue in Female Mice. Exp. Gerontol. 2019, 115, 155–164. [Google Scholar] [CrossRef]
- Seko, D.; Fujita, R.; Kitajima, Y.; Nakamura, K.; Imai, Y.; Ono, Y. Estrogen Receptor β Controls Muscle Growth and Regeneration in Young Female Mice. Stem Cell Rep. 2020, 15, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Yoh, K.; Ikeda, K.; Nagai, S.; Horie, K.; Takeda, S.; Inoue, S. Constitutive Activation of Estrogen Receptor α Signaling in Muscle Prolongs Exercise Endurance in Mice. Biochem. Biophys. Res. Commun. 2022, 628, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Collins, B.C.; Arpke, R.W.; Larson, A.A.; Baumann, C.W.; Xie, N.; Cabelka, C.A.; Nash, N.L.; Juppi, H.-K.; Laakkonen, E.K.; Sipilä, S.; et al. Estrogen Regulates the Satellite Cell Compartment in Females. Cell Rep. 2019, 28, 368–381.e6. [Google Scholar] [CrossRef] [PubMed]
- Norton, A.; Thieu, K.; Baumann, C.W.; Lowe, D.A.; Mansky, K.C. Estrogen Regulation of Myokines That Enhance Osteoclast Differentiation and Activity. Sci. Rep. 2022, 12, 15900. [Google Scholar] [CrossRef]
- Ikeda, K.; Tsukui, T.; Horie-Inoue, K.; Inoue, S. Conditional Expression of Constitutively Active Estrogen Receptor α in Osteoblasts Increases Bone Mineral Density in Mice. FEBS Lett. 2011, 585, 1303–1309. [Google Scholar] [CrossRef]
- Ikeda, K.; Tsukui, T.; Imazawa, Y.; Horie-Inoue, K.; Inoue, S. Conditional Expression of Constitutively Active Estrogen Receptor α in Chondrocytes Impairs Longitudinal Bone Growth in Mice. Biochem. Biophys. Res. Commun. 2012, 425, 912–917. [Google Scholar] [CrossRef]
- Nagai, S.; Ikeda, K.; Horie-Inoue, K.; Takeda, S.; Inoue, S. Estrogen Signaling Increases Nuclear Receptor Subfamily 4 Group A Member 1 Expression and Energy Production in Skeletal Muscle Cells. Endocr. J. 2018, 65, 1209–1218. [Google Scholar] [CrossRef]
- Chao, L.C.; Wroblewski, K.; Ilkayeva, O.R.; Stevens, R.D.; Bain, J.; Meyer, G.A.; Schenk, S.; Martinez, L.; Vergnes, L.; Narkar, V.A.; et al. Skeletal Muscle Nur77 Expression Enhances Oxidative Metabolism and Substrate Utilization. J. Lipid Res. 2012, 53, 2610–2619. [Google Scholar] [CrossRef]
- Heuer, B. Mitochondrial DNA: Unraveling the “Other” Genome. J. Am. Assoc. Nurse Pract. 2021, 33, 673–675. [Google Scholar] [CrossRef]
- Morganti, C.; Bonora, M.; Marchi, S.; Ferroni, L.; Gardin, C.; Wieckowski, M.R.; Giorgi, C.; Pinton, P.; Zavan, B. Citrate Mediates Crosstalk between Mitochondria and the Nucleus to Promote Human Mesenchymal Stem Cell In Vitro Osteogenesis. Cells 2020, 9, 1034. [Google Scholar] [CrossRef]
- Ikeda, K.; Shiba, S.; Horie-Inoue, K.; Shimokata, K.; Inoue, S. A Stabilizing Factor for Mitochondrial Respiratory Supercomplex Assembly Regulates Energy Metabolism in Muscle. Nat. Commun. 2013, 4, 2147. [Google Scholar] [CrossRef] [PubMed]
- Shiba, S.; Ikeda, K.; Horie-Inoue, K.; Nakayama, A.; Tanaka, T.; Inoue, S. Deficiency of COX7RP, a Mitochondrial Supercomplex Assembly Promoting Factor, Lowers Blood Glucose Level in Mice. Sci. Rep. 2017, 7, 7606. [Google Scholar] [CrossRef]
- Giguère, V. Transcriptional Control of Energy Homeostasis by the Estrogen-Related Receptors. Endocr. Rev. 2008, 29, 677–696. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, A.M.; Wilson, B.J.; Yang, X.-J.; Giguère, V. Phosphorylation-Dependent Sumoylation Regulates Estrogen-Related Receptor-Alpha and -Gamma Transcriptional Activity through a Synergy Control Motif. Mol. Endocrinol. 2008, 22, 570–584. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.B.; Laganière, J.; Giguère, V. A Single Nucleotide in an Estrogen-Related Receptor Alpha Site Can Dictate Mode of Binding and Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1alpha Activation of Target Promoters. Mol. Endocrinol. 2006, 20, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Cui, H. Emerging Roles of Estrogen-Related Receptors in the Brain: Potential Interactions with Estrogen Signaling. Int. J. Mol. Sci. 2018, 19, 1091. [Google Scholar] [CrossRef]
- Vanacker, J.M.; Pettersson, K.; Gustafsson, J.A.; Laudet, V. Transcriptional Targets Shared by Estrogen Receptor- Related Receptors (ERRs) and Estrogen Receptor (ER) Alpha, but Not by ERbeta. EMBO J. 1999, 18, 4270–4279. [Google Scholar] [CrossRef] [PubMed]
- Vernier, M.; Giguère, V. Aging, Senescence and Mitochondria: The PGC-1/ERR Axis. J. Mol. Endocrinol. 2021, 66, R1–R14. [Google Scholar] [CrossRef]
- Rangwala, S.M.; Wang, X.; Calvo, J.A.; Lindsley, L.; Zhang, Y.; Deyneko, G.; Beaulieu, V.; Gao, J.; Turner, G.; Markovits, J. Estrogen-Related Receptor Gamma Is a Key Regulator of Muscle Mitochondrial Activity and Oxidative Capacity. J. Biol. Chem. 2010, 285, 22619–22629. [Google Scholar] [CrossRef]
- Narkar, V.A.; Fan, W.; Downes, M.; Yu, R.T.; Jonker, J.W.; Alaynick, W.A.; Banayo, E.; Karunasiri, M.S.; Lorca, S.; Evans, R.M. Exercise and PGC-1α-Independent Synchronization of Type I Muscle Metabolism and Vasculature by ERRγ. Cell Metab. 2011, 13, 283–293. [Google Scholar] [CrossRef]
- Gan, Z.; Rumsey, J.; Hazen, B.C.; Lai, L.; Leone, T.C.; Vega, R.B.; Xie, H.; Conley, K.E.; Auwerx, J.; Smith, S.R.; et al. Nuclear Receptor/MicroRNA Circuitry Links Muscle Fiber Type to Energy Metabolism. J. Clin. Investig. 2013, 123, 2564–2575. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.-C.; Dufour, C.R.; Tam, I.S.; B’chir, W.; Giguère, V. Estrogen-Related Receptor-α Coordinates Transcriptional Programs Essential for Exercise Tolerance and Muscle Fitness. Mol. Endocrinol. 2014, 28, 2060–2071. [Google Scholar] [CrossRef] [PubMed]
- Villena, J.A.; Hock, M.B.; Chang, W.Y.; Barcas, J.E.; Giguère, V.; Kralli, A. Orphan Nuclear Receptor Estrogen-Related Receptor Alpha Is Essential for Adaptive Thermogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- LaBarge, S.; McDonald, M.; Smith-Powell, L.; Auwerx, J.; Huss, J.M. Estrogen-Related Receptor-α (ERRα) Deficiency in Skeletal Muscle Impairs Regeneration in Response to Injury. FASEB J. 2014, 28, 1082–1097. [Google Scholar] [CrossRef]
- Sopariwala, D.H.; Rios, A.S.; Park, M.K.; Song, M.S.; Kumar, A.; Narkar, V.A. Estrogen-Related Receptor Alpha Is an AMPK-Regulated Factor That Promotes Ischemic Muscle Revascularization and Recovery in Diet-Induced Obese Mice. FASEB Bioadv. 2022, 4, 602–618. [Google Scholar] [CrossRef]
- Fan, W.; He, N.; Lin, C.S.; Wei, Z.; Hah, N.; Waizenegger, W.; He, M.-X.; Liddle, C.; Yu, R.T.; Atkins, A.R.; et al. ERRγ Promotes Angiogenesis, Mitochondrial Biogenesis, and Oxidative Remodeling in PGC1α/β-Deficient Muscle. Cell Rep. 2018, 22, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Matsuura, T.R.; Wan, S.; Ryba, D.M.; Kim, J.U.; Won, K.J.; Lai, L.; Petucci, C.; Petrenko, N.; Musunuru, K.; et al. A Critical Role for Estrogen-Related Receptor Signaling in Cardiac Maturation. Circ. Res. 2020, 126, 1685–1702. [Google Scholar] [CrossRef]
- Wang, T.; McDonald, C.; Petrenko, N.B.; Leblanc, M.; Wang, T.; Giguere, V.; Evans, R.M.; Patel, V.v.; Pei, L. Estrogen-Related Receptor α (ERRα) and ERRγ Are Essential Coordinators of Cardiac Metabolism and Function. Mol. Cell Biol. 2015, 35, 1281–1298. [Google Scholar] [CrossRef]
- Vernier, M.; Dufour, C.R.; McGuirk, S.; Scholtes, C.; Li, X.; Bourmeau, G.; Kuasne, H.; Park, M.; St-Pierre, J.; Audet-Walsh, E.; et al. Estrogen-Related Receptors Are Targetable ROS Sensors. Genes Dev. 2020, 34, 544–559. [Google Scholar] [CrossRef]
- Di, W.; Lv, J.; Jiang, S.; Lu, C.; Yang, Z.; Ma, Z.; Hu, W.; Yang, Y.; Xu, B. PGC-1: The Energetic Regulator in Cardiac Metabolism. Curr. Issues Mol. Biol. 2018, 28, 29–46. [Google Scholar] [CrossRef]
- Gill, J.F.; Santos, G.; Schnyder, S.; Handschin, C. PGC-1α Affects Aging-Related Changes in Muscle and Motor Function by Modulating Specific Exercise-Mediated Changes in Old Mice. Aging Cell 2018, 17, e12697. [Google Scholar] [CrossRef] [PubMed]
- Melser, S.; Lavie, J.; Bénard, G. Mitochondrial Degradation and Energy Metabolism. Biochim. Biophys. Acta 2015, 1853, 2812–2821. [Google Scholar] [CrossRef] [PubMed]
- Vainshtein, A.; Tryon, L.D.; Pauly, M.; Hood, D.A. Role of PGC-1α during Acute Exercise-Induced Autophagy and Mitophagy in Skeletal Muscle. Am. J. Physiol. Cell Physiol. 2015, 308, C710–C719. [Google Scholar] [CrossRef] [PubMed]
- Vainshtein, A.; Desjardins, E.M.; Armani, A.; Sandri, M.; Hood, D.A. PGC-1α Modulates Denervation-Induced Mitophagy in Skeletal Muscle. Skelet. Muscle 2015, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Leone, T.C.; Lehman, J.J.; Finck, B.N.; Schaeffer, P.J.; Wende, A.R.; Boudina, S.; Courtois, M.; Wozniak, D.F.; Sambandam, N.; Bernal-Mizrachi, C.; et al. PGC-1alpha Deficiency Causes Multi-System Energy Metabolic Derangements: Muscle Dysfunction, Abnormal Weight Control and Hepatic Steatosis. PLoS Biol. 2005, 3, e101. [Google Scholar] [CrossRef] [PubMed]
- Tiidus, P.M.; Deller, M.; Liu, X.L. Oestrogen Influence on Myogenic Satellite Cells Following Downhill Running in Male Rats: A Preliminary Study. Acta Physiol. Scand. 2005, 184, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Enns, D.L.; Tiidus, P.M. Estrogen Influences Satellite Cell Activation and Proliferation Following Downhill Running in Rats. J. Appl. Physiol. 2008, 104, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Velders, M.; Schleipen, B.; Fritzemeier, K.H.; Zierau, O.; Diel, P. Selective Estrogen Receptor-β Activation Stimulates Skeletal Muscle Growth and Regeneration. FASEB J. 2012, 26, 1909–1920. [Google Scholar] [CrossRef]
- Knewtson, K.E.; Ohl, N.R.; Robinson, J.L. Estrogen Signaling Dictates Musculoskeletal Stem Cell Behavior: Sex Differences in Tissue Repair. Tissue Eng. Part. B Rev. 2022, 28, 789–812. [Google Scholar] [CrossRef]
- Feng, C.; Hu, J.; Liu, C.; Liu, S.; Liao, G.; Song, L.; Zeng, X. Association of 17-β Estradiol with Adipose-Derived Stem Cells: New Strategy to Produce Functional Myogenic Differentiated Cells with a Nano-Scaffold for Tissue Engineering. PLoS ONE 2016, 11, e0164918. [Google Scholar] [CrossRef]
- Murray, J.; Huss, J.M. Estrogen-Related Receptor α Regulates Skeletal Myocyte Differentiation via Modulation of the ERK MAP Kinase Pathway. Am. J. Physiol. Cell Physiol. 2011, 301, C630–C645. [Google Scholar] [CrossRef] [PubMed]
- Miki, K.; Deguchi, K.; Nakanishi-Koakutsu, M.; Lucena-Cacace, A.; Kondo, S.; Fujiwara, Y.; Hatani, T.; Sasaki, M.; Naka, Y.; Okubo, C.; et al. ERRγ Enhances Cardiac Maturation with T-Tubule Formation in Human IPSC-Derived Cardiomyocytes. Nat. Commun. 2021, 12, 3596. [Google Scholar] [CrossRef] [PubMed]
| Mouse | Experimental Condition | Phenotype | Other Phenotypes in Muscle | Reference |
|---|---|---|---|---|
| Ovarian-senesce by chemical, 4-vinylcyclohexene diepoxide (VCD) treatment followed by estrogen treatment for 8 weeks | In vitro muscle contractility test | Estrogen replacement increases muscle strength compared with no estrogen treatment mice. | No difference in soleus muscle size | [25] |
| OVX followed by estrogen administration for 10 weeks | Treadmill endurance test | Endurance is increased by estrogen administration. | Mitochondrial uncoupling protein 3 (UCP3) is upregulated by ovariectomy and downregulated by estrogen administration. | [26] |
| OVX for 24 weeks | Grip strength test | Grip force is decreased by OVX. | Increase in the proportion of fast twitch type fibers in the tibialis anterior muscle. This fiber-type shift was recovered by estradiol. Satellite cells were impaired in OVX mice. | [27] |
| OVX followed by estrogen administration for 2 weeks | Treadmill endurance test | Endurance is increased by estrogen administration. | Nitric oxide synthase activity is increased in females compared with males. | [29] |
| Mouse * | Experimental Condition | Phenotype | Other Phenotypes in Muscle | Reference |
|---|---|---|---|---|
| Muscle-specific knockout of ERα (MERKO) | In vitro muscular force and endurance test | Single muscle fibers from MERKO mice fatigued faster than fibers from control muscle. | Reduced oxygen consumption rates, excessive production of reactive oxygen species in mitochondria, and morphological abnormalities of mitochondria, indicating an impairment of fission-fusion dynamics. Reduction in mitophagy. | [57] |
| Muscle-specific knockout of ERα (skmERαKO) | In vitro muscle contractile test | Greater fatigability and impaired recovery from fatigue in muscles from skmERαKO mice | Phosphorylation of myosin regulatory light chain (RLC) was decreased in muscles from skmERαKO compared with WT mice. | [59] |
| Muscle specific estrogen receptor α knockout mice (skmERαKO) | Ex vivo or in vivo testing of muscle contractility | Smaller force and fatigability of soleus muscles. Less torque in in vivo plantar flexor muscle contractility. | [60] | |
| Muscle-specific ERβ-knockout (mERβKO) | Grip strength test | The absolute mean maximum strength was slightly decreased only in female KO mice compared with control mice. | Fast-type dominant muscle mass decreases in young female KO mice. There was no difference in running performance. | [61] |
| Muscle-specific constitutively active ERα transgenic (Mck-caERα) | Treadmill endurance test | Increased endurance | Genes related to lipid metabolism, insulin signaling, and growth factor signaling were upregulated. | [62] |
| Mouse * | Experimental Condition | Phenotype | Other Phenotypes in Muscle | Reference |
|---|---|---|---|---|
| Muscle-specific ERRγ and VP16ERRγ transgenic [ERRγ (N-TG) and VP16ERRγ (TG)] | Treadmill endurance test | Increased endurance | Decrease in muscle weight of glycolytic and mixed fiber muscles, increase in numbers of large mitochondria, and improved oxidative capacity and mitochondrial enzymatic function. | [79] |
| Heterozygotes knockout of ERRγ (HET) | Treadmill endurance test | Decreased endurance | Impaired mitochondrial oxidative metabolism. | [79] |
| Muscle-specific ERRγ transgenic (ERRGO) | Treadmill endurance test | Increased endurance | Increase in mitochondrial respiration, type I fiber specification, and vascularization. | [80] |
| Muscle–specific ERRγ/ERRβdouble knockout (ERRβ/γ dmKO) | Treadmill endurance test | Decreased endurance | miRNAs (miR-499 and miR-208b) and type I fiber-related genes were reduced, suggesting that ERRs are required for type I fiber formation. | [81] |
| ERRα knockout (ERRα KO) | Treadmill endurance test | Decreased endurance | Decreased muscle mass and reduced expression of many genes involved in mitochondrial oxidative metabolism | [82] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoh, K.; Ikeda, K.; Horie, K.; Inoue, S. Roles of Estrogen, Estrogen Receptors, and Estrogen-Related Receptors in Skeletal Muscle: Regulation of Mitochondrial Function. Int. J. Mol. Sci. 2023, 24, 1853. https://doi.org/10.3390/ijms24031853
Yoh K, Ikeda K, Horie K, Inoue S. Roles of Estrogen, Estrogen Receptors, and Estrogen-Related Receptors in Skeletal Muscle: Regulation of Mitochondrial Function. International Journal of Molecular Sciences. 2023; 24(3):1853. https://doi.org/10.3390/ijms24031853
Chicago/Turabian StyleYoh, Kenta, Kazuhiro Ikeda, Kuniko Horie, and Satoshi Inoue. 2023. "Roles of Estrogen, Estrogen Receptors, and Estrogen-Related Receptors in Skeletal Muscle: Regulation of Mitochondrial Function" International Journal of Molecular Sciences 24, no. 3: 1853. https://doi.org/10.3390/ijms24031853
APA StyleYoh, K., Ikeda, K., Horie, K., & Inoue, S. (2023). Roles of Estrogen, Estrogen Receptors, and Estrogen-Related Receptors in Skeletal Muscle: Regulation of Mitochondrial Function. International Journal of Molecular Sciences, 24(3), 1853. https://doi.org/10.3390/ijms24031853









