Separation of Antioxidants from Trace Fraction of Ribes himalense via Chromatographic Strategy and Their Antioxidant Activity Supported with Molecular Simulations
Abstract
:1. Introduction
2. Results
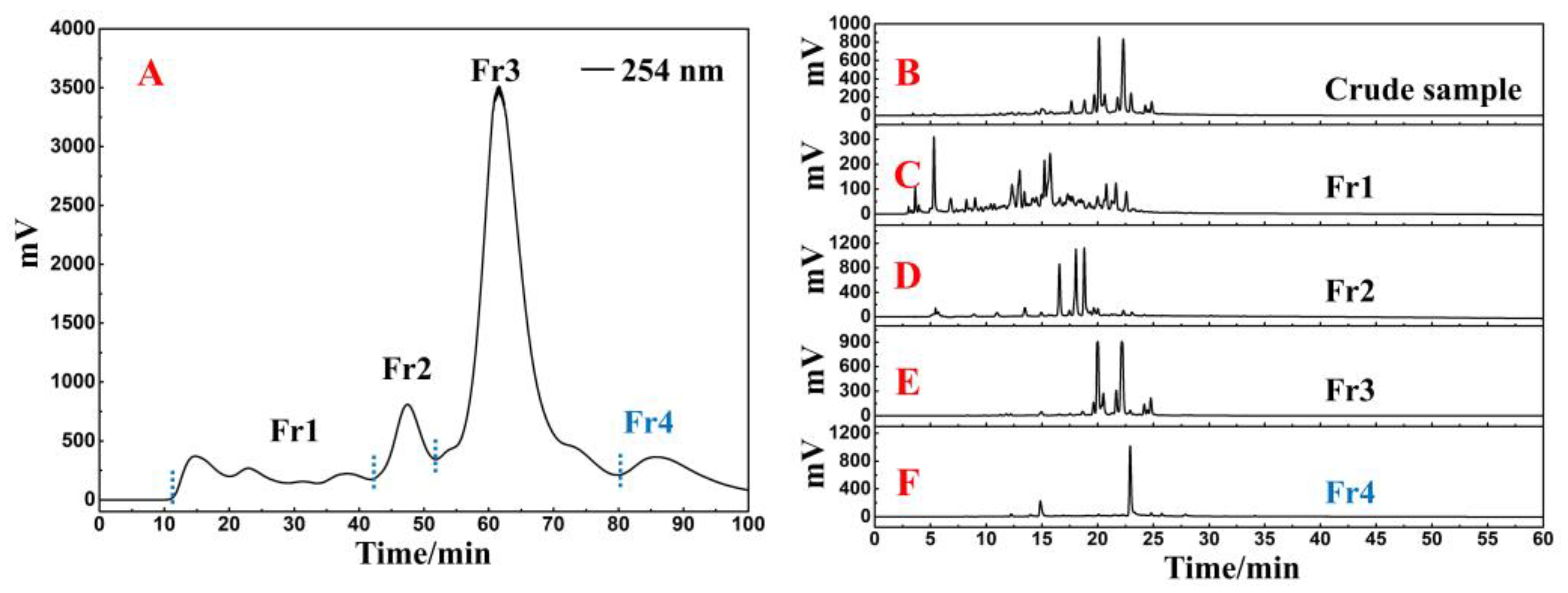
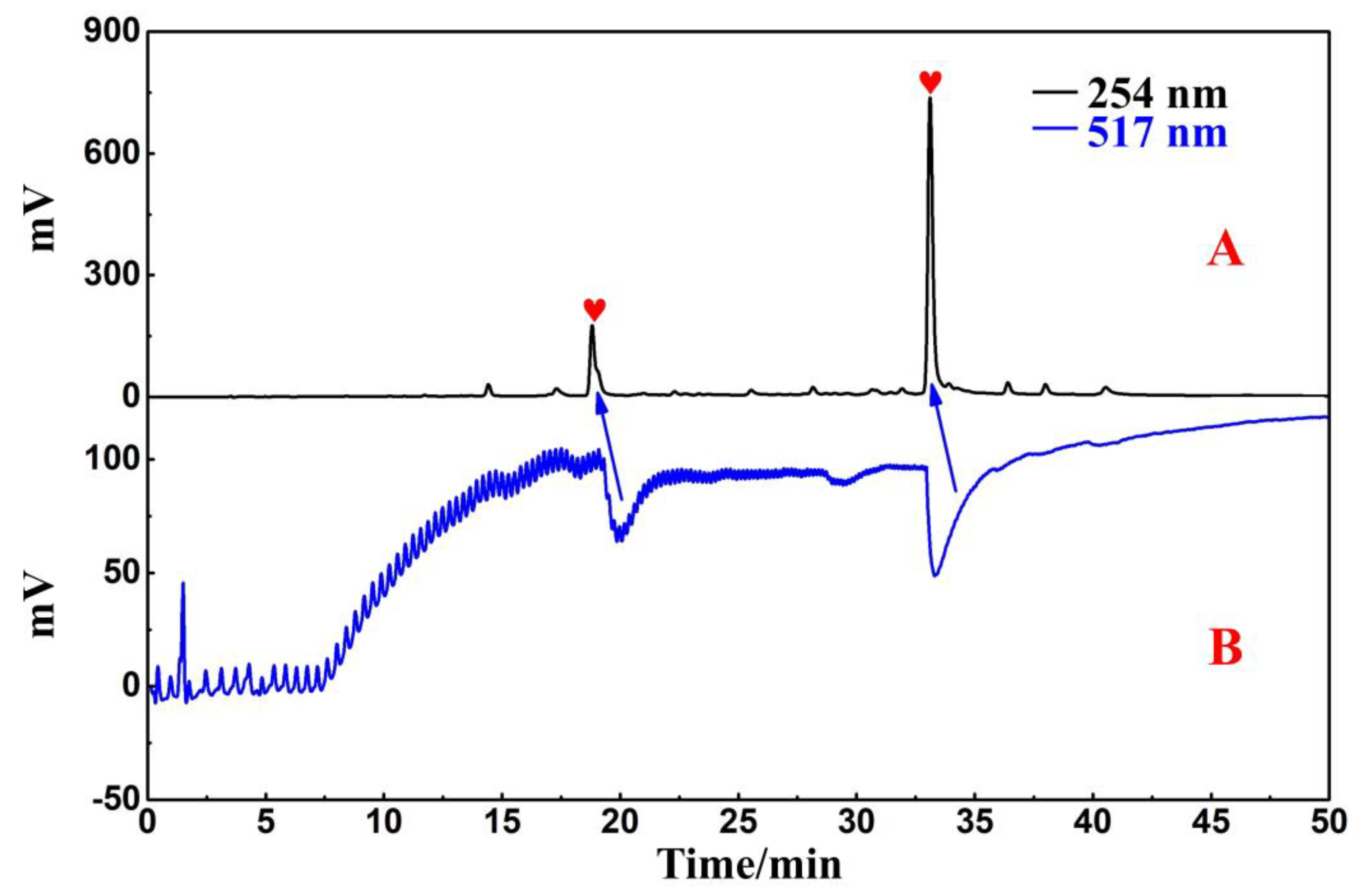
2.1. Polyamide-MPLC Enrichment and Antioxidant Recognition
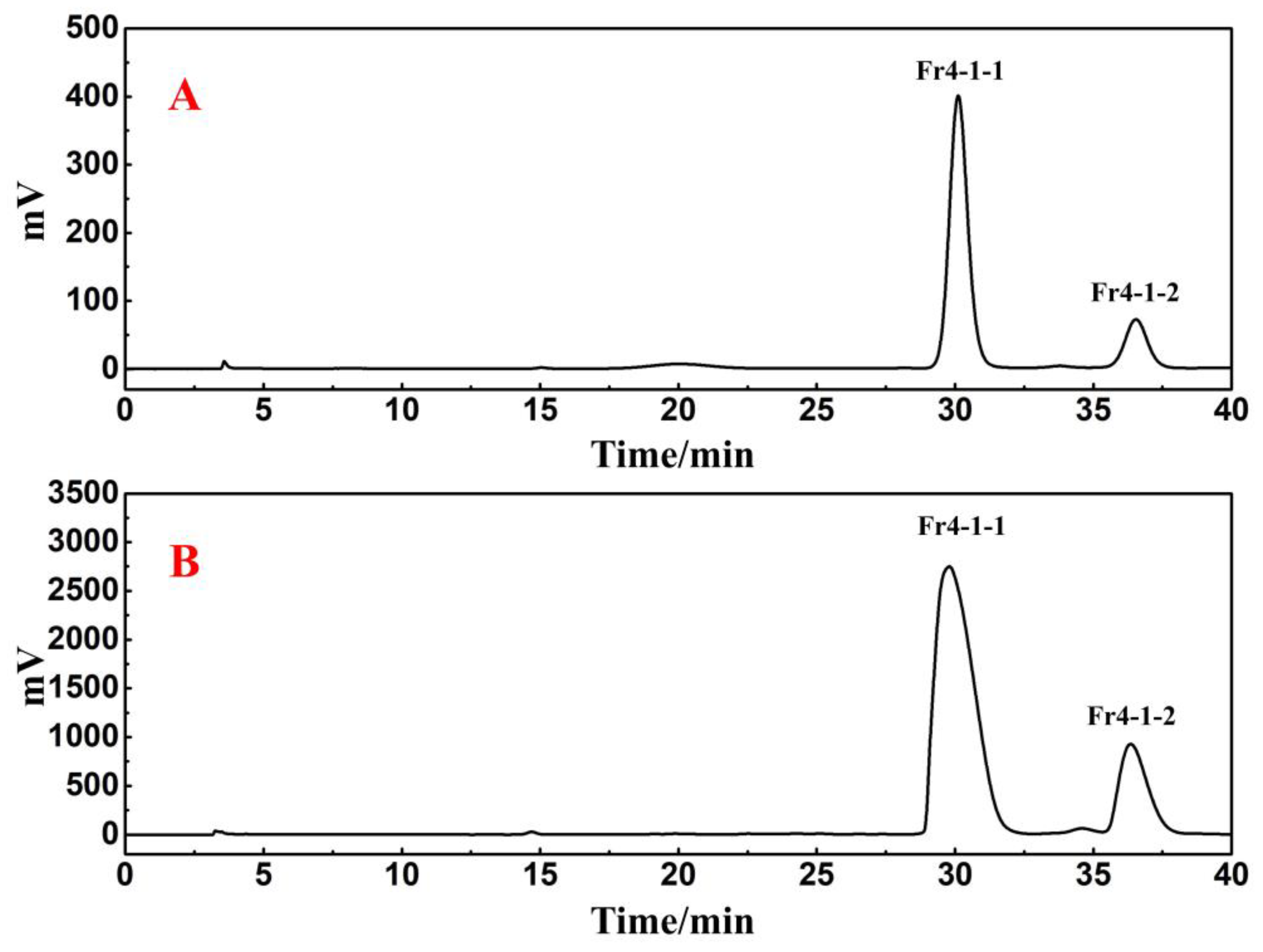
2.2. Directed Separation of Antioxidants from Fr4
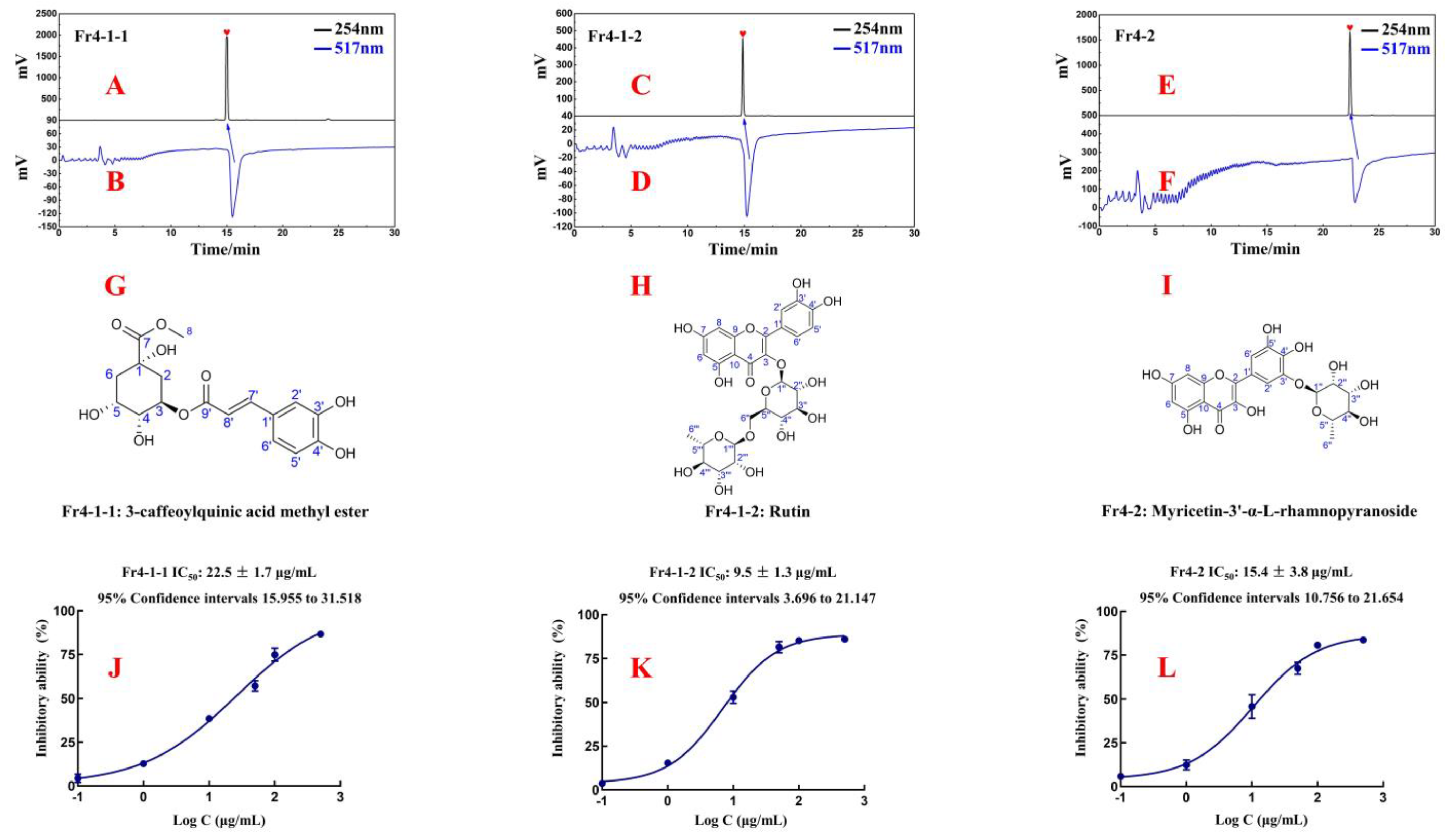
2.3. Purity, Structural Characterization, and Activity of Isolated Antioxidants
- Fr4-1-1: 3-caffeoylquinic acid methyl ester, light yellow powder, 43.3 mg, ESI-MS m/z 367.23, [M-H]−, calc. for C17H20O9, m/z 368.11. 1H NMR (600 MHz, DMSO-d6): 7.39 (1H, d, J = 15.9 Hz, H-7′), 7.02 (1H, d, J = 2.0 Hz, H-2′), 6.97 (1H, dd, J = 8.2, 2.0 Hz, H-6′), 6.77 (1H, d, J = 8.2 Hz, H-5′), 6.10 (1H, d, J = 15.9 Hz, H-8′), 5.01 (1H, dd, J = 9.1, 5.6 Hz, H-3), 3.87 (1H, m, H-4), 3.57 (1H, m, H-5), 3.55 (3H, s, H-7-CH3), 2.10 (2H, m, H-2a, 6a), 1.92 (1H, dd, J =13.6, 3.2 Hz, H-6b), and 1.76 (1H, dd, J = 12.5, 9.6 Hz, H-2b). 13C NMR(151 MHz, DMSO-d6): 173.6 (C-7), 165.4 (C-9′), 148.5 (C-4′), 145.6 (C-7′), 145.1 (C-3′), 125.4 (C-1′), 121.3 (C-6′), 115.8 (C-5′), 114.6 (C-2′), 113.8 (C-8′), 73.0 (C-1), 71.0 (C-5), 69.3 (C-4), 66.8 (C-3), 51.8 (7-O-CH3), and 37.3 (C-2), 35.0 (C-6). These data are consistent with the published data for 3-caffeoylquinic acid methyl ester [17,18].
- Fr4-1-2: rutin, yellow powder, 19.5 mg, HR-ESI-MS m/z 611.1633 [M+H]+, calc. for C27H30O16, m/z 610.1534. 1H NMR (600 MHz, MeOH-d4): 7.66 (1H, d, J = 2.1 Hz, H-2′), 7.62 (1H, dd, J = 8.4, 2.1 Hz, H-6′), 6.87 (1H, d, J = 8.4 Hz, H-5′), 6.39 (1H, d, J = 2.0 Hz, H-8), 6.20 (1H, d, J = 2.0 Hz, H-6), 5.10 (1H, d, J = 7.7 Hz, H-1″), 4.51 (1H, d, J = 1.5 Hz, H-1‴), 3.80~3.25 (10H, m, sugar protons), and 1.11 (3H, d, J = 6.2 Hz, H-6‴). 13C NMR (151 MHz, MeOH-d4): 179.4 (C-4), 166.0 (C-7), 163.0 (C-5), 159.3 (C-9), 158.5 (C-2), 149.8 (C-4′), 145.8 (C-3′), 135.6 (C-3), 123.6 (C-1′), 123.1 (C-6′), 117.5 (C-5′), 116.0 (C-2′), 105.6 (C-10), 104.7 (C-1″), 102.4 (C-1‴), 99.9 (C-6), 94.9 (C-8), 78.2 (C-5″), 77.2 (C-3″), 75.7 (C-2″), 73.9 (C-4‴), 72.2 (C-3‴), 72.1 (C-2‴), 71.4 (C-4″), 69.7 (C-5″), and 68.5 (C-6″), 17.9 (C-6‴). These data are consistent with the published data for rutin [19].
- Fr4-2: myricetin-3′-α-L-rhamnopyranoside, yellow powder, 377.2 mg, ESI-MS m/z 463.26 [M-H]−, calc. for C21H20O12, m/z 464.10). 1H NMR (600 MHz, DMSO-d6): 7.47 (1H, d, J = 2.0 Hz, H-2′), 7.46 (1H, d, J = 2.0 Hz, H-6′), 6.37 (1H, d, J = 2.0 Hz, H-8), 6.18 (1H, d, J = 2.0 Hz, H-6), 5.22 (1H, d, J = 1.3 Hz, H-1″), 3.77~3.28 (4H, m, sugar protons), and 1.16 (3H, d, J = 6.2 Hz, H-6″). 13C NMR (151 MHz, DMSO-d6): 175.9 (C-4), 163.9 (C-7), 160.8 (C-5), 156.1 (C-9), 146.4 (C-2), 146.0 (C-3′), 144.7 (C-5′), 138.6 (C-4′), 136.0 (C-3), 120.8 (C-1′), 110.1 (C-6′), 109.8 (C-2′), 103.0 (C-10), 100.3 (C-1″), 98.2 (C-6), 93.2 (C-8), 72.0 (C-4″), 70.3 (C-3″), 70.1 (C-2″), 69.5 (C-5″), and 17.9 (C-6″). The data are in agreement with those on myricetin-3′-α-L-rhamnopyranoside [20,21].
2.4. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sample Extraction, Polyamide-MPLC Pretreatment, and Activity Screening
4.3. Directed Separation of Antioxidants from Fr4
4.4. Activity and Purity of Antioxidants
4.5. Computer Simulation
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fu, K.; Xu, M.; Zhou, Y.; Li, X.; Wang, Z.; Liu, X.; Meng, X.; Zeng, Y.; Zhang, H. The Status Quo and Way Forwards on the Development of Tibetan Medicine and the Pharmacological Research of Tibetan Materia Medica. Pharmacol. Res. 2020, 155, 104688. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Long, X.; Luobu, T.; Wang, Q. Current Status of Research on Endophytes of Traditional Tibetan Medicinal Plant and Their Metabolites. 3 Biotech 2023, 13, 338. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lei, Y.; Dang, J.; Wang, W.; Zhang, J.; Mei, L.; Liu, Z.; Tao, Y.; Shao, Y. Preparative Isolation of 1,1-Diphenyl-2-Picrylhydrazyl Inhibitors from Ribes Himalense Using Medium-Pressure and Two-Dimensional Reversed-Phase/Reversed-Phase Liquid Chromatography Guided by an Online HPLC-1, 1-Diphenyl-2-Picrylhydrazyl Assay. J. Sep. Sci. 2021, 44, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, N.; Xu, W.; Zhou, H. Ribes himalense as Potential Source of Natural Bioactive Compounds: Nutritional, Phytochemical, and Antioxidant Properties. Food Sci. Nutr. 2021, 9, 2968–2984. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, N.; Xu, W.; Zhou, H. Genus Ribes Linn. (Grossulariaceae): A Comprehensive Review of Traditional Uses, Phytochemistry, Pharmacology and Clinical Applications. J. Ethnopharmacol. 2021, 276, 114166. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lei, Y.; Li, G.; Yuan, C.; Lv, Y.; Yu, S.; Shao, Y.; Dang, J. Three New Dihydroflavonols with Free Radical Scavenging Activity from Ribes Himalense Royle Ex Decne. Nat. Prod. Res. 2022, 36, 5490–5498. [Google Scholar] [CrossRef] [PubMed]
- Olla, S.; Siguri, C.; Fais, A.; Era, B.; Fantini, M.C.; Di Petrillo, A. Inhibitory Effect of Quercetin on Oxidative Endogen Enzymes: A Focus on Putative Binding Modes. Int. J. Mol. Sci. 2023, 24, 15391. [Google Scholar] [CrossRef] [PubMed]
- Newsholme, P.; Cruzat, V.F.; Keane, K.N.; Carlessi, R.; de Bittencourt, P.I.H., Jr. Molecular Mechanisms of ROS Production and Oxidative Stress in Diabetes. Biochem. J. 2016, 473, 4527–4550. [Google Scholar] [CrossRef]
- Yan, R.; Cao, Y.; Yang, B. HPLC-DPPH Screening Method for Evaluation of Antioxidant Compounds Extracted from Semen Oroxyli. Molecules 2014, 19, 4409–4417. [Google Scholar] [CrossRef]
- Dang, J.; Ma, J.; Dawa, Y.; Liu, C.; Ji, T.; Wang, Q. Preparative Separation of 1,1-Diphenyl-2-Picrylhydrazyl Inhibitors Originating from Saxifraga Sinomontana Employing Medium-Pressure Liquid Chromatography in Combination with Reversed-Phase Liquid Chromatography. RSC Adv. 2022, 11, 38739–38749. [Google Scholar] [CrossRef]
- Liu, C.; Lei, Y.; Liu, Y.; Guo, J.; Chen, X.; Tang, Y.; Dang, J.; Wu, M. An Integrated Strategy for Investigating Antioxidants from Ribes Himalense Royle Ex Decne and Their Potential Target Proteins. Antioxidants 2023, 12, 835. [Google Scholar] [CrossRef] [PubMed]
- Marlot, L.; Faure, K. Preparative Two Dimensional Separations Involving Liquid–Liquid Chromatography. J. Chromatogr. A 2017, 1494, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Dawa, Y.; Du, Y.; Wang, Q.; Chen, C.; Zou, D.; Qi, D.; Ma, J.; Dang, J. Targeted Isolation of 1,1-Diphenyl-2-Picrylhydrazyl Inhibitors from Saxifraga Atrata Using Medium- and High- Pressure Liquid Chromatography Combined with Online High Performance Liquid Chromatography–1,1-Diphenyl-2- Picrylhydrazyl Detection. J. Chromatogr. A 2021, 1635, 461690. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, H.; Shen, L.; Yao, L.; Ma, Y.; Yu, D.; Chen, J.; Li, P.; Chen, Y.; Zhang, C. Isolation and Purification of Six Iridoid Glycosides from Gardenia Jasminoides Fruit by Medium-Pressure Liquid Chromatography Combined with Macroporous Resin Chromatography. J. Sep. Sci. 2015, 38, 4119–4126. [Google Scholar] [CrossRef] [PubMed]
- Amin, S.A.; Ghosh, K.; Gayen, S.; Jha, T. Chemical-Informatics Approach to COVID-19 Drug Discovery: Monte Carlo Based QSAR, Virtual Screening and Molecular Docking Study of Some in-House Molecules as Papain-like Protease (PLpro) Inhibitors. J. Biomol. Struct. Dyn. 2021, 39, 4764–4773. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, S.; Wang, B.; Liu, F.; Xu, S.; Li, P.; Chen, Y. An Updated Review on Developing Small Molecule Kinase Inhibitors Using Computer-Aided Drug Design Approaches. Int. J. Mol. Sci. 2023, 24, 13953. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Nisar, M.F.; Wan, C. Characterization of Phenolic Constituents from Prunus cerasifera Ldb Leaves. J. Chem. 2020, 2020, e5976090. [Google Scholar] [CrossRef]
- Peng, L.-Y.; Mei, S.-X.; Jiang, B.; Zhou, H.; Sun, H.-D. Constituents from Lonicera japonica. Fitoterapia 2000, 71, 713–715. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Xing, H.; Lu, X.; Zhao, L.; Qu, K.; Bi, K. Evaluation of Antioxidant Activity of Ten Compounds in Different Tea Samples by Means of an On-Line HPLC–DPPH Assay. Food Res. Int. 2013, 53, 847–856. [Google Scholar] [CrossRef]
- Seo, C.; Ahn, E.-K.; Kang, J.-S.; Lee, J.-H.; Oh, J.S.; Hong, S.S. Excavasides A and B, Two New Flavonoid Glycosides from Clausena excavata Burm. f. (Rutaceae). Phytochem. Lett. 2017, 20, 93–97. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, D.; Deng, J. New Flavonoids from Lysimachia christinae Hance. Helv. Chim. Acta 2013, 96, 985–989. [Google Scholar] [CrossRef]
- Peng, X.; Tang, F.; Yang, Y.; Li, T.; Hu, X.; Li, S.; Wu, W.; He, K. Bidirectional Effects and Mechanisms of Traditional Chinese Medicine. J. Ethnopharmacol. 2022, 298, 115578. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Yang, L.; Qiu, S.; Zhang, A.-H.; Wang, X.-J. Efficacy Evaluation, Active Ingredients, and Multitarget Exploration of Herbal Medicine. Trends Endocrinol. Metab. 2023, 34, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Li, Y.-H.; Li, Z.; Lee, M.-R. Characterization of Effective Phytochemicals in Traditional Chinese Medicine by Mass Spectrometry. Mass Spectrom. Rev. 2023, 42, 1808–1827. [Google Scholar] [CrossRef] [PubMed]
- Molíková, M.; Jandera, P. Characterization of Stationary Phases for Reversed-Phase Chromatography. J. Sep. Sci. 2010, 33, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Long, H.; Liu, Y.; Zhang, Y.; Chen, W.; Tang, S. Preparation and Application of a Novel Imine-Linked Covalent Organic Framework@silica Composite for Reversed-Phase and Hydrophilic Interaction Chromatographic Separations. Anal. Chim. Acta 2023, 1276, 341635. [Google Scholar] [CrossRef]
- Baek, S.-Y.; Lee, S.; Kim, B. Separation of Hexabromocyclododecane Diastereomers: Application of C18 and Phenyl-Hexyl Ultra-Performance Liquid Chromatography Columns. J. Chromatogr. A 2017, 1488, 140–145. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Kajarabille, N.; Latunde-Dada, G.O. Programmed Cell-Death by Ferroptosis: Antioxidants as Mitigators. Int. J. Mol. Sci. 2019, 20, 4968. [Google Scholar] [CrossRef]
- Hano, C.; Tungmunnithum, D. Plant Polyphenols, More than Just Simple Natural Antioxidants: Oxidative Stress, Aging and Age-Related Diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef]
- Alam, F.; Mohammadin, K.; Shafique, Z.; Amjad, S.T.; bin Asad, M.H.H. Citrus Flavonoids as Potential Therapeutic Agents: A Review. Phytother. Res. 2022, 36, 1417–1441. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liang, S.; Ge, L.; Wan, H.; Wu, W.; Fei, J.; Wu, S.; Zhou, B.; Zeng, X. 4,5-Di-O-Caffeoylquinic Acid Methyl Ester Isolated from Lonicera japonica Thunb. Targets the Keap1/Nrf2 Pathway to Attenuate H2O2-Induced Liver Oxidative Damage in HepG2 Cells. Phytomedicine 2020, 70, 153219. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.d.N.; Jung, H.A.; Sohn, H.S.; Kim, H.M.; Choi, J.S. Potent α-Glucosidase and Protein Tyrosine Phosphatase 1B Inhibitors from Artemisia Capillaris. Arch. Pharm. Res. 2013, 36, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-Y.; Kim, J.Y.; Lee, Y.G.; Lee, H.J.; Shim, H.J.; Lee, J.H.; Kim, S.-J.; Ham, K.-S.; Moon, J.-H. Four New Dicaffeoylquinic Acid Derivatives from Glasswort (Salicornia herbacea L.) and Their Antioxidative Activity. Molecules 2016, 21, 1097. [Google Scholar] [CrossRef] [PubMed]
- Cruz, B.G.; dos Santos, H.S.; Bandeira, P.N.; Rodrigues, T.H.S.; Matos, M.G.C.; Nascimento, M.F.; de Carvalho, G.G.C.; Braz-Filho, R.; Teixeira, A.M.R.; Tintino, S.R.; et al. Evaluation of Antibacterial and Enhancement of Antibiotic Action by the Flavonoid Kaempferol 7-O-β-D-(6″-O-Cumaroyl)-Glucopyranoside Isolated from Croton Piauhiensis Müll. Microb. Pathog. 2020, 143, 104144. [Google Scholar] [CrossRef] [PubMed]
- Forli, S.; Huey, R.; Pique, M.E.; Sanner, M.F.; Goodsell, D.S.; Olson, A.J. Computational Protein–Ligand Docking and Virtual Drug Screening with the AutoDock Suite. Nat. Protoc. 2016, 11, 905–919. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Choi, M.; Oh, J.; Rhim, T.; Lee, M. Delivery of Hypoxia-Inducible Heme Oxygenase-1 Gene for Site-Specific Gene Therapy in the Ischemic Stroke Animal Model. Pharm. Res. 2016, 33, 2250–2258. [Google Scholar] [CrossRef]
- Vladimirova, O.; Soldan, S.; Su, C.; Kossenkov, A.; Ngalamika, O.; Tso, F.Y.; West, J.T.; Wood, C.; Lieberman, P.M. Elevated iNOS and 3′-Nitrotyrosine in Kaposi’s Sarcoma Tumors and Mouse Model. Tumour Virus Res. 2023, 15, 200259. [Google Scholar] [CrossRef]
- Petsouki, E.; Ender, S.; Sosa Cabrera, S.N.; Heiss, E.H. AMPK-Mediated Phosphorylation of Nrf2 at S374/S408/S433 Favors Its βTrCP2-Mediated Degradation in KEAP1-Deficient Cells. Antioxidants 2023, 12, 1586. [Google Scholar] [CrossRef]
- Damiano, S.; Forino, M.; De, A.; Vitali, L.A.; Lupidi, G.; Taglialatela-Scafati, O. Antioxidant and Antibiofilm Activities of Secondary Metabolites from Ziziphus Jujuba Leaves Used for Infusion Preparation. Food Chem. 2017, 230, 24–29. [Google Scholar] [CrossRef] [PubMed]


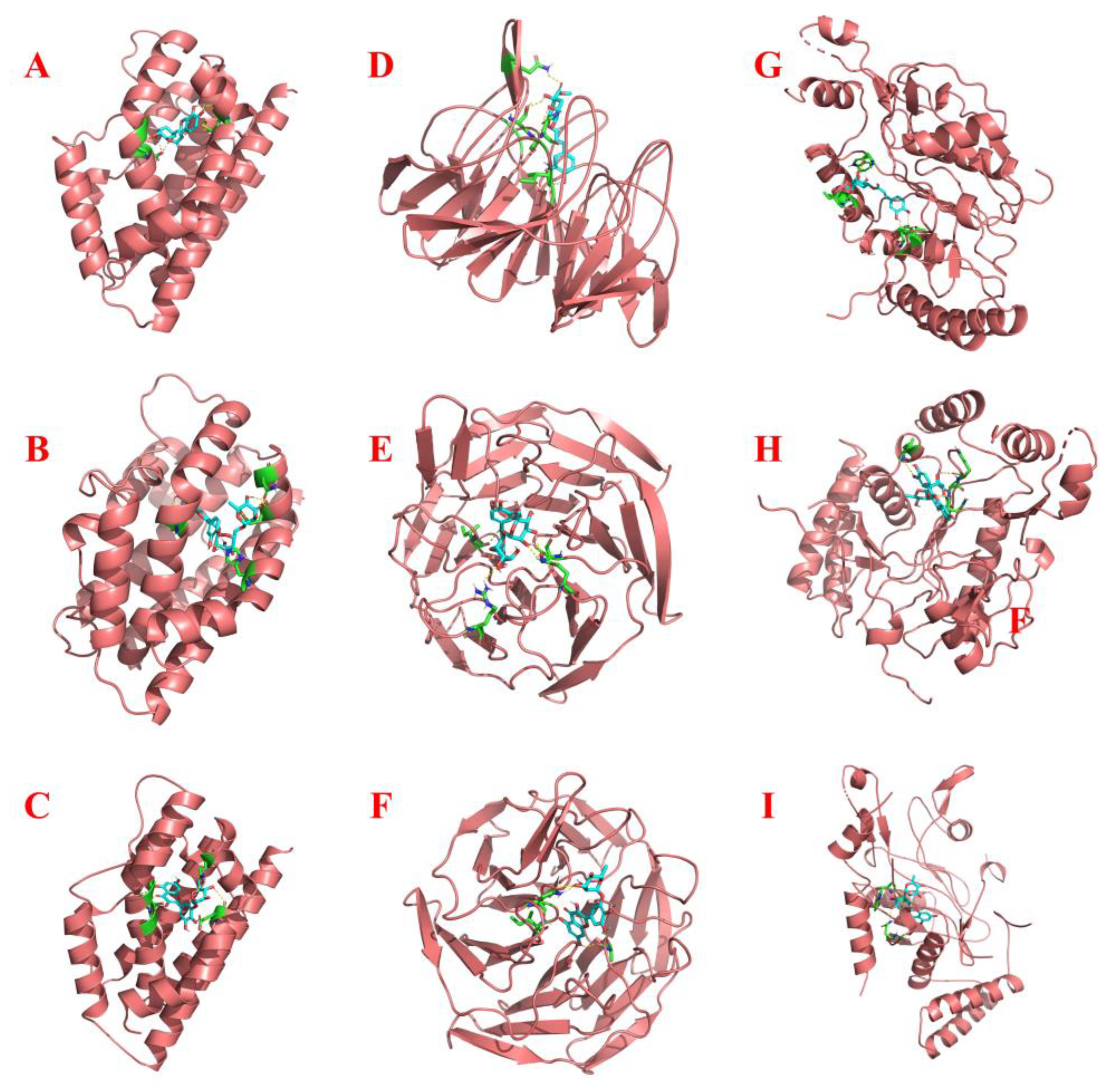
| Protein | Ligand | Number of Points | Center Grid Box | Spacing | Docking Score |
|---|---|---|---|---|---|
| HO-1 ID: 1N3U | Fr4-1-1 | X_dimension = 46 | X_center = 13.356 | 0.403 | −6.01 kcal/mol |
| Fr4-1-2 | Y_dimension = 40 | Y_center = 1.105 | −6.68 kcal/mol | ||
| Fr4-2 | Z_dimension = 36 | Z_center = 0.763 | −6.94 kcal/mol | ||
| Nrf2 ID: 4IQK | Fr4-1-1 | X_dimension = 34 | X_center = −45.033 | 0.375 | −7.01 kcal/mol |
| Fr4-1-2 | Y_dimension = 56 | Y_center = 2.477 | −5.73 kcal/mol | ||
| Fr4-2 | Z_dimension = 50 | Z_center = −15.44 | −6 kcal/mol | ||
| iNOS ID:2ORO | Fr4-1-1 | X_dimension = 40 | X_center = 64.041 | 0.375 | −8.47 kcal/mol |
| Fr4-1-2 | Y_dimension = 50 | Y_center = −13.171 | −6.49 kcal/mol | ||
| Fr4-2 | Z_dimension = 46 | Z_center = 50.069 | −8.54 kcal/mol |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, C.; Lei, Y.; Guo, J.; Chen, X.; Wu, M. Separation of Antioxidants from Trace Fraction of Ribes himalense via Chromatographic Strategy and Their Antioxidant Activity Supported with Molecular Simulations. Int. J. Mol. Sci. 2024, 25, 227. https://doi.org/10.3390/ijms25010227
Liu Y, Liu C, Lei Y, Guo J, Chen X, Wu M. Separation of Antioxidants from Trace Fraction of Ribes himalense via Chromatographic Strategy and Their Antioxidant Activity Supported with Molecular Simulations. International Journal of Molecular Sciences. 2024; 25(1):227. https://doi.org/10.3390/ijms25010227
Chicago/Turabian StyleLiu, Youyi, Chuang Liu, Yuqing Lei, Jingrou Guo, Xingyi Chen, and Minchen Wu. 2024. "Separation of Antioxidants from Trace Fraction of Ribes himalense via Chromatographic Strategy and Their Antioxidant Activity Supported with Molecular Simulations" International Journal of Molecular Sciences 25, no. 1: 227. https://doi.org/10.3390/ijms25010227
APA StyleLiu, Y., Liu, C., Lei, Y., Guo, J., Chen, X., & Wu, M. (2024). Separation of Antioxidants from Trace Fraction of Ribes himalense via Chromatographic Strategy and Their Antioxidant Activity Supported with Molecular Simulations. International Journal of Molecular Sciences, 25(1), 227. https://doi.org/10.3390/ijms25010227




