How Can Imbalance in Oral Microbiota and Immune Response Lead to Dental Implant Problems?
Abstract
:1. Introduction
2. Materials and Methods
Search Strategy, Study Selection, and Data Extraction
3. Diversity of Oral Microbiota and Its Interaction with the Immune System
4. Complications in Dental Implantology: Understanding the Interplay between the Immune System and Oral Microbiota Dysbiosis
4.1. Types of Treatments and Composition of Implants Used in Dentistry
- Enhanced osseointegration: To promote a faster and stronger bond between the implant and the jawbone, increasing implant stability and durability.
- Reduced healing time: Modifications can speed up the healing process, allowing for faster recovery and a shorter time to final reconstruction.
- Infection prevention: Modifications to incorporate antimicrobial properties are intended to reduce the risk of infections that may lead to implant failure.
- Improved biocompatibility: Surface modifications can increase the acceptance of the implant by surrounding tissues, reducing the risk of rejection.
- Improved aesthetics: Some modifications are intended to improve the appearance of implants, especially in visible areas of the mouth.
- Personalization: Customizing the implant to better fit the patient’s anatomy and specific clinical needs.
4.2. The Critical Role of the Immune System in the Osseointegration Process of Dental Implants
- Promotion of healing: After implantation, the immune system facilitates the healing of wounds at the site of implantation.
- Prevention of infections: Helps prevent potential infections that could interfere with the osseointegration process.
- Regulation of inflammation: Controlled inflammation is essential for osseointegration, but excessive inflammation can lead to implant failure.
- Supporting bone remodeling: Immune cells such as macrophages and osteoclasts are involved in bone remodeling, which is necessary for the integration of the implant with existing bone.
- The immune system plays a crucial role in maintaining homeostasis by regulating the balance between bone formation and resorption, a fundamental factor for implant stability. Numerous immune cells are engaged in these processes, including macrophages, neutrophils, osteoclasts, osteoblasts, dendritic cells, as well as T and B lymphocytes (Figure 8).
4.3. Types of Complications Observed in Dental Implantology and Etiology of Dental Failures
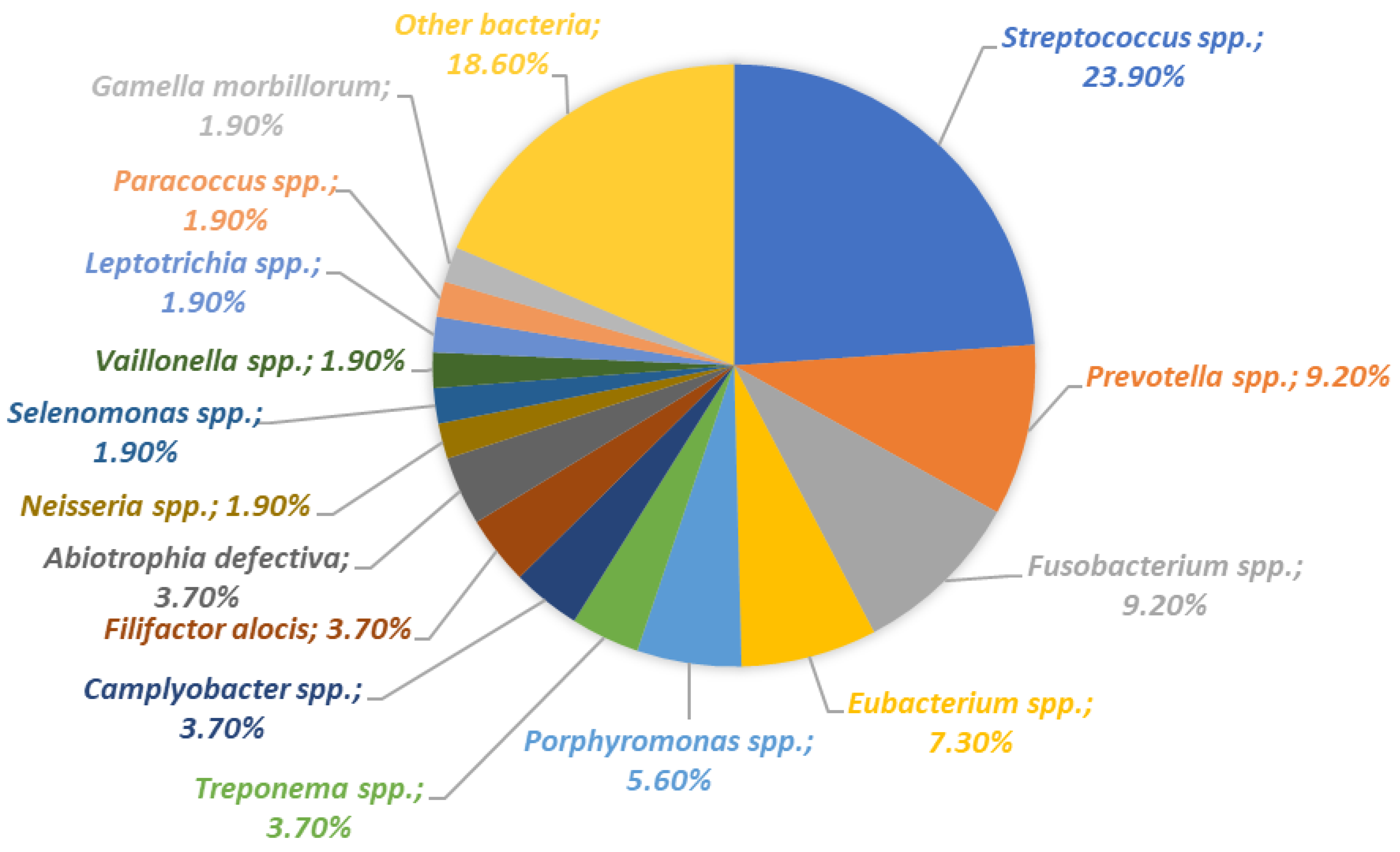
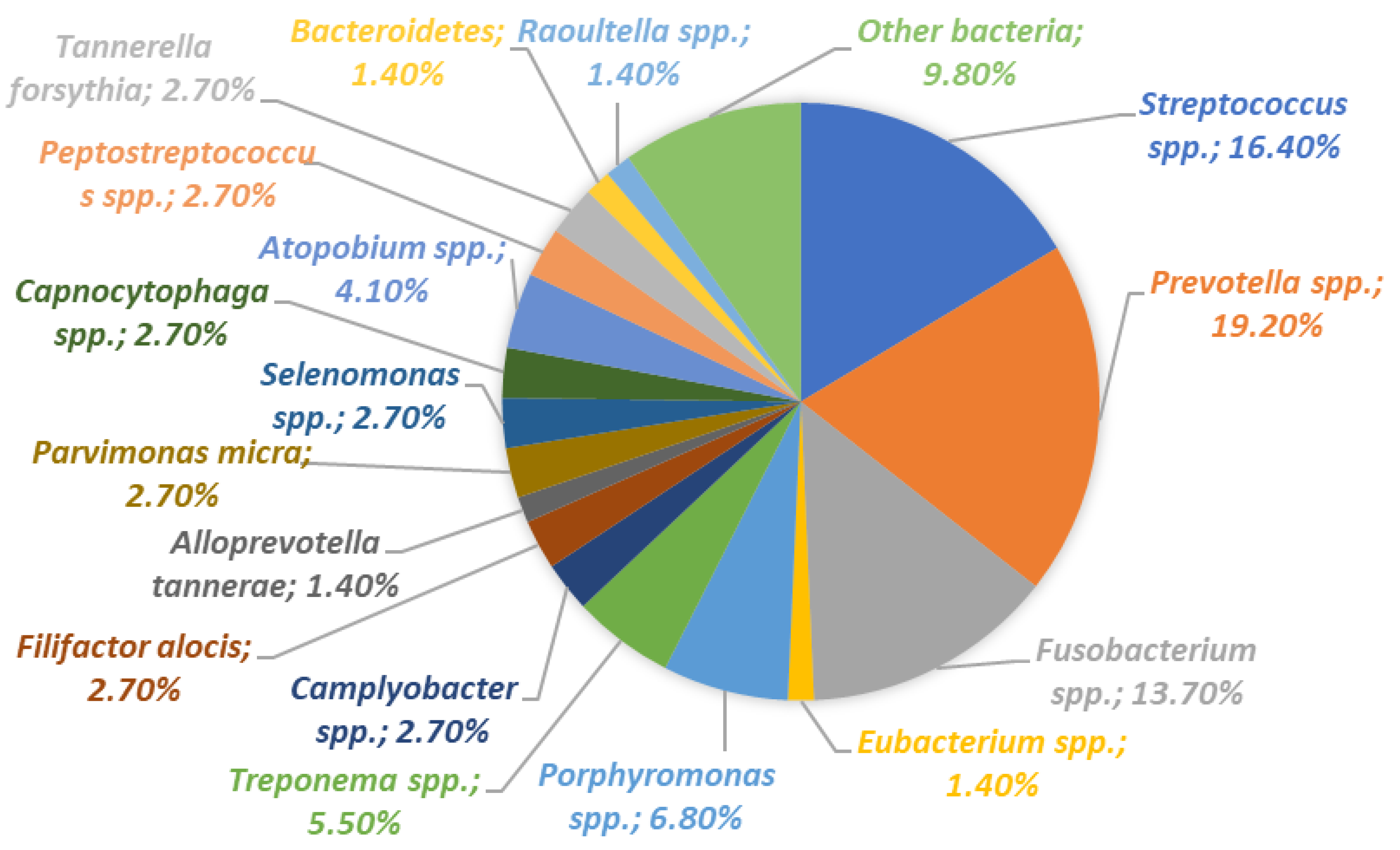
4.4. The Role of Oral Microbiota and the Immune System in the Course of Peri-Implantitis
5. Methods of Preventing Failures in Dental Implantology
5.1. Enhancing Dental Implant Safety: Strategies for Minimizing Bacterial Adhesion and Infection Risk through Material Modifications
5.2. Use of Probiotic Therapy
5.3. The Importance of Selected Nutrients in the Osseointegration Process
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thakkar, R. DDS Trends in Dental Implants 2022. Available online: https://connect.aaid-implant.org/blog/trends-in-dental-implants-2022 (accessed on 11 November 2023).
- Thiebot, N.; Hamdani, A.; Blanchet, F.; Dame, M.; Tawfik, S.; Mbapou, E.; Kaddouh, A.A.; Alantar, A. Implant Failure Rate and the Prevalence of Associated Risk Factors: A 6-Year Retrospective Observational Survey. J. Oral Med. Oral. Surg. 2022, 28, 19. [Google Scholar] [CrossRef]
- French, D.; Ofec, R.; Levin, L. Long Term Clinical Performance of 10,871 Dental Implants with up to 22 Years of Follow-up: A Cohort Study in 4247 Patients. Clin. Implant Dent. Relat. Res. 2021, 23, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Insua, A.; Monje, A.; Wang, H.-L.; Miron, R.J. Basis of Bone Metabolism around Dental Implants during Osseointegration and Peri-Implant Bone Loss. J. Biomed. Mater. Res. Part A 2017, 105, 2075–2089. [Google Scholar] [CrossRef] [PubMed]
- Baseri, M.; Radmand, F.; Hamedi, R.; Yousefi, M.; Kafil, H.S. Immunological Aspects of Dental Implant Rejection. BioMed Res. Int. 2020, 2020, 7279509. [Google Scholar] [CrossRef] [PubMed]
- Esimekara, J.-F.O.; Perez, A.; Courvoisier, D.S.; Scolozzi, P. Dental Implants in Patients Suffering from Autoimmune Diseases: A Systematic Critical Review. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e464–e473. [Google Scholar] [CrossRef] [PubMed]
- Radaelli, K.; Alberti, A.; Corbella, S.; Francetti, L. The Impact of Periimplantitis on Systemic Diseases and Conditions: A Review of the Literature. Int. J. Dent. 2021, 2021, 5536566. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Orlandi, M.; Suvan, J.; Harden, S.; Smith, J.; D’Aiuto, F. Association between Periimplantitis and Systemic Inflammation: A Systematic Review. Front. Immunol. 2023, 14, 1235155. [Google Scholar] [CrossRef]
- Xian, P.; Xuedong, Z.; Xin, X.; Yuqing, L.; Yan, L.; Jiyao, L.; Xiaoquan, S.; Shi, H.; Jian, X.; Ga, L. The Oral Microbiome Bank of China. Int. J. Oral Sci. 2018, 10, 16. [Google Scholar] [CrossRef]
- Vernon, J.J.; Raïf, E.M.; Aw, J.; Attenborough, E.; Jha, A.; Do, T. Dental Implant Surfaces and Their Interaction with the Oral Microbiome. Dent. Rev. 2022, 2, 100060. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Pellegrini, M.; Gallo, S.; Zampetti, P.; Scribante, A. Oral Microbiota in Patients with Peri-Implant Disease: A Narrative Review. Appl. Sci. 2022, 12, 3250. [Google Scholar] [CrossRef]
- Shahabouee, M.; Rismanchian, M.; Yaghini, J.; Babashahi, A.; Badrian, H.; Goroohi, H. Microflora around Teeth and Dental Implants. Dent. Res. J. 2012, 9, 215–220. [Google Scholar]
- Listl, S.; Fischer, L.; Giannakopoulos, N. An Economic Evaluation of Maxillary Implant Overdentures Based on Six vs. Four Implants. BMC Oral Health 2014, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Muzafferiy, F.; Anderson, A.C.; Wölber, J.P.; Ratka-Krüger, P.; Fretwurst, T.; Nelson, K.; Vach, K.; Hellwig, E. Shift of Microbial Composition of Peri-Implantitis-Associated Oral Biofilm as Revealed by 16S rRNA Gene Cloning. J. Med. Microbiol. 2018, 67, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.L.; Mark Welch, J.L.; Kauffman, K.M.; McLean, J.S.; He, X. The Oral Microbiome: Diversity, Biogeography and Human Health. Nat. Rev. Microbiol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.; Nagao, J.; Imayoshi, R.; Tanaka, Y. Importance of Diversity in the Oral Microbiota Including Candida Species Revealed by High-Throughput Technologies. Int. J. Dent. 2014, 2014, 454391. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between Microbiota and Immunity in Health and Disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Liu, Y.; Qv, W.; Ma, Y.; Zhang, Y.; Ding, C.; Chu, M.; Chen, F. The Interplay between Oral Microbes and Immune Responses. Front. Microbiol. 2022, 13, 1009018. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Wicherska-Pawłowska, K.; Wróbel, T.; Rybka, J. Toll-Like Receptors (TLRs), NOD-Like Receptors (NLRs), and RIG-I-Like Receptors (RLRs) in Innate Immunity. TLRs, NLRs, and RLRs Ligands as Immunotherapeutic Agents for Hematopoietic Diseases. Int. J. Mol. Sci. 2021, 22, 13397. [Google Scholar] [CrossRef]
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef] [PubMed]
- Sameer, A.S.; Nissar, S. Toll-Like Receptors (TLRs): Structure, Functions, Signaling, and Role of Their Polymorphisms in Colorectal Cancer Susceptibility. BioMed Res. Int. 2021, 2021, 1157023. [Google Scholar] [CrossRef] [PubMed]
- Le Noci, V.; Bernardo, G.; Bianchi, F.; Tagliabue, E.; Sommariva, M.; Sfondrini, L. Toll Like Receptors as Sensors of the Tumor Microbial Dysbiosis: Implications in Cancer Progression. Front. Cell Dev. Biol. 2021, 9, 732192. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Deng, D.; Buskermolen, J.K.; Roffel, S.; Janus, M.M.; Krom, B.P.; Crielaard, W.; Gibbs, S. Commensal and Pathogenic Biofilms Alter Toll-Like Receptor Signaling in Reconstructed Human Gingiva. Front. Cell Infect. Microbiol. 2019, 9, 282. [Google Scholar] [CrossRef]
- Lu, Q.; Ding, H.; Li, W. Role of Toll-like Receptors in Microbiota-Associated Gastrointestinal Cancer Metastasis. J. Cancer Res. Ther. 2013, 9 (Suppl. S7), S142–S149. [Google Scholar] [CrossRef]
- McClure, R.; Massari, P. TLR-Dependent Human Mucosal Epithelial Cell Responses to Microbial Pathogens. Front. Immunol. 2014, 5, 386. [Google Scholar] [CrossRef]
- Vestby, L.K.; Grønseth, T.; Simm, R.; Nesse, L.L. Bacterial Biofilm and Its Role in the Pathogenesis of Disease. Antibiotics 2020, 9, 59. [Google Scholar] [CrossRef]
- Kozak, M.; Pawlik, A. The Role of the Oral Microbiome in the Development of Diseases. Int. J. Mol. Sci. 2023, 24, 5231. [Google Scholar] [CrossRef]
- Kilian, M.; Chapple, I.L.C.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The Oral Microbiome—An Update for Oral Healthcare Professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef]
- Huang, R.; Li, M.; Gregory, R.L. Bacterial Interactions in Dental Biofilm. Virulence 2011, 2, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Radaic, A.; Kapila, Y.L. The Oralome and Its Dysbiosis: New Insights into Oral Microbiome-Host Interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Dental Plaque as a Biofilm and a Microbial Community—Implications for Health and Disease. BMC Oral Health 2006, 6, S14. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, M.; Costa, R.C.; Barão, V.A.R.; Villar, C.C.; Retamal-Valdes, B.; Feres, M.; Silva Souza, J.G. Oral Microorganisms and Biofilms: New Insights to Defeat the Main Etiologic Factor of Oral Diseases. Microorganisms 2022, 10, 2413. [Google Scholar] [CrossRef] [PubMed]
- Dhir, S. Biofilm and Dental Implant: The Microbial Link. J. Indian Soc. Periodontol. 2013, 17, 5–11. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The Oral Microbiota: Dynamic Communities and Host Interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for Combating Bacterial Biofilms: A Focus on Anti-Biofilm Agents and Their Mechanisms of Action. Virulence 2017, 9, 522–554. [Google Scholar] [CrossRef]
- Luo, Y.; Yang, Q.; Zhang, D.; Yan, W. Mechanisms and Control Strategies of Antibiotic Resistance in Pathological Biofilms. J. Microbiol. Biotechnol. 2021, 31, 1–7. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus Biofilm: An Emerging Battleground in Microbial Communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef]
- Camilleri, J.; Moliz, T.A.; Bettencourt, A.; Costa, J.; Martins, F.; Rabadijeva, D.; Rodriguez, D.; Visai, L.; Combes, C.; Farrugia, C.; et al. Standardization of antimicrobial testing of dental devices. Dent. Mater. 2020, 36, e59–e73. [Google Scholar] [CrossRef]
- Wawrzyk, A.; Rahnama, M.; Sofińska-Chmiel, W.; Wilczyński, S.; Gutarowska, B.; Konka, A.; Zeljas, D.; Łobacz, M. Analysis of the Microbiome on the Surface of Corroded Titanium Dental Implants in Patients with Periimplantitis and Diode Laser Irradiation as an Aid in the Implant Prosthetic Treatment: An Ex Vivo Study. Materials 2022, 15, 5890. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyk, A.; Łobacz, M.; Adamczuk, A.; Sofińska-Chmiel, W.; Rahnama, M. The Efficacy of a Diode Laser on Titanium Implants for the Reduction of Microorganisms That Cause Periimplantitis. Materials 2021, 14, 7215. [Google Scholar] [CrossRef]
- Rösing, C.K.; Fiorini, T.; Haas, A.N.; Muniz, F.W.M.G.; Oppermann, R.V.; Susin, C. The impact of maintenance on peri-implant health. Braz. Oral Res. 2019, 33 (Suppl. S1), e074. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, X.; Yu, C.; Wang, Y. Promising Therapeutic Strategies against Microbial Biofilm Challenges. Front. Cell Infect. Microbiol. 2020, 10, 359. [Google Scholar] [CrossRef] [PubMed]
- Cangui-Panchi, S.P.; Ñacato-Toapanta, A.L.; Enríquez-Martínez, L.J.; Salinas-Delgado, G.A.; Reyes, J.; Garzon-Chavez, D.; Machado, A. Battle Royale: Immune Response on Biofilms—Host-Pathogen Interactions. Curr. Res. Immunol. 2023, 4, 100057. [Google Scholar] [CrossRef] [PubMed]
- Ptasiewicz, M.; Grywalska, E.; Mertowska, P.; Korona-Głowniak, I.; Poniewierska-Baran, A.; Niedźwiedzka-Rystwej, P.; Chałas, R. Armed to the Teeth—The Oral Mucosa Immunity System and Microbiota. Int. J. Mol. Sci. 2022, 23, 882. [Google Scholar] [CrossRef] [PubMed]
- Perry, E.K.; Tan, M.-W. Bacterial Biofilms in the Human Body: Prevalence and Impacts on Health and Disease. Front. Cell. Infect. Microbiol. 2023, 13, 1237164. [Google Scholar] [CrossRef] [PubMed]
- Raafat, D.; Otto, M.; Reppschläger, K.; Iqbal, J.; Holtfreter, S. Fighting Staphylococcus Aureus Biofilms with Monoclonal Antibodies. Trends Microbiol. 2019, 27, 303–322. [Google Scholar] [CrossRef]
- Moser, C.; Jensen, P.Ø.; Thomsen, K.; Kolpen, M.; Rybtke, M.; Lauland, A.S.; Trøstrup, H.; Tolker-Nielsen, T. Immune Responses to Pseudomonas Aeruginosa Biofilm Infections. Front. Immunol. 2021, 12, 625597. [Google Scholar] [CrossRef]
- Moser, C.; Pedersen, H.T.; Lerche, C.J.; Kolpen, M.; Line, L.; Thomsen, K.; Høiby, N.; Jensen, P.Ø. Biofilms and Host Response—Helpful or Harmful. APMIS 2017, 125, 320–338. [Google Scholar] [CrossRef]
- Forner, L.; Nielsen, C.H.; Bendtzen, K.; Larsen, T.; Holmstrup, P. Increased Plasma Levels of IL-6 in Bacteremic Periodontis Patients after Scaling. J. Clin. Periodontol. 2006, 33, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Molina, A.; Buhlin, K.; Klinge, B. Periodontal Diseases and Association with Atherosclerotic Disease. Periodontology 2000 2020, 83, 66–89. [Google Scholar] [CrossRef] [PubMed]
- Ruff, W.E.; Greiling, T.M.; Kriegel, M.A. Host–Microbiota Interactions in Immune-Mediated Diseases. Nat. Rev. Microbiol. 2020, 18, 521–538. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Lo Giudice, A.; Polizzi, A.; Alibrandi, A.; Murabito, P.; Indelicato, F. Identification of the Different Salivary Interleukin-6 Profiles in Patients with Periodontitis: A Cross-Sectional Study. Arch. Oral Biol. 2021, 122, 104997. [Google Scholar] [CrossRef] [PubMed]
- Mattera, M.S.d.L.C.; Chiba, F.Y.; Lopes, F.L.; Tsosura, T.V.S.; Peres, M.A.; Brito, V.G.B.; de Oliveira, S.H.P.; Pereira, R.F.; Marani, F.; dos Santos, R.M.; et al. Effect of Maternal Periodontitis on GLUT4 and Inflammatory Pathway in Adult Offspring. J. Periodontol. 2019, 90, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Ren, Z.; Ang, L.; Huan, Z.; Jiang, J.; Xu, S.; Luo, Q.; Zhou, K.; Sun, X.; Zheng, S.; et al. Deep Sequencing Reveals Microbiota Dysbiosis of Tongue Coat in Patients with Liver Carcinoma. Sci. Rep. 2016, 6, 33142. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.W. Commentary: Oral Bacteria as Drivers for Colorectal Cancer. J. Periodontol. 2014, 85, 1155–1157. [Google Scholar] [CrossRef]
- Chang, J.S.; Tsai, C.-R.; Chen, L.-T.; Shan, Y.-S. Investigating the Association between Periodontal Disease and Risk of Pancreatic Cancer. Pancreas 2016, 45, 134–141. [Google Scholar] [CrossRef]
- Yusuf, E.; Wybo, I.; Piérard, D. Case Series of Patients with Fusobacterium Nucleatum Bacteremia with Emphasis on the Presence of Cancer. Anaerobe 2016, 39, 1–3. [Google Scholar] [CrossRef]
- García-García, M.; Mir-Mari, J.; Figueiredo, R.; Valmaseda-Castellón, E. Probing Single-Tooth Dental Implants with and without Prostheses: A Cross-Sectional Study Comparing Healthy and Peri-Implant Mucositis Sites. J. Clin. Periodontol. 2021, 48, 581–589. [Google Scholar] [CrossRef]
- Scheuber, S.; Hicklin, S.; Brägger, U. Implants versus Short-Span Fixed Bridges: Survival, Complications, Patients’ Benefits. A Systematic Review on Economic Aspects. Clin. Oral Implants Res. 2012, 23 (Suppl. S6), 50–62. [Google Scholar] [CrossRef] [PubMed]
- Bandiaky, O.N.; Lokossou, D.L.; Soueidan, A.; Le Bars, P.; Gueye, M.; Mbodj, E.B.; Le Guéhennec, L. Implant-Supported Removable Partial Dentures Compared to Conventional Dentures: A Systematic Review and Meta-Analysis of Quality of Life, Patient Satisfaction, and Biomechanical Complications. Clin. Exp. Dent. Res. 2022, 8, 294–312. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef] [PubMed]
- Tettamanti, L.; Andrisani, C.; Bassi, M.A.; Vinci, R.; Silvestre-Rangil, J.; Tagliabue, A. Immediate Loading Implants: Review of the Critical Aspects. Oral Implant. 2017, 10, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Upendran, A.; Gupta, N.; Salisbury, H.G. Dental Mini-Implants. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Chen, X.; Xie, L.; Chen, J.; Du, R.; Deng, F. Design and Fabrication of Custom-Made Dental Implants. J. Mech. Sci. Technol. 2012, 26, 1993–1998. [Google Scholar] [CrossRef]
- Aparicio, C.; Manresa, C.; Francisco, K.; Claros, P.; Alández, J.; González-Martín, O.; Albrektsson, T. Zygomatic Implants: Indications, Techniques and Outcomes, and the Zygomatic Success Code. Periodontol. 2000 2014, 66, 41–58. [Google Scholar] [CrossRef]
- Raikar, S.; Talukdar, P.; Kumari, S.; Panda, S.K.; Oommen, V.M.; Prasad, A. Factors Affecting the Survival Rate of Dental Implants: A Retrospective Study. J. Int. Soc. Prev. Community Dent. 2017, 7, 351–355. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, N.; Weber, D.D.S. Dental Implants. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Kochar, S.P.; Reche, A.; Paul, P. The Etiology and Management of Dental Implant Failure: A Review. Cureus 2022, 14, e30455. [Google Scholar] [CrossRef]
- Poli, P.P.; de Miranda, F.V.; Polo, T.O.B.; Santiago Júnior, J.F.; Lima Neto, T.J.; Rios, B.R.; Assunção, W.G.; Ervolino, E.; Maiorana, C.; Faverani, L.P. Titanium Allergy Caused by Dental Implants: A Systematic Literature Review and Case Report. Materials 2021, 14, 5239. [Google Scholar] [CrossRef]
- Sicilia, A.; Cuesta, S.; Coma, G.; Arregui, I.; Guisasola, C.; Ruiz, E.; Maestro, A. Titanium Allergy in Dental Implant. Patients: A Clinical Study on 1500 Consecutive Patients. Clin. Oral Implant. Res. 2008, 19, 823–835. [Google Scholar] [CrossRef]
- Chaturvedi, T. Allergy Related to Dental Implant and Its Clinical Significance. Clin. Cosmet. Investig. Dent. 2013, 5, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Hosoki, M.; Nishigawa, K.; Miyamoto, Y.; Ohe, G.; Matsuka, Y. Allergic Contact Dermatitis Caused by Titanium Screws and Dental Implants. J. Prosthodont. Res. 2016, 60, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, A.; Payne, A.G.T.; De Silva, R.K.; Duncan, W.J. Titanium Allergy: Could It Affect Dental Implant Integration? Clin. Oral. Implant. Res. 2011, 22, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Kligman, S.; Ren, Z.; Chung, C.-H.; Perillo, M.A.; Chang, Y.-C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef] [PubMed]
- Teo Wendy, Z.W.; Schalock, P.C. Hypersensitivity Reactions to Implanted Metal Devices: Facts and Fictions. J. Investig. Allergol. Clin. Immunol. 2016, 26, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Marwa, K.; Kondamudi, N.P. Type IV Hypersensitivity Reaction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Abbas, M.; Moussa, M.; Akel, H. Type I Hypersensitivity Reaction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Accioni, F.; Vázquez, J.; Merinero, M.; Begines, B.; Alcudia, A. Latest Trends in Surface Modification for Dental Implantology: Innovative Developments and Analytical Applications. Pharmaceutics 2022, 14, 455. [Google Scholar] [CrossRef] [PubMed]
- Matter, M.T.; Maliqi, L.; Keevend, K.; Guimond, S.; Ng, J.; Armagan, E.; Rottmar, M.; Herrmann, I.K. One-Step Synthesis of Versatile Antimicrobial Nano-Architected Implant Coatings for Hard and Soft Tissue Healing. ACS Appl. Mater. Interfaces 2021, 13, 33300–33310. [Google Scholar] [CrossRef]
- Ogle, O.E. Implant Surface Material, Design, and Osseointegration. Dent. Clin. N. Am. 2015, 59, 505–520. [Google Scholar] [CrossRef]
- Camargo, S.E.A.; Xia, X.; Fares, C.; Ren, F.; Hsu, S.-M.; Budei, D.; Aravindraja, C.; Kesavalu, L.; Esquivel-Upshaw, J.F. Nanostructured Surfaces to Promote Osteoblast Proliferation and Minimize Bacterial Adhesion on Titanium. Materials 2021, 14, 4357. [Google Scholar] [CrossRef]
- Amengual-Peñafiel, L.; Córdova, L.A.; Constanza Jara-Sepúlveda, M.; Brañes-Aroca, M.; Marchesani-Carrasco, F.; Cartes-Velásquez, R. Osteoimmunology Drives Dental Implant Osseointegration: A New Paradigm for Implant Dentistry. JPN Dent. Sci. Rev. 2021, 57, 12–19. [Google Scholar] [CrossRef]
- Albrektsson, T.; Tengvall, P.; Amengual, L.; Coli, P.; Kotsakis, G.A.; Cochran, D. Osteoimmune Regulation Underlies Oral Implant Osseointegration and Its Perturbation. Front. Immunol. 2023, 13, 1056914. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Sculean, A.; Bosshardt, D.D.; Miron, R.J. Macrophage Behavior and Interplay with Gingival Fibroblasts Cultured on Six Commercially Available Titanium, Zirconium, and Titanium-Zirconium Dental Implants. Clin. Oral Investig. 2019, 23, 3219–3227. [Google Scholar] [CrossRef] [PubMed]
- Fretwurst, T.; Buzanich, G.; Nahles, S.; Woelber, J.P.; Riesemeier, H.; Nelson, K. Metal Elements in Tissue with Dental Peri-Implantitis: A Pilot Study. Clin. Oral Implant. Res. 2016, 27, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, J.S.; Abd Rahman, N.A.; Ming, L.C.; Dhaliwal, S.K.S.; Knights, J.; Albuquerque Junior, R.F. Microbial Biofilm Decontamination on Dental Implant Surfaces: A Mini Review. Front. Cell Infect. Microbiol. 2021, 11, 736186. [Google Scholar] [CrossRef] [PubMed]
- Parithimarkalaignan, S.; Padmanabhan, T.V. Osseointegration: An Update. J. Indian Prosthodont. Soc. 2013, 13, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Bielemann, A.M.; Marcello-Machado, R.M.; Leite, F.R.M.; Martinho, F.C.; Chagas-Júnior, O.L.; Antoninha Del Bel Cury, A.; Faot, F. Comparison between Inflammation-Related Markers in Peri-Implant Crevicular Fluid and Clinical Parameters during Osseointegration in Edentulous Jaws. Clin. Oral Investig. 2018, 22, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Athanasou, N.A. The Pathobiology and Pathology of Aseptic Implant Failure. Bone Jt. Res. 2016, 5, 162–168. [Google Scholar] [CrossRef]
- Pajarinen, J.; Kouri, V.-P.; Jämsen, E.; Li, T.-F.; Mandelin, J.; Konttinen, Y.T. The Response of Macrophages to Titanium Particles Is Determined by Macrophage Polarization. Acta Biomater. 2013, 9, 9229–9240. [Google Scholar] [CrossRef]
- Hovav, A.-H. Dendritic Cells of the Oral Mucosa. Mucosal Immunol. 2014, 7, 27–37. [Google Scholar] [CrossRef]
- Arizon, M.; Nudel, I.; Segev, H.; Mizraji, G.; Elnekave, M.; Furmanov, K.; Eli-Berchoer, L.; Clausen, B.E.; Shapira, L.; Wilensky, A.; et al. Langerhans Cells Down-Regulate Inflammation-Driven Alveolar Bone Loss. Proc. Natl. Acad. Sci. USA 2012, 109, 7043–7048. [Google Scholar] [CrossRef]
- Heyman, O.; Koren, N.; Mizraji, G.; Capucha, T.; Wald, S.; Nassar, M.; Tabib, Y.; Shapira, L.; Hovav, A.-H.; Wilensky, A. Impaired Differentiation of Langerhans Cells in the Murine Oral Epithelium Adjacent to Titanium Dental Implants. Front. Immunol. 2018, 9, 1712. [Google Scholar] [CrossRef] [PubMed]
- Gooty, J.R.; Kannam, D.; Guntakala, V.R.; Palaparthi, R. Distribution of Dendritic Cells and Langerhans Cells in Peri-Implant Mucosa. Contemp. Clin. Dent. 2018, 9, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Abaricia, J.O.; Shah, A.H.; Musselman, R.M.; Olivares-Navarrete, R. Hydrophilic Titanium Surfaces Reduce Neutrophil Inflammatory Response and NETosis. Biomater. Sci. 2020, 8, 2289–2299. [Google Scholar] [CrossRef] [PubMed]
- Zizzi, A.; Aspriello, S.D.; Rubini, C.; Goteri, G. Peri-Implant Diseases and Host Inflammatory Response Involving Mast Cells: A Review. Int. J. Immunopathol. Pharmacol. 2011, 24, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Ding, Y.; Zhuang, J.; Sun, R.; Sun, H.; Bai, L. Osteoimmunomodulation Role of Exosomes Derived from Immune Cells on Osseointegration. Front. Bioeng. Biotechnol. 2022, 10, 989537. [Google Scholar] [CrossRef] [PubMed]
- Kordbacheh Changi, K.; Finkelstein, J.; Papapanou, P.N. Periimplantitis Prevalence, Incidence Rate, and Risk Factors: A Study of Electronic Health Records at a U.S. Dental School. Clin. Oral Implant. Res. 2019, 30, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.R.; Passi, D.; Singh, P.; Atri, M.; Mohan, S.; Sharma, A. Risks and Complications Associated with Dental Implant Failure: Critical Update. Natl. J. Maxillofac. Surg. 2020, 11, 14–19. [Google Scholar] [CrossRef]
- Chen, H.; Liu, N.; Xu, X.; Qu, X.; Lu, E. Smoking, Radiotherapy, Diabetes and Osteoporosis as Risk Factors for Dental Implant Failure: A Meta-Analysis. PLoS ONE 2013, 8, e71955. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A. Peri-Implant Diseases: Diagnosis and Risk Indicators. J. Clin. Periodontol. 2008, 35, 292–304. [Google Scholar] [CrossRef]
- Papi, P.; de Angelis, F.; Mencio, F.; Rosella, D.; Di Carlo, S.; Pompa, G. Implant Survival and Success Rates in Patients with Risk Factors: Results from a Long-Term Retrospective Study with a 10 to 18 Years Follow-Up. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 433–437. [Google Scholar]
- Alsaadi, G.; Quirynen, M.; Komárek, A.; Van Steenberghe, D. Impact of Local and Systemic Factors on the Incidence of Late Oral Implant Loss. Clin. Oral Implant. Res. 2008, 19, 670–676. [Google Scholar] [CrossRef]
- Kermalli, J.Y.; Deporter, D.A.; Atenafu, E.G.; Lam, E.W. A Retrospective Report on Three Implant Devices Used to Restore Posterior Partial Edentulism: Overall Performance and Changes in Crestal Bone Levels. Int. J. Periodontics Restor. Dent. 2014, 34, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, A.D.; Salame, Z.; Chrcanovic, B.R. Smoking and Dental Implants: A Systematic Review and Meta-Analysis. Medicina 2022, 58, 39. [Google Scholar] [CrossRef] [PubMed]
- Callaway, D.A.; Jiang, J.X. Reactive Oxygen Species and Oxidative Stress in Osteoclastogenesis, Skeletal Aging and Bone Diseases. J. Bone Miner. Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef] [PubMed]
- de Avila, E.D.; van Oirschot, B.A.; van den Beucken, J.J.J.P. Biomaterial-Based Possibilities for Managing Peri-Implantitis. J. Periodontal Res. 2020, 55, 165–173. [Google Scholar] [CrossRef]
- Zitzmann, N.U.; Berglundh, T. Definition and Prevalence of Peri-Implant Diseases. J. Clin. Periodontol. 2008, 35, 286–291. [Google Scholar] [CrossRef]
- Tabanella, G.; Nowzari, H.; Slots, J. Clinical and Microbiological Determinants of Ailing Dental Implants. Clin. Implant Dent. Relat. Res. 2009, 11, 24–36. [Google Scholar] [CrossRef]
- Derks, J.; Schaller, D.; Håkansson, J.; Wennström, J.L.; Tomasi, C.; Berglundh, T. Periimplantitis—Onset and Pattern of Progression. J. Clin. Periodontol. 2016, 43, 383–388. [Google Scholar] [CrossRef]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the Normal Bacterial Flora of the Oral Cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef]
- Mombelli, A.; van Oosten, M.A.C.; Schürch, E., Jr.; Lang, N.P. The Microbiota Associated with Successful or Failing Osseointegrated Titanium Implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef]
- Persson, G.R.; Renvert, S. Cluster of Bacteria Associated with Peri-Implantitis. Clin. Implant Dent. Relat. Res. 2014, 16, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R. New Concepts and New Weapons in Implant Infections. Int. J. Artif. Organs 2009, 32, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Venza, I.; Visalli, M.; Cucinotta, M.; De Grazia, G.; Teti, D.; Venza, M. Proinflammatory Gene Expression at Chronic Periodontitis and Periimplantitis Sites in Patients with or without Type 2 Diabetes. J. Periodontol. 2010, 81, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.H.; Russi, K.L.; Rocha, N.C.; Bastos, F.; Darrieux, M.; Parisotto, T.M.; Girardello, R. Host-Microbiome Interactions Regarding Periimplantitis and Dental Implant Loss. J. Transl. Med. 2022, 20, 425. [Google Scholar] [CrossRef] [PubMed]
- Mulla, M.; Mulla, M.; Hegde, S.; Koshy, A.V. In Vitro Assessment of the Effect of Probiotic Lactobacillus Reuteri on Periimplantitis Microflora. BMC Oral Health 2021, 21, 408. [Google Scholar] [CrossRef] [PubMed]
- Polymeri, A.; van der Horst, J.; Buijs, M.J.; Zaura, E.; Wismeijer, D.; Crielaard, W.; Loos, B.G.; Laine, M.L.; Brandt, B.W. Submucosal Microbiome of Peri-Implant Sites: A Cross-Sectional Study. J. Clin. Periodontol. 2021, 48, 1228–1239. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Yu, Y.; Xu, R.; Zhang, Z.; Liang, C.; Sun, H.; Deng, F.; Yu, X. The Temporal Shift of Peri-Implant Microbiota during the Biofilm Formation and Maturation in a Canine Model. Microb. Pathog. 2021, 158, 105100. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical Biocompatibilities of Titanium Alloys for Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, Properties, and Biomedical Applications of Gelatin Methacryloyl (GelMA) Hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef]
- Liu, Y.; Geever, L.M.; Kennedy, J.E.; Higginbotham, C.L.; Cahill, P.A.; McGuinness, G.B. Thermal Behavior and Mechanical Properties of Physically Crosslinked PVA/Gelatin Hydrogels. J. Mech. Behav. Biomed. Mater. 2010, 3, 203–209. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; Boot, W.; Dimas, K.; Malizos, K.; Hänsch, G.M.; Stuyck, J.; Gawlitta, D.; Romanò, C.L. Does Implant Coating with Antibacterial-Loaded Hydrogel Reduce Bacterial Colonization and Biofilm Formation in Vitro? Clin. Orthop. Relat. Res. 2014, 472, 3311–3323. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Feloutzis, A.; Brägger, U.; Lang, N.P. Treatment of Periimplantitis by Local Delivery of Tetracycline. Clinical, Microbiological and Radiological Results. Clin. Oral Implant. Res. 2001, 12, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Minamikawa, H.; Ikeda, T.; Att, W.; Hagiwara, Y.; Hirota, M.; Tabuchi, M.; Aita, H.; Park, W.; Ogawa, T. Photofunctionalization Increases the Bioactivity and Osteoconductivity of the Titanium Alloy Ti6Al4V. J. Biomed. Mater. Res. Part A 2014, 102, 3618–3630. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.R.; Sporer, S.; Poggie, R.A.; Della Valle, C.J.; Jacobs, J.J. Experimental and Clinical Performance of Porous Tantalum in Orthopedic Surgery. Biomaterials 2006, 27, 4671–4681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, L.; Qian, S.; Gu, Y.; Shi, J.; Lai, H. Improved Response of Human Gingival Fibroblasts to Titanium Coated with Micro-/Nano-Structured Tantalum. Int. J. Implant Dent. 2021, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Schildhauer, T.A.; Robie, B.; Muhr, G.; Köller, M. Bacterial Adherence to Tantalum Versus Commonly Used Orthopedic Metallic Implant Materials. J. Orthop. Trauma 2006, 20, 476. [Google Scholar] [CrossRef] [PubMed]
- Healthcare Engineering, J.O. Retracted: Relationship between Artificial Intelligence-Based General Anesthetics and Postoperative Cognitive Dysfunction. J. Healthc. Eng. 2022, 2022, 9829807. [Google Scholar] [CrossRef]
- Flichy-Fernández, A.J.; Ata-Ali, J.; Alegre-Domingo, T.; Candel-Martí, E.; Ata-Ali, F.; Palacio, J.R.; Peñarrocha-Diago, M. The Effect of Orally Administered Probiotic Lactobacillus Reuteri-Containing Tablets in Peri-Implant Mucositis: A Double-Blind Randomized Controlled Trial. J. Periodontal Res. 2015, 50, 775–785. [Google Scholar] [CrossRef]
- Morales, A.; Carvajal, P.; Silva, N.; Hernandez, M.; Godoy, C.; Rodriguez, G.; Cabello, R.; Garcia-Sesnich, J.; Hoare, A.; Diaz, P.I.; et al. Clinical Effects of Lactobacillus Rhamnosus in Non-Surgical Treatment of Chronic Periodontitis: A Randomized Placebo-Controlled Trial with 1-Year Follow-Up. J. Periodontol. 2016, 87, 944–952. [Google Scholar] [CrossRef]
- Homayouni Rad, A.; Pourjafar, H.; Mirzakhani, E. A Comprehensive Review of the Application of Probiotics and Postbiotics in Oral Health. Front. Cell. Infect. Microbiol. 2023, 13, 1120995. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xie, M.; Xie, Y.; Mei, F.; Lu, X.; Li, X.; Chen, L. The Roles of Osteocytes in Alveolar Bone Destruction in Periodontitis. J. Transl. Med. 2020, 18, 479. [Google Scholar] [CrossRef] [PubMed]
- Galofré, M.; Palao, D.; Vicario, M.; Nart, J.; Violant, D. Clinical and Microbiological Evaluation of the Effect of Lactobacillus Reuteri in the Treatment of Mucositis and Peri-Implantitis: A Triple-Blind Randomized Clinical Trial. J. Periodontal Res. 2018, 53, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Vacca, C.; Contu, M.P.; Rossi, C.; Ferrando, M.L.; Blus, C.; Szmukler-Moncler, S.; Scano, A.; Orrù, G. In Vitro Interactions between Streptococcus intermedius and Streptococcus salivarius K12 on a Titanium Cylindrical Surface. Pathogens 2020, 9, 1069. [Google Scholar] [CrossRef] [PubMed]
- Martorano-Fernandes, L.; Rodrigues, N.C.; de Souza Borges, M.H.; Cavalcanti, Y.W.; de Almeida, L.d.F.D. Interkingdom Interaction between C. albicans and S. salivarius on Titanium Surfaces. BMC Oral Health 2020, 20, 349. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, G.; Gimigliano, R.; Bianco, M.; de Sire, A.; Moretti, A.; Giusti, A.; Malavolta, N.; Migliaccio, S.; Migliore, A.; Napoli, N.; et al. Are Dietary Supplements and Nutraceuticals Effective for Musculoskeletal Health and Cognitive Function? A Scoping Review. J. Nutr. Health Aging 2017, 21, 527–538. [Google Scholar] [CrossRef]
- Montalvany-Antonucci, C.C.; Zicker, M.C.; Oliveira, M.C.; Macari, S.; Madeira, M.F.M.; Andrade, I.; Ferreira, A.V.M.; Silva, T.A. Diet versus Jaw Bones: Lessons from Experimental Models and Potential Clinical Implications. Nutrition 2018, 45, 59–67. [Google Scholar] [CrossRef]
- Fulgoni, V.L.; Keast, D.R.; Auestad, N.; Quann, E.E. Nutrients from Dairy Foods Are Difficult to Replace in Diets of Americans: Food Pattern Modeling and an Analyses of the National Health and Nutrition Examination Survey 2003–2006. Nutr. Res. 2011, 31, 759–765. [Google Scholar] [CrossRef]
- DePhillipo, N.N.; Aman, Z.S.; Kennedy, M.I.; Begley, J.P.; Moatshe, G.; LaPrade, R.F. Efficacy of Vitamin C Supplementation on Collagen Synthesis and Oxidative Stress After Musculoskeletal Injuries: A Systematic Review. Orthop. J. Sports Med. 2018, 6, 2325967118804544. [Google Scholar] [CrossRef]
- Wagner, F.; Schuder, K.; Hof, M.; Heuberer, S.; Seemann, R.; Dvorak, G. Does Osteoporosis Influence the Marginal Peri-Implant Bone Level in Female Patients? A Cross-Sectional Study in a Matched Collective. Clin. Implant Dent. Relat. Res. 2017, 19, 616–623. [Google Scholar] [CrossRef]
- Nastri, L.; Guida, L.; Annunziata, M.; Ruggiero, N.; Rizzo, A. Vitamin D Modulatory Effect on Cytokines Expression by Human Gingival Fibroblasts and Periodontal Ligament Cells. Minerva Stomatol. 2018, 67, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yu, T.; Yang, X.; Li, F.; Ma, L.; Yang, Y.; Liu, X.; Wang, Y.; Gong, P. Vitamin D3 and Insulin Combined Treatment Promotes Titanium Implant Osseointegration in Diabetes Mellitus Rats. Bone 2013, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Belluci, M.M.; Giro, G.; del Barrio, R.A.L.; Pereira, R.M.R.; Marcantonio, E., Jr.; Orrico, S.R.P. Effects of Magnesium Intake Deficiency on Bone Metabolism and Bone Tissue around Osseointegrated Implants. Clin. Oral Implant. Res. 2011, 22, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Valm, A.M. The Structure of Dental Plaque Microbial Communities in the Transition from Health to Dental Caries and Periodontal Disease. J. Mol. Biol. 2019, 431, 2957–2969. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, M.; Polizzi, A.; Santonocito, S.; Romano, A.; Lombardi, T.; Isola, G. Impact of Oral Microbiome in Periodontal Health and Periodontitis: A Critical Review on Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 5142. [Google Scholar] [CrossRef]
- Li, X.; Yu, C.; Zhang, B.; Shan, X.; Mao, W.; Zhang, Z.; Wang, C.; Jin, X.; Wang, J.; Zhao, H. The recovery of the microbial community after plaque removal depends on periodontal health status. NPJ Biofilms Microbiomes 2023, 9, 75. [Google Scholar] [CrossRef]
- Ghensi, P.; Manghi, P.; Zolfo, M.; Armanini, F.; Pasolli, E.; Bolzan, M.; Bertelle, A.; Dell’acqua, F.; Dellasega, E.; Waldner, R.; et al. Strong oral plaque microbiome signatures for dental implant diseases identified by strain-resolution metagenomics. NPJ Biofilms Microbiomes 2020, 6, 47. [Google Scholar] [CrossRef]
- Chalmers, N.I.; Palmer, R.J., Jr.; Cisar, J.O.; Kolenbrander, P.E. Characterization of a Streptococcus sp.-Veillonella sp. community micromanipulated from dental plaque. J. Bacteriol. 2008, 190, 8145–8154. [Google Scholar] [CrossRef]
- Larsen, T.; Fiehn, N.E. Dental biofilm infections—An update. APMIS 2017, 125, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Simón-Soro, A.; Mira, A. Solving the etiology of dental caries. Trends Microbiol. 2015, 23, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Páez, A.; Álvarez, M.; Belda-Ferre, P.; Rubido, S.; Mira, A.; Tomás, I. Detection of transient bacteraemia following dental extractions by 16S rDNA pyrosequencing: A pilot study. PLoS ONE 2013, 8, e57782. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahnama-Hezavah, M.; Mertowska, P.; Mertowski, S.; Skiba, J.; Krawiec, K.; Łobacz, M.; Grywalska, E. How Can Imbalance in Oral Microbiota and Immune Response Lead to Dental Implant Problems? Int. J. Mol. Sci. 2023, 24, 17620. https://doi.org/10.3390/ijms242417620
Rahnama-Hezavah M, Mertowska P, Mertowski S, Skiba J, Krawiec K, Łobacz M, Grywalska E. How Can Imbalance in Oral Microbiota and Immune Response Lead to Dental Implant Problems? International Journal of Molecular Sciences. 2023; 24(24):17620. https://doi.org/10.3390/ijms242417620
Chicago/Turabian StyleRahnama-Hezavah, Mansur, Paulina Mertowska, Sebastian Mertowski, Julia Skiba, Karol Krawiec, Michał Łobacz, and Ewelina Grywalska. 2023. "How Can Imbalance in Oral Microbiota and Immune Response Lead to Dental Implant Problems?" International Journal of Molecular Sciences 24, no. 24: 17620. https://doi.org/10.3390/ijms242417620
APA StyleRahnama-Hezavah, M., Mertowska, P., Mertowski, S., Skiba, J., Krawiec, K., Łobacz, M., & Grywalska, E. (2023). How Can Imbalance in Oral Microbiota and Immune Response Lead to Dental Implant Problems? International Journal of Molecular Sciences, 24(24), 17620. https://doi.org/10.3390/ijms242417620









