Artemisia annua L. Polyphenols Enhance the Anticancer Effect of β-Lapachone in Oxaliplatin-Resistant HCT116 Colorectal Cancer Cells
Abstract
1. Introduction
2. Results
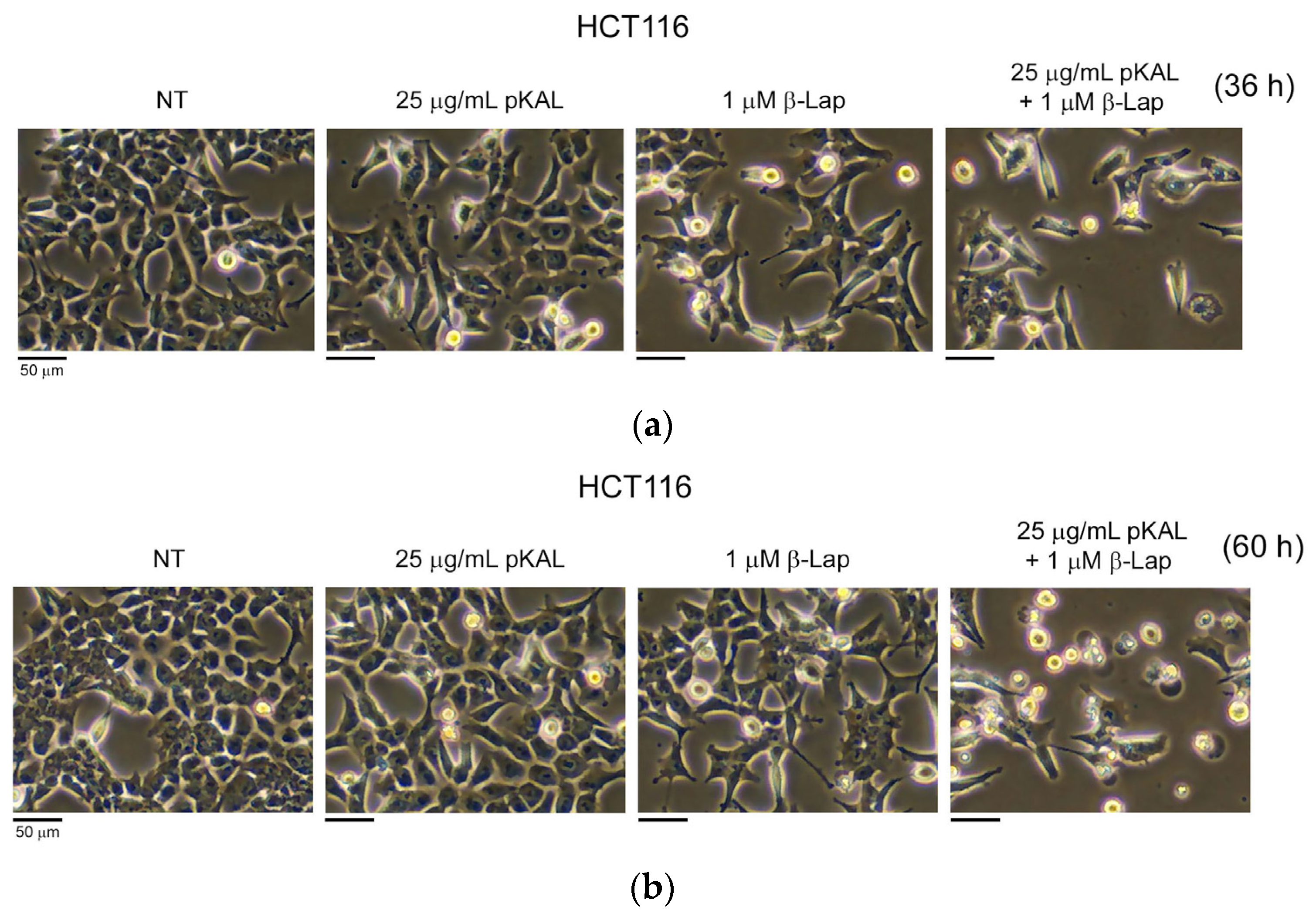
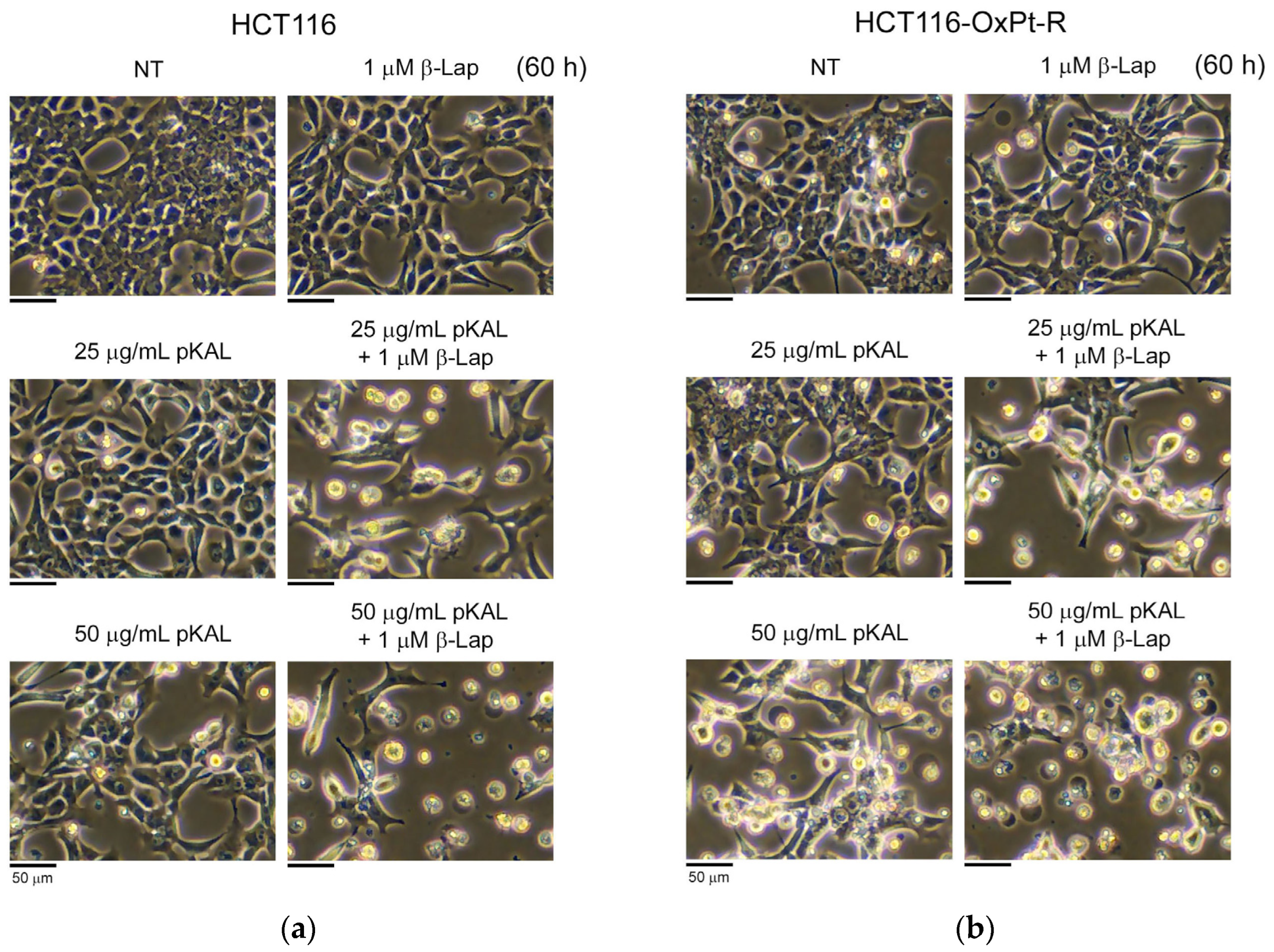
2.1. Anticancer Effect by Combined Treatment of pKAL and β-Lap in HCT116 Cells
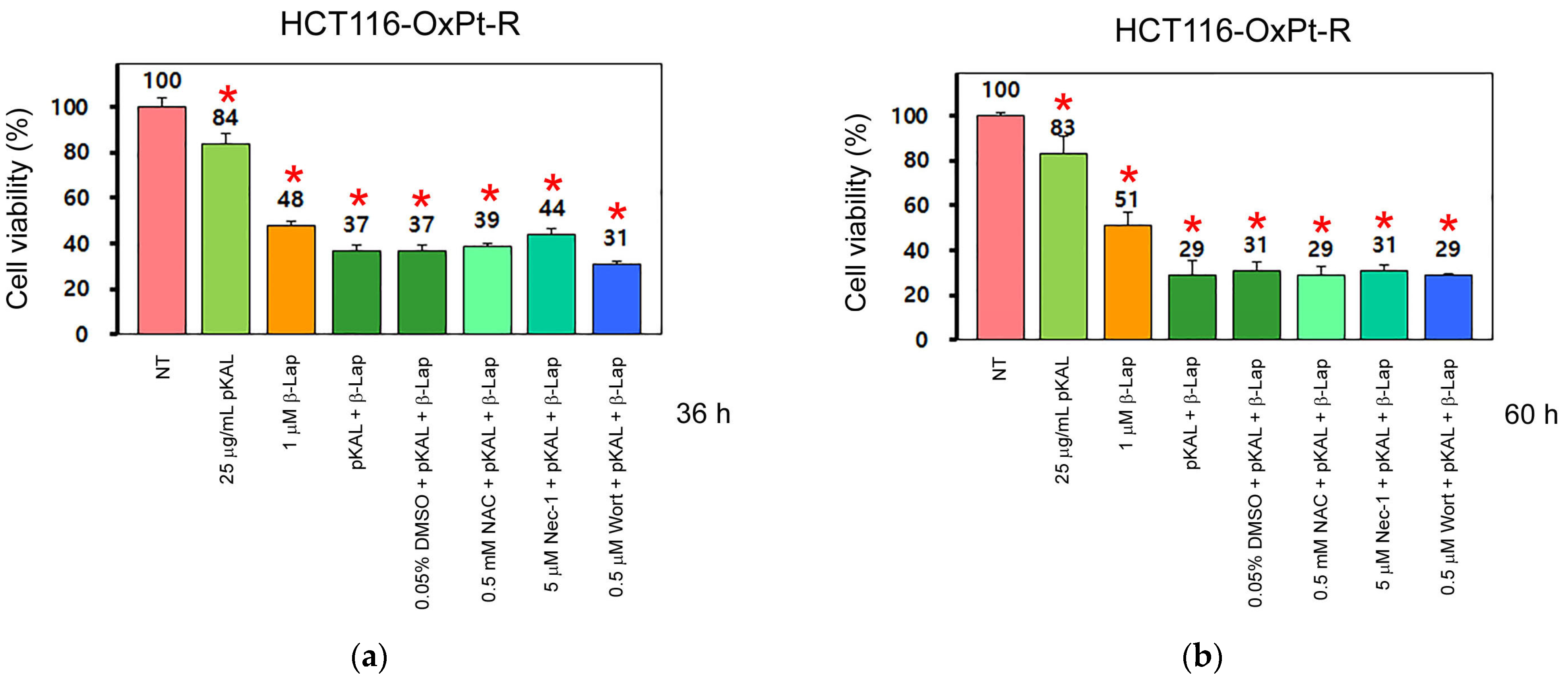
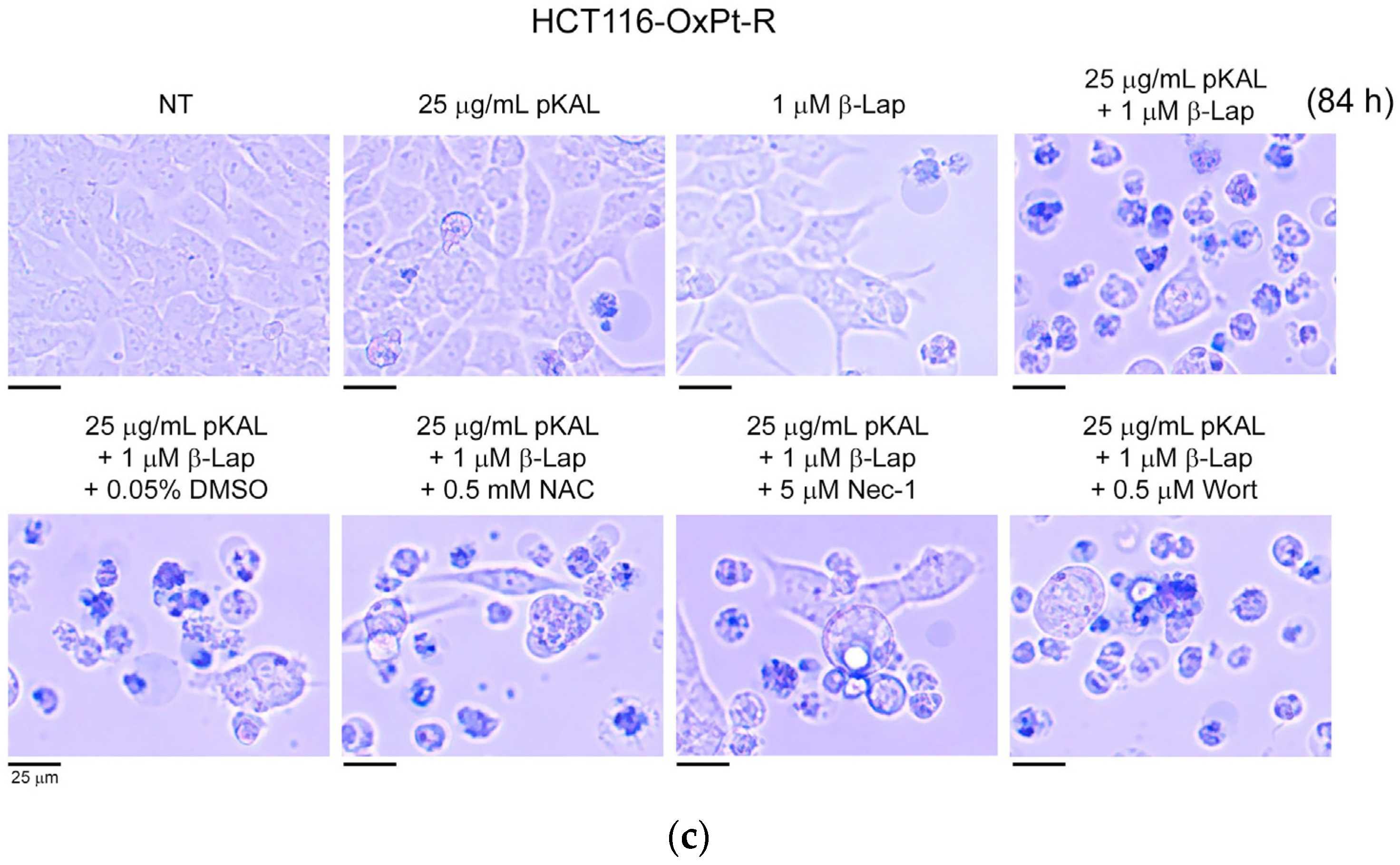
2.2. Anticancer Effect by Combined Treatment of pKAL and β-Lap in HCT116-OxPt-R Cells
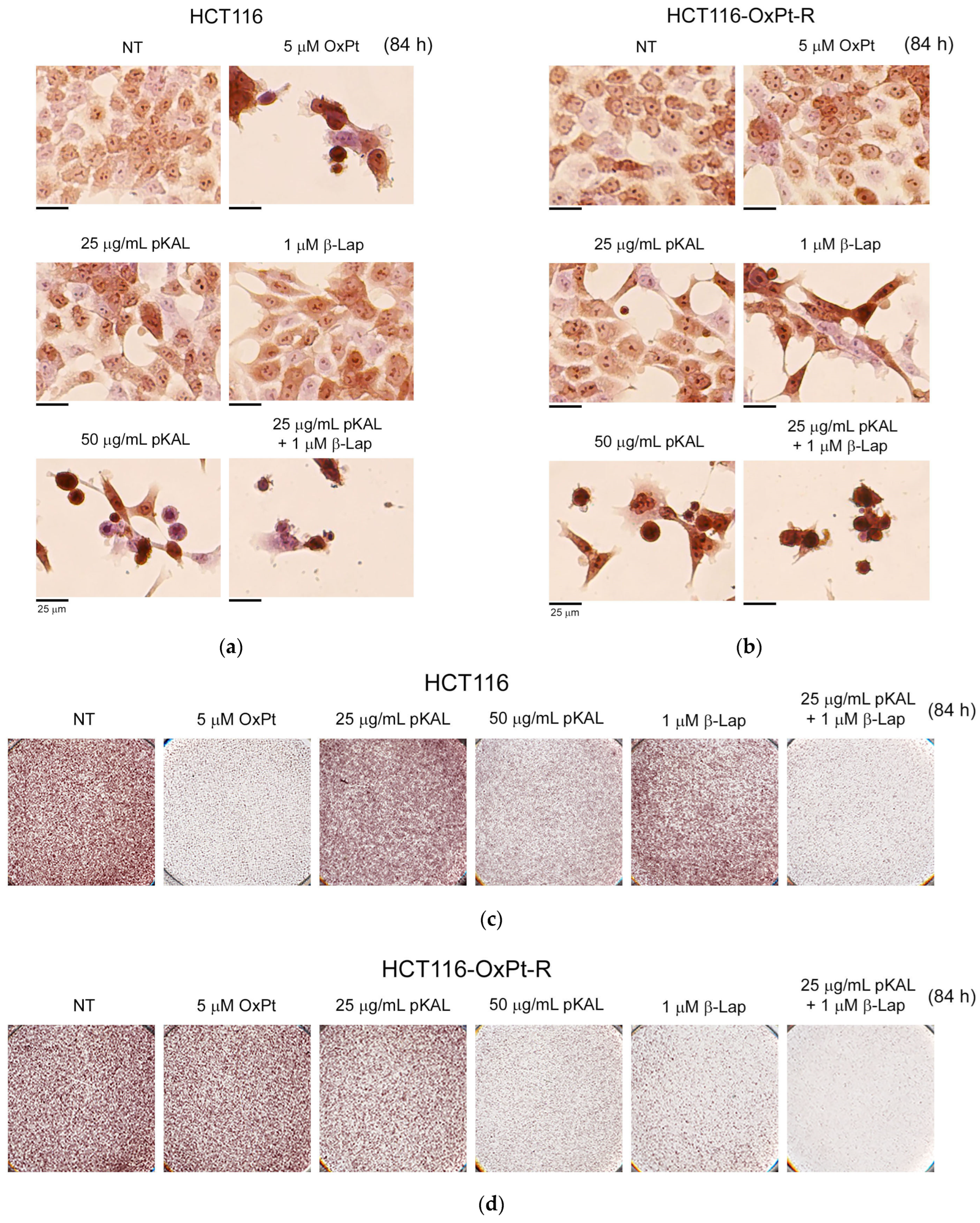
2.3. Comparison of Anticancer Effects between HCT116 and HCT116-OxPt-R Cells by Combined Treatment of pKAL and β-Lap
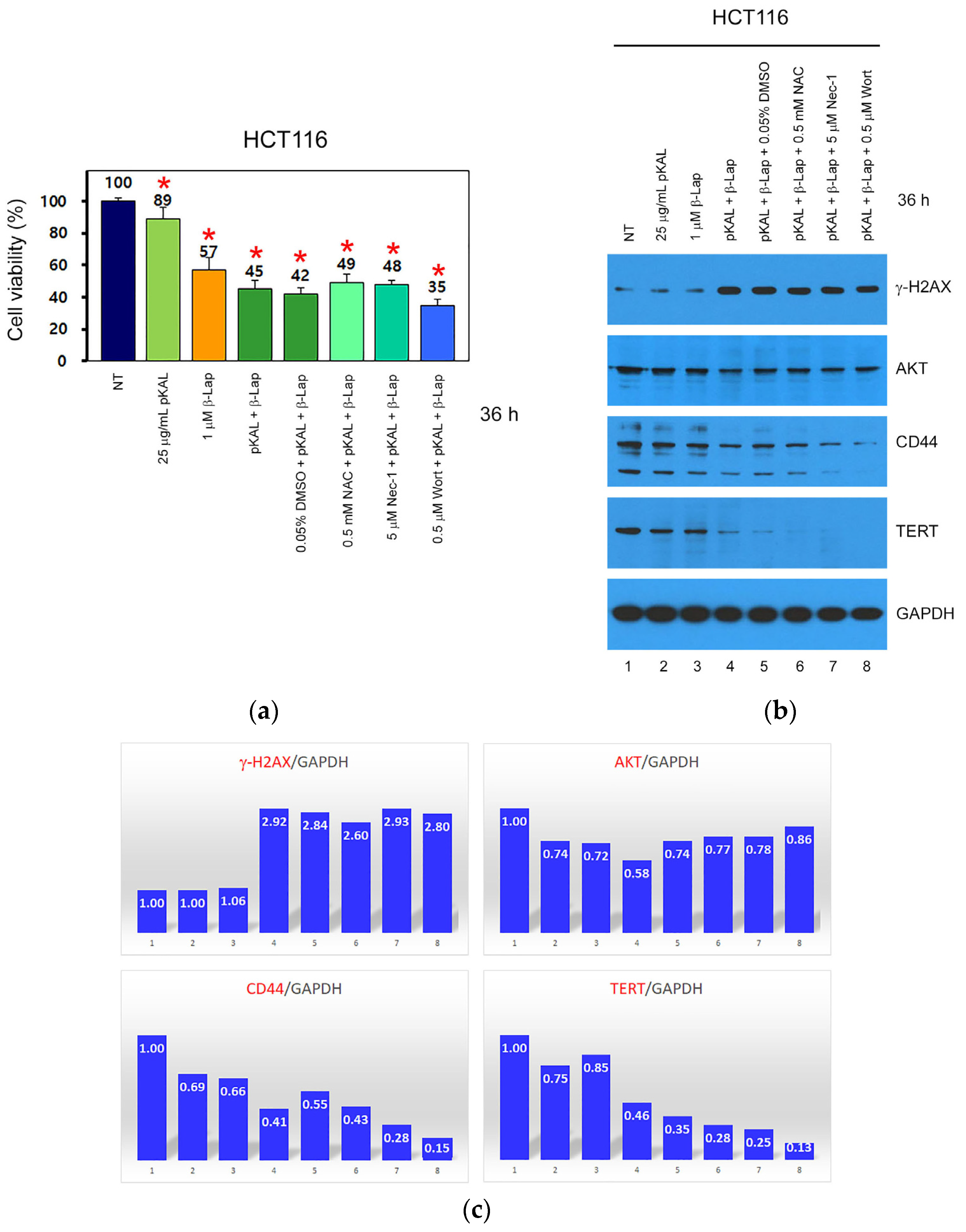
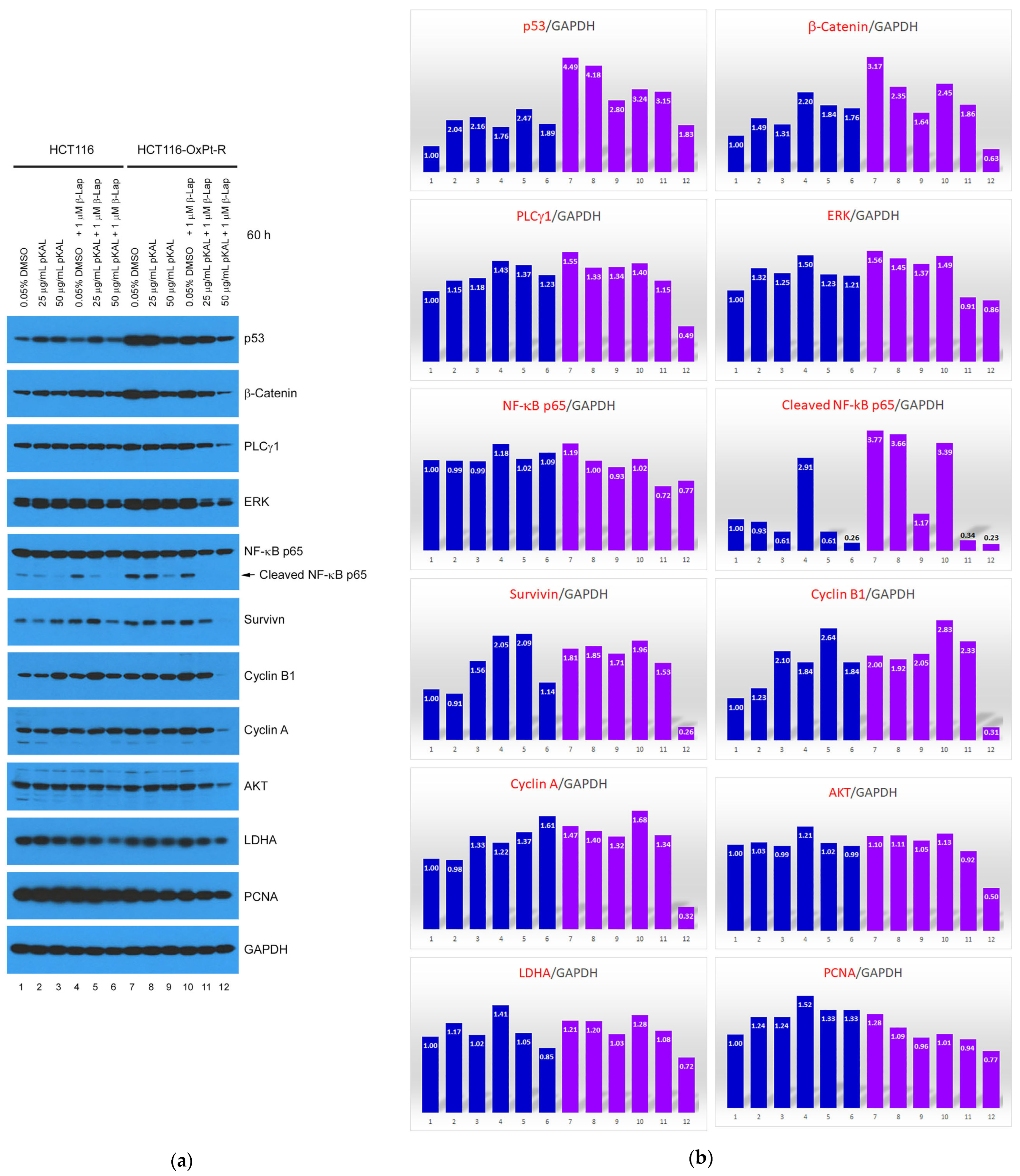
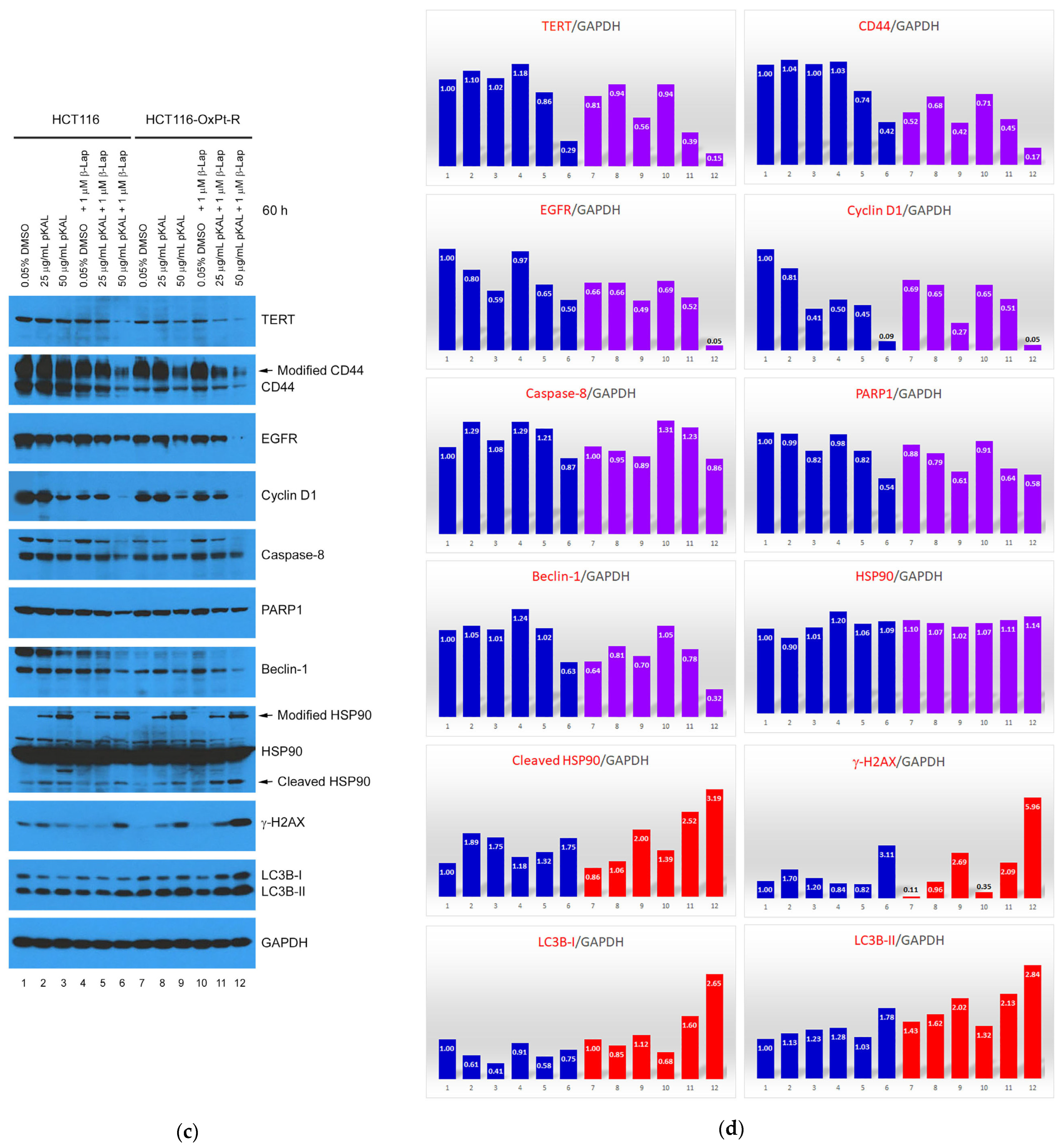
2.4. Anticancer Mechanism of Combined Treatment of pKAL and β-Lap in HCT116 and HCT116-OxPt-R Cells
2.5. Bioinformatics Analysis for21 Proteins Regulated by Combined Treatment of pKAL and β-Lap in HCT116-OxPt-R Cells
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. pKAL Compounds
4.3. Cell Culture
4.4. Phase-Contrast Microscopy
4.5. Cell Viability Analysis
4.6. Western Blot and Densitometry Analysis
4.7. Phase-Contrast Microscopy of Trypan Blue-Stained Cells
4.8. Phase-Contrast Microscopy of Hematoxylin-Stained Cells
4.9. Bioinformatics Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, S.; Xu, S.; Fang, J.; Jiang, H. The Protective Effect of Polyphenols for Colorectal Cancer. Front. Immunol. 2020, 11, 1407. [Google Scholar] [CrossRef]
- Bracci, L.; Fabbri, A.; Del Corno, M.; Conti, L. Dietary Polyphenols: Promising Adjuvants for Colorectal Cancer Therapies. Cancers 2021, 13, 4499. [Google Scholar] [CrossRef]
- Dana, P.M.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The role of polyphenols in overcoming cancer drug resistance: A comprehensive review. Cell. Mol. Biol. Lett. 2022, 27, 1. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jin, P.; Guan, Y.; Luo, M.; Wang, Y.; He, B.; Li, B.; He, K.; Cao, J.; Huang, C.; et al. Exploiting Polyphenol-Mediated Redox Reorientation in Cancer Therapy. Pharmaceuticals 2022, 15, 1540. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Reale, M.; Ullah, H.; Sureda, A.; Tejada, S.; Wang, Y.; Zhang, Z.J.; Xiao, J. Anti-cancer effects of polyphenols via targeting p53 signaling pathway: Updates and future directions. Biotechnol. Adv. 2020, 38, 107385. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.J.; Lee, W.S.; Paramanantham, A.; Kim, H.J.; Shin, S.C.; Kim, G.S.; Jung, J.M.; Ryu, C.H.; Hong, S.C.; Chung, K.H.; et al. p53 Enhances Artemisia annua L. Polyphenols-Induced Cell Death through Upregulation of p53-Dependent Targets and Cleavage of PARP1 and Lamin A/C in HCT116 Colorectal Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9315. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.J.; Paramanantham, A.; Kim, H.J.; Shin, S.C.; Kim, G.S.; Jung, J.M.; Ryu, C.H.; Hong, S.C.; Chung, K.H.; Kim, C.W.; et al. Artemisia annua L. Polyphenol-Induced Cell Death Is ROS-Independently Enhanced by Inhibition of JNK in HCT116 Colorectal Cancer Cells. Int. J. Mol. Sci. 2021, 22, 1366. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.; Sinkala, M. Cancer Stem Cell Marker CD44 Plays Multiple Key Roles in Human Cancers: Immune Suppression/Evasion, Drug Resistance, Epithelial-Mesenchymal Transition, and Metastasis. OMICS 2021, 25, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Sethi, K.; Sarkar, S.; Das, S.; Rajput, S.; Mazumder, A.; Roy, B.; Patra, S.; Mohanty, B.; El-Naggar, A.K.; Mandal, M. Expressions of CK-19, NF-kappaB, E-cadherin, beta-catenin and EGFR as diagnostic and prognostic markers by immunohistochemical analysis in thyroid carcinoma. J. Exp. Ther. Oncol. 2011, 9, 187–199. [Google Scholar]
- Gomes, C.L.; de Albuquerque Wanderley Sales, V.; Gomes de Melo, C.; Ferreira da Silva, R.M.; Vicente Nishimura, R.H.; Rolim, L.A.; Rolim Neto, P.J. Beta-lapachone: Natural occurrence, physicochemical properties, biological activities, toxicity and synthesis. Phytochemistry 2021, 186, 112713. [Google Scholar] [CrossRef]
- Gong, Q.; Hu, J.; Wang, P.; Li, X.; Zhang, X. A comprehensive review on beta-lapachone: Mechanisms, structural modifications, and therapeutic potentials. Eur. J. Med. Chem. 2021, 210, 112962. [Google Scholar] [CrossRef] [PubMed]
- Sunassee, S.N.; Veale, C.G.; Shunmoogam-Gounden, N.; Osoniyi, O.; Hendricks, D.T.; Caira, M.R.; de la Mare, J.A.; Edkins, A.L.; Pinto, A.V.; da Silva Junior, E.N.; et al. Cytotoxicity of lapachol, beta-lapachone and related synthetic 1,4-naphthoquinones against oesophageal cancer cells. Eur. J. Med. Chem. 2013, 62, 98–110. [Google Scholar] [CrossRef]
- Kim, I.; Kim, H.; Ro, J.; Jo, K.; Karki, S.; Khadka, P.; Yun, G.; Lee, J. Preclinical Pharmacokinetic Evaluation of beta-Lapachone: Characteristics of Oral Bioavailability and First-Pass Metabolism in Rats. Biomol. Ther. 2015, 23, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Pink, J.J.; Planchon, S.M.; Tagliarino, C.; Varnes, M.E.; Siegel, D.; Boothman, D.A. NAD(P)H:Quinone oxidoreductase activity is the principal determinant of beta-lapachone cytotoxicity. J. Biol. Chem. 2000, 275, 5416–5424. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Cho, J.Y. NQO1 is Required for beta-Lapachone-Mediated Downregulation of Breast-Cancer Stem-Cell Activity. Int. J. Mol. Sci. 2018, 19, 3813. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Jiang, L.; Fang, T.; Fang, F.; Liu, Y.; Zhao, Y.; You, Y.; Zhou, H.; Su, X.; Wang, J.; et al. Beta-Lapachone Selectively Kills Hepatocellular Carcinoma Cells by Targeting NQO1 to Induce Extensive DNA Damage and PARP1 Hyperactivation. Front. Oncol. 2021, 11, 747282. [Google Scholar] [CrossRef]
- Yu, H.Y.; Kim, S.O.; Jin, C.Y.; Kim, G.Y.; Kim, W.J.; Yoo, Y.H.; Choi, Y.H. Beta-lapachone-Induced Apoptosis of Human Gastric Carcinoma AGS Cells Is Caspase-Dependent and Regulated by the PI3K/Akt Pathway. Biomol. Ther. 2014, 22, 184–192. [Google Scholar] [CrossRef]
- Choi, B.T.; Cheong, J.; Choi, Y.H. Beta-Lapachone-induced apoptosis is associated with activation of caspase-3 and inactivation of NF-kappaB in human colon cancer HCT-116 cells. Anticancer Drugs 2003, 14, 845–850. [Google Scholar] [CrossRef]
- Park, E.J.; Min, K.J.; Lee, T.J.; Yoo, Y.H.; Kim, Y.S.; Kwon, T.K. Beta-Lapachone induces programmed necrosis through the RIP1-PARP-AIF-dependent pathway in human hepatocellular carcinoma SK-Hep1 cells. Cell Death Dis. 2014, 5, e1230. [Google Scholar] [CrossRef]
- Moon, D.O.; Kang, C.H.; Kim, M.O.; Jeon, Y.J.; Lee, J.D.; Choi, Y.H.; Kim, G.Y. Beta-lapachone (LAPA) decreases cell viability and telomerase activity in leukemia cells: Suppression of telomerase activity by LAPA. J. Med. Food 2010, 13, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Li, C.J.; Averboukh, L.; Pardee, A.B. Beta-Lapachone, a novel DNA topoisomerase I inhibitor with a mode of action different from camptothecin. J. Biol. Chem. 1993, 268, 22463–22468. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, P.; Bastow, K.F. Novel mechanism of cellular DNA topoisomerase II inhibition by the pyranonaphthoquinone derivatives alpha-lapachone and beta-lapachone. Cancer Chemother. Pharmacol. 2001, 47, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Choi, K.S.; Kwon, T.K. Beta-Lapachone-induced reactive oxygen species (ROS) generation mediates autophagic cell death in glioma U87 MG cells. Chem. Biol. Interact. 2011, 189, 37–44. [Google Scholar] [CrossRef]
- Planchon, S.M.; Wuerzberger, S.; Frydman, B.; Witiak, D.T.; Hutson, P.; Church, D.R.; Wilding, G.; Boothman, D.A. Beta-lapachone-mediated apoptosis in human promyelocytic leukemia (HL-60) and human prostate cancer cells: A p53-independent response. Cancer Res. 1995, 55, 3706–3711. [Google Scholar]
- Huang, L.; Pardee, A.B. Beta-lapachone induces cell cycle arrest and apoptosis in human colon cancer cells. Mol. Med. 1999, 5, 711–720. [Google Scholar] [CrossRef] [PubMed]
- D’Anneo, A.; Augello, G.; Santulli, A.; Giuliano, M.; di Fiore, R.; Messina, C.; Tesoriere, G.; Vento, R. Paclitaxel and beta-lapachone synergistically induce apoptosis in human retinoblastoma Y79 cells by downregulating the levels of phospho-Akt. J. Cell Physiol. 2010, 222, 433–443. [Google Scholar] [CrossRef]
- Kim, E.J.; Ji, I.M.; Ahn, K.J.; Choi, E.K.; Park, H.J.; Lim, B.U.; Song, C.W.; Park, H.J. Synergistic effect of ionizing radiation and beta-Lapachone against RKO human colon adenocarcinoma cells. Cancer Res. Treat. 2005, 37, 183–190. [Google Scholar] [CrossRef][Green Version]
- Falcone, A.; Ricci, S.; Brunetti, I.; Pfanner, E.; Allegrini, G.; Barbara, C.; Crinò, L.; Benedetti, G.; Evangelista, W.; Fanchini, L.; et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: The Gruppo Oncologico Nord Ovest. J. Clin. Oncol. 2007, 25, 1670–1676. [Google Scholar] [CrossRef]
- Cassidy, J.; Clarke, S.; Díaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.S.; Rivera, F.; et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 2006–2012. [Google Scholar] [CrossRef]
- Jung, E.J.; Kim, H.J.; Shin, S.C.; Kim, G.S.; Jung, J.M.; Hong, S.C.; Kim, C.W.; Lee, W.S. Beta-Lapachone Exerts Anticancer Effects by Downregulating p53, Lys-Acetylated Proteins, TrkA, p38 MAPK, SOD1, Caspase-2, CD44 and NPM in Oxaliplatin-Resistant HCT116 Colorectal Cancer Cells. Int. J. Mol. Sci. 2023, 24, 9867. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Cao, S.; Qiu, F.; Zhang, B. Traditional application and modern pharmacological research of Artemisia annua L. Pharmacol. Ther. 2020, 216, 107650. [Google Scholar] [CrossRef]
- Efferth, T. Cancer combination therapies with artemisinin-type drugs. Biochem. Pharmacol. 2017, 139, 56–70. [Google Scholar] [CrossRef]
- Kadioglu, O.; Chan, A.; Cong Ling Qiu, A.; Wong, V.K.W.; Colligs, V.; Wecklein, S.; Freund-Henni Rached, H.; Efferth, T.; Hsiao, W.W. Artemisinin Derivatives Target Topoisomerase 1 and Cause DNA Damage in Silico and in Vitro. Front. Pharmacol. 2017, 8, 711. [Google Scholar] [CrossRef]
- Li, P.C.; Lam, E.; Roos, W.P.; Zdzienicka, M.Z.; Kaina, B.; Efferth, T. Artesunate derived from traditional Chinese medicine induces DNA damage and repair. Cancer Res. 2008, 68, 4347–4351. [Google Scholar] [CrossRef] [PubMed]
- Berdelle, N.; Nikolova, T.; Quiros, S.; Efferth, T.; Kaina, B. Artesunate induces oxidative DNA damage, sustained DNA double-strand breaks, and the ATM/ATR damage response in cancer cells. Mol. Cancer Ther. 2011, 10, 2224–2233. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, F.W.; Saeed, M.E.; Schwarzkopf, J.; Efferth, T. Activity of Artemisia annua and artemisinin derivatives, in prostate carcinoma. Phytomedicine 2015, 22, 1223–1231. [Google Scholar] [CrossRef]
- Lang, S.J.; Schmiech, M.; Hafner, S.; Paetz, C.; Steinborn, C.; Huber, R.; Gaafary, M.E.; Werner, K.; Schmidt, C.Q.; Syrovets, T.; et al. Antitumor activity of an Artemisia annua herbal preparation and identification of active ingredients. Phytomedicine 2019, 62, 152962. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, K.; Yan, C.; Yin, Y.; He, S.; Qiu, L.; Li, G. Natural Polyphenols for Treatment of Colorectal Cancer. Molecules 2022, 27, 8810. [Google Scholar] [CrossRef]
- Vuoso, D.C.; D’Angelo, S.; Ferraro, R.; Caserta, S.; Guido, S.; Cammarota, M.; Porcelli, M.; Cacciapuoti, G. Annurca apple polyphenol extract promotes mesenchymal-to-epithelial transition and inhibits migration in triple-negative breast cancer cells through ROS/JNK signaling. Sci. Rep. 2020, 10, 15921. [Google Scholar] [CrossRef]
- Ko, Y.S.; Lee, W.S.; Panchanathan, R.; Joo, Y.N.; Choi, Y.H.; Kim, G.S.; Jung, J.M.; Ryu, C.H.; Shin, S.C.; Kim, H.J. Polyphenols from Artemisia annua L. Inhibit Adhesion and EMT of Highly Metastatic Breast Cancer Cells MDA-MB-231. Phytother. Res. 2016, 30, 1180–1188. [Google Scholar] [CrossRef]
- Vladu, A.F.; Ficai, D.; Ene, A.G.; Ficai, A. Combination Therapy Using Polyphenols: An Efficient Way to Improve Antitumoral Activity and Reduce Resistance. Int. J. Mol. Sci. 2022, 23, 10244. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.S.; Jung, E.J.; Go, S.I.; Jeong, B.K.; Kim, G.S.; Jung, J.M.; Hong, S.C.; Kim, C.W.; Kim, H.J.; Lee, W.S. Polyphenols Extracted from Artemisia annua L. Exhibit Anti-Cancer Effects on Radio-Resistant MDA-MB-231 Human Breast Cancer Cells by Suppressing Stem Cell Phenotype, beta-Catenin, and MMP-9. Molecules 2020, 25, 1916. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Desta, K.T.; Kim, G.S.; Lee, S.J.; Lee, W.S.; Kim, Y.H.; Jin, J.S.; Abd El-Aty, A.M.; Shin, H.C.; Shim, J.H.; et al. Polyphenolic profile and antioxidant effects of various parts of Artemisia annua L. Biomed. Chromatogr. 2016, 30, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.J.; Paramanantham, A.; Kim, H.J.; Shin, S.C.; Kim, G.S.; Jung, J.M.; Hong, S.C.; Chung, K.H.; Kim, C.W.; Lee, W.S. Identification of Growth Factors, Cytokines and Mediators Regulated by Artemisia annua L. Polyphenols (pKAL) in HCT116 Colorectal Cancer Cells: TGF-beta1 and NGF-beta Attenuate pKAL-Induced Anticancer Effects via NF-kappaB p65 Upregulation. Int. J. Mol. Sci. 2022, 23, 1598. [Google Scholar] [CrossRef]
- Atale, N.; Gupta, S.; Yadav, U.C.; Rani, V. Cell-death assessment by fluorescent and nonfluorescent cytosolic and nuclear staining techniques. J. Microsc. 2014, 255, 7–19. [Google Scholar] [CrossRef]
- Crowley, L.C.; Marfell, B.J.; Christensen, M.E.; Waterhouse, N.J. Measuring Cell Death by Trypan Blue Uptake and Light Microscopy. Cold Spring Harb. Protoc. 2016, 2016, prot087155. [Google Scholar] [CrossRef]

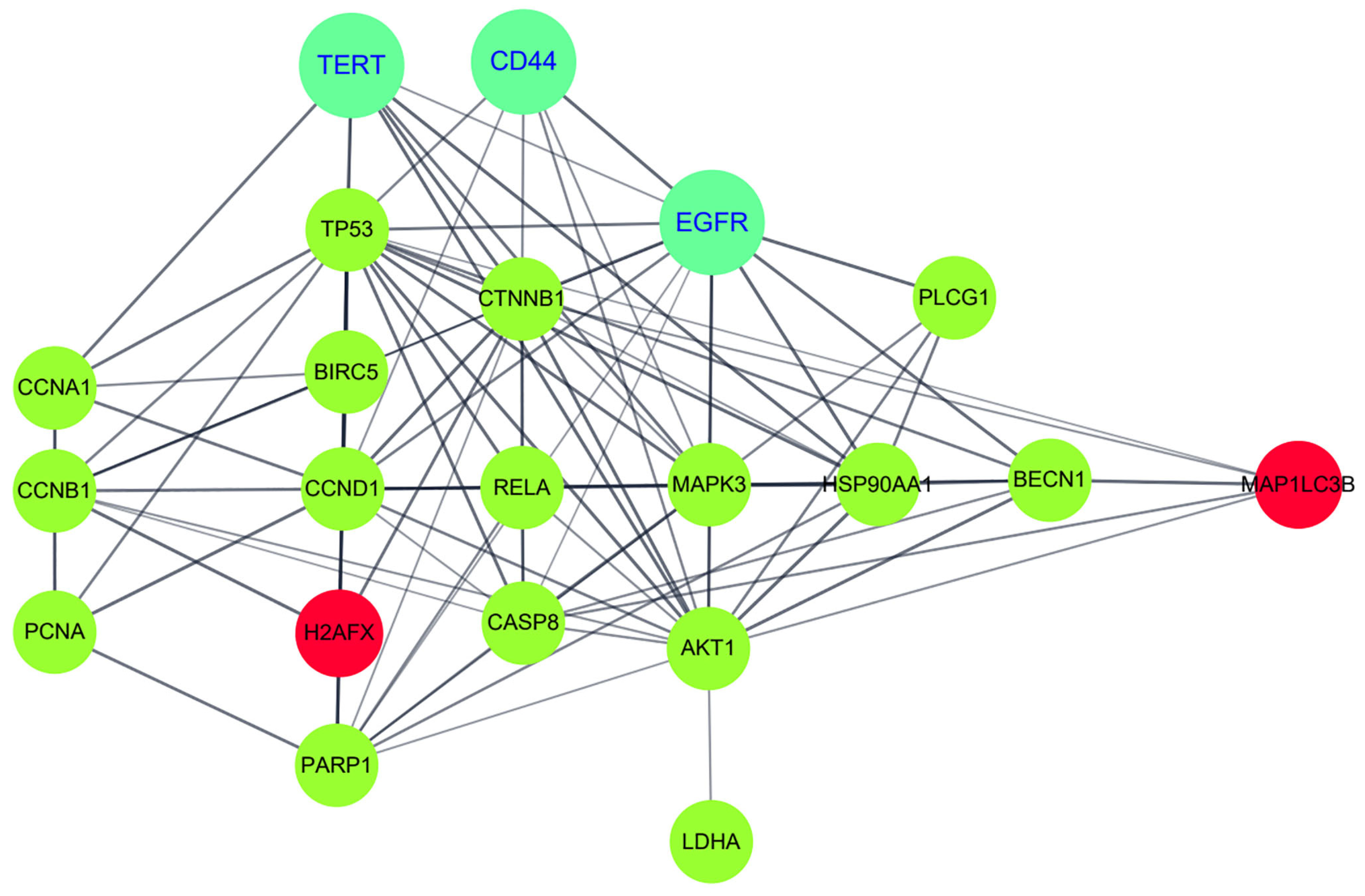
| Gene Symbol | Protein Name | UniProt ID | Keywords |
|---|---|---|---|
| AKT1 | RAC-alpha serine/threonine-protein kinase (AKT1) | P31749 | Kinase; Transferase; Apoptosis; Glycogen biosynthesis; Carbohydrate/Glucose/Glycogen metabolism |
| BECN1 | Beclin-1 | Q14457 | Autophagy; Apoptosis; Cell cycle; Cell division |
| BIRC5 | Baculoviral IAP repeat-containing protein 5 (Survivin) | O15392 | Thiol protease inhibitor; Repressor; Apoptosis; Cell cycle; Cell division; Chromosome partition |
| CASP8 | Caspase-8 | Q14790 | Hydrolase; Thiol protease; Apoptosis |
| CCNA1 | Cyclin-A1 (Cyclin A1) | P78396 | Cell cycle; Cell division; Mitosis |
| CCNB1 | G2/mitotic-specific cyclin-B1 (Cyclin B1) | P14635 | Cell cycle; Cell division; Mitosis |
| CCND1 | G1/S-specific cyclin-D1 (Cyclin D1) | P24385 | Repressor, Cell cycle; Cell division; DNA damage; Transcription |
| CD44 | CD44 antigen (CD44) | P16070 | Blood group antigen; Receptor; Cell adhesion |
| CTNNB1 | Catenin beta-1 (β-Catenin) | P35222 | Activator; Cell adhesion; Neurogenesis; Transcription; Wnt signaling pathway |
| EGFR | Epidermal growth factor receptor (EGFR) | P00533 | Receptor; Tyrosine-protein kinase; Transferase |
| H2AX/H2AFX | Histone H2AX (H2AX) | P16104 | Cell cycle; DNA damage; DNA recombination; DNA repair; Meiosis |
| HSP90AA1 | Heat shock protein 90-alpha (HSP90α) | P07900 | Chaperone; Hydrolase; Stress response |
| LDHA | L-lactate dehydrogenase A chain (LDHA) | P00338 | Oxidoreductase |
| MAP1LC3B | Microtubule-associated proteins 1A/1B light chain 3B (LC3B) | Q9GZQ8 | Autophagy; Ubl conjugation pathway |
| MAPK3 | Mitogen-activated protein kinase 3/p44-ERK1 (ERK1) | P27361 | Serine/threonine-protein kinase; Transferase; Apoptosis; Cell cycle |
| PARP1 | Poly [ADP-ribose] polymerase 1 (PARP1) | P09874 | Transferase; Apoptosis; DNA damage; DNA repair |
| PCNA | Proliferating cell nuclear antigen (PCNA) | P12004 | DNA damage; DNA repair; DNA replication |
| PLCG1 | Phospholipase C-gamma-1 (PLCγ1) | P19174 | Hydrolase; Transducer; Lipid degradation/metabolism |
| RELA | Nuclear factor NF-kappa-B p65 subunit (NF-κB p65) | Q04206 | Activator; Transcription |
| TERT | Telomerase reverse transcriptase (TERT) | O14746 | RNA-directed DNA polymerase; Transferase |
| TP53 | Cellular tumor antigen p53 (p53) | P04637 | Activator; Repressor; Apoptosis; Cell cycle; Necrosis |
| GO_BP_Term | p-Value | Gene symbols |
|---|---|---|
| GO:0043066~negative regulation of apoptotic process | 5.58 × 10−7 | BECN1, CTNNB1, BIRC5, AKT1, TP53, CD44, RELA, EGFR |
| GO:0001934~positive regulation of protein phosphorylation | 2.06 × 10−6 | HSP90AA1, CCND1, BIRC5, AKT1, EGFR, MAPK3 |
| GO:0071364~cellular response to epidermal growth factor stimulus | 1.21 × 10−5 | BECN1, AKT1, PLCG1, EGFR |
| GO:1900087~positive regulation of G1/S transition of mitotic cell cycle | 1.89 × 10−5 | CCND1, TERT, AKT1, EGFR |
| GO:1902895~positive regulation of pri-miRNA transcription from RNA polymerase II promoter | 2.12 × 10−5 | TERT, TP53, RELA, EGFR |
| GO:0042981~regulation of apoptotic process | 9.91 × 10−5 | HSP90AA1, CASP8, BIRC5, AKT1, TP53 |
| GO:0009410~response to xenobiotic stimulus | 1.16 × 10−4 | BECN1, HSP90AA1, CCND1, CTNNB1, TP53 |
| GO:0006974~cellular response to DNA damage stimulus | 2.01 × 10−4 | H2AX/H2AFX, CCND1, PARP1, TP53, MAPK3 |
| GO:0000423~macromitophagy | 2.09 × 10−4 | BECN1, MAP1LC3B, TP53 |
| GO:0044772~mitotic cell cycle phase transition | 2.75 × 10−4 | CCNA1, CCNB1, CCND1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, E.J.; Kim, H.J.; Shin, S.C.; Kim, G.S.; Jung, J.-M.; Hong, S.C.; Kim, C.W.; Lee, W.S. Artemisia annua L. Polyphenols Enhance the Anticancer Effect of β-Lapachone in Oxaliplatin-Resistant HCT116 Colorectal Cancer Cells. Int. J. Mol. Sci. 2023, 24, 17505. https://doi.org/10.3390/ijms242417505
Jung EJ, Kim HJ, Shin SC, Kim GS, Jung J-M, Hong SC, Kim CW, Lee WS. Artemisia annua L. Polyphenols Enhance the Anticancer Effect of β-Lapachone in Oxaliplatin-Resistant HCT116 Colorectal Cancer Cells. International Journal of Molecular Sciences. 2023; 24(24):17505. https://doi.org/10.3390/ijms242417505
Chicago/Turabian StyleJung, Eun Joo, Hye Jung Kim, Sung Chul Shin, Gon Sup Kim, Jin-Myung Jung, Soon Chan Hong, Choong Won Kim, and Won Sup Lee. 2023. "Artemisia annua L. Polyphenols Enhance the Anticancer Effect of β-Lapachone in Oxaliplatin-Resistant HCT116 Colorectal Cancer Cells" International Journal of Molecular Sciences 24, no. 24: 17505. https://doi.org/10.3390/ijms242417505
APA StyleJung, E. J., Kim, H. J., Shin, S. C., Kim, G. S., Jung, J.-M., Hong, S. C., Kim, C. W., & Lee, W. S. (2023). Artemisia annua L. Polyphenols Enhance the Anticancer Effect of β-Lapachone in Oxaliplatin-Resistant HCT116 Colorectal Cancer Cells. International Journal of Molecular Sciences, 24(24), 17505. https://doi.org/10.3390/ijms242417505











