The Use of Fungi of the Trichoderma Genus in Anaerobic Digestion: A Review
Abstract
:1. Introduction
2. The Use of Trichoderma Fungi in the Pre-Treatment of Lignocellulosic Biomass
2.1. Structure and Composition of Lignocellulosic Biomass
2.2. Methods of Pre-Treatment of Lignocellulosic Biomass
2.3. Application of Trichoderma Fungi in the Pre-Treatment of Lignocellulosic Biomass
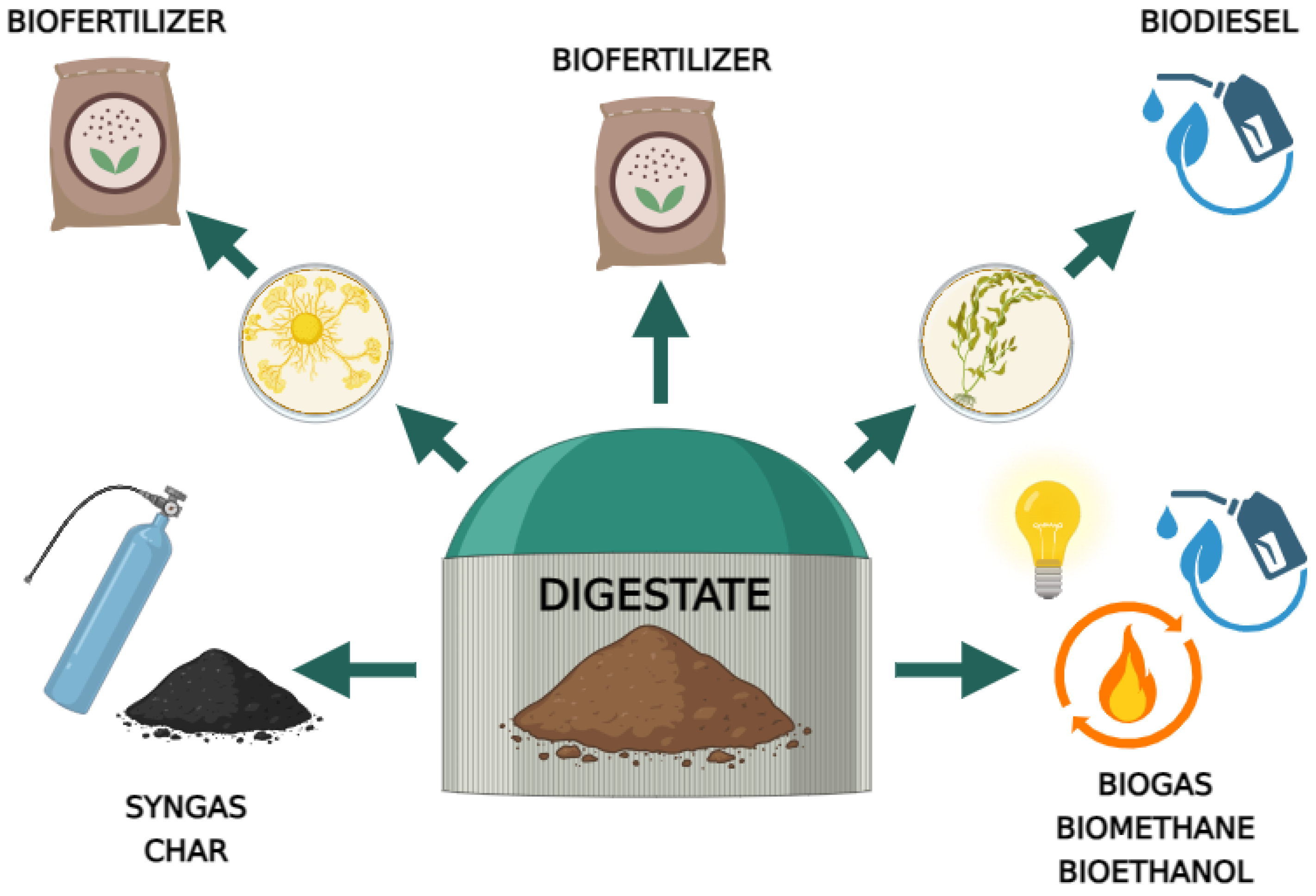
3. The Use of Digestate as an Organic Carrier for the Multiplication of Trichoderma Fungi
3.1. Structure and Composition of Digestate
3.2. Application of Trichoderma Fungi to the Digestate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Owusu, P.A.; Asumadu-Sarkodie, S. A review of renewable energy sources, sustainability issues and climate change mitigation. Cogent Eng. 2016, 3, 1167990. [Google Scholar] [CrossRef]
- Majeed, Y.; Khan, M.U.; Waseem, M.; Zahid, U.; Mahmood, F.; Majeed, F.; Sultan, M.; Raza, A. Renewable energy as an alternative source for energy management in agriculture. Energy Rep. 2023, 10, 344–359. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Sudharsana, T.; Jayamuthunagai, J.; Praveenkumar, R.; Chozhavendhan, S.; Iyyappan, J. Biogas production–A review on composition, fuel properties, feed stock and principles of anaerobic digestion. Renew. Sustain. Energy Rev. 2018, 90, 570–582. [Google Scholar] [CrossRef]
- Alalwan, H.A.; Alminshid, A.H.; Aljaafari, H.A.S. Promising Evolution of Biofuel Generations. Subject Review. Renew. Energy Focus 2019, 28, 127–139. [Google Scholar] [CrossRef]
- Martins, F.; Felgueiras, C.; Smitková, M. Fossil fuel energy consumption in European countries. Energy Procedia 2018, 153, 107–111. [Google Scholar] [CrossRef]
- Martins, F.; Felgueiras, C.; Smitkova, M.; Caetano, N. Analysis of Fossil Fuel Energy Consumption and Environmental Impacts in European Countries. Energies 2019, 12, 964. [Google Scholar] [CrossRef]
- Sayed, E.T.; Olabi, A.G.; Alami, A.H.; Radwan, A.; Mdallal, A.; Rezk, A.; Abdelkareem, M.A. Renewable Energy and Energy Storage Systems. Energies 2023, 16, 1415. [Google Scholar] [CrossRef]
- Perera, F.P. Multiple threats to child health from fossil fuel combustion: Impacts of air pollution and climate change. Environ. Health Perspect. 2017, 125, 141–148. [Google Scholar] [CrossRef]
- Perera, F. Pollution from Fossil-Fuel Combustion is the Leading Environmental Threat to Global Pediatric Health and Equity: Solutions Exist. Int. J. Environ. Res. Public Health 2018, 15, 16. [Google Scholar] [CrossRef]
- Communication from the Commission to the European Parliament, The European Council, The Council, The European Economic and Social Committee and The Committee of the Regions: The European Green Deal; European Commission: Brussels, Belgium, 2019; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2019%3A640%3AFIN (accessed on 29 October 2023).
- Gajdzik, B.; Wolniak, R.; Nagaj, R.; Grebski, W.W.; Romanyshyn, T. Barriers to Renewable Energy Source (RES) Installations as Determinants of Energy Consumption in EU Countries. Energies 2023, 16, 7364. [Google Scholar] [CrossRef]
- Mohtasham, J. Review article-renewable energies. Energy Procedia 2015, 74, 1289–1297. [Google Scholar] [CrossRef]
- Pavar, V.; Farooqui, S. The effectiveness of renewable energy technologies in reducing greenhouse gas emissions. South India J. Soc. Sci. 2023, 21, 150–160. [Google Scholar]
- Eurostat Statistics Explained. Renewable Energy Statistic: Share of Renewable Energy More Than Doubled between 2004 and 2021. 2023. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Renewable_energy_statistics#Share_of_renewable_energy_more_than_doubled_between_2004_and_2020 (accessed on 29 October 2023).
- Communication from the Commission to the European Parliament, The European Council, The Council, The European Economic and Social Committee and The Committee of the Regions: REPowerEU Plan; European Commission: Brussels, Belgium, 2022. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2022%3A230%3AFIN (accessed on 29 October 2023).
- Commission Welcomes Completion of Key ‘Fit for 55’ Legislation, Putting EU on Track to Exceed 2030 Targets; European Commission: Brussels, Belgium, 2023. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_23_4754 (accessed on 29 October 2023).
- Regulation (EU) 2021/1119 of the European Parliament and of the Council of 30 June 2021 establishing the framework for achieving climate neutrality and amending Regulations (EC) No 401/2009 and (EU) 2018/1999 (‘European Climate Law’). Off. J. Eur. Union 2021, 64, 1–17. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32021R1119 (accessed on 29 October 2023).
- Paris Agreement. Off. J. Eur. Union 2016, 59, 4–18. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:22016A1019(01) (accessed on 29 October 2023).
- Notton, G.; Nivet, M.-L.; Voyant, C.; Paoli, C.; Darras, C.; Motte, F.; Fouilloy, A. Intermittent and stochastic character of renewable energy sources: Consequences, cost of intermittence and benefit of forecasting. Renew. Sustain. Energy Rev. 2018, 87, 96–105. [Google Scholar] [CrossRef]
- Mignogna, D.; Ceci, P.; Cafaro, C.; Corazzi, G.; Avino, P. Production of Biogas and Biomethane as Renewable Energy Sources: A Review. Appl. Sci. 2023, 13, 10219. [Google Scholar] [CrossRef]
- Pfau, S.F.; Hagens, J.E.; Dankbaar, B. Biogas between renewable energy and bio-economy policies—Opportunities and constraints resulting from a dual role. Energy Sustain. Soc. 2017, 7, 17. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Lues, R. Anaerobic Digestion of Lignocellulosic Biomass: Substrate Characteristics (Challenge) and Innovation. Fermentation 2023, 9, 755. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Marzec-Grządziel, A.; Paluch, E.; Pilarski, K.; Wolna-Maruwka, A.; Kubiak, A.; Kałuża, T.; Kulupa, T. Biofilm Formation and Genetic Diversity of Microbial Communities in Anaerobic Batch Reactor with Polylactide (PLA) Addition. Int. J. Mol. Sci. 2023, 24, 10042. [Google Scholar] [CrossRef]
- Shrestha, S.; Fonoll, X.; Khanal, S.K.; Raskin, L. Biological Strategies for Enhanced Hydrolysis of Lignocellulosic Biomass during Anaerobic Digestion: Current Status and Future Perspectives. Bioresour. Technol. 2017, 245, 1245–1257. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Sarsaiya, S.; Patel, A.; Juneja, A.; Singh, R.P.; Yan, B.; Awasthi, S.K.; Jain, A.; Liu, T.; Duan, Y.; et al. Refining biomass residues for sustainable energy and bio-products: An assessment of technology, its importance, and strategic applications in circular bio-economy. Renew. Sustain. Energy Rev. 2020, 127, 109876. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Ahmed, N.A.; Ogunkunle, O. Optimization of biogas yield from lignocellulosic materials with different pretreatment methods: A review. Biotechnol. Biofuels 2021, 14, 159. [Google Scholar] [CrossRef] [PubMed]
- Kumar Khanal, S.; Lü, F.; Wong, J.W.C.; Wu, D.; Oechsner, H. Anaerobic digestion beyond biogas. Bioresour. Technol. 2021, 337, 125378. [Google Scholar] [CrossRef] [PubMed]
- Pilarska, A.A.; Pilarski, K.; Waliszewska, B.; Zborowska, M.; Witaszek, K.; Waliszewska, H.; Kolasiński, M.; Szwarc-Rzepka, K. Evaluation of bio-methane yields for high-energy organic waste and sewage sludge: A pilot-scale study for a wastewater treatment plant. Environ. Eng. Manag. J. 2019, 18, 2023–2034. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A Review of the Processes, Parameters, and Optimization of Anaerobic Digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef] [PubMed]
- Kougias, P.G.; Angelidaki, I. Biogas and its opportunities—A review Keywords. Front. Environ. Sci. Eng. 2018, 12, 14. [Google Scholar] [CrossRef]
- Náthia-Neves, G.; Berni, M.; Dragone, G.; Mussatto, S.I.; Forster-Carneiro, T. Anaerobic digestion process: Technological aspects and recent developments. Int. J. Environ. Sci. Technol. 2018, 15, 2033–2046. [Google Scholar] [CrossRef]
- Gerardi, M.H. Wastewater Microbiology Series: The Microbiology of Anaerobic Digesters; Wiley: New York, NY, USA, 2003. [Google Scholar]
- Atelge, M.; Krisa, D.; Kumar, G.; Eskicioglu, C.; Nguyen, D.D.; Chang, S.W.; Atabani, A.; Al-Muhtaseb, A.H.; Unalan, S. Biogas production from organic waste: Recent progress and perspectives. Waste Biomass Valorization 2020, 11, 1019–1040. [Google Scholar] [CrossRef]
- Yilmaz, A.; Ünvar, S.; Koçer, A.; Aygün, B. Factors affecting the production of biogas. Int. J. Sci. Eng. Res. 2018, 9, 59–62. [Google Scholar]
- Nsair, A.; Onen Cinar, S.; Alassali, A.; Abu Qdais, H.; Kuchta, K. Operational Parameters of Biogas Plants: A Review and Evaluation Study. Energies 2020, 13, 3761. [Google Scholar] [CrossRef]
- Pilarska, A.A.; Kulupa, T.; Kubiak, A.; Wolna-Maruwka, A.; Pilarski, K.; Niewiadomska, A. Anaerobic Digestion of Food Waste—A Short Review. Energies 2023, 16, 5742. [Google Scholar] [CrossRef]
- Angelidaki, I.; Treu, L.; Tsapekos, P.; Luo, G.; Campanaro, S.; Wenzel, H.; Kougias, P.G. Biogas upgrading and utilization: Current status and perspectives. Biotechnol. Adv. 2018, 36, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Kabeyi, M.J.B.; Olanrewaju, O.A. Biogas Production and Applications in the Sustainable Energy Transition. Energy 2022, 2022, 8750221. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, S. Biogas as a Powerhouse of Renewable Energy: A Review. J. Adv. Res. Altern. Energy Environ. Ecology 2023, 10, 1–5. [Google Scholar]
- Koupaie, E.H.; Dahadha, S.; Lakeh, A.A.B.; Azizi, A.; Elbeshbishy, E. Enzymatic pretreatment of lignocellulosic biomass for enhanced biomethane production-A review. J. Environ. Manag. 2019, 233, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Ometto, F.; Karlsson, A.; Ejlertsson, J.; Björn, A.V.; Shakeri, S.Y. Anaerobic digestion: An engineered biological process. In Substitute Natural Gas from Waste; Materazzi, M., Foscolo, P.U., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 63–74. [Google Scholar]
- Abraham, A.; Mathew, A.; Park, H.; Choi, O.; Sindhu, R.; Parameswaran, B.; Pandey, A.; Park, J.; Sang, B. Pretreatment strategies for enhanced biogas production from lignocellulosic biomass. Bioresour. Technol. 2020, 301, 122725. [Google Scholar] [CrossRef]
- Xu, N.; Liu, S.; Xin, F.; Zhou, J.; Jia, H.; Xu, J.; Jiang, M.; Don, W. Biomethane Production from Lignocellulose: Biomass Recalcitrance and Its Impacts on Anaerobic Digestion. Front. Bioeng. Biotechnol. 2019, 7, 191. [Google Scholar] [CrossRef]
- Agregán, R.; Lorenzo, J.M.; Kumar, M.; Shariati, M.A.; Khan, M.U.; Sarwar, A.; Sultan, M.; Rebezov, M.; Usman, M. Anaerobic Digestion of Lignocellulose Components: Challenges and Novel Approaches. Energies 2022, 15, 8413. [Google Scholar] [CrossRef]
- Witaszek, K.; Pilarski, K.; Niedbała, G.; Pilarska, A.A.; Herkowiak, M. Energy Efficiency of Comminution and Extrusion of Maize Substrates Subjected to Methane Fermentation. Energies 2020, 13, 1887. [Google Scholar] [CrossRef]
- Wagner, A.O.; Schwarzenauer, T.; Illmer, P. Improvement of methane generation capacity by aerobic pre-treatment of organic waste with a cellulolytic Trichoderma viride culture. J. Environ. Manag. 2013, 129, 357–360. [Google Scholar] [CrossRef]
- Zulkifli, Z.B.; Rasit, N.B.; Umor, N.A.; Ismail, S. The effect of A. Fumigatus SK1 and Trichoderma sp. on the biogas production from cow manure. Malays. J. Fundam. Appl. Sci. 2018, 14, 353–359. [Google Scholar] [CrossRef]
- Mahmoud, Y.G.; Awadalla, O.A.; Estafanous, A.N.; Etawy, W.A. The use of Phanerochaete chrysosporium, Trichoderma harzianum and Trichoderma viride for biogas production. Stud. Fungi 2020, 5, 368–380. [Google Scholar] [CrossRef]
- Sharma, S.; Kour, D.; Rana, K.L.; Dhiman, A.; Thakur, S.; Thakur, P.; Thakur, S.; Thakur, N.; Sudheer, S.; Yadav, N.; et al. Trichoderma: Biodiversity, ecological significances, and industrial applications. In Recent Advancement in White Biotechnology through Fungi; Springer: Berlin/Heidelberg, Germany, 2019; pp. 85–120. [Google Scholar]
- Japanis, F.G.; Vetaryan, S.; Raja, N.K.K.; Mokhtar, M.A.A.; Mohd Fishal, E.M. The Impact of Trichoderma spp. on Agriculture and Their Identification. Malays. Appl. Biol. 2022, 51, 1–15. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, A.; Wolna-Maruwka, A.; Pilarska, A.A.; Niewiadomska, A.; Piotrowska-Cyplik, A. Fungi of the Trichoderma Genus: Future Perspectives of Benefits in Sustainable Agriculture. Appl. Sci. 2023, 13, 6434. [Google Scholar] [CrossRef]
- Alias, C.; Bulgari, D.; Gobbi, E. It Works! Organic-Waste-Assisted Trichoderma spp. Solid-State Fermentation on Agricultural Digestate. Microorganisms 2022, 10, 164. [Google Scholar] [CrossRef]
- Bulgari, D.; Alias, C.; Peron, G.; Ribaudo, G.; Gianoncelli, A.; Savino, S.; Boureghda, H.; Bouznad, Z.; Monti, E.; Gobbi, E. Solid-State Fermentation of Trichoderma spp.: A New Way to Valorize the Agricultural Digestate and Produce Value-Added Bioproducts. J. Agric. Food Chem. 2023, 71, 3994–4004. [Google Scholar] [CrossRef]
- Czekała, W.; Jasinski, T.; Grzelak, M.; Witaszek, K.; Dach, J. Biogas Plant Operation: Digestate as the Valuable Product. Energies 2022, 15, 8275. [Google Scholar] [CrossRef]
- Paul, S.; Dutta, A. Challenges and Opportunities of Lignocellulosic Biomass for Anaerobic Digestion. Resour. Conserv. Recycl. 2018, 130, 164–174. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic digestion of lignocellulosic biomass: Challenges and opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Beltrán, J.U.; Hernández-De Lira, I.O.; Cruz-Santos, M.M.; Saucedo-Luevanos, A.; Hernández-Terán, F.; Balagurusamy, N. Insight into Pretreatment Methods of Lignocellulosic Biomass to Increase Biogas Yield: Current State, Challenges, and Opportunities. Appl. Sci. 2019, 9, 3721. [Google Scholar] [CrossRef]
- Bajpai, P. Structure of Lignocellulosic Biomass. In Pretreatment of Lignocellulosic Biomass for Biofuel Production; Springer: Singapore, 2016; pp. 7–12. [Google Scholar]
- Surendra, K.C.; Ogoshi, R.; Zaleski, H.M.; Hashimoto, A.G.; Khanal, S.K. High yielding tropical energy crops for bioenergy production: Effects of plant components, harvest years and locations on biomass composition. Bioresour. Technol. 2018, 251, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Kainthola, J.; Podder, A.; Fechner, M.; Goel, R. An Overview of Fungal Pretreatment Processes for Anaerobic Digestion: Applications, Bottlenecks and Future Needs. Bioresour. Technol. 2021, 321, 124397. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, W.; Mabon, R.; Broadbelt, L.J. A critical review on hemicellulose pyrolysis. Energy Technol. 2017, 5, 52–79. [Google Scholar] [CrossRef]
- Li, F.; Zhang, M.; Guo, K.; Hu, Z.; Zhang, R.; Feng, Y.; Yi, X.; Zou, W.; Wang, L.; Wu, C. High-level hemicellulosic arabinose predominately affects lignocellulose crystallinity for genetically enhancing both plant lodging resistance and biomass enzymatic digestibility in rice mutants. Plant Biotechnol. J. 2015, 13, 514–525. [Google Scholar] [CrossRef]
- Shahzadi, T.; Mehmood, S.; Irshad, M.; Anwar, Z.; Afroz, A.; Zeeshan, N.; Rashid, U.; Sughra, K. Advances in lignocellulosic biotechnology: A brief review on lignocellulosic biomass and cellulases. Adv. Biosci. Biotechnol. 2014, 5, 246–251. [Google Scholar] [CrossRef]
- Meenakshisundaram, S.; Fayeulle, A.; Léonard, E.; Ceballos, C.; Liu, X.; Pauss, A. Combined Biological and Chemical/Physicochemical Pretreatment Methods of Lignocellulosic Biomass for Bioethanol and Biomethane Energy Production—A Review. Appl. Microbiol. 2022, 2, 716–734. [Google Scholar] [CrossRef]
- Paudel, S.R.; Banjara, S.P.; Choi, O.K.; Park, K.Y.; Kim, Y.M.; Lee, J.W. Pretreatment of Agricultural Biomass for Anaerobic Digestion: Current State and Challenges. Bioresour. Technol. 2017, 245, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Poddar, B.J.; Nakhate, S.P.; Gupta, R.K.; Chavan, A.R.; Singh, A.K.; Khardenavis, A.A.; Purohit, H.J. A Comprehensive Review on the Pretreatment of Lignocellulosic Wastes for Improved Biogas Production by Anaerobic Digestion. Int. J. Environ. Sci. Technol. 2021, 19, 3429–3456. [Google Scholar] [CrossRef]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The Role of Pretreatment in Improving the Enzymatic Hydrolysis of Lignocellulosic Materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, K.; Drielak, E.; Varma, V.S.; Muthusamy, S.; Kumar, G. Updates on the Pretreatment of Lignocellulosic Feedstocks for Bioenergy Production—A Review. Biomass Convers. Biorefinery 2018, 8, 471–483. [Google Scholar] [CrossRef]
- Tsapekos, P.; Kougias, P.G.; Angelidaki, I. Biogas Production from Ensiled Meadow Grass; Effect of Mechanical Pretreatments and Rapid Determination of Substrate Biodegradability via Physicochemical Methods. Bioresour. Technol. 2015, 182, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Zeynali, R.; Khojastehpour, M.; Ebrahimi-Nik, M. Effect of ultrasonic pre-treatment on biogas yield and specific energy in anaerobic digestion of fruit and vegetable wholesale market wastes. Sustain. Environ. Res. 2017, 27, 259–264. [Google Scholar] [CrossRef]
- Zhao, B.-H.; Chen, J.; Yu, H.-Q.; Hu, Z.-H.; Yue, Z.-B.; Li, J. Optimization of Microwave Pretreatment of Lignocellulosic Waste for Enhancing Methane Production: Hyacinth as an Example. Front. Environ. Sci. Eng. 2017, 11, 17. [Google Scholar] [CrossRef]
- Patil, P.N.; Gogate, P.R.; Csoka, L.; Dregelyi-Kiss, A.; Horvath, M. Intensification of biogas production using pretreatment based on hydrodynamic cavitation. Ultrason. Sonochem. 2016, 30, 79–86. [Google Scholar] [CrossRef]
- Jaffar, M.; Pang, Y.; Yuan, H.; Zou, D.; Liu, Y.; Zhu, B.; Korai, M.R.; Li, X. Wheat straw pretreatment with KOH for enhancing biomethane production and fertilizer value in anaerobic digestion. Energy Resour. Environ. Technol. 2016, 3, 404–409. [Google Scholar] [CrossRef]
- Sarto, S.; Hildayati, R.; Syaichurrozi, I. Effect of Chemical Pretreatment Using Sulfuric Acid on Biogas Production from Water Hyacinth and Kinetics. Renew. Energy 2019, 132, 335–350. [Google Scholar] [CrossRef]
- Ostovareh, S.; Karimi, K.; Zamani, A. Efficient Conversion of Sweet Sorghum Stalks to Biogas and Ethanol Using Organosolv Pretreatment. Ind. Crops Prod. 2015, 66, 170–177. [Google Scholar] [CrossRef]
- Cardeña, R.; Moreno, G.; Bakonyi, P.; Buitrón, G. Enhancement of methane production from various microalgae cultures via novel ozonation pretreatment. Chem. Eng. J. 2017, 1, 948–954. [Google Scholar] [CrossRef]
- Rincón, B.; Rodríguez-Gutiérrez, G.; Bujalance, L.; Fernández-Bolaños, J.; Borja, R. Influence of a steam-explosion pre-treatment on the methane yield and kinetics of anaerobic digestion of two-phase olive mill solid waste or alperujo. Process Saf. Environ. Prot. 2016, 102, 361–369. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.R.; Khraisheh, M.A.M.; Shawaqfah, M. Enhancement of Biogas Production from Agricultural Wastes via Pre-Treatment with Advanced Oxidation Processes. Fuel 2019, 253, 964–974. [Google Scholar] [CrossRef]
- Shang, G.; Zhang, C.; Wang, F.; Qiu, L.; Guo, X.; Xu, F. Liquid hot water pretreatment to enhance the anaerobic digestion of wheat straw—Effects of temperature and retention time. Environ. Sci. Pollut. Res. 2019, 26, 29424–29434. [Google Scholar] [CrossRef] [PubMed]
- Phuttaro, C.; Sawatdeenarunat, C.; Surendra, K.C.; Boonsawang, P.; Chaiprapat, S.; Khanal, S.K. Anaerobic Digestion of Hydrothermally-Pretreated Lignocellulosic Biomass: Influence of Pretreatment Temperatures, Inhibitors and Soluble Organics on Methane Yield. Bioresour. Technol. 2019, 284, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Fu, S.; Yang, Z.; Lu, J.; Guo, R. Improved methane production from corn straw by microaerobic pretreatment with a pure bacteria system. Bioresour. Technol. 2018, 259, 18–23. [Google Scholar] [CrossRef]
- Zhao, J.; Ge, X.; Vasco-Correa, J.; Li, Y. Fungal pretreatment of unsterilized yard trimmings for enhanced methane production by solid-state anaerobic digestion. Bioresour. Technol. 2014, 158, 248–252. [Google Scholar] [CrossRef]
- Fu, S.F.; Wang, F.; Yuan, X.Z.; Yang, Z.M.; Luo, S.J.; Wang, C.S.; Guo, R.B. The thermophilic (55 °C) microaerobic pretreatment of corn straw for anaerobic digestion. Bioresour. Technol. 2015, 175, 203–208. [Google Scholar] [CrossRef]
- Ziemiński, K.; Kowalska-Wentel, M. Effect of enzymatic pretreatment on anaerobic co-digestion of sugar beet pulp silage and vinasse. Bioresour. Technol. 2015, 180, 274–280. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Poulsen, T.G.; Sheng, K. Fungal Pretreatment of Rice Straw with Pleurotus Ostreatus and Trichoderma Reesei to Enhance Methane Production under Solid-State Anaerobic Digestion. Appl. Energy 2016, 180, 661–671. [Google Scholar] [CrossRef]
- Deng, Y.; Dai, B.; Xu, J.; Liu, X.; Xu, J. Anaerobic co-digestion of rice straw and soybean straw to increase biogas production by pretreatment with Trichoderma reesei RUT C30. Environ. Prog. Sustain. Energy 2018, 37, 1050–1057. [Google Scholar] [CrossRef]
- Kovács, E.; Szűcs, C.; Farkas, A.; Szuhaj, M.; Maróti, G.; Bagi, Z.; Rákhely, G.; Kovács, K.L. Pretreatment of Lignocellulosic Biogas Substrates by Filamentous Fungi. J. Biotechnol. 2022, 360, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Ilo, O.P.; Nkomo, S.P.L.; Mkhize, N.M.; Mutanga, O.; Simatele, M.D. The effects of Trichoderma atroviride pretreatment on the biogas production from anaerobic digestion of water hyacinth. Energy Environ. 2022. [Google Scholar] [CrossRef]
- Mutschlechner, M.; Illmer, P.; Wagner, A.O. Biological pre-treatment: Enhancing biogas production using the highly cellulolytic fungus Trichoderma viride. Waste Manag. 2015, 43, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zheng, Z.; Cai, Y.; Zhao, Y.; Zhang, Y.; Gao, Y.; Cui, Z.; Wang, X. Accelerated biomethane production from lignocellulosic biomass: Pretreated by mixed enzymes secreted by Trichoderma viride and Aspergillus sp. Bioresour. Technol. 2020, 309, 123378. [Google Scholar] [CrossRef]
- Czekała, W.; Dach, J.; Dong, R.; Janczak, D.; Malińska, K.; Jóźwiakowski, K.; Smurzyńska, A.; Cieślik, M. Composting potential of the solid fraction of digested pulp produced by a biogas plant. Biosyst. Eng. 2017, 160, 25–29. [Google Scholar] [CrossRef]
- Logan, M.; Visvanathan, C. Management strategies for anaerobic digestate of organic fraction of municipal solid waste: Current status and future prospects. Waste Manag. Res. 2019, 37 (Suppl. S1), 27–39. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrere, H. New opportunities for agricultural digestate valorization: Current situation and perspectives. Energy Environ. Sci. 2015, 8, 2600–2621. [Google Scholar] [CrossRef]
- Lamolinara, B.; Pérez-Martínez, A.; Guardado-Yordi, E.; Guillén Fiallos, C.; Diéguez-Santana, K.; Ruiz-Mercado, G.J. Anaerobic Digestate Management, Environmental Impacts, and Techno-Economic Challenges. Waste Manag. 2022, 140, 14–30. [Google Scholar] [CrossRef]
- Koszel, M.; Kocira, A.; Lorencowicz, E. The evaluation of the use of biogas plant digestate as a fertilizer in alfalfa and spring wheat cultivation. Fresenius Environ. Bull. 2016, 25, 3258–3264. [Google Scholar]
- Panuccio, M.R.; Papalia, T.; Attinà, E.; Giuffrè, A.M.; Muscolo, A. Use of digestate as an alternative to mineral fertilizer: Effects on growth and crop quality. Arch. Agron. Soil Sci. 2019, 65, 700–711. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Mallamaci, C.; Attinà, E.; Muscolo, A. Using Digestate as Fertilizer for a Sustainable Tomato Cultivation. Sustainability 2021, 13, 1574. [Google Scholar] [CrossRef]
- Tiwary, A.; Williams, I.D.; Pant, D.C.; Kishore, V.V.N. Emerging Perspectives on Environmental Burden Minimisation Initiatives from Anaerobic Digestion Technologies for Community Scale Biomass Valorisation. Renew. Sustain. Energy Rev. 2015, 42, 883–901. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.J. Valorization of anaerobic digestion digestate: A prospect review. Bioresour. Technol. 2020, 323, 124626. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarska, A.A. The Problem of Weed Infestation of Agricultural Plantations vs. the Assumptions of the European Biodiversity Strategy. Agronomy 2022, 12, 1808. [Google Scholar] [CrossRef]
- Bulgari, D.; Renzetti, S.; Messgo-Moumene, S.; Monti, E.; Gobbi, E. Optimization of Esterase Production in Solid-State Fermentation of Agricultural Digestate. Fermentation 2023, 9, 524. [Google Scholar] [CrossRef]
- Escamilla-Alvarado, C.; Poggi-Varaldo, H.; Ponce-Noyola, M. Use of organic waste for the production of added-value holocellulases with Cellulomonas flavigena PR-22 and Trichoderma reesei MCG 80. Waste Manag. Res. 2013, 31, 849–858. [Google Scholar] [CrossRef]

| Pre-Treatment Methods | Biogas or Biomethane Yield | References | |
|---|---|---|---|
| Physical methods | milling | methane yield: 378.75 mL/g VS methane yield in control: 303 mL/g VS | [73] |
| ultrasound | biogas yield: 396 mL/g VS biogas yield in control: 139 mL/g VS | [74] | |
| microwave irradiation | methane yield: 221 mL/g TS methane yield in control: 137.18 mL/g TS | [75] | |
| high hydrostatic pressure | methane yield: 77.9 mL/g TS methane yield in control: 31.8 mL/g TS | [76] | |
| Chemical methods | potassium hydroxide | methane yield: 258 mL/g VS methane yield in control: 184 mL/g VS | [77] |
| sulfuric acid | biogas yield: 424.3 mL/g VS biogas yield in control: 183.32 mL/g VS | [78] | |
| ethanol | methane yield: 155.4 mL/g VS methane yield in control: 75.3 mL/g VS | [79] | |
| ozonolysis | methane yield: 432.7 mL/g VS methane yield in control: 260 mL/g VS | [80] | |
| Thermal methods | steam explosion | methane yield: 589 mL/g VS methane yield in control: 366 mL/g VS | [81] |
| advanced wet oxidation | methane yield: 289.2 mL/g VS methane yield in control: 220 mL/g VS | [82] | |
| liquid hot water | methane yield: 202.81 mL/g VS methane yield in control: 124.51 mL/g VS | [83] | |
| hydrothermal | methane yield: 248.2 mL/g VS methane yield in control: 183.85 mL/g VS | [84] | |
| Biological methods | Bacillus subtilis | methane yield: 270.8 mL/g VS methane yield in control: 230.7 mL/g VS | [85] |
| Ceriporiopsis subvermispora | methane yield: 44.6 L/kg VS methane yield in control: 20 L/kg VS | [86] | |
| consortium of thermophilic microorganisms | methane yield: 325.7 mL/g VS methane yield in control: 273.7 mL/g VS | [87] | |
| endoglucanase, xylanase and pectinase | biogas yield: 765.5 mL/g VS biogas yield in control: 529.1 mL/g VS methane yield: 465.4 mL/g VS methane yield in control: 295.2 mL/g VS | [88] | |
| Species of Trichoderma | Biogas or Biomethane Yield | References |
|---|---|---|
| Trichoderma atroviride | biogas yield: 223.4 mL/g VS biogas yield in control: 135 mL/g VS methane yield: 200 mL/g VS methane yield in control: 91.84 mL/g VS | [92] |
| Trichoderma viride | biogas yield: 703.7 mL/g VS biogas yield in control: 379.5 mL/g VS methane yield: 356.1 mL/g VS methane yield in control: 194.4 mL/g VS | [93] |
| Trichoderma viride | biogas yield: 790 mL/g VS biogas yield in control: 553.7 mL/g VS methane yield: 447.7 mL/g VS methane yield in control: 314.12 mL/g VS | [93] |
| Trichoderma viride | biogas yield: 840.9 mL/g VS biogas yield in control: 367.4 mL/g VS methane yield: 439.5 mL/g VS methane yield in control: 133.3 mL/g VS | [93] |
| Trichoderma viride | biogas yield: 1299.4 mL/g VS biogas yield in control: 688.3 mL/g VS methane yield: 722.6 mL/g VS methane yield in control: 312.3 mL/g VS | [93] |
| Trichoderma viride | methane yield: 419.63 mL/g TS methane yield in control: 389.13 mL/g TS | [94] |
| Trichoderma viride | biogas yield: 100.79 mL/g VS biogas yield in control: 66.16 mL/g VS methane yield: 23.42 mL/g VS methane yield in control: 11.41 mL/g VS | [46] |
| Trichoderma viride | biogas yield: 150.19 mL/g VS biogas yield in control: 66.16 mL/g VS methane yield: 47.36 mL/g VS methane yield in control: 11.41 mL/g VS | [46] |
| Trichoderma reesei | methane yield: 91.6 NmL/g TS methane yield in control: 9.4 NmL/g TS | [90] |
| Trichoderma reesei | methane yield: 90.1 NmL/g TS methane yield in control: 9.2 NmL/g TS | [90] |
| Trichoderma reesei | methane yield: 94.3 NmL/g TS methane yield in control: 10.4 NmL/g TS | [90] |
| Trichoderma reesei | methane yield: 214 L/kg VS methane yield in control: 127 L/kg VS | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubiak, A.; Pilarska, A.A.; Wolna-Maruwka, A.; Niewiadomska, A.; Panasiewicz, K. The Use of Fungi of the Trichoderma Genus in Anaerobic Digestion: A Review. Int. J. Mol. Sci. 2023, 24, 17576. https://doi.org/10.3390/ijms242417576
Kubiak A, Pilarska AA, Wolna-Maruwka A, Niewiadomska A, Panasiewicz K. The Use of Fungi of the Trichoderma Genus in Anaerobic Digestion: A Review. International Journal of Molecular Sciences. 2023; 24(24):17576. https://doi.org/10.3390/ijms242417576
Chicago/Turabian StyleKubiak, Adrianna, Agnieszka A. Pilarska, Agnieszka Wolna-Maruwka, Alicja Niewiadomska, and Katarzyna Panasiewicz. 2023. "The Use of Fungi of the Trichoderma Genus in Anaerobic Digestion: A Review" International Journal of Molecular Sciences 24, no. 24: 17576. https://doi.org/10.3390/ijms242417576
APA StyleKubiak, A., Pilarska, A. A., Wolna-Maruwka, A., Niewiadomska, A., & Panasiewicz, K. (2023). The Use of Fungi of the Trichoderma Genus in Anaerobic Digestion: A Review. International Journal of Molecular Sciences, 24(24), 17576. https://doi.org/10.3390/ijms242417576









