The Potential Role of Gossypetin in the Treatment of Diabetes Mellitus and Its Associated Complications: A Review
Abstract
:1. Introduction
1.1. Type 2 Diabetes Mellitus Complications
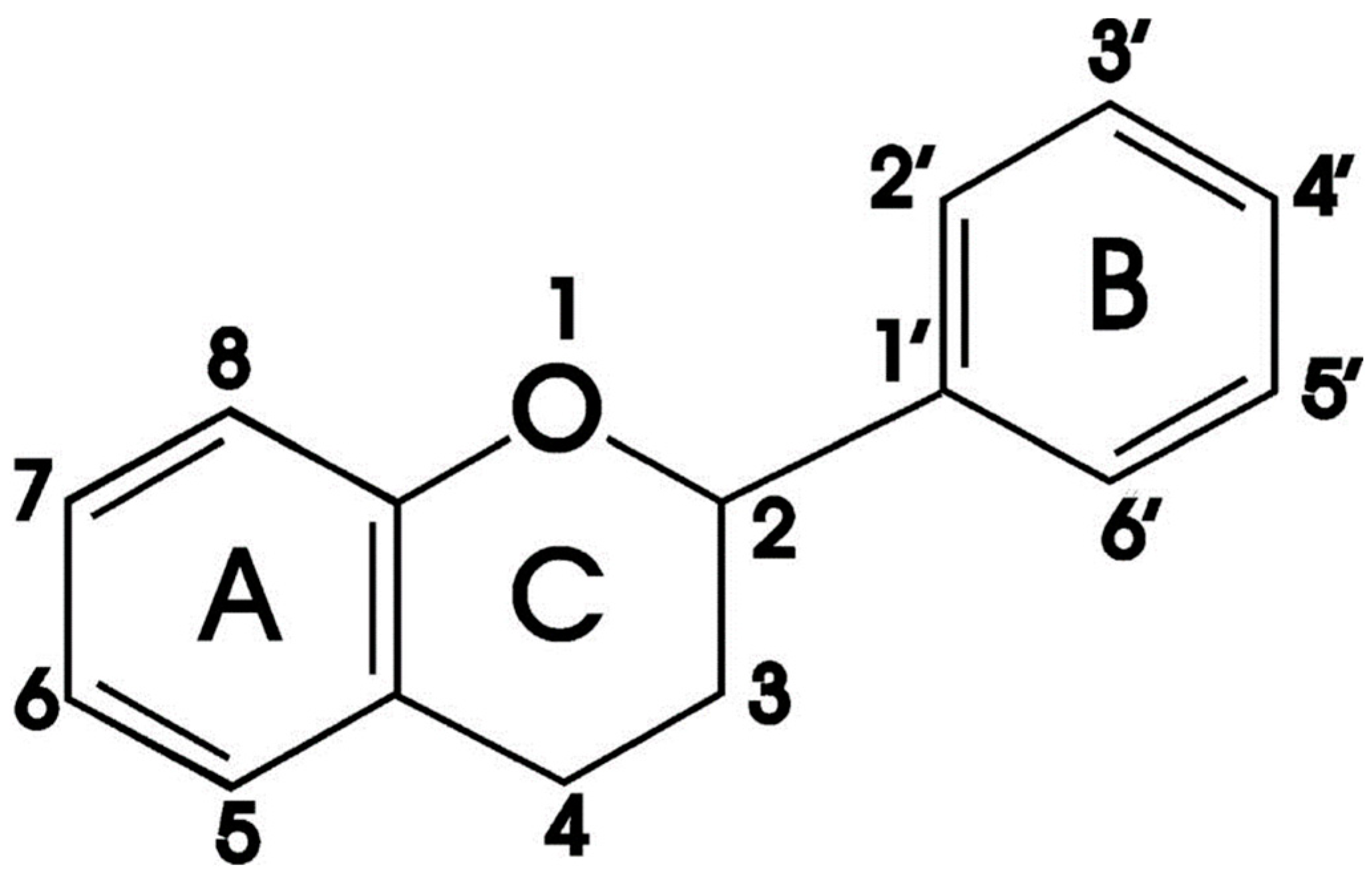
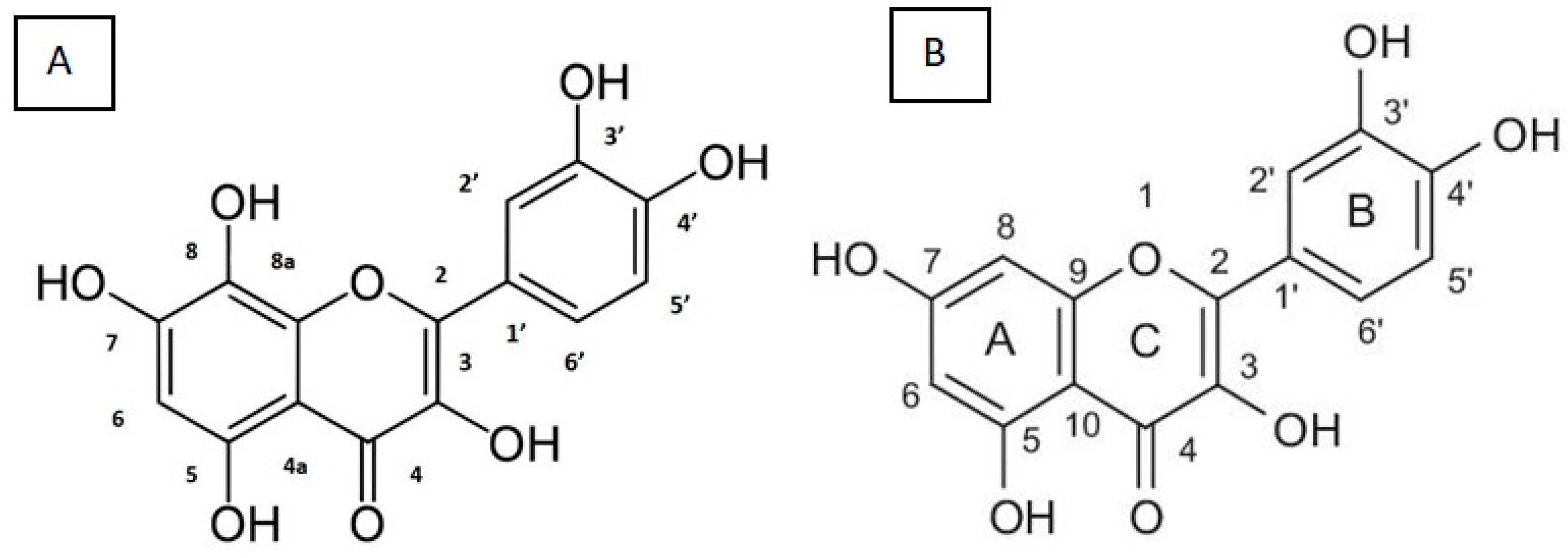
1.2. Flavonoids
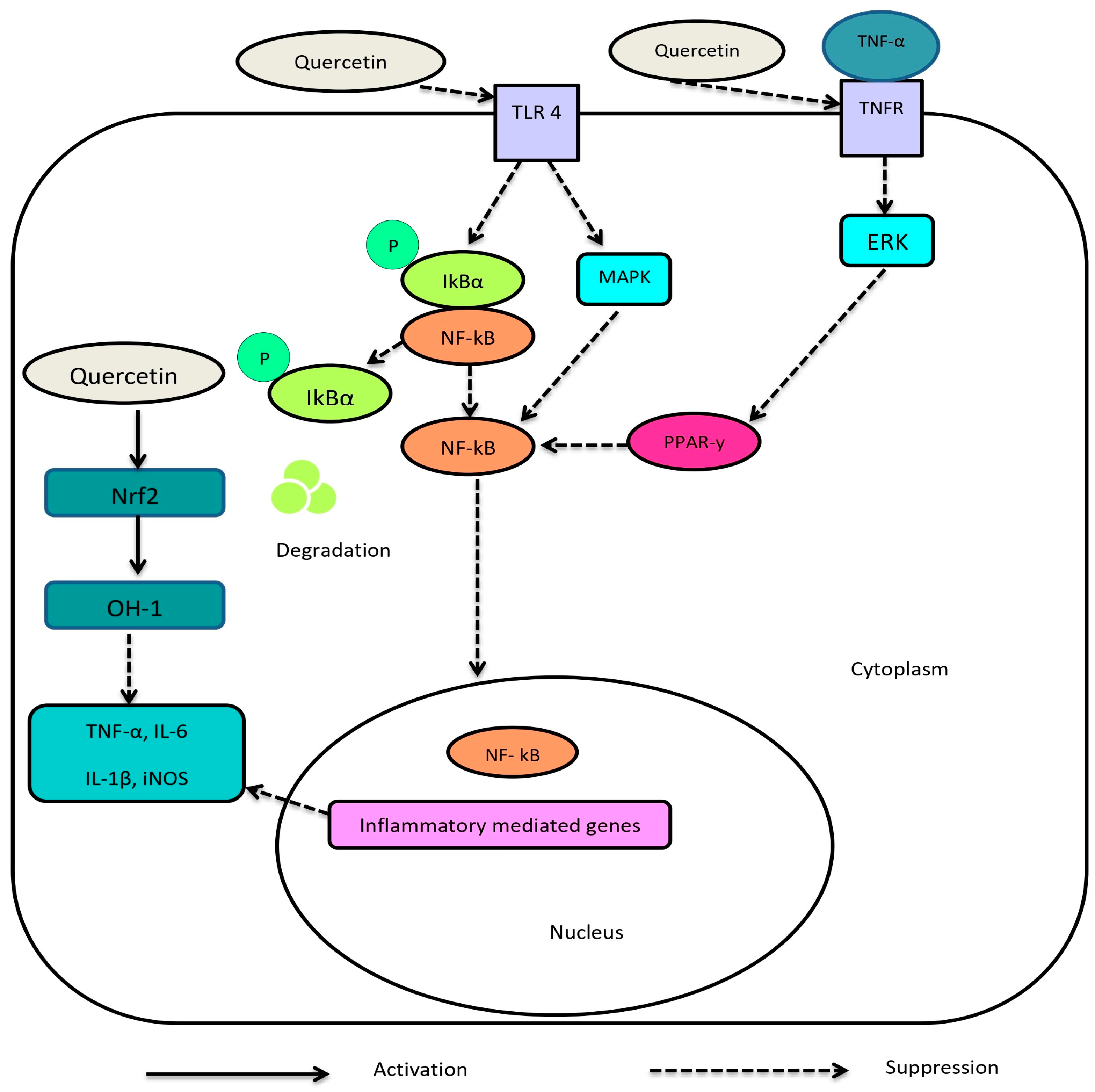
1.2.1. Quercetin
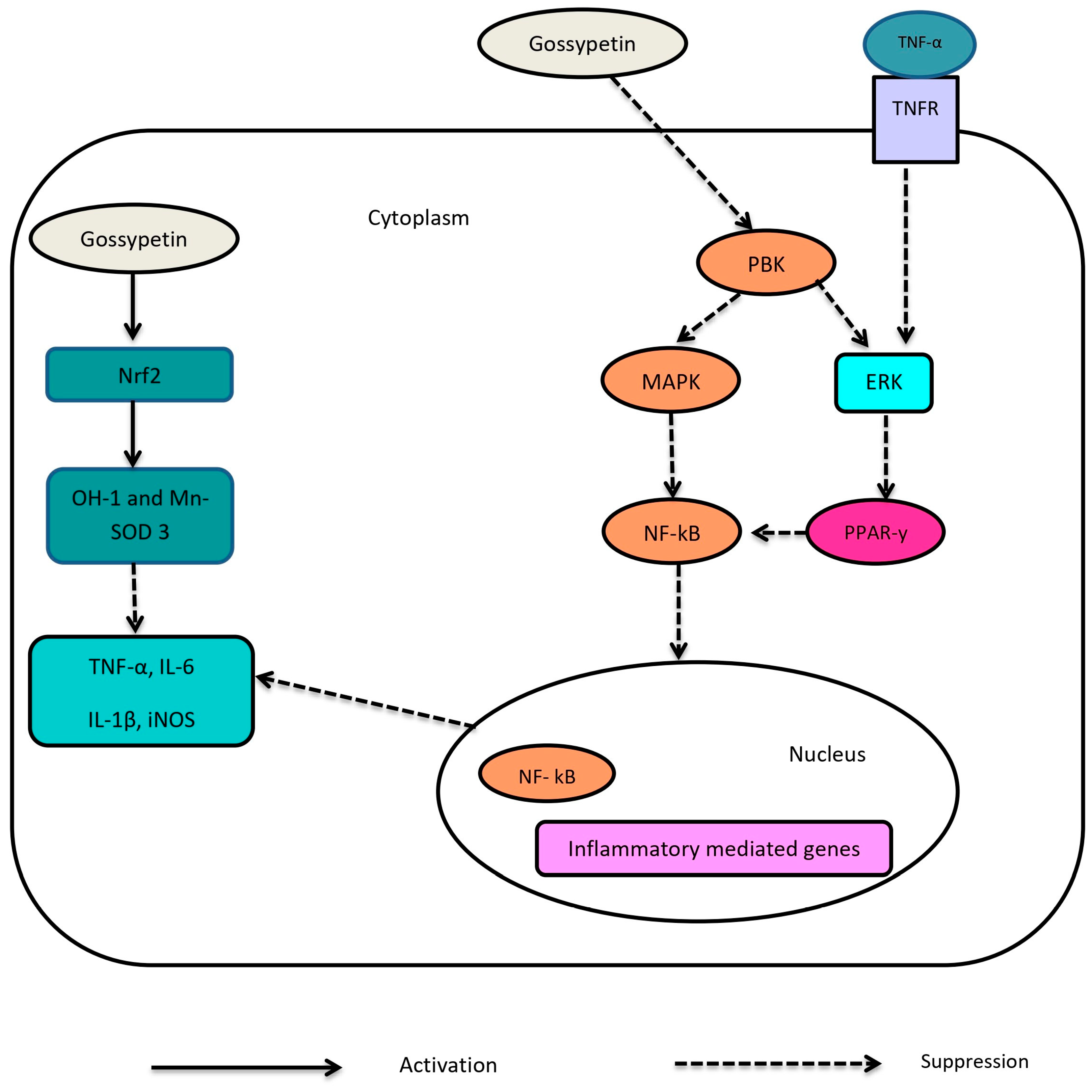
1.2.2. Gossypetin
1.3. Pharmacological Action of Gossypetin
1.3.1. Anti-Oxidant and Anti-Inflammatory Effects of Gossypetin
1.3.2. Anti-Atherosclerotic Effects of Gossypetin
1.3.3. Nephroprotective Effects of Gossypetin
1.3.4. Neuroprotective Effects of Gossypetin
1.3.5. Hepatoprotective Effects of Gossypetin
1.3.6. Reproprotective Effects of Gossypetin
1.4. Potential Use of Gossypetin in Diabetes Mellitus
1.5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erzse, A.; Stacey, N.; Chola, L.; Tugendhaft, A.; Freeman, M.; Hofman, K. The direct medical cost of type 2 diabetes mellitus in South Africa: A cost of illness study. Glob. Health Action 2019, 12, 1636611. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Wu, T.; Zhang, M.; Li, C.; Liu, Q.; Li, F. Prevalence, awareness and control of type 2 diabetes mellitus and risk factors in Chinese elderly population. BMC Public Health 2022, 22, 1382. [Google Scholar] [CrossRef] [PubMed]
- Federation, I.D. At a Glance|119(3) Mar 2011. Environ. Heal. Perspect. 2011, 119, a106–a109. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Rangel, E.B.; Rodrigues, C.O.; De Sa, J.R. Micro- and Macrovascular Complications in Diabetes Mellitus: Preclinical and Clinical Studies; Hindawi: London, UK, 2019. [Google Scholar]
- Chawla, A.; Chawla, R.; Jaggi, S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016, 20, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Sehrawat, A.; Mishra, J.; Sidhu, I.S.; Navik, U.; Khullar, N.; Kumar, S.; Bhatti, G.K.; Reddy, P.H. Oxidative stress in the pathophysiology of type 2 diabetes and related complications: Current therapeutics strategies and future perspectives. Free Radic. Biol. Med. 2022, 184, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Marín-Peñalver, J.J.; Martín-Timón, I.; Sevillano-Collantes, C.; Del Cañizo-Gómez, F.J. Update on the treatment of type 2 diabetes mellitus. World J. Diabetes 2016, 7, 354–395. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Lebelo, S.L. Antioxidant Effects and Mechanisms of Medicinal Plants and Their Bioactive Compounds for the Prevention and Treatment of Type 2 Diabetes: An Updated Review. Oxidative Med. Cell. Longev. 2020, 2020, 1356893. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- David, A.V.A.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84. [Google Scholar]
- Ansari, P.; Choudhury, S.T.; Seidel, V.; Rahman, A.B.; Aziz, M.A.; Richi, A.E.; Rahman, A.; Jafrin, U.H.; Hannan, J.; Abdel-Wahab, Y.H. Therapeutic potential of quercetin in the management of type-2 diabetes mellitus. Life 2022, 12, 1146. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.; Prabhakar, D.P.; Gupta, J. Identification of Structurally Similar Phytochemicals to Quercetin with High SIRT1 Binding Affinity and Improving Diabetic Wound Healing by Using In silico Approaches. Biointerface Res. Appl. Chem. 2021, 12, 7621–7632. [Google Scholar]
- Magar, R.T.; Sohng, J.K. A Review on Structure, Modifications and Structure-Activity Relation of Quercetin and Its Derivatives. J. Microbiol. Biotechnol. 2019, 30, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Banjarnahor, S.D.; Artanti, N. Antioxidant properties of flavonoids. Med. J. Indones. 2014, 23, 239–244. [Google Scholar] [CrossRef]
- Huang, K.; Liu, Z.; Kim, M.-O.; Kim, K.-R. Anticancer effects of gossypetin from Hibiscus sabdariffa in oral squamous cell carcinoma. J. Appl. Oral Sci. 2023, 31, e20230243. [Google Scholar] [CrossRef] [PubMed]
- Dutta, M.S.; Mahapatra, P.; Ghosh, A.; Basu, S. Estimation of the reducing power and electrochemical behavior of few flavonoids and polyhydroxybenzophenones substantiated by bond dissociation energy: A comparative analysis. Mol. Divers. 2022, 26, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.U.; Alvi, K.; Khan, H.A.; Imran, M.; Afsar, T.; Almajwal, A.; Amor, H.; Razak, S. Gossypetin mitigates doxorubicin-induced nephrotoxicity: A histopathological and biochemical evaluation. J. King Saud Univ.-Sci. 2023, 35, 102830. [Google Scholar] [CrossRef]
- Mustafa, S.; Anwar, H.; Ain, Q.u.; Ahmed, H.; Iqbal, S.; Ijaz, M.U. Therapeutic effect of gossypetin against paraquat-induced testicular damage in male rats: A histological and biochemical study. Environ. Sci. Pollut. Res. 2023, 30, 62237–62248. [Google Scholar] [CrossRef]
- Lin, H.-H. In vitro and in vivo atheroprotective effects of gossypetin against endothelial cell injury by induction of autophagy. Chem. Res. Toxicol. 2015, 28, 202–215. [Google Scholar] [CrossRef]
- Oh, E.; Lee, J.; Cho, S.; Kim, S.W.; Jo, K.W.; Shin, W.S.; Gwak, S.H.; Ha, J.; Jeon, S.Y.; Park, J.-H.; et al. Gossypetin prevents the progression of nonalcoholic steatohepatitis by regulating oxidative stress and AMP-activated protein kinase. Mol. Pharmacol. 2023, 104, 214–229. [Google Scholar] [CrossRef]
- Jejurkar, G.; Chavan, M. Therapeutic benefits of gossypin as an emerging phytoconstituents of Hibiscus spp.: A critical review. Future J. Pharm. Sci. 2023, 9, 95. [Google Scholar] [CrossRef]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef]
- Saisho, Y. β-cell dysfunction: Its critical role in prevention and management of type 2 diabetes. World J. Diabetes 2015, 6, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxidative Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.V.; Shaw, L.C.; Grant, M.B. Inflammation in the pathogenesis of microvascular complications in diabetes. Front. Endocrinol. 2012, 3, 170. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, S.; Dasu, M.R.; Jialal, I. Diabetes is a proinflammatory state: A translational perspective. Expert Rev. Endocrinol. Metab. 2010, 5, 19–28. [Google Scholar] [CrossRef]
- Kong, M.; Xie, K.; Lv, M.; Li, J.; Yao, J.; Yan, K.; Wu, X.; Xu, Y.; Ye, D. Anti-inflammatory phytochemicals for the treatment of diabetes and its complications: Lessons learned and future promise. Biomed. Pharmacother. 2021, 133, 110975. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H. Therapeutic Potential of Phenolic Compounds in Medicinal Plants—Natural Health Products for Human Health. Molecules 2023, 28, 1845. [Google Scholar] [PubMed]
- Dias, M.C.; Pinto, D.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Ekalu, A.; Habila, J.D. Flavonoids: Isolation, characterization, and health benefits. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 45. [Google Scholar] [CrossRef]
- Shehadeh, M.B.; Suaifan, G.; Abu-Odeh, A.M. Plants Secondary Metabolites as Blood Glucose-Lowering Molecules. Molecules 2021, 26, 4333. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Dong, M.; Guo, N.; Tian, J.; Lei, P.; Wang, S.; Yang, Y.; Shi, Y. Flavonoids improve type 2 diabetes mellitus and its complications: A review. Front. Nutr. 2023, 10, 1192131. [Google Scholar] [CrossRef]
- Dhanya, R. Quercetin for managing type 2 diabetes and its complications, an insight into multitarget therapy. Biomed. Pharmacother. 2022, 146, 112560. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Ozgen, S.; Kilinc, O.K.; Selamoğlu, Z. Antioxidant activity of quercetin: A mechanistic review. Turk. J. Agric.-Food Sci. Technol. 2016, 4, 1134–1138. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Shoorei, H.; Sasi, A.K.; Taheri, M.; Ayatollahi, S.A. The impact of the phytotherapeutic agent quercetin on expression of genes and activity of signaling pathways. Biomed. Pharmacother. 2021, 141, 111847. [Google Scholar] [CrossRef]
- Youl, E.; Bardy, G.; Magous, R.; Cros, G.; Sejalon, F.; Virsolvy, A.; Richard, S.; Quignard, J.F.; Gross, R.; Petit, P.; et al. Quercetin potentiates insulin secretion and protects INS-1 pancreatic β-cells against oxidative damage via the ERK1/2 pathway. Br. J. Pharmacol. 2010, 161, 799–814. [Google Scholar] [CrossRef]
- Hammes, H.-P. Diabetic retinopathy: Hyperglycaemia, oxidative stress and beyond. Diabetologia 2018, 61, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Charlton, A.; Garzarella, J.; Jandeleit-Dahm, K.A.; Jha, J.C. Oxidative stress and inflammation in renal and cardiovascular complications of diabetes. Biology 2020, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- De Nardo, D. Toll-like receptors: Activation, signalling and transcriptional modulation. Cytokine 2015, 74, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Srivastava, M.; Saqib, U.; Liu, D.; Faisal, S.M.; Sugathan, S.; Bishnoi, S.; Baig, M.S. Potential therapeutic targets for inflammation in toll-like receptor 4 (TLR4)-mediated signaling pathways. Int. Immunopharmacol. 2016, 40, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tao, B.; Wan, Y.; Sun, Y.; Wang, L.; Sun, J.; Li, C. Drug delivery based pharmacological enhancement and current insights of quercetin with therapeutic potential against oral diseases. Biomed. Pharmacother. 2020, 128, 110372. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-C.; Huang, W.-C.; Pang, J.-H.S.; Wu, Y.-H.; Cheng, C.-Y. Quercetin inhibits the production of IL-1β-induced inflammatory cytokines and chemokines in ARPE-19 cells via the MAPK and NF-κB signaling pathways. Int. J. Mol. Sci. 2019, 20, 2957. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.-C.; Martinez, K.; Xie, G.; Kennedy, A.; Bumrungpert, A.; Overman, A.; Jia, W.; McIntosh, M.K. Quercetin is equally or more effective than resveratrol in attenuating tumor necrosis factor-α–mediated inflammation and insulin resistance in primary human adipocytes. Am. J. Clin. Nutr. 2010, 92, 1511–1521. [Google Scholar] [CrossRef]
- Lai, P.B.; Zhang, L.; Yang, L.Y. Quercetin ameliorates diabetic nephropathy by reducing the expressions of transforming growth factor-β1 and connective tissue growth factor in streptozotocin-induced diabetic rats. Ren. Fail. 2012, 34, 83–87. [Google Scholar] [CrossRef]
- Yang, H.; Song, Y.; Liang, Y.N.; Li, R. Quercetin Treatment Improves Renal Function and Protects the Kidney in a Rat Model of Adenine-Induced Chronic Kidney Disease. Med. Sci. Monit. 2018, 24, 4760–4766. [Google Scholar] [CrossRef]
- Yi, H.; Peng, H.; Wu, X.; Xu, X.; Kuang, T.; Zhang, J.; Du, L.; Fan, G. The Therapeutic Effects and Mechanisms of Quercetin on Metabolic Diseases: Pharmacological Data and Clinical Evidence. Oxidative Med. Cell. Longev. 2021, 2021, 6678662. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O.; et al. Therapeutic Potential of Quercetin: New Insights and Perspectives for Human Health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Ji, W.; Wang, F.; Zhang, F.; Xue, P.; Cheng, M.; Sun, Y.; Wang, X.; Zhang, T. Quercetin inhibits inflammatory response induced by LPS from Porphyromonas gingivalis in human gingival fibroblasts via suppressing NF-κB signaling pathway. BioMed Res. Int. 2019, 2019, 6282635. [Google Scholar] [CrossRef] [PubMed]
- Krajka-Kuźniak, V.; Baer-Dubowska, W. Modulation of Nrf2 and NF-κB signaling pathways by naturally occurring compounds in relation to cancer prevention and therapy. Are combinations better than single compounds? Int. J. Mol. Sci. 2021, 22, 8223. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Manna, K.; Bose, C.; Sinha, M.; Das, D.K.; Kesh, S.B.; Chakrabarty, A.; Banerji, A.; Dey, S. Gossypetin, a naturally occurring hexahydroxy flavone, ameliorates gamma radiation-mediated DNA damage. Int. J. Radiat. Biol. 2013, 89, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Riaz, G.; Chopra, R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed. Pharmacother. 2018, 102, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus sabdariffa L.—A phytochemical and pharmacological review. Food Chem. 2014, 165, 424–443. [Google Scholar] [CrossRef] [PubMed]
- González-Stuart, A. Multifaceted therapeutic value of roselle (Hibiscus sabdariffa L.—Malvaceae). In Nutrients, Dietary Supplements, and Nutriceuticals Cost Analysis Versus Clinical Benefits; Humana Press: Totowa, NJ, USA, 2011; pp. 215–226. [Google Scholar] [CrossRef]
- Har Bhajan, S.; Bharati, K.A. 6-Enumeration of dyes. In Handbook of Natural Dyes and Pigments; Har Bhajan, S., Bharati, K.A., Eds.; Woodhead Publishing: Delhi, India, 2014. [Google Scholar]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Puthanveedu, V.; Muraleedharan, K. Study on structural detailing of gossypetin and its medicinal application in UV filtering, radical scavenging, and metal chelation open up through NCI, TD-DFT, QTAIM, ELF, and LOL analysis. Comput. Theor. Chem. 2023, 1225, 114126. [Google Scholar] [CrossRef]
- Samant, N.P.; Gupta, G.L. Gossypetin-based therapeutics for cognitive dysfunction in chronic unpredictable stress-exposed mice. Metab. Brain Dis. 2022, 37, 1527–1539. [Google Scholar] [CrossRef]
- Zheng, Y.; Chow, A. Production and characterization of a spray-dried hydroxypropyl-β-cyclodextrin/quercetin complex. Drug Dev. Ind. Pharm. 2009, 35, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Folli, F.; Corradi, D.; Fanti, P.; Davalli, A.; Paez, A.; Giaccari, A.; Perego, C.; Muscogiuri, G. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro-and macrovascular complications: Avenues for a mechanistic-based therapeutic approach. Curr. Diabetes Rev. 2011, 7, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-H.; Hsieh, M.-C.; Wang, C.-P.; Yu, P.-R.; Lee, M.-S.; Chen, J.-H. Anti-atherosclerotic effect of gossypetin on abnormal vascular smooth muscle cell proliferation and migration. Antioxidants 2021, 10, 1357. [Google Scholar] [CrossRef] [PubMed]
- Jo, K.W.; Lee, D.; Cha, D.G.; Oh, E.; Choi, Y.H.; Kim, S.; Park, E.S.; Kim, J.K.; Kim, K.-T. Gossypetin ameliorates 5xFAD spatial learning and memory through enhanced phagocytosis against Aβ. Alzheimer’s Res. Ther. 2022, 14, 158. [Google Scholar] [CrossRef] [PubMed]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45. [Google Scholar] [PubMed]
- Fischer, N.; Seo, E.-J.; Efferth, T. Prevention from radiation damage by natural products. Phytomedicine 2018, 47, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Devipriya, N.; Sudheer, A.R.; Srinivasan, M.; Menon, V.P. Quercetin ameliorates gamma radiation-induced DNA damage and biochemical changes in human peripheral blood lymphocytes. Mutat. Res. 2008, 654, 1–7. [Google Scholar] [CrossRef]
- Benzie, I.F.; Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: Concepts, procedures, limitations and applications. Meas. Antioxid. Act. Capacit. Recent Trends Appl. 2018, 77–106. [Google Scholar]
- Mounnissamy, V.; Gopal, V.; Gunasegaran, R.; Saraswathy, A. Antiinflammatory activity of gossypetin isolated from Hibiscus sabdariffa. Indian J. Heterocycl. Chem. 2002, 12, 85–86. [Google Scholar]
- Proença, C.; Rufino, A.T.; Santos, I.; Albuquerque, H.M.T.; Silva, A.M.S.; Fernandes, E.; Ferreira de Oliveira, J.M.P. Gossypetin Is a Novel Modulator of Inflammatory Cytokine Production and a Suppressor of Osteosarcoma Cell Growth. Antioxidants 2023, 12, 1744. [Google Scholar] [CrossRef]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; Aldasoro, M.; Victor, V.M.; Mauricio, M.D. Targeting early atherosclerosis: A focus on oxidative stress and inflammation. Oxidative Med. Cell. Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef] [PubMed]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. Pathophysiology of diabetic dyslipidemia. J. Atheroscler. Thromb. 2018, 25, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Khatana, C.; Saini, N.K.; Chakrabarti, S.; Saini, V.; Sharma, A.; Saini, R.V.; Saini, A.K. Mechanistic insights into the oxidized low-density lipoprotein-induced atherosclerosis. Oxidative Med. Cell. Longev. 2020, 2020, 5245308. [Google Scholar] [CrossRef] [PubMed]
- Casella, S.; Bielli, A.; Mauriello, A.; Orlandi, A. Molecular pathways regulating macrovascular pathology and vascular smooth muscle cells phenotype in type 2 diabetes. Int. J. Mol. Sci. 2015, 16, 24353–24368. [Google Scholar] [CrossRef] [PubMed]
- Jha, J.C.; Ho, F.; Dan, C.; Jandeleit-Dahm, K. A causal link between oxidative stress and inflammation in cardiovascular and renal complications of diabetes. Clin. Sci. 2018, 132, 1811–1836. [Google Scholar] [CrossRef] [PubMed]
- Bamanikar, S.; Bamanikar, A.A.; Arora, A. Study of Serum urea and Creatinine in Diabetic and nondiabetic patients in a tertiary teaching hospital. J. Med. Res. 2016, 2, 12–15. [Google Scholar] [CrossRef]
- Luft, F.C. Biomarkers and predicting acute kidney injury. Acta Physiol. 2021, 231, e13479. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutierrez-Grijalva, E.P.; Ambriz-Perez, D.L.; Heredia, J.B. Flavonoids as cytokine modulators: A possible therapy for inflammation-related diseases. Int. J. Mol. Sci. 2016, 17, 921. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Sahana, G.R.; Nagella, P.; Joseph, B.V.; Alessa, F.M.; Al-Mssallem, M.Q. Flavonoids as potential anti-inflammatory molecules: A review. Molecules 2022, 27, 2901. [Google Scholar] [CrossRef]
- Barone, E.; Di Domenico, F.; Perluigi, M.; Butterfield, D.A. The interplay among oxidative stress, brain insulin resistance and AMPK dysfunction contribute to neurodegeneration in type 2 diabetes and Alzheimer disease. Free Radic. Biol. Med. 2021, 176, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Gutierrez, E.; Muñoz-Arenas, G.; Treviño, S.; Espinosa, B.; Chavez, R.; Rojas, K.; Flores, G.; Díaz, A.; Guevara, J. Alzheimer’s disease and metabolic syndrome: A link from oxidative stress and inflammation to neurodegeneration. Synapse 2017, 71, e21990. [Google Scholar] [CrossRef]
- Antonelli, M.C.; Pallarés, M.E.; Ceccatelli, S.; Spulber, S. Long-term consequences of prenatal stress and neurotoxicants exposure on neurodevelopment. Prog. Neurobiol. 2017, 155, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Bisht, K.; Sharma, K.; Tremblay, M.-È. Chronic stress as a risk factor for Alzheimer’s disease: Roles of microglia-mediated synaptic remodeling, inflammation, and oxidative stress. Neurobiol. Stress 2018, 9, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-derived neurotrophic factor: A key molecule for memory in the healthy and the pathological brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef] [PubMed]
- Ortíz, B.M.; Emiliano, J.R.; Ramos-Rodríguez, E.; Martínez-Garza, S.; Macías-Cervantes, H.; Solorio-Meza, S.; Pereyra-Nobara, T.A. Brain-derived neurotrophic factor plasma levels and premature cognitive impairment/dementia in type 2 diabetes. World J. Diabetes 2016, 7, 615. [Google Scholar] [CrossRef] [PubMed]
- Gatckikh, I.V. Association of Serum BDNF with Severity of Cognitive Disorders in Patients with Type 2 Diabetes. Pers. Psychiatry Neurol. 2022, 2, 67–77. [Google Scholar] [CrossRef]
- Gulsheen; Kumar, A.; Sharma, A. Antianxiety and antidepressant activity guided isolation and characterization of gossypetin from Hibiscus sabdariffa Linn. calyces. J. Biol. Act. Prod. Nat. 2019, 9, 205–214. [Google Scholar] [CrossRef]
- Balogh, D.B.; Molnar, A.; Hosszu, A.; Lakat, T.; Hodrea, J.; Szabo, A.J.; Lenart, L.; Fekete, A. Antidepressant effect in diabetes-associated depression: A novel potential of RAAS inhibition. Psychoneuroendocrinology 2020, 118, 104705. [Google Scholar] [CrossRef]
- Gimenez, V.M.; Sanz, R.L.; Marón, F.J.M.; Ferder, L.; Manucha, W. Vitamin D-RAAS connection: An integrative standpoint into cardiovascular and neuroinflammatory disorders. Curr. Protein Pept. Sci. 2020, 21, 948–954. [Google Scholar] [CrossRef]
- Lima Giacobbo, B.; Doorduin, J.; Klein, H.C.; Dierckx, R.A.; Bromberg, E.; de Vries, E.F. Brain-derived neurotrophic factor in brain disorders: Focus on neuroinflammation. Mol. Neurobiol. 2019, 56, 3295–3312. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic factor BDNF, physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Ta, Q.T.H.; Nguyen, T.T.D.; Le, T.T.; Vo, V.G. Role of insulin resistance in the Alzheimer’s disease progression. Neurochem. Res. 2020, 45, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Singh, T.G. Insulin resistance and bioenergetic manifestations: Targets and approaches in Alzheimer’s disease. Life Sci. 2020, 262, 118401. [Google Scholar] [CrossRef]
- Jones, J.G. Hepatic glucose and lipid metabolism. Diabetologia 2016, 59, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Cheng, X.-f.; Liu, Y.; Lv, Q.-z.; Liu, G.-l.; Zhang, J.-g.; Li, X.-y. Potential nexus of non-alcoholic fatty liver disease and type 2 diabetes mellitus: Insulin resistance between hepatic and peripheral tissues. Front. Pharmacol. 2019, 9, 1566. [Google Scholar] [CrossRef]
- Mohamed, J.; Nafizah, A.N.; Zariyantey, A.; Budin, S. Mechanisms of diabetes-induced liver damage: The role of oxidative stress and inflammation. Sultan Qaboos Univ. Med. J. 2016, 16, e132. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of oxidative stress in pathophysiology of nonalcoholic fatty liver disease. Oxidative Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef]
- Ballestri, S.; Nascimbeni, F.; Romagnoli, D.; Lonardo, A. The independent predictors of non-alcoholic steatohepatitis and its individual histological features. Insulin resistance, serum uric acid, metabolic syndrome, alanine aminotransferase and serum total cholesterol are a clue to pathogenesis and candidate targets for treatment. Hepatol. Res. 2016, 46, 1074–1087. [Google Scholar]
- Yang, K.; Chen, J.; Zhang, T.; Yuan, X.; Ge, A.; Wang, S.; Xu, H.; Zeng, L.; Ge, J. Efficacy and safety of dietary polyphenol supplementation in the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis. Front. Immunol. 2022, 13, 949746. [Google Scholar] [CrossRef]
- Myint, M.; Oppedisano, F.; De Giorgi, V.; Kim, B.-M.; Marincola, F.M.; Alter, H.J.; Nesci, S. Inflammatory signaling in NASH driven by hepatocyte mitochondrial dysfunctions. J. Transl. Med. 2023, 21, 757. [Google Scholar] [CrossRef] [PubMed]
- Luangmonkong, T.; Suriguga, S.; Mutsaers, H.A.; Groothuis, G.M.; Olinga, P.; Boersema, M. Targeting oxidative stress for the treatment of liver fibrosis. Rev. Physiol. Biochem. Pharmacol. 2018, 175, 71–102. [Google Scholar] [PubMed]
- Eslamparast, T.; Eghtesad, S.; Poustchi, H.; Hekmatdoost, A. Recent advances in dietary supplementation, in treating non-alcoholic fatty liver disease. World J. Hepatol. 2015, 7, 204. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yin, G.; Li, Q.Q.; Zeng, Q.; Duan, J. Diabetes mellitus causes male reproductive dysfunction: A review of the evidence and mechanisms. In Vivo 2021, 35, 2503–2511. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Oliveira, P.F.; Sousa, M.; Silva, B.M.; Alves, M.G. Role of reactive oxygen species in diabetes-induced male reproductive dysfunction. In Oxidants, Antioxidants and Impact of the Oxidative Status in Male Reproduction; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–147. [Google Scholar]
- Chen, J.; Su, Y.; Lin, F.; Iqbal, M.; Mehmood, K.; Zhang, H.; Shi, D. Effect of paraquat on cytotoxicity involved in oxidative stress and inflammatory reaction: A review of mechanisms and ecological implications. Ecotoxicol. Environ. Saf. 2021, 224, 112711. [Google Scholar] [CrossRef] [PubMed]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Pickering, R.J.; Rosado, C.J.; Sharma, A.; Buksh, S.; Tate, M.; de Haan, J.B. Recent novel approaches to limit oxidative stress and inflammation in diabetic complications. Clin. Transl. Immunol. 2018, 7, e1016. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, A.; Kumar, A. Targeting oxidative stress through antioxidants in diabetes mellitus. J. Drug Target. 2018, 26, 766–776. [Google Scholar] [CrossRef]
- Wang, P.; Fiaschi-Taesch, N.M.; Vasavada, R.C.; Scott, D.K.; Garcia-Ocana, A.; Stewart, A.F. Diabetes mellitus—Advances and challenges in human β-cell proliferation. Nat. Rev. Endocrinol. 2015, 11, 201–212. [Google Scholar] [CrossRef]
- Entezari, M.; Hashemi, D.; Taheriazam, A.; Zabolian, A.; Mohammadi, S.; Fakhri, F.; Hashemi, M.; Hushmandi, K.; Ashrafizadeh, M.; Zarrabi, A.; et al. AMPK signaling in diabetes mellitus, insulin resistance and diabetic complications: A pre-clinical and clinical investigation. Biomed. Pharmacother. 2022, 146, 112563. [Google Scholar] [CrossRef] [PubMed]
- Joshi, T.; Singh, A.K.; Haratipour, P.; Sah, A.N.; Pandey, A.K.; Naseri, R.; Juyal, V.; Farzaei, M.H. Targeting AMPK signaling pathway by natural products for treatment of diabetes mellitus and its complications. J. Cell. Physiol. 2019, 234, 17212–17231. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, S.; Zhai, A.; Zhang, B.; Tian, G. AMPK-mediated regulation of lipid metabolism by phosphorylation. Biol. Pharm. Bull. 2018, 41, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Marino, A.; Hausenloy, D.J.; Andreadou, I.; Horman, S.; Bertrand, L.; Beauloye, C. AMP-activated protein kinase: A remarkable contributor to preserve a healthy heart against ROS injury. Free Radic. Biol. Med. 2021, 166, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, K.A.; Valentine, R.J.; Ruderman, N.B.; Saha, A.K. AMPK activation: A therapeutic target for type 2 diabetes? Diabetes Metab. Syndr. Obes. 2014, 7, 241–253. [Google Scholar] [PubMed]
- Misra, P.; Chakrabarti, R. The role of AMP kinase in diabetes. Indian J. Med. Res. 2007, 125, 389–398. [Google Scholar]
- Madhavi, Y.; Gaikwad, N.; Yerra, V.G.; Kalvala, A.K.; Nanduri, S.; Kumar, A. Targeting AMPK in diabetes and diabetic complications: Energy homeostasis, autophagy and mitochondrial health. Curr. Med. Chem. 2019, 26, 5207–5229. [Google Scholar] [CrossRef]
- Babiker, A.; Al Dubayee, M. Anti-diabetic medications: How to make a choice? Sudan. J. Paediatr. 2017, 17, 11. [Google Scholar] [CrossRef]
- Kumar, R.; Kerins, D.; Walther, T. Cardiovascular safety of anti-diabetic drugs. Eur. Heart J.-Cardiovasc. Pharmacother. 2016, 2, 32–43. [Google Scholar] [CrossRef]
- Blahova, J.; Martiniakova, M.; Babikova, M.; Kovacova, V.; Mondockova, V.; Omelka, R. Pharmaceutical drugs and natural therapeutic products for the treatment of type 2 diabetes mellitus. Pharmaceuticals 2021, 14, 806. [Google Scholar] [CrossRef]
- Francini, F.; Schinella, G.R.; Ríos, J.-L. Activation of AMPK by medicinal plants and natural products: Its role in type 2 diabetes mellitus. Mini Rev. Med. Chem. 2019, 19, 880–901. [Google Scholar] [CrossRef] [PubMed]
- Hurtová, M.; Biedermann, D.; Osifová, Z.; Cvačka, J.; Valentová, K.; Křen, V. Preparation of Synthetic and Natural Derivatives of Flavonoids Using Suzuki–Miyaura Cross-Coupling Reaction. Molecules 2022, 27, 967. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naidoo, K.; Khathi, A. The Potential Role of Gossypetin in the Treatment of Diabetes Mellitus and Its Associated Complications: A Review. Int. J. Mol. Sci. 2023, 24, 17609. https://doi.org/10.3390/ijms242417609
Naidoo K, Khathi A. The Potential Role of Gossypetin in the Treatment of Diabetes Mellitus and Its Associated Complications: A Review. International Journal of Molecular Sciences. 2023; 24(24):17609. https://doi.org/10.3390/ijms242417609
Chicago/Turabian StyleNaidoo, Karishma, and Andile Khathi. 2023. "The Potential Role of Gossypetin in the Treatment of Diabetes Mellitus and Its Associated Complications: A Review" International Journal of Molecular Sciences 24, no. 24: 17609. https://doi.org/10.3390/ijms242417609
APA StyleNaidoo, K., & Khathi, A. (2023). The Potential Role of Gossypetin in the Treatment of Diabetes Mellitus and Its Associated Complications: A Review. International Journal of Molecular Sciences, 24(24), 17609. https://doi.org/10.3390/ijms242417609






