Myrsinane-Type Diterpenes: A Comprehensive Review on Structural Diversity, Chemistry and Biological Activities
Abstract
:1. Introduction
2. Phytochemistry
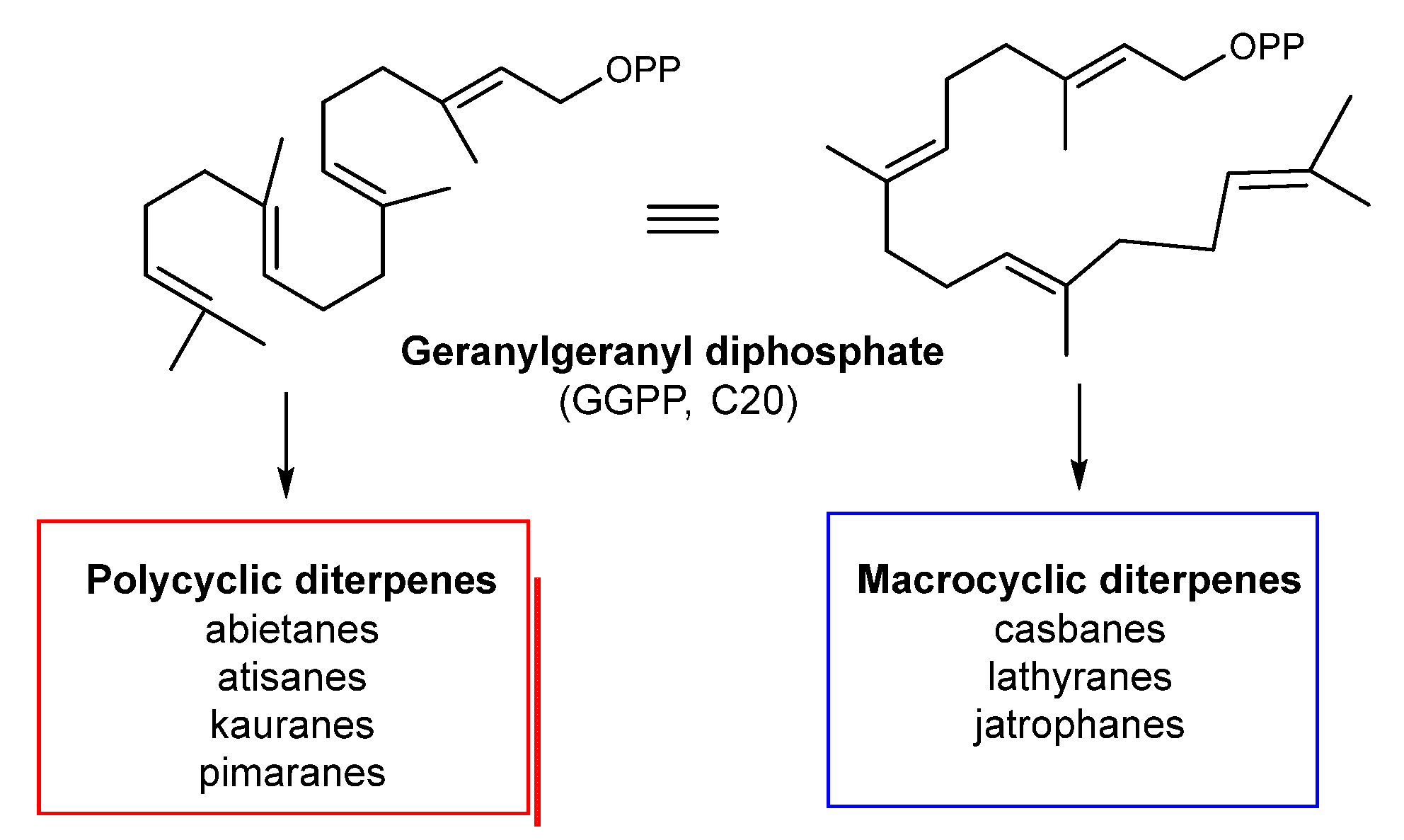
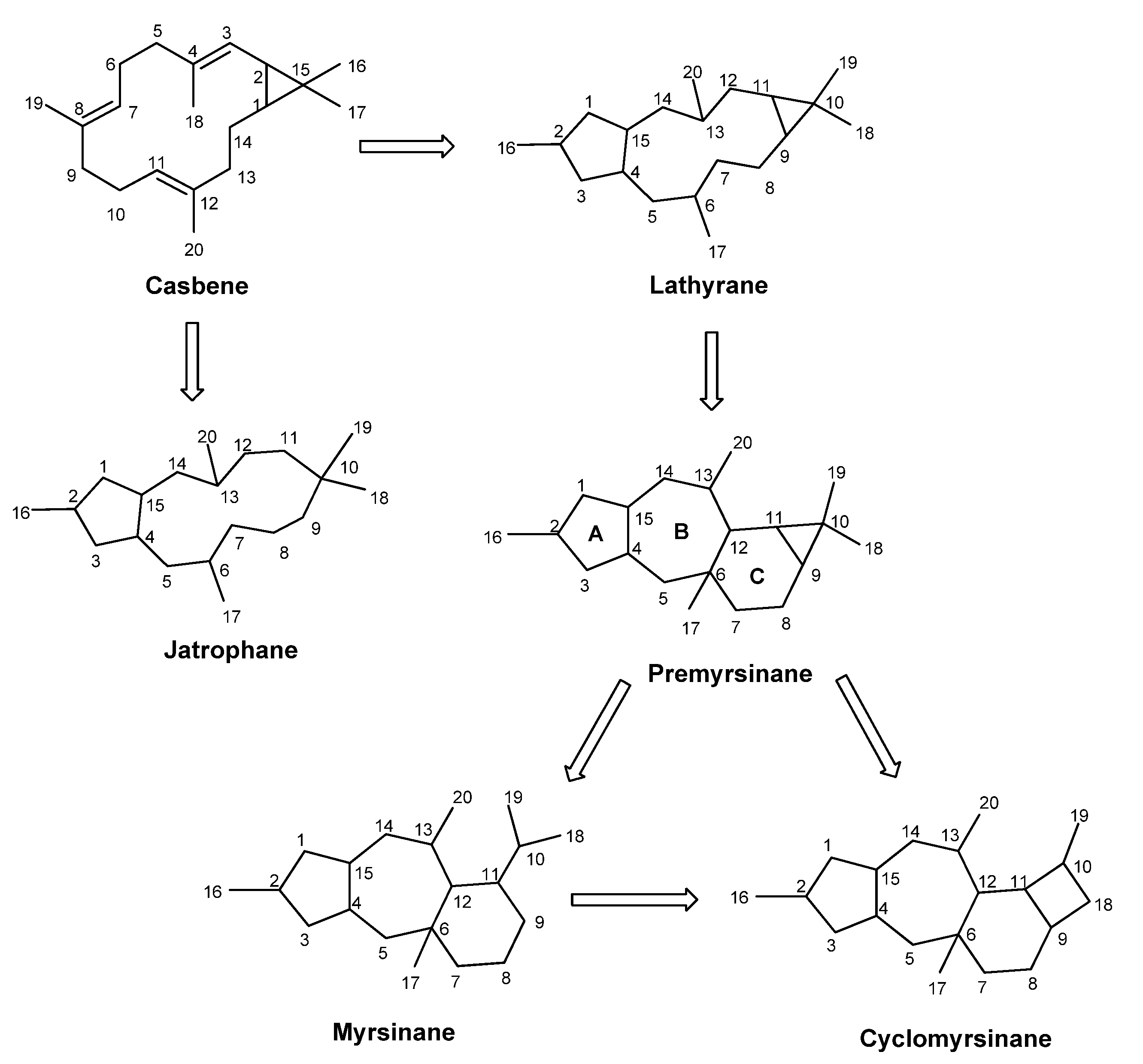
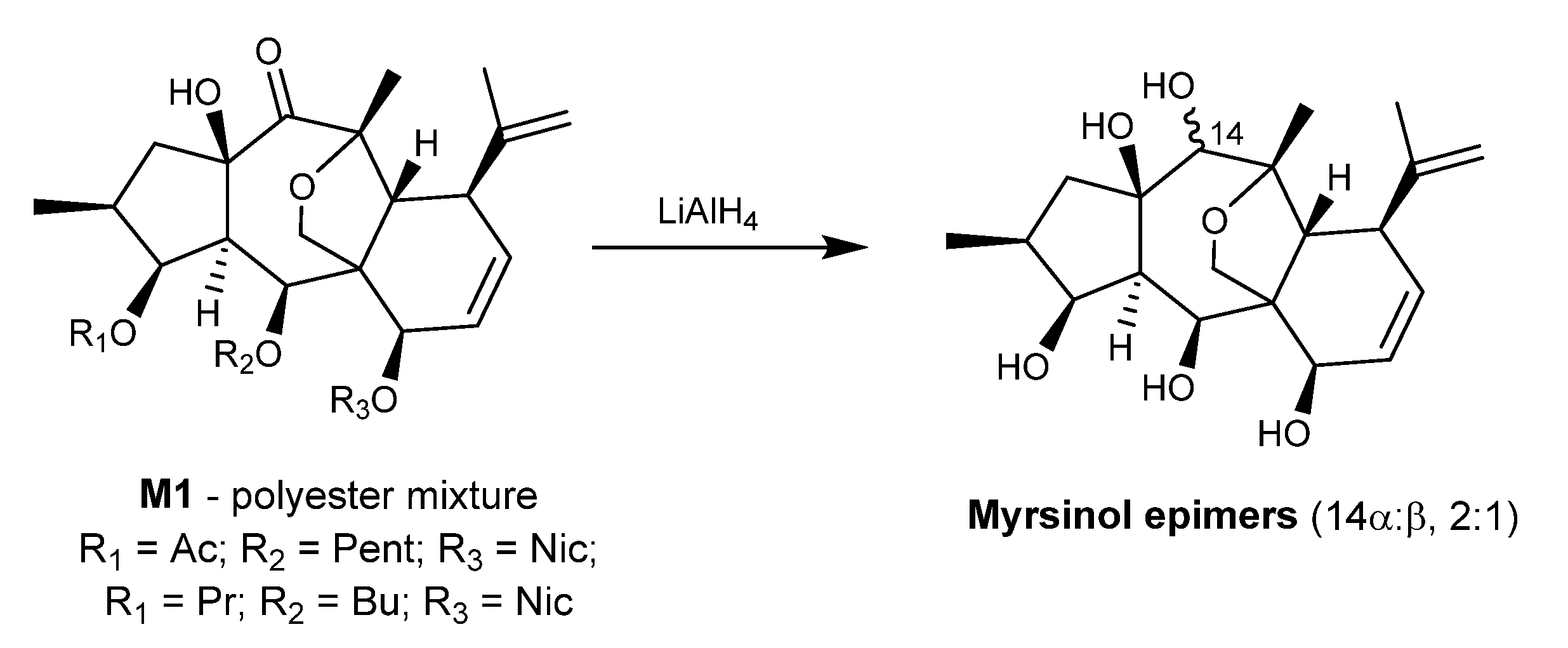
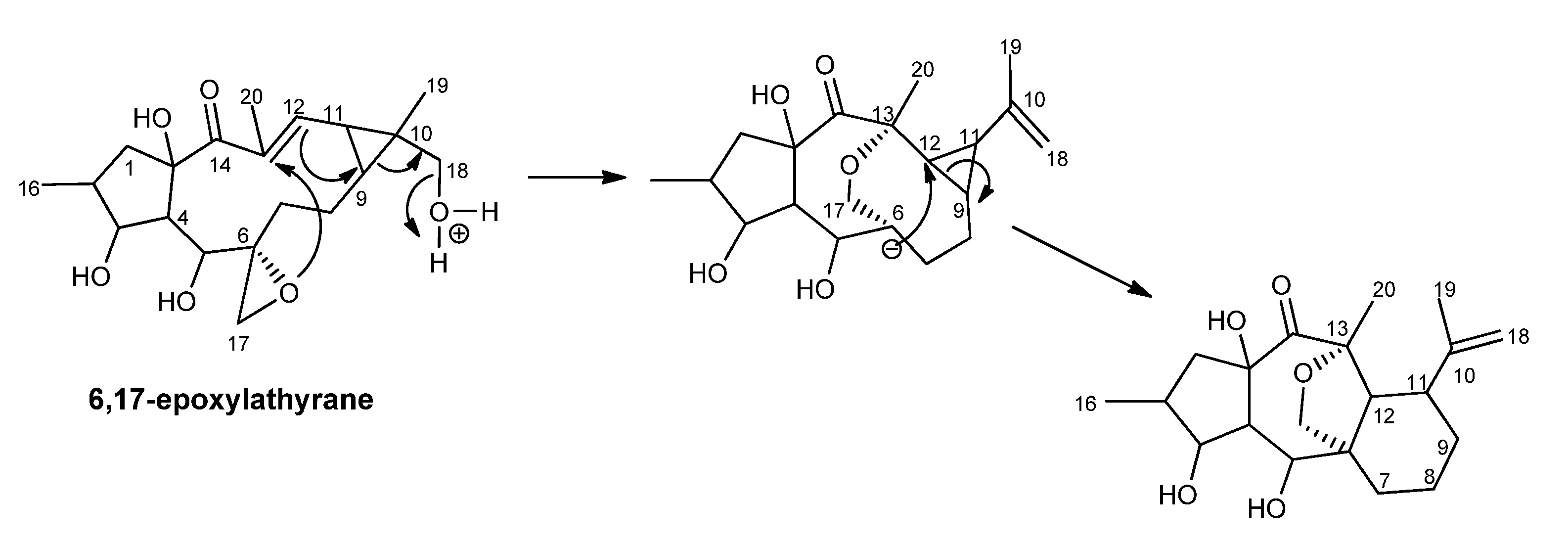
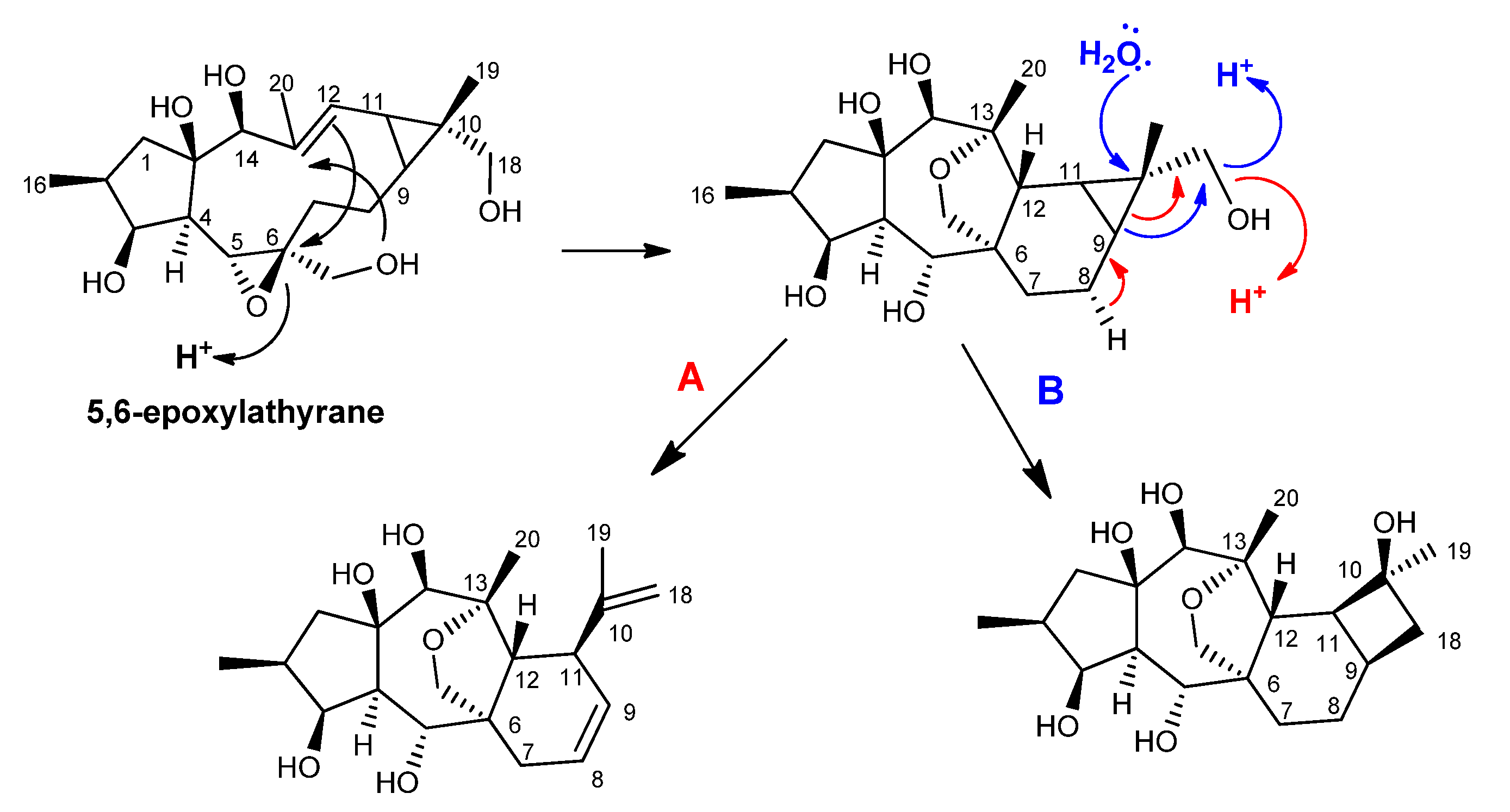
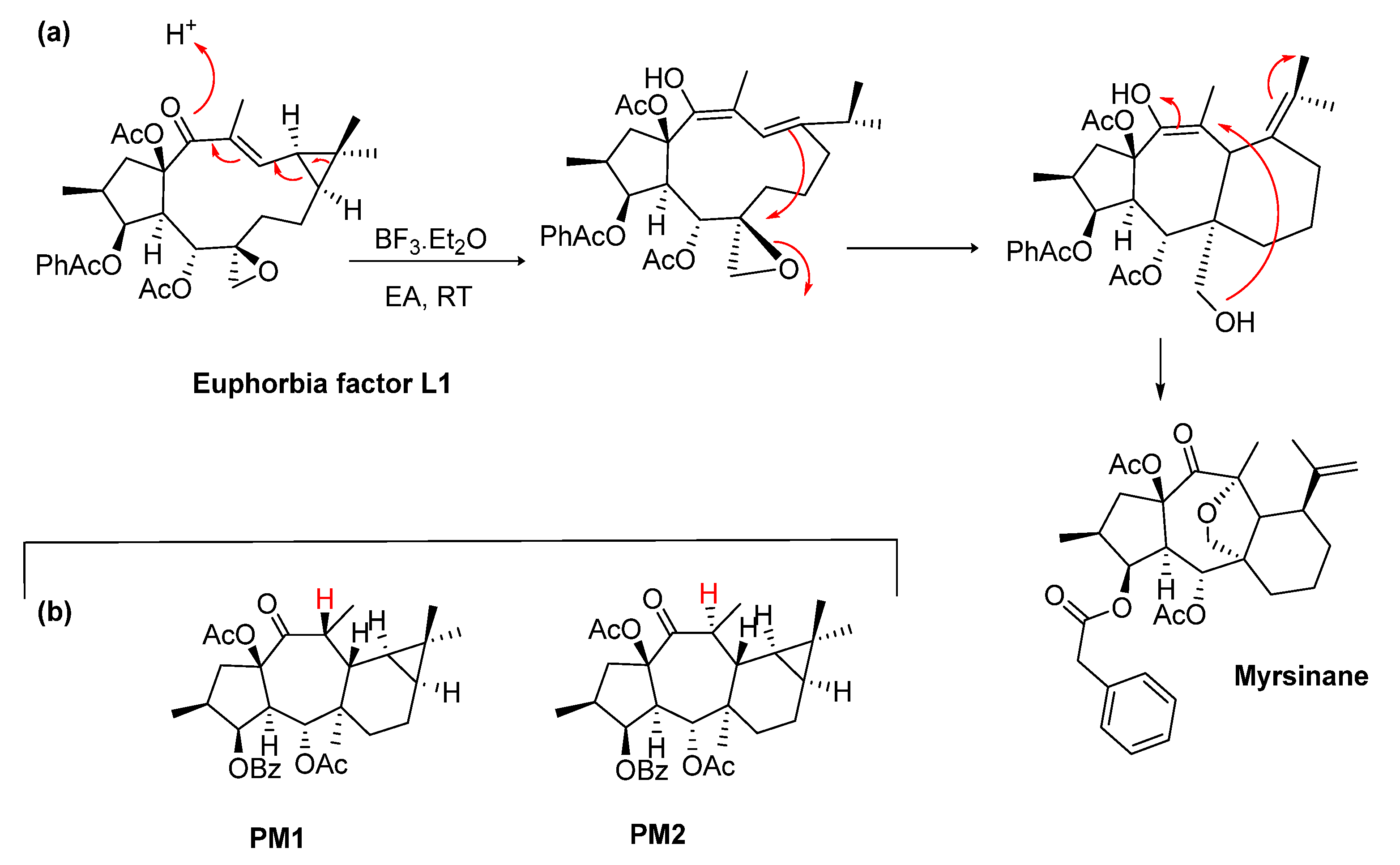
2.1. Biosynthetic Considerations
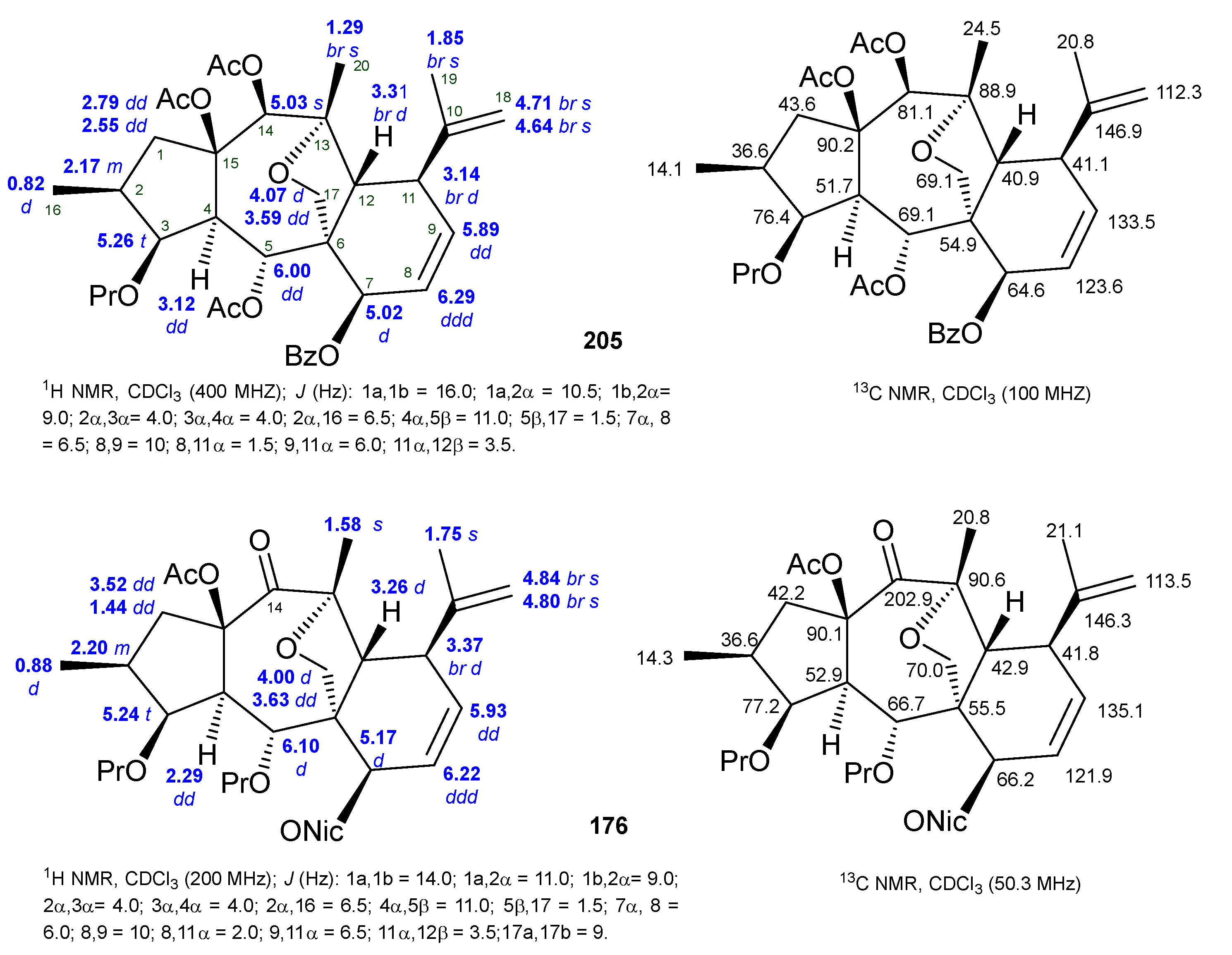
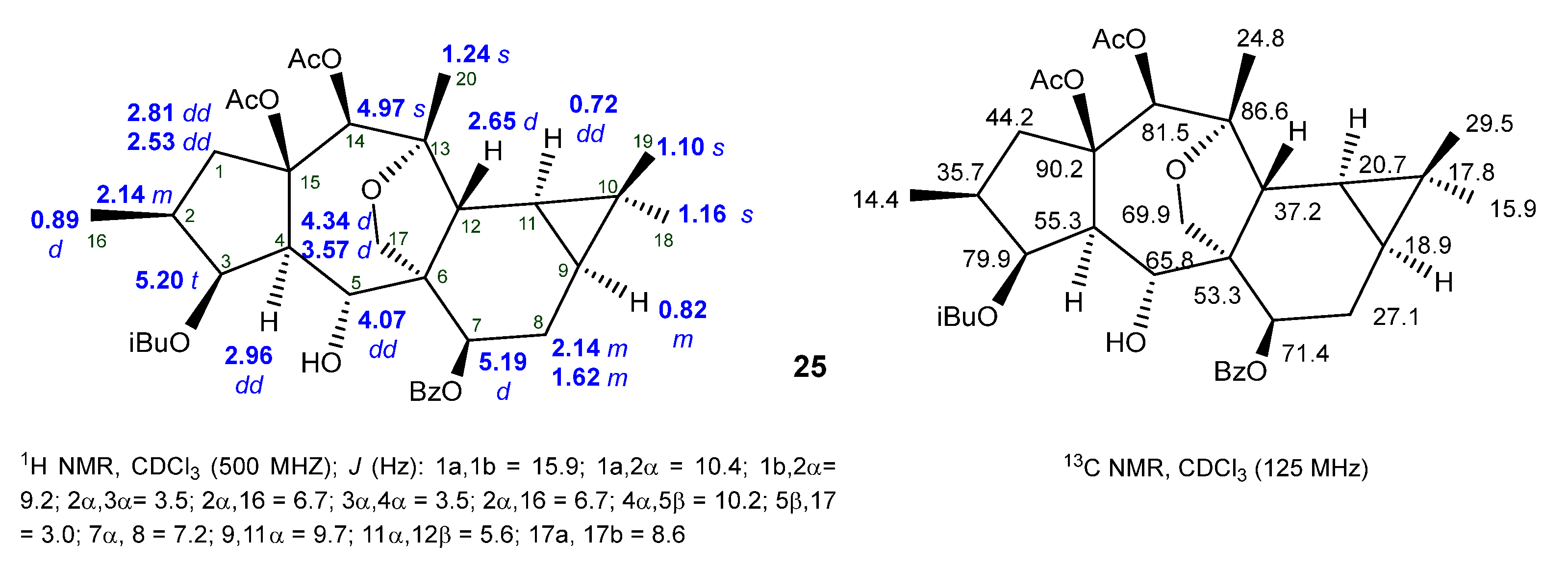
2.2. Isolation and Structural Identification
3. Distribution, Diversity, and Biological Activities of Myrsinane-Type Diterpenes from Euphorbia Species
3.1. E. falcata
3.2. E. prolifera Buch.-Ham
3.3. E. kopetdaghi (Prokh.) Prokh
3.4. E. sogdiana Popov
3.5. E. gedrosiaca Rech.f., Aellen & Esfand
3.6. E. decipiens Boiss. & Buhse
3.7. E. connata Boiss
3.8. E. sanctae-catharinae Fayed
3.9. E. pithyusa L.
3.10. E. cupanii
3.11. E. aellenii Rech
3.12. E. aleppica L.
3.13. E. lathyris L.
3.14. E. macrorrhiza C.A. Mey
3.15. E. boetica Boiss
3.16. Other Species
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Valentová, J.; Lintnerová, L.; Miklášová, N.; Oboňová, B.; Habala, L. Analogues of Anticancer Natural Products: Chiral Aspects. Int. J. Mol. Sci. 2023, 24, 5679. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural Products in Drug Discovery: Advances and Opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, L.B.; Lau, J. The Discovery and Development of Liraglutide and Semaglutide. Front. Endocrinol. 2019, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Aktas, O.; Küry, P.; Kieseier, B.; Hartung, H.P. Fingolimod Is a Potential Novel Therapy for Multiple Sclerosis. Nat. Rev. Neurol. 2010, 6, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Hersh, C.M.; Hara-Cleaver, C.; Rudick, R.A.; Cohen, J.A.; Bermel, R.A.; Ontaneda, D. Experience with Fingolimod in Clinical Practice. Int. J. Neurosci. 2015, 125, 678–685. [Google Scholar] [CrossRef]
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; Puente, T. de D. A Compressive Review about Taxol®: History and Future Challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef]
- Wani, M.C.; Horwitz, S.B. Nature as a Remarkable Chemist: A Personal Story of the Discovery and Development of Taxol®. Anticancer Drugs 2014, 25, 482–487. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Lawson, A.D.G.; Maccoss, M.; Heer, J.P. Importance of Rigidity in Designing Small Molecule Drugs to Tackle Protein-Protein Interactions (PPIs) through Stabilization of Desired Conformers. J. Med. Chem. 2018, 61, 4383–4389. [Google Scholar] [CrossRef]
- Baker, D.D.; Chu, M.; Oza, U.; Rajgarhia, V. The Value of Natural Products to Future Pharmaceutical Discovery. Nat. Prod. Rep. 2007, 24, 1225–1244. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.; Li, S.; Chu, W.; Yin, S. Euphorbia Diterpenoids: Isolation, Structure, Bioactivity, Biosynthesis, and Synthesis (2013–2021). Nat. Prod. Rep. 2022, 39, 2132–2174. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Iriti, M.; Vitalini, S.; Antolak, H.; Pawlikowska, E.; Kręgiel, D.; Sharifi-Rad, J.; Oyeleye, S.I.; Ademiluyi, A.O.; Czopek, K.; et al. Euphorbia-Derived Natural Products with Potential for Use in Health Maintenance. Biomolecules 2019, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Jedlowski, P.M. Ingenol Mebutate Is Associated with Increased Reporting Odds for Squamous Cell Carcinoma in Actinic Keratosis Patients, a Pharmacovigilance Study of the FDA Adverse Event Reporting System (FAERS). J. Cutan. Med. Surg. 2023, 27, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tang, P.; Zhu, M.; Wang, Y.; Sun, D.; Li, H.; Chen, L. Diterpenoids from the Genus Euphorbia: Structure and Biological Activity (2013–2019). Phytochemistry 2021, 190, 112846. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Sun, L.; Kong, C.H.; Mei, W.L.; Dai, H.F.; Xu, F.Q.; Huang, S.Z. Phytochemical and Pharmacological Review of Diterpenoids from the Genus Euphorbia Linn (2012–2021). J. Ethnopharmacol. 2022, 298, 115574. [Google Scholar] [CrossRef] [PubMed]
- Kemboi, D.; Siwe-noundou, X.; Krause, R.W.M.; Langat, M.K.; Tembu, V.J. Euphorbia Diterpenes: An Update of Isolation, Structure, Pharmacological Activities and Structure–Activity Relationship. Molecules 2021, 26, 5055. [Google Scholar] [CrossRef]
- Shi, Q.; Su, X.H.; Kiyota, H. Chemical and Pharmacological Research of the Plants in Genus Euphorbia. Chem. Rev. 2008, 108, 4295–4327. [Google Scholar] [CrossRef]
- Vasas, A.; Rédei, D.; Csupor, D.; Molnár, J.; Hohmann, J. Diterpenes from European Euphorbia Species Serving as Prototypes for Natural-Product-Based Drug Discovery. Eur. J. Org. Chem. 2012, 2012, 5115–5130. [Google Scholar] [CrossRef]
- Vasas, A.; Hohmann, J. Euphorbia Diterpenes: Isolation, Structure, Biological Activity, and Synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef]
- Appendino, G. Ingenane Diterpenoids. In Progress in the Chemistry of Organic Natural Products; Springer: Cham, Switzerland, 2016; Volume 102, pp. 1–90. [Google Scholar]
- Studies, B.; Jin, Y.; Shi, L.; Zhang, D.; Wei, H.; Si, Y.; Ma, G. A Review on Daphnane-Type Diterpenoids and Their Bioactive Studies. Molecules 2019, 24, 1842. [Google Scholar]
- Fattahian, M.; Ghanadian, M.; Ali, Z.; Khan, I.A. Jatrophane and Rearranged Jatrophane-Type Diterpenes: Biogenesis, Structure, Isolation, Biological Activity and SARs (1984–2019); Springer: Dordrecht, The Netherlands, 2020; Volume 19, ISBN 0123456789. [Google Scholar]
- Vela, F.; Ezzanad, A.; Hunter, A.C.; Macías-Sánchez, A.J.; Hernández-Galán, R. Pharmacological Potential of Lathyrane-Type Diterpenoids from Phytochemical Sources. Pharmaceuticals 2022, 15, 780. [Google Scholar] [CrossRef]
- Durán-Peña, M.J.; Botubol Ares, J.M.; Collado, I.G.; Hernández-Galán, R. Biologically Active Diterpenes Containing a Gem-Dimethylcyclopropane Subunit: An Intriguing Source of PKC Modulators. Nat. Prod. Rep. 2014, 31, 940–952. [Google Scholar] [CrossRef]
- Jian, B.; Zhang, H.; Liu, J. Structural Diversity and Biological Activities of Diterpenoids Derived from Euphorbia Fischeriana Steud. Molecules 2018, 23, 935. [Google Scholar] [CrossRef]
- Remy, S.; Litaudon, M. Macrocyclic Diterpenoids from Euphorbiaceae as a Source of Potent and Selective Inhibitors of Chikungunya Virus Replication. Molecules 2019, 24, 2336. [Google Scholar] [CrossRef]
- Fordjour, E.; Mensah, E.O.; Hao, Y.; Yang, Y.; Liu, X.; Li, Y.; Liu, C.L.; Bai, Z. Toward Improved Terpenoids Biosynthesis: Strategies to Enhance the Capabilities of Cell Factories. Bioresour. Bioprocess. 2022, 9, 6. [Google Scholar] [CrossRef]
- Chacón-Morales, P.A. Unprecedented Diterpene Skeletons Isolated from Vascular Plants in the Last Twenty Years (2001–2021). Phytochemistry 2022, 204, 113425. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Zhang, C. Sustainable Biosynthesis of Valuable Diterpenes in Microbes. Eng. Microbiol. 2023, 3, 100058. [Google Scholar] [CrossRef]
- Schulz, S. From Biosynthesis to Total Synthesis: Strategies and Tactics for Natural Products; Zografos, A., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Dewick, P.M. Medicinal Natural Products, A Biossynthetic Approach, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Hu, Z.; Liu, X.; Tian, M.; Ma, Y.; Jin, B.; Gao, W.; Cui, G.; Guo, J.; Huang, L. Recent Progress and New Perspectives for Diterpenoid Biosynthesis in Medicinal Plants. Med. Res. Rev. 2021, 41, 2971–2997. [Google Scholar] [CrossRef]
- Löffler, L.E.; Wirtz, C.; Fürstner, A. Collective Total Synthesis of Casbane Diterpenes: One Strategy, Multiple Targets. Angew. Chemie-Int. Ed. 2021, 60, 5316–5322. [Google Scholar] [CrossRef] [PubMed]
- Evans, F.J.; Taylor, S.E. Pro-Inflammatory, Tumour-Promoting and Anti-Tumour Diterpenes of the Plant Families Euphorbiaceae and Thymelaeaceae. In Fortschritte der Chemie Org. Naturstoffe; Springer: Vienna, Austria, 1983; Volume 44, pp. 1–99. [Google Scholar] [CrossRef]
- Rentzea, M.; Hecker, E. α-Ketol Rearrangement of Myrsinol to Isomyrsinol and Possible Biogenesis of the Myrsinane Framework. Tetrahedron Lett. 1982, 23, 1785–1788. [Google Scholar] [CrossRef]
- Jeske, F.; Jakupovic, J.; Berendsohn, W. Diterpenes from Euphorbia Seguieriana. Phytochemistry 1995, 40, 1743–1750. [Google Scholar] [CrossRef]
- Shi, Y.; Jia, Z.; Lahham, J.; Saleh, S. Diterpenoids from Euphorbia Aleppica Linn. Indian J. Chem.-Sect. B Org. Med. Chem. 1998, 37, 793–796. [Google Scholar]
- Rentzea, M.; Hecker, E.; Lotter, H. Neue Tetrazyklische, Polyfunktionelle Diterpenoide Aus Euphorbia Myrsinites L. Röntgenstrukturanalyse Und Stereochimie Des 14-Desoxo-14β-Hydroxymyrsinols. Tetrahedron Lett. 1982, 23, 1781–1784. [Google Scholar] [CrossRef]
- Shi, Y.; Jia, Z.; Lahham, J.; Saleh, S. Pentacyclic Diterpene Esters from Euphorbia Aleppica. Phytochemistry 1995, 40, 1219–1221. [Google Scholar] [CrossRef]
- Yang, L.; Shi, Y.P.; Jia, Z.J.; Saleh, S.; Lahham, J. Four Esters of a New Pentacyclic Diterpenoid of the Myrsinol Type from Euphorbia Aleppica. J. Nat. Prod. 1995, 58, 1883–1888. [Google Scholar] [CrossRef]
- Wu, D.; Sorg, B.; Hecker, E. New Myrsinol-Related Polyfunctional Pentacyclic Diterpene Esters from Roots of Euphorbia Prolifera. J. Nat. Prod. 1995, 58, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Cravotto, G.; Jareva, T. Epoxidation Studies on Lathyra-6 (17), 12-Dienes ؊ Revised Structure of the Euphorbia Factor L 1. Eur. J. Org. Chem. 2000, 6, 2933–2938. [Google Scholar] [CrossRef]
- Appendino, G.; Tron, G.C.; Jarevång, T.; Sterner, O. Unnatural Natural Products from the Transannular Cyclization of Lathyrane Diterpenes. Org. Lett. 2001, 3, 1609–1612. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, L.; Gao, F.; Zhou, X. Lewis Acid-Mediated Skeleton Transformation of Euphorbia Diterpenes: From Lathyrane to Euphoractane and Myrsinane. Fitoterapia 2019, 133, 212–218. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, N.; Wan, L.X.; Zhou, X.L.; Li, X.; Gao, F. Iron-Catalyzed Skeletal Conversion of Lathyrane to Premyrsinane Euphorbia Diterpenes and Their Cytotoxic Activities. J. Nat. Prod. 2021, 84, 1838–1842. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Fan, J.T.; Zeng, G.Z.; Tan, N.H. A New Tetracyclic Diterpene from Jatropha Curcas. Helv. Chim. Acta 2011, 94, 842–846. [Google Scholar] [CrossRef]
- Liu, J.Q.; Yang, Y.F.; Xia, J.J.; Li, X.Y.; Li, Z.R.; Zhou, L.; Qiu, M.H. Cytotoxic Diterpenoids from Jatropha curcas cv. Nigroviensrugosus CY Yang Roots. Phytochemistry 2015, 117, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.M.; Su, Z.Y.; Lou, L.L.; Zhu, J.Y.; Tang, G.H.; Gan, L.S.; Bu, X.Z.; Yin, S. Jatrocurcadiones A and B: Two Novel Diterpenoids with an Unusual 10,11-Seco-Premyrsinane Skeleton from Jatropha curcas. RSC Adv. 2015, 5, 62921–62925. [Google Scholar] [CrossRef]
- Flores-Giubi, E.; Geribaldi-Doldán, N.; Murillo-Carretero, M.; Castro, C.; Durán-Patrón, R.; MacÍas-Sánchez, A.J.; Hernández-Galán, R. Lathyrane, Premyrsinane, and Related Diterpenes from Euphorbia Boetica: Effect on in Vitro Neural Progenitor Cell Proliferation. J. Nat. Prod. 2019, 82, 2517–2528. [Google Scholar] [CrossRef] [PubMed]
- Vasas, A.; Forgo, P.; Orvos, P.; Tálosi, L.; Csorba, A.; Pinke, G.; Hohmann, J. Myrsinane, Premyrsinane, and Cyclomyrsinane Diterpenes from Euphorbia Falcata as Potassium Ion Channel Inhibitors with Selective G Protein-Activated Inwardly Rectifying Ion Channel (GIRK) Blocking Effects. J. Nat. Prod. 2016, 79, 1990–2004. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, B.; Yazdiniapour, Z.; Ghanadian, M.; Lanzotti, V. Cyclomyrsinane and Premyrsinane Diterpenes from Euphorbia Sogdiana Popov. Tetrahedron 2016, 72, 5394–5401. [Google Scholar] [CrossRef]
- Zolfaghari, B.; Farahani, A.; Jannesari, A.; Aghaei, M.; Ghanadian, M. New Cytotoxic Premyrsinane-Type Diterpenes from Euphorbia Aleppica Against Breast Cancer Cells. Iran. J. Pharm. Res. 2022, 21, 2–10. [Google Scholar] [CrossRef]
- Yazdiniapour, Z.; Mirian, M.; Zolfaghari, B.; Mehdifar, P.; Ghanadian, M.; Lanzotti, V. Myrsinane-Type Diterpenes from Euphorbia Gedrosiaca with Cell Growth Inhibitory Activity and Apoptotic Effects on Melanoma Cancer Cells. Fitoterapia 2022, 157, 105138. [Google Scholar] [CrossRef]
- Ghanadian, M.; Sadraei, H.; Yousuf, S.; Asghari, G.; Choudhary, M.I.; Jahed, M. New Diterpene Polyester and Phenolic Compounds from Pycnocycla Spinosa Decne. Ex Boiss with Relaxant Effects on KCl-Induced Contraction in Rat Ileum. Phytochem. Lett. 2014, 7, 57–61. [Google Scholar] [CrossRef]
- Shadi, S.; Saeidi, H.; Ghanadian, M.; Rahimnejad, M.R.; Aghaei, M.; Ayatollahi, S.M.; Iqbal Choudhary, M. New Macrocyclic Diterpenes from Euphorbia Connata Boiss. with Cytotoxic Activities on Human Breast Cancer Cell Lines. Nat. Prod. Res. 2015, 29, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Nothias-Esposito, M.; Nothias, L.F.; Da Silva, R.R.; Retailleau, P.; Zhang, Z.; Leyssen, P.; Roussi, F.; Touboul, D.; Paolini, J.; Dorrestein, P.C.; et al. Investigation of Premyrsinane and Myrsinane Esters in Euphorbia Cupanii and Euphobia Pithyusa with MS2LDA and Combinatorial Molecular Network Annotation Propagation. J. Nat. Prod. 2019, 82, 1459–1470. [Google Scholar] [CrossRef]
- Sulyok, E.; Vasas, A.; Rédei, D.; Forgo, P.; Kele, Z.; Pinke, G.; Hohmann, J. New Premyrsinane-Type Diterpene Polyesters from Euphorbia Falcata. Tetrahedron 2011, 67, 7289–7293. [Google Scholar] [CrossRef]
- Öksüz, S.; Gürek, F.; Gil, R.R.; Pengsuparp, T.; Pezzuto, J.M.; Cordell, G.A. Four Diterpene Esters from Euphorbia Myrsinites. Phytochemistry 1995, 38, 1457–1462. [Google Scholar] [CrossRef]
- Zhang, W.J.; Chen, D.F.; Hou, A.J. New Myrsinol Diterpenes from Euphorbia Prolifera. Chinese J. Chem. 2004, 22, 103–108. [Google Scholar] [CrossRef]
- Vasas, A.; Sulyok, E.; Martins, A.; Rédei, D.; Forgo, P.; Kele, Z.; Zupkó, I.; Molnár, J.; Pinke, G.; Hohmann, J. Cyclomyrsinane and Premyrsinane Diterpenes from Euphorbia Falcata Modulate Resistance of Cancer Cells to Doxorubicin. Tetrahedron 2012, 68, 1280–1285. [Google Scholar] [CrossRef]
- Chen, X.; Chen, X.; Liu, X.; Wink, M.; Ma, Y.; Guo, Y. A Myrsinol Diterpene Isolated from Euphorbia Prolifera Reverses Multidrug Resistance in Breast Cancer Cells. Pharmazie 2016, 71, 537–539. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X.; Li, T.; Xu, J.; Ma, Y. A Myrsinol Diterpene Isolated from a Traditional Herbal Medicine, LANGDU Reverses Multidrug Resistance in Breast Cancer Cells. J. Ethnopharmacol. 2016, 194, 1–5. [Google Scholar] [CrossRef]
- Xu, J.; Jin, D.; Guo, P.; Xie, C.; Fang, L.; Guo, Y. Three New Myrsinol Diterpenes from Euphorbia Prolifera and Their Neuroprotective Activities. Molecules 2012, 17, 9520–9528. [Google Scholar] [CrossRef]
- Xu, J.; Yang, B.; Fang, L.; Wang, S.; Guo, Y.; Yamakuni, T.; Ohizumi, Y. Four New Myrsinol Diterpenes from Euphorbia Prolifera. J. Nat. Med. 2013, 67, 333–338. [Google Scholar] [CrossRef]
- Xu, J.; Jin, D.Q.; Guo, Y.; Xie, C.; Ma, Y.; Yamakuni, T.; Ohizumi, Y. New Myrsinol Diterpenes from Euphorbia Prolifera and Their Inhibitory Activities on LPS-Induced NO Production. Bioorganic Med. Chem. Lett. 2012, 22, 3612–3618. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, L.; Wang, F.P. New Cytotoxic Myrsinane-Type Diterpenes from Euphorbia Prolifera. Helv. Chim. Acta 2010, 93, 746–752. [Google Scholar] [CrossRef]
- Xu, J.; Guo, Y.; Xie, C.; Li, Y.; Gao, J.; Zhang, T.; Hou, W.; Fang, L.; Gui, L. Bioactive Myrsinol Diterpenoids from the Roots of Euphorbia Prolifera. J. Nat. Prod. 2011, 74, 2224–2230. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Belloro, E.; Tron, G.C.; Jakupovic, J.; Ballero, M. Diterpenoids from Euphorbia Pithyusa Subsp. Cupanii. J. Nat. Prod. 1999, 62, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Riahi, F.; Dashti, N.; Ghanadian, M.; Aghaei, M.; Faez, F.; Jafari, S.M.; Zargar, N. Kopetdaghinanes, pro-Apoptotic Hemiacetialic Cyclomyrsinanes from Euphorbia Kopetdaghi. Fitoterapia 2020, 146, 104636. [Google Scholar] [CrossRef] [PubMed]
- Ghanadian, S.M.; Ayatollahi, A.M.; Mesaik, M.A.; Abdalla, O.M. New Immunosuppressive Cyclomyrsinol Diterpenes from Euphorbia Kopetdaghi Prokh. Nat. Prod. Res. 2013, 27, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Ayatollahi, A.M.; Ghanadian, M.; Mesaik, M.A.; Mohamed Abdella, O.; Afsharypuor, S.; Kobarfard, F.; Mirza-Taheri, M. New Myrsinane-Type Diterpenoids from Euphorbia Aellenii Rech. f. with Their Immunomodulatory Activity. J. Asian Nat. Prod. Res. 2010, 12, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, F.; Yazdiniapour, Z.; Ghanadian, M.; Zolfaghari, B.; Maleki, M.; Shafiee, F. Cytotoxicity and Apoptosis Assay of Novel Cyclomyrsinol Diterpenes against Breast Cancer Cell Lines. World J. Tradit. Chinese Med. 2022, 8, 273–277. [Google Scholar] [CrossRef]
- Ayatollahi, A.M.; Haji Molla Hoseini, M.; Ghanadian, S.M.; Kosari-Nasab, M.; Mami, F.; Yazdiniapoure, Z.; Zolfaghari, B.; Salari, A.A. TAMEC: A New Analogue of Cyclomyrsinol Diterpenes Decreases Anxiety- and Depression-like Behaviors in a Mouse Model of Multiple Sclerosis. Neurol. Res. 2017, 39, 1056–1065. [Google Scholar] [CrossRef]
- Ghanadian, M.; Choudhary, M.I.; Ayatollahi, A.M.; Mesaik, M.A.; Abdalla, O.M.; Afsharypour, S. New Cyclomyrsinol Diterpenes from Euphorbia Aellenii with Their Immunomodulatory Effects. J. Asian Nat. Prod. Res. 2013, 15, 22–29. [Google Scholar] [CrossRef]
- Yazdiniapour, Z.; Sohrabi, M.H.; Motinia, N.; Zolfaghari, B. Diterpenoids from Euphorbia Gedrosiaca as Potential Anti-Proliferative Agents against Breast Cancer Cells. Metabolites 2023, 13, 225. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.E.F.; Hamed, A.R.; Ibrahim, M.A.A.; Talat, Z.; Reda, E.H.; Abdel-Azim, N.S.; Hammouda, F.M.; Nakamura, S.; Matsuda, H.; Haggag, E.G.; et al. Euphosantianane A–D: Antiproliferative Premyrsinane Diterpenoids from the Endemic Egyptian Plant Euphorbia Sanctae-Catharinae. Molecules 2018, 23, 2221. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Jassbi, A.R. Two Pentacyclic Diterpene Esters from Euphorbia Decipiens. Phytochemistry 1998, 48, 1217–1220. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Jassbi, A.R. Three Tricyclic Diterpenoids from Euphorbia Decipiens. Planta Med. 1998, 64, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Husani, S.R.; Abbas, M.; Pan, Y.; Jassbi, A.R.; Asim, M.; Parvez, M.; Voelter, W.; Ahmad, V.U. Eight New Diterpenoids from Euphorbia Decipiens. Helv. Chim. Acta 2001, 84, 1980–1988. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Jassbi, R.; Parvez, M. Three New Diterpene Esters from Euphorbia Decipiens. Tetrahedron 1998, 54, 1573–1584. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Hussain, J.; Hussain, H.; Jassbi, A.R.; Ullah, F.; Lodhi, M.A.; Yasin, A.; Choudhary, M.I. First Natural Urease Inhibitor from Euphorbia Decipiens. Chem. Pharm. Bull. 2003, 51, 719–723. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Hussain, H.; Hussain, J.; Jassbi, A.R.; Bukhari, I.A.; Yasin, A.; Choudhary, M.I.; Dar, A. New Bioactive Diterpenoid from Euphorbia Decipiens. Z. Naturforsch. 2002, 57, 1066–1071. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Hussain, J.; Hussain, H.; Farooq, U.; Farmanullah, A.; Lodhi, M.A.; Choudhary, M.I. Two New Diterpene Polyesters from Euphorbia Decipiens. Nat. Prod. Res. 2005, 19, 267–274. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Hussain, H.; Bukhari, I.A.; Hussain, J.; Jassbi, A.R.; Dar, A. Antinociceptive Diterpene from Euphorbia Decipiens. Fitoterapia 2005, 76, 230–232. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Hussain, H.; Jassbi, A.R.; Hussain, J.; Bukhari, I.A.; Yasin, A.; Aziz, N.; Choudhary, M.I. New Bioactive Diterpene Polyesters from Euphorbia Decipiens. J. Nat. Prod. 2003, 66, 1221–1224. [Google Scholar] [CrossRef] [PubMed]
- Jassbi, A.R.; Fukushi, Y.; Tahara, S. Determination of Absolute Configuration of Decipinone, a Diterpenoid Ester with a Myrsinane-Type Carbon Skeleton, by NMR Spectroscopy. Helv. Chim. Acta 2002, 85, 1706–1713. [Google Scholar] [CrossRef]
- Lodhi, M.A.; Hussain, J.; Abbasi, M.A.; Jassbi, A.R.; Choudhary, M.I.; Ahmad, V.U. A New Bacillus Pasteurii Urease Inhibitor from Euphorbia Decipiens. J. Enzyme Inhib. Med. Chem. 2006, 21, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Abdolmohammadi, M.H.; Fallahian, F.; Ghanadian, M.; Mirjani, A.; Aghaei, M. New Diterpene Compound from Euphorbia Connate Boiss., 3,7,14,15-Tetraacetyl-5-Propanoyl-13(17)-Epoxy-8,10(18)-Myrsinadiene, Inhibits the Growth of Ovarian Cancer Cells by Promoting Mitochondrial-Mediated Apoptosis. Nutr. Cancer 2021, 73, 2030–2038. [Google Scholar] [CrossRef] [PubMed]
- Elshamy, A.I.; Mohamed, T.A.; Al-Rowaily, S.L.; Abd-ElGawad, A.M.; Dar, B.A.; Shahat, A.A.; Hegazy, M.E.F. Euphosantianane E-G: Three New Premyrsinane Type Diterpenoids from Euphorbia Sanctae-Catharinae with Contribution to Chemotaxonomy. Molecules 2019, 24, 2412. [Google Scholar] [CrossRef] [PubMed]
- Nothias-Scaglia, L.F.; Dumontet, V.; Neyts, J.; Roussi, F.; Costa, J.; Leyssen, P.; Litaudon, M.; Paolini, J. LC-MS2-Based Dereplication of Euphorbia Extracts with Anti-Chikungunya Virus Activity. Fitoterapia 2015, 105, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Nothias, L.F.; Retailleau, P.; Costa, J.; Roussi, F.; Neyts, J.; Leyssen, P.; Touboul, D.; Litaudon, M.; Paolini, J. Isolation of Premyrsinane, Myrsinane, and Tigliane Diterpenoids from Euphorbia Pithyusa Using a Chikungunya Virus Cell-Based Assay and Analogue Annotation by Molecular Networking. J. Nat. Prod. 2017, 80, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Ghanadian, S.M.; Ayatollahi, A.M.; Afsharypuor, S.; Javanmard, S.H.; Dana, N. New Mirsinane-Type Diterpenes from Euphorbia Microsciadia Boiss. with Inhibitory Effect on VEGF-Induced Angiogenesis. J. Nat. Med. 2013, 67, 327–332. [Google Scholar] [CrossRef]
- Öksüz, S.; Gürek, F.; Lin, L.Z.; Gil, R.R.; Pezzuto, J.M.; Cordell, G.A. Aleppicatines A and B from Euphorbia Aleppica. Phytochemistry 1996, 42, 473–478. [Google Scholar] [CrossRef]
- Wang, Q.; Zhen, Y.Q.; Gao, F.; Huang, S.; Zhou, X.L. Five New Diterpenoids from the Seeds of Euphorbia Lathyris. Chem. Biodivers. 2018, 15, 9–16. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Z.; Guo, Y.; Xie, H.; Zhang, Z.; Sun, D.; Li, H.; Chen, L. Diterpenoids from the Seeds of Euphorbia Lathyris and Their Anti-Inflammatory Activity. Bioorg. Chem. 2021, 112, 104944. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Chen, Q.-B.; Liu, Y.Q.; Xin, X.L.; Yili, A.; Aisa, H.A. Diterpenoid Constituents of Euphorbia Macrorrhiza. Phytochemistry 2016, 122, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.J.U.; Ascenso, J.R. Steroids and a Tetracyclic Diterpene from Euphorbia boetica. Phytochemistry 1999, 51, 439–444. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Zamanizadehnajari, S.; Tahara, S. Chemical Constituents of Euphorbia Marschalliana Boiss. Z. Naturforsch.-Sect. C J. Biosci. 2004, 59, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Jassbi, A.R.; Zahid, M.; Ali, Z.; Alam, N.; Akhtar, F.; Choudhary, M.I.; Ahmad, V.U. Three New Diterpenoids from Euphorbia Cheiradenia. Helv. Chim. Acta 2000, 83, 2751–2755. [Google Scholar] [CrossRef]
- Hussain, S.; Slevin, M.; Mesaik, M.A.; Choudhary, M.I.; Elosta, A.H.; Matou, S.; Ahmed, N.; West, D.; Gaffney, J. Cheiradone: A Vascular Endothelial Cell Growth Factor Receptor Antagonist. BMC Cell Biol. 2008, 29, 7. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.Q.; Wang, L.; Ouyang, W.-B.; Ma, Y.; Yu, M.; Zang, Z.; Zhao, Y.; Huang, S.; Zhao, Y. A New Myrinsol Diterpenoid Ester from Euphorbia Dracunculoides. Chem. Nat. Compd. 2016, 52, 1037–1040. [Google Scholar] [CrossRef]
- Wang, L.; Zang, Z.; Wang, Y.F.; Huang, S.X.; Cao, P.; Zhao, Y. Two New Myrinsol Diterpenoids from Euphorbia Dracunculoides Lam. Chin. Chem. Lett. 2015, 26, 121–123. [Google Scholar] [CrossRef]
- Wang, L.; Yang, J.; Chi, Y.Q.; Ouyang, W.-B.; Zang, Z.; Huang, S.X.; Cao, P.; Zhao, Y. A New Myrsinol-Type Diterpene Polyester from Euphorbia Dracunculoides Lam. Nat. Prod. Res. 2015, 29, 1406–1413. [Google Scholar] [CrossRef]
- Wang, L.; Ma, Y.T.; Sun, Q.Y.; Li, X.N.; Yan, Y.; Yang, J.; Yang, F.M.; Liu, F.Y.; Zang, Z.; Wu, X.H.; et al. Structurally Diversified Diterpenoids from Euphorbia Dracunculoides. Tetrahedron 2015, 71, 5484–5493. [Google Scholar] [CrossRef]
- Zarei, S.M.; Ayatollahi, A.M.; Ghanadian, M.; Kobarfard, F.; Aghaei, M.; Choudhary, M.I.; Fallahian, F. Unusual Ingenoids from Euphorbia Erythradenia Bioss. with pro-Apoptotic Effects. Fitoterapia 2013, 91, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Shokoohinia, Y.; Sajjadi, S.E.; Zolfaghari, B.; Chianese, G.; Appendino, G.; Taglialatela-Scafati, O. Diterpenoid (Poly)Esters and a Ring A-Seco-Phorboid from the Aerial Parts of Euphorbia Macroclada Boiss. Fitoterapia 2010, 81, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Öksüz, S.; Gürek, F.; Qiu, S.X.; Cordell, G.A. Diterpene Polyesters from Euphorbia Seguieriana. J. Nat. Prod. 1998, 61, 1198–1201. [Google Scholar] [CrossRef] [PubMed]
- Ayatollahi, S.A.; Shojaii, A.; Kobarfard, F.; Nori, M. Terpens from Aerial Parts of Euphorbia Splendida. J. Med. Plants Res. 2009, 3, 660–665. [Google Scholar]
- Ahmad, V.U.; Jassbi, A.R. New Diterpenoids from Euphorbia Teheranica. J. Nat. Prod. 1999, 62, 1016–1018. [Google Scholar] [CrossRef]
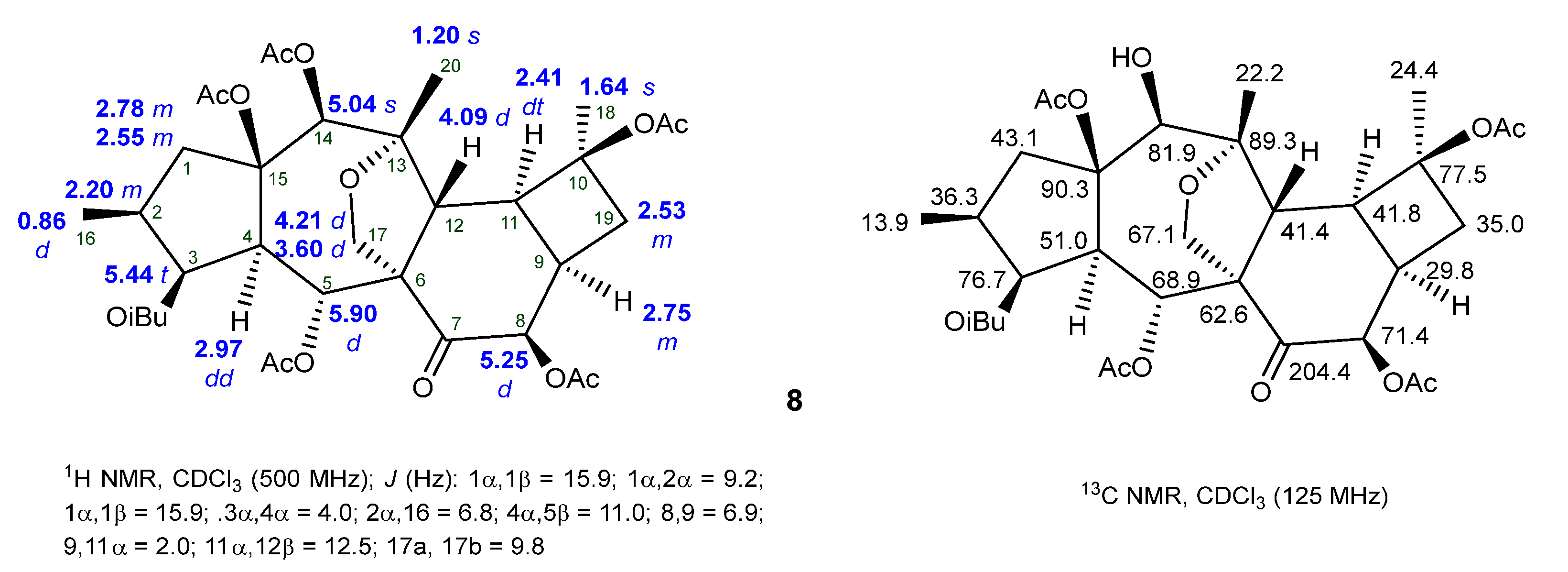
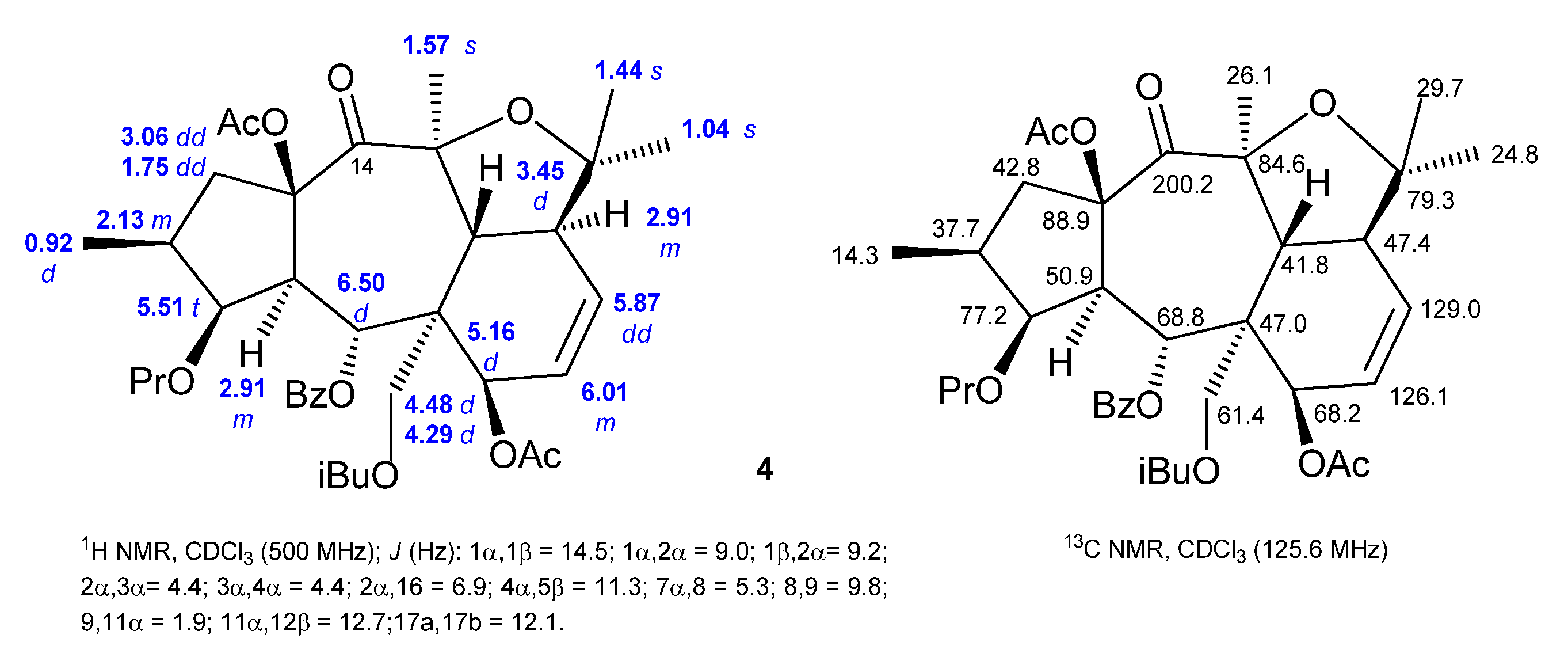
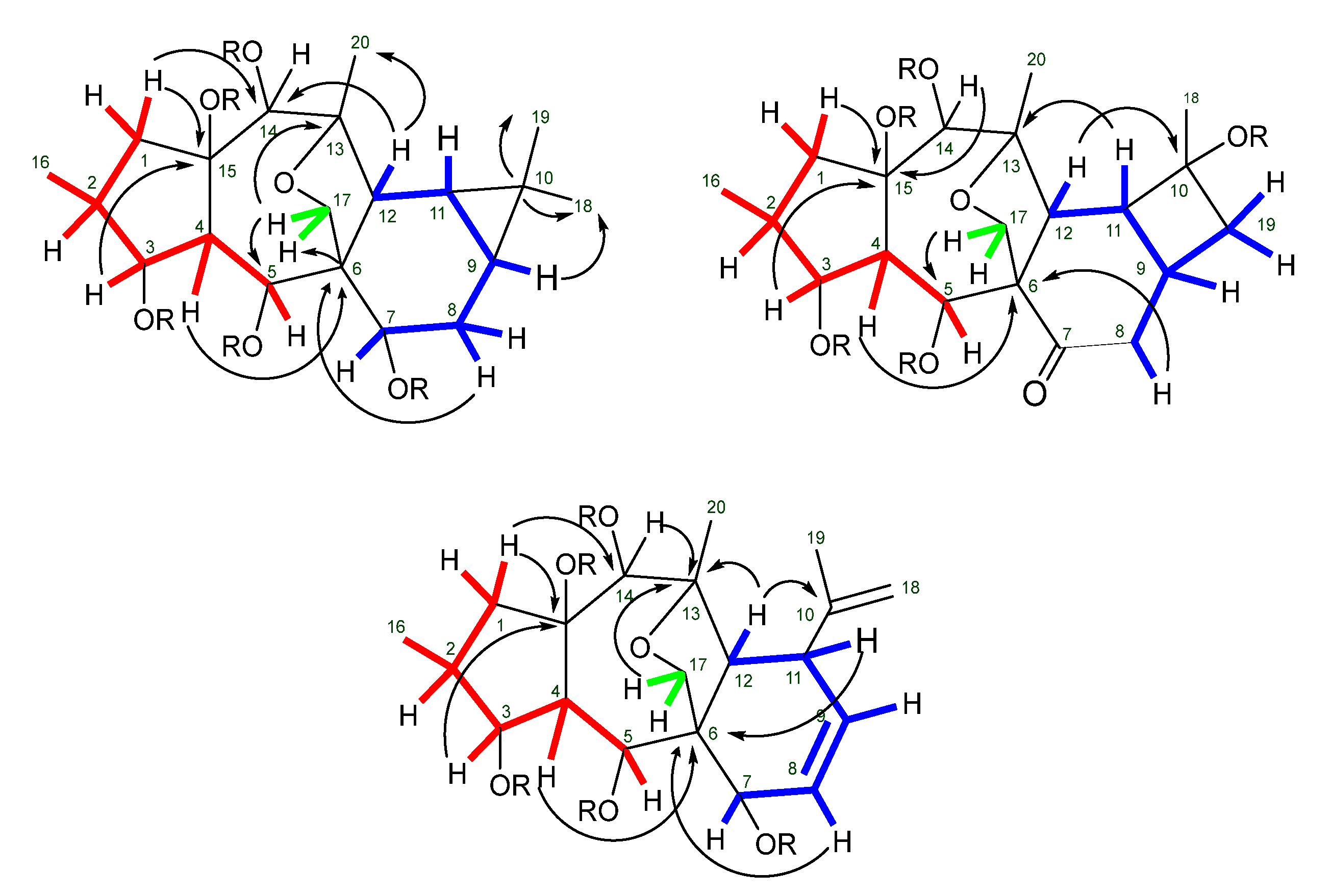
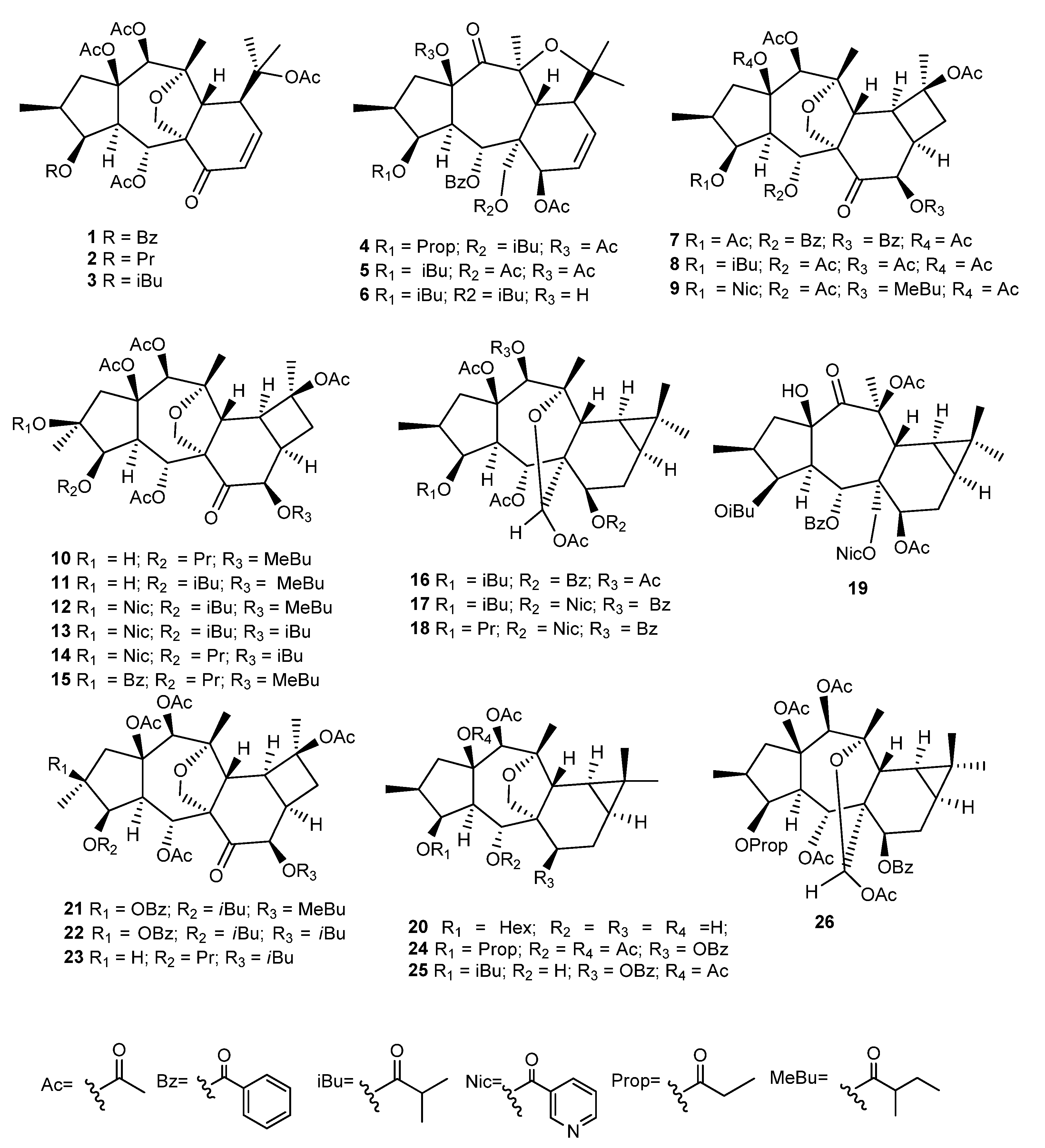
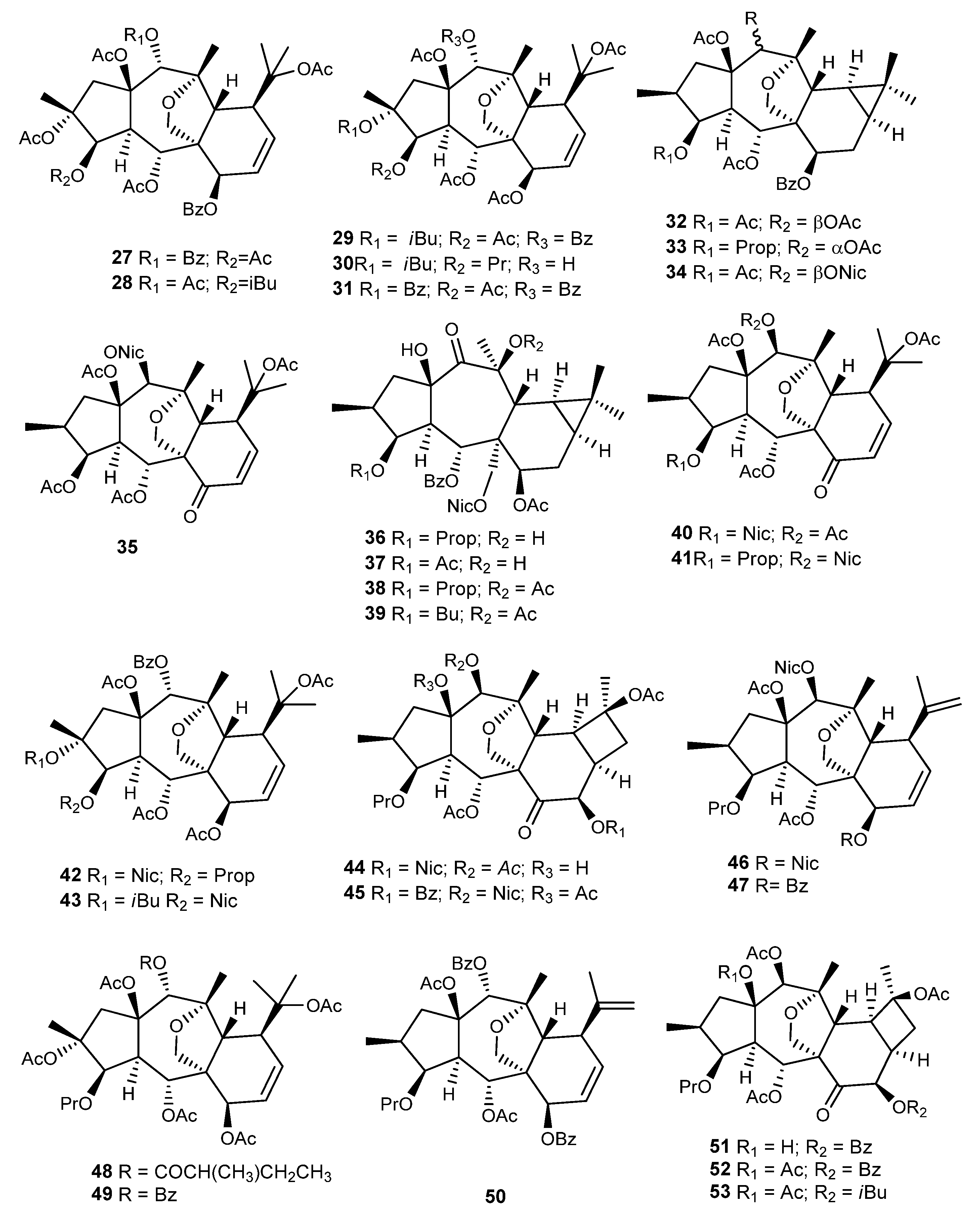
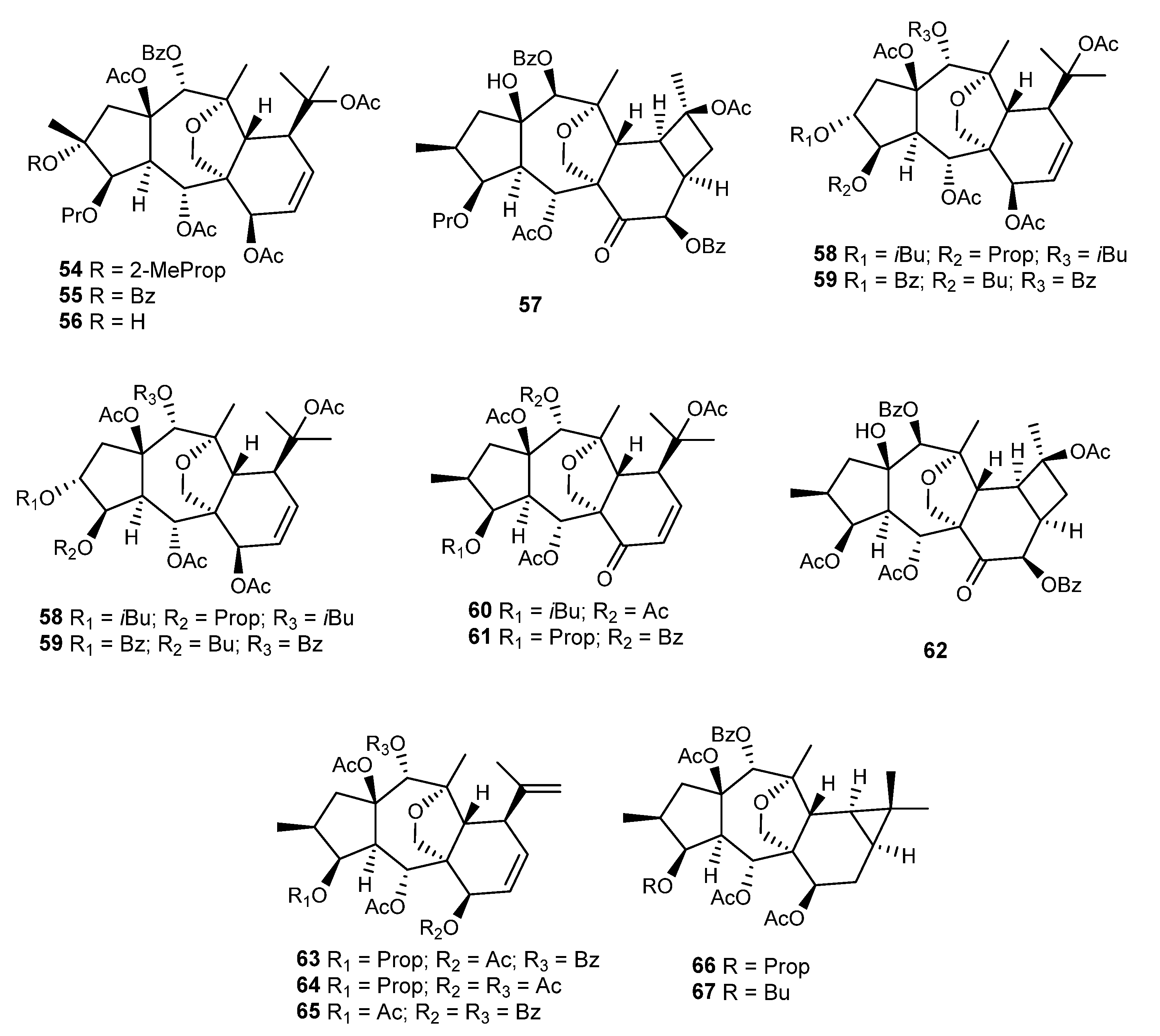
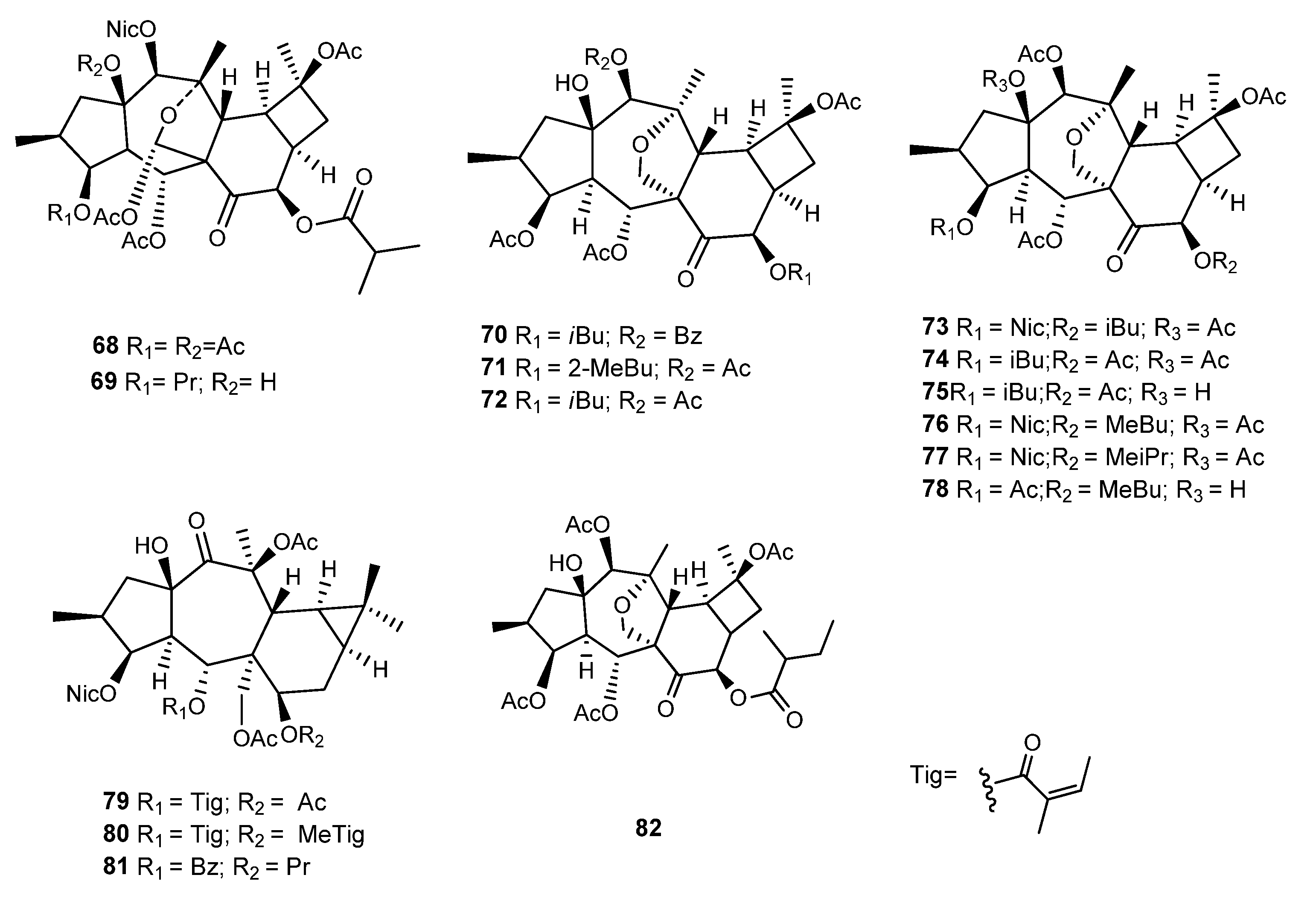



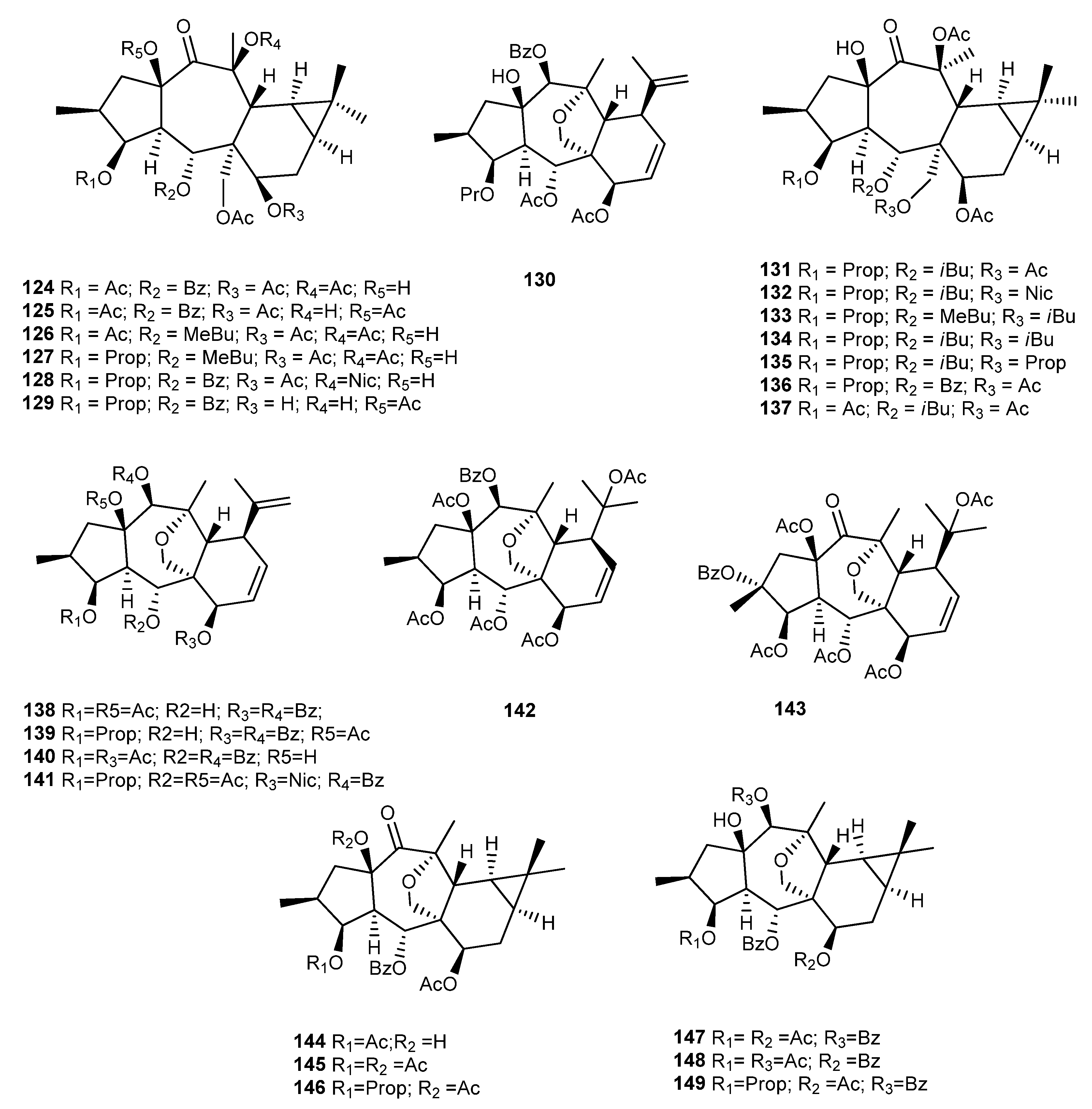
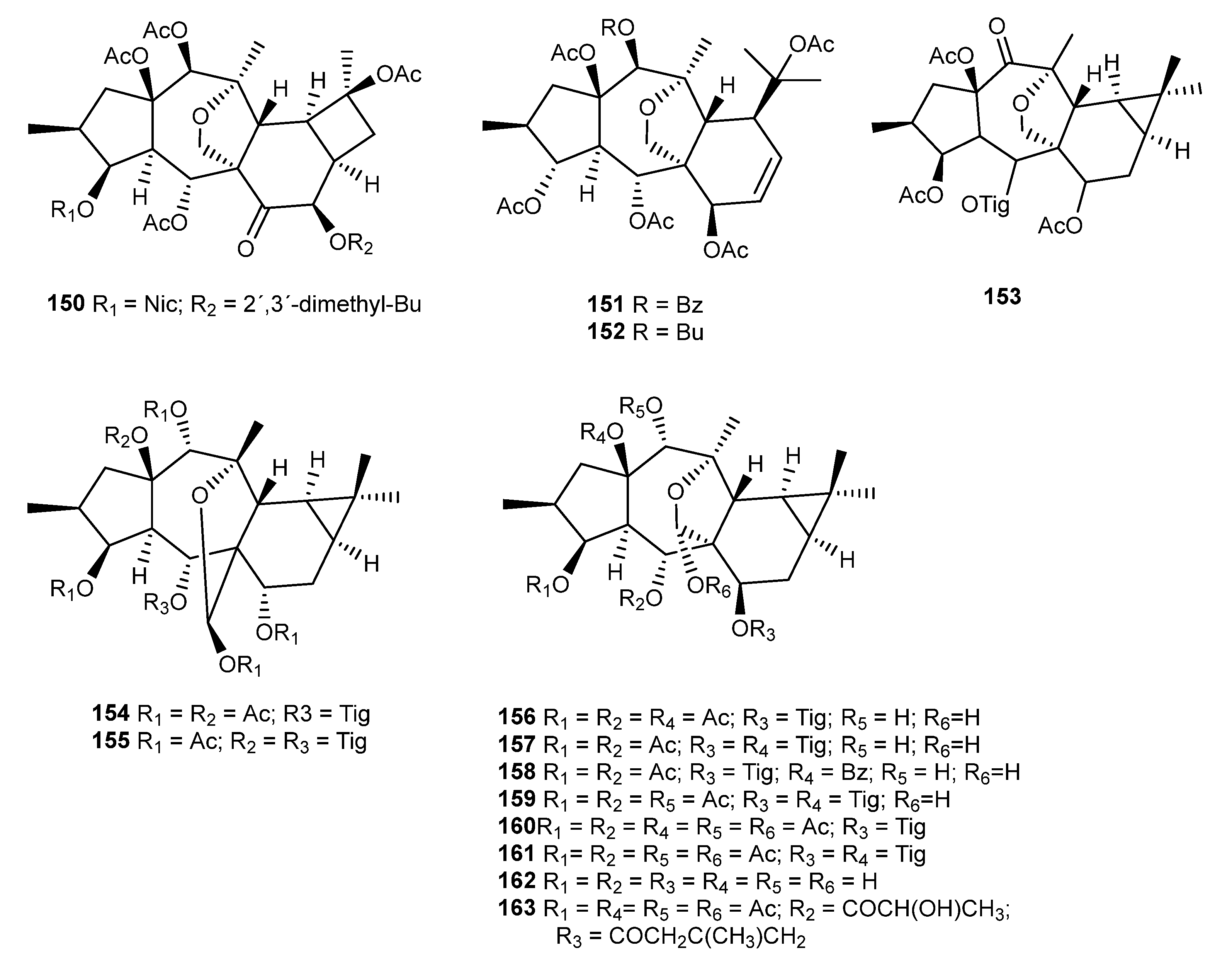
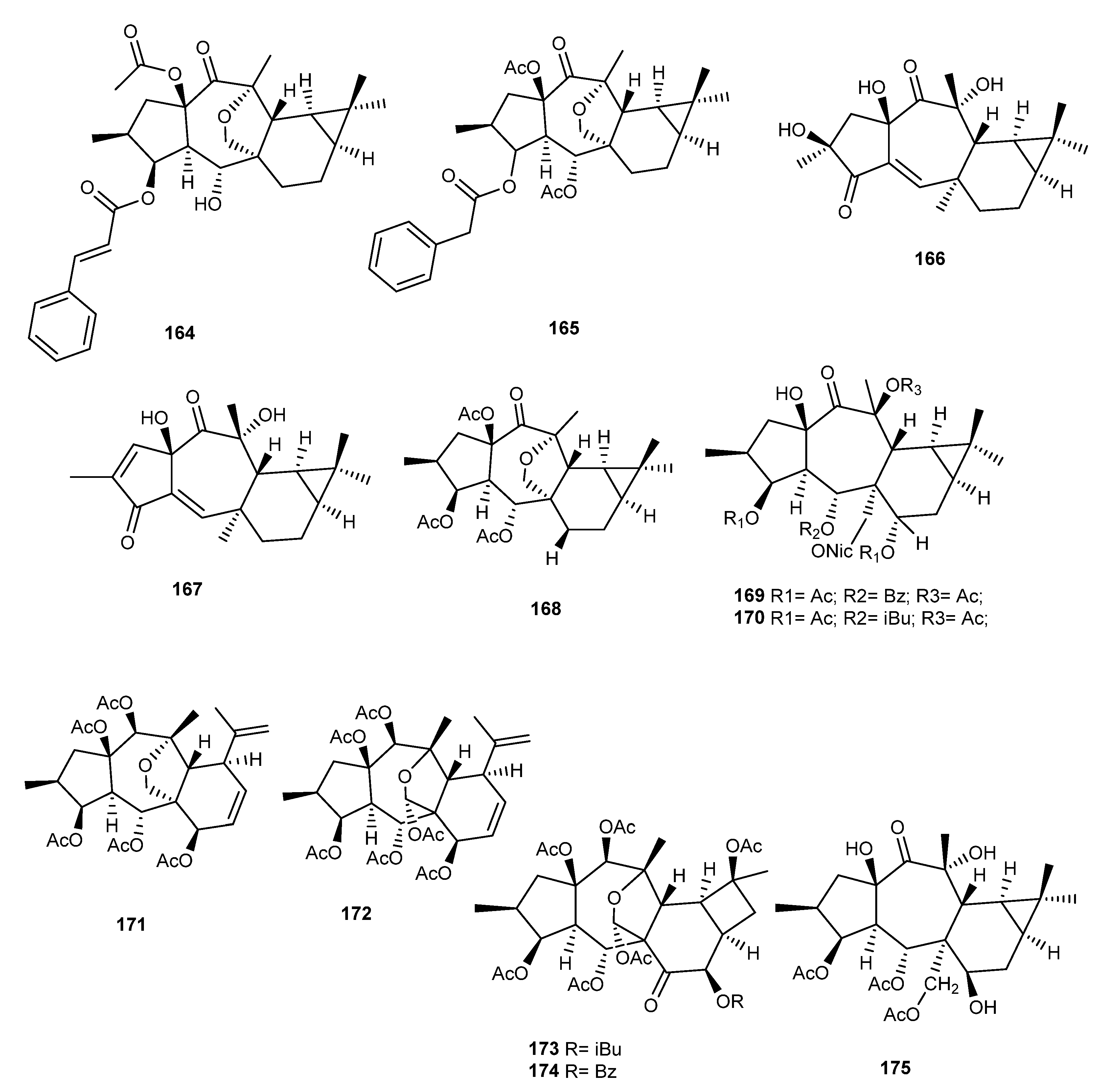
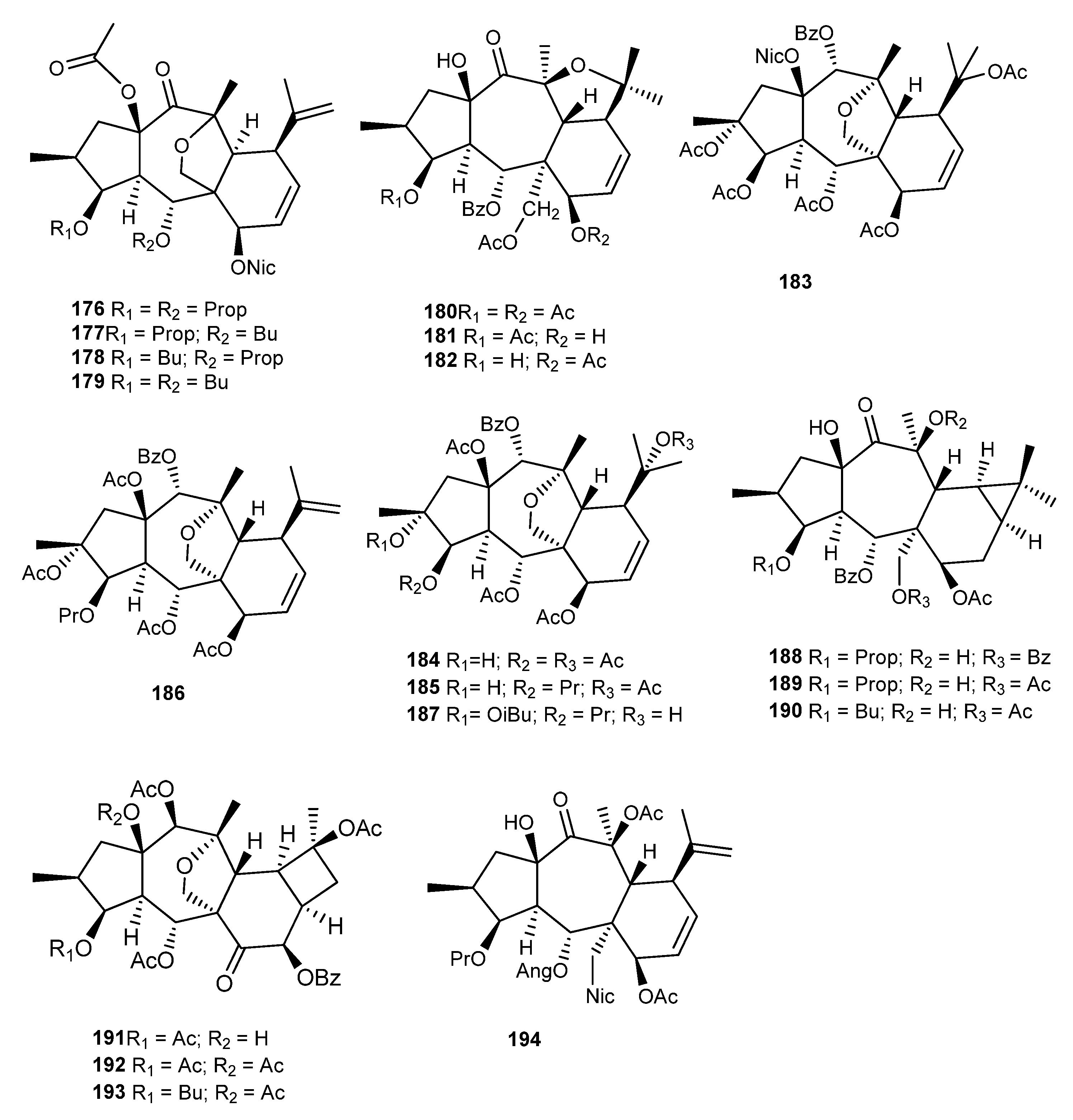
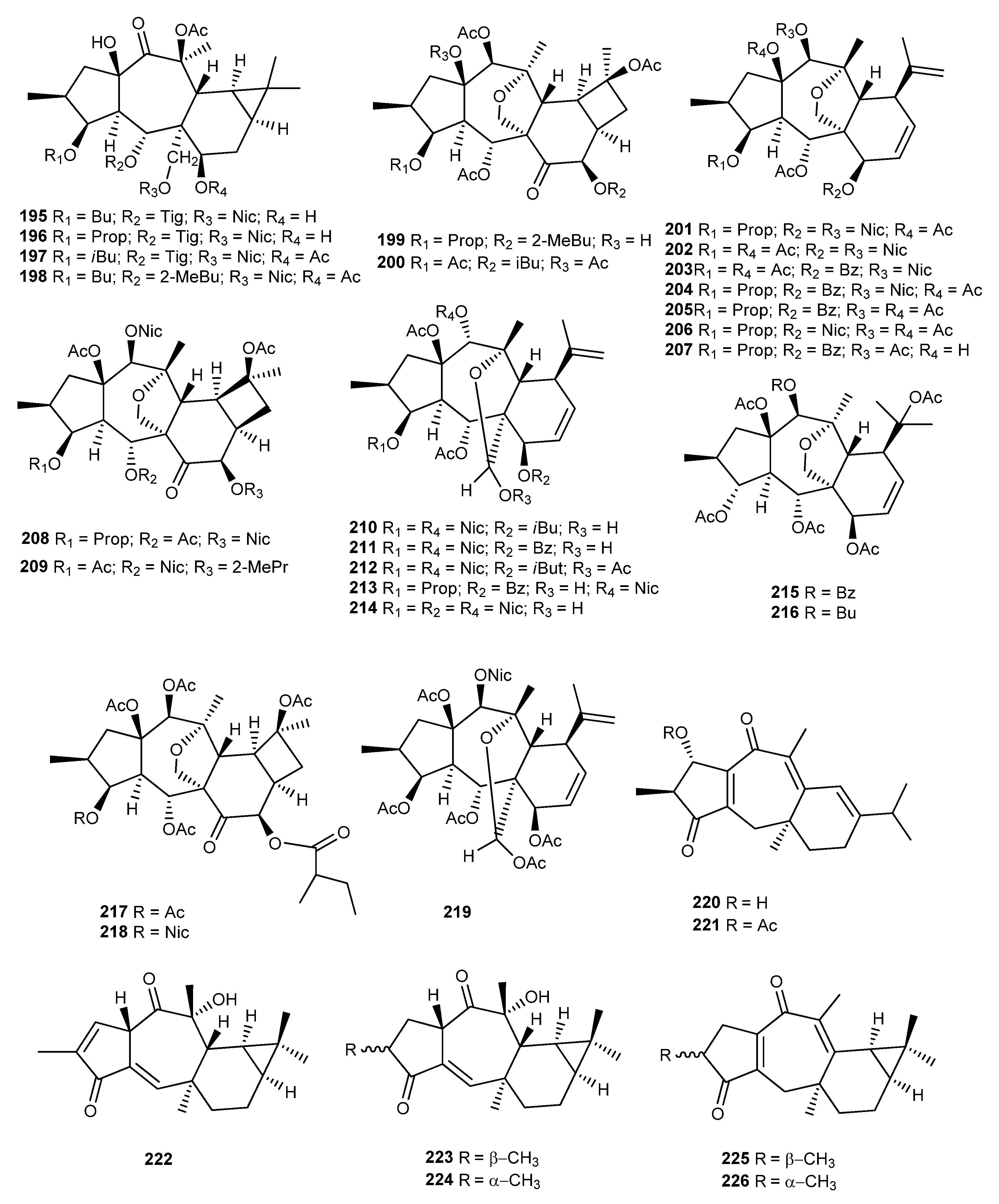
| Euphorbia spp. Extract (Plant Material) | Compounds | Biological Activity | Ref. |
| E. aellenii | |||
| CHF/ACET (aerial parts) | 200 |
| [75] |
| MeOH (whole plant) | 151 |
| [72] |
| E. aleppica | |||
| DCM/ACET (2:1) | 154 |
| [53] |
| E. connata | |||
| ACET (aerial parts) | 112, 113 |
| [56,89] |
| E. decipiens | |||
| ACET (whole plant) | 97, 99, 100, 105 |
| [82,83,86] |
| 98, 103, 104 |
| [82,84,86,88] | |
| ACET:CHF (whole plant) | 106 |
| [83,85] |
| CHF (whole plant) | 105, 106 |
| [85] |
| E. falcata | |||
| MeOH (whole fresh plant) | 1–3, 8, 9, 12, 14, 15, 20–26 |
| [51,61] |
| |||
| E. gedrosiaca | |||
| ACET:DCM (2:1) (whole plant) | 53, 83–85 |
| [54] |
| (aerial parts) | 49, 120, 131, 132, 136 |
| [76] |
| E. kopetdaghi | |||
| ACET:CHF (1:2) (aerial parts) | 70 |
| [71] |
| ACET:DCM (1:2) (aerial parts) | 68 |
| [70] |
| E. macrorrhiza | |||
| ACET (whole plant) | 167 |
| [97] |
| E. microsciadia | |||
| MeOH (aerial flowering parts) | 199 |
| [93] |
| E. myrsinites | |||
| EtOAc (aerial parts) | 176–179 |
| [59] |
| E. pithyusa | |||
| EtOAc(aerial parts) | 130 |
| [92] |
| E. prolifera | |||
| MeOH (dried roots) | 27, 28 |
| [62,63] |
| 29, 30 |
| [64] | |
| 36–44 |
| [66] | |
| 50, 54, 55, 59, 65–69, 205 |
| [68] | |
| EtOH 95% (roots) | 54 |
| [67] |
| E. santae catharinae | |||
| DCM:MeOH (1:1) (aerial parts) | 117–120 |
| [77] |
| E. sogdiana | |||
| ACET:DCM (2:1) (aerial parts) | 73–78, 79–81 |
| [52] |
| 72–74, 76, 78,151 |
| [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mendes, E.; Ramalhete, C.; Duarte, N. Myrsinane-Type Diterpenes: A Comprehensive Review on Structural Diversity, Chemistry and Biological Activities. Int. J. Mol. Sci. 2024, 25, 147. https://doi.org/10.3390/ijms25010147
Mendes E, Ramalhete C, Duarte N. Myrsinane-Type Diterpenes: A Comprehensive Review on Structural Diversity, Chemistry and Biological Activities. International Journal of Molecular Sciences. 2024; 25(1):147. https://doi.org/10.3390/ijms25010147
Chicago/Turabian StyleMendes, Eduarda, Cátia Ramalhete, and Noélia Duarte. 2024. "Myrsinane-Type Diterpenes: A Comprehensive Review on Structural Diversity, Chemistry and Biological Activities" International Journal of Molecular Sciences 25, no. 1: 147. https://doi.org/10.3390/ijms25010147
APA StyleMendes, E., Ramalhete, C., & Duarte, N. (2024). Myrsinane-Type Diterpenes: A Comprehensive Review on Structural Diversity, Chemistry and Biological Activities. International Journal of Molecular Sciences, 25(1), 147. https://doi.org/10.3390/ijms25010147