Cardiogenic Shock Integrated PHenotyping for Event Reduction: A Pilot Metabolomics Analysis
Highlights
- Therapeutic approaches in patients with cardiogenic shock hingers on the profiling of clinical and biomarkers phenotypes.
- There are no consistent data on the metabolomic profile and inflammatory status of patients with heart failure cardiogenic shock (HF-CS).
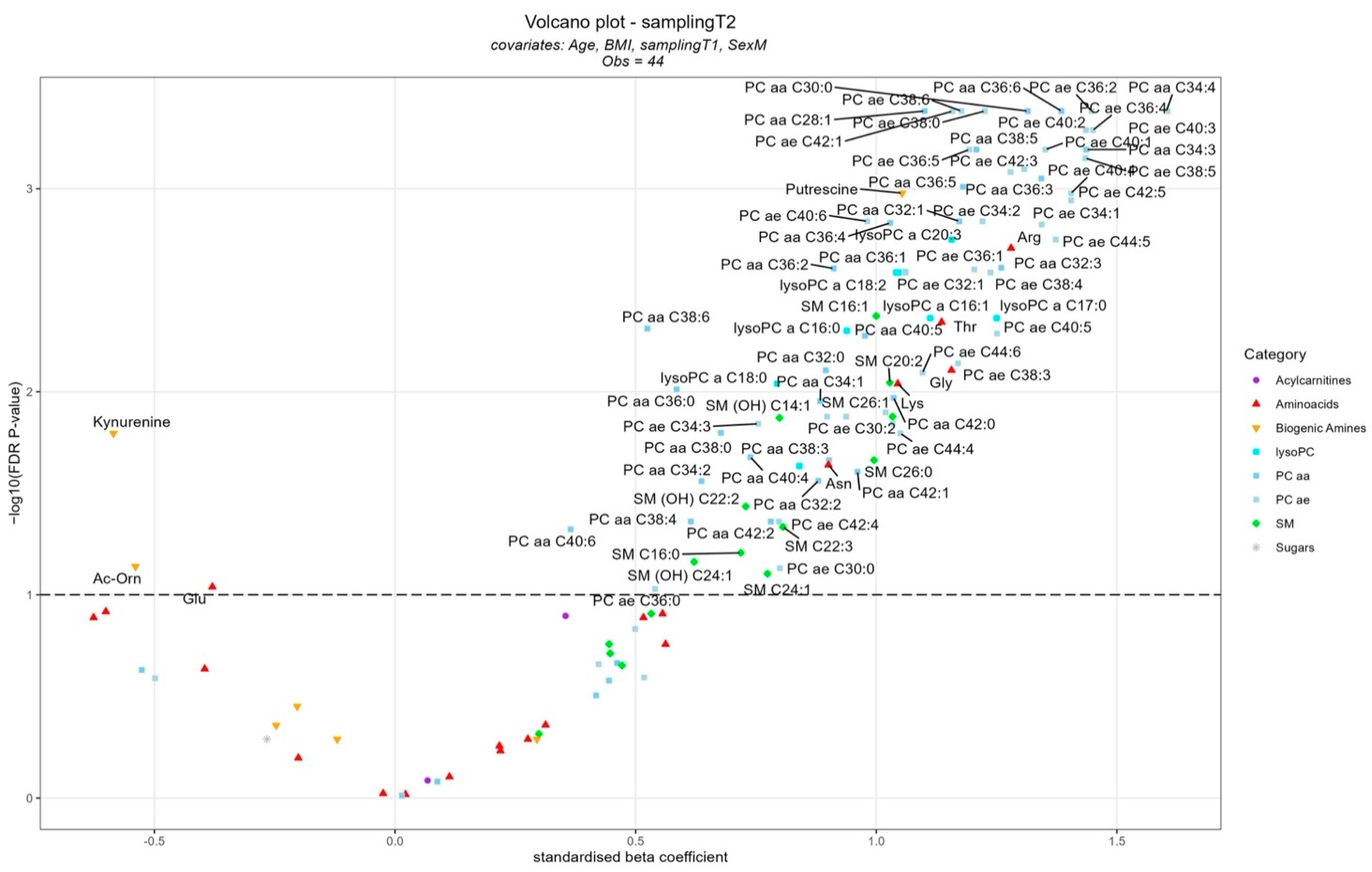
- Interleukin-6 and kynurenine could be considered early markers of a worsening sta-tus and prognosis in patients with HF-CS.
- The tryptophan–-kynurenine metabolites could affect the prognosis of HF-CS patients throughout inflammation-related pathways.
Abstract
:1. Introduction
2. Results
3. Methods and Experimental Design
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jentzer, J.C.; van Diepen, S.; Henry, T.D. Understanding How Cardiac Arrest Complicates the Analysis of Clinical Trials of Cardiogenic Shock. Circ. Cardiovasc. Qual. Outcomes 2020, 13, e006692. [Google Scholar] [CrossRef] [PubMed]
- Van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement from the American Heart Association. Circulation 2017, 136, e232–e268. [Google Scholar] [CrossRef] [PubMed]
- Morici, N.; Marini, C.; Sacco, A.; Tavazzi, G.; Cipriani, M.; Oliva, F.; Rota, M.; De Ferrari, G.M.; Campolo, J.; Frigerio, G.; et al. Early intra-aortic balloon pump in acute decompensated heart failure comoplicated by cardiogenic shock: Rationale and design of the randomized Altshock-2 trial. Am. Heart J. 2021, 233, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Biocrates-Life-Sciences-AG AbsoluteIDQ® p180 Kit. Available online: https://biocrates.com/absoluteidq-p180-kit/ (accessed on 31 January 2022).
- Frigerio, G.; Favero, C.; Savino, D.; Mercadante, R.; Albetti, B.; Dioni, L.; Vigna, L.; Bollati, V.; Pesatori, A.C.; Fustinoni, S. Plasma Metabolomic Profiling in 1391 Subjects with Overweight and Obesity from the SPHERE Study. Metabolites 2021, 11, 194. [Google Scholar] [CrossRef] [PubMed]
- Augustin, H.G.; Koh, G.Y.; Thurston, G.; Alitalo, K. Control of vascular morphogenesisand homeostasis through the Angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 2009, 10, 165–177. [Google Scholar] [CrossRef]
- Pang, Z.; Zhou, G.; Ewald, J.; Chang, L.; Hacariz, O.; Basu, N.; Xia, J. Using MetaboAnalyst 5.0 for LC-HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat. Protoc. 2022, 8, 1735–1761. [Google Scholar] [CrossRef]
- Gamble, J.R.; Drew, J.; Trezise, L.; Underwood, A.; Parsons, M.; Kasminkas, L.; Rudge, J.; Yancopoulos, G.; Vadas, M.A. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ. Res. 2000, 87, 603–607. [Google Scholar] [CrossRef]
- Benest, A.V.; Kruse, K.; Savant, S.; Thomas, M.; Laib, A.M.; Loos, E.K.; Fiedler, U.; Augustin, H.G. Angiopoietin-2 is critical for cytokine-induced vascular leakage. PLoS ONE 2013, 8, e70459. [Google Scholar] [CrossRef]
- Pöss, J.; Fuernau, G.; Denks, D.; Desch, S.; Eitel, I.; de Waha, S.; Link, A.; Schuler, G.; Adams, V.; Böhm, M.; et al. Angiopoietin-2 in acute myocardial infarction complicated by cardiogenic shock—A biomarker substudy of the IABP-SHOCK II-Trial. Eur. J. Heart Fail. 2015, 17, 1152–1160. [Google Scholar] [CrossRef]
- Chong, A.Y.; Caine, G.J.; Freestone, B.; Blann, A.D.; Lip, G.Y. Plasma Angiopoietin-1, Angiopoietin-2, and Angiopoietin receptor Tie-2 levels in congestive heart failure. J. Am. Coll. Cardiol. 2004, 43, 423–428. [Google Scholar] [CrossRef]
- Pöss, J.; Ukena, C.; Kindermann, I.; Ehrlich, P.; Fuernau, G.; Ewen, S.; Mahfoud, F.; Kriechbaum, S.; Böhm, M.; Link, A. Angiopoietin-2 and outcome in patients with acute decompensated heart failure. Clin. Res. Cardiol. 2015, 104, 380–387. [Google Scholar] [CrossRef] [PubMed]
- McGarrah, R.W.; Crown, S.B.; Zhang, G.F.; Shah, S.H.; Newgard, C.B. Cardiovascular Metabolomics. Circ. Res. 2018, 122, 1238–1258. [Google Scholar] [CrossRef] [PubMed]
- Patti, G.J.; Yanes, O.; Siuzdak, G. Innovation: Metabolomics: The apogee of the omics trilogy. Nat. Rev. Mol. Cell Biol. 2012, 13, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.; Labarthe, F.; Fortier, A.; Bouchard, B.; Thompson Legault, J.; Bolduc, V.; Rigal, O.; Chen, J.; Ducharme, A.; Crawford, P.A.; et al. Circulating acylcarnitine profile in human heart failure: A surrogate of fatty acid metabolic dysregulation in mitochondria and beyond. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H768–H781. [Google Scholar] [CrossRef] [PubMed]
- Diakos, N.A.; Navankasattusas, S.; Abel, E.D. Evidence of Glycolysis Up-Regulation and Pyruvate Mitochondrial Oxidation Mismatch During Mechanical Unloading of the Failing Human Heart: Implications for Cardiac Reloading and Conditioning. JACC Basic Transl. Sci. 2016, 1, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Lund, A.; Nordrehaug, J.E.; Slettom, G.; Solvang, S.E.H.; Pedersen, E.K.R.; Midttun, Ø.; Ulvik, A.; Ueland, P.M.; Nygard, O.; Giil, L.M. Plasma kynurenines and prognosis in patients with heart failure. PLoS ONE 2020, 15, e0227365. [Google Scholar]
- Dschietzig, T.B.; Kellner, K.H.; Sasse, K.; Boschann, F.; Klüsener, R.; Ruppert, J.; Armbruster, F.P.; Bankovic, D.; Meinitzer, A.; Mitrovic, V.; et al. Plasma kynurenine predicts severity and complications of heart failure and associates with established biochemical and clinical markers of disease. Kidney Blood Press. Res. 2019, 44, 765–776. [Google Scholar] [CrossRef]
- Zapolski, T.; Kamińska, A.; Kocki, T.; Wysokiński, A.; Urbanska, E.M. Aortic stiffness—Is kynurenic acid a novel marker? Cross-sectional study in patients with persistent atrial fibrillation. PLoS ONE 2020, 15, e0236413. [Google Scholar] [CrossRef]
- Sulo, G.; Vollset, S.E.; Nygard, O.; Midttun, O.; Ueland, P.M.; Eussen, S.; Pedersen, E.R.; Tell, G.S. Neopterin and kynurenine-tryptophan ratio as predictors of coronary events in older adults, the Hordaland Health Study. Int. J. Cardiol. 2013, 168, 1435–1440. [Google Scholar] [CrossRef]
- Zuo, H.; Ueland, P.M.; Ulvik, A.; Eussen, S.; Vollset, S.E.; Nygård, O.; Midttun, Ø.; Theofylaktopoulou, D.; Meyer, K.; Tell, G.S. Plasma biomarkers of inflammation, the kynurenine pathway, and risks of all-cause, cancer, and cardiovascular disease mortality. Am. J. Epidemiol. 2016, 183, 249–258. [Google Scholar] [CrossRef]
- Baumgartner, R.; Forteza, M.J.; Ketelhuth, D.F.J. The interplay between cytokines and the Kynurenine pathway in inflammation and atherosclerosis. Cytokine 2019, 122, 154148. [Google Scholar] [CrossRef] [PubMed]
| Variable | Number (%) |
|---|---|
| Male gender | 14 (78%) |
| Age | 50 (42–60) |
| BMI | 25 (23–27) |
| Hypertension | 3 (17%) |
| Diabetes | 2 (11.1%) |
| Ischemic etiology | 6 (33%) |
| On admission | |
| SCAI Stage | |
| B | 6 (33%) |
| C | 9 (50%) |
| D | 3 (17%) |
| Ejection fraction (%) | 20 (18–30) |
| Central venous pressure (mmHg) | 10 (4–15) |
| Wedge pressure (mmHg) | 19 (10–24) |
| Cardiac index L/min/m2 | 2 (1.4–2.5) |
| PAPm (mmHg) | 33 (26–35) |
| SOFA score | 5.5 (3–6) |
| Hb, gr/dl | 9 (8–10) |
| Serum creatinine, mg/dl | 1.7 (1.3–2.1) |
| ScVO2 (%) | 45 (41–60) |
| Lactates mmol/L | 3 (2.2–3.4) |
| During Hospital stay | |
| Epinephrine (17 patients) | |
| maximum dose, mcg/Kg/min | 0.05 (0.04–0.12) |
| total time of administration, days | 9 (5–20) |
| Sodium Nitroprusside (15 patients) | |
| maximum dose, mcg/Kg/min | 0.4 (0.3–0.5) |
| total time of administration, days | 5 (2–8) |
| Maximum inotropic score | 7 (4–12) |
| IABP | 13 (72%) |
| Total support time, days | 8 (5–19) |
| ECMO | 3 (17%) |
| Total support time, days | 7 (6–13) |
| Mechanical ventilation | 7 (39%) |
| NIV | 14 (78) |
| CRRT | 2 (11%) |
| In-hospital Outcome | |
| Heart transplantation | 8 (44%) |
| Left ventricular assist device | 1 (5%) |
| Death | 7 (44%) |
| Author | Year | Sample Size | Biomarkers | Outcome |
|---|---|---|---|---|
| Appoloni O Chest. 2004 Jun;125(6):2232-7 | 2004 | 33 patients (75% ischemic heart disease) | TNF- IL-6 IL-10 TGF- IFN- cytokine polymorphisms | 70% ICU mortality The rare TNF-2 allele of the TNF-promoter is a strong independent factor associated with better survival from cardiogenic shock. |
| Geppert A Crit Care Med. 2006 Aug;34(8):2035-42 | 2006 | 38 AMI-CS patients | IL-1 IL-6 IL-10 ICAM-1 E-selectin | 40% mortality at 30 days Association with IL6, no other markers. 200 pg/mL as the most valuable IL-6 cut-off concentration for predicting 30-day mortality with a specificity of 87% and a sensitivity of 74%. |
| Debrunner M Clin Res Cardiol. 2008 May;97(5):298-305 | 2008 | 41 AMI patients (19 with CS: 7 developed SIRS) | TNF-α IL-6 IL-1 Ra | 71% In-hospital mortality in group 3 (SIRS) IL-1Ra showed the most impressive changes. |
| Sleeper LA Am Heart J. 2010 Sep;160(3):443-50 | 2010 | 1217 AMI patients (294 from the randomized trial and 923 from the registry) | Creatinine ≥ 1.9 mg/dL | 57% in-hospital mortality at 30 days |
| Prondzinsky R Clin Res Cardiol. 2012 May;101(5):375-84 | 2012 | 40 AMI-CS patients | IL-1b IL-6 IL-7 IL-8 IL-10 | 32% mortality at 96 h The pro- and anti-inflammatory markers IL-6, IL-7, IL-8, and IL-10 showed a predictive power for mortality of infarct-related CS patients, while IL-1b did not discriminate. No IABP effect. |
| Link A Eur Heart J. 2013 Jun;34(22):1651-62 | 2013 | 96 CS patients (58% AMI) | Ang-1 Ang-2 | 37.5% mortality at 28 days. 61.5% mortality at 1 year Ang-2 level >2500 pg/mL at admission is an independent predictor for 1-year mortality (HR 2.11; 95% CI (1.03–4.36); p = 0.042) |
| Fuernau G Crit Care. 2014 Dec 21;18(6):713 | 2014 | 600 patients in the original trial (190 included: Leipzig cohort with blood sample available) AMI | OPG GDF-15 | 40.2% mortality at 30 days Multivariate: GDF-15, TIMI flow grade < 3 after PCI, age, LVEF, and serum lactate remained significant predictors of 30-day mortality. |
| Poss J Eur J Heart Fail. 2015 Nov;17(11):1152-60 | 2015 | 600 patients in the original trial (1890 included) AMI | Ang-2 IQR | 41% mortality at 30 days 53% mortality at 1 year Ang-2 was an independent predictor of 30-day and 1-year mortality (with creatinine, lactate, NTproBNP, FGF 23, SAPSII, age, EF) |
| Fuernau G Int J Cardiol. 2015 Jul 15; 191:159-66 | 2015 | 600 patients in the original trial (190 included: Leipzig cohort with blood sample available) AMI | Creatinine NGAL KIM1 CysC | 54% mortality at 1 year Creatinine demonstrated a better predictive performance at all 3 time points with respect to 1-year mortality in comparison to the other 3 biomarkers. |
| Poss J J Am Coll Cardiol. 2017 Apr 18;69(15):1913-1920 | 2017 | 600 patients in the original trial (480 included) AMI Validation in IABP shock II registry (188 pts) and Cardshock cohort (219 pts) | Glucose Creatinine Arterial Blood Lactate | 41% mortality at 30 days Age > 73 years, prior stroke, glucose at admission > 10.6 mmol/L (191 mg/dl), creatinine at admission > 132.6 mmol/l (1.5 mg/dl), thrombolysis in myocardial infarction flow grade < 3 after percutaneous coronary intervention, and arterial blood lactate at admission >5 mmol/l. |
| Tolppanen H Crit Care Med. 2017 Jul;45(7):e666-e673 | 2017 | 145 ACS-CS patients | sST2 NT-proBNP | 43% mortality at 30 days Combination of results for soluble ST2 and NT-proBNP provide early risk assessment beyond clinical variables of Cardshock score |
| Rueda F Eur Heart J. 2019 Aug 21;40(32):2684-2694 | 2019 | 48 AMI patients (Validation in 97 patients from Cardshock–71% ACS) | Quantitative proteomics analysis (QPC) | 37.1% mortality at 90 days 4-protein combination: the CS4P with proteins liver-type fatty acid-binding protein (L-FABP, P07148), beta-2-microglobulin (B2MG, P61769), fructose-bisphosphate aldolase B (ALDOB, P05062), and SerpinG1 as the best protein classifier to identify short-term mortality risk with an AUC of 0.83 (95% CI 0.74–0.89) |
| Peng Y Clin Chim Acta. 2020 Dec; 511:97-103 | 2020 | 707 of critically ill patients with CS (55% CAD) | Systemic immune-inflammatory index (SII) | 40% mortality at 30 days 50% mortality at 90 days 60% mortality at 1 year High-SII group independently associated with mortality (any time). Low-SII group (<82.85), 235 in the mid-SII group (82.8–111.7), and 236 in the high-SII group (>111.7) |
| Cuinet J Sci Rep. 2020 May 6;10(1):7639 | 2020 | 24 patients (12 ACS-CS—50% 6 HF-CS—25%) | WBC counts IL-1β IL-5 IL-6 IL-10 TNFα IFNγ MCP-1 Eotaxin (CCL11) | 21% In-hospital mortality Early (T1) neutrophilia and IL-6, IL-10, and MCP-1, rise of eosinophils over time. Most severe shock had reduced lymphocytes and monocytes at T2 and T3 |
| Ceglarek U Eur Heart J. 2021 Jun 21;42(24):2344-2352 | 2021 | 458 patients (derivation cohort of culprit shock, 152 patients among 458 were used for internal validation + 163 patients (validation cohort) IABP shock II trial) AMI | Lactate Cystatin C NT-proBNP IL-6 | 43.4% mortality at 30 days |
| Zhang Z Int J Gen Med. 2021 Aug 12; 14:4459-4468 | 2021 | 1487 patients (CAD 63.8%) | Lymphocyte to monocyte ratio (LMR) | Approximately 50% in-hospital mortality at 30 days Low-LMR group had a poor prognosis in crude cohort (HR: 1.40, 95% CI: 1.12–1.74, p = 0.003). After PSM, low-LMR group had a similar poor prognosis in crude cohort (HR: 1.31, 95% CI: 1.08–1.68, p = 0.016) compared with high-LMR group. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morici, N.; Frigerio, G.; Campolo, J.; Fustinoni, S.; Sacco, A.; Garatti, L.; Villanova, L.; Tavazzi, G.; Kapur, N.K.; Pappalardo, F. Cardiogenic Shock Integrated PHenotyping for Event Reduction: A Pilot Metabolomics Analysis. Int. J. Mol. Sci. 2023, 24, 17607. https://doi.org/10.3390/ijms242417607
Morici N, Frigerio G, Campolo J, Fustinoni S, Sacco A, Garatti L, Villanova L, Tavazzi G, Kapur NK, Pappalardo F. Cardiogenic Shock Integrated PHenotyping for Event Reduction: A Pilot Metabolomics Analysis. International Journal of Molecular Sciences. 2023; 24(24):17607. https://doi.org/10.3390/ijms242417607
Chicago/Turabian StyleMorici, Nuccia, Gianfranco Frigerio, Jonica Campolo, Silvia Fustinoni, Alice Sacco, Laura Garatti, Luca Villanova, Guido Tavazzi, Navin K. Kapur, and Federico Pappalardo. 2023. "Cardiogenic Shock Integrated PHenotyping for Event Reduction: A Pilot Metabolomics Analysis" International Journal of Molecular Sciences 24, no. 24: 17607. https://doi.org/10.3390/ijms242417607
APA StyleMorici, N., Frigerio, G., Campolo, J., Fustinoni, S., Sacco, A., Garatti, L., Villanova, L., Tavazzi, G., Kapur, N. K., & Pappalardo, F. (2023). Cardiogenic Shock Integrated PHenotyping for Event Reduction: A Pilot Metabolomics Analysis. International Journal of Molecular Sciences, 24(24), 17607. https://doi.org/10.3390/ijms242417607








