Monofluoromethylation of N-Heterocyclic Compounds
Abstract
:1. Introduction
2. Monofluoromethylation of N-Containing Heterocycles
2.1. Synthesis of Monofluoromethylated Aziridines
2.2. Monofluoromethylation of Five-Membered N-heterocycles
2.3. Monofluoromethylation of Six-Membered N-heterocycles
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heravi, M.M.; Zadsirjan, V. Prescribed drugs containing nitrogen heterocycles: An overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J. Heterocycles in Medicinal Chemistry. Molecules 2019, 24, 3839. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Kim, D.G.; Lee, A.; Kim, Y.M.; Cui, L.; Kim, S.; Choi, I. Synthesis and discovery of the first potent proteolysis targeting chimaera (PROTAC) degrader of AIMP2-DX2 as a lung cancer drug. J. Enzyme. Inhib. Med. Chem. 2023, 38, 51–66. [Google Scholar] [CrossRef]
- Barreca, M.; Spanò, V.; Rocca, R.; Bivacqua, R.; Gualtieri, G.; Raimondi, M.V.; Gaudio, E.; Bortolozzi, R.; Manfreda, L.; Bai, R.; et al. Identification of pyrrolo[3′,4′:3,4]cyclohepta[1,2-d][1,2]oxazoles as promising new candidates for the treatment of lymphomas. Eur. J. Med. Chem. 2023, 254, 115372. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, Y.; Chen, G.; Ji, Y.; Ma, Q.; Qiao, X.; Wu, S.; Zhou, L.; Bu, J.; Zhu, X.; et al. Targeting SOST using a small-molecule compound retards breast cancer bone metastasis. Mol. Cancer 2022, 21, 228. [Google Scholar] [CrossRef] [PubMed]
- Bivacqua, R.; Barreca, M.; Spanò, V.; Raimondi, M.V.; Romeo, I.; Alcaro, S.; Andrei, G.; Barraja, P.; Montalbano, A. Insight into non-nucleoside triazole-based systems as viral polymerases inhibitors. Eur. J. Med. Chem. 2023, 249, 115136. [Google Scholar] [CrossRef] [PubMed]
- Lang, K.D.; Kaur, R.; Arora, R.; Saini, B.; Arora, S. Nitrogen-Containing Heterocycles as Anticancer Agents: An Overview. Anticancer Agents Med. Chem. 2020, 20, 2150–2168. [Google Scholar] [CrossRef]
- Singh, P.K.; Silakari, O. Chapter 1—Multitargeting Heterocycles: Improved and Rational Chemical Probes for Multifactorial Diseases. In Key Heterocycle Cores for Designing Multitargeting Molecules; Silakari, O., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–29. [Google Scholar]
- Wei, X.; Wang, P.; Liu, F.; Ye, X.; Xiong, D. Drug Discovery Based on Fluorine-Containing Glycomimetics. Molecules 2023, 28, 6641. [Google Scholar] [CrossRef]
- Yin, R.; Huang, K.X.; Huang, L.A.; Ji, M.; Zhao, H.; Li, K.; Gao, A.; Chen, J.; Li, Z.; Liu, T.; et al. Indole-Based and Cyclopentenylindole-Based Analogues Containing Fluorine Group as Potential 18F-Labeled Positron Emission Tomography (PET) G-Protein Coupled Receptor 44 (GPR44) Tracers. Pharmaceuticals 2023, 16, 1203. [Google Scholar] [CrossRef]
- Miles, S.A.; Nillama, J.A.; Hunter, L. Tinker, Tailor, Soldier, Spy: The Diverse Roles That Fluorine Can Play within Amino Acid Side Chains. Molecules 2023, 28, 6192. [Google Scholar] [CrossRef]
- Shabir, G.; Saeed, A.; Zahid, W.; Naseer, F.; Riaz, Z.; Khalil, N.; Muneeba; Albericio, F. Chemistry and Pharmacology of Fluorinated Drugs Approved by the FDA (2016–2022). Pharmaceuticals 2023, 16, 1162. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Amata, S.; Pibiri, I.; Pace, A.; Buscemi, S.; Palumbo Piccionello, A. FDA-Approved Fluorinated Heterocyclic Drugs from 2016 to 2022. Int. J. Mol. Sci. 2023, 24, 7728. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Mei, H.; Dhawan, G.; Zhang, W.; Han, J.; Soloshonok, V.A. New Approved Drugs Appearing in the Pharmaceutical Market in 2022 Featuring Fragments of Tailor-Made Amino Acids and Fluorine. Molecules 2023, 28, 3651. [Google Scholar] [CrossRef] [PubMed]
- Tien Anh, D.; Hai Nam, N.; Kircher, B.; Baecker, D. The Impact of Fluorination on the Design of Histone Deacetylase Inhibitors. Molecules 2023, 28, 1973. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.-P.; Yu, C.-S. Recent Development of Radiofluorination of Boron Agents for Boron Neutron Capture Therapy of Tumor: Creation of 18F-Labeled C-F and B-F Linkages. Pharmaceuticals 2023, 16, 93. [Google Scholar] [CrossRef] [PubMed]
- Kawagoe, F.; Mototani, S.; Kittaka, A. Design and Synthesis of Fluoro Analogues of Vitamin D. Int. J. Mol. Sci. 2021, 22, 8191. [Google Scholar] [CrossRef]
- Dabur, M.; Loureiro, J.A.; Pereira, M.C. Fluorinated Molecules and Nanotechnology: Future ‘Avengers’ against the Alzheimer’s Disease? Int. J. Mol. Sci. 2020, 21, 2989. [Google Scholar] [CrossRef]
- Mishra, S.K.; Suryaprakash, N. Intramolecular Hydrogen Bonding Involving Organic Fluorine: NMR Investigations Corroborated by DFT-Based Theoretical Calculations. Molecules 2017, 22, 423. [Google Scholar] [CrossRef]
- Sandulenko, I.V.; Belozertseva, I.V.; Zvartau, E.E.; Zelentsova, M.V.; Ambartsumyan, A.A.; Smol’yakov, A.F.; Moiseev, S.K. C(21)-fluorinated thevinol scaffold for opioid ligands. 21,21,21-Trifluoro-6-O-nororvinols: Design, synthesis and analgesic activity. Eur. J. Med. Chem. 2023, 252, 115296. [Google Scholar] [CrossRef]
- Pietruś, W.; Kurczab, R.; Warszycki, D.; Bojarski, A.J.; Bajorath, J. Isomeric Activity Cliffs—A Case Study for Fluorine Substitution of Aminergic G Protein-Coupled Receptor Ligands. Molecules 2023, 28, 490. [Google Scholar] [CrossRef]
- Kovács, É.; Ali, H.; Minorics, R.; Traj, P.; Resch, V.; Paragi, G.; Bruszel, B.; Zupkó, I.; Mernyák, E. Synthesis and Antiproliferative Activity of Steroidal Diaryl Ethers. Molecules 2023, 28, 1196. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Marset, X.; Tostado, J.; Kircher, J.; She, Z.; Golz, C.; Mata, R.A.; Simon, M.; Alcarazo, M. 5-(Trifluorovinyl)dibenzothiophenium Triflate: Introducing the 1,1,2-Trifluoroethylene Tether in Drug-Like Structures. Chem. Eur. J. 2023, 29, e202203966. [Google Scholar] [CrossRef] [PubMed]
- Casa, S.; Henary, M. Synthesis and Applications of Selected Fluorine-Containing Fluorophores. Molecules 2021, 26, 1160. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.A.; Daré, J.K.; Freitas, M.P. Theoretical study of fluorinated bioisosteres of organochlorine compounds as effective and eco-friendly pesticides. Ecotoxicol. Environ. Saf. 2020, 199, 110679. [Google Scholar] [CrossRef] [PubMed]
- Selby, T.P.; Satterfield, A.D.; Puri, A.; Stevenson, T.M.; Travis, D.A.; Campbell, M.J.; Taggi, A.E.; Hughes, K.A.; Bereznak, J. Bioisosteric Tactics in the Discovery of Tetflupyrolimet: A New Mode-of-Action Herbicide. J. Agric. Food Chem. 2023, 71, 18197–18204. [Google Scholar] [CrossRef] [PubMed]
- Deneny, P.J.; Kumar, R.; Gaunt, M.J. Visible light-mediated radical fluoromethylation via halogen atom transfer activation of fluoroiodomethane. Chem. Sci. 2021, 12, 12812–12818. [Google Scholar] [CrossRef]
- Commare, B.; Schmitt, E.; Aribi, F.; Panossian, A.; Vors, J.-P.; Pazenok, S.; Leroux, F.R. Fluoroalkyl Amino Reagents (FARs): A General Approach towards the Synthesis of Heterocyclic Compounds Bearing Emergent Fluorinated Substituents. Molecules 2017, 22, 977. [Google Scholar] [CrossRef]
- Lu, X.-Y.; Gao, M.-T.; Yu, L.-J.; Pan, H.-Y.; Zhang, X.; Huang, R.; Yang, K.; Shui, F.-Y.; Song, Y.-W.; Yang, G.-X. Synthesis of fluorinated allylic alcohols via photoinduced decarboxylative cross-coupling of α-fluoroacrylic acids and alcohols. Org. Chem. Front. 2023, 10, 1788–1795. [Google Scholar] [CrossRef]
- Su, J.; Hu, X.; Huang, H.; Guo, Y.; Song, Q. Difluorocarbene enables to access 2-fluoroindoles from ortho-vinylanilines. Nature Commun. 2021, 12, 4986. [Google Scholar] [CrossRef]
- Zhou, L. Recent Advances in C-F Bond Cleavage Enabled by Visible Light Photoredox Catalysis. Molecules 2021, 26, 7051. [Google Scholar] [CrossRef]
- Purushotam; Bera, A.; Banerjee, D. Recent advances on non-precious metal-catalysed fluorination, difluoromethylation, trifluoromethylation, and perfluoroalkylation of N-heteroarenes. Org. Biomol. Chem. 2023, 21, 9298–9315. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Zhang, Z.; Fang, Y.; Zhu, L.; Li, C. Radical trifluoromethylation. Chem. Soc. Rev. 2021, 50, 6308–6319. [Google Scholar] [CrossRef] [PubMed]
- Bhaskaran, R.P.; Babu, B.P. Progress in Electrochemical Trifluoromethylation Reactions. Adv. Synth. Cat. 2020, 362, 5219–5237. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Huang, J.; Sun, K.; He, W.-M. Recent advances in transition-metal-free trifluoromethylation with Togni’s reagents. Org. Chem. Front. 2022, 9, 1152–1164. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, W.; Wang, F. Selective difluoromethylation and monofluoromethylation reactions. Chem. Commun. 2009, 48, 7465–7478. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ni, C. 2.6.2 Difluoro- and Fluoromethylation. In C-1 Building Blocks in Organic Synthesis 2; Georg Thieme Verlag KG: Stuttgart, Germany, 2014; Volume 2013/8. [Google Scholar]
- Adam, A.T.; Fronczek, F.R.; Colby, D.A. Synthesis of β-Fluoro-α,β-Unsaturated Amides from the Fragmentation of Morpholine 3,3,3-Trifluoropropanamide by Grignard Reagents. Org. Lett. 2020, 22, 2630–2633. [Google Scholar] [CrossRef] [PubMed]
- Mlostoń, G.; Shermolovich, Y.; Heimgartner, H. Synthesis of Fluorinated and Fluoroalkylated Heterocycles Containing at Least One Sulfur Atom via Cycloaddition Reactions. Materials 2022, 15, 7244. [Google Scholar] [CrossRef]
- Meanwell, N.A. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018, 61, 5822–5880. [Google Scholar] [CrossRef]
- Richardson, P. Applications of fluorine to the construction of bioisosteric elements for the purposes of novel drug discovery. Expert Opin. Drug Discov. 2021, 16, 1261–1286. [Google Scholar] [CrossRef]
- Meanwell, N.A. Applications of Bioisosteres in the Design of Biologically Active Compounds. J. Agric. Food Chem. 2023, 71, 18087–18122. [Google Scholar] [CrossRef]
- Baumann, M.; Baxendale, I.R. An overview of the synthetic routes to the best selling drugs containing 6-membered heterocycles. Beilstein J. Org. Chem. 2013, 9, 2265–2319. [Google Scholar] [CrossRef] [PubMed]
- Sietmann, J.; Ong, M.; Mück-Lichtenfeld, C.; Daniliuc, C.G.; Wahl, J.M. Desymmetrization of Prochiral Cyclobutanones via Nitrogen Insertion: A Concise Route to Chiral γ-Lactams. Angew. Chem. Int. Ed. 2021, 60, 9719–9723. [Google Scholar] [CrossRef] [PubMed]
- Winton, W.P.; Brooks, A.F.; Wong, K.K.; Scott, P.J.H.; Viglianti, B.L. Synthesis of 6-(Fluoromethyl)-19-norcholest-5(10)-en-3-ol, a Fluorinated Analogue of NP-59, using the Mild Fluorinating Reagent, TBAF(Pinacol)2. SynOpen 2019, 3, 55–58. [Google Scholar] [CrossRef]
- Salud, O.M. International Nonproprietary Names for Pharmaceutical Substances (INN). WHO Drug Inf. 2022, 36, 695. [Google Scholar]
- Rej, R.K.; Thomas, J.E., II; Acharyya, R.K.; Rae, J.M.; Wang, S. Targeting the Estrogen Receptor for the Treatment of Breast Cancer: Recent Advances and Challenges. J. Med. Chem. 2023, 66, 8339–8381. [Google Scholar] [CrossRef]
- Jhaveri, K.; O’Shaughnessy, J.; Andre, F.; Goetz, M.P.; Harbeck, N.; Martín, M.; Bidard, F.-C.; Thomas, Z.M.; Young, S.; Ismail-Khan, R.; et al. Abstract OT1-01-02: EMBER-4: A phase 3 adjuvant trial of imlunestrant vs standard endocrine therapy (ET) in patients with ER+, HER2- early breast cancer (EBC) with an increased risk of recurrence who have previously received 2 to 5 years of adjuvant ET. Cancer Res. 2023, 83 (Suppl. 5), OT1-01-02. [Google Scholar] [CrossRef]
- Jhaveri, K.L.; Jeselsohn, R.; Lim, E.; Hamilton, E.P.; Yonemori, K.; Beck, J.T.; Kaufman, P.A.; Sammons, S.; Bhave, M.A.; Saura, C.; et al. A phase 1a/b trial of imlunestrant (LY3484356), an oral selective estrogen receptor degrader (SERD) in ER-positive (ER+) advanced breast cancer (aBC) and endometrial endometrioid cancer (EEC): Monotherapy results from EMBER. J. Clin. Oncol. 2022, 40 (Suppl. 16), 1021. [Google Scholar] [CrossRef]
- Neven, P.; Stahl, N.; Losada, M.J.V.; Jimenez, M.M.; Kaufman, P.A.; Harbeck, N.; Hunt, K.; Carter, S.A.; Bidard, F.C.; Fasching, P.; et al. 273P A preoperative window-of-opportunity (WOO) study of imlunestrant in ER+, HER2- early breast cancer (EBC): Final analysis from EMBER-2. Ann. Oncol. 2023, 34, S292–S293. [Google Scholar] [CrossRef]
- Dicker, I.; Jeffrey, J.L.; Protack, T.; Lin, Z.; Cockett, M.; Chen, Y.; Sit, S.-Y.; Gartland, M.; Meanwell, N.A.; Regueiro-Ren, A.; et al. GSK3640254 Is a Novel HIV-1 Maturation Inhibitor with an Optimized Virology Profile. Antimicrob. Agents Chemother. 2022, 66, e01876-21. [Google Scholar] [CrossRef]
- Tamaki, N.; Hirata, K. Tumor hypoxia: A new PET imaging biomarker in clinical oncology. Int. J. Clin. Oncol. 2016, 21, 619–625. [Google Scholar] [CrossRef]
- Rajendran, J.G.; Mankoff, D.A.; O’Sullivan, F.; Peterson, L.M.; Schwartz, D.L.; Conrad, E.U.; Spence, A.M.; Muzi, M.; Farwell, D.G.; Krohn, K.A. Hypoxia and Glucose Metabolism in Malignant Tumors: Evaluation by [18F]Fluoromisonidazole and [18F]Fluorodeoxyglucose Positron Emission Tomography Imaging. Clin. Cancer. Res. 2004, 10, 2245–2252. [Google Scholar] [CrossRef] [PubMed]
- Saigal, N.; Pichika, R.; Easwaramoorthy, B.; Collins, D.; Christian, B.T.; Shi, B.; Narayanan, T.K.; Potkin, S.G.; Mukherjee, J. Synthesis and biologic evaluation of a novel serotonin 5-HT1A receptor radioligand, 18F-labeled mefway, in rodents and imaging by PET in a nonhuman primate. J. Nucl. Med. 2006, 47, 1697–1706. [Google Scholar] [PubMed]
- Choi, J.Y.; Kim, C.H.; Ryu, Y.H.; Seo, Y.B.; Truong, P.; Kim, E.J.; Choi, T.H.; Kang, J.; Lee, M.; Kim, D.G.; et al. Optimization of the radiosynthesis of [18F]MEFWAY for imaging brain serotonin 1A receptors by using the GE TracerLab FXFN-Pro module. J. Label Compd. Radiopharm. 2013, 56, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, T.; Ishida, R. Pharmacological studies on 6-amin0-2-flu0r0methyl-3-(O-tolyl)-4(3H)-quinazolinone (afloqualone), a new centrally acting muscle relaxant. (II) effects on the spinal reflex potential and the rigidity. Jap. J. Pharmac. 1982, 32, 427–438. [Google Scholar] [CrossRef]
- Sperandeo, A.; Ficola, U.; Quartuccio, N.; Kitson, S.; Mansi, L. Automated synthesis of [18F]fluorocholine using a modified GE TracerLab module. J. Diagn. Imaging Ther. 2014, 1, 49–58. [Google Scholar] [CrossRef]
- Ielo, L.; Touqeer, S.; Roller, A.; Langer, T.; Holzer, W.; Pace, V. Telescoped, Divergent, Chemoselective C1 and C1-C1 Homologation of Imine Surrogates: Access to Quaternary Chloro- and Halomethyl-Trifluoromethyl Aziridines. Angew. Chem. Int. Ed. 2019, 58, 2479–2484. [Google Scholar] [CrossRef]
- Monticelli, S.; Colella, M.; Pillari, V.; Tota, A.; Langer, T.; Holzer, W.; Degennaro, L.; Luisi, R.; Pace, V. Modular and Chemoselective Strategy for the Direct Access to α-Fluoroepoxides and Aziridines via the Addition of Fluoroiodomethyllithium to Carbonyl-Like Compounds. Org. Lett. 2019, 21, 584–588. [Google Scholar] [CrossRef]
- Hell, S.M.; Meyer, C.F.; Ortalli, S.; Sap, J.B.I.; Chen, X.; Gouverneur, V. Hydrofluoromethylation of alkenes with fluoroiodomethane and beyond. Chem. Sci. 2021, 12, 12149–12155. [Google Scholar] [CrossRef]
- Hajra, S.; Roy, S.; Maity, S.; Chatterjee, S. Reagent-Controlled Reversal of Regioselectivity in Nucleophilic Fluorination of Spiro-epoxyoxindole: Synthesis of 3-Fluoro-3-hydroxymethyloxindole and 3-Aryl-3-fluoromethyloxindole. J. Org. Chem. 2019, 84, 2252–2260. [Google Scholar] [CrossRef]
- Tang, X.-J.; Thomoson, C.S.; Dolbier, W.R., Jr. Photoredox-Catalyzed Tandem Radical Cyclization of N-Arylacrylamides: General Methods To Construct Fluorinated 3,3-Disubstituted 2-Oxindoles Using Fluoroalkylsulfonyl Chlorides. Org. Lett. 2014, 16, 4594–4597. [Google Scholar] [CrossRef]
- Koike, T.; Akita, M. Recent progress in photochemical radical di- and mono-fluoromethylation. Org. Biomol. Chem. 2019, 17, 5413–5419. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ding, T.; Jiang, L.; He, W.; Yi, W. Fluoromethoxymethylation of Nitrogen Heterocyclic Compounds with Fluoromethyl Iodide. J. Org. Chem. 2020, 85, 3993–4001. [Google Scholar] [CrossRef] [PubMed]
- Senatore, R.; Malik, M.; Spreitzer, M.; Holzer, W.; Pace, V. Direct and Chemoselective Electrophilic Monofluoromethylation of Heteroatoms (O-, S-, N-, P-, Se-) with Fluoroiodomethane. Org. Lett. 2020, 22, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Reichel, M.; Karaghiosoff, K. Monofluorinated Nitrogen Containing Heterocycles: Synthesis, Characterization and Fluorine Effect. Z. Anorg. Allg. Chem. 2020, 646, 1790–1794. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, L.; Shen, Q. Monofluoromethyl-Substituted Sulfonium Ylides: Electrophilic Monofluoromethylating Reagents with Broad Substrate Scopes. Angew. Chem. Int. Ed. 2017, 56, 9930–9934. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Liu, Y.; Lu, L.; Shen, Q. Monofluoromethyl-Substituted Sulfonium Ylides: Preparation, Structure-Reactivity Study and Substrate Scope†. Chin. J. Chem. 2020, 38, 1317–1331. [Google Scholar] [CrossRef]
- Qin, W.-B.; Liu, J.-J.; Huang, Z.; Li, X.; Xiong, W.; Chen, J.-Y.; Liu, G.-K. Bench-Stable S-(Monofluoromethyl)sulfonium Salts: Highly Efficient C- and O-Regioselective Monofluoromethylation of 1,3-Dicarbonyl Compounds. Eur. J. Org. Chem. 2020, 2020, 5862–5866. [Google Scholar] [CrossRef]
- Cao, Y.; Jiang, L.; Yi, W. Synthesis of Monofluoromethyl Selenoethers of Aryl and Alkyl from Organoselenocyanate via One-Pot Reaction. Adv. Synth. Cat. 2019, 361, 4360–4368. [Google Scholar] [CrossRef]
- Voltrová, S.; Filgas, J.; Slavíček, P.; Beier, P. Azidofluoromethane: Synthesis, stability and reactivity in [3 + 2] cycloadditions. Org. Chem. Front. 2020, 7, 10–13. [Google Scholar] [CrossRef]
- Zhang, W.; Zhu, L.; Hu, J. Electrophilic monofluoromethylation of O-, S-, and N-nucleophiles with chlorofluoromethane. Tetrahedron 2007, 63, 10569–10575. [Google Scholar] [CrossRef]
- Shen, X.; Zhou, M.; Ni, C.; Zhang, W.; Hu, J. Direct monofluoromethylation of O-, S-, N-, and P-nucleophiles with PhSO(NTs)CH2F: The accelerating effect of α-fluorine substitution. Chem. Sci. 2014, 5, 117–122. [Google Scholar] [CrossRef]
- Haupt, J.D.; Berger, M.; Waldvogel, S.R. Electrochemical Fluorocyclization of N-Allylcarboxamides to 2-Oxazolines by Hypervalent Iodine Mediator. Org. Lett. 2019, 21, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Winterson, B.; Rennigholtz, T.; Wirth, T. Flow electrochemistry: A safe tool for fluorine chemistry. Chem. Sci. 2021, 12, 9053–9059. [Google Scholar] [CrossRef] [PubMed]
- Sperga, A.; Kazia, A.; Veliks, J. Monofluorinated 5-membered rings via fluoromethylene transfer: Synthesis of monofluorinated isoxazoline N-oxides. Org. Biomol. Chem. 2021, 19, 2688–2691. [Google Scholar] [CrossRef] [PubMed]
- Parisi, G.; Colella, M.; Monticelli, S.; Romanazzi, G.; Holzer, W.; Langer, T.; Degennaro, L.; Pace, V.; Luisi, R. Exploiting a “Beast” in Carbenoid Chemistry: Development of a Straightforward Direct Nucleophilic Fluoromethylation Strategy. J. Am. Chem. Soc. 2017, 139, 13648–13651. [Google Scholar] [CrossRef] [PubMed]
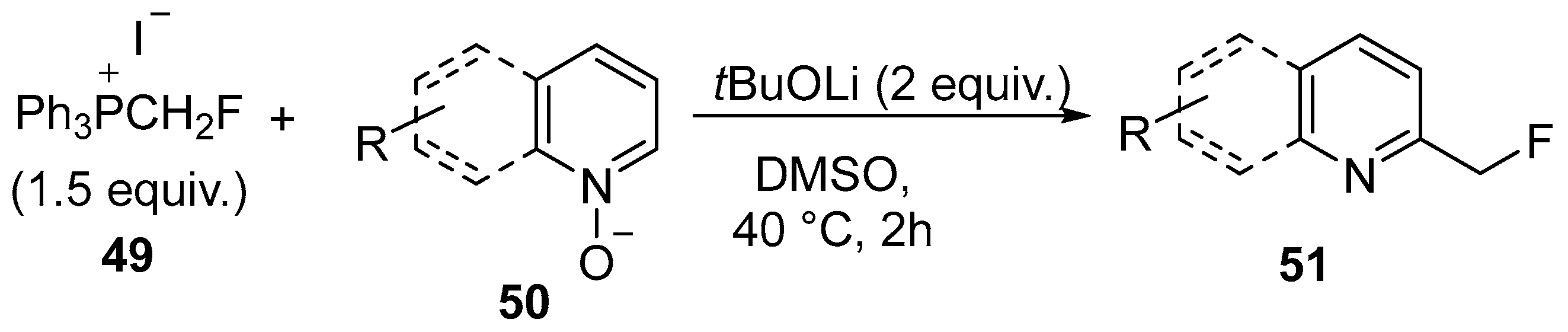
- Hu, C.-C.; Hu, W.-Q.; Xu, X.-H.; Qing, F.-L. 2-Position-selective CH fluoromethylation of six-membered heteroaryl N-oxides with (fluoromethyl)triphenylphosphonium iodide. J. Fluor. Chem. 2021, 242, 109695. [Google Scholar] [CrossRef]
- Du, Y.; Chen, S.; Huang, A.; Chen, Y.; Liu, Y.-L.; Song, G.; Tang, R.-Y.; Xu, H.; Yao, G.; Li, Z. Diversity-Oriented Synthesis of Fluoromethylated Arenes via Palladium-Catalyzed C–H Fluoromethylation of Aryl Iodides. Org. Lett. 2022, 24, 1341–1345. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Sheng, J.; Zhang, K.-F.; Zhang, Z.-Q.; Bian, K.-J.; Wang, X.-S. Nickel-catalyzed monofluoromethylation of (hetero)aryl bromides via reductive cross-coupling. Chem. Commun. 2019, 55, 7635–7638. [Google Scholar] [CrossRef]
- Rong, J.; Deng, L.; Tan, P.; Ni, C.; Gu, Y.; Hu, J. Radical Fluoroalkylation of Isocyanides with Fluorinated Sulfones by Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2016, 55, 2743–2747. [Google Scholar] [CrossRef]
- Guo, C.; Liu, Z.; Li, X.; Han, X.; Li, Y.; Liu, H.; Zhang, L.; Li, X.; Dong, Y. Silver-catalyzed monofluoroalkylation of heteroarenes with α-fluorocarboxylic acids: An insight into the solvent effect. Chem. Commun. 2022, 58, 1147–1150. [Google Scholar] [CrossRef]
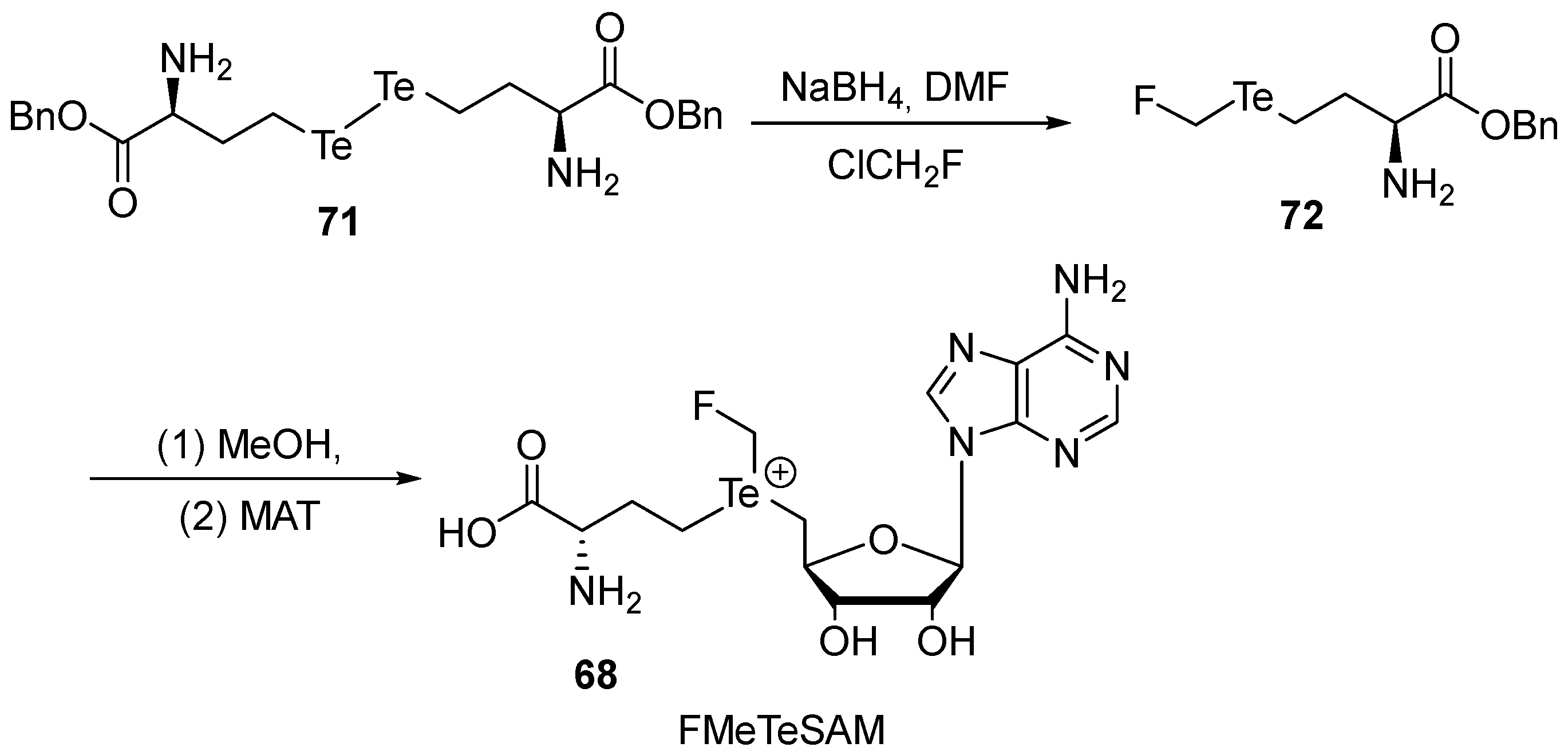
- Neti, S.S.; Wang, B.; Iwig, D.F.; Onderko, E.L.; Booker, S.J. Enzymatic Fluoromethylation Enabled by the S-Adenosylmethionine Analog Te-Adenosyl-L-(fluoromethyl)homotellurocysteine. ACS Cent. Sci. 2023, 9, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liao, C.; Bauer, C.; Seebeck, F.P. Fluorinated S-Adenosylmethionine as a Reagent for Enzyme-Catalyzed Fluoromethylation. Angew. Chem. Int. Ed. 2021, 60, 27178–27183. [Google Scholar] [CrossRef] [PubMed]

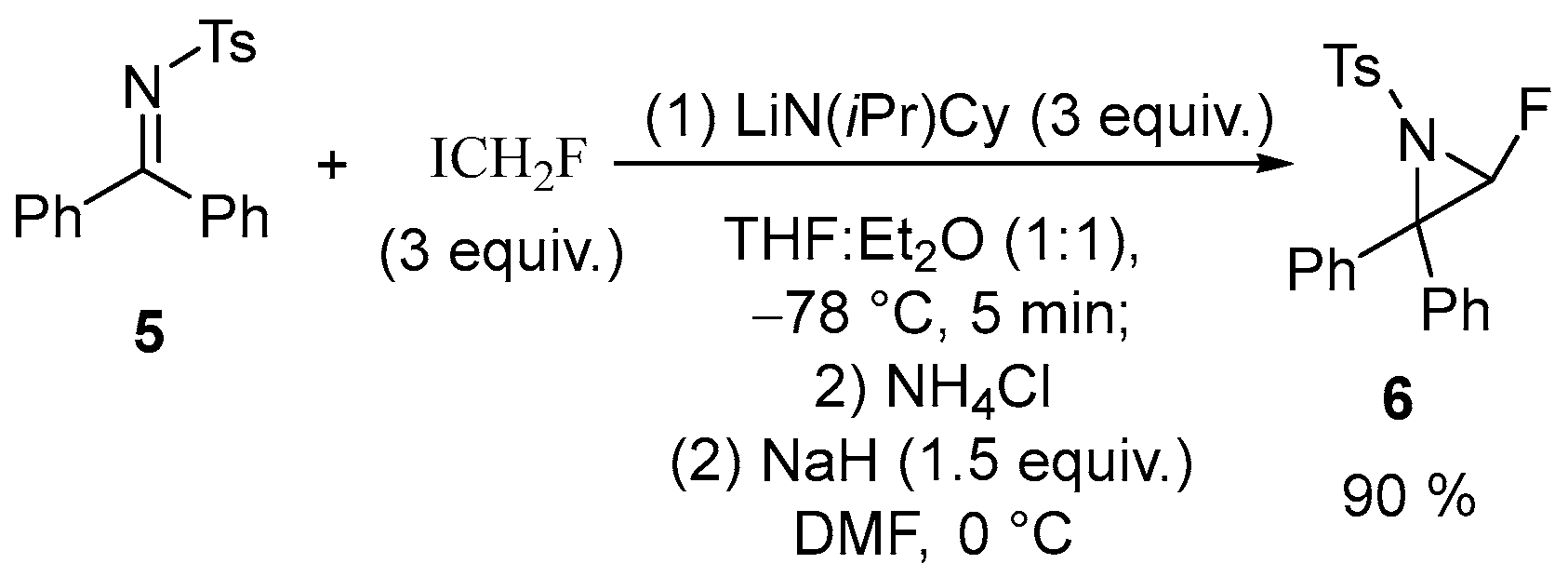
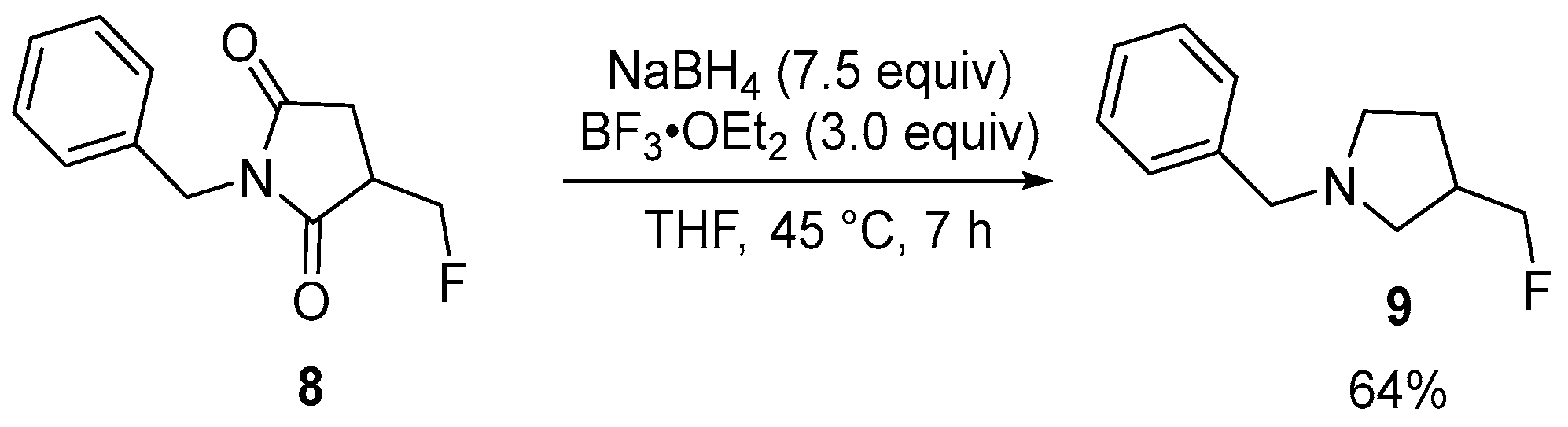


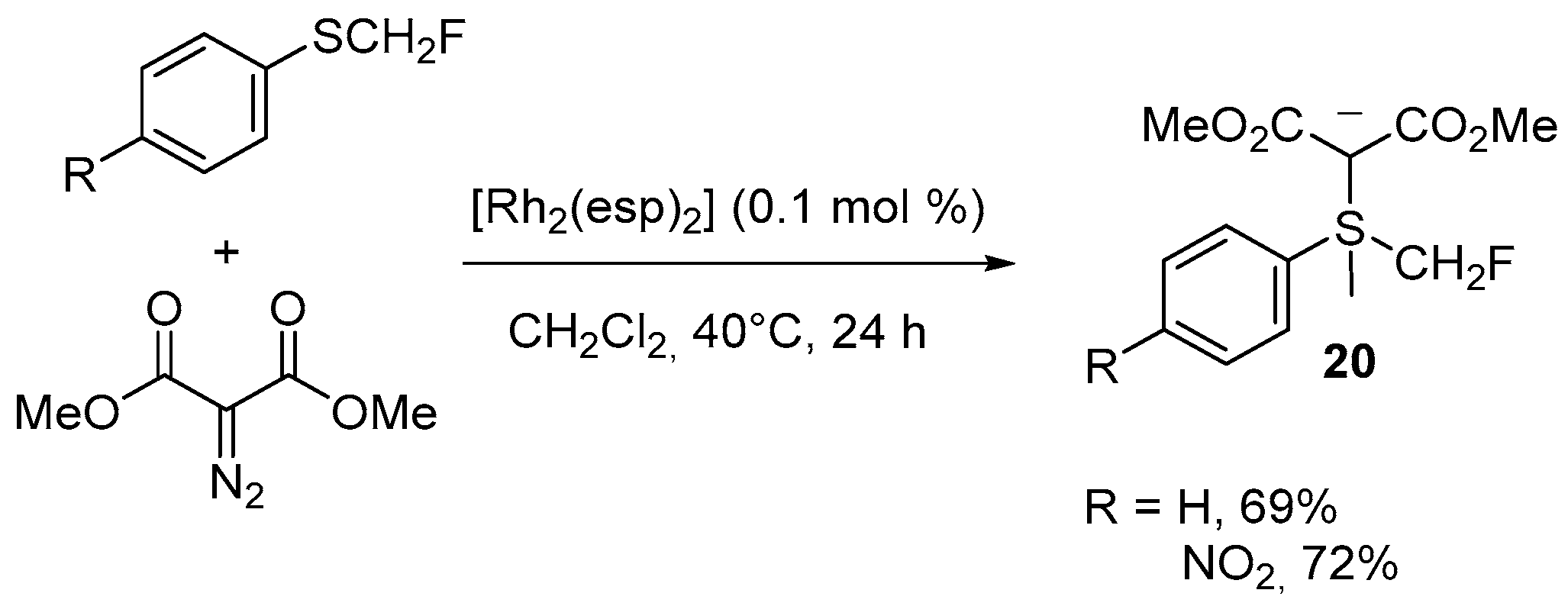
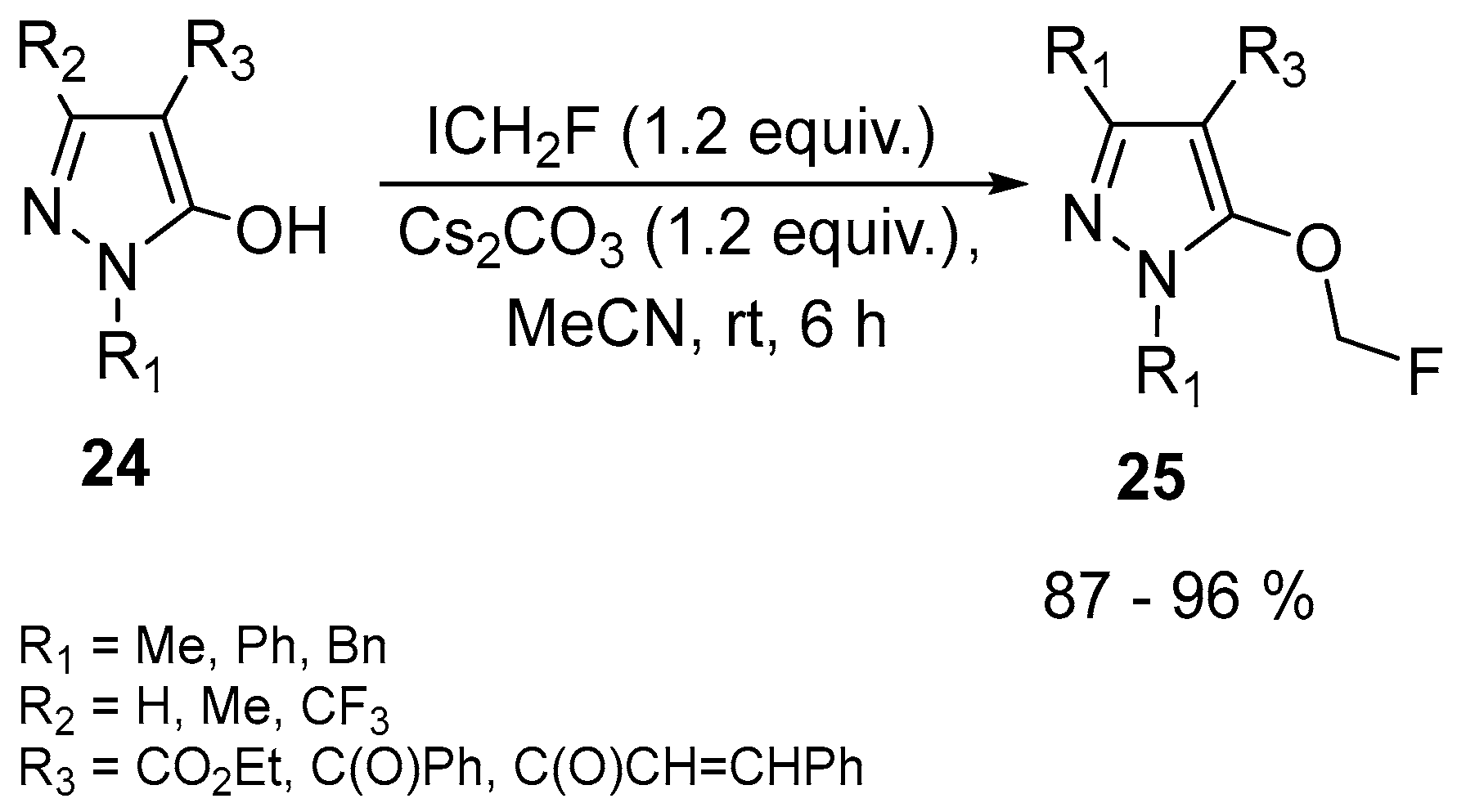
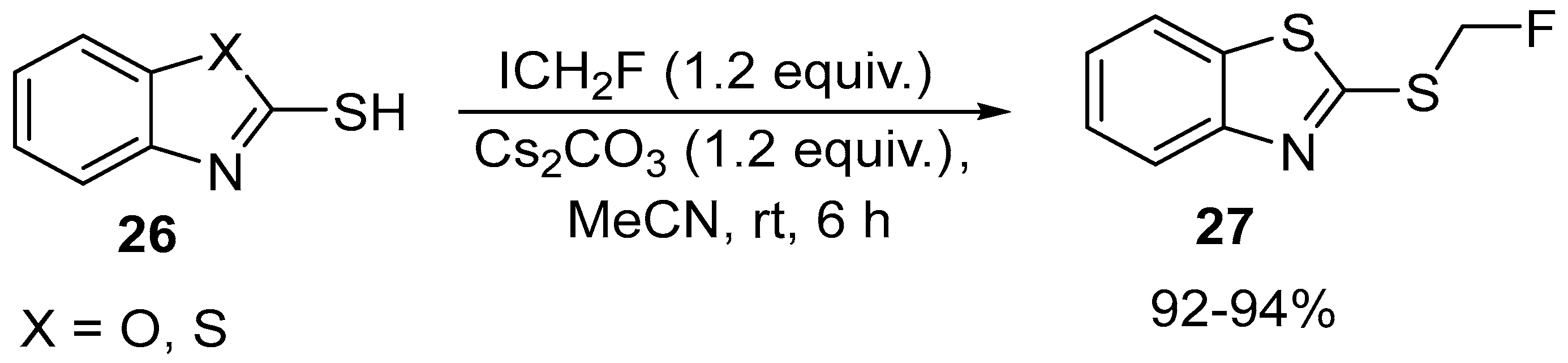



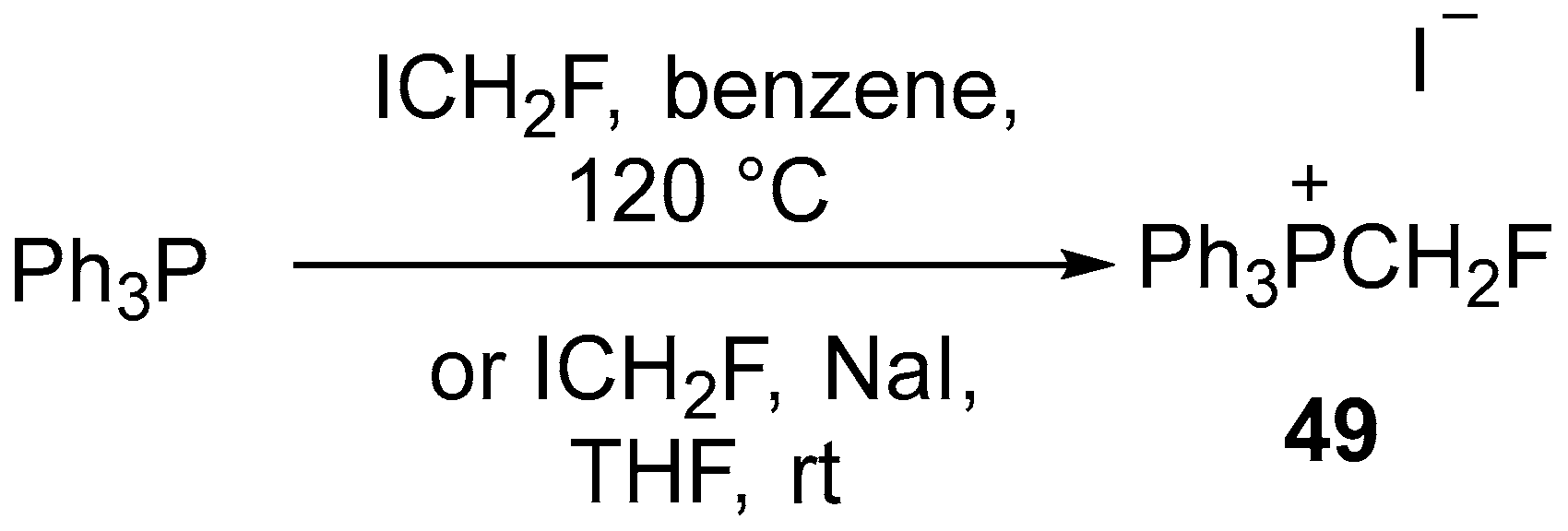

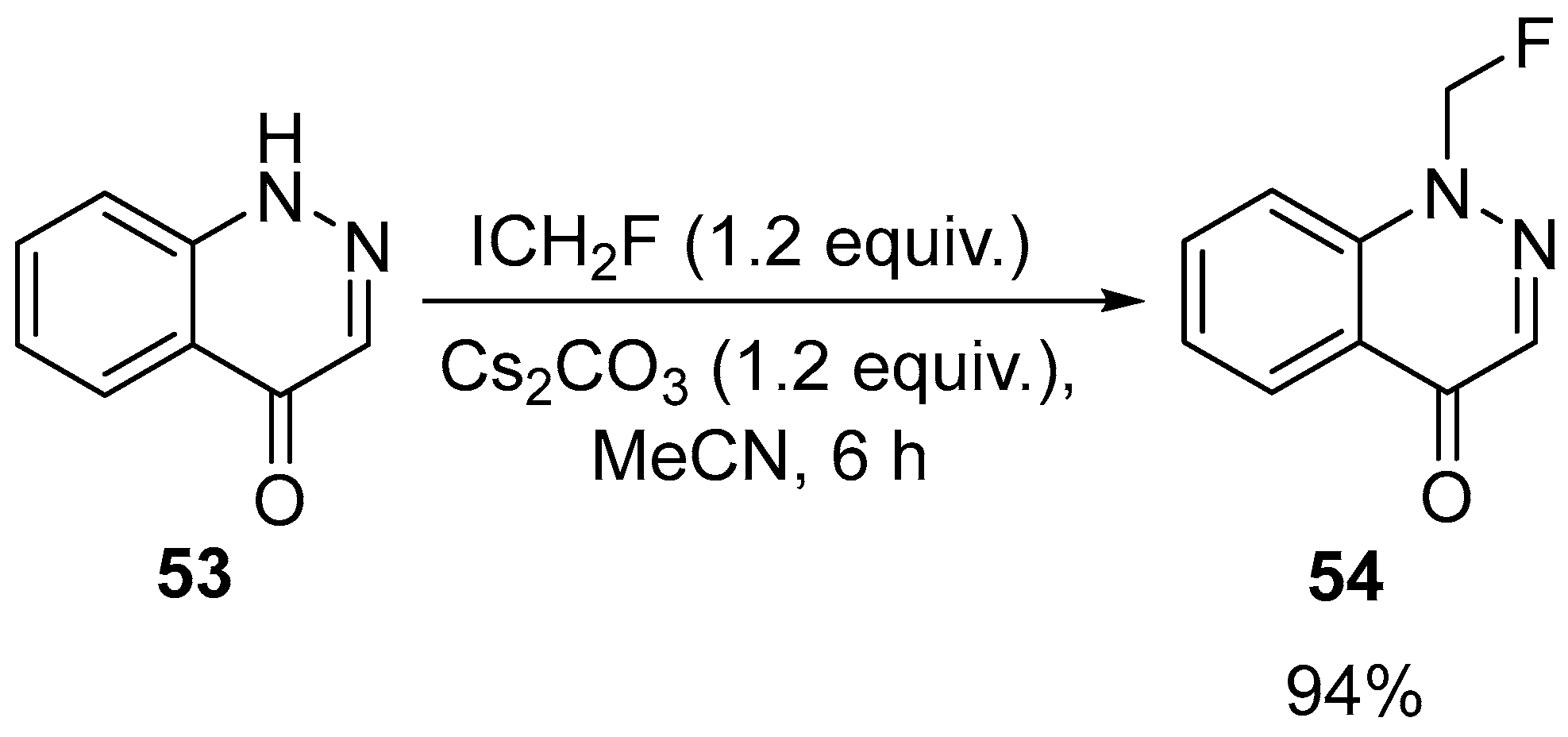
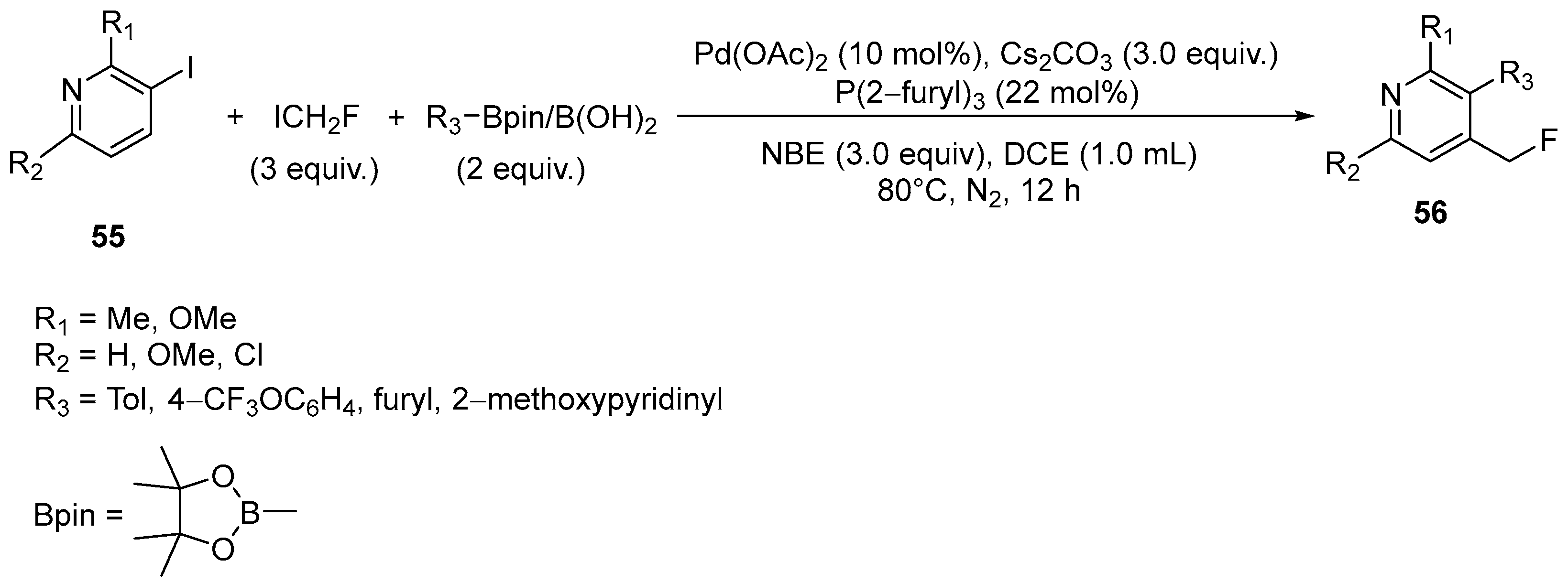
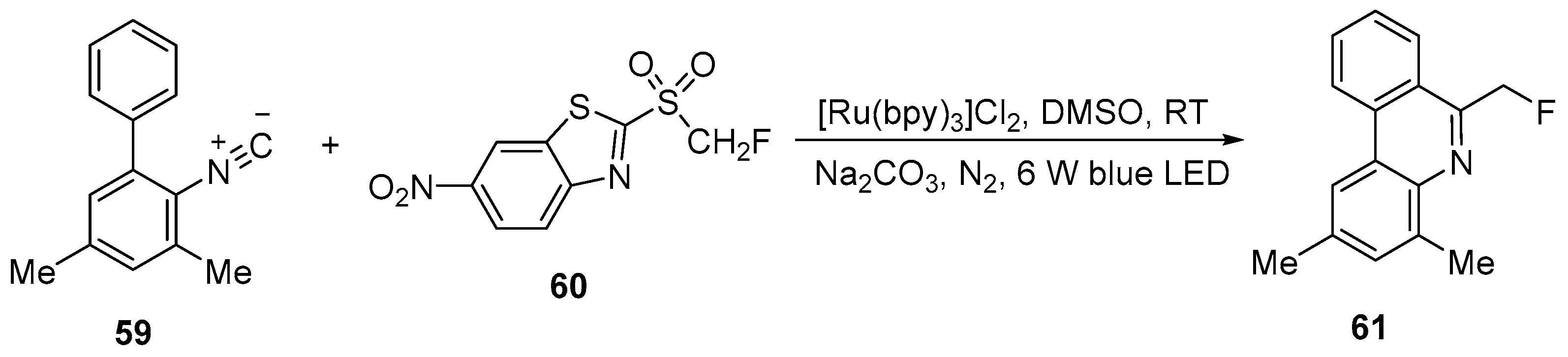
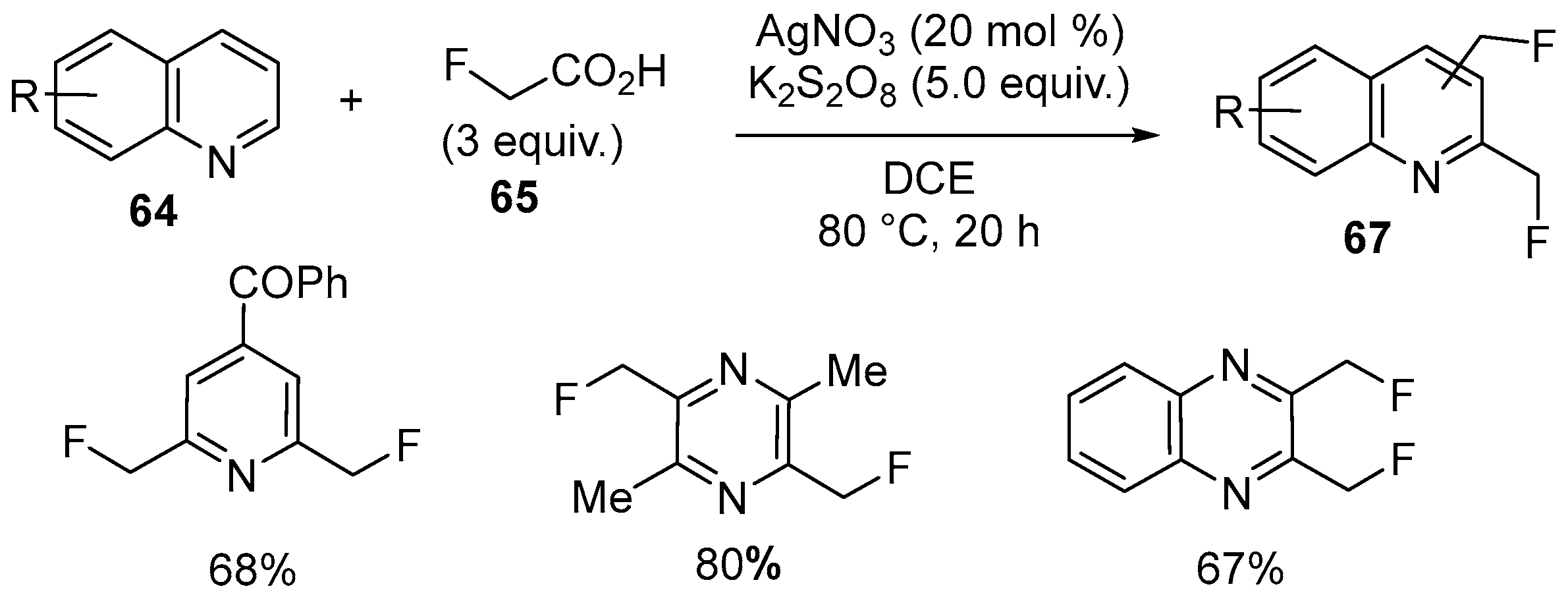

| Structure | Therapeutic Use | Approval Status |
|---|---|---|
Carmegliptin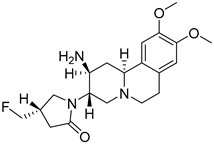 | Inhibitor of dipeptidyl peptidase 4 (DPP-4), with hypoglycemic activity for monotherapy or in combination with other oral antihyperglycemic agents for the treatment of Type 2 diabetes | Investigational drug; second phase of clinical trials [43,44]; |
Fluticasone furoate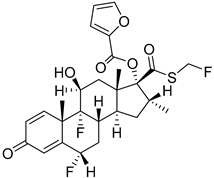 | Corticosteroid with potent anti-inflammatory, anti-allergic action | 2007 |
Sevoflurane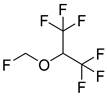 | General anesthetic | 1995 |
Sirpefenicol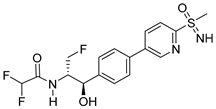 | Antibacterial agent | Investigational veterinary drug; |
Norcholestenol fluoromethyl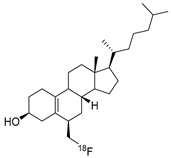 | Fluorinated analog of the scintiscanning/SPECT agent for PET imaging | Investigational PET imaging agent [45]; |
Imlunestant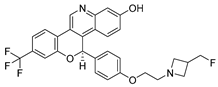 | Orally active ER-α degrader, antiestrogen and antineoplastic agent for the treatment of ER-positive (ER+) breast cancers; | Investigational drug [46,47,48,49,50]; third phase of clinical trials; |
Fipravirimat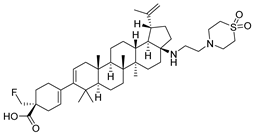 | Anti-HIV/AIDS; maturation inhibitors; | Investigational drug [51]; |
18F-FMISO or fluoromisonidazole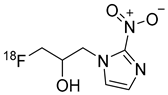 | Radiopharmaceutical for PET imaging of hypoxia | Investigational drug [52,53]; |
Mefway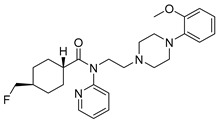 | Serotonin 5-HT1A receptor antagonist; PET radiotracer | Investigational drug [54,55]; |
Florfenicol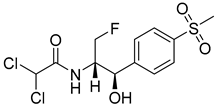 | Antibiotic | 1996 |
Cefluprenam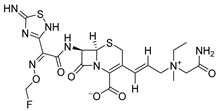 | Anti-bacterial agent of broad spectrum; fourth generation cephalosporin; Highly effective against bacterial pneumonia; | 1997 |
Afloqualone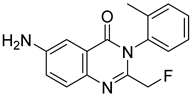 | Sedative and muscle-relaxant | Investigational drug [56]; |
Fluorocholine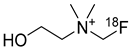 | Oncologic PET tracer | Investigational drug [57]; |
| Scheme № | Product | Substrate | CH2F-Source, Conditions | Ref. |
|---|---|---|---|---|
| Three-Membered N-heterocycles | ||||
| 1 | α-Fluoromethyl-α-CF3 aziridine | Trifluoroacetimidoyl chloride (TFAIC) | ICH2F; MeLi-LiBr THF, −78 °C, 1 h | [58] |
| 2 | α-Fluoroaziridine | N-tosyl-diphenyl ketimine | ICH2F; LiN(iPr)Cy, | [59] |
| Five-Membered N-heterocycles | ||||
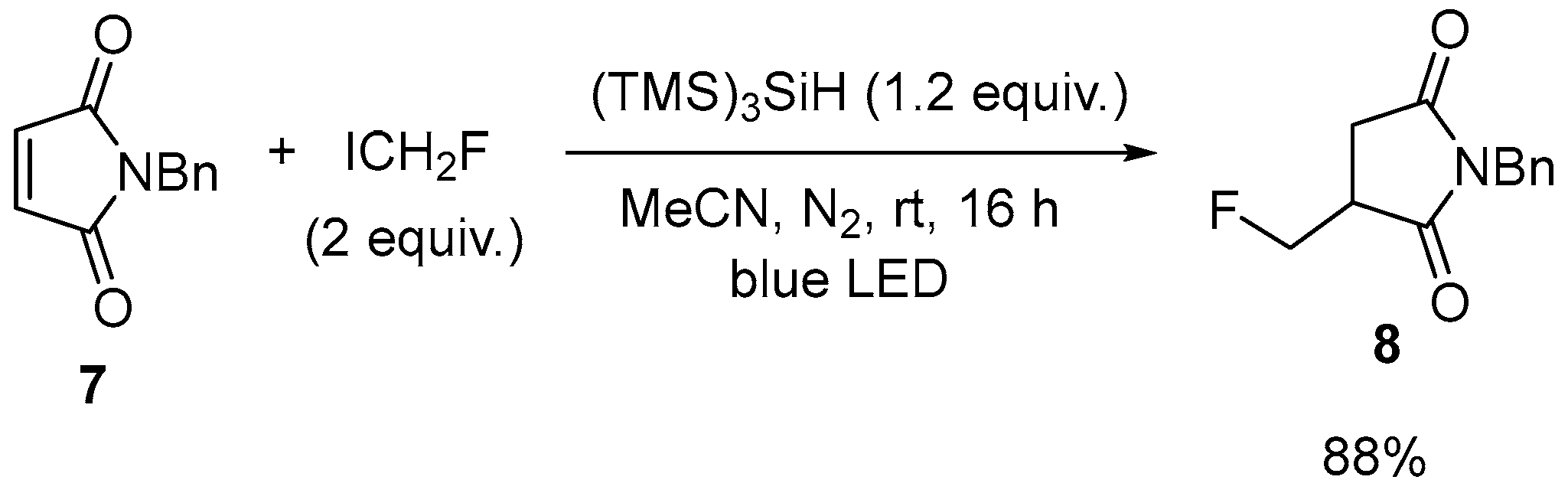
| 3 | 3-Fluoromethylated N-benzylpyrrolidines | N-Benzylmaleimide | ICH2F; (Me3Si)3SiH, blue LED | [60] |
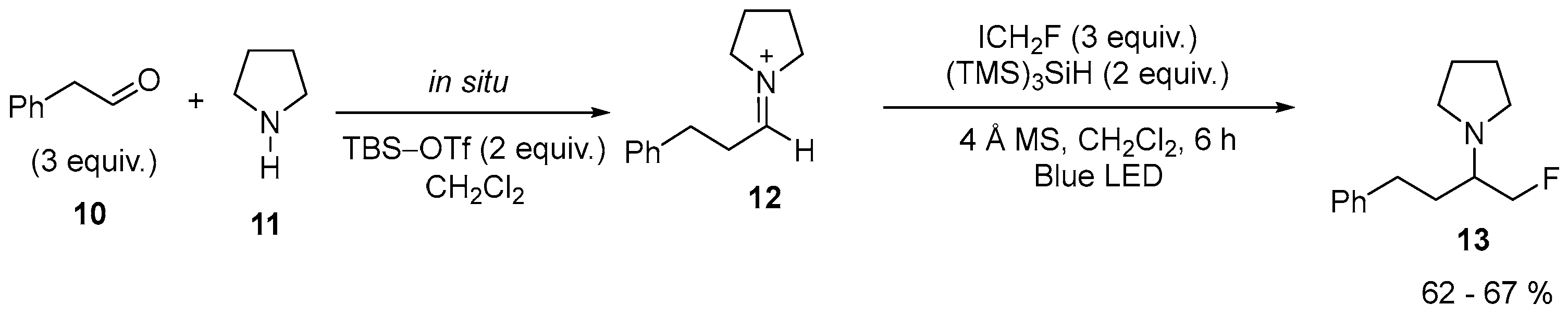
| 4 | 1-(1-Fluoro-4-phenylbutan-2-yl)pyrrolidine | Pyrrolidine | ICH2F; 2-phenylacetaldehyde, (Me3Si)3SiH, blue LED | [27] |
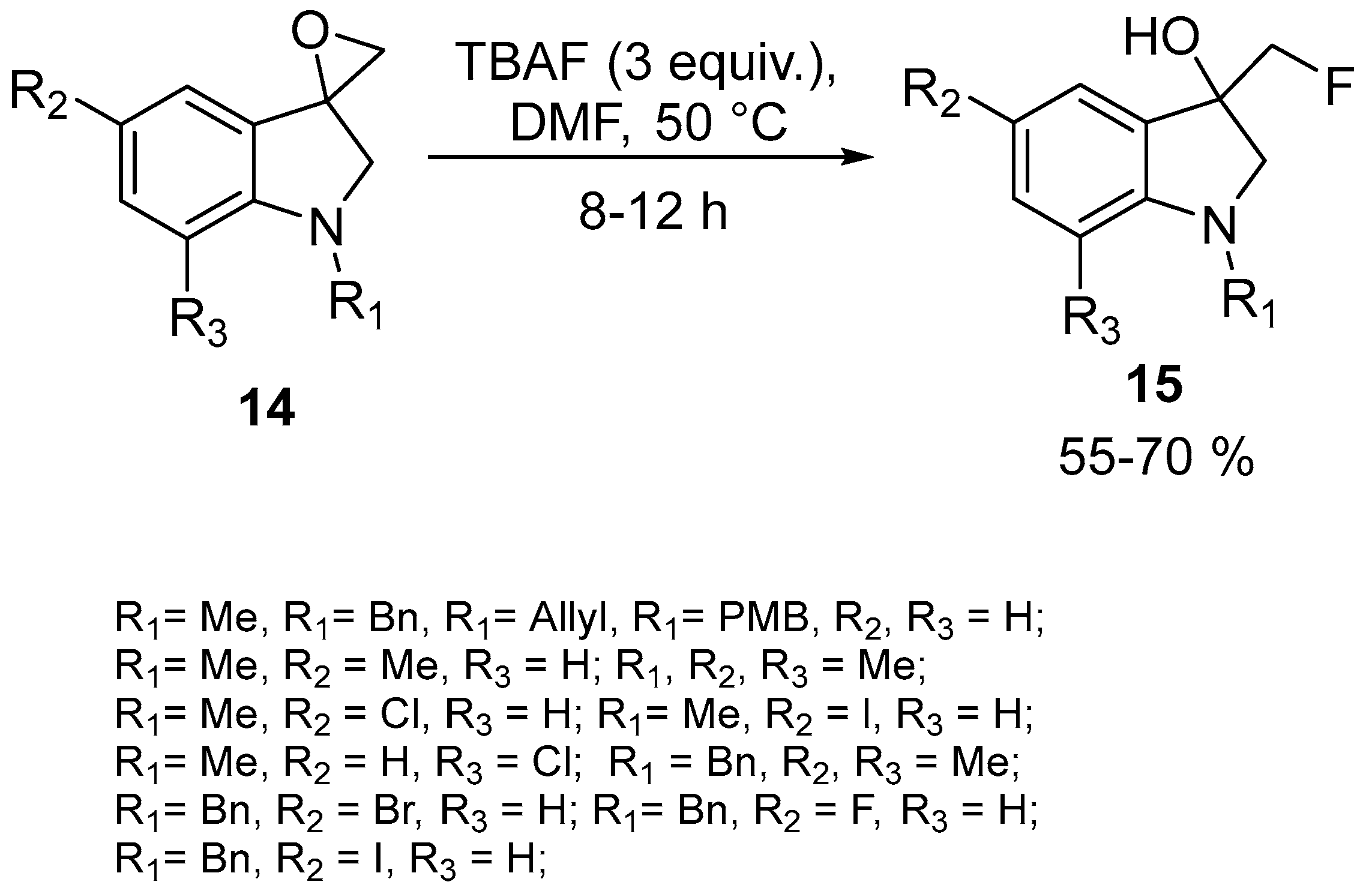
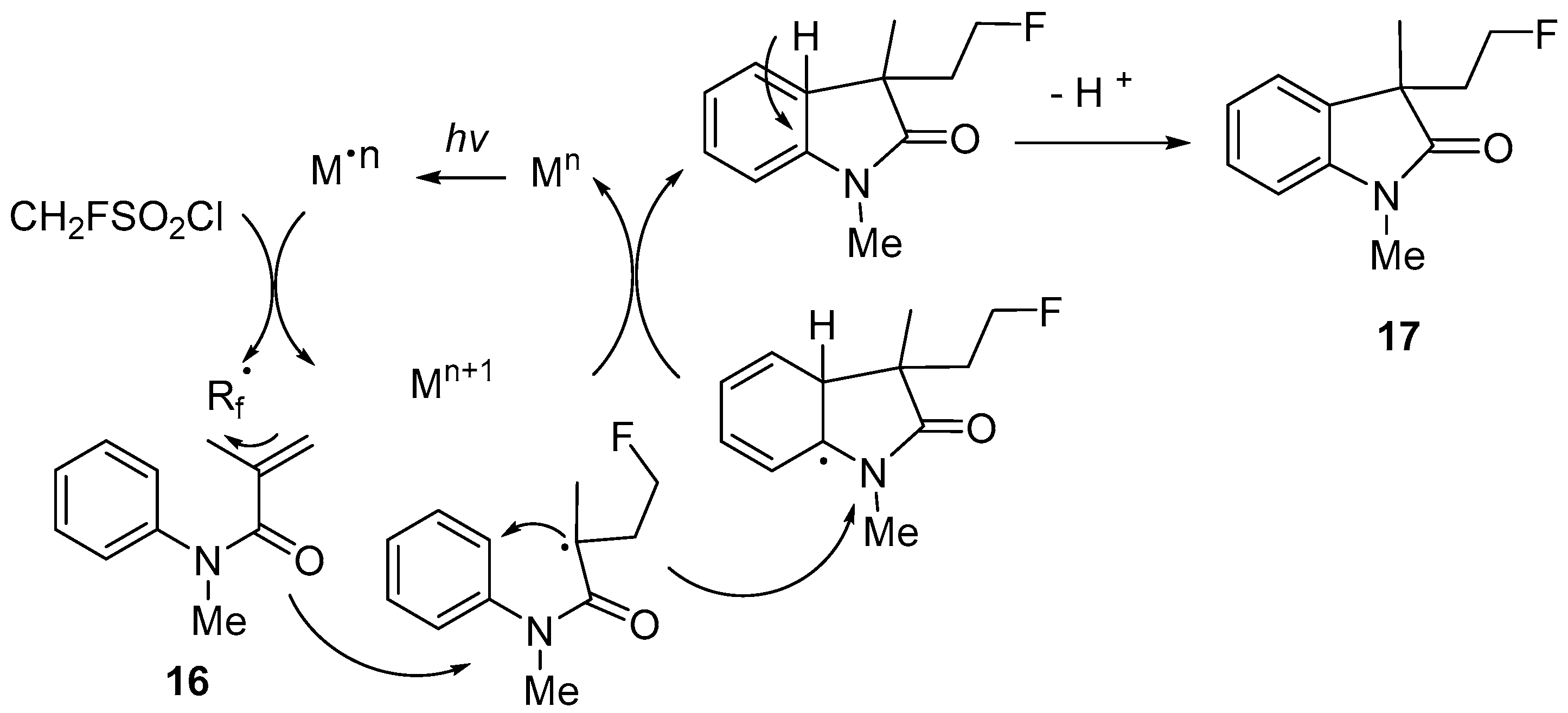
| 6 | 3-Fluoromethylated indolines | Spiro-epoxyoxindolines | TBAF | [61] |
| 7 | 3-(2-Fluoroethyl)indolin-2-ones | N-Methyl-N-phenylmethacrylamides | FCH2SO2Cl; K2HPO4 fac-Ir(ppy)3 (1 mol%) | [62,63] |
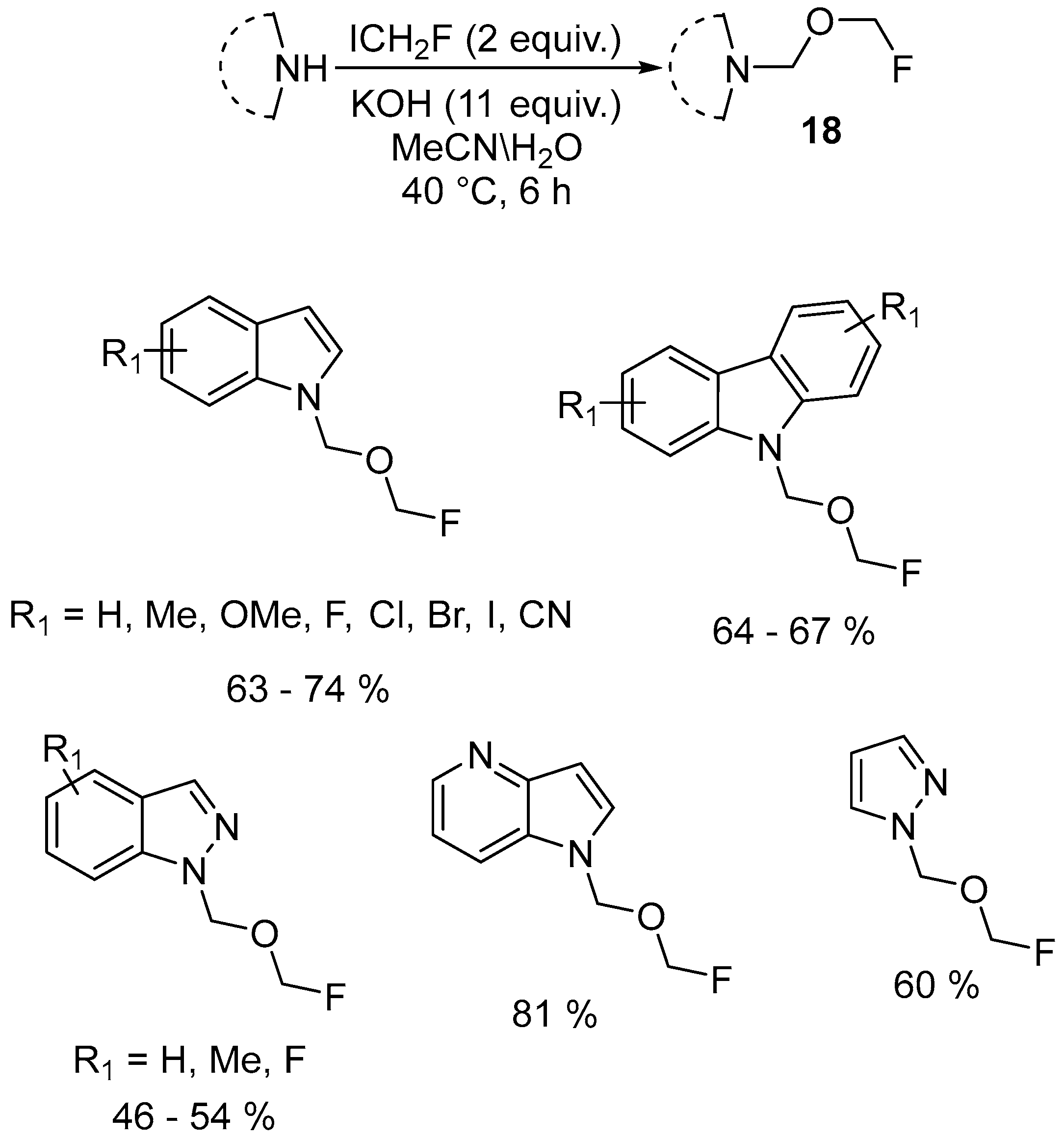
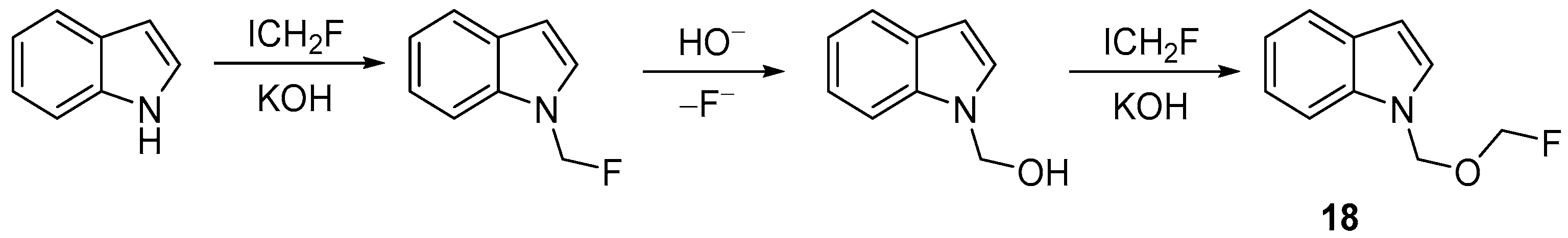
| 9 | N-Fluoromethoxymethylated indoles, carbazoles, 1H-indazoles and pyrazoles | Indoles, carbazoles, 1H-indazoles and pyrazoles | ICH2F; KOH MeCN/H2O 40 °C, 6 h | [64] |
| 11 | N-fluoromethylated Theophylline, Cimetidine, Phenytoin (hydantoin), imidazole, indazoles, phthalimide | Theophylline, Cimetidine, Phenytoin (hydantoin), Imidazole, Indazole, phthalimide | ICH2F, Cs2CO3. Theophylline, Cimetidine, Phenytoin (hydantoin), Imidazole, Indazole, phthalimide | [65] |
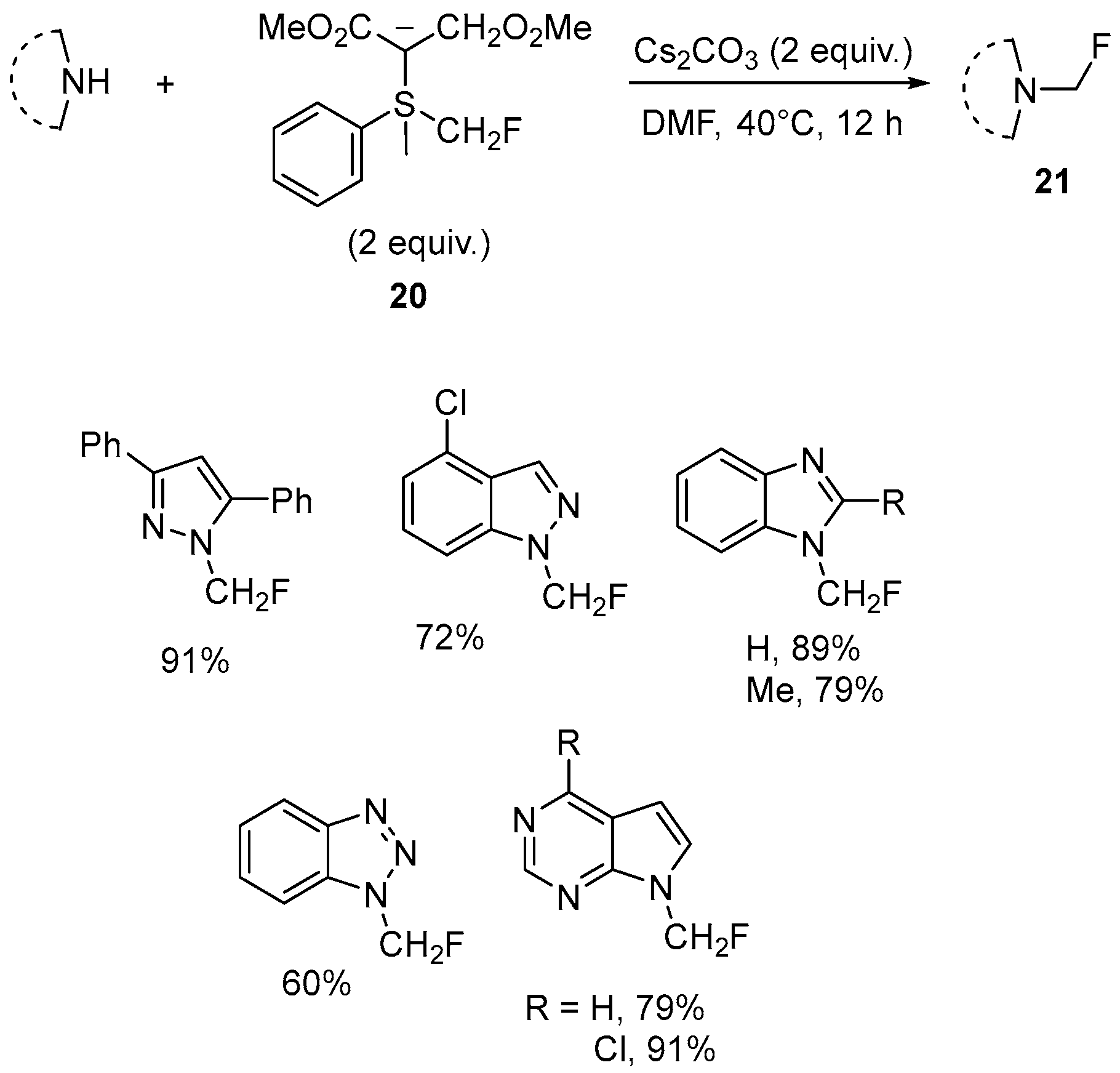
| 12 | N-Fluoromethylated pyrazole, indazole, benzoimidazole, benzotriazole, 7H-pyrrolo[2,3-d]pyrimidine | 1H-pyrazole, 1H-indazole, benzoimidazole, benzotriazole, 7H-pyrrolo[2,3-d]pyrimidine | Monofluoromethyl(aryl)sulfonium bis(carbomethoxy)methylide; Cs2CO3, DMF; 40 °C, 12 h | [67,68,69] |
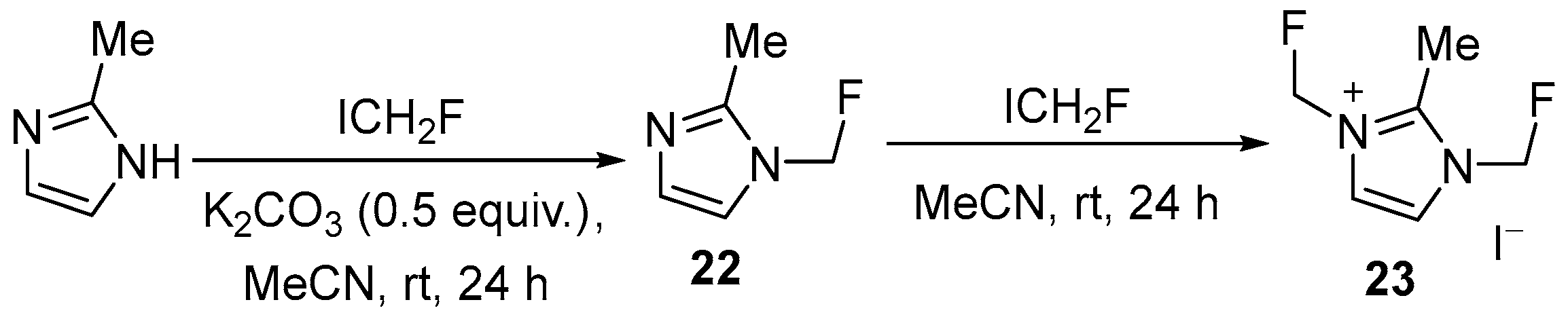
| 14 | 1-Fluoromethyl-2-methyl imidazole; bis(fluoromethyl)-2-methylimidazolium iodide | 2-Methyl imidazole | ICH2F; K2CO3, MeCN, rt | [66] |
| 15 | O-fluoromethylated N-substituted 5-hydroxypyrazoles | N-substituted 5-hydroxypyrazoles | ICH2F, Cs2CO3, MeCN 6 h, rt | [65] |
| 16 | 2-((Fluoromethyl)thio)benzo[d]thiazole, 2-((Fluoromethyl)thio)benzo[d]thiazole | 2-thio-benzooxazole and 2-thio-benzothiazole | ICH2F, Cs2CO3, MeCN 6 h, rt | [65] |
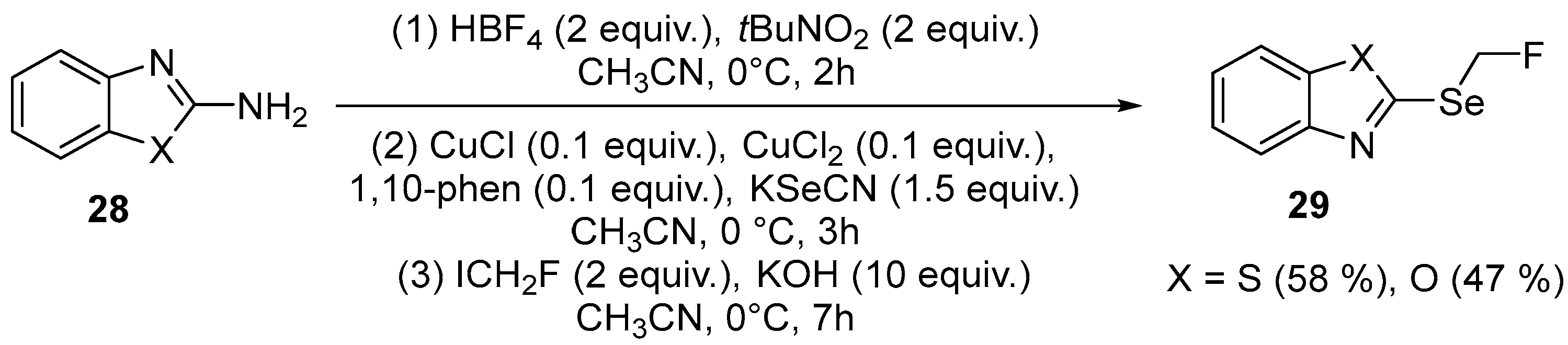
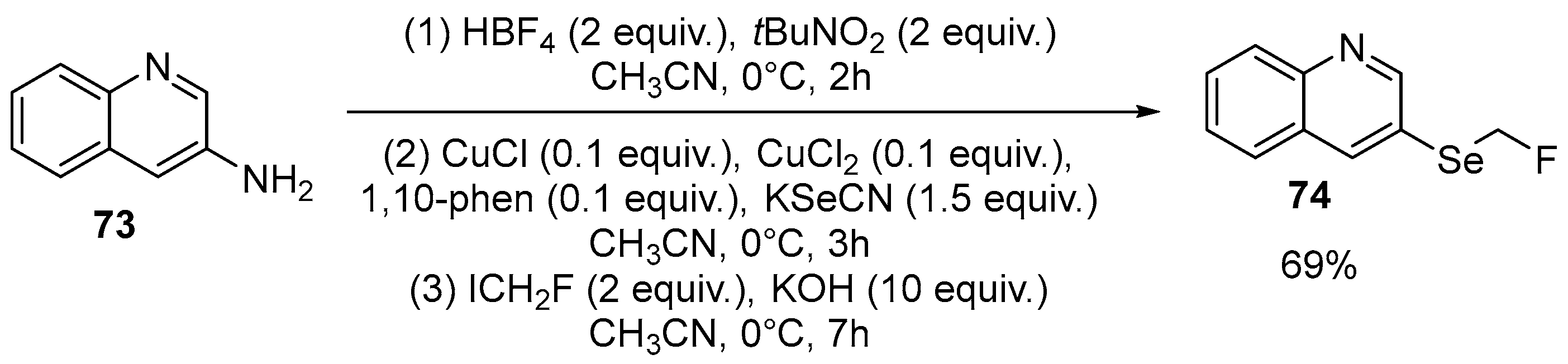
| 17 | 2-((fluoromethyl)selanyl)benzooxazole, 2-((fluoromethyl)selanyl) benzothiazole | 2-(Amimo)benzooxazole, 2-(Amimo)benzothiazole | ICH2F; KSeCN, KOH | [70] |
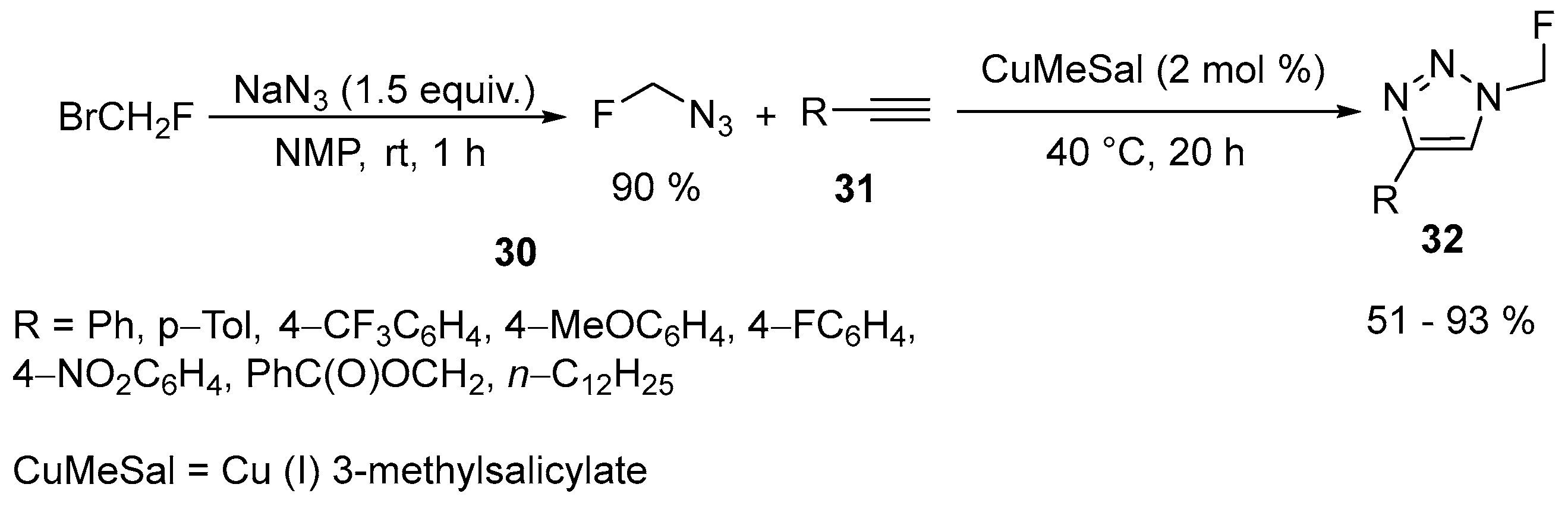
| 19 | 4-Substituted N-fluoromethyl-1,2,3-triazoles | Terminal alkynes | Azidofluoromethane; NMP, Cu (I) 3-methylsalicylate, rt, 1 h | [71] |
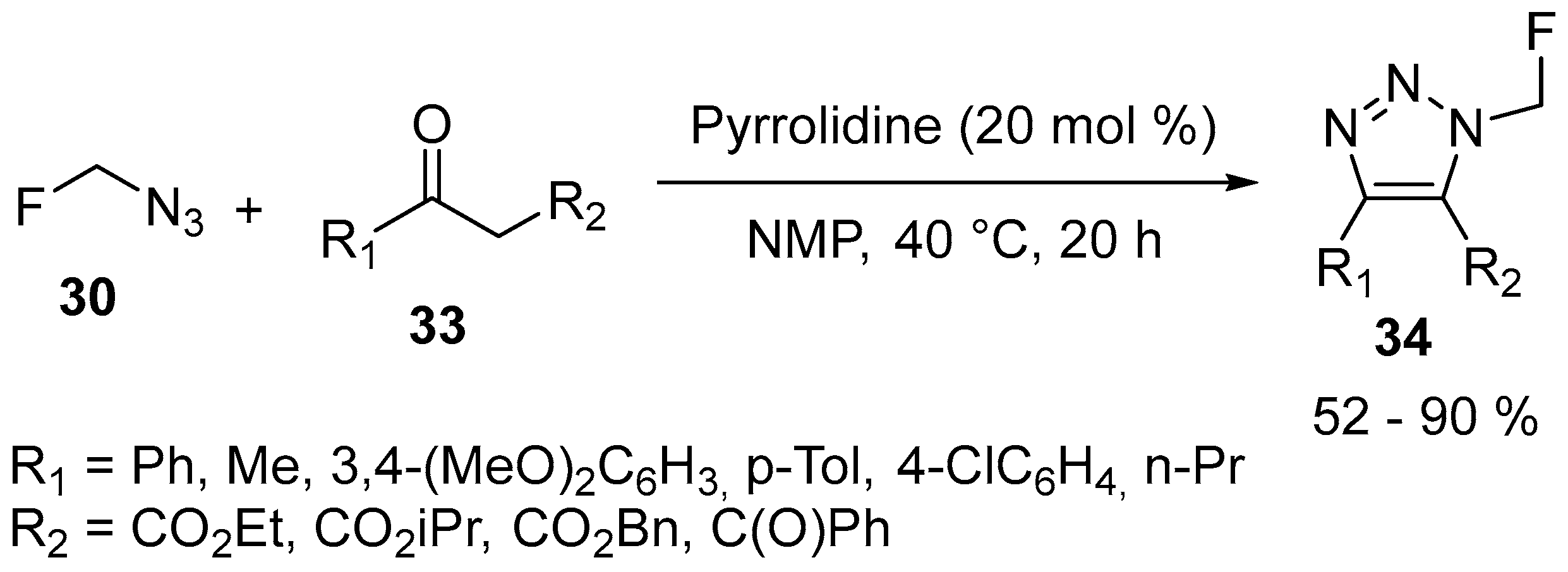
| 20 | 4,5-Disubstituted N-fluoromethyl-1,2,3-triazole | β-Ketoesters or 1,3-Diones | Azidofluoromethane; pyrrolidine, 40 °C, 20 h | [71] |
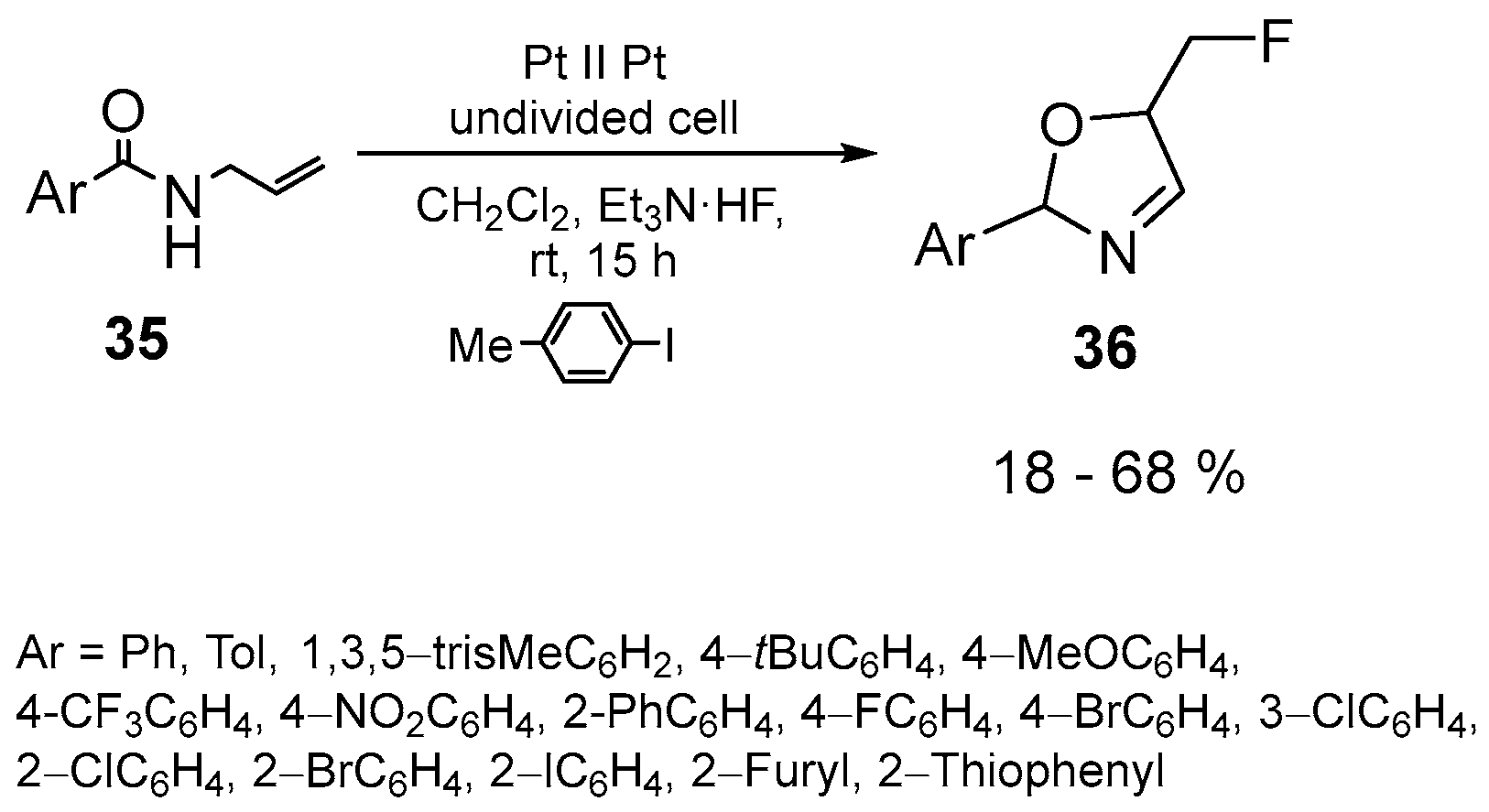
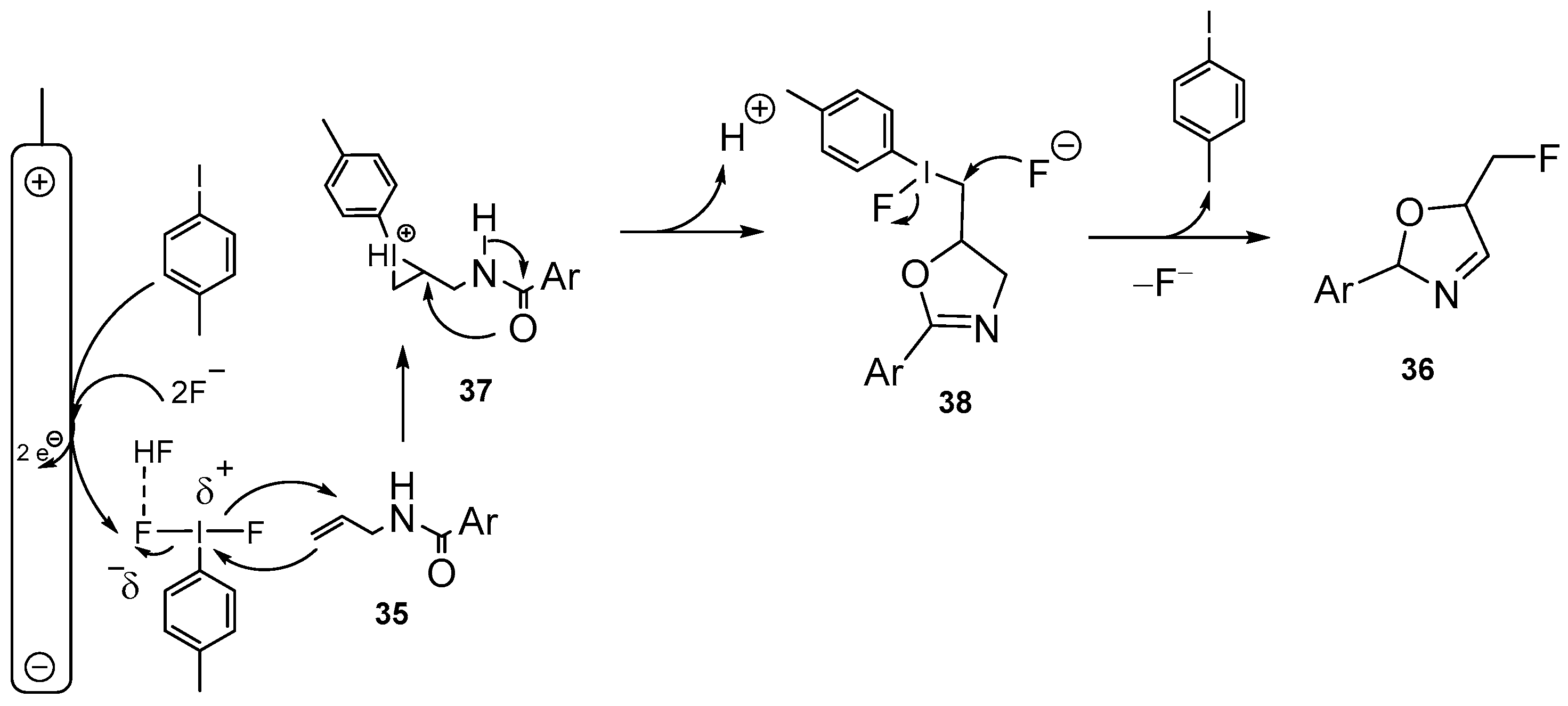
| 21 | 5-Fluoromethyl-2-oxazolines | N-Allylcarboxamides | Et3N·5HF; Electrochemical conditions; 4-Methyliodobenzene, 4-tert-butyliodobenzene; CH2Cl2, rt, 15 h | [74,75] |
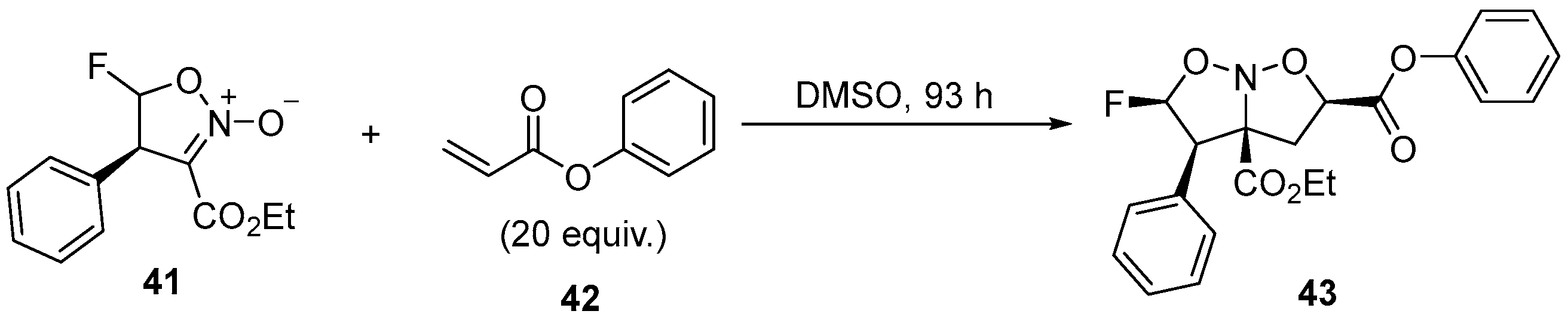
| 23 | 5-Monofluorinated isoxazoline N-oxides | 2-Nitroacrylates | Monofluoromethyl sulfides; NaH, 40 °C, 20 h | [76] |
| Six-Membered N-heterocycles | ||||
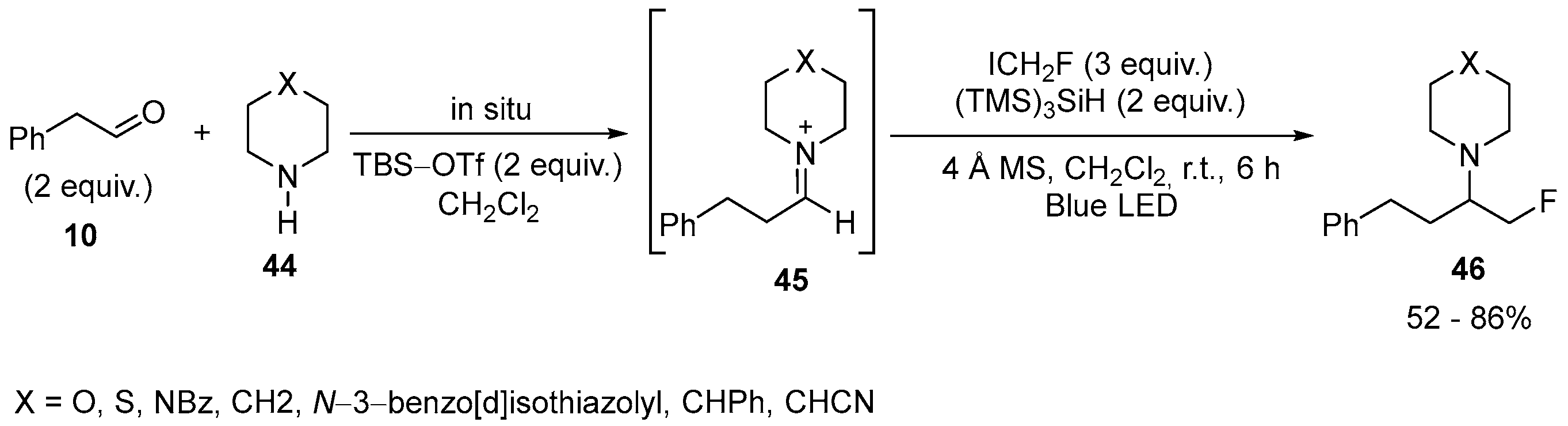
| 25 | 1-(1-Fluoro-4-phenylbutan-2-yl) pyperidine, piperazines, morpholine, thiomorpholine | 2-phenylacetaldehyde Pyperidine, piperazines, morpholine, thiomorpholine | ICH2F; tBudimethylsilyltrifluoromethanesulfonate (TBS-OTf) (TMS)3SiH; 4 Å MS, CH2Cl2, r.t., 6 h Blue LED | [27] |
| 26 | 1-Benzyl-4-(fluoromethyl)piperidin-4-ol | Piperidin-4-one | ICH2F; MeLi-LiBr THF, −78 °C, 1 h | [77] |
| 28 | C2-fluoromethylated pyridines, 2,2′-bipyridine, quinoline and phenanthridine-N-oxides | Pyridines, 2,2′-bipyridine, quinoline and phenanthridine-N-oxides | (fluoromethyl)triphenylphosphonium iodide | [78] |
| 30 | 1-(Fluoromethyl)cinnolin-4(1H)-one | 4(1H)-cinnolinone | ICH2F, Cs2CO3, MeCN 6 h, rt | [65] |
| 31 | Ortho-fluoromethylated pyridines | 3-Iodo-2,5-disabstituted pyridines | ICH2F; Pinacol arylboronic esters (RBpin) Pd(OAc)2 (10 mol%), Cs2CO3 (3.0 equiv.) P(2-furyl)3 (22 mol%) | [79] |
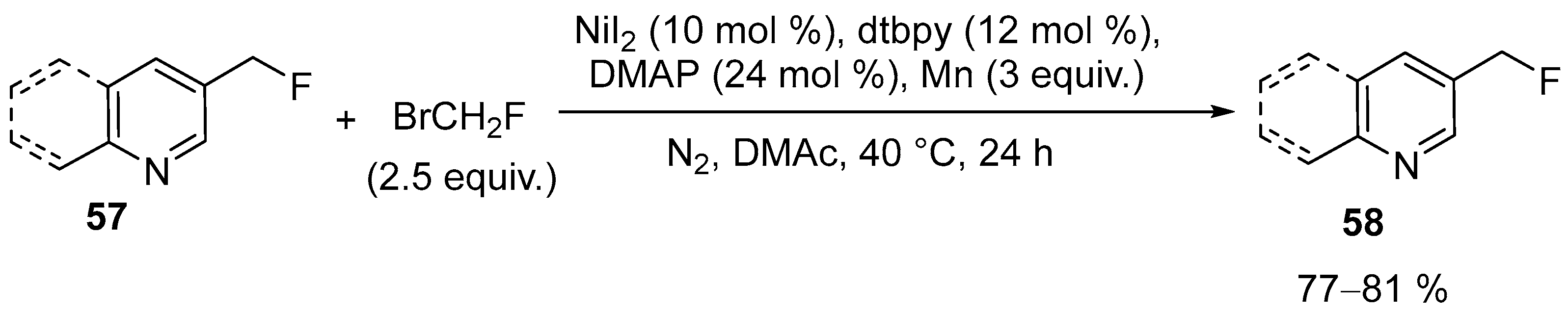
| 32 | 3-(Fluoromethyl)pyridine; 3-(Fluoromethyl)quinoline | Pyridine, quinoline | BrCH2F; NiI2; dtbpy (4,4-Di-tert-butyl-2,2-dipyridine); DMAP (4-dimethylaminopyridine); N2, DMAc, 40 °C, 24 h | [80] |
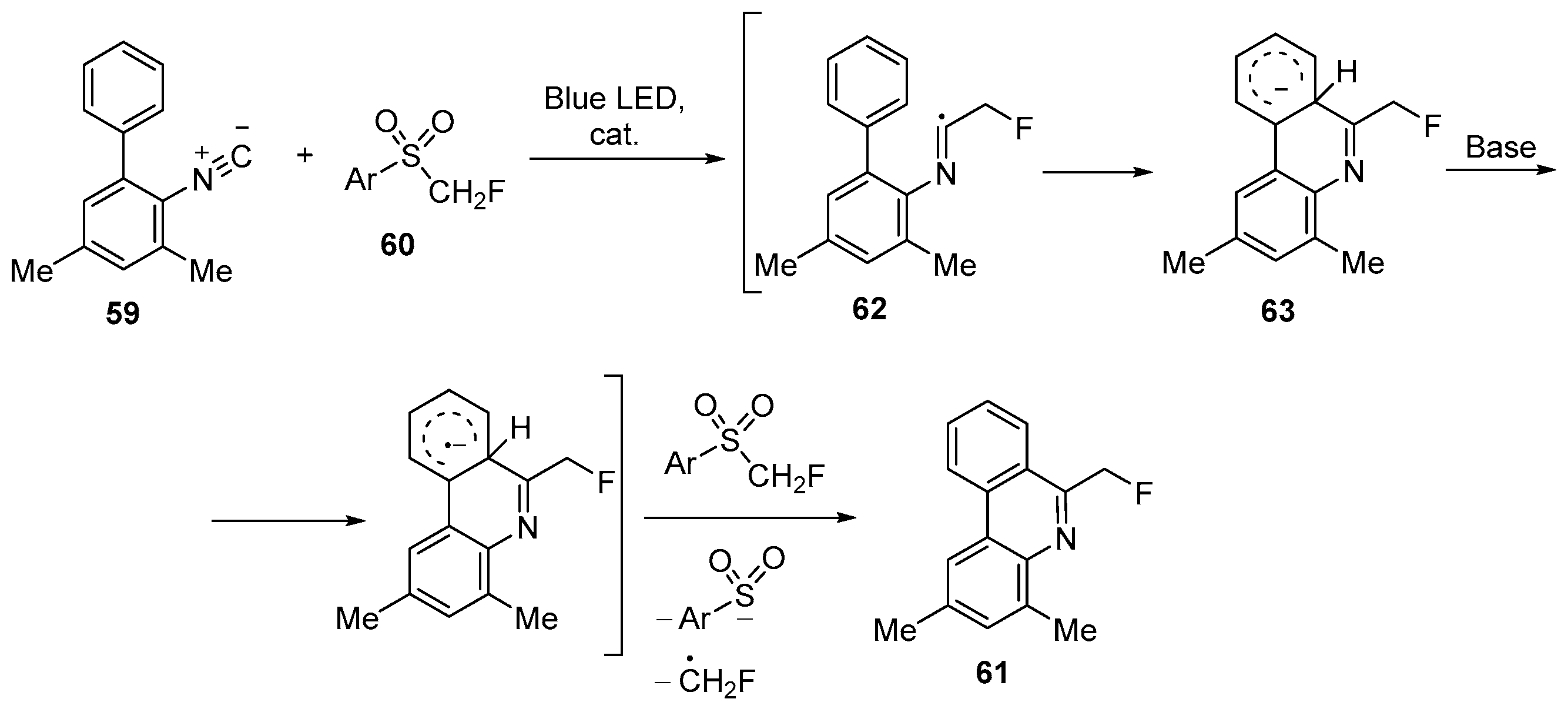
| 33 | 6-Fluoromethylphenanthridine | 2-Isocyano-3,5-dimethyl-1,1′-biphenyl | 2-((fluoromethyl)sulfonyl)-6-nitrobenzothiazole; [Ru(bpy)3]Cl2, DMSO, rt, Na2CO3, N2, Blue LED | [81] |
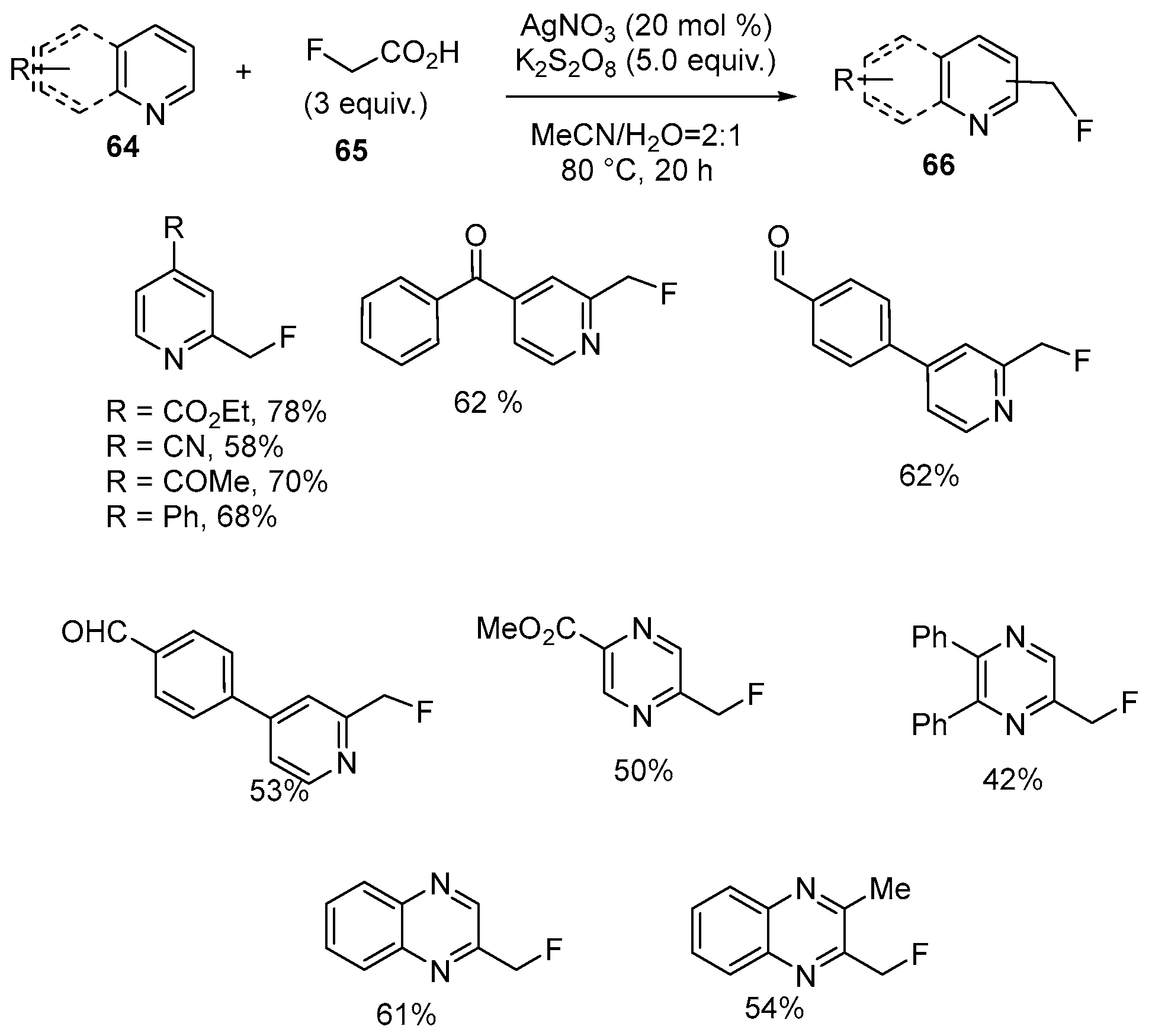
| 35 | 2-Monofluoromethylated pyridines, pyrazines and quinoxalines | Pyridines, pyrazines and quinoxalines | 2-fluoroacetic acid; AgNO3, K2S2O8, MeCN/H2O = 2:1 80 °C, 20 h | [82] |
| 36 | 2,6-Bis(monofluoromethyl)pyridine; 2,5-Bis(monofluoromethyl) pyrazines; 2,3-Bis(monofluoromethyl)quinoxalines | Pyridines, pyrazines and quinoxalines | 2-fluoroacetic acid; AgNO3, K2S2O8, DCE, 80 °C, 20 h | [82] |
| 39 | 3-((Fluoromethyl)selanyl)quinoline | Quinoline-3-amine | ICH2F; HBF4/tBuCN CH3CN, 0 °C, 2 h; CuCl/CuCl2/1,10-phen/ KSeCN CH3CN, 0 °C, 3 h; KOH CH3CN, 0 °C, 7 h | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moskalik, M.Y. Monofluoromethylation of N-Heterocyclic Compounds. Int. J. Mol. Sci. 2023, 24, 17593. https://doi.org/10.3390/ijms242417593
Moskalik MY. Monofluoromethylation of N-Heterocyclic Compounds. International Journal of Molecular Sciences. 2023; 24(24):17593. https://doi.org/10.3390/ijms242417593
Chicago/Turabian StyleMoskalik, Mikhail Yu. 2023. "Monofluoromethylation of N-Heterocyclic Compounds" International Journal of Molecular Sciences 24, no. 24: 17593. https://doi.org/10.3390/ijms242417593
APA StyleMoskalik, M. Y. (2023). Monofluoromethylation of N-Heterocyclic Compounds. International Journal of Molecular Sciences, 24(24), 17593. https://doi.org/10.3390/ijms242417593






