Discovery and Characterization of MaK: A Novel Knottin Antimicrobial Peptide from Monochamus alternatus
Abstract
:1. Introduction
2. Results
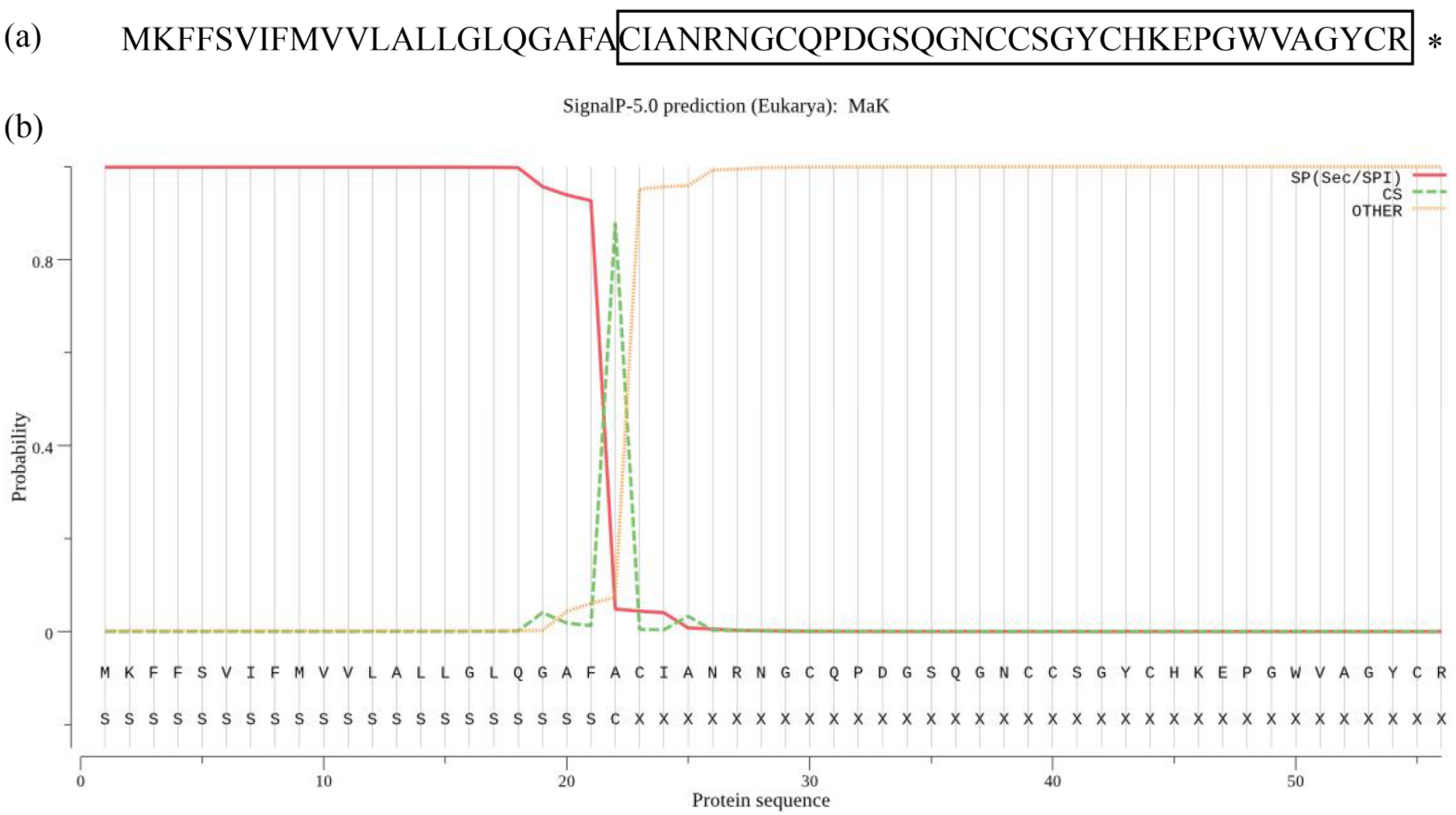
2.1. The Structure of Knottin-Type Peptide MaK
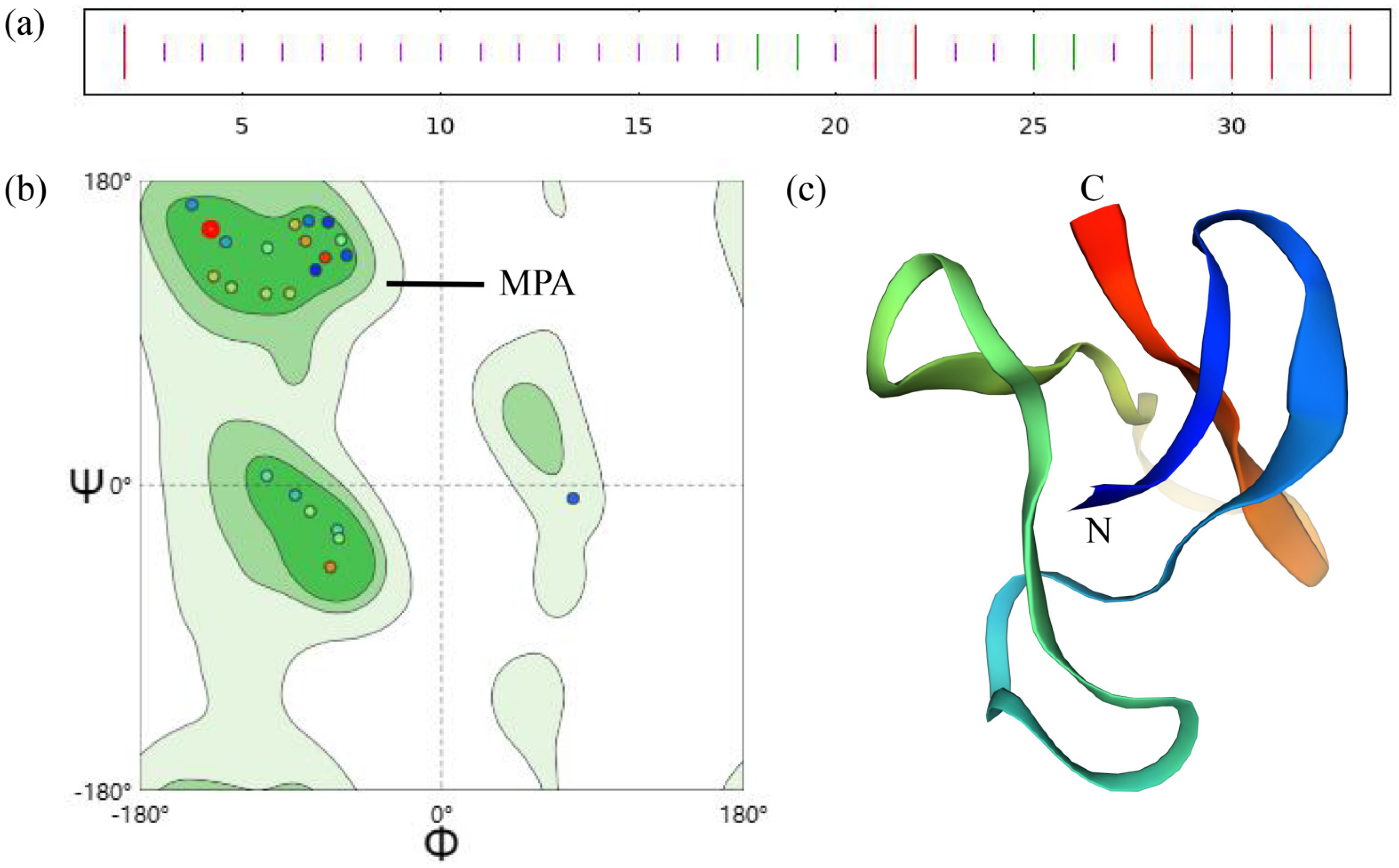
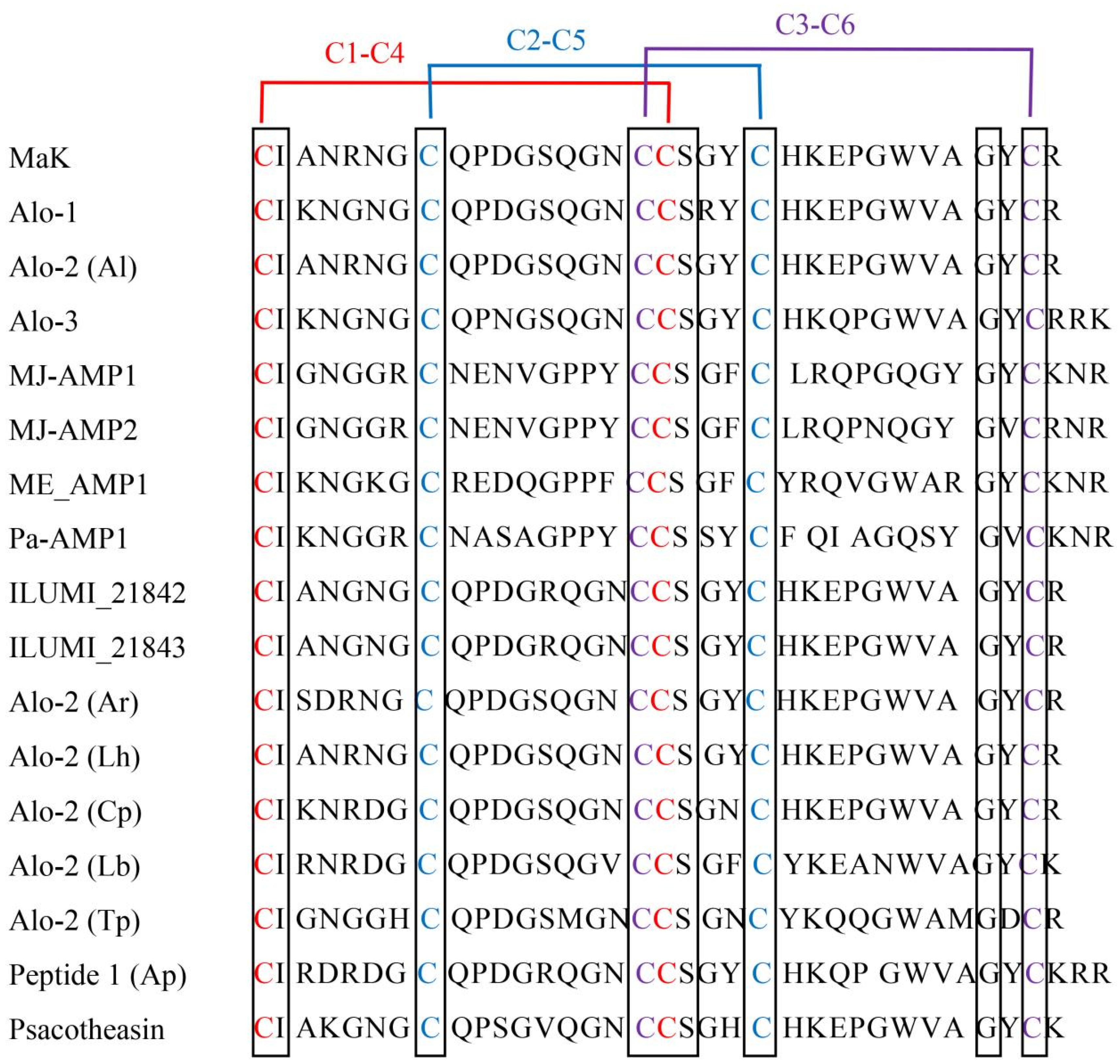
2.2. Structural Analysis and Homology Alignment of Knottin-Type Peptide MaK
2.3. Peptide Synthesis
2.4. Antimicrobial Activity Testing of Knottin-Type Peptide MaK
2.5. Nematocidal Activity Testing of Knottin-Type Peptide MaK
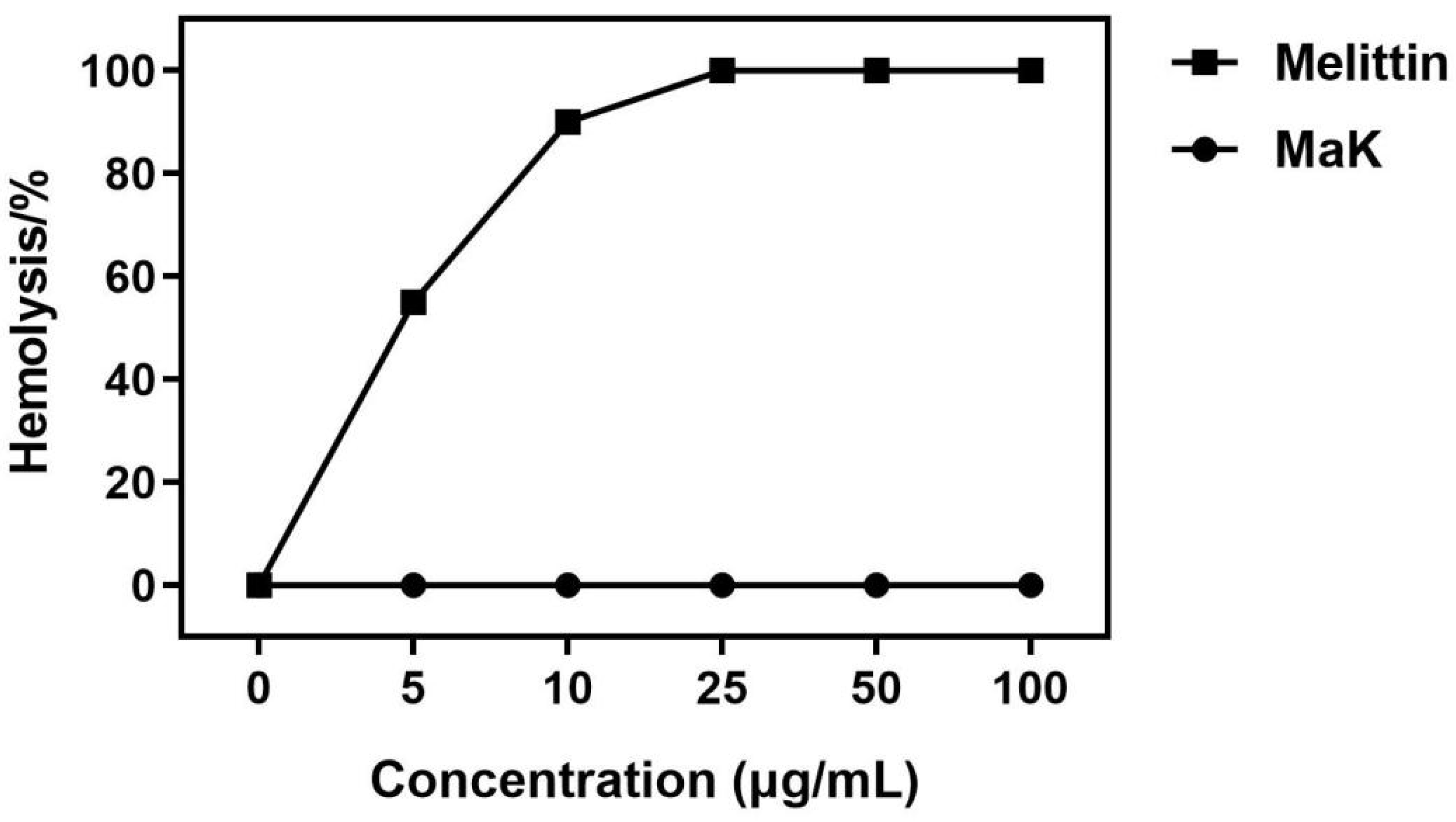
2.6. Hemolytic Activity Assay of Knottin-Type Peptide MaK
2.7. Thermal Stability Assessment of Knottin-Type Peptide MaK
3. Discussion
4. Materials and Methods
4.1. Transcriptomic Data Filtering and Sequence Analysis
4.2. Structural Analysis and Homology Comparison of Knottin-Type Peptide MaK
4.3. Peptide Synthesis
4.4. Measurement of the Antimicrobial Activity of Knottin-Type Peptide MaK
4.5. The Nematocidal Action of Knottin-Type Peptide MaK
4.6. Hemolytic Activity Assay of Knottin-Type Peptide MaK
4.7. Thermal Stability Testing of Knottin-Type Peptide MaK
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Togashi, K.; Arakawa, Y. Horizontal transmission of Bursaphelenchus xylophilus between sexes of Monochamus alternatus. J. Nematol. 2003, 35, 7–16. [Google Scholar] [PubMed]
- Futai, K. Pine wood nematode, Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef]
- Gao, R.; Liu, L.; Zhao, L.; Cui, S. Potentially suitable geographical area for Monochamus alternatus under current and future climatic scenarios based on optimized maxent model. Insects 2023, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Kirino, H.; Maehara, N.; Shinya, R. How did Bursaphelenchus nematodes acquire a specific relationship with their beetle vectors, Monochamus? Front. Physiol. 2023, 14, 1209695. [Google Scholar] [CrossRef] [PubMed]
- Maehara, N.; Kanzaki, N.; Aikawa, T.; Nakamura, K. Potential vector switching in the evolution of Bursaphelenchus xylophilus group nematodes (Nematoda: Aphelenchoididae). Ecol. Evol. 2020, 10, 14320–14329. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Weng, M.; Sun, Y.; Carballar-Lejarazú, R.; Wu, S.; Lian, C. Bacillus thuringiensis toxins with nematocidal activity against the pinewood nematode Bursaphelenchus xylophilus. J. Invertebr. Pathol. 2022, 189, 107726. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, H.; Ding, X.; Wang, L.; Wang, X.; Chen, F. The detection of pine wilt disease: A literature review. Int. J. Mol. Sci. 2022, 23, 10797. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Jeong, S.G.; Choi, I.S.; Yang, J.E.; Lee, K.H.; Kim, J.; Kim, J.C.; Kim, J.S.; Park, H.W. Mechanisms of insecticidal action of Metarhizium anisopliae on adult Japanese pine sawyer beetles (Monochamus alternatus). ACS Omega 2020, 5, 25312–25318. [Google Scholar] [CrossRef]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef]
- Neyen, C.; Poidevin, M.; Roussel, A.; Lemaitre, B. Tissue and ligand specific sensing of gram-negative infection in drosophila by PGRP-LC isoforms and PGRP-LE. J. Immunol. 2012, 189, 1886–1897. [Google Scholar] [CrossRef]
- Ciulla, M.G.; Gelain, F. Structure-activity relationships of antibacterial peptides. Microb. Biotechnol. 2023, 16, 757–777. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Wang, A.; Li, Y.; Cai, Z.; Lemaitre, B.; Zhang, H. The dual oxidase gene BdDuox regulates the intestinal bacterial community homeostasis of Bactrocera dorsalis. ISME J. 2016, 10, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Candian, V.; Savio, C.; Meneguz, M.; Gasco, L.; Tedeschi, R. Effect of the rearing diet on gene expression of antimicrobial peptides in Hermetia illucens (Diptera: Stratiomyidae). Insect Sci. 2023, 30, 933–946. [Google Scholar] [CrossRef] [PubMed]
- Dekmak, A.S.; Yang, X.; Zu Dohna, H.; Buchon, N.; Osta, M.A. The route of infection influences the contribution of key immunity genes to antibacterial defense in Anopheles gambiae. J. Innate Immun. 2021, 13, 107–126. [Google Scholar] [CrossRef]
- Morejon, B.; Michel, K. A zone-of-inhibition assay to screen for humoral antimicrobial activity in mosquito hemolymph. Front. Cell. Infect. Microbiol. 2023, 13, 891577. [Google Scholar] [CrossRef]
- Vizioli, J.; Richman, A.M.; Uttenweiler-Joseph, S.; Blass, C.; Bulet, P. The defensin peptide of the malaria vector mosquito Anopheles gambiae: Antimicrobial activities and expression in adult mosquitoes. Insect Biochem. Mol. Biol. 2001, 31, 241–248. [Google Scholar] [CrossRef]
- Rhode, C.; Greenwood, M.P. Antimicrobial peptide gene expression and the microbiome in Black Soldier Fly. Insect Sci. 2023, 30, 1017–1021. [Google Scholar] [CrossRef]
- Wang, H.; Meng, X.L.; Xu, J.P.; Wang, J.; Wang, H.; Ma, C.W. Production, purification, and characterization of the cecropin from Plutella xylostella, pxCECA1, using an intein induced self cleavable system in Escherichia coli. Appl. Microbiol. Biotechnol. 2012, 94, 1031–1039. [Google Scholar] [CrossRef]
- Fehlbaum, P.; Bulet, P.; Chernysh, S.; Briand, J.P.; Roussel, J.P.; Letellier, L.; Hetru, C.; Hoffmann, J.A. Structure-activity analysis of thanatin, a 21-residue inducible insect defense peptide with sequence homology to frog skin antimicrobial peptides. Proc. Natl. Acad. Sci. USA 1996, 93, 1221–1225. [Google Scholar] [CrossRef]
- Date-Ito, A.; Kasahara, K.; Sawai, H.; Chigusa, S.I. Rapid evolution of the male-specific antibacterial protein andropin gene in Drosophila. J. Mol. Evol. 2002, 54, 665–670. [Google Scholar] [CrossRef]
- Steiner, H.; Hultmark, D.; Engström, A.; Bennich, H.; Boman, H.G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 292: 246–248 1981. J. Immunol. 2009, 182, 6635–6637. [Google Scholar] [PubMed]
- Moore, S.J.; Hayden Gephart, M.G.; Bergen, J.M.; Su, Y.S.; Rayburn, H.; Scott, M.P.; Cochran, J.R. Engineered knottin peptide enables noninvasive optical imaging of intracranial medulloblastoma. Proc. Natl. Acad. Sci. USA 2013, 110, 14598–14603. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.J.; Leung, C.L.; Norton, H.K.; Cochran, J.R. Engineering agatoxin, a cystine-knot peptide from spider venom, as a molecular probe for in vivo tumor imaging. PLoS ONE 2013, 8, e60498. [Google Scholar] [CrossRef] [PubMed]
- Attah, F.A.; Lawal, B.A.; Yusuf, A.B.; Adedeji, O.J.; Folahan, J.T.; Akhigbe, K.O.; Roy, T.; Lawal, A.A.; Ogah, N.B.; Olorundare, O.E.; et al. Nutritional and pharmaceutical applications of under-explored knottin peptide-rich phytomedicines. Plants 2022, 11, 3271. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Stanger, K.; Kaluarachchi, H.; Maurer, T.; Ciepla, P.; Chalouni, C.; Franke, Y.; Hannoush, R.N. Cellular uptake of a cystine-knot peptide and modulation of its intracellular trafficking. Sci. Rep. 2016, 6, 35179. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.P.G.; Walker, A.A.; King, G.F.; Rossi, G.D. Immunosuppressive, antimicrobial and insecticidal activities of inhibitor cystine knot peptides produced by teratocytes of the endoparasitoid wasp Cotesia flavipes (Hymenoptera: Braconidae). Insect Sci. 2023, 30, 1105–1117. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, F.H.; Meissner, G.O.; Herzig, V.; Justa, H.C.; Dias, B.C.; Trevisan-Silva, D.; Gremski, L.H.; Gremski, W.; Senff-Ribeiro, A.; Chaim, O.M.; et al. Insecticidal activity of a recombinant knottin peptide from Loxosceles intermedia venom and recognition of these peptides as a conserved family in the genus. Insect Mol. Biol. 2017, 26, 25–34. [Google Scholar] [CrossRef]
- Layer, P.; Stanghellini, V. Review article: Linaclotide for the management of irritable bowel syndrome with constipation. Aliment. Pharmacol. Ther. 2014, 39, 371–384. [Google Scholar] [CrossRef]
- D’Souza, C.; Henriques, S.T.; Wang, C.K.; Cheneval, O.; Chan, L.Y.; Bokil, N.J.; Sweet, M.J.; Craik, D.J. Using the MCoTI-II cyclotide scaffold to design a stable cyclic peptide antagonist of SET, a protein overexpressed in human cancer. Biochemistry 2016, 55, 396–405. [Google Scholar] [CrossRef]
- Huang, Y.H.; Henriques, S.T.; Wang, C.K.; Thorstholm, L.; Daly, N.L.; Kaas, Q.; Craik, D.J. Design of substrate-based BCR-ABL kinase inhibitors using the cyclotide scaffold. Sci. Rep. 2015, 5, 12974. [Google Scholar] [CrossRef]
- Ji, Y.; Majumder, S.; Millard, M.; Borra, R.; Bi, T.; Elnagar, A.Y.; Neamati, N.; Shekhtman, A.; Camarero, J.A. In vivo activation of the p53 tumor suppressor pathway by an engineered cyclotide. J. Am. Chem. Soc. 2013, 135, 11623–11633. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Rehm, F.B.H.; Yap, K.; Zdenek, C.N.; Harding, M.D.; Fry, B.G.; Durek, T.; Craik, D.J.; de Veer, S.J. Cystine knot peptides with tuneable activity and mechanism. Angew. Chem. (Int. Ed. Engl.) 2022, 61, e202200951. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.S.; Lee, J.; Hwang, B.; Nam, S.H.; Yun, E.Y.; Kim, S.R.; Lee, D.G. Isolation and characterization of Psacotheasin, a novel knottin-type antimicrobial peptide, from Psacothea hilaris. J. Microbiol. Biotechnol. 2010, 20, 708–711. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wei, X.; Sui, Z.; Guo, M.; Geng, J.; Xiao, J.; Huang, D. Preparation of antioxidant and antibacterial chitosan film from Periplaneta americana. Insects 2021, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Peng, L.Y.; Wang, Z.C.; Wang, W.; Zhu, Z.; Huang, X.H.; Chen, L.B.; Song, Q.S.; Bao, Y.Y. Identification of novel antimicrobial peptides from rice planthopper, Nilaparvata lugens. Insect Biochem. Mol. Biol. 2019, 113, 103215. [Google Scholar] [CrossRef] [PubMed]
- Maaroufi, H.; Potvin, M.; Cusson, M.; Levesque, R.C. Novel antimicrobial anionic cecropins from the spruce budworm feature a poly-L-aspartic acid C-terminus. Proteins 2021, 89, 1205–1215. [Google Scholar] [CrossRef] [PubMed]
- Vilcinskas, A.; Mukherjee, K.; Vogel, H. Expansion of the antimicrobial peptide repertoire in the invasive ladybird Harmonia axyridis. Proc. Biol. Sci. 2013, 280, 20122113. [Google Scholar]
- Álvarez-Baz, G.; Fernández-Bravo, M.; Pajares, J.; Quesada-Moraga, E. Potential of native Beauveria pseudobassiana strain for biological control of pine wood nematode vector Monochamus galloprovincialis. J. Invertebr. Pathol. 2015, 132, 48–56. [Google Scholar] [CrossRef]
- Huang, T.; Lin, Q.; Qian, X.; Zheng, Y.; Yao, J.; Wu, H.; Li, M.; Jin, X.; Pan, X.; Zhang, L.; et al. Nematicidal Activity of Cry1Ea11 from Bacillus thuringiensis BRC-XQ12 against the pine wood nematode (Bursaphelenchus xylophilus). Phytopathology 2018, 108, 44–51. [Google Scholar] [CrossRef]
- Kim, S.H.; Yun, H.Y.; Jeong, H.J. Generation of a novel antibody against BxPrx, a diagnostic marker of pine wilt disease. Mol. Biol. Rep. 2023, 50, 4715–4721. [Google Scholar] [CrossRef]
- Kolmar, H. Biological diversity and therapeutic potential of natural and engineered cystine knot miniproteins. Curr. Opin. Pharmacol. 2009, 9, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Sommerhoff, C.P.; Avrutina, O.; Schmoldt, H.U.; Gabrijelcic-Geiger, D.; Diederichsen, U.; Kolmar, H. Engineered cystine knot miniproteins as potent inhibitors of human mast cell tryptase beta. J. Mol. Biol. 2010, 395, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, S.E.; Currier, N.V.; Bergen, J.M.; Cochran, J.R. Cystine-knot peptides: Emerging tools for cancer imaging and therapy. Expert Rev. Proteom. 2014, 11, 561–572. [Google Scholar] [CrossRef] [PubMed]
- De Veer, S.J.; Kan, M.W.; Craik, D.J. Cyclotides: From structure to function. Chem. Rev. 2019, 119, 12375–12421. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.; Hwang, J.S.; Lee, J.; Lee, D.G. Antifungal properties and mode of action of Psacotheasin, a novel knottin-type peptide derived from Psacothea hilaris. Biochem. Biophys. Res. Commun. 2010, 400, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Arguelles, J.; Lee, J.; Cardenas, L.V.; Govind, S.; Singh, S. In silico analysis of a Drosophila parasitoid venom peptide reveals prevalence of the cation-polar-cation clip motif in knottin proteins. Pathogens 2023, 12, 143. [Google Scholar] [CrossRef]
- Kintzing, J.R.; Cochran, J.R. Engineered knottin peptides as diagnostics, therapeutics, and drug delivery vehicles. Curr. Opin. Chem. Biol. 2016, 34, 143–150. [Google Scholar] [CrossRef]
- Huang, J.; Guo, Y.; Weng, X.; Wu, S. Comparative transcriptome analysis of stage-specific changes in gene expression during larval development in Monochamus alternatus hope. Forests 2021, 12, 1312. [Google Scholar] [CrossRef]
- Lee, J.H.; Chung, H.; Shin, Y.P.; Kim, I.W.; Natarajan, S.; Veerappan, K.; Seo, M.; Park, J.; Hwang, J.S. Transcriptome analysis of Psacothea hilaris: De novo assembly and antimicrobial peptide prediction. Insects 2020, 11, 676. [Google Scholar] [CrossRef]
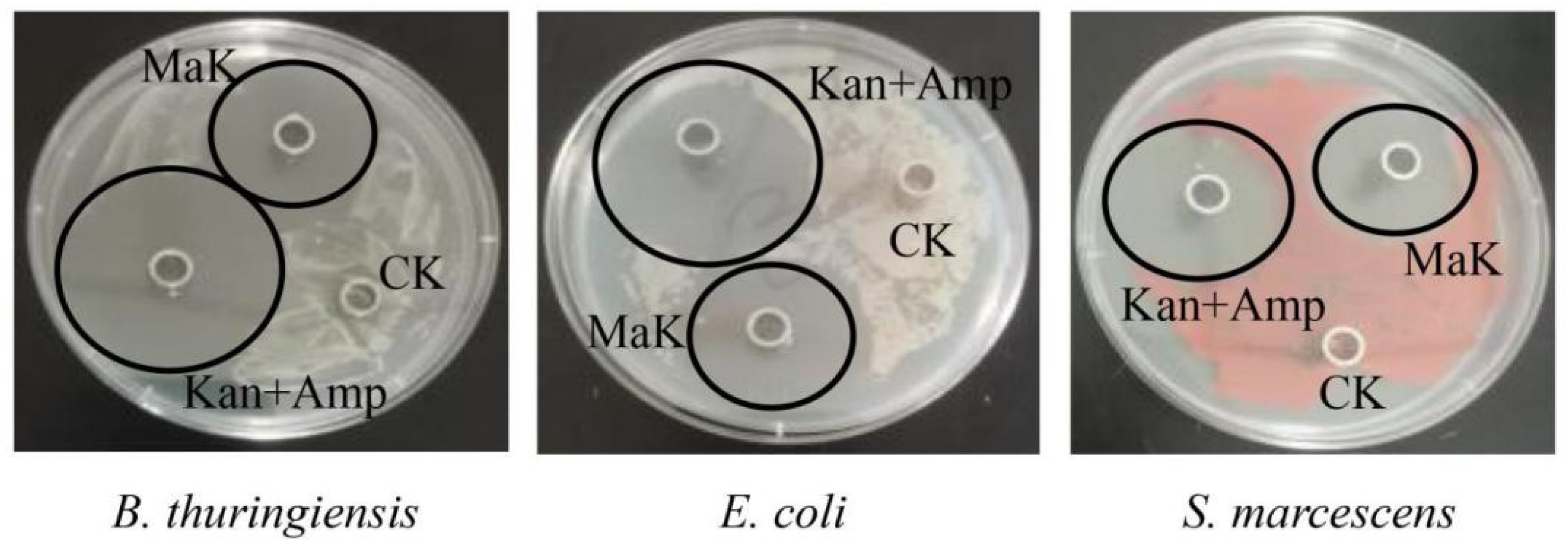


| Bacterial Strains | MIC (µg/mL) | ||
|---|---|---|---|
| MaK | Kan + Amp | ||
| Gram-positive | B. thuringiensis | 36.29 | <36.29 |
| Gram-negative | E. coli | 36.29 | <36.29 |
| S. marcescens | 72.58 | <36.29 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Zhou, T.; Hu, X.; Zhu, Y.; Shi, Z.; Chen, S.; Liu, Y.; Weng, X.; Zhang, F.; Wu, S. Discovery and Characterization of MaK: A Novel Knottin Antimicrobial Peptide from Monochamus alternatus. Int. J. Mol. Sci. 2023, 24, 17565. https://doi.org/10.3390/ijms242417565
Han X, Zhou T, Hu X, Zhu Y, Shi Z, Chen S, Liu Y, Weng X, Zhang F, Wu S. Discovery and Characterization of MaK: A Novel Knottin Antimicrobial Peptide from Monochamus alternatus. International Journal of Molecular Sciences. 2023; 24(24):17565. https://doi.org/10.3390/ijms242417565
Chicago/Turabian StyleHan, Xiaohong, Tong Zhou, Xinran Hu, Yukun Zhu, Zengzeng Shi, Shi Chen, Yunfei Liu, Xiaoqian Weng, Feiping Zhang, and Songqing Wu. 2023. "Discovery and Characterization of MaK: A Novel Knottin Antimicrobial Peptide from Monochamus alternatus" International Journal of Molecular Sciences 24, no. 24: 17565. https://doi.org/10.3390/ijms242417565
APA StyleHan, X., Zhou, T., Hu, X., Zhu, Y., Shi, Z., Chen, S., Liu, Y., Weng, X., Zhang, F., & Wu, S. (2023). Discovery and Characterization of MaK: A Novel Knottin Antimicrobial Peptide from Monochamus alternatus. International Journal of Molecular Sciences, 24(24), 17565. https://doi.org/10.3390/ijms242417565






