MLLT11 Regulates Endometrial Stroma Cell Adhesion, Proliferation and Survival in Ectopic Lesions of Women with Advanced Endometriosis
Abstract
1. Introduction
2. Results
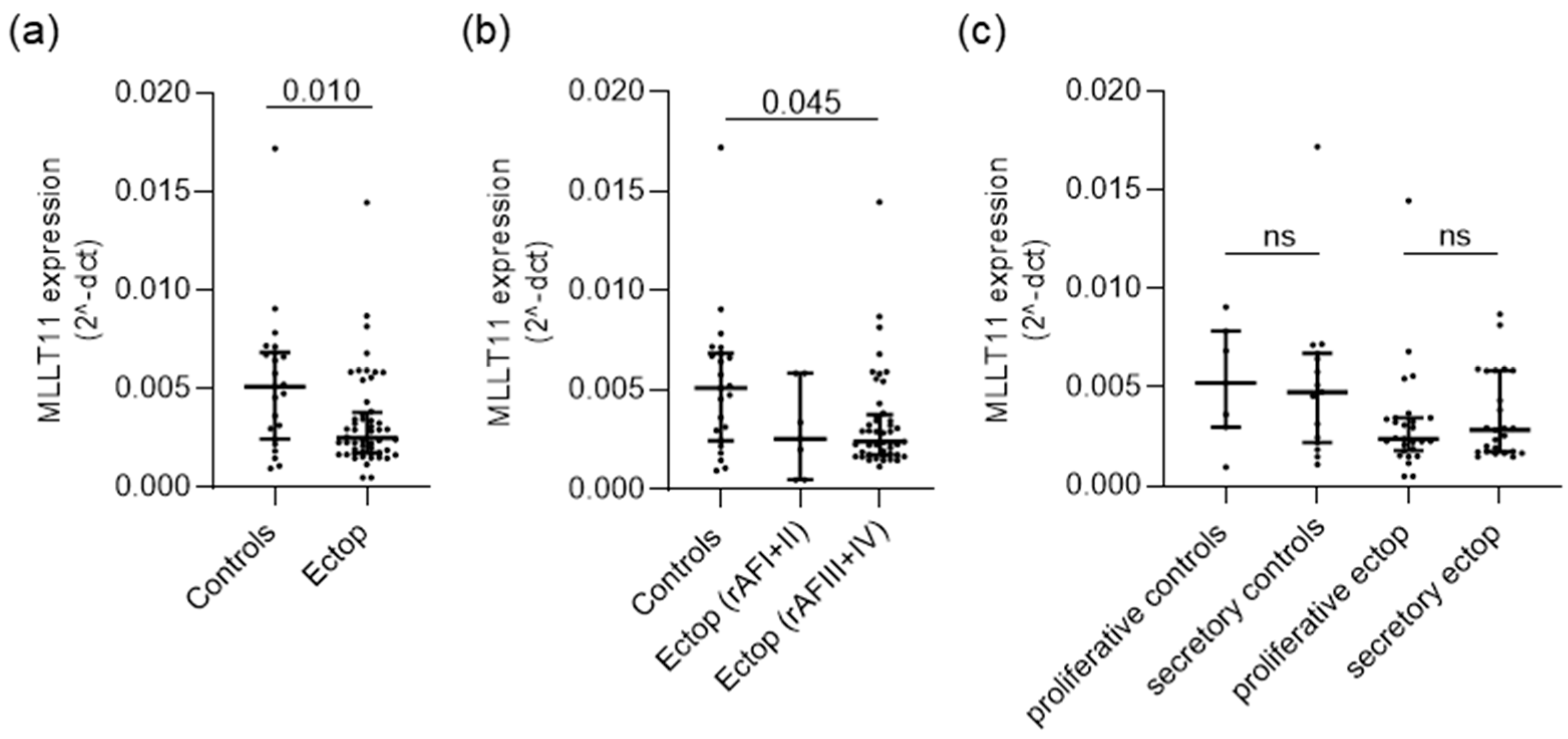
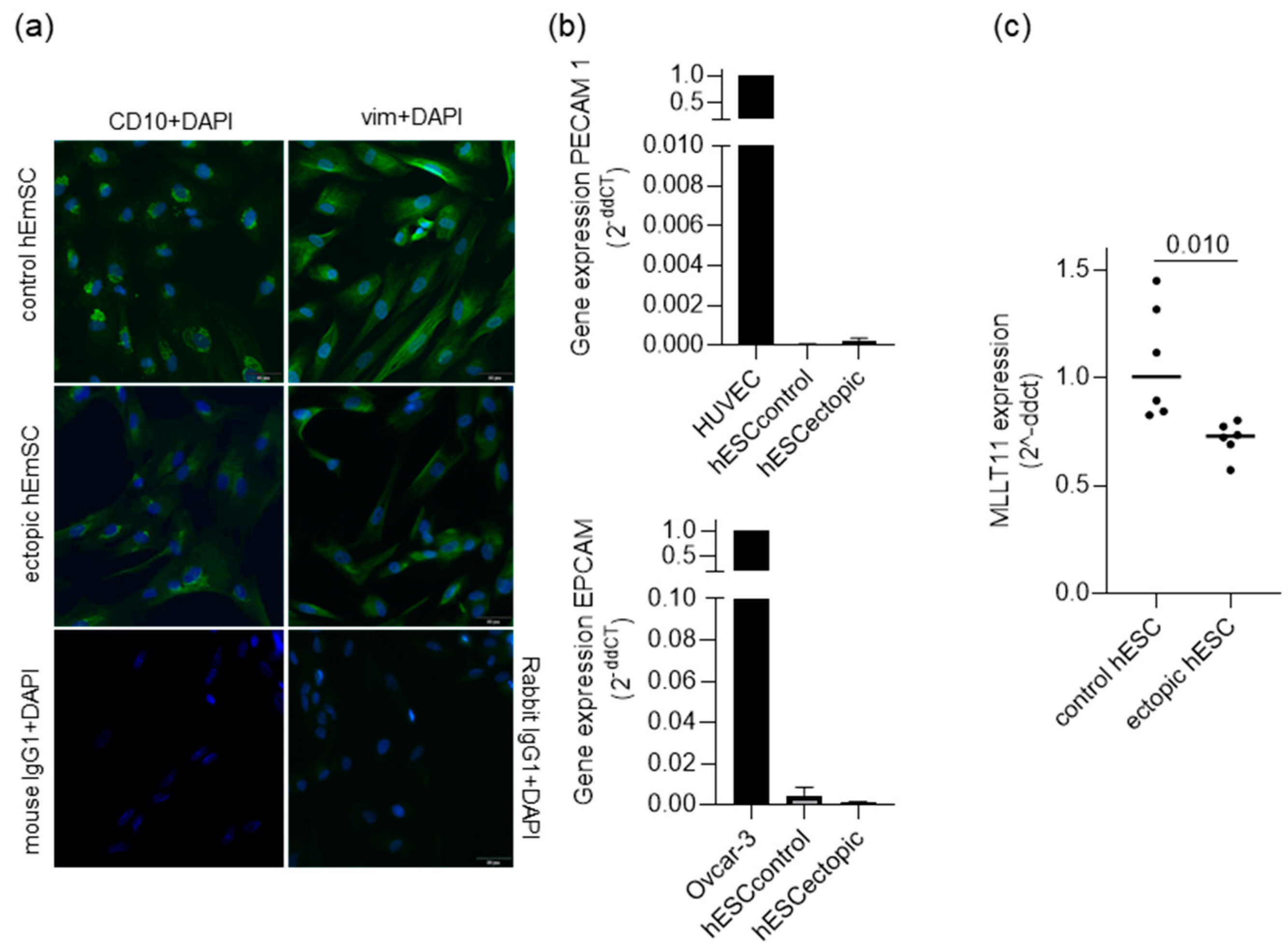
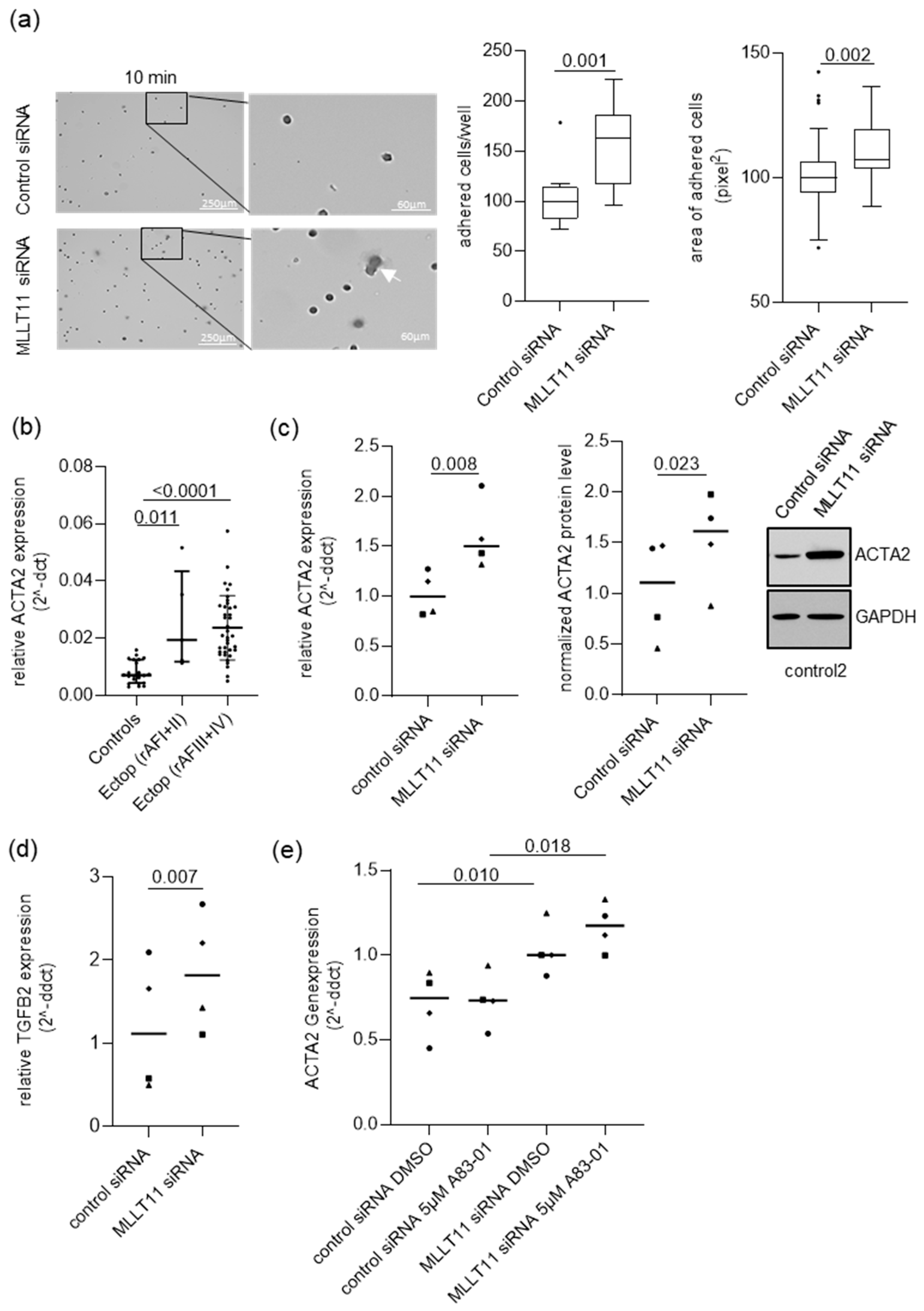
2.1. MLLT11 Is Downregulated in Ectopic Lesions of Women with Stage III and IV Endometriosis
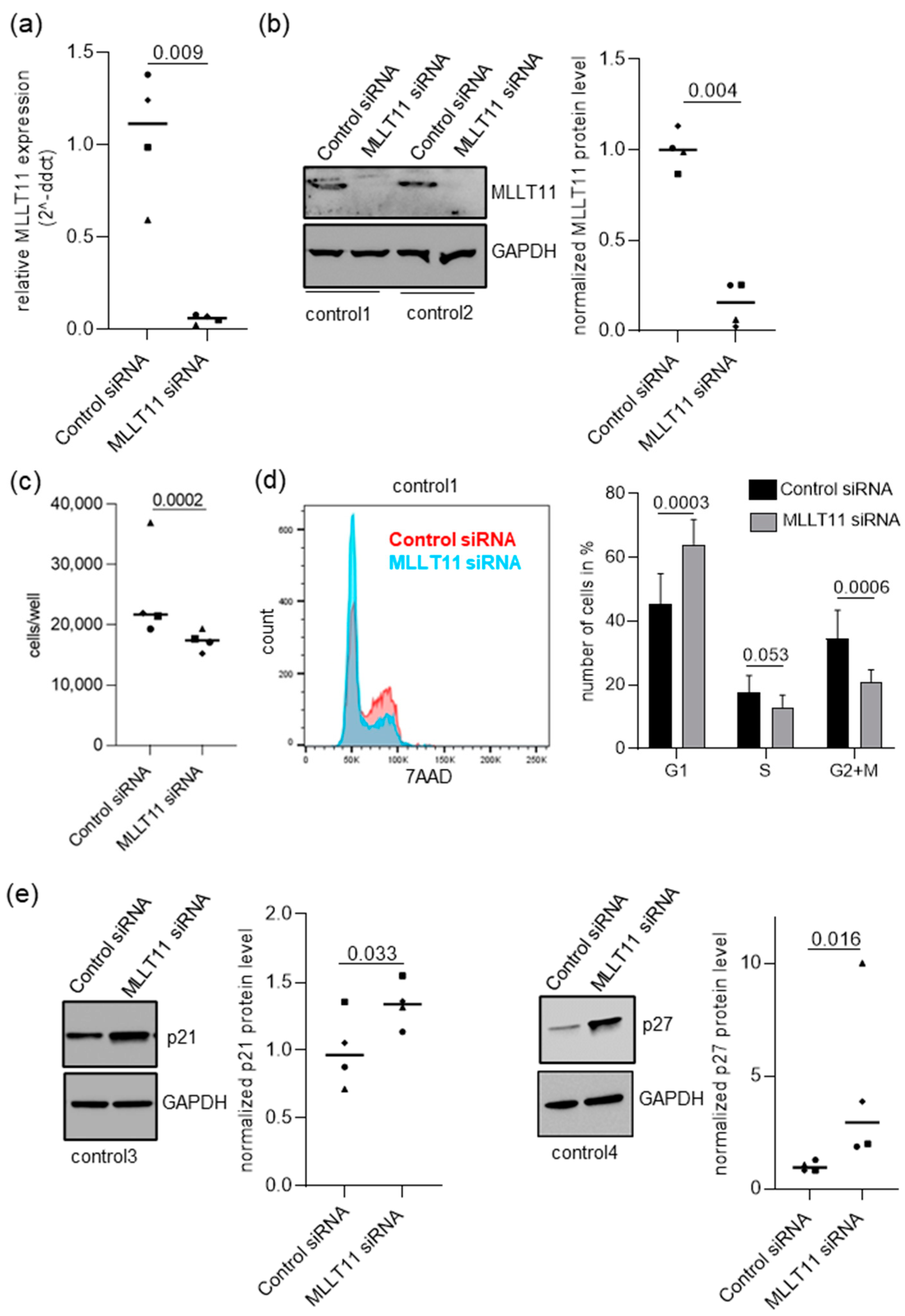
2.2. MLLT11 Knockdown Impairs Cells Proliferation in Primary Endometrial Stromal Cells of Women without Endometriosis
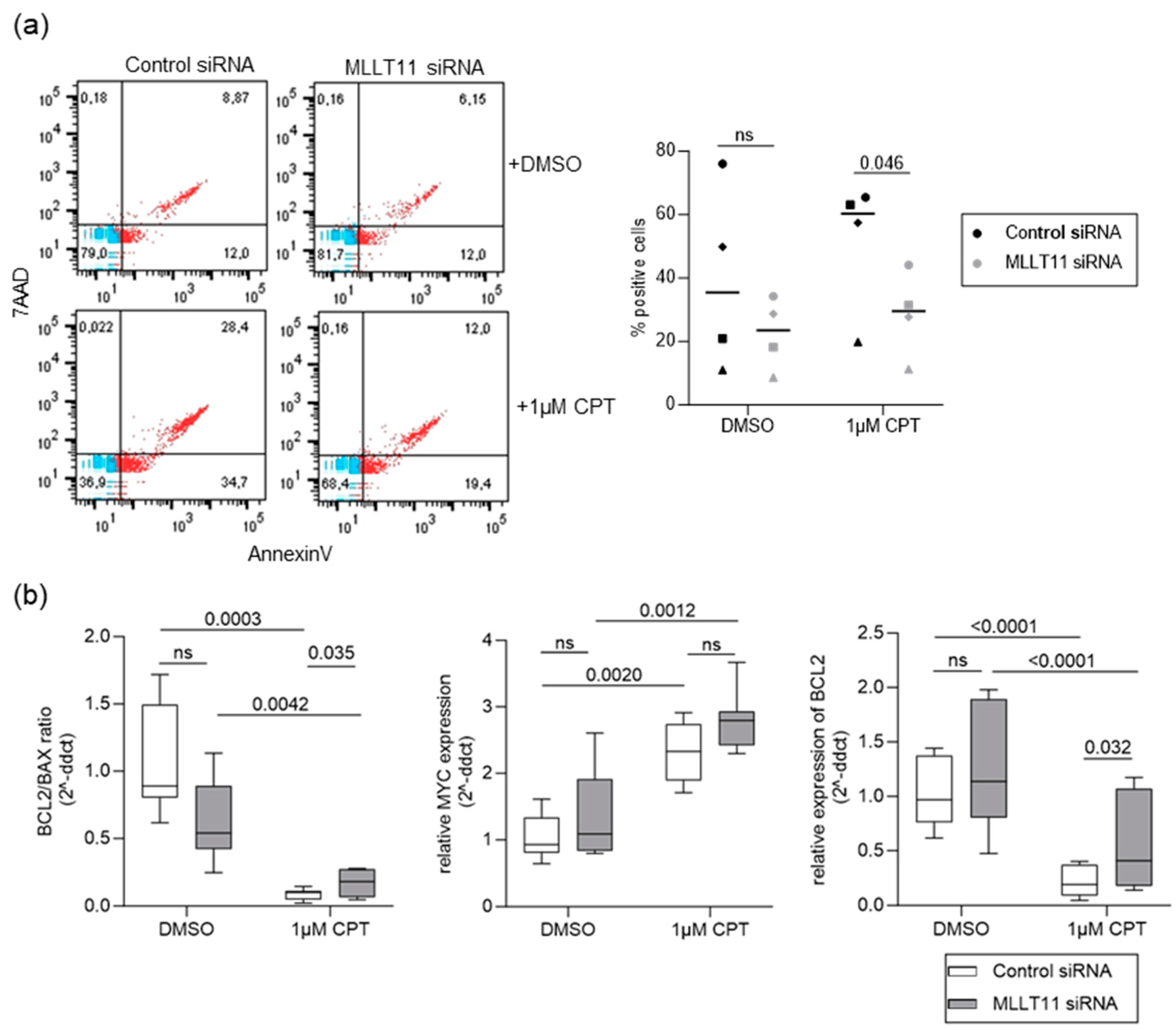
2.3. MLLT11 Mediates Camptothecin-Induced Apoptosis in Primary Endometrial Stroma Cells
2.4. MLLT11 Suppresses Adhesion in Primary Endometrial Stroma Cells of Women without Endometriosis
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. RNA Isolation
4.3. Quantitative Reverse Transcription PCR (RT-qPCR)
4.4. Immunohistochemistry
4.5. Primary Endometrial Stromal Cell (hESC) Cultures
4.6. Immunofluorescence
4.7. MLLT11 Knockdown
4.8. Protein Isolation and Western Blot
4.9. Flow Cytometry
4.10. Proliferation Assay
4.11. Matrigel Invasion Assay
4.12. Adhesion Assay
4.13. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shafrir, A.L.; Farland, L.V.; Shah, D.K.; Harris, H.R.; Kvaskoff, M.; Zondervan, K.; Missmer, S.A. Risk for and consequences of endometriosis: A critical epidemiologic review. Best. Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.K.; Chapron, C.; Giudice, L.C.; Laufer, M.R.; Leyland, N.; Missmer, S.A.; Singh, S.S.; Taylor, H.S. Clinical diagnosis of endometriosis: A call to action. Am. J. Obstet. Gynecol. 2019, 220, 354.e1–354.e12. [Google Scholar] [CrossRef] [PubMed]
- Klemmt, P.A.B.; Starzinski-Powitz, A. Molecular and Cellular Pathogenesis of Endometriosis. Curr. Womens Health Rev. 2018, 14, 106–116. [Google Scholar] [CrossRef]
- Van Gorp, T.; Amant, F.; Neven, P.; Vergote, I.; Moerman, P. Endometriosis and the development of malignant tumours of the pelvis. A review of literature. Best. Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Flores, I.; Rivera, E.; Ruiz, L.A.; Santiago, O.I.; Vernon, M.W.; Appleyard, C.B. Molecular profiling of experimental endometriosis identified gene expression patterns in common with human disease. Fertil. Steril. 2007, 87, 1180–1199. [Google Scholar] [CrossRef]
- Abramiuk, M.; Grywalska, E.; Malkowska, P.; Sierawska, O.; Hrynkiewicz, R.; Niedzwiedzka-Rystwej, P. The Role of the Immune System in the Development of Endometriosis. Cells 2022, 11, 2028. [Google Scholar] [CrossRef]
- Sundqvist, J.; Andersson, K.L.; Scarselli, G.; Gemzell-Danielsson, K.; Lalitkumar, P.G. Expression of adhesion, attachment and invasion markers in eutopic and ectopic endometrium: A link to the aetiology of endometriosis. Hum. Reprod. 2012, 27, 2737–2746. [Google Scholar] [CrossRef]
- Scotti, S.; Regidor, P.A.; Schindler, A.E.; Winterhager, E. Reduced proliferation and cell adhesion in endometriosis. Mol. Hum. Reprod. 2000, 6, 610–617. [Google Scholar] [CrossRef]
- Bhyan, S.B.; Zhao, L.; Wee, Y.; Liu, Y.; Zhao, M. Genetic links between endometriosis and cancers in women. PeerJ 2019, 7, e8135. [Google Scholar] [CrossRef]
- Saavalainen, L.; Lassus, H.; But, A.; Tiitinen, A.; Harkki, P.; Gissler, M.; Pukkala, E.; Heikinheimo, O. Risk of Gynecologic Cancer According to the Type of Endometriosis. Obstet. Gynecol. 2018, 131, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Banz, C.; Ungethuem, U.; Kuban, R.J.; Diedrich, K.; Lengyel, E.; Hornung, D. The molecular signature of endometriosis-associated endometrioid ovarian cancer differs significantly from endometriosis-independent endometrioid ovarian cancer. Fertil. Steril. 2010, 94, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Tiberio, P.; Lozneanu, L.; Angeloni, V.; Cavadini, E.; Pinciroli, P.; Callari, M.; Carcangiu, M.L.; Lorusso, D.; Raspagliesi, F.; Pala, V.; et al. Involvement of AF1q/MLLT11 in the progression of ovarian cancer. Oncotarget 2017, 8, 23246–23264. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tse, W.; Zhu, W.; Chen, H.S.; Cohen, A. A novel gene, AF1q, fused to MLL in t(1;11) (q21;q23), is specifically expressed in leukemic and immature hematopoietic cells. Blood 1995, 85, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Strunk, C.J.; Platzbecker, U.; Thiede, C.; Schaich, M.; Illmer, T.; Kang, Z.; Leahy, P.; Li, C.; Xie, X.; Laughlin, M.J.; et al. Elevated AF1q expression is a poor prognostic marker for adult acute myeloid leukemia patients with normal cytogenetics. Am. J. Hematol. 2009, 84, 308–309. [Google Scholar] [CrossRef] [PubMed]
- Tiberio, P.; Cavadini, E.; Callari, M.; Daidone, M.G.; Appierto, V. AF1q: A novel mediator of basal and 4-HPR-induced apoptosis in ovarian cancer cells. PLoS ONE 2012, 7, e39968. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Schlederer, M.; Schreiber, M.; Ice, R.; Merkel, O.; Bilban, M.; Hofbauer, S.; Kim, S.; Addison, J.; Zou, J.; et al. AF1q is a novel TCF7 co-factor which activates CD44 and promotes breast cancer metastasis. Oncotarget 2015, 6, 20697–20710. [Google Scholar] [CrossRef]
- Liao, J.; Chen, H.; Qi, M.; Wang, J.; Wang, M. MLLT11-TRIL complex promotes the progression of endometrial cancer through PI3K/AKT/mTOR signaling pathway. Cancer Biol. Ther. 2022, 23, 211–224. [Google Scholar] [CrossRef]
- Jin, H.; Sun, W.; Zhang, Y.; Yan, H.; Liufu, H.; Wang, S.; Chen, C.; Gu, J.; Hua, X.; Zhou, L.; et al. MicroRNA-411 Downregulation Enhances Tumor Growth by Upregulating MLLT11 Expression in Human Bladder Cancer. Mol. Ther. Nucleic Acids 2018, 11, 312–322. [Google Scholar] [CrossRef]
- Kadletz, L.C.; Brkic, F.F.; Jank, B.J.; Schneider, S.; Cede, J.; Seemann, R.; Gruber, E.S.; Gurnhofer, E.; Heiduschka, G.; Kenner, L. AF1q Expression Associates with CD44 and STAT3 and Impairs Overall Survival in Adenoid Cystic Carcinoma of the Head and Neck. Pathol. Oncol. Res. 2020, 26, 1287–1292. [Google Scholar] [CrossRef]
- Chen, L.; Xiong, Z.; Zhao, H.; Teng, C.; Liu, H.; Huang, Q.; Wanggou, S.; Li, X. Identification of the novel prognostic biomarker, MLLT11, reveals its relationship with immune checkpoint markers in glioma. Front. Oncol. 2022, 12, 889351. [Google Scholar] [CrossRef] [PubMed]
- Stanton-Turcotte, D.; Hsu, K.; Moore, S.A.; Yamada, M.; Fawcett, J.P.; Iulianella, A. Mllt11 Regulates Migration and Neurite Outgrowth of Cortical Projection Neurons during Development. J. Neurosci. 2022, 42, 3931–3948. [Google Scholar] [CrossRef] [PubMed]
- Toki, T.; Shimizu, M.; Takagi, Y.; Ashida, T.; Konishi, I. CD10 is a marker for normal and neoplastic endometrial stromal cells. Int. J. Gynecol. Pathol. 2002, 21, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Raisova, M.; Hossini, A.M.; Eberle, J.; Riebeling, C.; Wieder, T.; Sturm, I.; Daniel, P.T.; Orfanos, C.E.; Geilen, C.C. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J. Investig. Dermatol. 2001, 117, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Co, N.N.; Tsang, W.P.; Wong, T.W.; Cheung, H.H.; Tsang, T.Y.; Kong, S.K.; Kwok, T.T. Oncogene AF1q enhances doxorubicin-induced apoptosis through BAD-mediated mitochondrial apoptotic pathway. Mol. Cancer Ther. 2008, 7, 3160–3168. [Google Scholar] [CrossRef] [PubMed]
- Poncelet, C.; Leblanc, M.; Walker-Combrouze, F.; Soriano, D.; Feldmann, G.; Madelenat, P.; Scoazec, J.Y.; Darai, E. Expression of cadherins and CD44 isoforms in human endometrium and peritoneal endometriosis. Acta Obstet. Gynecol. Scand. 2002, 81, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Dugina, V.; Ballestrem, C.; Wehrle-Haller, B.; Chaponnier, C. Alpha-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol. Biol. Cell 2003, 14, 2508–2519. [Google Scholar] [CrossRef]
- Lee, H.W.; Park, Y.M.; Lee, S.J.; Cho, H.J.; Kim, D.H.; Lee, J.I.; Kang, M.S.; Seol, H.J.; Shim, Y.M.; Nam, D.H.; et al. Alpha-smooth muscle actin (ACTA2) is required for metastatic potential of human lung adenocarcinoma. Clin. Cancer Res. 2013, 19, 5879–5889. [Google Scholar] [CrossRef]
- Mytilinaiou, M.; Bano, A.; Nikitovic, D.; Berdiaki, A.; Voudouri, K.; Kalogeraki, A.; Karamanos, N.K.; Tzanakakis, G.N. Syndecan-2 is a key regulator of transforming growth factor beta 2/Smad2-mediated adhesion in fibrosarcoma cells. IUBMB Life 2013, 65, 134–143. [Google Scholar] [CrossRef]
- Kurosaka, D.; Muraki, Y.; Inoue, M.; Katsura, H. TGF-beta 2 increases alpha-smooth muscle actin expression in bovine retinal pigment epithelial cells. Curr. Eye Res. 1996, 15, 1144–1147. [Google Scholar] [CrossRef]
- Parcelier, A.; Maharzi, N.; Delord, M.; Robledo-Sarmiento, M.; Nelson, E.; Belakhdar-Mekid, H.; Pla, M.; Kuranda, K.; Parietti, V.; Goodhardt, M.; et al. AF1q/MLLT11 regulates the emergence of human prothymocytes through cooperative interaction with the Notch signaling pathway. Blood 2011, 118, 1784–1796. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Clark, J.; Iulianella, A. MLLT11/AF1q is differentially expressed in maturing neurons during development. Gene Expr. Patterns 2014, 15, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Beliard, A.; Noel, A.; Foidart, J.M. Reduction of apoptosis and proliferation in endometriosis. Fertil. Steril. 2004, 82, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Delbandi, A.A.; Mahmoudi, M.; Shervin, A.; Akbari, E.; Jeddi-Tehrani, M.; Sankian, M.; Kazemnejad, S.; Zarnani, A.H. Eutopic and ectopic stromal cells from patients with endometriosis exhibit differential invasive, adhesive, and proliferative behavior. Fertil. Steril. 2013, 100, 761–769. [Google Scholar] [CrossRef]
- Yang, H.; Hu, T.; Hu, P.; Qi, C.; Qian, L. miR-143-3p inhibits endometriotic stromal cell proliferation and invasion by inactivating autophagy in endometriosis. Mol. Med. Rep. 2021, 23, 356. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Canis, M.; Murakami, T.; Dechelotte, P.; Bruhat, M.A.; Okamura, K. Expression of the cyclin-dependent kinase inhibitor p27Kip1 in eutopic endometrium and peritoneal endometriosis. Fertil. Steril. 2001, 75, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Meola, J.; Rosa e Silva, J.C.; Dentillo, D.B.; da Silva, W.A., Jr.; Veiga-Castelli, L.C.; Bernardes, L.A.; Ferriani, R.A.; de Paz, C.C.; Giuliatti, S.; Martelli, L. Differentially expressed genes in eutopic and ectopic endometrium of women with endometriosis. Fertil. Steril. 2010, 93, 1750–1773. [Google Scholar] [CrossRef] [PubMed]
- Braza-Boils, A.; Mari-Alexandre, J.; Gilabert, J.; Sanchez-Izquierdo, D.; Espana, F.; Estelles, A.; Gilabert-Estelles, J. MicroRNA expression profile in endometriosis: Its relation to angiogenesis and fibrinolytic factors. Hum. Reprod. 2014, 29, 978–988. [Google Scholar] [CrossRef]
- Co, N.N.; Tsang, W.P.; Tsang, T.Y.; Yeung, C.L.; Yau, P.L.; Kong, S.K.; Kwok, T.T. AF1q enhancement of gamma irradiation-induced apoptosis by up-regulation of BAD expression via NF-kappaB in human squamous carcinoma A431 cells. Oncol. Rep. 2010, 24, 547–554. [Google Scholar]
- McMahon, S.B. MYC and the control of apoptosis. Cold Spring Harb. Perspect. Med. 2014, 4, a014407. [Google Scholar] [CrossRef]
- Proestling, K.; Birner, P.; Gamperl, S.; Nirtl, N.; Marton, E.; Yerlikaya, G.; Wenzl, R.; Streubel, B.; Husslein, H. Enhanced epithelial to mesenchymal transition (EMT) and upregulated MYC in ectopic lesions contribute independently to endometriosis. Reprod. Biol. Endocrinol. 2015, 13, 75. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, C.; Sueyoshi, Y.; Ema, M.; Mori, Y.; Takaishi, K.; Hisatomi, H. Induction of oxidative stress by anticancer drugs in the presence and absence of cells. Oncol. Lett. 2017, 14, 6066–6070. [Google Scholar] [CrossRef] [PubMed]
- Flor, A.; Wolfgeher, D.; Li, J.; Hanakahi, L.A.; Kron, S.J. Lipid-derived electrophiles mediate the effects of chemotherapeutic topoisomerase I poisons. Cell Chem. Biol. 2021, 28, 776–787.e8. [Google Scholar] [CrossRef] [PubMed]
- Cacciottola, L.; Donnez, J.; Dolmans, M.M. Can Endometriosis-Related Oxidative Stress Pave the Way for New Treatment Targets? Int. J. Mol. Sci. 2021, 22, 7138. [Google Scholar] [CrossRef] [PubMed]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid. Med. Cell Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, H.; Cestari, B.A.; Meola, J.; Podgaec, S. Higher Oxidative Stress in Endometriotic Lesions Upregulates Senescence-Associated p16(ink4a) and beta-Galactosidase in Stromal Cells. Int. J. Mol. Sci. 2023, 24, 914. [Google Scholar] [CrossRef]
- Sotnikova, N.Y.; Antsiferova, Y.S.; Posiseeva, L.V.; Shishkov, D.N.; Posiseev, D.V.; Filippova, E.S. Mechanisms regulating invasiveness and growth of endometriosis lesions in rat experimental model and in humans. Fertil. Steril. 2010, 93, 2701–2705. [Google Scholar] [CrossRef]
- Yerlikaya, G.; Balendran, S.; Prostling, K.; Reischer, T.; Birner, P.; Wenzl, R.; Kuessel, L.; Streubel, B.; Husslein, H. Comprehensive study of angiogenic factors in women with endometriosis compared to women without endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 204, 88–98. [Google Scholar] [CrossRef]
- Anaf, V.; Simon, P.; Fayt, I.; Noel, J. Smooth muscles are frequent components of endometriotic lesions. Hum. Reprod. 2000, 15, 767–771. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, Y.; Xu, H.; Hill, C.; Ewing, R.M.; He, D.; Zhang, X.; Wang, Y. Bioinformatic analysis reveals the importance of epithelial-mesenchymal transition in the development of endometriosis. Sci. Rep. 2020, 10, 8442. [Google Scholar] [CrossRef]
- Zhang, Q.; Duan, J.; Olson, M.; Fazleabas, A.; Guo, S.W. Cellular Changes Consistent with Epithelial-Mesenchymal Transition and Fibroblast-to-Myofibroblast Transdifferentiation in the Progression of Experimental Endometriosis in Baboons. Reprod. Sci. 2016, 23, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Chen, Y.; Suo, L.; Chen, H.; Zhu, L.; Wan, G.; Han, X. Activin a promotes myofibroblast differentiation of endometrial mesenchymal stem cells via STAT3-dependent Smad/CTGF pathway. Cell Commun. Signal 2019, 17, 45. [Google Scholar] [CrossRef]
- Fassbender, A.; Rahmioglu, N.; Vitonis, A.F.; Vigano, P.; Giudice, L.C.; D’Hooghe, T.M.; Hummelshoj, L.; Adamson, G.D.; Becker, C.M.; Missmer, S.A.; et al. World Endometriosis Research Foundation Endometriosis Phenome and Biobanking Harmonisation Project: IV. Tissue collection, processing, and storage in endometriosis research. Fertil. Steril. 2014, 102, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Proestling, K.; Yotova, I.; Gamperl, S.; Hauser, C.; Wenzl, R.; Schneeberger, C.; Szabo, L.; Mairhofer, M.; Husslein, H.; Kuessel, L. Enhanced expression of TACE contributes to elevated levels of sVCAM-1 in endometriosis. Mol. Hum. Reprod. 2019, 25, 76–87. [Google Scholar] [CrossRef]
- Tulac, S.; Overgaard, M.T.; Hamilton, A.E.; Jumbe, N.L.; Suchanek, E.; Giudice, L.C. Dickkopf-1, an inhibitor of Wnt signaling, is regulated by progesterone in human endometrial stromal cells. J. Clin. Endocrinol. Metab. 2006, 91, 1453–1461. [Google Scholar] [CrossRef]
- Yotova, I.Y.; Quan, P.; Leditznig, N.; Beer, U.; Wenzl, R.; Tschugguel, W. Abnormal activation of Ras/Raf/MAPK and RhoA/ROCKII signalling pathways in eutopic endometrial stromal cells of patients with endometriosis. Hum. Reprod. 2011, 26, 885–897. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proestling, K.; Husslein, H.; Hudson, Q.J.; Witzmann-Stern, M.; Widmar, B.; Bagó-Horváth, Z.; Sandrieser, L.; Perricos, A.; Wenzl, R.; Yotova, I. MLLT11 Regulates Endometrial Stroma Cell Adhesion, Proliferation and Survival in Ectopic Lesions of Women with Advanced Endometriosis. Int. J. Mol. Sci. 2024, 25, 439. https://doi.org/10.3390/ijms25010439
Proestling K, Husslein H, Hudson QJ, Witzmann-Stern M, Widmar B, Bagó-Horváth Z, Sandrieser L, Perricos A, Wenzl R, Yotova I. MLLT11 Regulates Endometrial Stroma Cell Adhesion, Proliferation and Survival in Ectopic Lesions of Women with Advanced Endometriosis. International Journal of Molecular Sciences. 2024; 25(1):439. https://doi.org/10.3390/ijms25010439
Chicago/Turabian StyleProestling, Katharina, Heinrich Husslein, Quanah James Hudson, Matthias Witzmann-Stern, Barbara Widmar, Zsuzsanna Bagó-Horváth, Lejla Sandrieser, Alexandra Perricos, René Wenzl, and Iveta Yotova. 2024. "MLLT11 Regulates Endometrial Stroma Cell Adhesion, Proliferation and Survival in Ectopic Lesions of Women with Advanced Endometriosis" International Journal of Molecular Sciences 25, no. 1: 439. https://doi.org/10.3390/ijms25010439
APA StyleProestling, K., Husslein, H., Hudson, Q. J., Witzmann-Stern, M., Widmar, B., Bagó-Horváth, Z., Sandrieser, L., Perricos, A., Wenzl, R., & Yotova, I. (2024). MLLT11 Regulates Endometrial Stroma Cell Adhesion, Proliferation and Survival in Ectopic Lesions of Women with Advanced Endometriosis. International Journal of Molecular Sciences, 25(1), 439. https://doi.org/10.3390/ijms25010439






