Advances in Understanding the Mechanism of Cap-Independent Cucurbit Aphid-Borne Yellows Virus Protein Synthesis
Abstract
:1. Introduction
2. Results
2.1. The 5′-UTR Is Required for CABYV 3′-CITE Activities

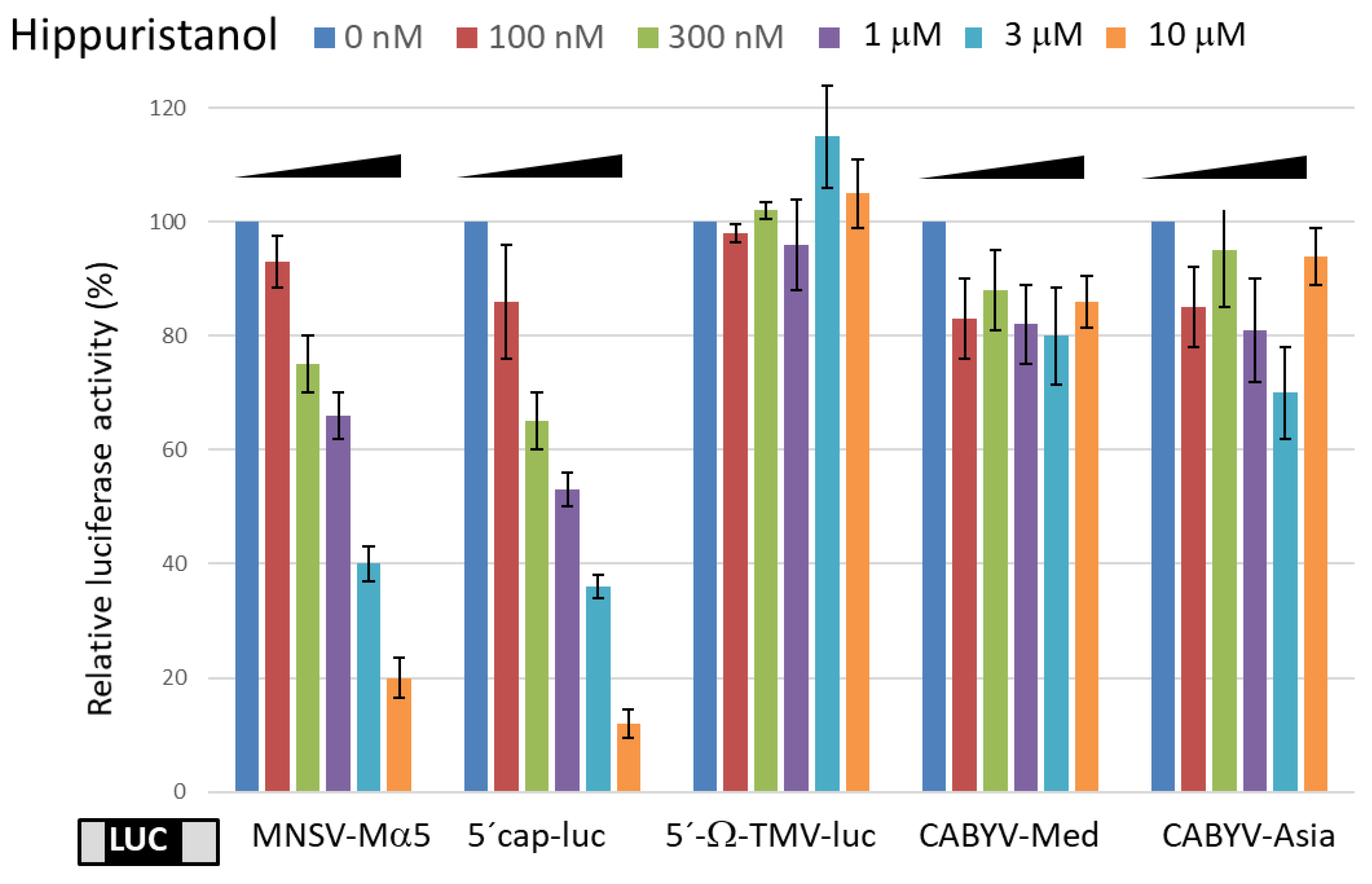
2.2. EIF4A Inhibition Does Not Affect CABYV 3′-CITE Activities
2.3. Arabidopsis Proteins Involved in CABYV 3′-CITE-Mediated Translation Activity
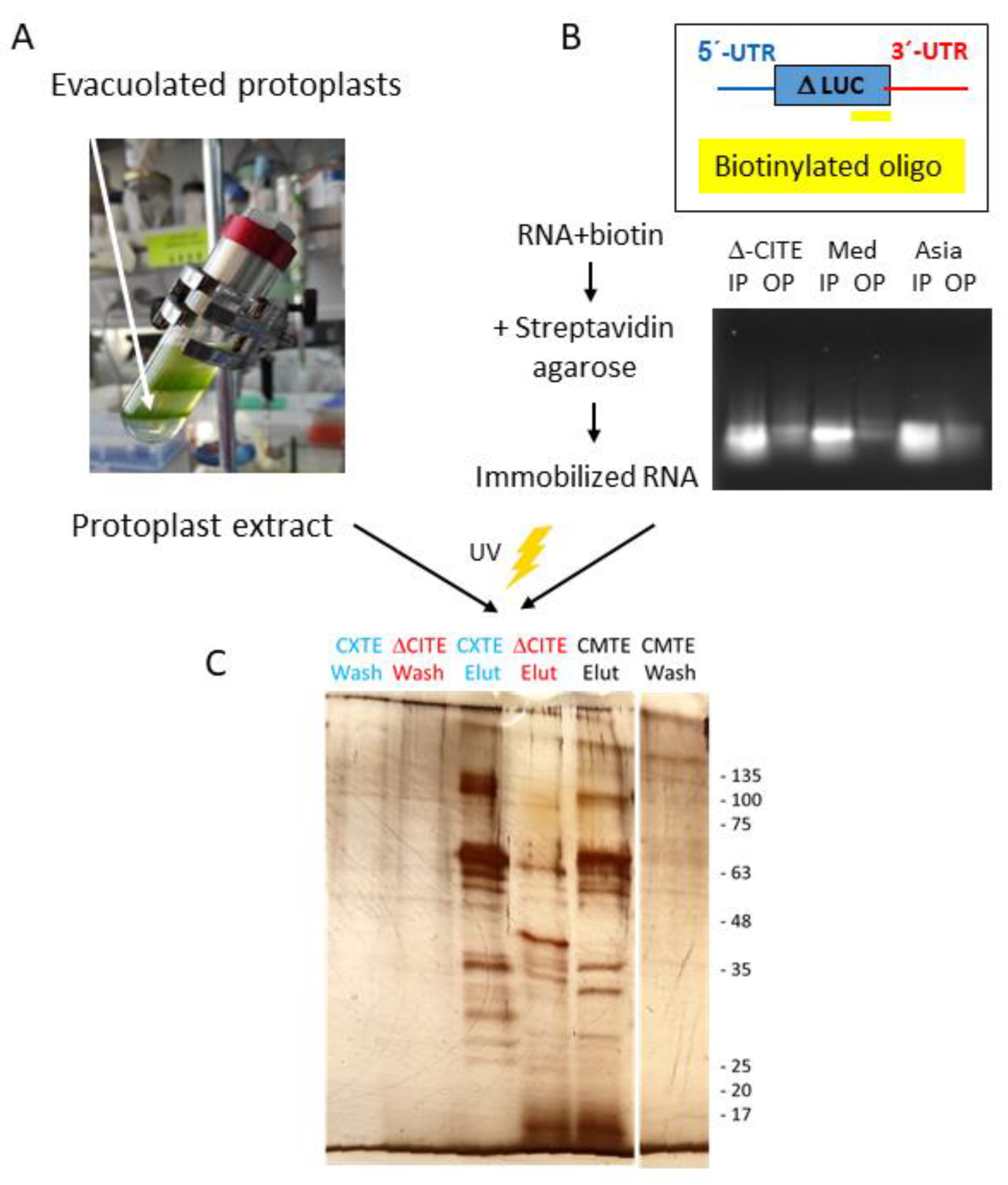
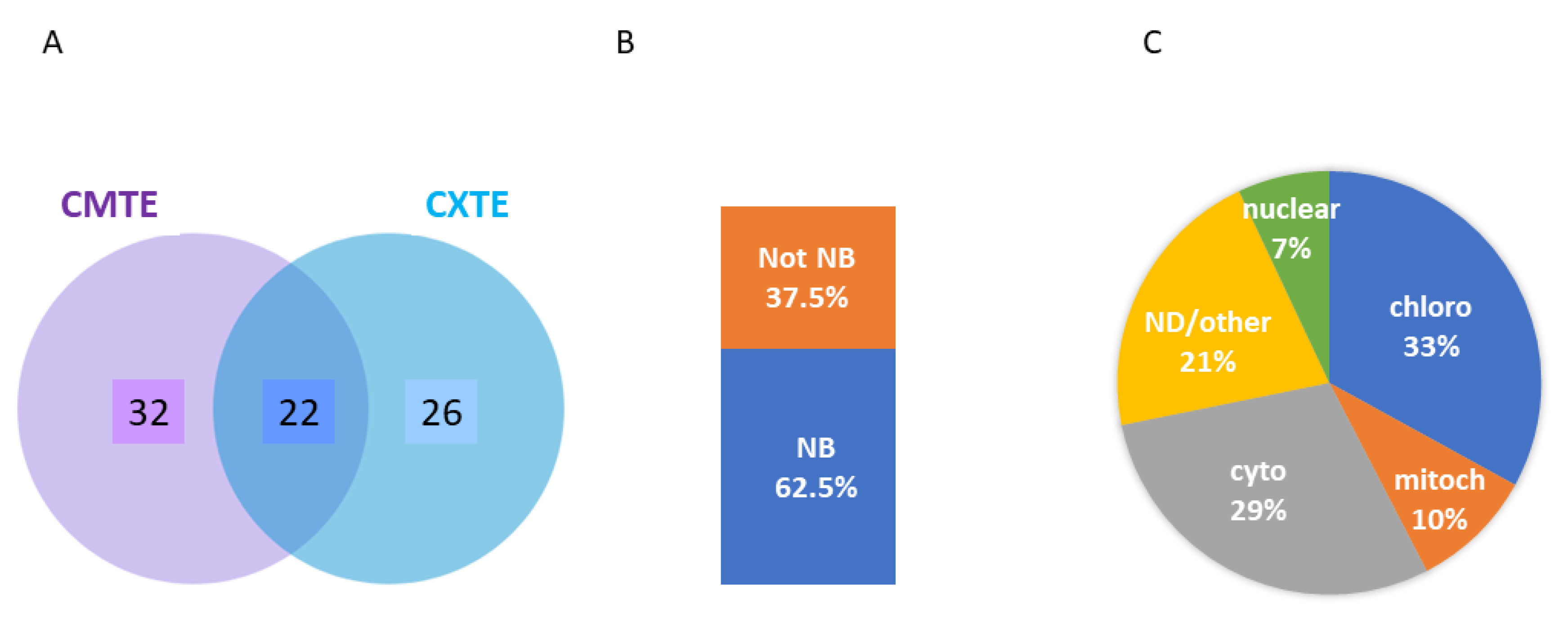
2.4. Capture of Melon Proteins Binding to the CABYV 3′-CITEs
3. Discussion
4. Materials and Methods
4.1. RNA Constructs
4.2. In Vivo and In Vitro Translation Assays
4.3. Purification of Evacuolated Protoplasts and Extract Preparation
4.4. Capture of RNA-Binding Proteins from Vacuolated Protoplast Extracts: RNA Centric Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Regenmortel, M.H.V.; Fauquet, C.M.; Bishop, D.H.L.; Carstens, E.B.; Estes, M.K.; Lemon, S.M.; Maniliff, J.; Mayo, M.A.; McGeoch, D.J.; Pringle, C.R.; et al. Virus Taxonomy:Seventh Report of the International Committee on Taxonomy of Viruses; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Dreher, T.W.; Miller, W.A. Translational Control in Positive Strand RNA Plant Viruses. Virology 2006, 344, 185–197. [Google Scholar] [CrossRef]
- Kneller, E.L.P.; Rakotondrafara, A.M.; Miller, W.A. Cap-Independent Translation of Plant Viral RNAs. Virus Res. 2006, 119, 63–75. [Google Scholar] [CrossRef]
- Miras, M.; Miller, W.A.; Truniger, V.; Aranda, M.A. Non-Canonical Translation in Plant RNA Viruses. Front. Plant Sci. 2017, 8, 494. [Google Scholar] [CrossRef]
- Miras, M.; Sempere, R.N.; Kraft, J.J.; Miller, W.A.; Aranda, M.A.; Truniger, V. Interfamilial Recombination between Viruses Led to Acquisition of a Novel Translation-Enhancing RNA Element That Allows Resistance Breaking. New Phytol. 2014, 202, 233–246. [Google Scholar] [CrossRef]
- Simon, A.E.; Miller, W.A. 3′ Cap-Independent Translation Enhancers of Plant Viruses. Annu. Rev. Microbiol. 2013, 67, 21–42. [Google Scholar] [CrossRef]
- Truniger, V.; Miras, M.; Aranda, M.A. Structural and Functional Diversity of Plant Virus 3′-Cap-Independent Translation Enhancers (3′-CITEs). Front. Plant Sci. 2017, 8, 2047. [Google Scholar] [CrossRef]
- Truniger, V.; Nieto, C.; Gonzalez-Ibeas, D.; Aranda, M. Mechanism of Plant EIF4E-Mediated Resistance against a Carmovirus (Tombusviridae): Cap-Independent Translation of a Viral RNA Controlled in Cis by an (a)Virulence Determinant. Plant J. 2008, 56, 716–727. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, Q.; Miller, W.A.; Goss, D.J. Eukaryotic Translation Initiation Factor 4G (EIF4G) Coordinates Interactions with EIF4A, EIF4B, and EIF4E in Binding and Translation of the Barley Yellow Dwarf Virus 3′ Cap-Independent Translation Element (BTE). J. Biol. Chem. 2017, 292, 5921–5931. [Google Scholar] [CrossRef]
- Kraft, J.J.; Treder, K.; Peterson, M.S.; Miller, W.A. Cation-Dependent Folding of 3′ Cap-Independent Translation Elements Facilitates Interaction of a 17-Nucleotide Conserved Sequence with EIF4G. Nucleic Acids Res. 2013, 41, 3398–3413. [Google Scholar] [CrossRef]
- Treder, K.; Kneller, E.L.P.; Allen, E.M.; Wang, Z.; Browning, K.S.; Miller, W.A. The 3′ Cap-Independent Translation Element of Barley Yellow Dwarf Virus Binds EIF4F via the EIF4G Subunit to Initiate Translation. RNA 2008, 14, 134–147. [Google Scholar] [CrossRef]
- Miras, M.; Truniger, V.; Querol-Audi, J.; Aranda, M.A. Analysis of the Interacting Partners EIF4F and 3′-CITE Required for Melon Necrotic Spot Virus Cap-Independent Translation. Mol. Plant Pathol. 2017, 18, 635–648. [Google Scholar] [CrossRef]
- Nicholson, B.L.; Wu, B.; Chevtchenko, I.; White, K.A. Tombusvirus Recruitment of Host Translational Machinery via the 3′ UTR. RNA 2010, 16, 1402–1419. [Google Scholar] [CrossRef]
- Wang, Z.; Treder, K.; Miller, W.A. Structure of a Viral Cap-Independent Translation Element That Functions via High Affinity Binding to the EIF4E Subunit of EIF4F. J. Biol. Chem. 2009, 284, 14189–14202. [Google Scholar] [CrossRef]
- Gazo, B.M.; Murphy, P.; Gatchel, J.R.; Browning, K.S. A Novel Interaction of Cap-Binding Protein Complexes Eukaryotic Initiation Factor (EIF) 4F and EIF(Iso)4F with a Region in the 3’-Untranslated Region of Satellite Tobacco Necrosis Virus. J. Biol. Chem. 2004, 279, 13584–13592. [Google Scholar] [CrossRef]
- Nicholson, B.L.; Zaslaver, O.; Mayberry, L.K.; Browning, K.S.; White, K.A. Tombusvirus Y-Shaped Translational Enhancer Forms a Complex with EIF4F and Can Be Functionally Replaced by Heterologous Translational Enhancers. J. Virol. 2013, 87, 1872–1883. [Google Scholar] [CrossRef]
- Stupina, V.A.; Meskauskas, A.; McCormack, J.C.; Yingling, Y.G.; Shapiro, B.A.; Dinman, J.D.; Simon, A.E. The 3’ Proximal Translational Enhancer of Turnip Crinkle Virus Binds to 60S Ribosomal Subunits. RNA 2008, 14, 2379–2393. [Google Scholar] [CrossRef]
- Zuo, X.; Wang, J.; Yu, P.; Eyler, D.; Xu, H.; Starich, M.R.; Tiede, D.M.; Simon, A.E.; Kasprzak, W.; Schwieters, C.D.; et al. Solution Structure of the Cap-Independent Translational Enhancer and Ribosome-Binding Element in the 3′ UTR of Turnip Crinkle Virus. Proc. Natl. Acad. Sci. USA 2010, 107, 1385. [Google Scholar] [CrossRef]
- Gao, F.; Kasprzak, W.; Stupina, V.A.; Shapiro, B.A.; Simon, A.E.; VA, S.; BA, S.; AE, S. A Ribosome-Binding, 3′ Translational Enhancer Has a T-Shaped Structure and Engages in a Long-Distance RNA-RNA Interaction. J. Virol. 2012, 86, 9828. [Google Scholar] [CrossRef]
- Miras, M.; Rodríguez-Hernández, A.M.; Romero-López, C.; Berzal-Herranz, A.; Colchero, J.; Aranda, M.A.; Truniger, V. A Dual Interaction between the 5′- And 3′-Ends of the Melon Necrotic Spot Virus (MNSV) RNA Genome Is Required for Efficient Cap-Independent Translation. Front. Plant Sci. 2018, 9, 625. [Google Scholar] [CrossRef]
- Guo, L.; Allen, E.M.; Miller, W.A.; WA, M.; Miller, W.A.; Hall, B.; Wang, R.N.A. Base-Pairing between Untranslated Regions Facilitates Translation of Uncapped, Nonpolyadenylated Viral RNA. Mol. Cell 2001, 7, 1103–1109. [Google Scholar] [CrossRef]
- Chattopadhyay, M.; Shi, K.; Yuan, X.; Simon, A.E.; AE, S. Long-Distance Kissing Loop Interactions between a 3′ Proximal Y-Shaped Structure and Apical Loops of 5′ Hairpins Enhance Translation of Saguaro Cactus Virus. Virology 2011, 417, 113–125. [Google Scholar] [CrossRef]
- Blanco-Pérez, M.; Pérez-Cañamás, M.; Ruiz, L.; Hernández, C. Efficient Translation of Pelargonium Line Pattern Virus RNAs Relies on a TED-like 3′-Translational Enhancer That Communicates with the Corresponding 5′-Region through a Long-Distance RNA-RNA Interaction. PLoS ONE 2016, 11, e0152593. [Google Scholar] [CrossRef]
- Nicholson, B.L.; White, K.A. Context-Influenced Cap-Independent Translation of Tombusvirus MRNAs in Vitro. Virology 2008, 380, 203–212. [Google Scholar] [CrossRef]
- Fabian, M.R.; White, K.A. 5’-3’ RNA-RNA Interaction Facilitates Cap- and Poly(A) Tail-Independent Translation of Tomato Bushy Stunt Virus MRNA: A Potential Common Mechanism for Tombusviridae. J. Biol. Chem. 2004, 279, 28862–28872. [Google Scholar] [CrossRef]
- Fabian, M.R.; White, K.A. Analysis of a 3′-Translation Enhancer in a Tombusvirus: A Dynamic Model for RNA-RNA Interactions of MRNA Termini. RNA 2006, 12, 1304–1314. [Google Scholar] [CrossRef]
- Stupina, V.A.; Yuan, X.; Meskauskas, A.; Dinman, J.D.; Simon, A.E. Ribosome Binding to a 5′ Translational Enhancer Is Altered in the Presence of the 3′ Untranslated Region in Cap-Independent Translation of Turnip Crinkle Virus. J. Virol. 2011, 85, 4638–4653. [Google Scholar] [CrossRef]
- Gao, F.; Kasprzak, W.K.; Szarko, C.; Shapiro, B.A.; Simon, A.E. The 3′ Untranslated Region of Pea Enation Mosaic Virus Contains Two T-Shaped, Ribosome-Binding, Cap-Independent Translation Enhancers. J. Virol. 2014, 88, 11696–11712. [Google Scholar] [CrossRef]
- Gao, F.; Gulay, S.P.; Kasprzak, W.; Dinman, J.D.; Shapiro, B.A.; Simon, A.E.; Jonathan, D.; Shapiro, B.A.; Simon, A.E. The Kissing-Loop T-Shaped Structure Translational Enhancer of Pea Enation Mosaic Virus Can Bind Simultaneously to Ribosomes and a 5′ Proximal Hairpin. J. Virol. 2013, 87, 11987–12002. [Google Scholar] [CrossRef]
- Gao, F.; Simon, A.E. Differential Use of 3’CITEs by the Subgenomic RNA of Pea Enation Mosaic Virus 2. Virology 2017, 510, 194–204. [Google Scholar] [CrossRef]
- Sõmera, M.; Fargette, D.; Hébrard, E.; Sarmiento, C. ICTV Virus Taxonomy Profile: Solemoviridae 2021. J. Gen. Virol. 2021, 102, 001707. [Google Scholar] [CrossRef]
- Taliansky, M.; Mayo, M.A.; Barker, H. Potato Leafroll Virus: A Classic Pathogen Shows Some New Tricks. Mol. Plant Pathol. 2003, 4, 81–89. [Google Scholar] [CrossRef]
- Reinbold, C.; Lacombe, S.; Ziegler-Graff, V.; Scheidecker, D.; Wiss, L.; Beuve, M.; Caranta, C.; Brault, V. Closely Related Poleroviruses Depend on Distinct Translation Initiation Factors to Infect Arabidopsis Thaliana. Mol. Plant-Microbe Interact. 2013, 26, 257. [Google Scholar] [CrossRef]
- Kassem, M.A.; Juarez, M.; Gómez, P.; Mengual, C.M.; Sempere, R.N.; Plaza, M.; Elena, S.F.; Moreno, A.; Fereres, A.; Aranda, M.A. Genetic Diversity and Potential Vectors and Reservoirs of Cucurbit Aphid-Borne Yellows Virus in Southeastern Spain. Phytopathology 2013, 103, 1188–1197. [Google Scholar] [CrossRef]
- Miras, M.; Aranda, M.A.; Truniger, V. Different RNA Elements Control Viral Protein Synthesis in Polerovirus Isolates Evolved in Separate Geographical Regions. Int. J. Mol. Sci. 2022, 23, 12503. [Google Scholar] [CrossRef]
- Shirokikh, N.E.; Spirin, A.S. Poly(A) Leader of Eukaryotic MRNA Bypasses the Dependence of Translation on Initiation Factors. Proc. Natl. Acad. Sci. USA 2008, 105, 10738–10743. [Google Scholar] [CrossRef]
- Martinez-Salas, E. Insights into Structural and Mechanistic Features of Viral IRES Elements. Front. Microbiol. 2018, 8, 2629. [Google Scholar] [CrossRef]
- Johnson, P.Z.; Simon, A.E. RNAcanvas: Interactive Drawing and Exploration of Nucleic Acid Structures. Nucleic Acids Res. 2023, 51, W501–W508. [Google Scholar] [CrossRef]
- Cencic, R.; Pelletier, J. Hippuristanol—A Potent Steroid Inhibitor of Eukaryotic Initiation Factor 4A. Translation 2016, 4, e1137381. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Mayberry, L.K.; Browning, K.S.; Rakotondrafara, A.M. The Triticum Mosaic Virus 5’ Leader Binds to Both EIF4G and EIFiso4G for Translation. PLoS ONE 2017, 12, e0169602. [Google Scholar] [CrossRef] [PubMed]
- Sakharov, P.A.; Agalarov, S.C. Free Initiation Factors EIF4A and EIF4B Are Dispensable for Translation Initiation on Uncapped MRNAs. Biochemistry 2016, 81, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Agalarov, S.C.; Sakharov, P.A.; Fattakhova, D.K.; Sogorin, E.A.; Spirin, A.S. Internal Translation Initiation and EIF4F/ATP-Independent Scanning of MRNA by Eukaryotic Ribosomal Particles. Sci. Rep. 2014, 4, 4438. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Treder, K.; Miller, W.A. Untranslated Regions of Diverse Plant Viral RNAs Vary Greatly in Translation Enhancement Efficiency. BMC Biotechnol. 2012, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.; Brault, V.; Klein, D.; Weyens, G.U.Y.; Lefèbvre, M.; Ziegler-graff, V.; Gilmer, D. Divergence of Host Range and Biological Properties between Natural Isolate and Full-Length Infectious CDNA Clone of the Beet Mild Yellowing Virus 2ITB. Mol. Plant Pathol. 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Belostotsky, D.A. Unexpected Complexity of Poly(A)-Binding Protein Gene Families in Flowering Plants: Three Conserved Lineages That Are at Least 200 Million Years Old and Possible Auto- and Cross-Regulation. Genetics 2003, 163, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, M.; Nishikiori, M.; Tomita, K.; Yoshioka, N.; Kozuka, R.; Naito, S.; Ishikawa, M. The Arabidopsis Cucumovirus Multiplication 1 and 2 Loci Encode Translation Initiation Factors 4E and 4G. J. Virol. 2004, 78, 6102–6111. [Google Scholar] [CrossRef]
- Alonso, J.M.; Stepanova, A.N.; Leisse, T.J.; Kim, C.J.; Chen, H.; Shinn, P.; Stevenson, D.K.; Zimmerman, J.; Barajas, P.; Cheuk, R.; et al. Genome-Wide Insertional Mutagenesis of Arabidopsis Thaliana. Science 2003, 301, 653–657. [Google Scholar] [CrossRef]
- Bach-Pages, M.; Homma, F.; Kourelis, J.; Kaschani, F.; Mohammed, S.; Kaiser, M.; van der Hoorn, R.A.L.; Castello, A.; Preston, G.M. Discovering the RNA-Binding Proteome of Plant Leaves with an Improved RNA Interactome Capture Method. Biomolecules 2020, 10, 661. [Google Scholar] [CrossRef]
- Molho, M.; Prasanth, K.R.; Pogany, J.; Nagy, P.D. Targeting Conserved Co-Opted Host Factors to Block Virus Replication: Using Allosteric Inhibitors of the Cytosolic Hsp70s to Interfere with Tomato Bushy Stunt Virus Replication. Virology 2021, 563, 1–19. [Google Scholar] [CrossRef]
- Hinnebusch, A.G. Molecular Mechanism of Scanning and Start Codon Selection in Eukaryotes. Microbiol. Mol. Biol. Rev. 2011, 75, 434–467. [Google Scholar] [CrossRef]
- Dhungel, P.; Cao, S.; Yang, Z. The 5’-Poly(A) Leader of Poxvirus MRNA Confers a Translational Advantage That Can Be Achieved in Cells with Impaired Cap-Dependent Translation. PLoS Pathog. 2017, 13, e1006602. [Google Scholar] [CrossRef]
- Gilbert, W.V.; Zhou, K.; Butler, T.K.; Doudna, J.A. Cap-Independent Translation Is Required for Starvation-Induced Differentiation in Yeast. Science 2007, 317, 1224–1227. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Greene, G.H.; Yoo, H.; Liu, L.; Marqués, J.; Motley, J.; Dong, X. Global Translational Reprogramming Is a Fundamental Layer of Immune Regulation in Plants. Nature 2017, 545, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X.; Greene, G.H.; Xu, G.; Dong, X. PABP/Purine-Rich Motif as an Initiation Module for Cap-Independent Translation in Pattern-Triggered Immunity. Cell 2022, 185, 3186–3200.e17. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, G.S.; Webb, C.O.; Gilbert, G.S.; Webb, C.O. Phylogenetic Signal in Plant Pathogen—Host Range. Proc. Natl. Acad. Sci. USA 2007, 104, 4979–4983. [Google Scholar] [CrossRef]
- Terenin, I.M.; Andreev, D.E.; Dmitriev, S.E.; Shatsky, I.N. A Novel Mechanism of Eukaryotic Translation Initiation That Is Neither m 7 G-Cap-, nor IRES-Dependent. Nucleic Acids Res. 2013, 41, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Macovei, A.; Sevilla, N.R.; Cantos, C.; Jonson, G.B.; Slamet-Loedin, I.; Čermák, T.; Voytas, D.F.; Choi, I.R.; Chadha-Mohanty, P. Novel Alleles of Rice EIF4G Generated by CRISPR/Cas9-Targeted Mutagenesis Confer Resistance to Rice Tungro Spherical Virus. Plant Biotechnol. J. 2018, 16, 1918–1927. [Google Scholar] [CrossRef]
- Wang, W.; Ma, S.; Hu, P.; Ji, Y.; Sun, F. Genome Editing of Rice EIF4G Loci Confers Partial Resistance to Rice Black-Streaked Dwarf Virus. Viruses 2021, 13, 2100. [Google Scholar] [CrossRef]
- Svitkin, Y.V.; Pause, A.; Haghighat, A.; Pyronnet, S.; Witherell, G.; Belsham, G.J.; Sonenberg, N. The Requirement for Eukaryotic Initiation Factor 4A (ElF4A) in Translation Is in Direct Proportion to the Degree of MRNA 5′ Secondary Structure. RNA 2001, 7, 382–394. [Google Scholar] [CrossRef]
- Zhang, Z.; Boonen, K.; Ferrari, P.; Schoofs, L.; Janssens, E.; Van Noort, V.; Rolland, F.; Geuten, K. UV Crosslinked MRNA - Binding Proteins Captured from Leaf Mesophyll Protoplasts. Plant Methods 2016, 12, 42. [Google Scholar] [CrossRef]
- Harries, P.A.; Park, J.W.; Sasaki, N.; Ballard, K.D.; Maule, A.J.; Nelson, R.S. Differing Requirements for Actin and Myosin by Plant Viruses for Sustained Intercellular Movement. Proc. Natl. Acad. Sci. USA 2009, 106, 17594–17599. [Google Scholar] [CrossRef]
- Abbink, T.E.; Peart, J.R.; Mos, T.N.; Baulcombe, D.C.; Bol, J.F.; Linthorst, H.J. Silencing of a Gene Encoding a Protein Component of the Oxygen-Evolving Complex of Photosystem II Enhances Virus Replication in Plants. Virology 2002, 295, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lyu, S.; Liu, Y.; Luo, M.; Shi, S.; Deng, S. Cauliflower Mosaic Virus P6 Dysfunctions Histone Deacetylase Hd2c to Promote Virus Infection. Cells 2021, 10, 2278. [Google Scholar] [CrossRef] [PubMed]
- Von der Haar, T.; McCarthy, J.E.G. Intracellular Translation Initiation Factor Levels in Saccharomyces Cerevisiae and Their Role in Cap-Complex Function. Mol. Microbiol. 2002, 46, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Kishor, A.; White, E.J.F.; Matsangos, A.E.; Yan, Z.; Tandukar, B.; Wilson, G.M. Hsp70’s RNA-Binding and MRNA-Stabilizing Activities Are Independent of Its Protein Chaperone Functions. J. Biol. Chem. 2017, 292, 14122–14133. [Google Scholar] [CrossRef] [PubMed]
- Lubkowska, A.; Pluta, W.; Strońska, A.; Lalko, A. Role of Heat Shock Proteins (Hsp70 and Hsp90) in Viral Infection. Int. J. Mol. Sci. 2021, 22, 9366. [Google Scholar] [CrossRef] [PubMed]
- Taguwa, S.; Yeh, M.T.; Rainbolt, T.K.; Nayak, A.; Shao, H.; Gestwicki, J.E.; Andino, R.; Frydman, J. Zika Virus Dependence on Host Hsp70 Provides a Protective Strategy against Infection and Disease. Cell Rep. 2019, 26, 906–920.e3. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.S.; Shin, K.S.; Oh, S.H.; Kang, S.M.; Won, S.J.; Hwang, S.B. Nonstructural 5A Protein of Hepatitis C Virus Regulates Heat Shock Protein 72 for Its Own Propagation. J. Viral Hepat. 2012, 19, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Men, R.; Dan, X.; Chen, Y.; Li, H.; Chen, G.; Zee, B.; Wang, M.H.T.; He, M.L. Hsc70 Regulates the IRES Activity and Serves as an Antiviral Target of Enterovirus A71 Infection. Antivir. Res. 2018, 150, 39–46. [Google Scholar] [CrossRef]
- Su, Y.S.; Hwang, L.H.; Chen, C.J. Heat Shock Protein A6, a Novel HSP70, Is Induced During Enterovirus A71 Infection to Facilitate Internal Ribosomal Entry Site-Mediated Translation. Front. Microbiol. 2021, 12, 664955. [Google Scholar] [CrossRef]
- Wang, F.; Qiu, Y.; Zhang, H.M.; Hanson, P.; Ye, X.; Zhao, G.; Xie, R.; Tong, L.; Yang, D. Heat Shock Protein 70 Promotes Coxsackievirus B3 Translation Initiation and Elongation via Akt-MTORC1 Pathway Depending on Activation of P70S6K and Cdc2. Cell. Microbiol. 2017, 19, e12725. [Google Scholar] [CrossRef]
- Tanguay, R.L.; Gallie, D.R. Isolation and Characterization of the 102-Kilodalton RNA-Binding Protein That Binds to the 5′ and 3′ Translational Enhancers of Tobacco Mosaic Virus RNA. J. Biol. Chem. 1996, 271, 14316–14322. [Google Scholar] [CrossRef] [PubMed]
- Wells, S.E.; Hillner, P.E.; Vale, R.D.; Sachs, A.B. Circularization of MRNA by Eukaryotic Translation Initiation Factors. Mol. Cell 1998, 2, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis Mesophyll Protoplasts: A Versatile Cell System for Transient Gene Expression Analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.; Nieto, C.; Moriones, E.; Truniger, V.; Aranda, M.A. Molecular Characterization of a Melon Necrotic Spot Virus Strain That Overcomes the Resistance in Melon and Nonhost Plants. Mol. Plant-Microbe Interact. 2004, 17, 668. [Google Scholar] [CrossRef]
- Griesbach, R.J.; Sink, K. Evacuolation of Mesophyll Protoplasts. Plant Sci. Lett. 1983, 30, 297–301. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truniger, V.; Pechar, G.S.; Aranda, M.A. Advances in Understanding the Mechanism of Cap-Independent Cucurbit Aphid-Borne Yellows Virus Protein Synthesis. Int. J. Mol. Sci. 2023, 24, 17598. https://doi.org/10.3390/ijms242417598
Truniger V, Pechar GS, Aranda MA. Advances in Understanding the Mechanism of Cap-Independent Cucurbit Aphid-Borne Yellows Virus Protein Synthesis. International Journal of Molecular Sciences. 2023; 24(24):17598. https://doi.org/10.3390/ijms242417598
Chicago/Turabian StyleTruniger, Verónica, Giuliano Sting Pechar, and Miguel A. Aranda. 2023. "Advances in Understanding the Mechanism of Cap-Independent Cucurbit Aphid-Borne Yellows Virus Protein Synthesis" International Journal of Molecular Sciences 24, no. 24: 17598. https://doi.org/10.3390/ijms242417598
APA StyleTruniger, V., Pechar, G. S., & Aranda, M. A. (2023). Advances in Understanding the Mechanism of Cap-Independent Cucurbit Aphid-Borne Yellows Virus Protein Synthesis. International Journal of Molecular Sciences, 24(24), 17598. https://doi.org/10.3390/ijms242417598






