Green Biologics: Harnessing the Power of Plants to Produce Pharmaceuticals
Abstract
1. Introduction
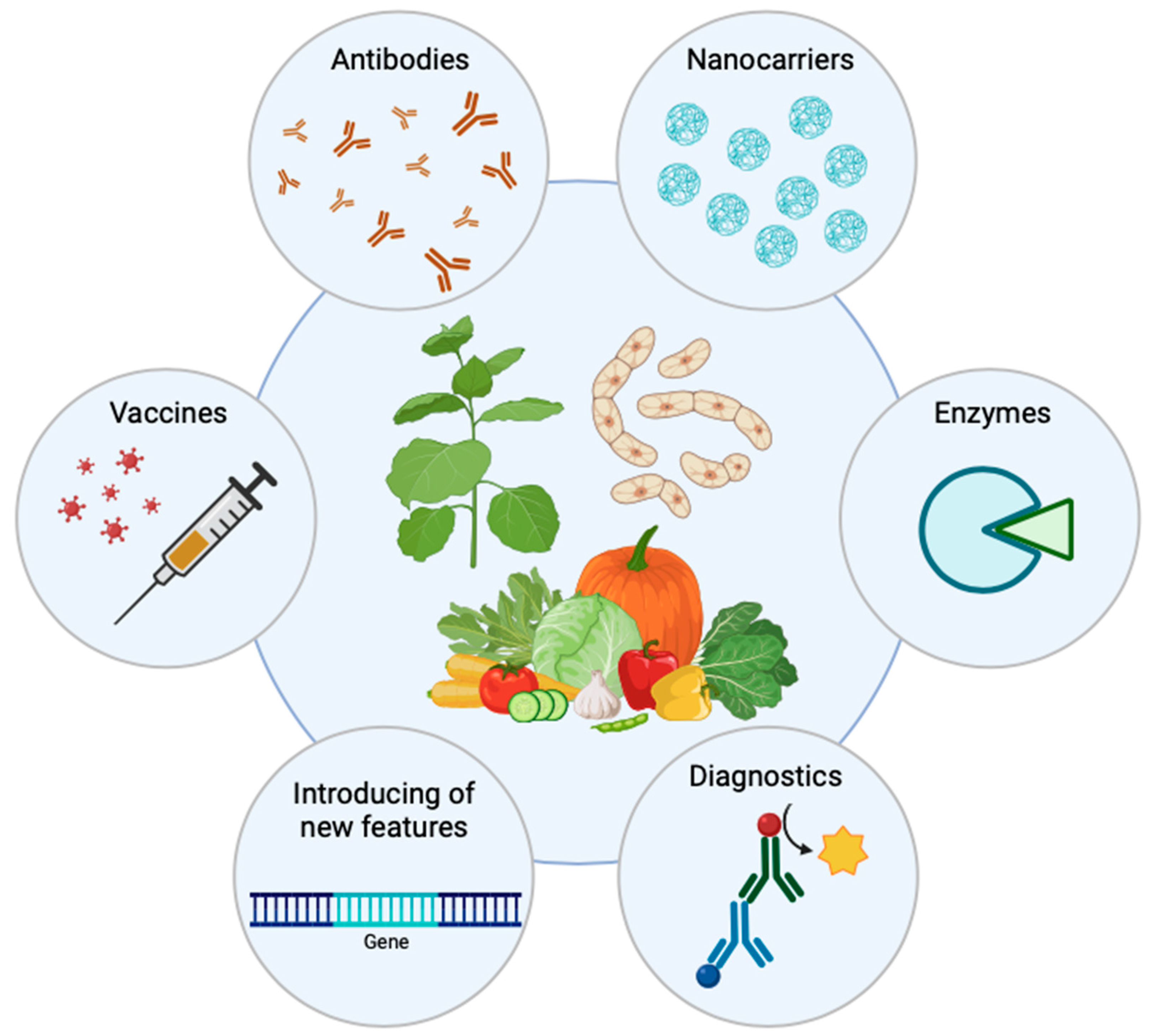
2. Types of Plant-Derived Biologics
2.1. Antibodies and Antibody Fragments
2.2. Vaccines and VLPs
2.3. Therapeutic Enzymes
2.4. Receptor Modulators
2.5. Small Molecules
2.6. Bioactive Proteins from Plants
3. Strengths, Weaknesses, Opportunities, and Threats (SWOT) Analysis of Biologics
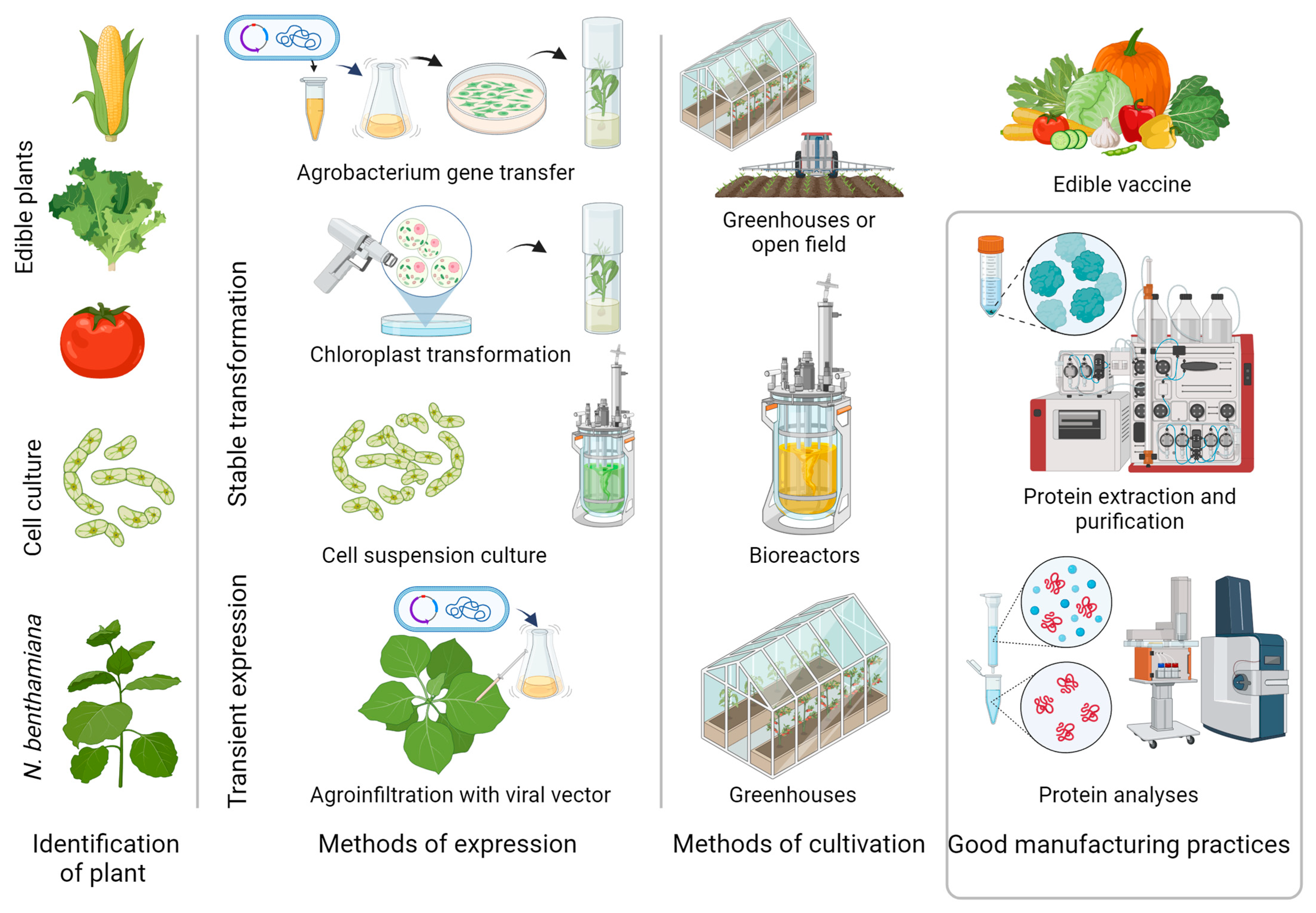
4. Manufacturing of Plant-Based Production Systems
5. Challenges in Producing and Using Plant-Derived Biologics
5.1. Complex Protein Expression
5.2. Post-Translational Modifications
5.3. Post-Translational Gene Silencing (PTGS)
5.4. Proteolytic Degradation of Recombinant Proteins
5.5. Downstream Processing
6. Novel Strategies for Improving Production and Efficacy
6.1. Protein Engineering
6.2. Synthetic Biology Approaches
6.3. Metabolic Engineering
6.4. Advancing Plant Molecular Biology
6.5. Field Scale-Up and Commercialization
7. Applications of Plant-Derived Recombinant Proteins
7.1. Therapeutic Applications of Plant-Derived Recombinant Proteins for Human and Animal Health
7.2. Biologics Produced in Space
7.3. Industrial and Agricultural Applications of Plant-Derived Recombinant Proteins
8. Regulatory Considerations for Plant-Derived Biologics
8.1. Current Regulatory Landscape
- Source of Plant Expression System: Detailed scrutiny of plant species, genetic modifications, and expression vectors used in production.
- Characterization of Biologics: Thorough evaluation of structural attributes, purity, potency, and stability.
- Variability in Expression: Implementation of measures to ensure consistent production and quality despite inherent variability within plants.
- Co-expression of Plant-Specific Proteins/Allergens: Rigorous characterization is required to identify and mitigate potential risks associated with unintended co-expression.
8.2. Future Regulatory Requirements
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvarez, M.A. Plants for Health: From Secondary Metabolites to Molecular Farming Chapter 1. In Plant Biotechnology for Health: From Secondary Metabolites to Molecular Farming; Alvarez, M.A., Ed.; Springer International Publishing: Cham, Switzerland, 2014; pp. 1–2. ISBN 978-3-319-05771-2. [Google Scholar]
- Rybicki, E.P.; Chikwamba, R.; Koch, M.; Rhodes, J.I.; Groenewald, J.-H. Plant-Made Therapeutics: An Emerging Platform in South Africa. Biotechnol. Adv. 2012, 30, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Sil, B.; Jha, S. Plants: The Future Pharmaceutical Factory. Am. J. Plant Sci. 2014, 5, 319–327. [Google Scholar] [CrossRef][Green Version]
- Buyel, J.F. Plant Molecular Farming—Integration and Exploitation of Side Streams to Achieve Sustainable Biomanufacturing. Front. Plant Sci. 2019, 9, 1893. [Google Scholar] [CrossRef] [PubMed]
- Zahmanova, G.; Aljabali, A.A.; Takova, K.; Toneva, V.; Tambuwala, M.M.; Andonov, A.P.; Lukov, G.L.; Minkov, I. The Plant Viruses and Molecular Farming: How Beneficial They Might Be for Human and Animal Health? Int. J. Mol. Sci. 2023, 24, 1533. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaraj, B.; Bulaon, C.J.I.; Phoolcharoen, W. Plant Molecular Farming: A Viable Platform for Recombinant Biopharmaceutical Production. Plants 2020, 9, E842. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Buyel, J.F. Molecular Farming—The Slope of Enlightenment. Biotechnol. Adv. 2020, 40, 107519. [Google Scholar] [CrossRef]
- Chung, Y.H.; Church, D.; Koellhoffer, E.C.; Osota, E.; Shukla, S.; Rybicki, E.P.; Pokorski, J.K.; Steinmetz, N.F. Integrating Plant Molecular Farming and Materials Research for Next-Generation Vaccines. Nat. Rev. Mater 2022, 7, 372–388. [Google Scholar] [CrossRef]
- Buyel, J.F.; Twyman, R.M.; Fischer, R. Extraction and Downstream Processing of Plant-Derived Recombinant Proteins. Biotechnol. Adv. 2015, 33, 902–913. [Google Scholar] [CrossRef]
- Buyel, J.F.; Stöger, E.; Bortesi, L. Targeted Genome Editing of Plants and Plant Cells for Biomanufacturing. Transgenic Res. 2021, 30, 401–426. [Google Scholar] [CrossRef]
- Wiltschi, B.; Cernava, T.; Dennig, A.; Galindo Casas, M.; Geier, M.; Gruber, S.; Haberbauer, M.; Heidinger, P.; Herrero Acero, E.; Kratzer, R.; et al. Enzymes Revolutionize the Bioproduction of Value-Added Compounds: From Enzyme Discovery to Special Applications. Biotechnol. Adv. 2020, 40, 107520. [Google Scholar] [CrossRef]
- Schillberg, S.; Finnern, R. Plant Molecular Farming for the Production of Valuable Proteins—Critical Evaluation of Achievements and Future Challenges. J. Plant Physiol. 2021, 258–259, 153359. [Google Scholar] [CrossRef]
- Nandi, S.; Kwong, A.T.; Holtz, B.R.; Erwin, R.L.; Marcel, S.; McDonald, K.A. Techno-Economic Analysis of a Transient Plant-Based Platform for Monoclonal Antibody Production. MABS 2016, 8, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Santi, L.; Zhang, C. Plant-Made Biologics. Biomed Res. Int. 2014, 2014, 418064. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lai, H. Plant-Derived Virus-like Particles as Vaccines. Hum. Vaccines Immunother. 2013, 9, 26–49. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.N.; Chan, A.T.C. Biologics and Biosimilars: What, Why and How? ESMO Open 2017, 2, e000180. [Google Scholar] [CrossRef]
- Zimran, A.; Gonzalez-Rodriguez, D.E.; Abrahamov, A.; Cooper, P.A.; Varughese, S.; Giraldo, P.; Petakov, M.; Tan, E.S.; Chertkoff, R. Long-Term Safety and Efficacy of Taliglucerase Alfa in Pediatric Gaucher Disease Patients Who Were Treatment-Naïve or Previously Treated with Imiglucerase. Blood Cells Mol. Dis. 2018, 68, 163–172. [Google Scholar] [CrossRef]
- van der Veen, S.J.; Hollak, C.E.M.; van Kuilenburg, A.B.P.; Langeveld, M. Developments in the Treatment of Fabry Disease. J Inherit. Metab. Dis. 2020, 43, 908–921. [Google Scholar] [CrossRef]
- Daniell, H.; Nair, S.K.; Esmaeili, N.; Wakade, G.; Shahid, N.; Ganesan, P.K.; Islam, M.R.; Shepley-McTaggart, A.; Feng, S.; Gary, E.N.; et al. Debulking SARS-CoV-2 in Saliva Using Angiotensin Converting Enzyme 2 in Chewing Gum to Decrease Oral Virus Transmission and Infection. Mol. Ther. 2022, 30, 1966–1978. [Google Scholar] [CrossRef]
- SemBioSys. Eligible to Proceed With Phase I/II Plant-Produced Insulin Trial After Submission of IND. Available online: https://www.ots.at/presseaussendung/OTE_20080916_OTE0007/sembiosys-eligible-to-proceed-with-phase-iii-plant-produced-insulin-trial-after-submission-of-ind (accessed on 17 September 2023).
- Ward, B.J.; Séguin, A.; Couillard, J.; Trépanier, S.; Landry, N. Phase III: Randomized Observer-Blind Trial to Evaluate Lot-to-Lot Consistency of a New Plant-Derived Quadrivalent Virus like Particle Influenza Vaccine in Adults 18–49 Years of Age. Vaccine 2021, 39, 1528–1533. [Google Scholar] [CrossRef]
- Health Canada Medicago Covifenz COVID-19 Vaccine. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/vaccines/medicago.html (accessed on 10 September 2023).
- iBio. iBio Reports Successful Preclinical Immunization Studies with Next-Gen Nucleocapsid COVID-19 Vaccine Candidate. Available online: https://ir.ibioinc.com/news-events/press-releases/detail/163/ibio-reports-successful-preclinical-immunization-studies (accessed on 17 September 2023).
- Shanmugaraj, B.; Khorattanakulchai, N.; Paungpin, W.; Akkhawattanangkul, Y.; Manopwisedjaroen, S.; Thitithanyanont, A.; Phoolcharoen, W. Immunogenicity and Efficacy of Recombinant Subunit SARS-CoV-2 Vaccine Candidate in the Syrian Hamster Model. Biotechnol. Rep. 2023, 37, e00779. [Google Scholar] [CrossRef] [PubMed]
- Govea-Alonso, D.O.; Malla, A.; Bolaños-Martínez, O.C.; Vimolmangkang, S.; Rosales-Mendoza, S. An Algae-Made RBD from SARS-CoV-2 Is Immunogenic in Mice. Pharmaceuticals 2022, 15, 1298. [Google Scholar] [CrossRef] [PubMed]
- Vermij, P.; Waltz, E. USDA Approves the First Plant-Based Vaccine. Nat. Biotechnol. 2006, 24, 234. [Google Scholar]
- Magnusdottir, A.; Vidarsson, H.; Björnsson, J.M.; Örvar, B.L. Barley Grains for the Production of Endotoxin-Free Growth Factors. Trends Biotechnol. 2013, 31, 572–580. [Google Scholar] [CrossRef]
- Edgue, G.; Twyman, R.M.; Beiss, V.; Fischer, R.; Sack, M. Antibodies from Plants for Bionanomaterials. WIREs Nanomed. Nanobiotechnology 2017, 9, e1462. [Google Scholar] [CrossRef] [PubMed]
- PREVAIL II Writing Group; Multi-National PREVAIL II Study Team; Davey, R.T., Jr.; Dodd, L.; Proschan, M.A.; Neaton, J.; Neuhaus Nordwall, J.; Koopmeiners, J.S.; Beigel, J.; Tierney, J.; et al. A Randomized, Controlled Trial of ZMapp for Ebola Virus Infection. N. Engl. J. Med. 2016, 375, 1448–1456. [Google Scholar] [CrossRef]
- Ma, J.K.-C.; Drossard, J.; Lewis, D.; Altmann, F.; Boyle, J.; Christou, P.; Cole, T.; Dale, P.; van Dolleweerd, C.J.; Isitt, V.; et al. Regulatory Approval and a First-in-Human Phase I Clinical Trial of a Monoclonal Antibody Produced in Transgenic Tobacco Plants. Plant Biotechnol. J. 2015, 13, 1106–1120. [Google Scholar] [CrossRef]
- Shanmugaraj, B.; Rattanapisit, K.; Manopwisedjaroen, S.; Thitithanyanont, A.; Phoolcharoen, W. Monoclonal Antibodies B38 and H4 Produced in Nicotiana Benthamiana Neutralize SARS-CoV-2 in Vitro. Front. Plant Sci. 2020, 11, 589995. [Google Scholar] [CrossRef]
- Liu, C.; Morrow, K.J., Jr. Biosimilars of Monoclonal Antibodies: A Practical Guide to Manufacturing, Preclinical, and Clinical Development; John Wiley & Sons: New York, NY, USA, 2016; ISBN 1-118-66231-8. [Google Scholar]
- Oldstone, M.B.A.; Rose Oldstone, M. Chapter 5—ZMapp: The Ethics of Decision Making. In Ebola’s Curse; Oldstone, M.B.A., Rose Oldstone, M., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 49–62. ISBN 978-0-12-813888-5. [Google Scholar]
- Thuenemann, E.C.; Lenzi, P.; Love, A.J.; Taliansky, M.; Bécares, M.; Zuñiga, S.; Enjuanes, L.; Zahmanova, G.G.; Minkov, I.N.; Matić, S.; et al. The Use of Transient Expression Systems for the Rapid Production of Virus-like Particles in Plants. Curr. Pharm. Des. 2013, 19, 5564–5573. [Google Scholar] [CrossRef]
- Zahmanova, G.; Takova, K.; Valkova, R.; Toneva, V.; Minkov, I.; Andonov, A.; Lukov, G.L. Plant-Derived Recombinant Vaccines against Zoonotic Viruses. Life 2022, 12, 156. [Google Scholar] [CrossRef]
- Zahmanova, G.; Naimov, S.; Mazalovska, M.; Valkova, R.; Minkov, I. Transient Expression of Modified Hepatitis B Capsid Protein in Nicotiana Benthamiana Plants for Viral Nanoparticles Production. J. BioScience Biotechnol. 2014, 2014, 11–16. [Google Scholar]
- Ponndorf, D.; Meshcheriakova, Y.; Thuenemann, E.C.; Dobon Alonso, A.; Overman, R.; Holton, N.; Dowall, S.; Kennedy, E.; Stocks, M.; Lomonossoff, G.P.; et al. Plant-Made Dengue Virus-like Particles Produced by Co-Expression of Structural and Non-Structural Proteins Induce a Humoral Immune Response in Mice. Plant Biotechnol. J. 2021, 19, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Peyret, H.; Steele, J.F.C.; Jung, J.-W.; Thuenemann, E.C.; Meshcheriakova, Y.; Lomonossoff, G.P. Producing Vaccines against Enveloped Viruses in Plants: Making the Impossible, Difficult. Vaccines 2021, 9, 780. [Google Scholar] [CrossRef] [PubMed]
- Balke, I.; Zeltins, A. Recent Advances in the Use of Plant Virus-Like Particles as Vaccines. Viruses 2020, 12, 270. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Beiss, V.; Steinmetz, N.F. Cowpea Mosaic Virus Nanoparticles and Empty Virus-Like Particles Show Distinct but Overlapping Immunostimulatory Properties. J. Virol. 2019, 93, e00129-19. [Google Scholar] [CrossRef] [PubMed]
- Mardanova, E.S.; Takova, K.H.; Toneva, V.T.; Zahmanova, G.G.; Tsybalova, L.M.; Ravin, N.V. A Plant-Based Transient Expression System for the Rapid Production of Highly Immunogenic Hepatitis E Virus-like Particles. Biotechnol. Lett. 2020, 42, 2441–2446. [Google Scholar] [CrossRef]
- Marsian, J.; Lomonossoff, G.P. Molecular Pharming—VLPs Made in Plants. Curr. Opin. Biotechnol. 2016, 37, 201–206. [Google Scholar] [CrossRef]
- Nikitin, N.; Vasiliev, Y.; Kovalenko, A.; Ryabchevskaya, E.; Kondakova, O.; Evtushenko, E.; Karpova, O. Plant Viruses as Adjuvants for Next-Generation Vaccines and Immunotherapy. Vaccines 2023, 11, 1372. [Google Scholar] [CrossRef]
- Chung, Y.H.; Cai, H.; Steinmetz, N.F. Viral Nanoparticles for Drug Delivery, Imaging, Immunotherapy, and Theranostic Applications. Adv. Drug Deliv. Rev. 2020, 156, 214–235. [Google Scholar] [CrossRef]
- Shaaltiel, Y.; Bartfeld, D.; Hashmueli, S.; Baum, G.; Brill-Almon, E.; Galili, G.; Dym, O.; Boldin-Adamsky, S.A.; Silman, I.; Sussman, J.L.; et al. Production of Glucocerebrosidase with Terminal Mannose Glycans for Enzyme Replacement Therapy of Gaucher’s Disease Using a Plant Cell System. Plant Biotechnol. J. 2007, 5, 579–590. [Google Scholar] [CrossRef]
- Tekoah, Y.; Tzaban, S.; Kizhner, T.; Hainrichson, M.; Gantman, A.; Golembo, M.; Aviezer, D.; Shaaltiel, Y. Glycosylation and Functionality of Recombinant β-Glucocerebrosidase from Various Production Systems. Biosci. Rep. 2013, 33, e00071. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Daniell, H. Oral Delivery of Therapeutic Proteins Bioencapsulated in Plant Cells: Preclinical and Clinical Advances. Curr. Opin. Colloid Interface Sci. 2021, 54, 101452. [Google Scholar] [CrossRef] [PubMed]
- Fry, S.C. Plant Cell Wall Polymers. In Biofuels and Bioenergy; John Wiley & Sons, Ltd.: London, UK, 2017; pp. 59–87. ISBN 978-1-118-35055-3. [Google Scholar]
- Xiao, Y.; Kwon, K.-C.; Hoffman, B.E.; Kamesh, A.; Jones, N.T.; Herzog, R.W.; Daniell, H. Low Cost Delivery of Proteins Bioencapsulated in Plant Cells to Human Non-Immune or Immune Modulatory Cells. Biomaterials 2016, 80, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, A.; Mandal, A.K.; Dwivedi, K.; Kumar, V. A Cross Talk between the Immunization and Edible Vaccine: Current Challenges and Future Prospects. Life Sci. 2020, 261, 118343. [Google Scholar] [CrossRef] [PubMed]
- Iacobucci, I.; Hay Mele, B.; Cozzolino, F.; Monaco, V.; Cimmaruta, C.; Monti, M.; Andreotti, G.; Monticelli, M. Enzyme Replacement Therapy for FABRY Disease: Possible Strategies to Improve Its Efficacy. Int. J. Mol. Sci. 2023, 24, 4548. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, Y.A.; Zeidner, K.M.; Gordon, R.E.; Desnick, R.J. Fabry Disease: Preclinical Studies Demonstrate the Effectiveness of Alpha-Galactosidase A Replacement in Enzyme-Deficient Mice. Am. J. Hum. Genet 2001, 68, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Umer, M.; Kalra, D.K. Treatment of Fabry Disease: Established and Emerging Therapies. Pharmaceuticals 2023, 16, 320. [Google Scholar] [CrossRef]
- Peng, L.-H.; Gu, T.-W.; Xu, Y.; Dad, H.A.; Liu, J.-X.; Lian, J.-Z.; Huang, L.-Q. Gene Delivery Strategies for Therapeutic Proteins Production in Plants: Emerging Opportunities and Challenges. Biotechnol. Adv. 2022, 54, 107845. [Google Scholar] [CrossRef]
- Eidenberger, L.; Kogelmann, B.; Steinkellner, H. Plant-Based Biopharmaceutical Engineering. Nat. Rev. Bioeng. 2023, 1, 426–439. [Google Scholar] [CrossRef]
- Sirko, A.; Vaněk, T.; Góra-Sochacka, A.; Redkiewicz, P. Recombinant Cytokines from Plants. Int. J. Mol. Sci. 2011, 12, 3536–3552. [Google Scholar] [CrossRef]
- Rabindran, S.; Stevenson, N.; Roy, G.; Fedorkin, O.; Skarjinskaia, M.; Ensley, B.; Yusibov, V. Plant-Produced Human Growth Hormone Shows Biological Activity in a Rat Model. Biotechnol. Prog. 2009, 25, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Ikura, K.; Ueda, M.; Sasaki, R. Characterization of a Human Glycoprotein (Erythropoietin) Produced in Cultured Tobacco Cells. Plant Mol. Biol. 1995, 27, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Cheong, H. Expression and Production of Recombinant Human Interleukin-2 in Potato Plants. Protein Expr. Purif. 2002, 25, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Zhu, H.; Menassa, R.; Gyenis, L.; Richman, A.; Brandle, J. Elastin-like Polypeptide Fusions Enhance the Accumulation of Recombinant Proteins in Tobacco Leaves. Transgenic Res. 2007, 16, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Ortega, A.; Sandoval-Montes, C.; de Olivera-Flores, T.J.; Santos-Argumedo, L.; Gómez-Lim, M.Á. Expression of Functional Interleukin-12 from Mouse in Transgenic Tomato Plants. Transgenic Res. 2005, 14, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.J.; Brandsma, M.; Yin, Z.; Wang, A.; Jevnikar, A.M.; Ma, S. A Novel Platform for Biologically Active Recombinant Human Interleukin-13 Production. Plant Biotechnol. J. 2008, 6, 504–515. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, Y.-H.; Lin, Y.-M.; Rao, Q.; Zheng, G.-G.; Wu, K.-F. Expression and Production of Bioactive Human Interleukin-18 in Transgenic Tobacco Plants. Biotechnol. Lett. 2003, 25, 1629–1635. [Google Scholar] [CrossRef]
- Farran, I.; Río-Manterola, F.; Iñiguez, M.; Gárate, S.; Prieto, J.; Mingo-Castel, A.M. High-Density Seedling Expression System for the Production of Bioactive Human Cardiotrophin-1, a Potential Therapeutic Cytokine, in Transgenic Tobacco Chloroplasts. Plant Biotechnol. J. 2008, 6, 516–527. [Google Scholar] [CrossRef]
- Shin, Y.-J.; Hong, S.-Y.; Kwon, T.-H.; Jang, Y.-S.; Yang, M.-S. High Level of Expression of Recombinant Human Granulocyte-Macrophage Colony Stimulating Factor in Transgenic Rice Cell Suspension Culture. Biotechnol. Bioeng. 2003, 82, 778–783. [Google Scholar] [CrossRef]
- James, E.A.; Wang, C.; Wang, Z.; Reeves, R.; Shin, J.H.; Magnuson, N.S.; Lee, J.M. Production and Characterization of Biologically Active Human GM-CSF Secreted by Genetically Modified Plant Cells. Protein. Expr. Purif. 2000, 19, 131–138. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, N.-S.; Kwon, T.-H.; Jang, Y.-S.; Yang, M.-S. Increased Production of Human Granulocyte-Macrophage Colony Stimulating Factor (hGM-CSF) by the Addition of Stabilizing Polymer in Plant Suspension Cultures. J. Biotechnol. 2002, 96, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-S.; Kim, T.-G.; Jang, Y.-S.; Shin, Y.-J.; Kwon, T.-H.; Yang, M.-S. Amylase Gene Silencing by RNA Interference Improves Recombinant hGM-CSF Production in Rice Suspension Culture. Plant Mol. Biol. 2008, 68, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Ohya, K.; Itchoda, N.; Ohashi, K.; Onuma, M.; Sugimoto, C.; Matsumura, T. Expression of Biologically Active Human Tumor Necrosis Factor-Alpha in Transgenic Potato Plant. J. Interferon. Cytokine Res. 2002, 22, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Arlen, P.A.; Falconer, R.; Cherukumilli, S.; Cole, A.; Cole, A.M.; Oishi, K.K.; Daniell, H. Field Production and Functional Evaluation of Chloroplast-Derived Interferon-Alpha2b. Plant Biotechnol. J. 2007, 5, 511–525. [Google Scholar] [CrossRef] [PubMed]
- Potula, H.H.S.K.; Kathuria, S.R.; Ghosh, A.K.; Maiti, T.K.; Dey, S. Transient Expression, Purification and Characterization of Bioactive Human Fibroblast Growth Factor 8b in Tobacco Plants. Transgenic Res. 2008, 17, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.; Ruiz, G.; Denes, B.; Sandberg, L.; Langridge, W. Optimization of Codon Composition and Regulatory Elements for Expression of Human Insulin like Growth Factor-1 in Transgenic Chloroplasts and Evaluation of Structural Identity and Function. BMC Biotechnol. 2009, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Paddon, C.J.; Westfall, P.J.; Pitera, D.J.; Benjamin, K.; Fisher, K.; McPhee, D.; Leavell, M.D.; Tai, A.; Main, A.; Eng, D.; et al. High-Level Semi-Synthetic Production of the Potent Antimalarial Artemisinin. Nature 2013, 496, 528–532. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, Y.; Jia, H.; Han, Y.; Zheng, X.; Wang, M.; Feng, W. From Plant to Yeast—Advances in Biosynthesis of Artemisinin. Molecules 2022, 27, 6888. [Google Scholar] [CrossRef]
- Owen, C.; Patron, N.J.; Huang, A.; Osbourn, A. Harnessing Plant Metabolic Diversity. Curr. Opin. Chem. Biol. 2017, 40, 24–30. [Google Scholar] [CrossRef]
- Gerasymenko, I.; Sheludko, Y.; Fräbel, S.; Staniek, A.; Warzecha, H. Chapter Sixteen—Combinatorial Biosynthesis of Small Molecules in Plants: Engineering Strategies and Tools. In Methods in Enzymology; Schmidt-Dannert, C., Quin, M.B., Eds.; Metabolons and Supramolecular Enzyme Assemblies; Academic Press: Cambridge, MA, USA, 2019; Volume 617, pp. 413–442. [Google Scholar]
- Reed, J.; Osbourn, A. Engineering Terpenoid Production through Transient Expression in Nicotiana Benthamiana. Plant Cell Rep. 2018, 37, 1431–1441. [Google Scholar] [CrossRef]
- Stephenson, M.J.; Reed, J.; Brouwer, B.; Osbourn, A. Transient Expression in Nicotiana Benthamiana Leaves for Triterpene Production at a Preparative Scale. J. Vis. Exp. 2018, 138, e58169. [Google Scholar] [CrossRef]
- Reed, J.; Orme, A.; El-Demerdash, A.; Owen, C.; Martin, L.B.B.; Misra, R.C.; Kikuchi, S.; Rejzek, M.; Martin, A.C.; Harkess, A.; et al. Elucidation of the Pathway for Biosynthesis of Saponin Adjuvants from the Soapbark Tree. Science 2023, 379, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Caputi, L.; Franke, J.; Farrow, S.C.; Chung, K.; Payne, R.M.E.; Nguyen, T.-D.; Dang, T.-T.T.; Soares Teto Carqueijeiro, I.; Koudounas, K.; Dugé de Bernonville, T.; et al. Missing Enzymes in the Biosynthesis of the Anticancer Drug Vinblastine in Madagascar Periwinkle. Science 2018, 360, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Grzech, D.; Hong, B.; Caputi, L.; Sonawane, P.D.; O’Connor, S.E. Engineering the Biosynthesis of Late-Stage Vinblastine Precursors Precondylocarpine Acetate, Catharanthine, Tabersonine in Nicotiana Benthamiana. ACS Synth. Biol. 2022, 12, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Chun-Ting Liu, J.; De La Pena, R.; Tocol, C.; Sattely, E.S. Reconstitution of Early Paclitaxel Biosynthetic Network. bioRxiv 2023, 9, 559859. [Google Scholar] [CrossRef]
- Breitenbach Barroso Coelho, L.C.; Marcelino dos Santos Silva, P.; Felix de Oliveira, W.; de Moura, M.C.; Viana Pontual, E.; Soares Gomes, F.; Guedes Paiva, P.M.; Napoleão, T.H.; dos Santos Correia, M.T. Lectins as Antimicrobial Agents. J. Appl. Microbiol. 2018, 125, 1238–1252. [Google Scholar] [CrossRef]
- Yau, T.; Dan, X.; Ng, C.C.W.; Ng, T.B. Lectins with Potential for Anti-Cancer Therapy. Molecules 2015, 20, 3791–3810. [Google Scholar] [CrossRef]
- Mazalovska, M.; Kouokam, J.C. Plant-Derived Lectins as Potential Cancer Therapeutics and Diagnostic Tools. BioMed Res. Int. 2020, 2020, e1631394. [Google Scholar] [CrossRef]
- Fu, L.; Zhou, C.; Yao, S.; Yu, J.; Liu, B.; Bao, J. Plant Lectins: Targeting Programmed Cell Death Pathways as Antitumor Agents. Int. J. Biochem. Cell Biol. 2011, 43, 1442–1449. [Google Scholar] [CrossRef]
- Mazalovska, M.; Kouokam, J.C. Transiently Expressed Mistletoe Lectin II in Nicotiana Benthamiana Demonstrates Anticancer Activity In Vitro. Molecules 2020, 25, 2562. [Google Scholar] [CrossRef]
- Rup, B.; Alon, S.; Amit-Cohen, B.-C.; Almon, E.B.; Chertkoff, R.; Tekoah, Y.; Rudd, P.M. Immunogenicity of Glycans on Biotherapeutic Drugs Produced in Plant Expression Systems—The Taliglucerase Alfa Story. PLoS ONE 2017, 12, e0186211. [Google Scholar] [CrossRef] [PubMed]
- Wilson, I.B.; Harthill, J.E.; Mullin, N.P.; Ashford, D.A.; Altmann, F. Core Alpha1,3-Fucose Is a Key Part of the Epitope Recognized by Antibodies Reacting against Plant N-Linked Oligosaccharides and Is Present in a Wide Variety of Plant Extracts. Glycobiology 1998, 8, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Bencúrová, M.; Borth, N.; Ferko, B.; Jensen-Jarolim, E.; Altmann, F.; Hantusch, B. Immunoglobulin G Specifically Binding Plant N-Glycans with High Affinity Could Be Generated in Rabbits but Not in Mice. Glycobiology 2006, 16, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, W. Human IgE Antibodies Against Cross-Reactive Carbohydrate Determinants. In Anticarbohydrate Antibodies: From Molecular Basis to Clinical Application; Kosma, P., Müller-Loennies, S., Eds.; Springer: Vienna, Austria, 2012; pp. 181–202. ISBN 978-3-7091-0870-3. [Google Scholar]
- Aviezer, D.; Brill-Almon, E.; Shaaltiel, Y.; Hashmueli, S.; Bartfeld, D.; Mizrachi, S.; Liberman, Y.; Freeman, A.; Zimran, A.; Galun, E. A Plant-Derived Recombinant Human Glucocerebrosidase Enzyme—A Preclinical and Phase I Investigation. PLoS ONE 2009, 4, e4792. [Google Scholar] [CrossRef] [PubMed]
- Rybicki, E.P. Plant-Made Vaccines for Humans and Animals. Plant Biotechnol. J. 2010, 8, 620–637. [Google Scholar] [CrossRef] [PubMed]
- Tacket, C.O.; Mason, H.S.; Losonsky, G.; Estes, M.K.; Levine, M.M.; Arntzen, C.J. Human Immune Responses to a Novel Norwalk Virus Vaccine Delivered in Transgenic Potatoes. J. Infect. Dis. 2000, 182, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Tacket, C.O.; Mason, H.S.; Losonsky, G.; Clements, J.D.; Levine, M.M.; Arntzen, C.J. Immunogenicity in Humans of a Recombinant Bacterial Antigen Delivered in a Transgenic Potato. Nat. Med. 1998, 4, 607–609. [Google Scholar] [CrossRef]
- Tacket, C.O.; Pasetti, M.F.; Edelman, R.; Howard, J.A.; Streatfield, S. Immunogenicity of Recombinant LT-B Delivered Orally to Humans in Transgenic Corn. Vaccine 2004, 22, 4385–4389. [Google Scholar] [CrossRef]
- Kapusta, J.; Modelska, A.; Pniewski, T.; Figlerowicz, M.; Jankowski, K.; Lisowa, O.; Plucienniczak, A.; Koprowski, H.; Legocki, A.B. Oral Immunization of Human with Transgenic Lettuce Expressing Hepatitis B Surface Antigen. Adv. Exp. Med. Biol. 2001, 495, 299–303. [Google Scholar] [CrossRef]
- Wang, X.; Sherman, A.; Liao, G.; Leong, K.W.; Daniell, H.; Terhorst, C.; Herzog, R.W. Mechanism of Oral Tolerance Induction to Therapeutic Proteins. Adv. Drug Deliv. Rev. 2013, 65, 759–773. [Google Scholar] [CrossRef]
- Fukuda, K.; Ishida, W.; Harada, Y.; Wakasa, Y.; Takagi, H.; Takaiwa, F.; Fukushima, A. Prevention of Allergic Conjunctivitis in Mice by a Rice-Based Edible Vaccine Containing Modified Japanese Cedar Pollen Allergens. Br. J. Ophthalmol. 2015, 99, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Sherman, A.; Su, J.; Lin, S.; Wang, X.; Herzog, R.W.; Daniell, H. Suppression of Inhibitor Formation against FVIII in a Murine Model of Hemophilia A by Oral Delivery of Antigens Bioencapsulated in Plant Cells. Blood 2014, 124, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.W.; Zhao, D.L.; Yin, Z.Q.; Mukherjee, R.; Singh, B.; Qin, H.Y.; Stiller, C.R.; Jevnikar, A.M. Transgenic Plants Expressing Autoantigens Fed to Mice to Induce Oral Immune Tolerance. Nat. Med. 1997, 3, 793–796. [Google Scholar] [CrossRef] [PubMed]
- Tsekoa, T.L.; Singh, A.A.; Buthelezi, S.G. Molecular Farming for Therapies and Vaccines in Africa. Curr. Opin. Biotechnol. 2020, 61, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.C.-J.; Yao, J.; Zhang, W. The Health Impact Fund: Making the Case for Engagement with Pharmaceutical Laboratories in Brazil, Russia, India, and China. Glob. Health 2021, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Buyel, J.F. Plants as Sources of Natural and Recombinant Anti-Cancer Agents. Biotechnol. Adv. 2018, 36, 506–520. [Google Scholar] [CrossRef]
- Ma, J.K.-C.; Chikwamba, R.; Sparrow, P.; Fischer, R.; Mahoney, R.; Twyman, R.M. Plant-Derived Pharmaceuticals--the Road Forward. Trends Plant Sci. 2005, 10, 580–585. [Google Scholar] [CrossRef]
- Shanmugaraj, B.; Malla, A.; Bulaon, C.J.I.; Phoolcharoen, W.; Phoolcharoen, N. Harnessing the Potential of Plant Expression System towards the Production of Vaccines for the Prevention of Human Papillomavirus and Cervical Cancer. Vaccines 2022, 10, 2064. [Google Scholar] [CrossRef]
- Shanmugaraj, B.; Siriwattananon, K.; Malla, A.; Phoolcharoen, W. Potential for Developing Plant-Derived Candidate Vaccines and Biologics against Emerging Coronavirus Infections. Pathogens 2021, 10, 1051. [Google Scholar] [CrossRef]
- Su, H.; van Eerde, A.; Rimstad, E.; Bock, R.; Branza-Nichita, N.; Yakovlev, I.A.; Clarke, J.L. Plant-Made Vaccines against Viral Diseases in Humans and Farm Animals. Front. Plant Sci. 2023, 14, 1170815. [Google Scholar] [CrossRef]
- Hu, H.; Steinmetz, N.F. Development of a Virus-Like Particle-Based Anti-HER2 Breast Cancer Vaccine. Cancers 2021, 13, 2909. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Beiss, V.; Wang, C.; Wang, L.; Steinmetz, N.F. Plant Viral Nanoparticle Conjugated with Anti-PD-1 Peptide for Ovarian Cancer Immunotherapy. Int. J. Mol. Sci. 2021, 22, 9733. [Google Scholar] [CrossRef] [PubMed]
- Chariou, P.L.; Beiss, V.; Ma, Y.; Steinmetz, N.F. In Situ Vaccine Application of Inactivated CPMV Nanoparticles for Cancer Immunotherapy. Mater. Adv. 2021, 2, 1644–1656. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Beiss, V.; Ho, G.W.; Fields, J.; Steinmetz, N.F.; Fiering, S. In Situ Vaccination with Cowpea Mosaic Virus Elicits Systemic Antitumor Immunity and Potentiates Immune Checkpoint Blockade. J. Immunother. Cancer 2022, 10, e005834. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Ortega-Rivera, O.A.; Volckaert, B.A.; Jung, E.; Zhao, Z.; Steinmetz, N.F. Viral Nanoparticle Vaccines against S100A9 Reduce Lung Tumor Seeding and Metastasis. Proc. Natl. Acad. Sci. USA 2023, 120, e2221859120. [Google Scholar] [CrossRef]
- Chen, Q.; Davis, K.R. The Potential of Plants as a System for the Development and Production of Human Biologics. F1000Res 2016, 5, 912. [Google Scholar] [CrossRef]
- Göritzer, K.; Grandits, M.; Grünwald-Gruber, C.; Figl, R.; Mercx, S.; Navarre, C.; Ma, J.K.-C.; Teh, A.Y.-H. Engineering the N-Glycosylation Pathway of Nicotiana Tabacum for Molecular Pharming Using CRISPR/Cas9. Front. Plant Sci. 2022, 13, 1003065. [Google Scholar] [CrossRef]
- Strasser, R.; Altmann, F.; Mach, L.; Glössl, J.; Steinkellner, H. Generation of Arabidopsis Thaliana Plants with Complex N-Glycans Lacking Beta1,2-Linked Xylose and Core Alpha1,3-Linked Fucose. FEBS Lett. 2004, 561, 132–136. [Google Scholar] [CrossRef]
- Castilho, A.; Strasser, R.; Stadlmann, J.; Grass, J.; Jez, J.; Gattinger, P.; Kunert, R.; Quendler, H.; Pabst, M.; Leonard, R.; et al. In Planta Protein Sialylation through Overexpression of the Respective Mammalian Pathway. J. Biol. Chem. 2010, 285, 15923–15930. [Google Scholar] [CrossRef]
- Streatfield, S.J. Approaches to Achieve High-Level Heterologous Protein Production in Plants. Plant Biotechnol. J. 2007, 5, 2–15. [Google Scholar] [CrossRef]
- Jutras, P.V.; D’Aoust, M.-A.; Couture, M.M.-J.; Vézina, L.-P.; Goulet, M.-C.; Michaud, D.; Sainsbury, F. Modulating Secretory Pathway pH by Proton Channel Co-Expression Can Increase Recombinant Protein Stability in Plants. Biotechnol. J. 2015, 10, 1478–1486. [Google Scholar] [CrossRef] [PubMed]
- Jutras, P.V.; Dodds, I.; van der Hoorn, R.A. Proteases of Nicotiana Benthamiana: An Emerging Battle for Molecular Farming. Curr. Opin. Biotechnol. 2020, 61, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Vamvaka, E.; Twyman, R.M.; Murad, A.M.; Melnik, S.; Teh, A.Y.-H.; Arcalis, E.; Altmann, F.; Stoger, E.; Rech, E.; Ma, J.K.C.; et al. Rice Endosperm Produces an Underglycosylated and Potent Form of the HIV-Neutralizing Monoclonal Antibody 2G12. Plant Biotechnol. J. 2016, 14, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Scotti, N.; Alagna, F.; Ferraiolo, E.; Formisano, G.; Sannino, L.; Buonaguro, L.; De Stradis, A.; Vitale, A.; Monti, L.; Grillo, S.; et al. High-Level Expression of the HIV-1 Pr55gag Polyprotein in Transgenic Tobacco Chloroplasts. Planta 2009, 229, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, J.J.; Verdonck, F.; Ehrström, A.; Peltola, M.; Siljander-Rasi, H.; Nuutila, A.M.; Oksman-Caldentey, K.-M.; Teeri, T.H.; Cox, E.; Goddeeris, B.M.; et al. F4 (K88) Fimbrial Adhesin FaeG Expressed in Alfalfa Reduces F4+ Enterotoxigenic Escherichia Coli Excretion in Weaned Piglets. Vaccine 2006, 24, 2387–2394. [Google Scholar] [CrossRef] [PubMed]
- Lamphear, B.J.; Jilka, J.M.; Kesl, L.; Welter, M.; Howard, J.A.; Streatfield, S.J. A Corn-Based Delivery System for Animal Vaccines: An Oral Transmissible Gastroenteritis Virus Vaccine Boosts Lactogenic Immunity in Swine. Vaccine 2004, 22, 2420–2424. [Google Scholar] [CrossRef]
- Thanavala, Y.; Mahoney, M.; Pal, S.; Scott, A.; Richter, L.; Natarajan, N.; Goodwin, P.; Arntzen, C.J.; Mason, H.S. Immunogenicity in Humans of an Edible Vaccine for Hepatitis B. PNAS 2005, 102, 3378–3382. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, N. On the Way to Commercializing Plant Cell Culture Platform for Biopharmaceuticals: Present Status and Prospect. Pharm. Bioprocess. 2014, 2, 499–518. [Google Scholar] [CrossRef]
- Mor, T.S. Molecular Pharming’s Foot in the FDA’s Door: Protalix’s Trailblazing Story. Biotechnol. Lett. 2015, 37, 2147–2150. [Google Scholar] [CrossRef]
- Gasdaska, J.R.; Spencer, D.; Dickey, L. Advantages of Therapeutic Protein Production in the Aquatic Plant Lemna. Bioprocess. J. 2003, 2, 49–56. [Google Scholar] [CrossRef]
- Buntru, M.; Vogel, S.; Spiegel, H.; Schillberg, S. Tobacco BY-2 Cell-Free Lysate: An Alternative and Highly-Productive Plant-Based in Vitro Translation System. BMC Biotechnol. 2014, 14, 37. [Google Scholar] [CrossRef] [PubMed]
- Bally, J.; Jung, H.; Mortimer, C.; Naim, F.; Philips, J.G.; Hellens, R.; Bombarely, A.; Goodin, M.M.; Waterhouse, P.M. The Rise and Rise of Nicotiana Benthamiana: A Plant for All Reasons. Annu. Rev. Phytopathol. 2018, 56, 405–426. [Google Scholar] [CrossRef] [PubMed]
- Molina-Hidalgo, F.J.; Vazquez-Vilar, M.; D’Andrea, L.; Demurtas, O.C.; Fraser, P.; Giuliano, G.; Bock, R.; Orzáez, D.; Goossens, A. Engineering Metabolism in Nicotiana Species: A Promising Future. Trends. Biotechnol. 2021, 39, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Schiermeyer, A. Optimizing Product Quality in Molecular Farming. Curr. Opin. Biotechnol. 2020, 61, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Mardanova, E.S.; Blokhina, E.A.; Tsybalova, L.M.; Peyret, H.; Lomonossoff, G.P.; Ravin, N.V. Efficient Transient Expression of Recombinant Proteins in Plants by the Novel pEff Vector Based on the Genome of Potato Virus X. Front. Plant Sci. 2017, 8, 247. [Google Scholar] [CrossRef] [PubMed]
- Klimyuk, V.; Pogue, G.; Herz, S.; Butler, J.; Haydon, H. Production of Recombinant Antigens and Antibodies in Nicotiana Benthamiana Using ‘Magnifection’ Technology: GMP-Compliant Facilities for Small- and Large-Scale Manufacturing. In Plant Viral Vectors; Palmer, K., Gleba, Y., Eds.; Current Topics in Microbiology and Immunology; Springer: Berlin, Heidelberg, 2014; pp. 127–154. ISBN 978-3-642-40829-8. [Google Scholar]
- Goodin, M.M.; Zaitlin, D.; Naidu, R.A.; Lommel, S.A. Nicotiana Benthamiana: Its History and Future as a Model for Plant-Pathogen Interactions. Mol. Plant Microbe Interact. 2008, 21, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Palmer, K.; Gleba, Y. Plant Viral Vectors; Springer: Berlin/Heidelberg, Germany, 2013; Volume 375, ISBN 3-642-40829-X. [Google Scholar]
- Takamatsu, N.; Ishikawa, M.; Meshi, T.; Okada, Y. Expression of Bacterial Chloramphenicol Acetyltransferase Gene in Tobacco Plants Mediated by TMV-RNA. EMBO J. 1987, 6, 307–311. [Google Scholar] [CrossRef]
- Howell, S.H.; Walker, L.L.; Dudley, R.K. Cloned Cauliflower Mosaic Virus DNA Infects Turnips (Brassica Rapa). Science 1980, 208, 1265–1267. [Google Scholar] [CrossRef]
- Lico, C.; Chen, Q.; Santi, L. Viral Vectors for Production of Recombinant Proteins in Plants. J. Cell Physiol. 2008, 216, 366–377. [Google Scholar] [CrossRef]
- Grimsley, N.; Hohn, B.; Hohn, T.; Walden, R. “Agroinfection,” an Alternative Route for Viral Infection of Plants by Using the Ti Plasmid. Proc. Natl. Acad. Sci. USA 1986, 83, 3282–3286. [Google Scholar] [CrossRef]
- Giritch, A.; Marillonnet, S.; Engler, C.; van Eldik, G.; Botterman, J.; Klimyuk, V.; Gleba, Y. Rapid High-Yield Expression of Full-Size IgG Antibodies in Plants Coinfected with Noncompeting Viral Vectors. Proc. Natl. Acad. Sci. USA 2006, 103, 14701–14706. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Thuenemann, E.C.; Sainsbury, F.; Lomonossoff, G.P. Virus-Derived Vectors for the Expression of Multiple Proteins in Plants. In Recombinant Proteins from Plants: Methods and Protocols; MacDonald, J., Kolotilin, I., Menassa, R., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2016; pp. 39–54. ISBN 978-1-4939-3289-4. [Google Scholar]
- Peyret, H.; Lomonossoff, G.P. When Plant Virology Met Agrobacterium: The Rise of the Deconstructed Clones. Plant Biotechnol. J. 2015, 13, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Marillonnet, S.; Giritch, A.; Gils, M.; Kandzia, R.; Klimyuk, V.; Gleba, Y. In Planta Engineering of Viral RNA Replicons: Efficient Assembly by Recombination of DNA Modules Delivered by Agrobacterium. Proc. Natl. Acad. Sci. USA 2004, 101, 6852–6857. [Google Scholar] [CrossRef] [PubMed]
- Pinneh, E.C.; van Dolleweerd, C.J.; Göritzer, K.; Drake, P.M.W.; Ma, J.K.-C.; Teh, A.Y.-H. Multiple Gene Expression in Plants Using MIDAS-P, a Versatile Type II Restriction-Based Modular Expression Vector. Biotechnol. Bioeng. 2022, 119, 1660–1672. [Google Scholar] [CrossRef] [PubMed]
- Castilho, A.; Neumann, L.; Daskalova, S.; Mason, H.S.; Steinkellner, H.; Altmann, F.; Strasser, R. Engineering of Sialylated Mucin-Type O-Glycosylation in Plants. J. Biol. Chem. 2012, 287, 36518–36526. [Google Scholar] [CrossRef] [PubMed]
- Castilho, A.; Gattinger, P.; Grass, J.; Jez, J.; Pabst, M.; Altmann, F.; Gorfer, M.; Strasser, R.; Steinkellner, H. N-Glycosylation Engineering of Plants for the Biosynthesis of Glycoproteins with Bisected and Branched Complex N-Glycans. Glycobiology 2011, 21, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Stoddard, T.J.; Demorest, Z.L.; Lavoie, P.-O.; Luo, S.; Clasen, B.M.; Cedrone, F.; Ray, E.E.; Coffman, A.P.; Daulhac, A.; et al. Multiplexed, Targeted Gene Editing in Nicotiana Benthamiana for Glyco-Engineering and Monoclonal Antibody Production. Plant Biotechnol. J. 2016, 14, 533–542. [Google Scholar] [CrossRef]
- Jansing, J.; Sack, M.; Augustine, S.M.; Fischer, R.; Bortesi, L. CRISPR/Cas9-Mediated Knockout of Six Glycosyltransferase Genes in Nicotiana Benthamiana for the Production of Recombinant Proteins Lacking β-1,2-Xylose and Core α-1,3-Fucose. Plant Biotechnol. J. 2019, 17, 350–361. [Google Scholar] [CrossRef]
- Heenatigala, P.P.M.; Yang, J.; Bishopp, A.; Sun, Z.; Li, G.; Kumar, S.; Hu, S.; Wu, Z.; Lin, W.; Yao, L.; et al. Development of Efficient Protocols for Stable and Transient Gene Transformation for Wolffia Globosa Using Agrobacterium. Front. Chem. 2018, 6, 227. [Google Scholar] [CrossRef]
- Jones, H.D.; Sparks, C.A. Stable Transformation of Plants. Methods Mol. Biol. 2009, 513, 111–130. [Google Scholar] [CrossRef]
- Daniell, H.; Jin, S.; Zhu, X.-G.; Gitzendanner, M.A.; Soltis, D.E.; Soltis, P.S. Green Giant—A Tiny Chloroplast Genome with Mighty Power to Produce High-Value Proteins: History and Phylogeny. Plant Biotechnol. J. 2021, 19, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Daniell, H. Chloroplast Vector Systems for Biotechnology Applications. Plant Physiol. 2007, 145, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Meyers, B.; Zaltsman, A.; Lacroix, B.; Kozlovsky, S.V.; Krichevsky, A. Nuclear and Plastid Genetic Engineering of Plants: Comparison of Opportunities and Challenges. Biotechnol. Adv. 2010, 28, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Warnock, J.N.; Daigre, C.; Al-Rubeai, M. Introduction to Viral Vectors. Methods Mol. Biol. 2011, 737, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Ueki, S.; Magori, S.; Lacroix, B.; Citovsky, V. Transient Gene Expression in Epidermal Cells of Plant Leaves by Biolistic DNA Delivery. Methods Mol. Biol. 2013, 940, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Nosaki, S.; Hoshikawa, K.; Ezura, H.; Miura, K. Transient Protein Expression Systems in Plants and Their Applications. Plant Biotechnol. 2021, 38, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Hellens, R.P.; Allan, A.C.; Friel, E.N.; Bolitho, K.; Grafton, K.; Templeton, M.D.; Karunairetnam, S.; Gleave, A.P.; Laing, W.A. Transient Expression Vectors for Functional Genomics, Quantification of Promoter Activity and RNA Silencing in Plants. Plant Methods 2005, 1, 13. [Google Scholar] [CrossRef]
- Santos, R.B.; Abranches, R.; Fischer, R.; Sack, M.; Holland, T. Putting the Spotlight Back on Plant Suspension Cultures. Front. Plant Sci. 2016, 7, 297. [Google Scholar] [CrossRef]
- Borisjuk, N.V.; Borisjuk, L.G.; Logendra, S.; Petersen, F.; Gleba, Y.; Raskin, I. Production of Recombinant Proteins in Plant Root Exudates. Nat. Biotechnol. 1999, 17, 466–469. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of Genome Editing Technology in the Targeted Therapy of Human Diseases: Mechanisms, Advances and Prospects. Sig. Transduct. Target Ther. 2020, 5, 1. [Google Scholar] [CrossRef]
- Strasser, R. Plant Glycoengineering for Designing Next-Generation Vaccines and Therapeutic Proteins. Biotechnol. Adv. 2023, 67, 108197. [Google Scholar] [CrossRef] [PubMed]
- Kalyanpur, M. Downstream Processing in the Biotechnology Industry. Mol. Biotechnol 2002, 22, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, A. Continuous Downstream Processing of Biopharmaceuticals. Trends Biotechnol. 2013, 31, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.L.; Langer, R. Formulation and Delivery of Proteins and Peptides: Design and Development Strategies; ACS Publications: Washington, DC, USA, 1994; ISBN 1947-5918. [Google Scholar]
- Anselmo, A.C.; Gokarn, Y.; Mitragotri, S. Non-Invasive Delivery Strategies for Biologics. Nat. Rev. Drug Discov. 2019, 18, 19–40. [Google Scholar] [CrossRef] [PubMed]
- Sarrion-Perdigones, A.; Vazquez-Vilar, M.; Palací, J.; Castelijns, B.; Forment, J.; Ziarsolo, P.; Blanca, J.; Granell, A.; Orzaez, D. GoldenBraid 2.0: A Comprehensive DNA Assembly Framework for Plant Synthetic Biology. Plant Physiol. 2013, 162, 1618–1631. [Google Scholar] [CrossRef] [PubMed]
- van Dolleweerd, C.J.; Teh, A.Y.-H.; Banyard, A.C.; Both, L.; Lotter-Stark, H.C.T.; Tsekoa, T.; Phahladira, B.; Shumba, W.; Chakauya, E.; Sabeta, C.T.; et al. Engineering, Expression in Transgenic Plants and Characterisation of E559, a Rabies Virus-Neutralising Monoclonal Antibody. J. Infect. Dis. 2014, 210, 200–208. [Google Scholar] [CrossRef]
- Benchabane, M.; Goulet, C.; Rivard, D.; Faye, L.; Gomord, V.; Michaud, D. Preventing Unintended Proteolysis in Plant Protein Biofactories. Plant Biotechnol. J. 2008, 6, 633–648. [Google Scholar] [CrossRef]
- Palaniswamy, H.; Syamaladevi, D.P.; Mohan, C.; Philip, A.; Petchiyappan, A.; Narayanan, S. Vacuolar Targeting of R-Proteins in Sugarcane Leads to Higher Levels of Purifiable Commercially Equivalent Recombinant Proteins in Cane Juice. Plant Biotechnol. J. 2016, 14, 791–807. [Google Scholar] [CrossRef]
- Margolin, E.; Oh, Y.J.; Verbeek, M.; Naude, J.; Ponndorf, D.; Meshcheriakova, Y.A.; Peyret, H.; van Diepen, M.T.; Chapman, R.; Meyers, A.E.; et al. Co-Expression of Human Calreticulin Significantly Improves the Production of HIV Gp140 and Other Viral Glycoproteins in Plants. Plant Biotechnol. J. 2020, 18, 2109–2117. [Google Scholar] [CrossRef]
- Shin, Y.-J.; König-Beihammer, J.; Vavra, U.; Schwestka, J.; Kienzl, N.F.; Klausberger, M.; Laurent, E.; Grünwald-Gruber, C.; Vierlinger, K.; Hofner, M.; et al. N-Glycosylation of the SARS-CoV-2 Receptor Binding Domain Is Important for Functional Expression in Plants. Front. Plant Sci. 2021, 12, 689104. [Google Scholar] [CrossRef]
- Sainsbury, F. Innovation in Plant-Based Transient Protein Expression for Infectious Disease Prevention and Preparedness. Curr Opin Biotechnol 2020, 61, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, X.; Fan, B.; Zhu, C.; Chen, Z. Maximizing the Production of Recombinant Proteins in Plants: From Transcription to Protein Stability. Int. J. Mol. Sci. 2022, 23, 13516. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Matsumura, T. Repression of the DCL2 and DCL4 Genes in Nicotiana Benthamiana Plants for the Transient Expression of Recombinant Proteins. J. Biosci. Bioeng. 2017, 124, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Atsumi, G. CRISPR/Cas9-Mediated Knockout of the RDR6 Gene in Nicotiana Benthamiana for Efficient Transient Expression of Recombinant Proteins. Planta 2019, 250, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Ludman, M.; Burgyán, J.; Fátyol, K. Crispr/Cas9 Mediated Inactivation of Argonaute 2 Reveals Its Differential Involvement in Antiviral Responses. Sci. Rep. 2017, 7, 1010. [Google Scholar] [CrossRef] [PubMed]
- Boivin, E.B.; Lepage, E.; Matton, D.P.; De Crescenzo, G.; Jolicoeur, M. Transient Expression of Antibodies in Suspension Plant Cell Suspension Cultures Is Enhanced When Co-Transformed with the Tomato Bushy Stunt Virus P19 Viral Suppressor of Gene Silencing. Biotechnol. Prog. 2010, 26, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Garabagi, F.; Gilbert, E.; Loos, A.; McLean, M.D.; Hall, J.C. Utility of the P19 Suppressor of Gene-Silencing Protein for Production of Therapeutic Antibodies in Nicotiana Expression Hosts. Plant Biotechnol. J. 2012, 10, 1118–1128. [Google Scholar] [CrossRef]
- Pillay, P.; Schlüter, U.; van Wyk, S.; Kunert, K.J.; Vorster, B.J. Proteolysis of Recombinant Proteins in Bioengineered Plant Cells. Bioengineered 2014, 5, 15–20. [Google Scholar] [CrossRef]
- Puchol Tarazona, A.A.; Maresch, D.; Grill, A.; Bakalarz, J.; Torres Acosta, J.A.; Castilho, A.; Steinkellner, H.; Mach, L. Identification of Two Subtilisin-like Serine Proteases Engaged in the Degradation of Recombinant Proteins in Nicotiana Benthamiana. FEBS Lett. 2021, 595, 379–388. [Google Scholar] [CrossRef]
- Chauhan, V.M.; Zhang, H.; Dalby, P.A.; Aylott, J.W. Advancements in the Co-Formulation of Biologic Therapeutics. J. Control. Release 2020, 327, 397–405. [Google Scholar] [CrossRef]
- Daugherty, A.L.; Mrsny, R.J. Formulation and Delivery Issues for Monoclonal Antibody Therapeutics. Adv. Drug Deliv. Rev. 2006, 58, 686–706. [Google Scholar] [CrossRef] [PubMed]
- Łojewska, E.; Kowalczyk, T.; Olejniczak, S.; Sakowicz, T. Extraction and Purification Methods in Downstream Processing of Plant-Based Recombinant Proteins. Protein. Expr. Purif. 2016, 120, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Castells-Graells, R.; Lomonossoff, G.P. Plant-based Production Can Result in Covalent Cross-linking of Proteins. Plant Biotechnol. J. 2021, 19, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Liang, Y.; Zhong, X.; Pan, Z.; Huang, L.; Zhang, H.; Xu, Y.; Zhou, W.; Liu, Z. Codon Optimization with Deep Learning to Enhance Protein Expression. Sci. Rep. 2020, 10, 17617. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Timko, M.P. Improving Protein Quantity and Quality—The Next Level of Plant Molecular Farming. Int. J. Mol. Sci. 2022, 23, 1326. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, D.T.; Lian, J.; Zhao, H. Protein Design for Pathway Engineering. J. Struct. Biol. 2014, 185, 234–242. [Google Scholar] [CrossRef]
- Yu, K.; Ang, K.S.; Lee, D.-Y. Synthetic Gene Design Using Codon Optimization On-Line (COOL). Methods Mol. Biol. 2017, 1472, 13–34. [Google Scholar] [CrossRef]
- Wang, X.; Karki, U.; Abeygunaratne, H.; UnnoldCofre, C.; Xu, J. Plant Cell-Secreted Stem Cell Factor Stimulates Expansion and Differentiation of Hematopoietic Stem Cells. Process Biochem. 2021, 100, 39–48. [Google Scholar] [CrossRef]
- Boehm, V.; Haberman, N.; Ottens, F.; Ule, J.; Gehring, N.H. 3′ UTR Length and Messenger Ribonucleoprotein Composition Determine Endocleavage Efficiencies at Termination Codons. Cell Rep. 2014, 9, 555–568. [Google Scholar] [CrossRef]
- Diamos, A.G.; Mason, H.S. Chimeric 3′ Flanking Regions Strongly Enhance Gene Expression in Plants. Plant Biotechnol. J. 2018, 16, 1971–1982. [Google Scholar] [CrossRef]
- Diamos, A.G.; Rosenthal, S.H.; Mason, H.S. 5′ and 3′ Untranslated Regions Strongly Enhance Performance of Geminiviral Replicons in Nicotiana Benthamiana Leaves. Front Plant Sci. 2016, 7, 200. [Google Scholar] [CrossRef] [PubMed]
- Brockman, I.M.; Prather, K.L.J. Dynamic Metabolic Engineering: New Strategies for Developing Responsive Cell Factories. Biotechnol. J. 2015, 10, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Munshi, A.; Sharma, V. Chapter 8—Omics and Edible Vaccines. In Omics Technologies and Bio-Engineering; Barh, D., Azevedo, V., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 129–141. ISBN 978-0-12-815870-8. [Google Scholar]
- Roychowdhury, R.; Das, S.P.; Gupta, A.; Parihar, P.; Chandrasekhar, K.; Sarker, U.; Kumar, A.; Ramrao, D.P.; Sudhakar, C. Multi-Omics Pipeline and Omics-Integration Approach to Decipher Plant’s Abiotic Stress Tolerance Responses. Genes 2023, 14, 1281. [Google Scholar] [CrossRef] [PubMed]
- Amer, B.; Baidoo, E.E.K. Omics-Driven Biotechnology for Industrial Applications. Front. Bioeng. Biotechnol. 2021, 9, 613307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, C.; Yu, C.; Dong, J.; Hu, J. Integration of Multi-Omics Technologies for Crop Improvement: Status and Prospects. Front Bioinform 2022, 2, 1027457. [Google Scholar] [CrossRef] [PubMed]
- Buntru, M.; Vogel, S.; Stoff, K.; Spiegel, H.; Schillberg, S. A Versatile Coupled Cell-Free Transcription–Translation System Based on Tobacco BY-2 Cell Lysates. Biotechnol. Bioeng. 2015, 112, 867–878. [Google Scholar] [CrossRef] [PubMed]
- LenioBio. Available online: https://www.leniobio.com/ (accessed on 30 November 2023).
- Vasilev, N.; Smales, C.M.; Schillberg, S.; Fischer, R.; Schiermeyer, A. Developments in the Production of Mucosal Antibodies in Plants. Biotechnol. Adv. 2016, 34, 77–87. [Google Scholar] [CrossRef]
- Schillberg, S.; Raven, N.; Fischer, R.; Twyman, R.M.; Schiermeyer, A. Molecular Farming of Pharmaceutical Proteins Using Plant Suspension Cell and Tissue Cultures. Curr. Pharm. Des. 2013, 19, 5531–5542. [Google Scholar] [CrossRef]
- Spiegel, H.; Stöger, E.; Twyman, R.M.; Buyel, J.F. Current Status and Perspectives of the Molecular Farming Landscape. In Molecular Pharming; John Wiley & Sons, Ltd.: London, UK, 2018; pp. 1–23. ISBN 978-1-118-80151-2. [Google Scholar]
- Schillberg, S.; Raven, N.; Spiegel, H.; Rasche, S.; Buntru, M. Critical Analysis of the Commercial Potential of Plants for the Production of Recombinant Proteins. Front. Plant Sci. 2019, 10, 720. [Google Scholar] [CrossRef]
- Rybicki, E.P. Plant Molecular Farming of Virus-like Nanoparticles as Vaccines and Reagents. WIREs Nanomed. Nanobiotechnology 2020, 12, e1587. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.; Singh, A. Plant Molecular Farming: A Marvelous Biotechnological Approach in Agricultural Production. In Agriculture, Livestock Production and Aquaculture: Advances for Smallholder Farming Systems Volume 2; Kumar, A., Kumar, P., Singh, S.S., Trisasongko, B.H., Rani, M., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 97–111. ISBN 978-3-030-93262-6. [Google Scholar]
- Benvenuto, E.; Broer, I.; D’Aoust, M.-A.; Hitzeroth, I.; Hundleby, P.; Menassa, R.; Oksman-Caldentey, K.-M.; Peyret, H.; Salgueiro, S.; Saxena, P.; et al. Plant Molecular Farming in the Wake of the Closure of Medicago Inc. Nat. Biotechnol. 2023, 41, 893–894. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, V.; Barani, M.; Amiri, M.S.; Taghavizadeh Yazdi, M.E.; Hassanisaadi, M.; Rahdar, A.; Varma, R.S. Applications of Plant-Based Nanoparticles in Nanomedicine: A Review. Sustain. Chem. Pharm. 2022, 25, 100606. [Google Scholar] [CrossRef]
- Jantan, I.; Ahmad, W.; Bukhari, S.N.A. Plant-Derived Immunomodulators: An Insight on Their Preclinical Evaluation and Clinical Trials. Front. Plant Sci. 2015, 6, 655. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, S.B.; Samanta, D. Engineering Protein-Based Therapeutics through Structural and Chemical Design. Nat. Commun. 2023, 14, 2411. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic Peptides: Current Applications and Future Directions. Sig. Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- McCormick, A.A. Tobacco Derived Cancer Vaccines for Non-Hodgkin’s Lymphoma: Perspectives and Progress. Hum. Vaccin. 2011, 7, 305–312. [Google Scholar] [CrossRef]
- Boonyayothin, W.; Kobtrakul, K.; Khositanon, P.; Vimolmangkang, S.; Phoolcharoen, W. Development of a Plant-Produced Recombinant Monoclonal Antibody against Δ-9-Tetrahydrocannabinol (Δ9-THC) for Immunoassay Application. Biotechnol. Rep. 2022, 34, e00725. [Google Scholar] [CrossRef]
- Jugler, C.; Grill, F.J.; Eidenberger, L.; Karr, T.L.; Grys, T.E.; Steinkellner, H.; Lake, D.F.; Chen, Q. Humanization and Expression of IgG and IgM Antibodies in Plants as Potential Diagnostic Reagents for Valley Fever. Front. Plant Sci. 2022, 13, 925008. [Google Scholar] [CrossRef]
- Capell, T.; Twyman, R.M.; Armario-Najera, V.; Ma, J.K.-C.; Schillberg, S.; Christou, P. Potential Applications of Plant Biotechnology against SARS-CoV-2. Trends Plant Sci. 2020, 25, 635–643. [Google Scholar] [CrossRef]
- He, J.; Lai, H.; Brock, C.; Chen, Q. A Novel System for Rapid and Cost-Effective Production of Detection and Diagnostic Reagents of West Nile Virus in Plants. J. Biomed. Biotechnol. 2011, 2012, e106783. [Google Scholar] [CrossRef]
- Takova, K.; Koynarski, T.; Minkov, G.; Toneva, V.; Mardanova, E.; Ravin, N.; Lukov, G.L.; Zahmanova, G. Development and Optimization of an Enzyme Immunoassay to Detect Serum Antibodies against the Hepatitis E Virus in Pigs, Using Plant-Derived ORF2 Recombinant Protein. Vaccines 2021, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Mazalovska, M.; Varadinov, N.; Koynarski, T.; Minkov, I.; Teoharov, P.; Lomonossoff, G.P.; Zahmanova, G. Detection of Serum Antibodies to Hepatitis E Virus Based on HEV Genotype 3 ORF2 Capsid Protein Expressed in Nicotiana Benthamiana. Ann. Lab. Med. 2017, 37, 313–319. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stander, J.; Mbewana, S.; Meyers, A.E. Plant-Derived Human Vaccines: Recent Developments. BioDrugs 2022, 36, 573–589. [Google Scholar] [CrossRef] [PubMed]
- Yusibov, V.; Rabindran, S. Recent Progress in the Development of Plant Derived Vaccines. Expert. Rev. Vaccines 2008, 7, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Mardanova, E.S.; Kotlyarov, R.Y.; Stuchinskaya, M.D.; Nikolaeva, L.I.; Zahmanova, G.; Ravin, N.V. High-Yield Production of Chimeric Hepatitis E Virus-Like Particles Bearing the M2e Influenza Epitope and Receptor Binding Domain of SARS-CoV-2 in Plants Using Viral Vectors. Int. J. Mol. Sci. 2022, 23, 15684. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-W.; Zahmanova, G.; Minkov, I.; Lomonossoff, G.P. Plant-Based Expression and Characterization of SARS-CoV-2 Virus-like Particles Presenting a Native Spike Protein. Plant Biotechnol. J. 2022, 20, 1363–1372. [Google Scholar] [CrossRef]
- Pang, E.L.; Peyret, H.; Ramirez, A.; Loh, H.-S.; Lai, K.-S.; Fang, C.-M.; Rosenberg, W.M.; Lomonossoff, G.P. Epitope Presentation of Dengue Viral Envelope Glycoprotein Domain III on Hepatitis B Core Protein Virus-Like Particles Produced in Nicotiana Benthamiana. Front. Plant Sci. 2019, 10, 455. [Google Scholar] [CrossRef]
- Thuenemann, E.C.; Meyers, A.E.; Verwey, J.; Rybicki, E.P.; Lomonossoff, G.P. A Method for Rapid Production of Heteromultimeric Protein Complexes in Plants: Assembly of Protective Bluetongue Virus-like Particles. Plant Biotechnol. J. 2013, 11, 839–846. [Google Scholar] [CrossRef]
- Zahmanova, G.G.; Mazalovska, M.; Takova, K.H.; Toneva, V.T.; Minkov, I.N.; Mardanova, E.S.; Ravin, N.V.; Lomonossoff, G.P. Rapid High-Yield Transient Expression of Swine Hepatitis E ORF2 Capsid Proteins in Nicotiana Benthamiana Plants and Production of Chimeric Hepatitis E Virus-Like Particles Bearing the M2e Influenza Epitope. Plants 2020, 9, 29. [Google Scholar] [CrossRef]
- Noris, E.; Poli, A.; Cojoca, R.; Rittà, M.; Cavallo, F.; Vaglio, S.; Matic, S.; Landolfo, S. A Human Papillomavirus 8 E7 Protein Produced in Plants Is Able to Trigger the Mouse Immune System and Delay the Development of Skin Lesions. Arch. Virol. 2011, 156, 587–595. [Google Scholar] [CrossRef]
- Blokhina, E.A.; Mardanova, E.S.; Stepanova, L.A.; Tsybalova, L.M.; Ravin, N.V. Plant-Produced Recombinant Influenza A Virus Candidate Vaccine Based on Flagellin Linked to Conservative Fragments of M2 Protein and Hemagglutintin. Plants 2020, 9, 162. [Google Scholar] [CrossRef] [PubMed]
- Streatfield, S.J. Plant-Based Vaccines for Animal Health. Rev. Sci. Tech. 2005, 24, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Santi, L. Plant Derived Veterinary Vaccines. Vet. Res. Commun. 2009, 33, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Zahmanova, G.; Takova, K.; Tonova, V.; Koynarski, T.; Lukov, L.L.; Minkov, I.; Pishmisheva, M.; Kotsev, S.; Tsachev, I.; Baymakova, M.; et al. The Re-Emergence of Hepatitis E Virus in Europe and Vaccine Development. Viruses 2023, 15, 1558. [Google Scholar] [CrossRef] [PubMed]
- Oluwayelu, D.O.; Adebiyi, A.I. Plantibodies in Human and Animal Health: A Review. Afr. Health Sci. 2016, 16, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Zahmanova, G.; Falzarano, D.; Naimov, S.; Kostova, M.; Boncheva, R.; Dukiandjiev, S.; Minkov, I. Anton Andonov Oral Immunization With Truncated Hepatitis B Virus Nucleocapsid Expressed In Transgenic Potatoes. Comptes. Rendus. De L’acade’mie Bulg. Des. Sci. 2008, 61, 1293–1300. [Google Scholar]
- Dubey, K.K.; Luke, G.A.; Knox, C.; Kumar, P.; Pletschke, B.I.; Singh, P.K.; Shukla, P. Vaccine and Antibody Production in Plants: Developments and Computational Tools. Brief. Funct. Genom. 2018, 17, 295–307. [Google Scholar] [CrossRef]
- Steele, J.F.C.; Peyret, H.; Saunders, K.; Castells-Graells, R.; Marsian, J.; Meshcheriakova, Y.; Lomonossoff, G.P. Synthetic Plant Virology for Nanobiotechnology and Nanomedicine. WIREs Nanomed. Nanobiotechnology 2017, 9, e1447. [Google Scholar] [CrossRef]
- Czapar, A.E.; Steinmetz, N.F. Plant Viruses and Bacteriophages for Drug Delivery in Medicine and Biotechnology. Curr. Opin. Chem. Biol. 2017, 38, 108–116. [Google Scholar] [CrossRef]
- Chariou, P.L.; Steinmetz, N.F. Delivery of Pesticides to Plant Parasitic Nematodes Using Tobacco Mild Green Mosaic Virus as a Nanocarrier. ACS Nano 2017, 11, 4719–4730. [Google Scholar] [CrossRef]
- Cao, J.; Guenther, R.H.; Sit, T.L.; Lommel, S.A.; Opperman, C.H.; Willoughby, J.A. Development of Abamectin Loaded Plant Virus Nanoparticles for Efficacious Plant Parasitic Nematode Control. ACS Appl. Mater. Interfaces 2015, 7, 9546–9553. [Google Scholar] [CrossRef] [PubMed]
- Shelar, A.; Nile, S.H.; Singh, A.V.; Rothenstein, D.; Bill, J.; Xiao, J.; Chaskar, M.; Kai, G.; Patil, R. Recent Advances in Nano-Enabled Seed Treatment Strategies for Sustainable Agriculture: Challenges, Risk Assessment, and Future Perspectives. Nano-Micro Lett. 2023, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- McNulty, M.J.; Xiong, Y. (Mary); Yates, K.; Karuppanan, K.; Hilzinger, J.M.; Berliner, A.J.; Delzio, J.; Arkin, A.P.; Lane, N.E.; Nandi, S.; et al. Molecular Pharming to Support Human Life on the Moon, Mars, and Beyond. Crit. Rev. Biotechnol. 2021, 41, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Jegannathan, K.R.; Nielsen, P.H. Environmental Assessment of Enzyme Use in Industrial Production—A Literature Review. J. Clean. Prod. 2013, 42, 228–240. [Google Scholar] [CrossRef]
- Stein, H.; Wilensky, M.; Tsafrir, Y.; Rosenthal, M.; Amir, R.; Avraham, T.; Ofir, K.; Dgany, O.; Yayon, A.; Shoseyov, O. Production of Bioactive, Post-Translationally Modified, Heterotrimeric, Human Recombinant Type-I Collagen in Transgenic Tobacco. Biomacromolecules 2009, 10, 2640–2645. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Gührs, K.H.; Grosse, F.; Conrad, U. Production of Spider Silk Proteins in Tobacco and Potato. Nat. Biotechnol. 2001, 19, 573–577. [Google Scholar] [CrossRef]
- Weichert, N.; Hauptmann, V.; Menzel, M.; Schallau, K.; Gunkel, P.; Hertel, T.C.; Pietzsch, M.; Spohn, U.; Conrad, U. Transglutamination Allows Production and Characterization of Native-Sized ELPylated Spider Silk Proteins from Transgenic Plants. Plant Biotechnol. J. 2014, 12, 265–275. [Google Scholar] [CrossRef]
- Kučuk, N.; Primožič, M.; Knez, Ž.; Leitgeb, M. Sustainable Biodegradable Biopolymer-Based Nanoparticles for Healthcare Applications. Int. J. Mol. Sci. 2023, 24, 3188. [Google Scholar] [CrossRef]
- Tschofen, M.; Knopp, D.; Hood, E.; Stöger, E. Plant Molecular Farming: Much More than Medicines. Annu. Rev. Anal. Chem. 2016, 9, 271–294. [Google Scholar] [CrossRef]
- Velázquez-De Lucio, B.S.; Hernández-Domínguez, E.M.; Villa-García, M.; Díaz-Godínez, G.; Mandujano-Gonzalez, V.; Mendoza-Mendoza, B.; Álvarez-Cervantes, J. Exogenous Enzymes as Zootechnical Additives in Animal Feed: A Review. Catalysts 2021, 11, 851. [Google Scholar] [CrossRef]
- Nellore, R. Regulatory Considerations for Biosimilars. Perspect. Clin. Res. 2010, 1, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, C.F.; Wang, X.-Z.M.; Conlon, H.D.; Anderson, S.; Ryan, A.M.; Bose, A. Biosimilars: Key Regulatory Considerations and Similarity Assessment Tools. Biotechnol. Bioeng. 2017, 114, 2696–2705. [Google Scholar] [CrossRef] [PubMed]
- Minghetti, P.; Rocco, P.; Cilurzo, F.; Vecchio, L.D.; Locatelli, F. The Regulatory Framework of Biosimilars in the European Union. Drug Discov. Today 2012, 17, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Downey, R.K. Environmental Effects of Transgenic Plants: The Scope and Adequacy of Regulation. Crop. Sci. 2003, 43, 447–449. [Google Scholar] [CrossRef]
- Vulto, A.G.; Jaquez, O.A. The Process Defines the Product: What Really Matters in Biosimilar Design and Production? Rheumatol. 2017, 56, iv14–iv29. [Google Scholar] [CrossRef]
- Morrow, T.; Felcone, L.H. Defining the Difference: What Makes Biologics Unique. Biotechnol. Healthc. 2004, 1, 24. [Google Scholar]
- EFSA Panel on Genetically Modified Organisms (GMO); Mullins, E.; Bresson, J.-L.; Dalmay, T.; Dewhurst, I.C.; Epstein, M.M.; George Firbank, L.; Guerche, P.; Hejatko, J.; Naegeli, H.; et al. Scientific Opinion on Development Needs for the Allergenicity and Protein Safety Assessment of Food and Feed Products Derived from Biotechnology. EFSA J. 2022, 20, e07044. [Google Scholar] [CrossRef]
- Giraldo, P.A.; Shinozuka, H.; Spangenberg, G.C.; Cogan, N.O.I.; Smith, K.F. Safety Assessment of Genetically Modified Feed: Is There Any Difference From Food? Front. Plant Sci. 2019, 10, 1592. [Google Scholar] [CrossRef]
- Zagalo, D.M.; Simões, S.; Sousa, J. Regulatory Science Approach in Pharmaceutical Development of Follow-on Versions of Non-Biological Complex Drug Products. J. Pharm. Sci. 2022, 111, 2687–2713. [Google Scholar] [CrossRef]
- Drezner, D.W. Globalization, Harmonization, and Competition: The Different Pathways to Policy Convergence. J. Eur. Public Policy 2005, 12, 841–859. [Google Scholar] [CrossRef]
- WHO. WHO Global Model Regulatory Framework for Medical Devices Including in Vitro Diagnostic Medical Devices. Available online: https://www.who.int/publications-detail-redirect/9789241512350 (accessed on 7 September 2023).
| Biologic/Application | Plant/Expression System | Status/Research Findings/Reference/Website Links | Company |
|---|---|---|---|
| Therapeutics | |||
| β-Glucocerebrosidase Gaucher’s disease, enzyme replacement | Daucus carota, carrot cell culture, stable gene expression ProCellEx® | Elelyso™ has been approved by the USDA and EMA as the first biologic on the market [18]. https://protalix.com/ (accessed 10 October 2023) | Protalix BioTherapeutics Inc., Karmiel, Israel/Pfizer |
| Pegunigalsidase alfa Fabry disease | ProCellEx® Stable gene expression | ELFABRIOTM has been approved for the USA and EU [19]. https://protalix.com/ (accessed on 10 October 2023) | Protalix BioTherapeutics Inc., Israel |
| Clinical-grade plant material of the virus-trapping proteins CTB-ACE2 SARS-CoV-2 | Lactuca sativa, Lettuce stable chloroplast expression | Clinical trial phase I/II of chewing gum containing proteins CTB-ACE2 (angiotensin-converting enzyme 2 fused to the non-toxic cholera toxin subunit B) [20]. | University of Pennsylvania |
| Uricase (PRX 115) Severe Gout | ProCellEx® Stable gene expression | Clinical trial phase I https://protalix.com/( accessed on 10 October 2023) | Protalix BioTherapeutics Inc., Carmiel, Israel |
| Insulin Diabetes | Helianthus annuus, (sunflower)/stable gene expression | Clinical trial phase I/II [21] http://www.sembiosys.com/ (accessed on 9 October 2023) | SemBioSys Genetics Inc., Calgary, Alberta, Canada; in 2012, SemBioSys terminated its operation |
| Lactoferrin VEN120 Inflammatory bowel disease VEN BETA E. coli gastroenteritis | Oryza sativa, Transgenic rice seeds, cell culture media | Products on the market https://ventria.com/ (accessed on 9 October 2023) | Ventria Bioscience, Junction City, KS, USA |
| Allergens bioparticles | N. benthamiana Transient expression | Production of high-quality (“natural-like”) allergens and other sophisticated proteins for pharmaceutical purposes https://angany.com/ (accessed on 9 October 2023) | Angani Inc., Québec, QC, Canada |
| Vaccines | |||
| Influenza VLPs vaccine Seasonal flue | N. benthamiana Transient expression | Clinical trial phase III [22] | Medicago Inc., Quebec City, QC, Canada; the company closed in 2023. |
| Covifenz® SARS-CoV-2 vaccine | N. benthamiana Transient expression | Authorized for use by Canada Health after successfully completed clinical trials [23]. | Medicago Inc., Quebec City, QC, Canada |
| KBP-201 with CpG oligonucleotides SARS-CoV-2 vaccine | N. benthamiana Transient expression | Clinical trial phase 1/2 https://kbio.com/ (accessed on 10 October 2023) | Kentucky BioProcessing, Owensboro, KY, USA |
| IBIO-201 IBIO-202 SARS-CoV-2 vaccine | N. benthamiana Transient expression | Preclinical studies [24] | iBio Inc., Bryan, TX, USA |
| Baiya SARS-CoV-2 Vax 1 SARS-CoV-2 vaccine | N. benthamiana Transient expression | Clinical trial phase 1 ongoing [25] https://baiyaphytopharm.com/ (accessed on 10 October 2023) | Baiya Phytopharm Co., Ltd., Bangkok, Thailand |
| SARS-CoV-2 RBD vaccine | Chlamydomonas reinhardtii, algae | SARS-CoV-2 RBD was evaluated as an oral immunogen in mice. The test immunogen was stable in freeze-dried algae biomass and able to induce mucosal responses [26]. | - |
| HERBAVAC™ CSF Green Marker Classical swine fever virus (CSFV) | N. benthamiana Transient expression | Registered by the World Organization for Animal Health (WOAH) http://bioapp.co.kr/eng/ (accessed on 10 October 2023) | BioApplications Inc., Pohang, Republic of Korea |
| Newcastle disease vaccine (in poultry) | N. tabacum sell suspension culture, stable gene expression | The first vaccine produced in plants approved by the US Food and Drug Administration for application in poultry [27]. | Dow AgroSciences LLC, Benton County, IN, USA |
| Oral delivery platform of vaccines | Chlamydomonas reinhardtii, TransAlge technology | Edible vaccine https://www.transalgae.com/( accessed on 9 October 2023) | TransAlgae, Rehovot, Israel |
| Others | |||
| Growth factors, cytokines, lectins anti-CD25 antibody | N. benthamiana Transient expression | Research reagents on the market https://ibioinc.com// (accessed on 9 October 2023) | IBio Inc., Bryan, TX, USA |
| Antibodies, viral proteins, VLPs | N. benthamiana Transient expression | Research reagents on the market https://capebiologix.com (accessed on 9 October 2023) | Cape Bio Pharma, South Africa, Cape Town, Africa |
| Antibodies, enzymes, cytokines VLPs, viral proteins | N. benthamiana Transient expression | Research reagents on the market https://www.leafexpressionsystems.com/ (accessed on 10 October 2023) | Leaf Expression Systems, Norwich, UK |
| Diagnostic antibodies, cytokines, growth factors | N. benthamiana Transient expression | Research reagents on the market https://www.agrenvec.es/ (accessed on 9 October 2023) | Agrenvec, Madrid, Spain |
| Plant virus-like particles, Alternanthera Mosaic Virus | N. benthamiana Transient expression | Research reagents on the market https://www.diamante.tech/ (accessed on 9 October 2023) | Diamante Società Benefit, Verona, Italy |
| Growth factors | Hordeum vulgare/Barley grains, stable gene expression | Cosmetics [28] https://www.orfgenetics.com/ (accessed on 10 October 2023) | ORF Genetics, Kópavogur, Iceland |
| Enzymes | Zea mays, Corn, stable gene expression | Industry https://www.infiniteenzymes.com/ (accessed on 10 October 2023) https://www.greenlab.com/#in-production (accessed on 10 October 2023) | Infiniteenzyme Inc., Jonesboro, AR, USA Greenlab, Inc., Jutland, Denmark |
| Therapeutic antibodies | |||
| Anti-Human rAntibody (BLX-301) Non-Hodgkin’s lymphoma | Lemna minor, Duckweed, LEX system Stable gene expression | Phase II; The product and duckweed production system has been abandoned. | Biolex Inc., Pittsboro, NC, USA; the company declared bankruptcy in 2012 [29] |
| ZMapp™ Anti-Ebola monoclonal antibodies | N. benthamiana Transient expression | In randomized, controlled trial, although the estimated effect of ZMapp appeared to be beneficial, the result did not meet the prespecified statistical threshold for efficacy [30]. https://mappbio.com (accessed on 10 October 2023) | Mapp Biotherapeutics, Inc., San Diego, CA, USA |
| P2G12 HIV-neutralizing human monoclonal antibody 2G12 | Nicotiana tabacum cv. Petit Havana cv. SR1, stable gene expression | Phase I clinical trial [31] | Pharma-Planta consortium, Fraunhofer IME, Schmallenberg, Germany |
| Anti-Spike antibody (mAb B38, H4) SARS-CoV-2 | N. benthamiana Transient expression | Both mAb B38 and H4 demonstrated specific binding to receptor binding domain (RBD) of SARS-CoV-2 and exhibited efficient virus neutralization activity in vitro [32].https://baiyaphytopharm.com/ (accessed on 10 October 2023) | Baiya Phytopahrn, Bangkok, Thailand |
| Plant-made monoclonal antibody against ricin exposure | vivoXPRESS® plant-based manufacturing system | www.antoxacorp.com (accessed on 10 October 2023) www.swiftpharma.eu (accessed on 10 October 2023) | AntoXa Corporation, Toronto, Ontario, Canada and SwiftPharma, Belgium |
| Strengths | Weaknesses |
| Low cost: Plants can be grown at a relatively low cost compared to other expression systems. Profit can be made if the production of recombinant protein is high, the downstream processing is efficient, and there is an opportunity to scale up the production for a short period of time. | Time consuming: The production process of plant-derived biologics can be time-consuming. |
| Scalability: Plant-based biologic production can be easily scaled up to meet demand. | Variable yields: The yields of plant-derived biologics can be highly variable. |
| Complex molecules: Plants can produce complex biological molecules with post-translational modifications. | Regulatory considerations: Plant-derived biologics are subject to regulatory scrutiny and require approval from regulatory agencies. |
| Safety: Plant-derived biologics are considered safe for human consumption and do not pose a risk of contamination. | Protein degradation: Proteases present in plants can degrade proteins during production. |
| Opportunities | Threats |
| Alternative to traditional expression systems: Plant-derived biologics have clinically improved profiles. | Intellectual property: Intellectual property rights can be a barrier to development and commercialization. |
| Unmet medical needs: Plant-derived biologics can address unmet medical needs, such as low-cost vaccines for developing countries. | Competition: The field of plant-derived biologics is highly competitive. |
| Diversification: Using plant-derived biologics diversifies biological production sources. | Public perception: The use of genetically modified plants may face skepticism. |
| Method | Description | Advantages | Disadvantages |
|---|---|---|---|
| Stable nuclear transformation [151,152] | Stable integration of the gene of interest into the plant genome, enabling long-term and consistent protein production. Agrobacterium or biolistics-mediated delivery of transgenes. | Potential for large-scale production. Suitable for biologics with high demand or requiring complex PTMs. The use of edible plant species for oral delivery and cereals for long storage at ambient temperature. | The time-consuming process of plant transformation and regeneration. Relatively lower protein yield. Potential position effect and gene silencing. Regulatory considerations and public concerns regarding GMOs. |
| Stable chloroplast transformation [153,154,155] | Each plant cell has 10,000 copies of the chloroplast genomes, which can stably integrate the gene of interest using a biolistic method of delivery. | The recombinant protein can be expressed at very high levels, up to 45% of the TP; there is no reported gene silencing; toxic proteins can be expressed successfully; more than one gene can be expressed, facilitating the production of complex proteins; and no gene flow. | A time-consuming process with low transformation frequencies, the formation of inclusion bodies, and challenges during the purification of recombinant proteins. Regulatory considerations. |
| Viral Vectors [156] | Utilization of viral vectors, such as TMV or CPMV, to enhance protein expression levels by leveraging the viral replication machinery within plants. | Increased protein yields compared to non-viral expression systems. Compatibility with both transient and stable expression approaches. | Risk of viral contamination and potential biosafety concerns. Additional steps are required for viral vector construction and handling. Potential for adverse effects on plant growth. |
| Transient Expression [157,158,159] | Rapid production of target proteins by introducing the gene of interest into plants using binary vector-based plasmids and agroinfiltration or viral vectors. | Quick and high-yield protein production. Suitable for rapid response scenarios. Flexibility and versatility in terms of the biological molecules that can be produced. | The transient nature of expression requires repeated plant agroinfiltration for continuous production. |
| Plant cell cultures [160] | Production of recombinant proteins in plant cell suspension cultures. | Potential for easy scale-up for manufacturing under aseptic conditions using classical fermentation technology. Low risk of contamination. The same regulatory requirements as mammalian cell production systems. | Slower growth and lower yields compared to microbes and mammalian cells; overall cost is medium. Plant cell cultures are characterized by heterogenicity. |
| Hairy roots Rhizobium rhizogenes [161] | Rhizosecretion of recombinant proteins in the hydroponic medium. | Secretion of the proteins into medium, facilitated purification, and improved product homogeneity. | Protein degradation, high proteolytic activity, and GMO regulatory considerations. |
| Gene Editing [162] | Precise modification of plant genomes using gene editing technologies, such as CRISPR/Cas9, to optimize protein production and reduce proteolytic degradation. | Targeted modification of specific genes or regulatory elements to enhance protein expression. Potential for multiplex gene editing to improve multiple traits simultaneously. | Technical complexity and optimization required for gene editing experiments. Potential for off-target effects and unintended genomic modifications. Regulatory considerations for GMOs. |
| Glycoengineering [163] | Elimination of unwanted glycan modifications and expression of glycosylation enzymes to provide the required specific glycans. | Production of recombinant glycoproteins with human-type glycans that resemble natural glycosylation. Eliminate unwanted glycan modifications. | Some plant species do not tolerate the engineering of glycan processing pathway, N-glycan heterogenicity, or GMO safety risks. |
| Downstream Processing [164,165] | Implementation of purification strategies to effectively remove plant-specific contaminants, ensuring stability and quality of the final product. | Improved purity and removal of unwanted plant-specific contaminants. Optimization of downstream processing for specific biological molecules. | Additional processing steps, costs, and requirements. Need for customized purification methods for different biological molecules. |
| Formulation and Delivery [166,167] | Development of innovative formulation and delivery methods to improve stability, bioavailability, and targeted delivery of plant-derived biologics. | Improved stability during storage and transportation. Enhanced bioavailability and efficacy in the target tissues or cells. Targeted delivery to specific organs or cellular compartments. | Additional costs associated with formulation and delivery systems. Potential challenges in achieving targeted delivery to specific sites. |
| Regulatory Considerations for Plant-Derived Biologics |
|---|
| 1. Source of plant expression system: Detailed information on plant species, genetic modifications, and expression vectors used in production. |
| 2. Characterization of biologics: Thorough evaluation of structure, purity, potency, and stability. |
| 3. Variability in expression: Measures to ensure consistent production and quality of biologics despite inherent variability within plants. |
| 4. Co-expression of plant-specific proteins/allergens: Identification and mitigation of potential risks associated with unintended co-expression. |
| 5. Environmental impact: Risk assessment to evaluate the potential environmental effects of cultivation and production processes. |
| 6. Case-by-case evaluation: Tailoring regulatory requirements based on the specific characteristics of each plant-derived biologic. |
| 7. Global harmonization: Collaboration between regulatory authorities and industry stakeholders to establish international guidelines and standards. |
| 8. Good Manufacturing Practice: Compliance with GMP guidelines to ensure consistent quality and safety during manufacturing processes. |
| 9. Preclinical and clinical data: Submission of comprehensive preclinical and clinical data to establish safety and efficacy profiles of plant-derived biologics. |
| 10. Post-marketing surveillance: Monitoring and reporting of adverse events and safety data following the commercialization of plant-derived biologics. |
| 11. Intellectual property rights: Consideration of intellectual property protection for novel plant-derived biologics and their manufacturing processes. |
| 12. Labeling and product information: Clear and accurate labeling to provide information on indications, dosage, administration, and potential risks associated with the use of plant-derived biologics. |
| 13. Risk management plan: Development of a risk management plan to identify and address potential risks throughout the lifecycle of plant-derived biologics. |
| 14. Regulatory updates and advancements: Staying informed about evolving regulations, guidelines, and advancements in the field of plant-derived biologics. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahmanova, G.; Aljabali, A.A.A.; Takova, K.; Minkov, G.; Tambuwala, M.M.; Minkov, I.; Lomonossoff, G.P. Green Biologics: Harnessing the Power of Plants to Produce Pharmaceuticals. Int. J. Mol. Sci. 2023, 24, 17575. https://doi.org/10.3390/ijms242417575
Zahmanova G, Aljabali AAA, Takova K, Minkov G, Tambuwala MM, Minkov I, Lomonossoff GP. Green Biologics: Harnessing the Power of Plants to Produce Pharmaceuticals. International Journal of Molecular Sciences. 2023; 24(24):17575. https://doi.org/10.3390/ijms242417575
Chicago/Turabian StyleZahmanova, Gergana, Alaa A. A. Aljabali, Katerina Takova, George Minkov, Murtaza M. Tambuwala, Ivan Minkov, and George P. Lomonossoff. 2023. "Green Biologics: Harnessing the Power of Plants to Produce Pharmaceuticals" International Journal of Molecular Sciences 24, no. 24: 17575. https://doi.org/10.3390/ijms242417575
APA StyleZahmanova, G., Aljabali, A. A. A., Takova, K., Minkov, G., Tambuwala, M. M., Minkov, I., & Lomonossoff, G. P. (2023). Green Biologics: Harnessing the Power of Plants to Produce Pharmaceuticals. International Journal of Molecular Sciences, 24(24), 17575. https://doi.org/10.3390/ijms242417575









