Copper in Gynecological Diseases
Abstract
:1. Introduction
1.1. Copper Metabolism
1.1.1. Copper Uptake
1.1.2. Copper Distribution
1.1.3. Copper Excretion
1.2. Copper Homeostasis
1.3. Copper and Pathogenesis
1.3.1. Copper and Cell Proliferation
1.3.2. Copper and Angiogenesis
1.3.3. Copper and Metastasis
2. Copper in Gynecological Diseases
2.1. Ovarian Diseases
2.1.1. Ovarian Cancer
2.1.2. Polycystic Ovary Syndrome
2.2. Uterine Diseases
2.2.1. Uterine Cervix Cancer
2.2.2. Endometrial Cancer
2.2.3. ‘Benign’ Diseases
3. Therapeutic Strategies
3.1. Copper Chelators
3.1.1. D-penicillamine
3.1.2. Trientine
3.1.3. Tetrathiomolybdate
| Disease | Trial Phase | Intervention | Trial ID | Status | Study Completion |
|---|---|---|---|---|---|
| Breast Cancer | Phase 2 | TM | NCT00195091 | Active, not recruiting | 06/2025 |
| Wilson’s Disease | Phase 2 | ALXN1840 | NCT04422431 | Completed | 05/2023 |
| EOC, TC, PPC | Phase 1–2 | Trientine 2HC + PLD + carboplatin | NCT03480750 | Completed | 12/2019 |
| Advanced cancers | Phase 1 | Trientine 4HC + carboplatin | NCT01178112 | Completed | 08/2014 |
| EOC, TC, PPC | Phase 2 | Elesclomol + paclitaxel | NCT00888615 | Completed | 08/2016 |
| CC | Phase 2 | 64CuII(atsm) | NCT00794339 | Terminated | 12/2011 |
| CIN | Phase 2 | Curcumin | NCT04266275 | Not yet recruiting | 03/2025 |
| CC | Phase 1–2 | Curcumin + radiotherapy | NCT05947513 | Not yet recruiting | 11/2024 |
| CC | Phase 2 | Curcumin | NCT04294836 | Withdrawn | 12/2023 |
| EDT | Phase 2 | Curcumin | NCT04493476 | Unknown Status | 12/2022 |
| CC, EC | Phase 2 | Pembrolizumab + radiation + curcumin + immune modulatory cocktail | NCT03192059 | Completed | 06/2021 |
| EC | Phase 2 | Curcumin | NCT02017353 | Completed | 10/2016 |
3.2. Copper Ionophores
3.2.1. Disulfiram and Dithiocarbamates
3.2.2. Clioquinol
3.2.3. Elesclomol and Derivatives
3.2.4. Bis(thiosemicarbazones)
3.3. New Therapeutic Strategies
3.3.1. Cu-Based Nanoparticles
3.3.2. Natural Compounds Derived from Plants
Curcumin
Coumarins
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Akt | Protein kinase B |
| ALDH | Aldehyde dehydrogenase |
| ALXN1840 | Bis-choline tetrathiomolybdate |
| ARID1A | AT-rich interactive domain-containing protein 1A |
| Arp | Actin-related proteins |
| ATOX1 | Antioxidant chaperone 1 |
| ATP | Adenosine triphosphate |
| ATP7A | Copper-transporting ATPase alpha |
| ATP7B | Copper-transporting ATPase beta |
| BRAF | Serine/threonine-protein kinase B-raf |
| CA-9 | Carbonic anhydrase 9 |
| Cas9 | CRISPR-associated protein 9 |
| CC | Cervical cancer |
| CCDC | Coiled-coil domain containing protein |
| CCO | Cytochrome C oxidase |
| CCS | Copper chaperone for superoxide dismutase |
| CD31 | Cluster of differentiation 31 |
| CDDP | Cisplatin |
| CIN | Cervical intraepithelial neoplasia |
| CMRGs | Copper-metabolism related genes |
| COMMD | Copper metabolism MURR1 domain-containing protein |
| COX11 | CCO copper chaperone 11 |
| COX17 | CCO copper chaperone 17 |
| COX19 | CCO assembly factor 19 |
| Cp | Ceruloplasmin |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| CTR1 | Copper transporter 1 |
| CTR2 | Copper transporter 2 |
| Cu | Copper |
| Cu IUDs | Copper intrauterine devices |
| CuS NPs | Copper sulfide nanoparticles |
| CXCR | C-X-C motif chemokine receptor |
| DβH | Dopamine-β-hydroxylase |
| DCYTB | Duodenal cytochrome B |
| DHA | Docosahexaenoic acid |
| DMT1 | Divalent metal transporter 1 |
| DSF | Disulfiram |
| EC | Endometrial cancer |
| ECM | Extracellular matrix |
| EDT | Endometriosis |
| EGFR | Epidermal growth factor receptor |
| EMT | Epithelial-mesenchymal transition |
| EOC | Epithelial ovarian cancer |
| ERK | Extracellular signal-regulated kinase |
| FDX1 | Ferredoxin-1 |
| FGF | Fibroblast growth factor |
| GSH | Glutathione |
| 2HC | Dihydrochloride |
| 4HC | Tetrahydrochloride |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| HO-1 | Heme oxygenase 1 |
| HOMA-IR | Homeostatic model assessment for insulin resistance |
| HPV | Human papillomavirus |
| HREs | Hypoxia response elements |
| ICAM | Intercellular adhesion molecule |
| IKKs | Inhibitory kappa B kinases |
| IL | Interleukin |
| IMS | Mitochondrial intermembrane space |
| IR | Insulin resistance |
| JNK | c-Jun N-terminal kinase |
| LC3 | Microtubule-associated protein light chain 3 |
| LDH | Lactate dehydrogenase |
| LOX | Lysyl oxidase |
| LOXL | LOX-like proteins |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MEK | Mitogen-activated protein kinase kinase |
| MEMO1 | Mediator of cell motility 1 |
| MMP | Matrix metalloproteinase |
| MT | Metallothionein |
| MTF1 | Metal-regulatory transcription factor 1 |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NF-κB | Nuclear factor kappa B |
| NO | Nitric oxide |
| NPs | Nanoparticles |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NSAID | Non-steroidal anti-inflammatory drug |
| OC | Ovarian cancer |
| p53 | Tumor protein p53 |
| PARP | Poly (ADP-ribose) polymerase |
| PBT2 | 5,7-dichloro-2-[(dimethylamino)methyl]-8-hydroxyquinoline |
| PCOS | Polycystic ovary syndrome |
| PDGF | Platelet-derived growth factor |
| PI3K | Phosphoinositide 3-kinase |
| PLD | Pegylated liposomal doxorubicin |
| PPAR | Peroxisome proliferator-activated receptor |
| PPC | Primary peritoneal cancer |
| RAF | Rapidly accelerated fibrosarcoma |
| ROS | Reactive oxygen species |
| SCO1 | Synthesis of cytochrome C oxidase 1 |
| SDF-1 | Stromal cell-derived factor 1 |
| SOD | Superoxide dismutase |
| STAT | Signal transducer and activator of transcription |
| STEAP | Six-transmembrane epithelial antigen of the prostate |
| TC | Fallopian tube cancer |
| TCA | Tricarboxylic acid |
| TGF-β | Transforming growth factor beta |
| TGN | Trans-Golgi network |
| TM | Ammonium tetrathiomolybdate |
| TMD | Transmembrane domain |
| TNF | Tumor necrosis factor |
| TNFR | TNF receptor |
| TRAMP | Transgenic adenocarcinoma of the mouse prostate |
| ULK | Unc-51 like autophagy activating kinase |
| VCAM | Vascular cell adhesion protein |
| VEGF | Vascular endothelial growth factor |
| VEGFR | VEGF receptor |
| WASH | Wiskott–Aldrich syndrome protein and SCAR homolog |
| XIAP | X-linked inhibitor of apoptosis protein |
References
- Nevitt, T.; Öhrvik, H.; Thiele, D.J. Charting the Travels of Copper in Eukaryotes from Yeast to Mammals. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2012, 1823, 1580–1593. [Google Scholar] [CrossRef]
- Trumbo, P.; Yates, A.A.; Schlicker, S.; Poos, M. Dietary Reference Intakes. J. Am. Diet. Assoc. 2001, 101, 294–301. [Google Scholar] [CrossRef]
- Moshfegh, A.J.; Goldman, J.D.; Rhodes, D.G.; Friday, J.E. Usual Nutrient Intake from Food and Beverages, by Gender and Age, What We Eat In America, NHANES 2017-March 2020 Prepandemic. 2023. Available online: www.ars.usda.gov/nea/bhnrc/fsrg (accessed on 11 November 2023).
- Myint, Z.W.; Oo, T.H.; Thein, K.Z.; Tun, A.M.; Saeed, H. Copper Deficiency Anemia. Ann. Hematol. 2018, 97, 1527–1534. [Google Scholar] [CrossRef]
- Grochowski, C.; Blicharska, E.; Baj, J.; Mierzwińska, A.; Brzozowska, K.; Forma, A.; Maciejewski, R. Serum Iron, Magnesium, Copper, and Manganese Levels in Alcoholism: A Systematic Review. Molecules 2019, 24, 1361. [Google Scholar] [CrossRef]
- Feng, Y.; Zeng, J.-W.; Ma, Q.; Zhang, S.; Tang, J.; Feng, J.-F. Serum Copper and Zinc Levels in Breast Cancer: A Meta-Analysis. J. Trace Elem. Med. Biol. 2020, 62, 126629. [Google Scholar] [CrossRef]
- Linder, M.C. Ceruloplasmin and Other Copper Binding Components of Blood Plasma and Their Functions: An Update. Metallomics 2016, 8, 887–905. [Google Scholar] [CrossRef]
- Tsang, T.; Davis, C.I.; Brady, D.C. Copper Biology. Curr. Biol. 2021, 31, R421–R427. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, Y.; Yang, Y.; Peng, Y.; Li, C. Copper Metabolism in Saccharomyces Cerevisiae: An Update. Biometals 2021, 34, 3–14. [Google Scholar] [CrossRef]
- Ge, E.J.; Bush, A.I.; Casini, A.; Cobine, P.A.; Cross, J.R.; DeNicola, G.M.; Dou, Q.P.; Franz, K.J.; Gohil, V.M.; Gupta, S.; et al. Connecting Copper and Cancer: From Transition Metal Signalling to Metalloplasia. Nat. Rev. Cancer 2022, 22, 102–113. [Google Scholar] [CrossRef]
- Grubman, A.; White, A.R. Copper as a Key Regulator of Cell Signalling Pathways. Expert Rev. Mol. Med. 2014, 16, e11. [Google Scholar] [CrossRef]
- Shanbhag, V.C.; Gudekar, N.; Jasmer, K.; Papageorgiou, C.; Singh, K.; Petris, M.J. Copper Metabolism as a Unique Vulnerability in Cancer. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2021, 1868, 118893. [Google Scholar] [CrossRef]
- Shawki, A.; Anthony, S.R.; Nose, Y.; Engevik, M.A.; Niespodzany, E.J.; Barrientos, T.; Öhrvik, H.; Worrell, R.T.; Thiele, D.J.; Mackenzie, B. Intestinal DMT1 Is Critical for Iron Absorption in the Mouse but Is Not Required for the Absorption of Copper or Manganese. Am. J. Physiol. Liver Physiol. 2015, 309, G635–G647. [Google Scholar] [CrossRef]
- Wyman, S.; Simpson, R.J.; McKie, A.T.; Sharp, P.A. Dcytb (Cybrd1) Functions as Both a Ferric and a Cupric Reductase in Vitro. FEBS Lett. 2008, 582, 1901–1906. [Google Scholar] [CrossRef]
- Ozumi, K.; Sudhahar, V.; Kim, H.W.; Chen, G.-F.; Kohno, T.; Finney, L.; Vogt, S.; McKinney, R.D.; Ushio-Fukai, M.; Fukai, T. Role of Copper Transport Protein Antioxidant 1 in Angiotensin II–Induced Hypertension: A Key Regulator of Extracellular Superoxide Dismutase. Hypertension 2012, 60, 476–486. [Google Scholar] [CrossRef]
- Nose, Y.; Wood, L.K.; Kim, B.-E.; Prohaska, J.R.; Fry, R.S.; Spears, J.W.; Thiele, D.J. Ctr1 Is an Apical Copper Transporter in Mammalian Intestinal Epithelial Cells in Vivo That Is Controlled at the Level of Protein Stability. J. Biol. Chem. 2010, 285, 32385–32392. [Google Scholar] [CrossRef]
- Zimnicka, A.M.; Maryon, E.B.; Kaplan, J.H. Human Copper Transporter HCTR1 Mediates Basolateral Uptake of Copper into Enterocytes: Implications for Copper Homeostasis. J. Biol. Chem. 2007, 282, 26471–26480. [Google Scholar] [CrossRef]
- Galler, T.; Lebrun, V.; Raibaut, L.; Faller, P.; Wezynfeld, N.E. How Trimerization of CTR1 N-Terminal Model Peptides Tunes Cu-Binding and Redox-Chemistry. Chem. Commun. 2020, 56, 12194–12197. [Google Scholar] [CrossRef]
- Schushan, M.; Barkan, Y.; Haliloglu, T.; Ben-Tal, N. Cα-Trace Model of the Transmembrane Domain of Human Copper Transporter 1, Motion and Functional Implications. Proc. Natl. Acad. Sci. USA 2010, 107, 10908–10913. [Google Scholar] [CrossRef]
- Nose, Y.; Kim, B.-E.; Thiele, D.J. Ctr1 Drives Intestinal Copper Absorption and Is Essential for Growth, Iron Metabolism, and Neonatal Cardiac Function. Cell Metab. 2006, 4, 235–244. [Google Scholar] [CrossRef]
- Kuo, Y.-M.; Zhou, B.; Cosco, D.; Gitschier, J. The Copper Transporter CTR1 Provides an Essential Function in Mammalian Embryonic Development. Proc. Natl. Acad. Sci. USA 2001, 98, 6836–6841. [Google Scholar] [CrossRef]
- Lelièvre, P.; Sancey, L.; Coll, J.-L.; Deniaud, A.; Busser, B. The Multifaceted Roles of Copper in Cancer: A Trace Metal Element with Dysregulated Metabolism, but Also a Target or a Bullet for Therapy. Cancers 2020, 12, 3594. [Google Scholar] [CrossRef]
- Lutsenko, S.; Barnes, N.L.; Bartee, M.Y.; Dmitriev, O.Y. Function and Regulation of Human Copper-Transporting ATPases. Physiol. Rev. 2007, 87, 1011–1046. [Google Scholar] [CrossRef]
- Chen, L.; Min, J.; Wang, F. Copper Homeostasis and Cuproptosis in Health and Disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef]
- Ramos, D.; Mar, D.; Ishida, M.; Vargas, R.; Gaite, M.; Montgomery, A.; Linder, M.C. Mechanism of Copper Uptake from Blood Plasma Ceruloplasmin by Mammalian Cells. PLoS ONE 2016, 11, e0149516. [Google Scholar] [CrossRef]
- Moriya, M.; Ho, Y.-H.; Grana, A.; Nguyen, L.; Alvarez, A.; Jamil, R.; Ackland, M.L.; Michalczyk, A.; Hamer, P.; Ramos, D.; et al. Copper Is Taken up Efficiently from Albumin and A2-Macroglobulin by Cultured Human Cells by More than One Mechanism. Am. J. Physiol. Physiol. 2008, 295, C708–C721. [Google Scholar] [CrossRef]
- Pierson, H.; Yang, H.; Lutsenko, S. Copper Transport and Disease: What Can We Learn from Organoids? Annu. Rev. Nutr. 2019, 39, 75–94. [Google Scholar] [CrossRef]
- Heaton, D.N.; George, G.N.; Garrison, G.; Winge, D.R. The Mitochondrial Copper Metallochaperone Cox17 Exists as an Oligomeric, Polycopper Complex. Biochemistry 2001, 40, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Calvo, J.; Jung, H.; Meloni, G. Copper Metallothioneins. IUBMB Life 2017, 69, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Nývltová, E.; Dietz, J.V.; Seravalli, J.; Khalimonchuk, O.; Barrientos, A. Coordination of Metal Center Biogenesis in Human Cytochrome c Oxidase. Nat. Commun. 2022, 13, 3615. [Google Scholar] [CrossRef] [PubMed]
- Horng, Y.-C.; Cobine, P.A.; Maxfield, A.B.; Carr, H.S.; Winge, D.R. Specific Copper Transfer from the Cox17 Metallochaperone to Both Sco1 and Cox11 in the Assembly of Yeast Cytochrome C Oxidase. J. Biol. Chem. 2004, 279, 35334–35340. [Google Scholar] [CrossRef]
- Zischka, H.; Einer, C. Mitochondrial Copper Homeostasis and Its Derailment in Wilson Disease. Int. J. Biochem. Cell Biol. 2018, 102, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Skopp, A.; Boyd, S.D.; Ullrich, M.S.; Liu, L.; Winkler, D.D. Copper-Zinc Superoxide Dismutase (Sod1) Activation Terminates Interaction between Its Copper Chaperone (Ccs) and the Cytosolic Metal-Binding Domain of the Copper Importer Ctr1. Biometals 2019, 32, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Bertinato, J.; L’Abbé, M.R. Copper Modulates the Degradation of Copper Chaperone for Cu, Zn Superoxide Dismutase by the 26 S Proteosome. J. Biol. Chem. 2003, 278, 35071–35078. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Inesi, G.; Pilankatta, R.; Tadini-Buoninsegni, F. Biochemical Characterization of P-Type Copper ATPases. Biochem. J. 2014, 463, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Jayakanthan, S.; Braiterman, L.T.; Hasan, N.M.; Unger, V.M.; Lutsenko, S. Human Copper Transporter ATP7B (Wilson Disease Protein) Forms Stable Dimers in Vitro and in Cells. J. Biol. Chem. 2017, 292, 18760–18774. [Google Scholar] [CrossRef]
- Polishchuk, E.V.; Concilli, M.; Iacobacci, S.; Chesi, G.; Pastore, N.; Piccolo, P.; Paladino, S.; Baldantoni, D.; van IJzendoorn, S.C.D.; Chan, J.; et al. Wilson Disease Protein ATP7B Utilizes Lysosomal Exocytosis to Maintain Copper Homeostasis. Dev. Cell 2014, 29, 686–700. [Google Scholar] [CrossRef]
- Hamza, I.; Prohaska, J.; Gitlin, J.D. Essential Role for Atox1 in the Copper-Mediated Intracellular Trafficking of the Menkes ATPase. Proc. Natl. Acad. Sci. USA 2003, 100, 1215–1220. [Google Scholar] [CrossRef]
- Maryon, E.B.; Molloy, S.A.; Kaplan, J.H. Cellular Glutathione Plays a Key Role in Copper Uptake Mediated by Human Copper Transporter 1. Am. J. Physiol. Physiol. 2013, 304, C768–C779. [Google Scholar] [CrossRef]
- Singleton, W.C.J.; McInnes, K.T.; Cater, M.A.; Winnall, W.R.; McKirdy, R.; Yu, Y.; Taylor, P.E.; Ke, B.-X.; Richardson, D.R.; Mercer, J.F.B.; et al. Role of Glutaredoxin1 and Glutathione in Regulating the Activity of the Copper-Transporting P-Type ATPases, ATP7A and ATP7B. J. Biol. Chem. 2010, 285, 27111–27121. [Google Scholar] [CrossRef]
- Jiang, X.; Chen, J.; Bajić, A.; Zhang, C.; Song, X.; Carroll, S.L.; Cai, Z.-L.; Tang, M.; Xue, M.; Cheng, N.; et al. Quantitative Real-Time Imaging of Glutathione. Nat. Commun. 2017, 8, 16087. [Google Scholar] [CrossRef]
- Harvey, L.J.; Ashton, K.; Hooper, L.; Casgrain, A.; Fairweather-Tait, S.J. Methods of Assessment of Copper Status in Humans: A Systematic Review. Am. J. Clin. Nutr. 2009, 89, 2009S–2024S. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Popiołek, Ł.; Kocot, J. The Many “Faces” of Copper in Medicine and Treatment. Biometals 2014, 27, 611–621. [Google Scholar] [CrossRef]
- Mercer, J.F.B.; Barnes, N.; Stevenson, J.; Strausak, D.; Llanos, R.M. Copper-Induced Trafficking of the Cu-ATPases: A Key Mechanism for Copper Homeostasis. Biometals 2003, 16, 175–184. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, Toxicity and Oxidative Stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting Copper in Cancer Therapy: “Copper That Cancer”. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Tosco, A.; Fontanella, B.; Danise, R.; Cicatiello, L.; Grober, O.; Ravo, M.; Weisz, A.; Marzullo, L. Molecular Bases of Copper and Iron Deficiency-Associated Dyslipidemia: A Microarray Analysis of the Rat Intestinal Transcriptome. Genes Nutr. 2010, 5, 1–8. [Google Scholar] [CrossRef]
- Bonham, M.; O’Connor, J.M.; Hannigan, B.M.; Strain, J.J. The Immune System as a Physiological Indicator of Marginal Copper Status? Br. J. Nutr. 2002, 87, 393–403. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological Relevance and Mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Molloy, S.A.; Kaplan, J.H. Copper-Dependent Recycling of HCTR1, the Human High Affinity Copper Transporter. J. Biol. Chem. 2009, 284, 29704–29713. [Google Scholar] [CrossRef]
- Clifford, R.J.; Maryon, E.B.; Kaplan, J.H. Dynamic Internalization and Recycling of a Metal Ion Transporter: Cu Homeostasis and CTR1, the Human Cu+ Uptake System. J. Cell Sci. 2016, 129, 1711–1721. [Google Scholar] [CrossRef]
- Liang, Z.D.; Tsai, W.-B.; Lee, M.-Y.; Savaraj, N.; Kuo, M.T. Specificity Protein 1 (Sp1) Oscillation Is Involved in Copper Homeostasis Maintenance by Regulating Human High-Affinity Copper Transporter 1 Expression. Mol. Pharmacol. 2012, 81, 455–464. [Google Scholar] [CrossRef]
- Kuo, Y.-M.; Gybina, A.A.; Pyatskowit, J.W.; Gitschier, J.; Prohaska, J.R. Copper Transport Protein (Ctr1) Levels in Mice Are Tissue Specific and Dependent on Copper Status. J. Nutr. 2006, 136, 21–26. [Google Scholar] [CrossRef]
- Öhrvik, H.; Logeman, B.; Turk, B.; Reinheckel, T.; Thiele, D.J. Cathepsin Protease Controls Copper and Cisplatin Accumulation via Cleavage of the Ctr1 Metal-Binding Ectodomain. J. Biol. Chem. 2016, 291, 13905–13916. [Google Scholar] [CrossRef]
- Logeman, B.L.; Wood, L.K.; Lee, J.; Thiele, D.J. Gene Duplication and Neo-Functionalization in the Evolutionary and Functional Divergence of the Metazoan Copper Transporters Ctr1 and Ctr2. J. Biol. Chem. 2017, 292, 11531–11546. [Google Scholar] [CrossRef]
- Chen, G.-F.; Sudhahar, V.; Youn, S.-W.; Das, A.; Cho, J.; Kamiya, T.; Urao, N.; McKinney, R.D.; Surenkhuu, B.; Hamakubo, T.; et al. Copper Transport Protein Antioxidant-1 Promotes Inflammatory Neovascularization via Chaperone and Transcription Factor Function. Sci. Rep. 2015, 5, 14780. [Google Scholar] [CrossRef]
- Itoh, S.; Kim, H.W.; Nakagawa, O.; Ozumi, K.; Lessner, S.M.; Aoki, H.; Akram, K.; McKinney, R.D.; Ushio-Fukai, M.; Fukai, T. Novel Role of Antioxidant-1 (Atox1) as a Copper-Dependent Transcription Factor Involved in Cell Proliferation. J. Biol. Chem. 2008, 283, 9157–9167. [Google Scholar] [CrossRef]
- Kamiya, T.; Takeuchi, K.; Fukudome, S.; Hara, H.; Adachi, T. Copper Chaperone Antioxidant-1, Atox-1, Is Involved in the Induction of SOD3 in THP-1 Cells. Biometals 2018, 31, 61–68. [Google Scholar] [CrossRef]
- Palmiter, R.D. Regulation of Metallothionein Genes by Heavy Metals Appears to Be Mediated by a Zinc-Sensitive Inhibitor That Interacts with a Constitutively Active Transcription Factor, MTF-1. Proc. Natl. Acad. Sci. USA 1994, 91, 1219–1223. [Google Scholar] [CrossRef]
- Song, M.O.; Mattie, M.D.; Lee, C.-H.; Freedman, J.H. The Role of Nrf1 and Nrf2 in the Regulation of Copper-Responsive Transcription. Exp. Cell Res. 2014, 322, 39–50. [Google Scholar] [CrossRef]
- Hartwig, C.; Zlatic, S.A.; Wallin, M.; Vrailas-Mortimer, A.; Fahrni, C.J.; Faundez, V. Trafficking Mechanisms of P-Type ATPase Copper Transporters. Curr. Opin. Cell Biol. 2019, 59, 24–33. [Google Scholar] [CrossRef]
- Ojha, R.; Prasad, A.N. Menkes Disease: What a Multidisciplinary Approach Can Do. J. Multidiscip. Healthc. 2016, 9, 371–385. [Google Scholar] [CrossRef]
- Dev, S.; Kruse, R.L.; Hamilton, J.P.; Lutsenko, S. Wilson Disease: Update on Pathophysiology and Treatment. Front. Cell Dev. Biol. 2022, 10, 871877. [Google Scholar] [CrossRef]
- Członkowska, A.; Litwin, T.; Dusek, P.; Ferenci, P.; Lutsenko, S.; Medici, V.; Rybakowski, J.K.; Weiss, K.H.; Schilsky, M.L. Wilson Disease. Nat. Rev. Dis. Prim. 2018, 4, 21. [Google Scholar] [CrossRef]
- Gromadzka, G.; Tarnacka, B.; Flaga, A.; Adamczyk, A. Copper Dyshomeostasis in Neurodegenerative Diseases—Therapeutic Implications. Int. J. Mol. Sci. 2020, 21, 9259. [Google Scholar] [CrossRef]
- Gil-Bea, F.J.; Aldanondo, G.; Lasa-Fernández, H.; de Munain, A.L.; Vallejo-Illarramendi, A. Insights into the Mechanisms of Copper Dyshomeostasis in Amyotrophic Lateral Sclerosis. Expert Rev. Mol. Med. 2017, 19, e7. [Google Scholar] [CrossRef]
- Chen, X.; Cai, Q.; Liang, R.; Zhang, D.; Liu, X.; Zhang, M.; Xiong, Y.; Xu, M.; Liu, Q.; Li, P.; et al. Copper Homeostasis and Copper-Induced Cell Death in the Pathogenesis of Cardiovascular Disease and Therapeutic Strategies. Cell Death Dis. 2023, 14, 105. [Google Scholar] [CrossRef]
- Pal, I.; Dey, S.G. The Role of Heme and Copper in Alzheimer’s Disease and Type 2 Diabetes Mellitus. JACS Au 2023, 3, 657–681. [Google Scholar] [CrossRef]
- Tang, X.; Yan, Z.; Miao, Y.; Ha, W.; Li, Z.; Yang, L.; Mi, D. Copper in Cancer: From Limiting Nutrient to Therapeutic Target. Front. Oncol. 2023, 13, 1209156. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Sun, G. Targeting Copper Metabolism: A Promising Strategy for Cancer Treatment. Front. Pharmacol. 2023, 14, 1203447. [Google Scholar] [CrossRef]
- Michalczyk, K.; Cymbaluk-Płoska, A. The Role of Zinc and Copper in Gynecological Malignancies. Nutrients 2020, 12, 3732. [Google Scholar] [CrossRef]
- Barresi, V.; Trovato-Salinaro, A.; Spampinato, G.; Musso, N.; Castorina, S.; Rizzarelli, E.; Condorelli, D.F. Transcriptome Analysis of Copper Homeostasis Genes Reveals Coordinated Upregulation of SLC 31A1, SCO 1, and COX 11 in Colorectal Cancer. FEBS Open Bio. 2016, 6, 794–806. [Google Scholar] [CrossRef]
- Mulware, S.J. Comparative Trace Elemental Analysis in Cancerous and Noncancerous Human Tissues Using PIXE. J. Biophys. 2013, 2013, 192026. [Google Scholar] [CrossRef]
- Ishida, S.; Andreux, P.; Poitry-Yamate, C.; Auwerx, J.; Hanahan, D. Bioavailable Copper Modulates Oxidative Phosphorylation and Growth of Tumors. Proc. Natl. Acad. Sci. USA 2013, 110, 19507–19512. [Google Scholar] [CrossRef]
- Lopez, J.; Ramchandani, D.; Vahdat, L. Copper Depletion as a Therapeutic Strategy in Cancer. Met. Ions Life Sci. 2019, 19, 303–330. [Google Scholar] [CrossRef]
- Zowczak, M.; Iskra, M.; Torliński, L.; Cofta, S. Analysis of Serum Copper and Zinc Concentrations in Cancer Patients. Biol. Trace Elem. Res. 2001, 82, 1–8. [Google Scholar] [CrossRef]
- Gupte, A.; Mumper, R.J. Elevated Copper and Oxidative Stress in Cancer Cells as a Target for Cancer Treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Yaman, M.; Kaya, G.; Simsek, M. Comparison of Trace Element Concentrations in Cancerous and Noncancerous Human Endometrial and Ovary Tissues. Int. J. Gynecol. Cancer 2007, 17, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Zimnicka, A.M.; Tang, H.; Guo, Q.; Kuhr, F.K.; Oh, M.-J.; Wan, J.; Chen, J.; Smith, K.A.; Fraidenburg, D.R.; Choudhury, M.S.R.; et al. Upregulated Copper Transporters in Hypoxia-Induced Pulmonary Hypertension. PLoS ONE 2014, 9, e90544. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, X.; Li, S.; Xie, W.; Guo, J. Emerging Roles of the Copper-CTR1 Axis in Tumorigenesis. Mol. Cancer Res. 2022, 20, 1339–1353. [Google Scholar] [CrossRef]
- United Nations; Department of Economic and Social Affairs. Population Division. In Contraceptive Use by Method 2019: Data Booklet; United Nations: New York, NY, USA, 2019; ISBN 978-92-1-148329-1. [Google Scholar]
- Crandell, L.; Mohler, N. A Literature Review of the Effects of Copper Intrauterine Devices on Blood Copper Levels in Humans. Nurs. Womens Health 2021, 25, 71–81. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Q.; Sun, H.; Hu, Y.; Wang, Z. Chronic Systemic Toxicity Study of Copper Intrauterine Devices in Female Wistar Rats. Med. Sci. Monit. 2017, 23, 3961–3970. [Google Scholar] [CrossRef]
- Boutry, J.; Tissot, S.; Ujvari, B.; Capp, J.-P.; Giraudeau, M.; Nedelcu, A.M.; Thomas, F. The Evolution and Ecology of Benign Tumors. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2022, 1877, 188643. [Google Scholar] [CrossRef]
- Li, Y.; Liang, R.; Zhang, X.; Wang, J.; Shan, C.; Liu, S.; Li, L.; Zhang, S. Copper Chaperone for Superoxide Dismutase Promotes Breast Cancer Cell Proliferation and Migration via ROS-Mediated MAPK/ERK Signaling. Front. Pharmacol. 2019, 10, 356. [Google Scholar] [CrossRef]
- Wang, J.; Luo, C.; Shan, C.; You, Q.; Lu, J.; Elf, S.; Zhou, Y.; Wen, Y.; Vinkenborg, J.L.; Fan, J.; et al. Inhibition of Human Copper Trafficking by a Small Molecule Significantly Attenuates Cancer Cell Proliferation. Nat. Chem. 2015, 7, 968–979. [Google Scholar] [CrossRef]
- Pham, V.N.; Chang, C.J. Metalloallostery and Transition Metal Signaling: Bioinorganic Copper Chemistry Beyond Active Sites. Angew. Chemie. 2023, 62, e202213644. [Google Scholar] [CrossRef]
- Brady, D.C.; Crowe, M.S.; Greenberg, D.N.; Counter, C.M. Copper Chelation Inhibits BRAFV600E-Driven Melanomagenesis and Counters Resistance to BRAFV600E and MEK1/2 Inhibitors. Cancer Res. 2017, 77, 6240–6252. [Google Scholar] [CrossRef] [PubMed]
- Polishchuk, E.V.; Merolla, A.; Lichtmannegger, J.; Romano, A.; Indrieri, A.; Ilyechova, E.Y.; Concilli, M.; De Cegli, R.; Crispino, R.; Mariniello, M.; et al. Activation of Autophagy, Observed in Liver Tissues from Patients with Wilson Disease and from ATP7B-Deficient Animals, Protects Hepatocytes from Copper-Induced Apoptosis. Gastroenterology 2019, 156, 1173–1189. [Google Scholar] [CrossRef] [PubMed]
- Tsang, T.; Posimo, J.M.; Gudiel, A.A.; Cicchini, M.; Feldser, D.M.; Brady, D.C. Copper Is an Essential Regulator of the Autophagic Kinases ULK1/2 to Drive Lung Adenocarcinoma. Nat. Cell Biol. 2020, 22, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Adjei, A.A. Targeting Angiogenesis in Cancer Therapy: Moving beyond Vascular Endothelial Growth Factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef] [PubMed]
- McAuslan, B.R.; Reilly, W. Endothelial Cell Phagokinesis in Response to Specific Metal Ions. Exp. Cell Res. 1980, 130, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Urso, E.; Maffia, M. Behind the Link between Copper and Angiogenesis: Established Mechanisms and an Overview on the Role of Vascular Copper Transport Systems. J. Vasc. Res. 2015, 52, 172–196. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Ye, F.; Xue, W.; Zhou, Z.; Kang, Y.J. Copper Regulation of Hypoxia-Inducible Factor-1 Activity. Mol. Pharmacol. 2009, 75, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Abelman, S.; Yano, N.; Ribeiro, J.R.; Singh, R.K.; Tipping, M.; Moore, R.G. Tetrathiomolybdate Inhibits Mitochondrial Complex IV and Mediates Degradation of Hypoxia-Inducible Factor-1α in Cancer Cells. Sci. Rep. 2015, 5, 14296. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Kleer, C.G.; Van Golen, K.L.; Irani, J.; Bottema, K.M.; Bias, C.; De Carvalho, M.; Mesri, E.A.; Robins, D.M.; Dick, R.D.; et al. Copper Deficiency Induced by Tetrathiomolybdate Suppresses Tumor Growth and Angiogenesis. Cancer Res. 2002, 62, 4854–4859. [Google Scholar] [PubMed]
- Pan, Q.; Bao, L.W.; Merajver, S.D. Tetrathiomolybdate Inhibits Angiogenesis and Metastasis through Suppression of the NFκB Signaling Cascade. Mol. Cancer Res. 2003, 1, 701–706. [Google Scholar] [PubMed]
- Denoyer, D.; Clatworthy, S.A.S.; Cater, M.A. Copper Complexes in Cancer Therapy. In Metal Ions in Life Sciences; Europe PMC: London, UK, 2018; Volume 18, pp. 469–506. ISBN 9783110470734. [Google Scholar]
- Das, A.; Ash, D.; Fouda, A.Y.; Sudhahar, V.; Kim, Y.-M.; Hou, Y.; Hudson, F.Z.; Stansfield, B.K.; Caldwell, R.B.; McMenamin, M.; et al. Cysteine Oxidation of Copper Transporter CTR1 Drives VEGFR2 Signalling and Angiogenesis. Nat. Cell Biol. 2022, 24, 35–50. [Google Scholar] [CrossRef]
- Narayanan, G.; Vuyyuru, H.; Muthuvel, B.; Konerirajapuram Natrajan, S. CTR1 Silencing Inhibits Angiogenesis by Limiting Copper Entry into Endothelial Cells. PLoS ONE 2013, 8, e71982. [Google Scholar] [CrossRef]
- Kohno, T.; Urao, N.; Ashino, T.; Sudhahar, V.; McKinney, R.D.; Hamakubo, T.; Iwanari, H.; Ushio-Fukai, M.; Fukai, T. Novel Role of Copper Transport Protein Antioxidant-1 in Neointimal Formation after Vascular Injury. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 805–813. [Google Scholar] [CrossRef]
- Ash, D.; Sudhahar, V.; Youn, S.-W.; Okur, M.N.; Das, A.; O’Bryan, J.P.; McMenamin, M.; Hou, Y.; Kaplan, J.H.; Fukai, T.; et al. The P-Type ATPase Transporter ATP7A Promotes Angiogenesis by Limiting Autophagic Degradation of VEGFR2. Nat. Commun. 2021, 12, 3091. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M.; Kaplan, J.H. Copper Transporters and Copper Chaperones: Roles in Cardiovascular Physiology and Disease. Am. J. Physiol. Physiol. 2018, 315, C186–C201. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New Insights into the Mechanisms of Epithelial–Mesenchymal Transition and Implications for Cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, J.; Yang, H.; Wu, C.; Dang, X.; Liu, Y. Copper Depletion Inhibits CoCl2-Induced Aggressive Phenotype of MCF-7 Cells via Downregulation of HIF-1 and Inhibition of Snail/Twist-Mediated Epithelial-Mesenchymal Transition. Sci. Rep. 2015, 5, 12410. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Ge, G. Lysyl Oxidase, Extracellular Matrix Remodeling and Cancer Metastasis. Cancer Microenviron. 2012, 5, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Cao, D.-F.; Yin, X.-X.; Zhou, H.-H.; Mao, X.-Y. Lysyl Oxidases: Emerging Biomarkers and Therapeutic Targets for Various Diseases. Biomed. Pharmacother. 2020, 131, 110791. [Google Scholar] [CrossRef]
- El-Haibi, C.P.; Bell, G.W.; Zhang, J.; Collmann, A.Y.; Wood, D.; Scherber, C.M.; Csizmadia, E.; Mariani, O.; Zhu, C.; Campagne, A.; et al. Critical Role for Lysyl Oxidase in Mesenchymal Stem Cell-Driven Breast Cancer Malignancy. Proc. Natl. Acad. Sci. USA 2012, 109, 17460–17465. [Google Scholar] [CrossRef]
- Barker, H.E.; Chang, J.; Cox, T.R.; Lang, G.; Bird, D.; Nicolau, M.; Evans, H.R.; Gartland, A.; Erler, J.T. LOXL2-Mediated Matrix Remodeling in Metastasis and Mammary Gland Involution. Cancer Res. 2011, 71, 1561–1572. [Google Scholar] [CrossRef]
- Osawa, T.; Ohga, N.; Akiyama, K.; Hida, Y.; Kitayama, K.; Kawamoto, T.; Yamamoto, K.; Maishi, N.; Kondoh, M.; Onodera, Y.; et al. Lysyl Oxidase Secreted by Tumour Endothelial Cells Promotes Angiogenesis and Metastasis. Br. J. Cancer 2013, 109, 2237–2247. [Google Scholar] [CrossRef]
- Semenza, G.L. Molecular Mechanisms Mediating Metastasis of Hypoxic Breast Cancer Cells. Trends Mol. Med. 2012, 18, 534–543. [Google Scholar] [CrossRef]
- Pez, F.; Dayan, F.; Durivault, J.; Kaniewski, B.; Aimond, G.; Le Provost, G.S.; Deux, B.; Clézardin, P.; Sommer, P.; Pouysségur, J.; et al. The HIF-1-Inducible Lysyl Oxidase Activates HIF-1 via the Akt Pathway in a Positive Regulation Loop and Synergizes with HIF-1 in Promoting Tumor Cell Growth. Cancer Res. 2011, 71, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, G.; Nalvarte, I.; Smirnova, T.; Vecchi, M.; Aceto, N.; Doelemeyer, A.; Frei, A.; Lienhard, S.; Wyckoff, J.; Hess, D.; et al. Memo Is a Copper-Dependent Redox Protein with an Essential Role in Migration and Metastasis. Sci. Signal. 2014, 7, ra56. [Google Scholar] [CrossRef] [PubMed]
- Lukanović, D.; Herzog, M.; Kobal, B.; Černe, K. The Contribution of Copper Efflux Transporters ATP7A and ATP7B to Chemoresistance and Personalized Medicine in Ovarian Cancer. Biomed. Pharmacother. 2020, 129, 110401. [Google Scholar] [CrossRef] [PubMed]
- Mok, S.C.; Wong, K.K.; Lu, K.H.; Munger, K.; Nagymanyoki, Z. Molecular Basis of Gynecologic Diseases. In Essential Concepts in Molecular Pathology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 409–424. ISBN 9780128132579. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2022. CA. Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Peng, H.; Qi, X.; Wu, M.; Zhao, X. Targeted Therapies in Gynecological Cancers: A Comprehensive Review of Clinical Evidence. Signal Transduct. Target. Ther. 2020, 5, 137. [Google Scholar] [CrossRef]
- Savant, S.S.; Sriramkumar, S.; O’Hagan, H.M. The Role of Inflammation and Inflammatory Mediators in the Development, Progression, Metastasis, and Chemoresistance of Epithelial Ovarian Cancer. Cancers 2018, 10, 251. [Google Scholar] [CrossRef]
- Ritch, S.J.; Telleria, C.M. The Transcoelomic Ecosystem and Epithelial Ovarian Cancer Dissemination. Front. Endocrinol. 2022, 13, 886533. [Google Scholar] [CrossRef]
- Nayak, S.B.; Bhat, V.R.; Mayya, S.S. Serum Copper, Ceruloplasmin and Thiobarbituric Acid Reactive Substance Status in Patients with Ovarian Cancer. Indian J. Physiol. Pharmacol. 2004, 48, 486–488. [Google Scholar]
- Korun, Z.E.U.; Erdem, M.; Erdem, A.; Onan, A.; Bozkurt, N.; Öktem, M.; Biberoğlu, K. Use of Serum Copper and Zinc Levels in the Diagnostic Evaluation of Endometrioma and Epithelial Ovarian Carcinoma. Česká Gynekol. 2023, 88, 279–286. [Google Scholar] [CrossRef]
- Lin, S.; Yang, H. Ovarian Cancer Risk According to Circulating Zinc and Copper Concentrations: A Meta-Analysis and Mendelian Randomization Study. Clin. Nutr. 2021, 40, 2464–2468. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, L.J.; Desanto, K.; Teal, S.B.; Sheeder, J.; Guntupalli, S.R. Intrauterine Device Use and Ovarian Cancer Risk: A Systematic Review and Meta-Analysis. Obstet. Gynecol. 2019, 134, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, X.; Gao, F.; Chi, H.; Zhang, J.; Xia, Z.; Cheng, C.; Liu, J. Identification of Copper Metabolism-Related Subtypes and Establishment of the Prognostic Model in Ovarian Cancer. Front. Endocrinol. 2023, 14, 1145797. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Harter, P.; Leary, A.; Lorusso, D.; Miller, R.E.; Pothuri, B.; Ray-Coquard, I.; Tan, D.S.P.; Bellet, E.; Oaknin, A.; et al. Newly Diagnosed and Relapsed Epithelial Ovarian Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Christie, E.L.; Bowtell, D.D.L. Acquired Chemotherapy Resistance in Ovarian Cancer. Ann. Oncol. 2017, 28, viii13–viii15. [Google Scholar] [CrossRef] [PubMed]
- Pignata, S.; Pisano, C.; Di Napoli, M.; Cecere, S.C.; Tambaro, R.; Attademo, L. Treatment of Recurrent Epithelial Ovarian Cancer. Cancer 2019, 125, 4609–4615. [Google Scholar] [CrossRef] [PubMed]
- Katano, K.; Kondo, A.; Safaei, R.; Holzer, A.; Samimi, G.; Mishima, M.; Kuo, Y.-M.; Rochdi, M.; Howell, S.B. Acquisition of Resistance to Cisplatin Is Accompanied by Changes in the Cellular Pharmacology of Copper. Cancer Res. 2002, 62, 6559–6565. [Google Scholar]
- Ishida, S.; McCormick, F.; Smith-McCune, K.; Hanahan, D. Enhancing Tumor-Specific Uptake of the Anticancer Drug Cisplatin with a Copper Chelator. Cancer Cell 2010, 17, 574–583. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Choi, C.H.; Do, I.-G.; Song, S.Y.; Lee, W.; Park, H.S.; Song, T.J.; Kim, M.K.; Kim, T.-J.; Lee, J.-W.; et al. Prognostic Value of the Copper Transporters, CTR1 and CTR2, in Patients with Ovarian Carcinoma Receiving Platinum-Based Chemotherapy. Gynecol. Oncol. 2011, 122, 361–365. [Google Scholar] [CrossRef]
- Samimi, G.; Safaei, R.; Katano, K.; Holzer, A.K.; Rochdi, M.; Tomioka, M.; Goodman, M.; Howell, S.B. Increased Expression of the Copper Efflux Transporter ATP7A Mediates Resistance to Cisplatin, Carboplatin, and Oxaliplatin in Ovarian Cancer Cells. Clin. Cancer Res. 2004, 10, 4661–4669. [Google Scholar] [CrossRef]
- Dolgova, N.V.; Nokhrin, S.; Yu, C.H.; George, G.N.; Dmitriev, O.Y. Copper Chaperone Atox1 Interacts with the Metal-Binding Domain of Wilson’s Disease Protein in Cisplatin Detoxification. Biochem. J. 2013, 454, 147–156. [Google Scholar] [CrossRef] [PubMed]
- E Palm-Espling, M.; Lundin, C.; Bjorn, E.; Naredi, P.; Wittung-Stafshede, P. Interaction between the Anticancer Drug Cisplatin and the Copper Chaperone Atox1 in Human Melanoma Cells. Protein Pept. Lett. 2014, 21, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Bompiani, K.M.; Tsai, C.-Y.; Achatz, F.P.; Liebig, J.K.; Howell, S.B. Copper Transporters and Chaperones CTR1, CTR2, ATOX1, and CCS as Determinants of Cisplatin Sensitivity. Metallomics 2016, 8, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.; Mateen, S.; Ahmad, R.; Moin, S. A Brief Insight into the Etiology, Genetics, and Immunology of Polycystic Ovarian Syndrome (PCOS). J. Assist. Reprod. Genet. 2022, 39, 2439–2473. [Google Scholar] [CrossRef] [PubMed]
- Kiel, I.A.; Lionett, S.; Parr, E.B.; Jones, H.; Røset, M.A.H.; Salvesen, Ø.; Vanky, E.; Moholdt, T. Improving Reproductive Function in Women with Polycystic Ovary Syndrome with High-Intensity Interval Training (IMPROV-IT): Study Protocol for a Two-Centre, Three-Armed Randomised Controlled Trial. BMJ Open 2020, 10, e034733. [Google Scholar] [CrossRef]
- Wang, Z.; Zhai, D.; Zhang, D.; Bai, L.; Yao, R.; Yu, J.; Cheng, W.; Yu, C. Quercetin Decreases Insulin Resistance in a Polycystic Ovary Syndrome Rat Model by Improving Inflammatory Microenvironment. Reprod. Sci. 2017, 24, 682–690. [Google Scholar] [CrossRef]
- Torshizi, F.F.; Chamani, M.; Khodaei, H.R.; Sadeghi, A.A.; Hejazi, S.H.; Heravi, R.M. Therapeutic Effects of Organic Zinc on Reproductive Hormones, Insulin Resistance and MTOR Expression, as a Novel Component, in a Rat Model of Polycystic Ovary Syndrome. Iran. J. Basic Med. Sci. 2020, 23, 36. [Google Scholar] [CrossRef]
- Palomba, S.; De Wilde, M.A.; Falbo, A.; Koster, M.P.H.; La Sala, G.B.; Fauser, B.C.J.M. Pregnancy Complications in Women with Polycystic Ovary Syndrome. Hum. Reprod. Update 2015, 21, 575–592. [Google Scholar] [CrossRef]
- Naderpoor, N.; Shorakae, S.; Joham, A.; Boyle, J.; De Courten, B.; Teede, H.J. Obesity and Polycystic Ovary Syndrome. Minerva Endocrinol. 2014, 40, 37–51. [Google Scholar]
- Chen, C.; Jing, G.; Li, Z.; Juan, S.; Bin, C.; Jie, H. Insulin Resistance and Polycystic Ovary Syndrome in a Chinese Population. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2017. [Google Scholar] [CrossRef]
- Ollila, M.-M.; West, S.; Keinänen-Kiukaanniemi, S.; Jokelainen, J.; Auvinen, J.; Puukka, K.; Ruokonen, A.; Järvelin, M.-R.; Tapanainen, J.S.; Franks, S.; et al. Overweight and Obese but Not Normal Weight Women with PCOS Are at Increased Risk of Type 2 Diabetes Mellitus—A Prospective, Population-Based Cohort Study. Hum. Reprod. 2017, 32, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Berni, T.R.; Morgan, C.L.; Rees, D.A. Women with Polycystic Ovary Syndrome Have an Increased Risk of Major Cardiovascular Events: A Population Study. J. Clin. Endocrinol. Metab. 2021, 106, e3369–e3380. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.A.; Azizia, M.M.; Hardiman, P.J. Risk of Endometrial, Ovarian and Breast Cancer in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2014, 20, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Joham, A.E.; Norman, R.J.; Stener-Victorin, E.; Legro, R.S.; Franks, S.; Moran, L.J.; Boyle, J.; Teede, H.J. Polycystic Ovary Syndrome. Lancet Diabetes Endocrinol. 2022, 10, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Günalan, E.; Yaba, A.; Yılmaz, B. The Effect of Nutrient Supplementation in the Management of Polycystic Ovary Syndrome-Associated Metabolic Dysfunctions: A Critical Review. J. Turkish Ger. Gynecol. Assoc. 2018, 19, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Dapas, M.; Lin, F.T.J.; Nadkarni, G.N.; Sisk, R.; Legro, R.S.; Urbanek, M.; Hayes, M.G.; Dunaif, A. Distinct Subtypes of Polycystic Ovary Syndrome with Novel Genetic Associations: An Unsupervised, Phenotypic Clustering Analysis. PLoS Med. 2020, 17, e1003132. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Zhang, F.; Han, L.; Zhu, B.; Liu, X. Serum Copper Level and Polycystic Ovarian Syndrome: A Meta-Analysis. Gynecol. Obstet. Investig. 2021, 86, 239–246. [Google Scholar] [CrossRef]
- Yin, J.; Hong, X.; Ma, J.; Bu, Y.; Liu, R. Serum Trace Elements in Patients with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 572384. [Google Scholar] [CrossRef]
- Mohmmed, A.H.; Awad, N.A.; AL-Fartosy, A.J.M. Study of Trace Elements Selenium, Copper, Zinc and Manganese Level in Polycystic Ovary Syndrome (PCOS). Int. J. Res. Appl. Sci. Biotechnol. 2019, 6, 16–22. [Google Scholar] [CrossRef]
- Kanafchian, M.; Esmaeilzadeh, S.; Mahjoub, S.; Rahsepar, M.; Ghasemi, M. Status of Serum Copper, Magnesium, and Total Antioxidant Capacity in Patients with Polycystic Ovary Syndrome. Biol. Trace Elem. Res. 2020, 193, 111–117. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, W.; Guo, Y.; Zheng, B.; Li, H.; Chen, J.; Zhang, W. High Copper Levels in Follicular Fluid Affect Follicle Development in Polycystic Ovary Syndrome Patients: Population-Based and in Vitro Studies. Toxicol. Appl. Pharmacol. 2019, 365, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, Y.; Lin, C.; Huang, Q.; Lei, D.; Hu, Y. Serum Macroelement and Microelement Concentrations in Patients with Polycystic Ovary Syndrome: A Cross-Sectional Study. Biol. Trace Elem. Res. 2017, 176, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Spritzer, P.M.; Lecke, S.B.; Fabris, V.C.; Ziegelmann, P.K.; Amaral, L. Blood Trace Element Concentrations in Polycystic Ovary Syndrome: Systematic Review and Meta-Analysis. Biol. Trace Elem. Res. 2017, 175, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Wang, L.; Guo, Z.; Sun, L.; Wang, L.; Wang, C.; Zuo, Z.; Qiu, H. Association of Serum Heavy Metals and Trace Element Concentrations with Reproductive Hormone Levels and Polycystic Ovary Syndrome in a Chinese Population. Biol. Trace Elem. Res. 2015, 167, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Celik, C.; Bastu, E.; Abali, R.; Alpsoy, S.; Guzel, E.C.; Aydemir, B.; Yeh, J. The Relationship between Copper, Homocysteine and Early Vascular Disease in Lean Women with Polycystic Ovary Syndrome. Gynecol. Endocrinol. 2013, 29, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Mehde, A.A.; Resan, A.K. Study of Several Biochemical Features in Sera of Patients with Polycystic Ovaries and Compared with the Control Group. Aust. J. Basic Appl. Sci. 2014, 8, 620–627. [Google Scholar]
- Sharif, M.E.; Adam, I.; Ahmed, M.A.; Rayis, D.A.; Hamdan, H.Z. Serum Level of Zinc and Copper in Sudanese Women with Polycystic Ovarian Syndrome. Biol. Trace Elem. Res. 2017, 180, 23–27. [Google Scholar] [CrossRef]
- Khalaf, B.H.; Ouda, M.H.; Alghurabi, H.S.; Shubbar, A.S. Zinc and Copper Levels and Their Correlation with Polycystic Ovary Syndrome Biochemical Changes. Int. J. Pharm. Sci. Res 2018, 9, 3036–3041. [Google Scholar] [CrossRef]
- Schmalbrock, L.J.; Weiss, G.; Rijntjes, E.; Reinschissler, N.; Sun, Q.; Schenk, M.; Schomburg, L. Pronounced Trace Element Variation in Follicular Fluids of Subfertile Women Undergoing Assisted Reproduction. Nutrients 2021, 13, 4134. [Google Scholar] [CrossRef]
- Chakraborty, P.; Ghosh, S.; Goswami, S.K.; Kabir, S.N.; Chakravarty, B.; Jana, K. Altered Trace Mineral Milieu Might Play an Aetiological Role in the Pathogenesis of Polycystic Ovary Syndrome. Biol. Trace Elem. Res. 2013, 152, 9–15. [Google Scholar] [CrossRef]
- Bizoń, A.; Tchórz, A.; Madej Pawełand Leśniewski, M.; Wójtowicz, M.; Piwowar, A.; Franik, G. The Activity of Superoxide Dismutase, Its Relationship with the Concentration of Zinc and Copper and the Prevalence of Rs2070424 Superoxide Dismutase Gene in Women with Polycystic Ovary Syndrome—Preliminary Study. J. Clin. Med. 2022, 11, 2548. [Google Scholar] [CrossRef] [PubMed]
- Kirmizi, D.A.; Baser, E.; Turksoy, V.A.; Kara, M.; Yalvac, E.S.; Gocmen, A.Y. Are Heavy Metal Exposure and Trace Element Levels Related to Metabolic and Endocrine Problems in Polycystic Ovary Syndrome? Biol. Trace Elem. Res. 2020, 198, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Sun, Y.; Zhao, A.; Cai, X.; Yu, A.; Xu, Q.; Liu, W.; Zhang, N.; Wu, S.; Chen, Y.; et al. High Dietary Copper Intake Induces Perturbations in the Gut Microbiota and Affects Host Ovarian Follicle Development. Ecotoxicol. Environ. Saf. 2023, 255, 114810. [Google Scholar] [CrossRef] [PubMed]
- Ojha, P.S.; Maste, M.M.; Tubachi, S.; Patil, V.S. Human Papillomavirus and Cervical Cancer: An Insight Highlighting Pathogenesis and Targeting Strategies. Virus Dis. 2022, 33, 132–154. [Google Scholar] [CrossRef] [PubMed]
- Bruni, L.; Albero, G.; Serrano, B.; Mena, M.; Collado, J.; Gómez, D.; Muñoz, J.; Bosch, F.; de Sanjosé, S. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World. Summ. Rep. 10 March 2023. Available online: https://hpvcentre.net/statistics/reports/XWX.pdf (accessed on 11 November 2023).
- Kamolratanakul, S.; Pitisuttithum, P. Human Papillomavirus Vaccine Efficacy and Effectiveness against Cancer. Vaccines 2021, 9, 1413. [Google Scholar] [CrossRef] [PubMed]
- Wentzensen, N.; Schiffman, M.; Palmer, T.; Arbyn, M. Triage of HPV Positive Women in Cervical Cancer Screening. J. Clin. Virol. 2016, 76, S49–S55. [Google Scholar] [CrossRef] [PubMed]
- Preci, D.P.; Almeida, A.; Weiler, A.L.; Franciosi, M.L.M.; Cardoso, A.M. Oxidative Damage and Antioxidants in Cervical Cancer. Int. J. Gynecol. Cancer 2021, 31, 265–271. [Google Scholar] [CrossRef]
- Averbach, S.; Silverberg, M.J.; Leyden, W.; Smith-McCune, K.; Raine-Bennett, T.; Sawaya, G.F. Recent Intrauterine Device Use and the Risk of Precancerous Cervical Lesions and Cervical Cancer. Contraception 2018, 98, 130–134. [Google Scholar] [CrossRef]
- Skorstengaard, M.; Lynge, E.; Napolitano, G.; Blaakær, J.; Bor, P. Risk of Precancerous Cervical Lesions in Women Using a Hormone-Containing Intrauterine Device and Other Contraceptives: A Register-Based Cohort Study from Denmark. Hum. Reprod. 2021, 36, 1796–1807. [Google Scholar] [CrossRef]
- Cunzhi, H.; Jiexian, J.; Xianwen, Z.; Jingang, G.; Shumin, Z.; Lili, D. Serum and Tissue Levels of Six Trace Elements and Copper/Zinc Ratio in Patients with Cervical Cancer and Uterine Myoma. Biol. Trace Elem. Res. 2003, 94, 113–122. [Google Scholar] [CrossRef]
- Naidu, M.S.K.; Suryakar, A.N.; Swami, S.C.; Katkam, R.V.; Kumbar, K.M. Oxidative Stress and Antioxidant Status in Cervical Cancer Patients. Indian J. Clin. Biochem. 2007, 22, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Shi, M.; Zhao, Y. Association between Serum Copper Levels and Cervical Cancer Risk: A Meta-Analysis. Biosci. Rep. 2018, 38, BSR20180161. [Google Scholar] [CrossRef]
- Okonkwo, C.A.; Amegor, F.O.; Gbolade, J.O. Relationship between Trace Elements and Major Gynaecological Malignancies. Asian J. Med. Sci. 2013, 5, 124–127. [Google Scholar] [CrossRef]
- Hijam, D.; Dubey, A.; Laishram, V.; Jaichand, L.; Devi, T.I. Serum Copper Levels in Different Stages of Cervical Cancer in Manipur. Int. J. Med. Res. Prof. 2016, 24, 194–197. [Google Scholar] [CrossRef]
- Shah, S.; Kalal, B.S. Oxidative Stress in Cervical Cancer and Its Response to Chemoradiation. Turkish J. Obstet. Gynecol. 2019, 16, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.A.; Fleming, G.F.; Lastra, R.R.; Lee, N.K.; Moroney, J.W.; Son, C.H.; Tatebe, K.; Veneris, J.L. Current Recommendations and Recent Progress in Endometrial Cancer. CA. Cancer J. Clin. 2019, 69, 258–279. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lheureux, S.; Oza, A.M. Treatment Strategies for Endometrial Cancer: Current Practice and Perspective. Curr. Opin. Obstet. Gynecol. 2017, 29, 47–58. [Google Scholar] [CrossRef]
- Atakul, T.; Altinkaya, S.O.; Abas, B.I.; Yenisey, C. Serum Copper and Zinc Levels in Patients with Endometrial Cancer. Biol. Trace Elem. Res. 2020, 195, 46–54. [Google Scholar] [CrossRef]
- Rzymski, P.; Niedzielski, P.; Rzymski Pawełand Tomczyk, K.; Kozak, L.; Poniedziałek, B. Metal Accumulation in the Human Uterus Varies by Pathology and Smoking Status. Fertil. Steril. 2016, 105, 1511–1518. [Google Scholar] [CrossRef]
- Wieder-Huszla, S.; Chudecka-Głaz, A.; Cymbaluk-Płoska, A.; Karakiewicz, B.; Bosiacki, M.; Chlubek, D.; Jurczak, A. Evaluation of the Concentration of Selected Elements in Patients with Cancer of the Reproductive Organs with Respect to Treatment Stage—Preliminary Study. Nutrients 2022, 14, 2368. [Google Scholar] [CrossRef]
- Michalczyk, K.; Kapczuk, P.; Kupnicka, P.; Witczak, G.; Michalczyk, B.; Bosiacki, M.; Chlubek, D.; Cymbaluk-Płoska, A. Assessment of Serum Zn, Cu, Mn, and Fe Concentration in Women with Endometrial Cancer and Different Endometrial Pathologies. Nutrients 2023, 15, 3605. [Google Scholar] [CrossRef] [PubMed]
- Bahamondes, L.; Bahamondes, M.V.; Shulman, L.P. Non-Contraceptive Benefits of Hormonal and Intrauterine Reversible Contraceptive Methods. Hum. Reprod. Update 2015, 21, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Raz, N.; Feinmesser, L.; Moore, O.; Haimovich, S. Endometrial Polyps: Diagnosis and Treatment Options—A Review of Literature. Minim. Invasive Ther. Allied Technol. 2021, 30, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Ciebiera, M.; Bariani, M.V.; Ali, M.; Elkafas, H.; Boyer, T.G.; Al-Hendy, A. Comprehensive Review of Uterine Fibroids: Developmental Origin, Pathogenesis, and Treatment. Endocr. Rev. 2022, 43, 678–719. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis Is a Chronic Systemic Disease: Clinical Challenges and Novel Innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.J. Physiological Aspects of Female Fertility: Role of the Environment, Modern Lifestyle, and Genetics. Physiol. Rev. 2016, 96, 873–909. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Kaunitz, A.M.; Sanchez-Ramos, L.; Rhatigan, R.M. The Oncogenic Potential of Endometrial Polyps: A Systematic Review and Meta-Analysis. Obstet. Gynecol. 2010, 116, 1197–1205. [Google Scholar] [CrossRef]
- Peng, X.; Li, T.; Xia, E.; Xia, C.; Liu, Y.; Yu, D. A Comparison of Oestrogen Receptor and Progesterone Receptor Expression in Endometrial Polyps and Endometrium of Premenopausal Women. J. Obstet. Gynaecol. 2009, 29, 340–346. [Google Scholar] [CrossRef]
- Liu, Z.; Kuokkanen, S.; Pal, L. Steroid Hormone Receptor Profile of Premenopausal Endometrial Polyps. Reprod. Sci. 2010, 17, 377–383. [Google Scholar] [CrossRef]
- Yin, P.; Ono, M.; Moravek, M.B.; Coon, J.S.; Navarro, A.; Monsivais, D.; Dyson, M.T.; Druschitz, S.A.; Malpani, S.S.; Serna, V.A.; et al. Human Uterine Leiomyoma Stem/Progenitor Cells Expressing CD34 and CD49b Initiate Tumors in Vivo. J. Clin. Endocrinol. Metab. 2015, 100, E601–E606. [Google Scholar] [CrossRef]
- Mas, A.; Stone, L.; O’Connor, P.M.; Yang, Q.; Kleven, D.; Simon, C.; Walker, C.L.; Al-Hendy, A. Developmental Exposure to Endocrine Disruptors Expands Murine Myometrial Stem Cell Compartment as a Prerequisite to Leiomyoma Tumorigenesis. Stem Cells 2017, 35, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Jiang, T.; Wang, X.; Yin, T.; Shen, L.; Zhang, Z.; Zou, W.; Liu, Y.; Zong, K.; Liang, D.; et al. Serum Essential Trace Element Status in Women and the Risk of Endometrial Diseases: A Case-Control Study. Biol. Trace Elem. Res. 2023, 201, 2151–2161. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, B.K.; Evliyaoğlu, Ö.; Yorgancı, A.; Özyer, Ş.; Üstün, Y.E. Serum Concentrations of Heavy Metals in Women with Endometrial Polyps. J. Obstet. Gynaecol. 2020, 40, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Flores, I.; Rivera, E.; Ruiz, L.A.; Santiago, O.I.; Vernon, M.W.; Appleyard, C.B. Molecular Profiling of Experimental Endometriosis Identified Gene Expression Patterns in Common with Human Disease. Fertil. Steril. 2007, 87, 1180–1199. [Google Scholar] [CrossRef] [PubMed]
- Turgut, A.I.; Ozler, A.; Goruk, N.Y.; Tunc, S.Y.; Evliyaoglu, O.; Gul, T. Copper, Ceruloplasmin and Oxidative Stress in Patients with Advanced-Stage Endometriosis. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1472–1478. [Google Scholar] [PubMed]
- Pollack, A.Z.; Louis, G.M.B.; Chen, Z.; Peterson, C.M.; Sundaram, R.; Croughan, M.S.; Sun, L.; Hediger, M.L.; Stanford, J.B.; Varner, M.W.; et al. Trace Elements and Endometriosis: The ENDO Study. Reprod. Toxicol. 2013, 42, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Delsouc, M.B.; Ghersa, F.; Ramírez, D.; Della Vedova, M.C.; Gil, R.A.; Vallcaneras, S.S.; Casais, M. Endometriosis Progression in Tumor Necrosis Factor Receptor P55-Deficient Mice: Impact on Oxidative/Nitrosative Stress and Metallomic Profile. J. Trace Elem. Med. Biol. 2019, 52, 157–165. [Google Scholar] [CrossRef]
- Ngô, C.; Chéreau, C.; Nicco, C.; Weill, B.; Chapron, C.; Batteux, F. Reactive Oxygen Species Controls Endometriosis Progression. Am. J. Pathol. 2009, 175, 225–234. [Google Scholar] [CrossRef]
- Tsang, C.K.; Chen, M.; Cheng, X.; Qi, Y.; Chen, Y.; Das, I.; Li, X.; Vallat, B.; Fu, L.-W.; Qian, C.-N.; et al. SOD1 Phosphorylation by MTORC1 Couples Nutrient Sensing and Redox Regulation. Mol. Cell 2018, 70, 502–515. [Google Scholar] [CrossRef]
- McKinnon, B.D.; Kocbek, V.; Nirgianakis, K.; Bersinger, N.A.; Mueller, M.D. Kinase Signalling Pathways in Endometriosis: Potential Targets for Non-Hormonal Therapeutics. Hum. Reprod. Update 2016, 22, 382–403. [Google Scholar] [CrossRef]
- Klevay, L.M.; Christopherson, D.M. Copper Deficiency Halves Serum Dehydroepiandrosterone in Rats. J. Trace Elem. Med. Biol. 2000, 14, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.K.; Gupta, P.S.P.; Nandi, S.; Mondal, S.; Ippala, J.R.; Mor, A.; Mishra, A.; Tripathi, S.K. Effect of in Vitro Copper Supplementation on Granulosa Cell Estradiol Synthesis and Associated Genes. Indian J. Anim. Res. 2018, 52, 652–657. [Google Scholar] [CrossRef]
- Delsouc, M.B.; Conforti, R.A.; Vitale, D.L.; Alaniz, L.; Pacheco, P.; Andujar, S.; Vallcaneras, S.S.; Casais, M. Antiproliferative and Antiangiogenic Effects of Ammonium Tetrathiomolybdate in a Model of Endometriosis. Life Sci. 2021, 287, 120099. [Google Scholar] [CrossRef] [PubMed]
- Conforti, R.A.; Delsouc, M.B.; Zabala, A.S.; Vallcaneras, S.S.; Casais, M. The Copper Chelator Ammonium Tetrathiomolybdate Inhibits the Progression of Experimental Endometriosis in TNFR1-Deficient Mice. Sci. Rep. 2023, 13, 10354. [Google Scholar] [CrossRef] [PubMed]
- Vallcaneras, S.; Ghersa, F.; Bastón, J.; Delsouc, M.B.; Meresman, G.; Casais, M. TNFRp55 Deficiency Promotes the Development of Ectopic Endometriotic-like Lesions in Mice. J. Endocrinol. 2017, 234, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Ghersa, F.; Delsouc, M.B.; Goyeneche, A.A.; Vallcaneras, S.S.; Meresman, G.; Telleria, C.M.; Casais, M. Reduced Inflammatory State Promotes Reinnervation of Endometriotic-like Lesions in TNFRp55 Deficient Mice. Mol. Hum. Reprod. 2019, 25, 385–396. [Google Scholar] [CrossRef]
- Li, Y. Copper Homeostasis: Emerging Target for Cancer Treatment. IUBMB Life 2020, 72, 1900–1908. [Google Scholar] [CrossRef]
- Babak, M.V.; Ahn, D. Modulation of Intracellular Copper Levels as the Mechanism of Action of Anticancer Copper Complexes: Clinical Relevance. Biomedicines 2021, 9, 852. [Google Scholar] [CrossRef]
- Kim, J.-J.; Kim, Y.-S.; Kumar, V. Heavy Metal Toxicity: An Update of Chelating Therapeutic Strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef]
- Peisach, J.; Blumberg, W.E. A Mechanism for the Action of Penicillamine in the Treatment of Wilson’s Disease. Mol. Pharmacol. 1969, 5, 200–209. [Google Scholar]
- Kumar, V.; Singh, A.P.; Wheeler, N.; Galindo, C.L.; Kim, J.-J. Safety Profile of D-Penicillamine: A Comprehensive Pharmacovigilance Analysis by FDA Adverse Event Reporting System. Expert Opin. Drug Saf. 2021, 20, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Saura, R.; Hirohata, K.; Ziff, M. Inhibition of Human Endothelial Cell Proliferation in Vitro and Neovascularization in Vivo by D-Penicillamine. J. Clin. Investig. 1989, 83, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Crowe, A.; Jackaman, C.; Beddoes, K.M.; Ricciardo, B.; Nelson, D.J. Rapid Copper Acquisition by Developing Murine Mesothelioma: Decreasing Bioavailable Copper Slows Tumor Growth, Normalizes Vessels and Promotes T Cell Infiltration. PLoS ONE 2013, 8, e73684. [Google Scholar] [CrossRef] [PubMed]
- Mammoto, T.; Jiang, A.; Jiang, E.; Panigrahy, D.; Kieran, M.W.; Mammoto, A. Role of Collagen Matrix in Tumor Angiogenesis and Glioblastoma Multiforme Progression. Am. J. Pathol. 2013, 183, 1293–1305. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jo, S.; Kim, I.-G.; Kim, R.-K.; Kahm, Y.-J.; Jung, S.-H.; Lee, J.H. Effect of Copper Chelators via the TGF-β Signaling Pathway on Glioblastoma Cell Invasion. Molecules 2022, 27, 8851. [Google Scholar] [CrossRef]
- Chen, S.-J.; Kuo, C.-C.; Pan, H.-Y.; Tsou, T.-C.; Yeh, S.-C.; Chang, J.-Y. Mechanistic Basis of a Combination D-Penicillamine and Platinum Drugs Synergistically Inhibits Tumor Growth in Oxaliplatin-Resistant Human Cervical Cancer Cells In Vitro and In Vivo. Biochem. Pharmacol. 2015, 95, 28–37. [Google Scholar] [CrossRef]
- Horn, N.; Møller, L.B.; Nurchi, V.M.; Aaseth, J. Chelating Principles in Menkes and Wilson Diseases: Choosing the Right Compounds in the Right Combinations at the Right Time. J. Inorg. Biochem. 2019, 190, 98–112. [Google Scholar] [CrossRef]
- Weiss, K.H.; Thurik, F.; Gotthardt, D.N.; Schäfer, M.; Teufel, U.; Wiegand, F.; Merle, U.; Ferenci-Foerster, D.; Maieron, A.; Stauber, R.; et al. Efficacy and Safety of Oral Chelators in Treatment of Patients with Wilson Disease. Clin. Gastroenterol. Hepatol. 2013, 11, 1028–1035. [Google Scholar] [CrossRef]
- Yoshii, J.; Yoshiji, H.; Kuriyama, S.; Ikenaka, Y.; Noguchi, R.; Okuda, H.; Tsujinoue, H.; Nakatani, T.; Kishida, H.; Nakae, D.; et al. The Copper-Chelating Agent, Trientine, Suppresses Tumor Development and Angiogenesis in the Murine Hepatocellular Carcinoma Cells. Int. J. Cancer 2001, 94, 768–773. [Google Scholar] [CrossRef]
- Moriguchi, M.; Nakajima, T.; Kimura, H.; Watanabe, T.; Takashima, H.; Mitsumoto, Y.; Katagishi, T.; Okanoue, T.; Kagawa, K. The Copper Chelator Trientine Has an Antiangiogenic Effect against Hepatocellular Carcinoma, Possibly through Inhibition of Interleukin-8 Production. Int. J. Cancer 2002, 102, 445–452. [Google Scholar] [CrossRef]
- Hayashi, M.; Nishiya, H.; Chiba, T.; Endoh, D.; Kon, Y.; Okui, T. Trientine, a Copper-Chelating Agent, Induced Apoptosis in Murine Fibrosarcoma Cells in Vivo and in Vitro. J. Vet. Med. Sci. 2007, 69, 137–142. [Google Scholar] [CrossRef]
- Liu, J.; Guo, L.; Yin, F.; Zheng, X.; Chen, G.; Wang, Y. Characterization and Antitumor Activity of Triethylene Tetramine, a Novel Telomerase Inhibitor. Biomed. Pharmacother. 2008, 62, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Guterres, A.N.; Villanueva, J. Targeting Telomerase for Cancer Therapy. Oncogene 2020, 39, 5811–5824. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-F.; Kuo, M.T.; Liu, Y.-S.; Cheng, Y.-M.; Wu, P.-Y.; Chou, C.-Y. A Dose Escalation Study of Trientine plus Carboplatin and Pegylated Liposomal Doxorubicin in Women with a First Relapse of Epithelial Ovarian, Tubal, and Peritoneal Cancer within 12 Months after Platinum-Based Chemotherapy. Front. Oncol. 2019, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, W.S.; Lewis, A.H.; Watson, S.J. The Teart Pastures of Somerset: I. The Cause and Cure of Teartness. J. Agric. Sci. 1943, 33, 44–51. [Google Scholar] [CrossRef]
- Bickel, H.; Neale, F.C.; Hall, G. A Clinical and Biochemical Study of Hepatolenticular Degeneration (Wilson’s Disease). QJM An Int. J. Med. 1957, 26, 527–558. [Google Scholar]
- Dick, A.T.; Dewey, D.W.; Gawthorne, J.M. Thiomolybdates and the Copper–Molybdenum–Sulphur Interaction in Ruminant Nutrition. J. Agric. Sci. 1975, 85, 567–568. [Google Scholar] [CrossRef]
- Brewer, G.J.; Askari, F.; Lorincz, M.T.; Carlson, M.; Schilsky, M.; Kluin, K.J.; Hedera, P.; Moretti, P.; Fink, J.K.; Tankanow, R.; et al. Treatment of Wilson Disease with Ammonium Tetrathiomolybdate: IV. Comparison of Tetrathiomolybdate and Trientine in a Double-Blind Study of Treatment of the Neurologic Presentation of Wilson Disease. Arch. Neurol. 2006, 63, 521. [Google Scholar] [CrossRef]
- Cox, C.; Teknos, T.N.; Barrios, M.; Brewer, G.J.; Dick, R.D.; Merajver, S.D. The Role of Copper Suppression as an Antiangiogenic Strategy in Head and Neck Squamous Cell Carcinoma. Laryngoscope 2001, 111, 696–701. [Google Scholar] [CrossRef]
- Khan, M.K.; Miller, M.W.; Taylor, J.; Gill, N.K.; Dick, R.D.; Van Goled, K.; Brewert, G.J.; Merajver, S.D. Radiotherapy and Antiangiogenic TM in Lung Cancer. Neoplasia 2002, 4, 164–170. [Google Scholar] [CrossRef]
- Van Golen, K.L.; Bao, L.; Brewert, G.J.; Pienta, K.J.; Kamradt, J.M.; Livant, D.L.; Merajver, S.D. Suppression of Tumor Recurrence and Metastasis by a Combination of the PHSCN Sequence and the Antiangiogenic Compound Tetrathiomolybdate in Prostate Carcinoma. Neoplasia 2002, 4, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Lange, T.S.; Singh, R.K.; Brard, L.; Moore, R.G. Tetrathiomolybdate Sensitizes Ovarian Cancer Cells to Anticancer Drugs Doxorubicin, Fenretinide, 5-Fluorouracil and Mitomycin C. BMC Cancer 2012, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.; Willis, A.; Kornhauser, N.; Ward, M.M.; Lee, S.B.; Nackos, E.; Seo, B.R.; Chuang, E.; Cigler, T.; Moore, A.; et al. Influencing the Tumor Microenvironment: A Phase II Study of Copper Depletion Using Tetrathiomolybdate in Patients with Breast Cancer at High Risk for Recurrence and in Preclinical Models of Lung Metastases. Clin. Cancer Res. 2017, 23, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, H.M.; Xue, Y.; Robinson, C.D.; Canalizo-Hernández, M.A.; Marvin, R.G.; Kelly, R.A.; Mondragón, A.; Penner-Hahn, J.E.; O’Halloran, T.V. Tetrathiomolybdate Inhibits Copper Trafficking Proteins through Metal Cluster Formation. Science 2010, 327, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Juarez, J.C.; Betancourt, O.; Pirie-Shepherd, S.R.; Guan, X.; Price, M.L.; Shaw, D.E.; Mazar, A.P.; Doñate, F. Copper Binding by Tetrathiomolybdate Attenuates Angiogenesis and Tumor Cell Proliferation through the Inhibition of Superoxide Dismutase 1. Clin. Cancer Res. 2006, 12, 4974–4982. [Google Scholar] [CrossRef]
- Baldari, S.; Di Rocco, G.; Heffern, M.C.; Su, T.A.; Chang, C.J.; Toietta, G. Effects of Copper Chelation on BRAFV600E Positive Colon Carcinoma Cells. Cancers 2019, 11, 659. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Tsang, T.; Anderson, G.R.; Posimo, J.M.; Brady, D.C. Inhibition of BCL2 Family Members Increases the Efficacy of Copper Chelation in BRAFV600E-Driven Melanoma. Cancer Res. 2020, 80, 1387–1400. [Google Scholar] [CrossRef]
- Ryumon, S.; Okui, T.; Kunisada, Y.; Kishimoto, K.; Shimo, T.; Hasegawa, K.; Ibaragi, S.; Akiyama, K.; Thu Ha, N.T.; Monsur Hassan, N.M.; et al. Ammonium Tetrathiomolybdate Enhances the Antitumor Effect of Cisplatin via the Suppression of ATPase Copper Transporting Beta in Head and Neck Squamous Cell Carcinoma. Oncol. Rep. 2019, 42, 2611–2621. [Google Scholar] [CrossRef]
- Schneider, B.J.; Lee, J.S.-J.; Hayman, J.A.; Chang, A.C.; Orringer, M.B.; Pickens, A.; Pan, C.C.; Merajver, S.D.; Urba, S.G. Pre-Operative Chemoradiation Followed by Post-Operative Adjuvant Therapy with Tetrathiomolybdate, a Novel Copper Chelator, for Patients with Resectable Esophageal Cancer. Investig. New Drugs 2013, 31, 435–442. [Google Scholar] [CrossRef]
- Kim, K.K.; Han, A.; Yano, N.; Ribeiro, J.R.; Lokich, E.; Singh, R.K.; Moore, R.G. Tetrathiomolybdate Mediates Cisplatin-Induced P38 Signaling and EGFR Degradation and Enhances Response to Cisplatin Therapy in Gynecologic Cancers. Sci. Rep. 2015, 5, 1–11. [Google Scholar] [CrossRef]
- Kim, K.K.; Kawar, N.M.; Singh, R.K.; Lange, T.S.; Brard, L.; Moore, R.G. Tetrathiomolybdate Induces Doxorubicin Sensitivity in Resistant Tumor Cell Lines. Gynecol. Oncol. 2011, 122, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Nan, L.; Yuan, W.; Guodong, C.; Yonghui, H. Multitargeting Strategy Using Tetrathiomolybdate and Lenvatinib: Maximizing Antiangiogenesis Activity in a Preclinical Liver Cancer Model. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Agents) 2023, 23, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Rogers, P.A.W.; Adamson, G.D.; Al-Jefout, M.; Becker, C.M.; D’Hooghe, T.M.; Dunselman, G.A.J.; Fazleabas, A.; Giudice, L.C.; Horne, A.W.; Hull, M.L.; et al. Research Priorities for Endometriosis. Reprod. Sci. 2017, 24, 202–226. [Google Scholar] [CrossRef] [PubMed]
- Richter, O.N.; Dorn, C.; Rösing, B.; Flaskamp, C.; Ulrich, U. Tumor Necrosis Factor Alpha Secretion by Peritoneal Macrophages in Patients with Endometriosis. Arch. Gynecol. Obstet. 2005, 271, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.P.; Ding, J.; Dmowski, W.P. Peritoneal Fluid-Mediated Enhancement of Eutopic and Ectopic Endometrial Cell Proliferation Is Dependent on Tumor Necrosis Factor-α in Women with Endometriosis. Fertil. Steril. 2002, 78, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Li, F.; Qin, Z. TNF Receptor 2 Makes Tumor Necrosis Factor a Friend of Tumors. Front. Immunol. 2018, 9, 1170. [Google Scholar] [CrossRef] [PubMed]
- Gough, P.; Myles, I.A. Tumor Necrosis Factor Receptors: Pleiotropic Signaling Complexes and Their Differential Effects. Front. Immunol. 2020, 11, 585880. [Google Scholar] [CrossRef]
- Rivas, M.A.; Carnevale, R.P.; Proietti, C.J.; Rosemblit, C.; Beguelin, W.; Salatino, M.; Charreau, E.H.; Frahm, I.; Sapia, S.; Brouckaert, P.; et al. TNFα Acting on TNFR1 Promotes Breast Cancer Growth via P42/P44 MAPK, JNK, Akt and NF-ΚB-Dependent Pathways. Exp. Cell Res. 2008, 314, 509–529. [Google Scholar] [CrossRef]
- Islimye, M.; Kilic, S.; Zulfikaroglu, E.; Topcu, O.; Zergeroglu, S.; Batioglu, S. Regression of Endometrial Autografts in a Rat Model of Endometriosis Treated with Etanercept. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 159, 184–189. [Google Scholar] [CrossRef]
- Oliveri, V. Selective Targeting of Cancer Cells by Copper Ionophores: An Overview. Front. Mol. Biosci. 2022, 9, 841814. [Google Scholar] [CrossRef]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting Cancer Cells by ROS-Mediated Mechanisms: A Radical Therapeutic Approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Reznik, E.; Stokes, M.E.; Krishnamoorthy, L.; Bos, P.H.; Song, Y.; Quartararo, C.E.; Pagano, N.C.; Carpizo, D.R.; DeCarvalho, A.C.; et al. Copper-Binding Small Molecule Induces Oxidative Stress and Cell-Cycle Arrest in Glioblastoma-Patient-Derived Cells. Cell Chem. Biol. 2018, 25, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper Induces Cell Death by Targeting Lipoylated TCA Cycle Proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.A.N.; Chen, D.I.; Zhang, X.I.A.; Cui, Q.; Fan, Y.; Bi, C.; Dou, Q.P. Molecular Study on Copper-Mediated Tumor Proteasome Inhibition and Cell Death. Int. J. Oncol. 2010, 37, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Denoyer, D.; Pearson, H.B.; Clatworthy, S.A.S.; Smith, Z.M.; Francis, P.S.; Llanos, R.M.; Volitakis, I.; Phillips, W.A.; Meggyesy, P.M.; Masaldan, S.; et al. Copper as a Target for Prostate Cancer Therapeutics: Copper-Ionophore Pharmacology and Altering Systemic Copper Distribution. Oncotarget 2016, 7, 37064–37080. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Li, X.; Ren, Y.; Zhang, X. Disulfiram: A Novel Repurposed Drug for Cancer Therapy. Cancer Chemother. Pharmacol. 2021, 87, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Lewison, E.F. Spontaneous Regression of Breast Cancer. Prog. Clin. Biol. Res. 1977, 12, 47–53. [Google Scholar]
- Ekinci, E.; Rohondia, S.; Khan, R.; Dou, Q.P. Repurposing Disulfiram as an Anti-Cancer Agent: Updated Review on Literature and Patents. Recent Pat. Anticancer. Drug Discov. 2019, 14, 113–132. [Google Scholar] [CrossRef]
- Jia, Y.; Huang, T. Overview of Antabuse®(Disulfiram) in Radiation and Cancer Biology. Cancer Manag. Res. 2021, 13, 4095–4101. [Google Scholar] [CrossRef]
- Kannappan, V.; Ali, M.; Small, B.; Rajendran, G.; Elzhenni, S.; Taj, H.; Wang, W.; Dou, Q.P. Recent Advances in Repurposing Disulfiram and Disulfiram Derivatives as Copper-Dependent Anticancer Agents. Front. Mol. Biosci. 2021, 8, 741316. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Wu, C.; Wang, L.; Chen, Z.-S.; Cui, W. The Combination of Disulfiram and Copper for Cancer Treatment. Drug Discov. Today 2020, 25, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, L.-H.; Zhang, H.-T.; Wang, Y.-T.; Liu, S.; Zhou, W.-L.; Yuan, X.-Z.; Li, T.-Y.; Wu, C.-F.; Yang, J.-Y. Disulfiram Combined with Copper Inhibits Metastasis and Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma through the NF-ΚB and TGF-β Pathways. J. Cell. Mol. Med. 2018, 22, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Caminear, M.W.; Harrington, B.S.; Kamdar, R.D.; Kruhlak, M.J.; Annunziata, C.M. Disulfiram Transcends ALDH Inhibitory Activity When Targeting Ovarian Cancer Tumor-Initiating Cells. Front. Oncol. 2022, 12, 762820. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Yang, Z.; Sehouli, J.; Kaufmann, A.M. Blockade of ALDH in Cisplatin-Resistant Ovarian Cancer Stem Cells in Vitro Synergistically Enhances Chemotherapy-Induced Cell Death. Curr. Oncol. 2022, 29, 2808–2822. [Google Scholar] [CrossRef] [PubMed]
- Dinavahi, S.S.; Bazewicz, C.G.; Gowda, R. Aldehyde Dehydrogenase Inhibitors for Cancer Therapeutics. Trends Pharmacol. Sci. 2019, 40, 774–789. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.A.; Bai, S.; McLean, K.; Yang, K.; Griffith, K.; Thomas, D.; Ginestier, C.; Johnston, C.; Kueck, A.; Reynolds, R.K.; et al. Aldehyde Dehydrogenase in Combination with CD133 Defines Angiogenic Ovarian Cancer Stem Cells That Portend Poor Patient Survival. Cancer Res. 2011, 71, 3991–4001. [Google Scholar] [CrossRef]
- Çelik, Ö.; Erşahin, A.; Acet, M.; Çelik, N.; Baykuş, Y.; Deniz, R.; Özerol, E.; Özerol, İ. Disulfiram, as a Candidate NF-Kappa B and Proteasome Inhibitor, Prevents Endometriotic Implant Growing in a Rat Model of Endometriosis. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4380–4389. [Google Scholar]
- Meraz-Torres, F.; Plöger, S.; Garbe, C.; Niessner, H.; Sinnberg, T. Disulfiram as a Therapeutic Agent for Metastatic Malignant Melanoma—Old Myth or New Logos? Cancers 2020, 12, 3538. [Google Scholar] [CrossRef]
- Jiao, Y.; Hannafon, B.N.; Zhang, R.R.; Fung, K.-M.; Ding, W.-Q. Docosahexaenoic Acid and Disulfiram Act in Concert to Kill Cancer Cells: A Mutual Enhancement of Their Anticancer Actions. Oncotarget 2017, 8, 17908–17920. [Google Scholar] [CrossRef]
- Tang, B.; Wu, M.; Zhang, L.; Jian, S.; Lv, S.; Lin, T.; Zhu, S.; Liu, L.; Wang, Y.; Yi, Z.; et al. Combined Treatment of Disulfiram with PARP Inhibitors Suppresses Ovarian Cancer. Front. Oncol. 2023, 13, 1154073. [Google Scholar] [CrossRef]
- Du, R.; Sun, F.; Li, K.; Qi, J.; Zhong, W.; Wang, W.; Sun, Q.; Deng, Q.; Wang, H.; Nie, J.; et al. Proteomics Analysis Revealed Smad3 as A Potential Target of the Synergistic Anti-Tumor Activity of Disulfiram and Cisplatin in Ovarian Cancer. Anticancer. Agents Med. Chem. 2023, 23, 1754–1764. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guan, X.; Wang, M.; Wang, N.; Chen, Y.; Li, B.; Xu, Z.; Fu, F.; Du, C.; Zheng, Z. Disulfiram/Copper Induces Antitumor Activity against Gastric Cancer via the ROS/MAPK and NPL4 Pathways. Bioengineered 2022, 13, 6579–6589. [Google Scholar] [CrossRef] [PubMed]
- Safi, R.; Nelson, E.R.; Chitneni, S.K.; Franz, K.J.; George, D.J.; Zalutsky, M.R.; McDonnell, D.P. Copper Signaling Axis as a Target for Prostate Cancer Therapeutics. Cancer Res. 2014, 74, 5819–5831. [Google Scholar] [CrossRef] [PubMed]
- Lun, X.; Wells, J.C.; Grinshtein, N.; King, J.C.; Hao, X.; Dang, N.-H.; Wang, X.; Aman, A.; Uehling, D.; Datti, A.; et al. Disulfiram When Combined with Copper Enhances the Therapeutic Effects of Temozolomide for the Treatment of Glioblastoma. Clin. Cancer Res. 2016, 22, 3860–3875. [Google Scholar] [CrossRef] [PubMed]
- Oliveri, V. Biomedical Applications of Copper Ionophores. Coord. Chem. Rev. 2020, 422, 213474. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Cui, W.; Yuan, X.; Lin, L.; Cao, Q.; Wang, N.; Li, Y.; Guo, W.; Zhang, X.; et al. Targeting ALDH1A1 by Disulfiram/Copper Complex Inhibits Non-Small Cell Lung Cancer Recurrence Driven by ALDH-Positive Cancer Stem Cells. Oncotarget 2016, 7, 58516–58530. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wang, S.; Li, R.; Chen, K.; He, L.; Deng, M.; Kannappan, V.; Zha, J.; Dong, H.; Wang, W. Disulfiram/Copper Selectively Eradicates AML Leukemia Stem Cells in Vitro and in Vivo by Simultaneous Induction of ROS-JNK and Inhibition of NF-ΚB and Nrf2. Cell Death Dis. 2017, 8, e2797. [Google Scholar] [CrossRef]
- Serra, R.; Zhao, T.; Huq, S.; Gorelick, N.L.; Casaos, J.; Cecia, A.; Mangraviti, A.; Eberhart, C.; Bai, R.; Olivi, A.; et al. Disulfiram and Copper Combination Therapy Targets NPL4, Cancer Stem Cells and Extends Survival in a Medulloblastoma Model. PLoS ONE 2021, 16, e0251957. [Google Scholar] [CrossRef]
- Sun, T.; Yang, W.; Toprani, S.M.; Guo, W.; He, L.; DeLeo, A.B.; Ferrone, S.; Zhang, G.; Wang, E.; Lin, Z.; et al. Induction of Immunogenic Cell Death in Radiation-Resistant Breast Cancer Stem Cells by Repurposing Anti-Alcoholism Drug Disulfiram. Cell Commun. Signal. 2020, 18, 36. [Google Scholar] [CrossRef]
- Falls-Hubert, K.C.; Butler, A.L.; Gui, K.; Anderson, M.; Li, M.; Stolwijk, J.M.; Rodman III, S.N.; Solst, S.R.; Tomanek-Chalkley, A.; Searby, C.C.; et al. Disulfiram Causes Selective Hypoxic Cancer Cell Toxicity and Radio-Chemo-Sensitization via Redox Cycling of Copper. Free Radic. Biol. Med. 2020, 150, 1–11. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, X.; Lin, L.; Wang, H.; He, E.; Wang, G.; Zhao, Q. The Disulfiram/Copper Complex Induces Apoptosis and Inhibits Tumour Growth in Human Osteosarcoma by Activating the ROS/JNK Signalling Pathway. J. Biochem. 2021, 170, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhai, Q.; Li, M.; Huang, S.; Sun, Z.; Yan, Z.; Li, J.; Li, L.; Li, Y. Anti-Cancer Effects of Disulfiram in Cervical Cancer Cell Lines Are Mediated by Both Autophagy and Apoptosis. Bull. Exp. Biol. Med. 2022, 172, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.D.; Sakla, A.P.; Shankaraiah, N. An Insight into Medicinal Attributes of Dithiocarbamates: Bird’s Eye View. Bioorg. Chem. 2020, 105, 104346. [Google Scholar] [CrossRef] [PubMed]
- Wykowski, R.; Fuentefria, A.M.; de Andrade, S.F. Antimicrobial Activity of Clioquinol and Nitroxoline: A Scoping Review. Arch. Microbiol. 2022, 204, 535. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.-Q.; Liu, B.; Vaught, J.L.; Yamauchi, H.; Lind, S.E. Anticancer Activity of the Antibiotic Clioquinol. Cancer Res. 2005, 65, 3389–3395. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cui, Q.C.; Yang, H.; Barrea, R.A.; Sarkar, F.H.; Sheng, S.; Yan, B.; Reddy, G.P.V.; Dou, Q.P. Clioquinol, a Therapeutic Agent for Alzheimer’s Disease, Has Proteasome-Inhibitory, Androgen Receptor-Suppressing, Apoptosis-Inducing, and Antitumor Activities in Human Prostate Cancer Cells and Xenografts. Cancer Res. 2007, 67, 1636–1644. [Google Scholar] [CrossRef]
- Tuller, E.R.; Brock, A.L.; Yu, H.; Lou, J.R.; Benbrook, D.M.; Ding, W.-Q. PPARα Signaling Mediates the Synergistic Cytotoxicity of Clioquinol and Docosahexaenoic Acid in Human Cancer Cells. Biochem. Pharmacol. 2009, 77, 1480–1486. [Google Scholar] [CrossRef]
- Cater, M.A.; Haupt, Y. Clioquinol Induces Cytoplasmic Clearance of the X-Linked Inhibitor of Apoptosis Protein (XIAP): Therapeutic Indication for Prostate Cancer. Biochem. J. 2011, 436, 481–491. [Google Scholar] [CrossRef]
- Mao, X.; Li, X.; Sprangers, R.; Wang, X.; Venugopal, A.; Wood, T.; Zhang, Y.; Kuntz, D.A.; Coe, E.; Trudel, S.; et al. Clioquinol Inhibits the Proteasome and Displays Preclinical Activity in Leukemia and Myeloma. Leukemia 2009, 23, 585–590. [Google Scholar] [CrossRef]
- Cao, B.; Li, J.; Zhou, X.; Juan, J.; Han, K.; Zhang, Z.; Kong, Y.; Wang, J.; Mao, X. Clioquinol Induces Pro-Death Autophagy in Leukemia and Myeloma Cells by Disrupting the MTOR Signaling Pathway. Sci. Rep. 2014, 4, 5749. [Google Scholar] [CrossRef]
- Barrea, R.A.; Chen, D.; Irving, T.C.; Dou, Q.P. Synchrotron X-Ray Imaging Reveals a Correlation of Tumor Copper Speciation with Clioquinol’s Anticancer Activity. J. Cell. Biochem. 2009, 108, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Filiz, G.; Caragounis, A.; Crouch, P.J.; White, A.R. Clioquinol Promotes Cancer Cell Toxicity through Tumor Necrosis Factor α Release from Macrophages. J. Pharmacol. Exp. Ther. 2008, 324, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Bareggi, S.R.; Cornelli, U. Clioquinol: Review of Its Mechanisms of Action and Clinical Uses in Neurodegenerative Disorders. CNS Neurosci. Ther. 2012, 18, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Khan, H.; Abdullah, Y.; Dou, Q.P. Feasibility of Repurposing Clioquinol for Cancer Therapy. Recent Pat. Anticancer. Drug Discov. 2020, 15, 14–31. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Taggart, J.E.; Zhang, X.; Benbrook, D.M.; Lind, S.E.; Ding, W.-Q. Nitroxoline (8-Hydroxy-5-Nitroquinoline) Is More a Potent Anti-Cancer Agent than Clioquinol (5-Chloro-7-Iodo-8-Quinoline). Cancer Lett. 2011, 312, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Summers, K.L.; Dolgova, N.V.; Gagnon, K.B.; Sopasis, G.J.; James, A.K.; Lai, B.; Sylvain, N.J.; Harris, H.H.; Nichol, H.K.; George, G.N.; et al. PBT2 Acts through a Different Mechanism of Action than Other 8-Hydroxyquinolines: An X-ray Fluorescence Imaging Study. Metallomics 2020, 12, 1979–1994. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sun, L.; Koya, K.; Tatsuta, N.; Xia, Z.; Korbut, T.; Du, Z.; Wu, J.; Liang, G.; Jiang, J.; et al. Syntheses and Antitumor Activities of N′ 1, N′ 3-Dialkyl-N′ 1, N′ 3-Di-(Alkylcarbonothioyl) Malonohydrazide: The Discovery of Elesclomol. Bioorganic Med. Chem. Lett. 2013, 23, 5070–5076. [Google Scholar] [CrossRef] [PubMed]
- Kwan, S.-Y.; Cheng, X.; Tsang, Y.T.M.; Choi, J.-S.; Kwan, S.-Y.; Izaguirre, D.I.; Kwan, H.-S.; Gershenson, D.M.; Wong, K.-K. Loss of ARID1A Expression Leads to Sensitivity to ROS-Inducing Agent Elesclomol in Gynecologic Cancer Cells. Oncotarget 2016, 7, 56933–56943. [Google Scholar] [CrossRef]
- Buccarelli, M.; D’Alessandris, Q.G.; Matarrese, P.; Mollinari, C.; Signore, M.; Cappannini, A.; Martini, M.; D’Aliberti, P.; De Luca, G.; Pedini, F.; et al. Elesclomol-Induced Increase of Mitochondrial Reactive Oxygen Species Impairs Glioblastoma Stem-like Cell Survival and Tumor Growth. J. Exp. Clin. Cancer Res. 2021, 40, 228. [Google Scholar] [CrossRef]
- Nagai, M.; Vo, N.H.; Ogawa, L.S.; Chimmanamada, D.; Inoue, T.; Chu, J.; Beaudette-Zlatanova, B.C.; Lu, R.; Blackman, R.K.; Barsoum, J.; et al. The Oncology Drug Elesclomol Selectively Transports Copper to the Mitochondria to Induce Oxidative Stress in Cancer Cells. Free Radic. Biol. Med. 2012, 52, 2142–2150. [Google Scholar] [CrossRef]
- Hasinoff, B.B.; Yadav, A.A.; Patel, D.; Wu, X. The Cytotoxicity of the Anticancer Drug Elesclomol Is Due to Oxidative Stress Indirectly Mediated through Its Complex with Cu (II). J. Inorg. Biochem. 2014, 137, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, P.; Detappe, A.; Cai, K.; Keys, H.R.; Brune, Z.; Ying, W.; Thiru, P.; Reidy, M.; Kugener, G.; Rossen, J.; et al. Mitochondrial Metabolism Promotes Adaptation to Proteotoxic Stress. Nat. Chem. Biol. 2019, 15, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.A.; Patel, D.; Wu, X.; Hasinoff, B.B. Molecular Mechanisms of the Biological Activity of the Anticancer Drug Elesclomol and Its Complexes with Cu (II), Ni (II) and Pt (II). J. Inorg. Biochem. 2013, 126, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ling, X.; Sun, Y.; Liu, L.; Liu, L.; Wang, X.; Lu, C.; Ren, C.; Han, X.; Yu, Z. FDX1 Enhances Endometriosis Cell Cuproptosis via G6PD-Mediated Redox Homeostasis. Apoptosis 2023, 28, 1128–1140. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Banno, K.; Okawa, R.; Yanokura, M.; Iijima, M.; Irie-Kunitomi, H.; Nakamura, K.; Iida, M.; Adachi, M.; Umene, K.; et al. ARID1A Gene Mutation in Ovarian and Endometrial Cancers. Oncol. Rep. 2016, 35, 607–613. [Google Scholar] [CrossRef]
- Nie, X.; Chen, H.; Xiong, Y.; Chen, J.; Liu, T. Anisomycin Has a Potential Toxicity of Promoting Cuproptosis in Human Ovarian Cancer Stem Cells by Attenuating YY1/Lipoic Acid Pathway Activation. J. Cancer 2022, 13, 3503–3514. [Google Scholar] [CrossRef]
- Harrington, B.S.; Ozaki, M.K.; Caminear, M.W.; Hernandez, L.F.; Jordan, E.; Kalinowski, N.J.; Goldlust, I.S.; Guha, R.; Ferrer, M.; Thomas, C.; et al. Drugs Targeting Tumor-Initiating Cells Prolong Survival in a Post-Surgery, Post-Chemotherapy Ovarian Cancer Relapse Model. Cancers 2020, 12, 1645. [Google Scholar] [CrossRef]
- Monk, B.J.; Kauderer, J.T.; Moxley, K.M.; Bonebrake, A.J.; Dewdney, S.B.; Secord, A.A.; Ueland, F.R.; Johnston, C.M.; Aghajanian, C. A Phase II Evaluation of Elesclomol Sodium and Weekly Paclitaxel in the Treatment of Recurrent or Persistent Platinum-Resistant Ovarian, Fallopian Tube or Primary Peritoneal Cancer: An NRG Oncology/Gynecologic Oncology Group Study. Gynecol. Oncol. 2018, 151, 422–427. [Google Scholar] [CrossRef]
- Hedley, D.; Shamas-Din, A.; Chow, S.; Sanfelice, D.; Schuh, A.C.; Brandwein, J.M.; Seftel, M.D.; Gupta, V.; Yee, K.W.L.; Schimmer, A.D. A Phase I Study of Elesclomol Sodium in Patients with Acute Myeloid Leukemia. Leuk. Lymphoma 2016, 57, 2437–2440. [Google Scholar] [CrossRef]
- O’Day, S.; Gonzalez, R.; Lawson, D.; Weber, R.; Hutchins, L.; Anderson, C.; Haddad, J.; Kong, S.; Williams, A.; Jacobson, E. Phase II, Randomized, Controlled, Double-Blinded Trial of Weekly Elesclomol plus Paclitaxel versus Paclitaxel Alone for Stage IV Metastatic Melanoma. J. Clin. Oncol. 2009, 27, 5452–5458. [Google Scholar] [CrossRef]
- O’Day, S.J.; Eggermont, A.M.M.; Chiarion-Sileni, V.; Kefford, R.; Grob, J.J.; Mortier, L.; Robert, C.; Schachter, J.; Testori, A.; Mackiewicz, J.; et al. Final Results of Phase III SYMMETRY Study: Randomized, Double-Blind Trial of Elesclomol plus Paclitaxel versus Paclitaxel Alone as Treatment for Chemotherapy-Naive Patients with Advanced Melanoma. J. Clin. Oncol. 2013, 31, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Zhou, C.; Lu, L.; Liu, B.; Ding, Y. Elesclomol: A Copper Ionophore Targeting Mitochondrial Metabolism for Cancer Therapy. J. Exp. Clin. Cancer Res. 2022, 41, 271. [Google Scholar] [CrossRef] [PubMed]
- Helsel, M.E.; Franz, K.J. Pharmacological Activity of Metal Binding Agents That Alter Copper Bioavailability. Dalt. Trans. 2015, 44, 8760–8770. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Donnelly, P.S.; Zimmermann, M.; Wedd, A.G. Transfer of Copper between Bis (Thiosemicarbazone) Ligands and Intracellular Copper-Binding Proteins. Insights into Mechanisms of Copper Uptake and Hypoxia Selectivity. Inorg. Chem. 2008, 47, 4338–4347. [Google Scholar] [CrossRef]
- Cater, M.A.; Pearson, H.B.; Wolyniec, K.; Klaver, P.; Bilandzic, M.; Paterson, B.M.; Bush, A.I.; Humbert, P.O.; La Fontaine, S.; Donnelly, P.S.; et al. Increasing Intracellular Bioavailable Copper Selectively Targets Prostate Cancer Cells. ACS Chem. Biol. 2013, 8, 1621–1631. [Google Scholar] [CrossRef]
- Donnelly, P.S.; Liddell, J.R.; Lim, S.; Paterson, B.M.; Cater, M.A.; Savva, M.S.; Mot, A.I.; James, J.L.; Trounce, I.A.; White, A.R.; et al. An Impaired Mitochondrial Electron Transport Chain Increases Retention of the Hypoxia Imaging Agent Diacetylbis (4-Methylthiosemicarbazonato) CopperII. Proc. Natl. Acad. Sci. USA 2012, 109, 47–52. [Google Scholar] [CrossRef]
- Holland, J.P.; Barnard, P.J.; Collison, D.; Dilworth, J.R.; Edge, R.; Green, J.C.; McInnes, E.J.L. Spectroelectrochemical and Computational Studies on the Mechanism of Hypoxia Selectivity of Copper Radiopharmaceuticals. Chem. Eur. J. 2008, 14, 5890–5907. [Google Scholar] [CrossRef]
- Paterson, B.M.; Donnelly, P.S. Copper Complexes of Bis (Thiosemicarbazones): From Chemotherapeutics to Diagnostic and Therapeutic Radiopharmaceuticals. Chem. Soc. Rev. 2011, 40, 3005–3018. [Google Scholar] [CrossRef]
- Boschi, A.; Martini, P.; Janevik-Ivanovska, E.; Duatti, A. The Emerging Role of Copper-64 Radiopharmaceuticals as Cancer Theranostics. Drug Discov. Today 2018, 23, 1489–1501. [Google Scholar] [CrossRef]
- Lewis, J.S.; Laforest, R.; Buettner, T.L.; Song, S.-K.; Fujibayashi, Y.; Connett, J.M.; Welch, M.J. Copper-64-Diacetyl-Bis (N 4-Methylthiosemicarbazone): An Agent for Radiotherapy. Proc. Natl. Acad. Sci. USA 2001, 98, 1206–1211. [Google Scholar] [CrossRef]
- Dehdashti, F.; Grigsby, P.W.; Lewis, J.S.; Laforest, R.; Siegel, B.A.; Welch, M.J. Assessing Tumor Hypoxia in Cervical Cancer by PET with 60Cu-Labeled Diacetyl-Bis (N4-Methylthiosemicarbazone). J. Nucl. Med. 2008, 49, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, P.W.; Malyapa, R.S.; Higashikubo, R.; Schwarz, J.K.; Welch, M.J.; Huettner, P.C.; Dehdashti, F. Comparison of Molecular Markers of Hypoxia and Imaging with 60 Cu-ATSM in Cancer of the Uterine Cervix. Mol. Imaging Biol. 2007, 9, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.S.; Laforest, R.; Dehdashti, F.; Grigsby, P.W.; Welch, M.J.; Siegel, B.A. An Imaging Comparison of 64Cu-ATSM and 60Cu-ATSM in Cancer of the Uterine Cervix. J. Nucl. Med. 2008, 49, 1177–1182. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in Copper Complexes as Anticancer Agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.K.; Singh, A.; Singh, V.K.; Singh, M.P. Cancer Nanotechnology: A New Revolution for Cancer Diagnosis and Therapy. Curr. Drug Metab. 2019, 20, 416–429. [Google Scholar] [CrossRef]
- Ameh, T.; Sayes, C.M. The Potential Exposure and Hazards of Copper Nanoparticles: A Review. Environ. Toxicol. Pharmacol. 2019, 71, 103220. [Google Scholar] [CrossRef]
- Dou, L.; Zhang, X.; Zangeneh, M.M.; Zhang, Y. Efficient Biogenesis of Cu2O Nanoparticles Using Extract of Camellia Sinensis Leaf: Evaluation of Catalytic, Cytotoxicity, Antioxidant, and Anti-Human Ovarian Cancer Properties. Bioorg. Chem. 2021, 106, 104468. [Google Scholar] [CrossRef]
- Tabrez, S.; Khan, A.U.; Mirza, A.A.; Suhail, M.; Jabir, N.R.; Zughaibi, T.A.; Alam, M. Biosynthesis of Copper Oxide Nanoparticles and Its Therapeutic Efficacy against Colon Cancer. Nanotechnol. Rev. 2022, 11, 1322–1331. [Google Scholar] [CrossRef]
- Zhao, H.; Maruthupandy, M.; Al-mekhlafi, F.A.; Chackaravarthi, G.; Ramachandran, G.; Chelliah, C.K. Biological Synthesis of Copper Oxide Nanoparticles Using Marine Endophytic Actinomycetes and Evaluation of Biofilm Producing Bacteria and A549 Lung Cancer Cells. J. King Saud Univ. 2022, 34, 101866. [Google Scholar] [CrossRef]
- Zughaibi, T.A.; Jabir, N.R.; Khan, A.U.; Khan, M.S.; Tabrez, S. Screening of Cu4O3 NPs Efficacy and Its Anticancer Potential against Cervical Cancer. Cell Biochem. Funct. 2023, 41, 1174–1187. [Google Scholar] [CrossRef]
- Chen, H.; Feng, X.; Gao, L.; Mickymaray, S.; Paramasivam, A.; Abdulaziz Alfaiz, F.; Almasmoum, H.A.; Ghaith, M.M.; Almaimani, R.A.; Aziz Ibrahim, I.A. Inhibiting the PI3K/AKT/MTOR Signalling Pathway with Copper Oxide Nanoparticles from Houttuynia Cordata Plant: Attenuating the Proliferation of Cervical Cancer Cells. Artif. Cells Nanomed. Biotechnol. 2021, 49, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Goswami, U.; Dutta, A.; Raza, A.; Kandimalla, R.; Kalita, S.; Ghosh, S.S.; Chattopadhyay, A. Transferrin–Copper Nanocluster–Doxorubicin Nanoparticles as Targeted Theranostic Cancer Nanodrug. ACS Appl. Mater. Interfaces 2018, 10, 3282–3294. [Google Scholar] [CrossRef] [PubMed]
- Aboeita, N.M.; Fahmy, S.A.; El-Sayed, M.M.H.; Azzazy, H.M.E.-S.; Shoeib, T. Enhanced Anticancer Activity of Nedaplatin Loaded onto Copper Nanoparticles Synthesized Using Red Algae. Pharmaceutics 2022, 14, 418. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Sun, Q.; Yu, Z.; Gao, X.; Pan, W.; Wan, X.; Tang, B. Nuclear-Targeted Photothermal Therapy Prevents Cancer Recurrence with near-Infrared Triggered Copper Sulfide Nanoparticles. ACS Nano 2018, 12, 5197–5206. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Melancon, M.; Stafford, R.J.; Li, J.; Nick, A.M.; Tian, M.; Sood, A.K.; Li, C. Precision Nanomedicine Using Dual PET and MR Temperature Imaging–Guided Photothermal Therapy. J. Nucl. Med. 2016, 57, 1778–1783. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Murad, W.; Mubin, S.; Ullah, O.; Rehman, N.U.; Rahman, M.H. Multiple Health Benefits of Curcumin and Its Therapeutic Potential. Environ. Sci. Pollut. Res. 2022, 29, 43732–43744. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liang, S.; Geng, H.; Xiong, M.; Li, M.; Su, Q.; Jia, F.; Zhao, Y.; Wang, K.; Jiang, J.; et al. Proteomics Revealed the Crosstalk between Copper Stress and Cuproptosis, and Explored the Feasibility of Curcumin as Anticancer Copper Ionophore. Free Radic. Biol. Med. 2022, 193, 638–647. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Pourhanifeh, M.H.; Darvish, M.; Tabatabaeian, J.; Fard, M.R.; Mottaghi, R.; Azadchehr, M.J.; Jahanshahi, M.; Sahebkar, A.; Mirzaei, H. Therapeutic Role of Curcumin and Its Novel Formulations in Gynecological Cancers. J. Ovarian Res. 2020, 13, 130. [Google Scholar] [CrossRef]
- Greish, K.; Pittalà, V.; Taurin, S.; Taha, S.; Bahman, F.; Mathur, A.; Jasim, A.; Mohammed, F.; El-Deeb, I.M.; Fredericks, S.; et al. Curcumin–Copper Complex Nanoparticles for the Management of Triple-Negative Breast Cancer. Nanomaterials 2018, 8, 884. [Google Scholar] [CrossRef]
- Luo, C.-Q.; Xing, L.; Cui, P.-F.; Qiao, J.-B.; He, Y.-J.; Chen, B.-A.; Jin, L.; Jiang, H.-L. Curcumin-Coordinated Nanoparticles with Improved Stability for Reactive Oxygen Species-Responsive Drug Delivery in Lung Cancer Therapy. Int. J. Nanomed. 2017, 12, 855–869. [Google Scholar] [CrossRef]
- Sirohi, V.K.; Popli, P.; Sankhwar, P.; Kaushal, J.B.; Gupta, K.; Manohar, M.; Dwivedi, A. Curcumin Exhibits Anti-Tumor Effect and Attenuates Cellular Migration via Slit-2 Mediated down-Regulation of SDF-1 and CXCR4 in Endometrial Adenocarcinoma Cells. J. Nutr. Biochem. 2017, 44, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Gao, Q.; Chen, K.; Wang, Y.; Chen, L.; Li, X.U. Curcumin Suppresses Migration and Invasion of Human Endometrial Carcinoma Cells. Oncol. Lett. 2015, 10, 1297–1302. [Google Scholar] [CrossRef]
- Sun, M.-X.; Yu, F.; Gong, M.-L.; Fan, G.-L.; Liu, C.-X. Effects of Curcumin on the Role of MMP-2 in Endometrial Cancer Cell Proliferation and Invasion. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5033–5041. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, F.; Shafiee, M.; Banikazemi, Z.; Pourhanifeh, M.H.; Khanbabaei, H.; Shamshirian, A.; Moghadam, S.A.; ArefNezhad, R.; Sahebkar, A.; Avan, A.; et al. Curcumin Inhibits NF-KB and Wnt/β-Catenin Pathways in Cervical Cancer Cells. Pathol. Pract. 2019, 215, 152556. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Kim, H.S.; Jung, E.-J.; Lee, J.Y.; Tsang, B.K.; Lim, J.M.; Song, Y.S. Curcumin Induces ER Stress-Mediated Apoptosis through Selective Generation of Reactive Oxygen Species in Cervical Cancer Cells. Mol. Carcinog. 2016, 55, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qi, M.; Li, X.; Wang, J.; Wang, M. Curcumin: A Natural Organic Component That Plays a Multi-Faceted Role in Ovarian Cancer. J. Ovarian Res. 2023, 16, 47. [Google Scholar] [CrossRef]
- Reddy, P.S.; Begum, N.; Mutha, S.; Bakshi, V. Beneficial Effect of Curcumin in Letrozole Induced Polycystic Ovary Syndrome. Asian Pac. J. Reprod. 2016, 5, 116–122. [Google Scholar] [CrossRef]
- Mohammadi, S.; Kayedpoor, P.; Karimzadeh-Bardei, L.; Nabiuni, M. The Effect of Curcumin on TNF-α, IL-6 and CRP Expression in a Model of Polycystic Ovary Syndrome as an Inflammation State. J. Reprod. Infertil. 2017, 18, 352–360. [Google Scholar]
- Abuelezz, N.Z.; Shabana, M.E.; Abdel-Mageed, H.M.; Rashed, L.; Morcos, G.N.B. Nanocurcumin Alleviates Insulin Resistance and Pancreatic Deficits in Polycystic Ovary Syndrome Rats: Insights on PI3K/AkT/MTOR and TNF-α Modulations. Life Sci. 2020, 256, 118003. [Google Scholar] [CrossRef]
- Jamilian, M.; Foroozanfard, F.; Kavossian, E.; Aghadavod, E.; Shafabakhsh, R.; Hoseini, A.; Asemi, Z. Effects of Curcumin on Body Weight, Glycemic Control and Serum Lipids in Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Nutr. ESPEN 2020, 36, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Sohaei, S.; Amani, R.; Tarrahi, M.J.; Ghasemi-Tehrani, H. The Effects of Curcumin Supplementation on Glycemic Status, Lipid Profile and Hs-CRP Levels in Overweight/Obese Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Complement. Ther. Med. 2019, 47, 102201. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Lee, E.N.; Park, J.K.; Lee, J.-R.; Kim, J.-H.; Choi, H.-J.; Kim, B.-S.; Lee, H.-W.; Lee, K.-S.; Yoon, S. Curcumin Attenuates TNF-α-Induced Expression of Intercellular Adhesion Molecule-1, Vascular Cell Adhesion Molecule-1 and Proinflammatory Cytokines in Human Endometriotic Stromal Cells. Phyther. Res. 2012, 26, 1037–1047. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Mei, S.; Cheng, W.; Ni, Z.; Yu, C. Curcumin Treats Endometriosis in Mice by the HIF Signaling Pathway. Am. J. Transl. Res. 2022, 14, 2184–2198. [Google Scholar] [PubMed]
- Chowdhury, I.; Banerjee, S.; Driss, A.; Xu, W.; Mehrabi, S.; Nezhat, C.; Sidell, N.; Taylor, R.N.; Thompson, W.E. Curcumin Attenuates Proangiogenic and Proinflammatory Factors in Human Eutopic Endometrial Stromal Cells through the NF-ΚB Signaling Pathway. J. Cell. Physiol. 2019, 234, 6298–6312. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Wei, Y.-X.; Zhou, Q.; Zhang, Y.; Guo, X.-P.; Zhang, J. Inhibitory Effect of Curcumin in Human Endometriosis Endometrial Cells via Downregulation of Vascular Endothelial Growth Factor. Mol. Med. Rep. 2017, 16, 5611–5617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, H.; Yu, Z.; Peng, H.-Y.; Zhang, C. Curcumin Inhibits Endometriosis Endometrial Cells by Reducing Estradiol Production. Iran. J. Reprod. Med. 2013, 11, 415–422. [Google Scholar] [PubMed]
- Jana, S.; Paul, S.; Swarnakar, S. Curcumin as Anti-Endometriotic Agent: Implication of MMP-3 and Intrinsic Apoptotic Pathway. Biochem. Pharmacol. 2012, 83, 797–804. [Google Scholar] [CrossRef]
- Fadin, M.; Nicoletti, M.C.; Pellizzato, M.; Accardi, M.; Baietti, M.G.; Fratter, A. Effectiveness of the Integration of Quercetin, Turmeric, and N-Acetylcysteine in Reducing Inflammation and Pain Associated with Endometriosis. In-Vitro and in-Vivo Studies. Minerva Ginecol. 2020, 72, 285–291. [Google Scholar] [CrossRef]
- Meresman, G.F.; Götte, M.; Laschke, M.W. Plants as Source of New Therapies for Endometriosis: A Review of Preclinical and Clinical Studies. Hum. Reprod. Update 2021, 27, 367–392. [Google Scholar] [CrossRef]
- Zhu, J.-J.; Jiang, J.-G. Pharmacological and Nutritional Effects of Natural Coumarins and Their Structure–Activity Relationships. Mol. Nutr. Food Res. 2018, 62, e1701073. [Google Scholar] [CrossRef] [PubMed]
- Pivetta, T.; Valletta, E.; Ferino, G.; Isaia, F.; Pani, A.; Vascellari, S.; Castellano, C.; Demartin, F.; Cabiddu, M.G.; Cadoni, E. Novel Coumarins and Related Copper Complexes with Biological Activity: DNA Binding, Molecular Docking and in Vitro Antiproliferative Activity. J. Inorg. Biochem. 2017, 177, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Zafar, A.; Naseem, I. Redox Cycling of Copper by Coumarin-Di (2-Picolyl) Amine Hybrid Molecule Leads to ROS-Mediated Modulation of Redox Scavengers, DNA Damage and Cell Death in Diethylnitrosamine Induced Hepatocellular Carcinoma. Bioorg. Chem. 2020, 99, 103818. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, I.; Babak, M.V.; Spengler, G.; Hammerstad, M.; Popovic-Bijelic, A.; Shova, S.; Büchel, G.E.; Darvasiova, D.; Rapta, P.; Arion, V.B. Coumarin-Based Triapine Derivatives and Their Copper (II) Complexes: Synthesis, Cytotoxicity and MR2 RNR Inhibition Activity. Biomolecules 2021, 11, 862. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Tang, J.; Gu, Z.; Sun, L.; Wei, H.; Wang, Y.; Yang, S.; Chi, X.; Xu, L. Crystal Structure, in Vitro Cytotoxicity, DNA Binding and DFT Calculations of New Copper (II) Complexes with Coumarin-Amide Ligand. J. Inorg. Biochem. 2023, 238, 112030. [Google Scholar] [CrossRef]
- An, G.; Park, S.; Lee, M.; Lim, W.; Song, G. Antiproliferative Effect of 4-Methylumbelliferone in Epithelial Ovarian Cancer Cells Is Mediated by Disruption of Intracellular Homeostasis and Regulation of PI3K/AKT and MAPK Signaling. Pharmaceutics 2020, 12, 640. [Google Scholar] [CrossRef]
- Liang, J.; Zhou, J.; Xu, Y.; Huang, X.; Wang, X.; Huang, W.; Li, H. Osthole Inhibits Ovarian Carcinoma Cells through LC3-Mediated Autophagy and GSDME-Dependent Pyroptosis except for Apoptosis. Eur. J. Pharmacol. 2020, 874, 172990. [Google Scholar] [CrossRef]
- Che, Y.; Li, J.; Li, Z.; Li, J.; Wang, S.; Yan, Y.; Zou, K.; Zou, L. Osthole Enhances Antitumor Activity and Irradiation Sensitivity of Cervical Cancer Cells by Suppressing ATM/NF-ΚB Signaling. Oncol. Rep. 2018, 40, 737–747. [Google Scholar] [CrossRef]
- Su, J.; Zhang, F.; Li, X.; Liu, Z. Osthole Promotes the Suppressive Effects of Cisplatin on NRF2 Expression to Prevent Drug-Resistant Cervical Cancer Progression. Biochem. Biophys. Res. Commun. 2019, 514, 510–517. [Google Scholar] [CrossRef]
- Zhu, M.-L.; Li, J.-C.; Wang, L.; Zhong, X.; Zhang, Y.-W.; Tan, R.-Z.; Wang, H.-L.; Fan, J.-M.; Wang, L. Decursin Inhibits the Growth of HeLa Cervical Cancer Cells through PI3K/Akt Signaling. J. Asian Nat. Prod. Res. 2021, 23, 584–595. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, L.; Sun, X.; Zeng, F.; Pan, Q.; Xue, M. Scopoletin Exerts Anticancer Effects on Human Cervical Cancer Cell Lines by Triggering Apoptosis, Cell Cycle Arrest, Inhibition of Cell Invasion and PI3K/AKT Signalling Pathway. J BUON 2019, 24, 997–1002. [Google Scholar] [PubMed]
- Ma, T.; Liu, P.; Wei, J.; Zhao, M.; Yao, X.; Luo, X.; Xu, S. Imperatorin Alleviated Endometriosis by Inhibiting the Activation of PI3K/Akt/NF-ΚB Pathway in Rats. Life Sci. 2021, 274, 119291. [Google Scholar] [CrossRef] [PubMed]
- Abizadeh, M.; Novin, M.G.; Amidi, F.; Ziaei, S.A.; Abdollahifar, M.A.; Nazarian, H. Potential of Auraptene in Improvement of Oocyte Maturation, Fertilization Rate, and Inflammation in Polycystic Ovary Syndrome Mouse Model. Reprod. Sci. 2020, 27, 1742–1751. [Google Scholar] [CrossRef]
- Kim, Y.H.; Lee, S.Y.; Kim, E.Y.; Kim, K.H.; Koong, M.K.; Lee, K.A. The Antioxidant Auraptene Improves Aged Oocyte Quality and Embryo Development in Mice. Antioxidants 2023, 12, 87. [Google Scholar] [CrossRef] [PubMed]
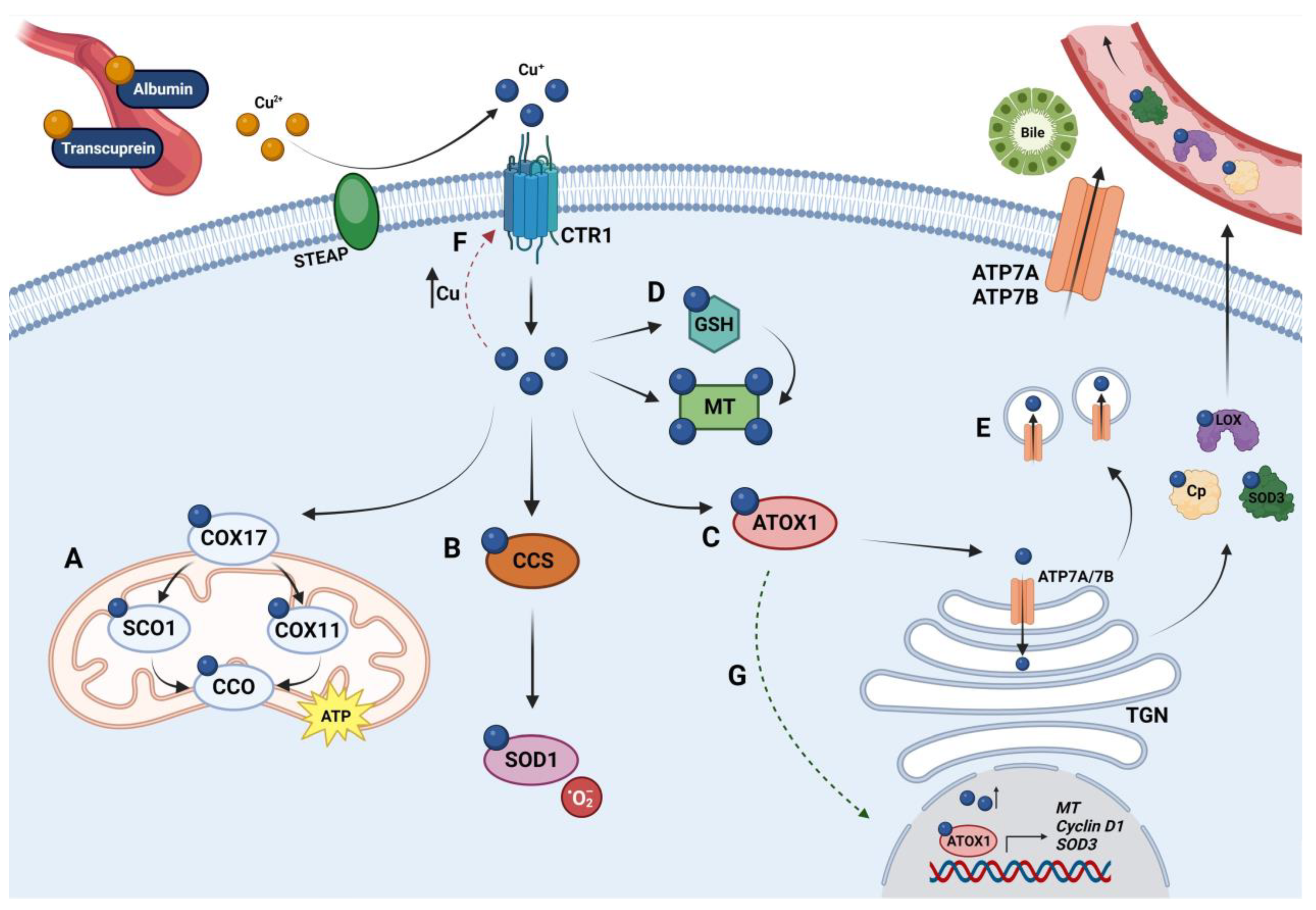
| Cuproenzyme | Function |
|---|---|
| LOX | Required for the formation of the extracellular matrix. |
| SOD | Catalyzes the conversion of superoxide radicals to molecular oxygen and hydrogen peroxide. |
| Cp | Multicopper ferroxidase; principal Cu carrier in serum. |
| Hephaestin | Multicopper ferroxidase. It supports the transportation of Fe released from intestinal enterocytes. |
| CCO | Electron transfer protein. It catalyzes ATP production. |
| Tyrosinase | Catalyzes phenol oxidation; it is required for melanin synthesis, a fundamental pigment for hair, skin, and eyes. |
| DβH | Oxidoreductase. It catalyzes the conversion of dopamine to epinephrine. |
| MEK | Kinases that belong to the mitogen-activated protein kinase cascade and that mainly promote cell proliferation and survival. |
| ULK1/2 | Autophagy-initiating kinases. |
| MEMO1 | Regulation of cell motility and ROS production. |
| Compound Name | Chemical Formula | Structural Formula 1 |
|---|---|---|
| D-penicillamine | C5H11NO2S |  |
| Trientine | C6H18N4 | 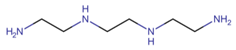 |
| Tetrathiomolybdate | MoS4 | 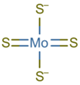 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Conforti, R.A.; Delsouc, M.B.; Zorychta, E.; Telleria, C.M.; Casais, M. Copper in Gynecological Diseases. Int. J. Mol. Sci. 2023, 24, 17578. https://doi.org/10.3390/ijms242417578
Conforti RA, Delsouc MB, Zorychta E, Telleria CM, Casais M. Copper in Gynecological Diseases. International Journal of Molecular Sciences. 2023; 24(24):17578. https://doi.org/10.3390/ijms242417578
Chicago/Turabian StyleConforti, Rocío A., María B. Delsouc, Edith Zorychta, Carlos M. Telleria, and Marilina Casais. 2023. "Copper in Gynecological Diseases" International Journal of Molecular Sciences 24, no. 24: 17578. https://doi.org/10.3390/ijms242417578
APA StyleConforti, R. A., Delsouc, M. B., Zorychta, E., Telleria, C. M., & Casais, M. (2023). Copper in Gynecological Diseases. International Journal of Molecular Sciences, 24(24), 17578. https://doi.org/10.3390/ijms242417578






