In Vitro and In Vivo Imaging-Based Evaluation of Doxorubicin Anticancer Treatment in Combination with the Herbal Medicine Black Cohosh
Abstract
:1. Introduction
2. Results
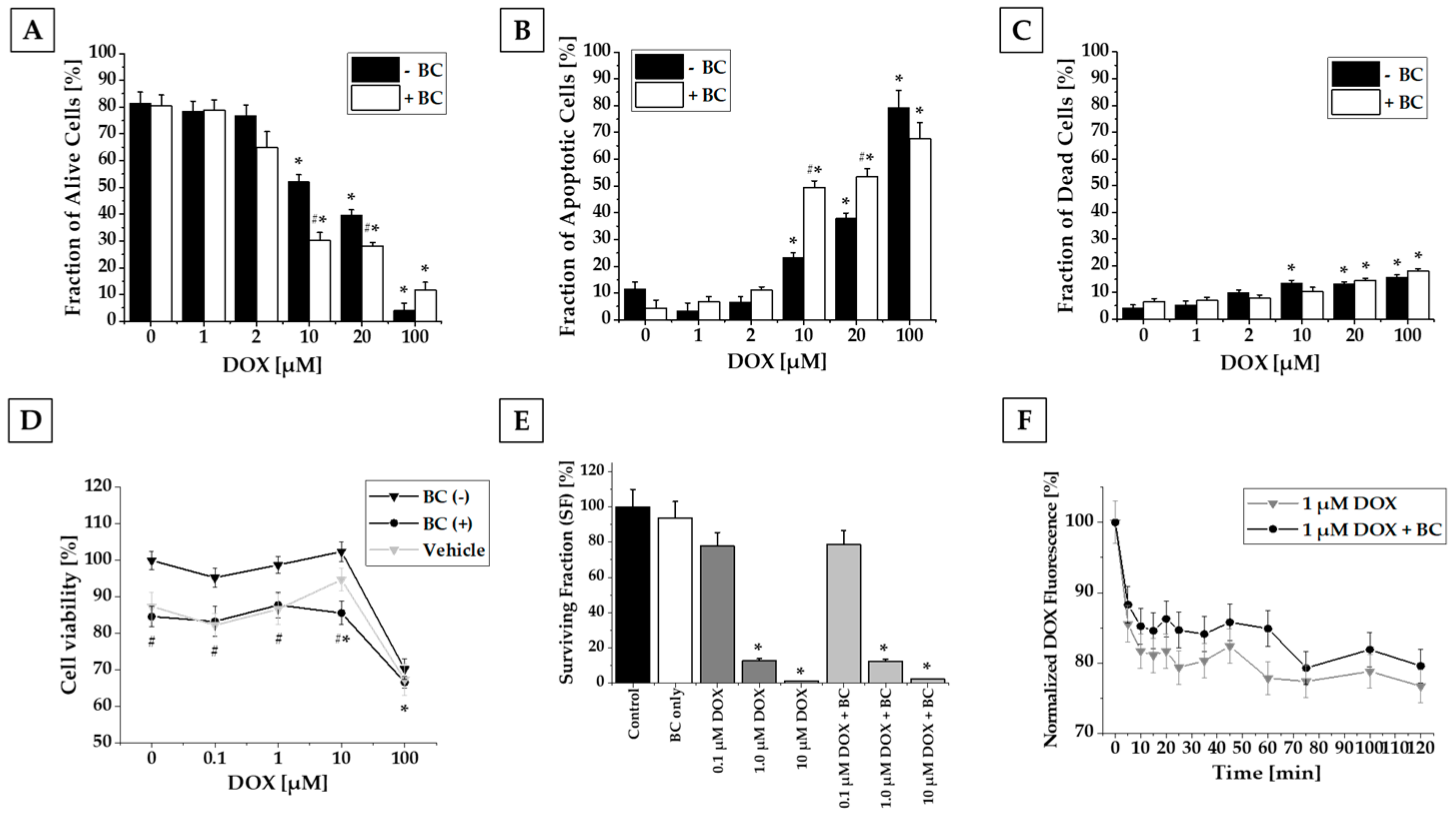
2.1. Results of In Vitro Studies
2.2. Results of In Vivo Studies
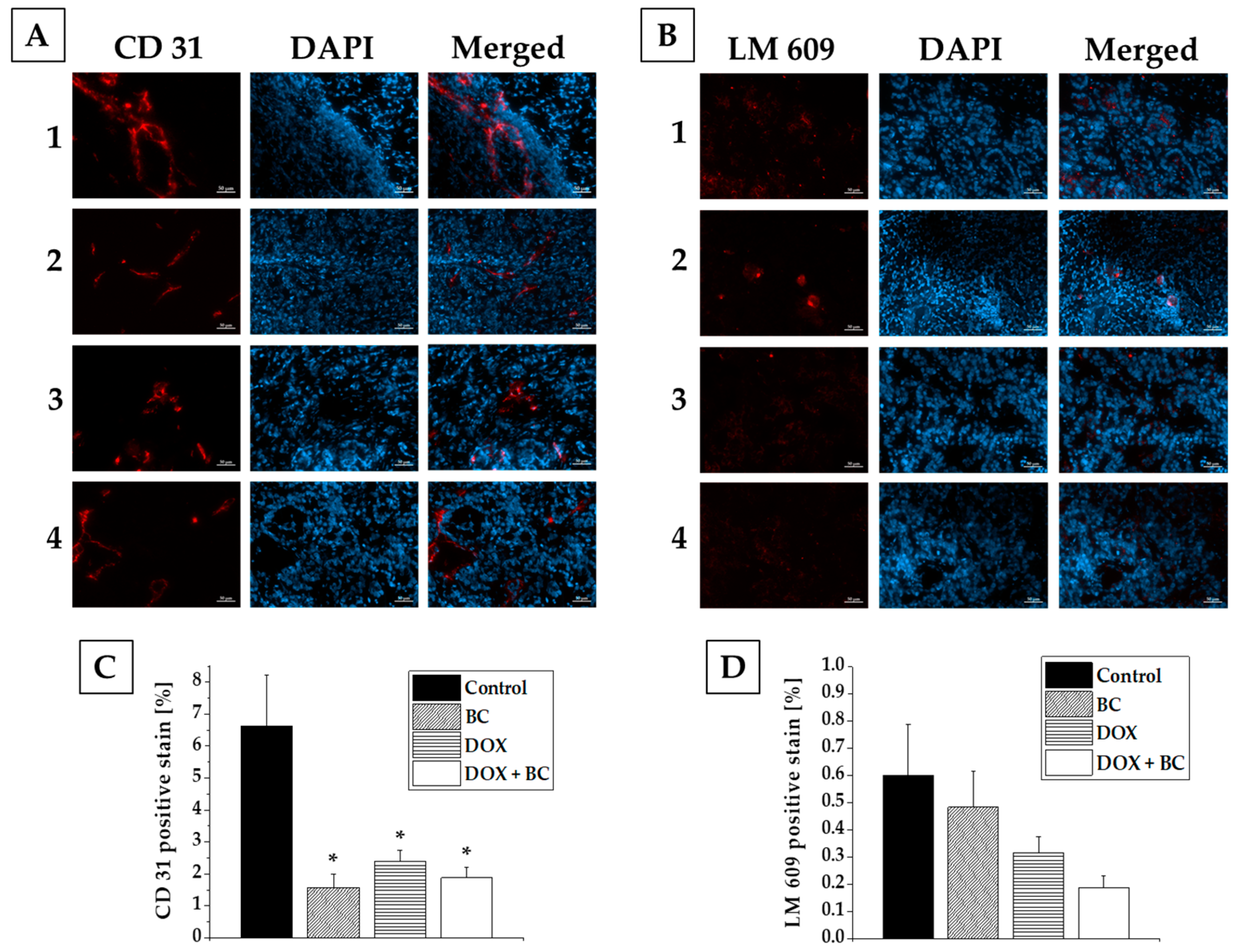
2.3. Results of Ex Vivo Studies
3. Discussion
4. Materials and Methods
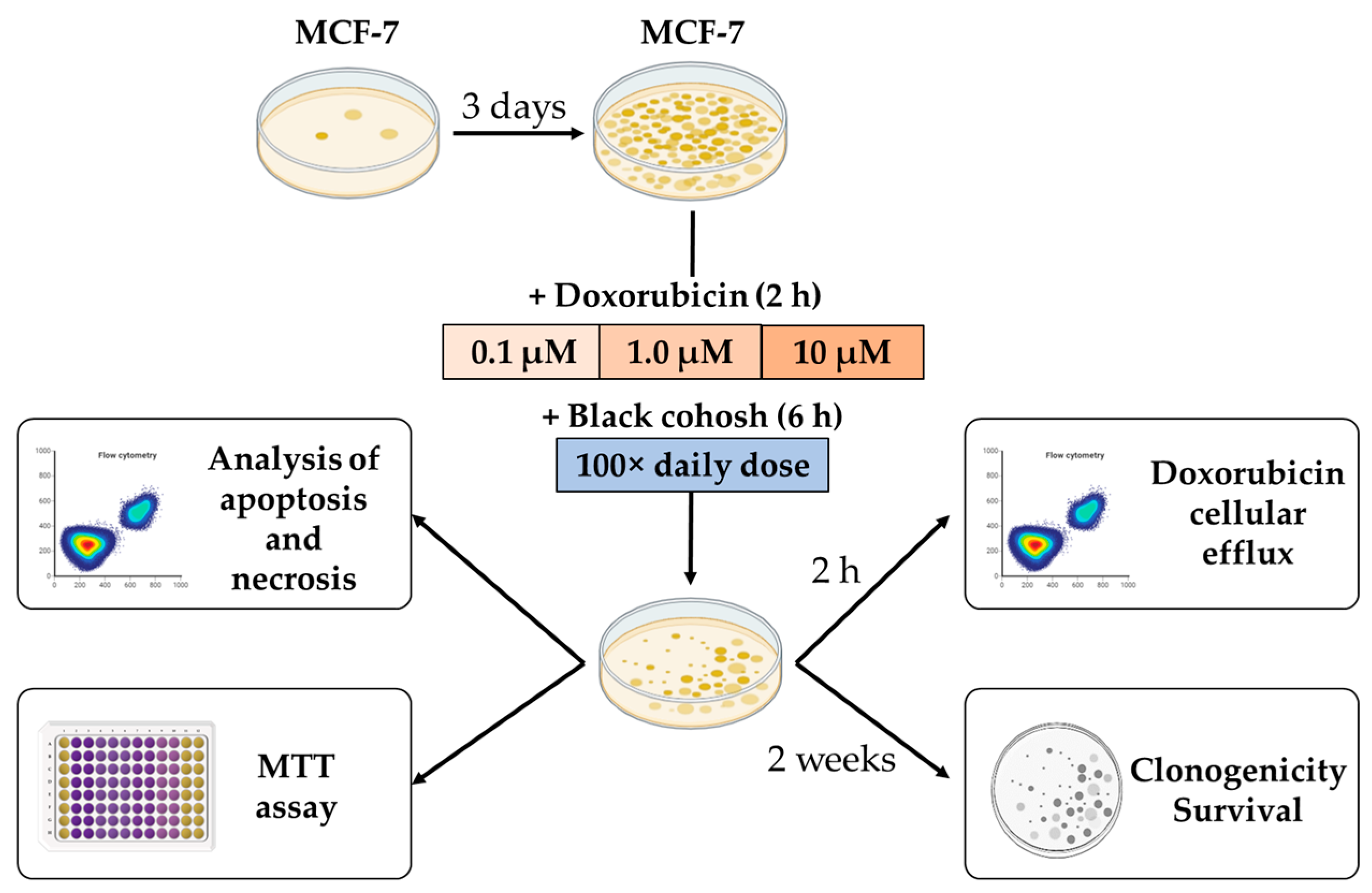
4.1. In Vitro Studies
4.1.1. Cell Culture
4.1.2. Cell Viability Studies
4.1.3. Flow Cytometric Analysis of Apoptosis and Necrosis
4.1.4. Cell Survival Studies
4.1.5. Doxorubicin Efflux Studies
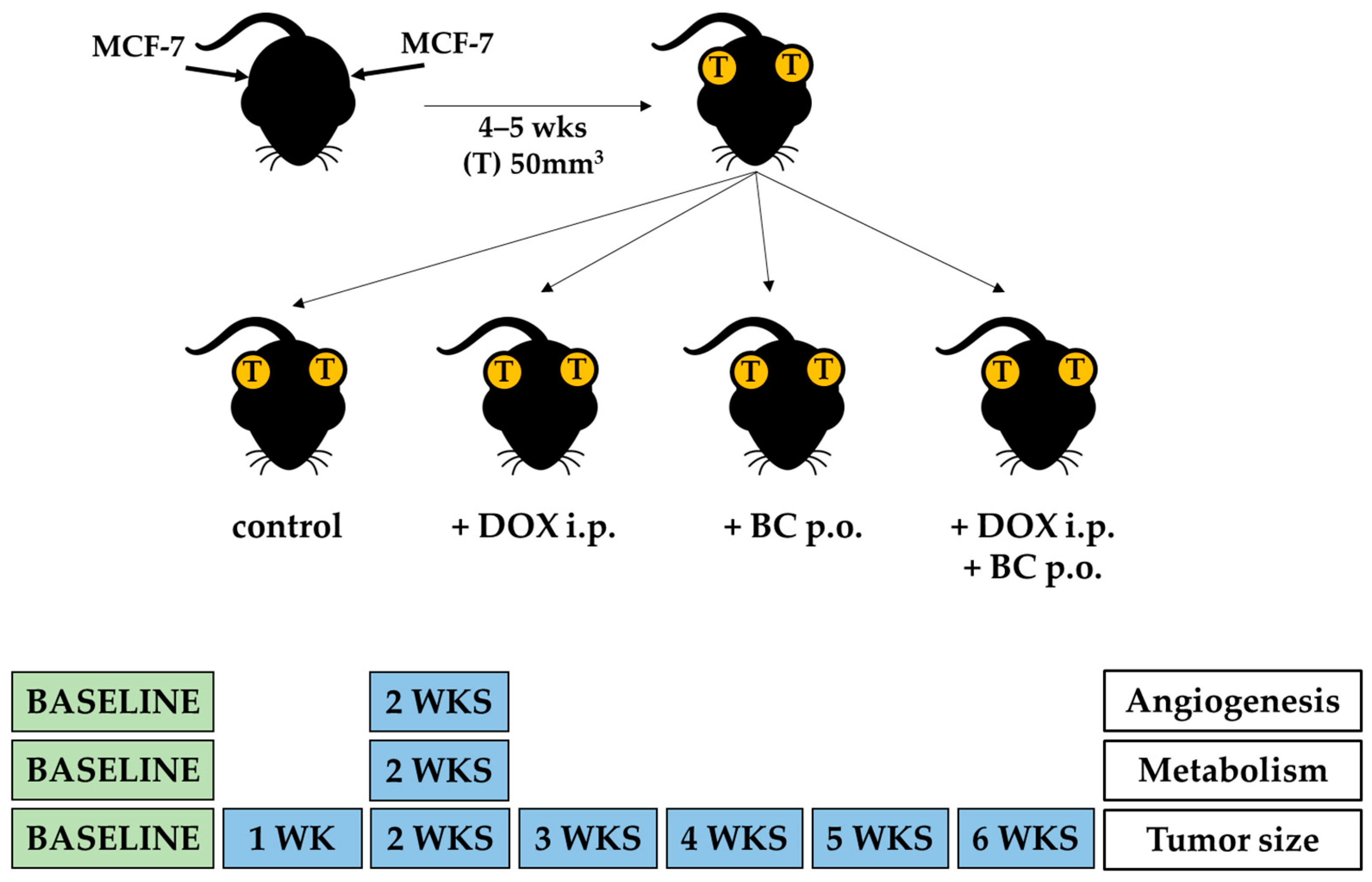
4.2. In Vivo Studies
4.2.1. In Vivo Imaging of MCF-7 Xenografts
4.2.2. Image Analysis
4.3. Ex Vivo Studies
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barnes, P.M.; Bloom, B.; Nahin, R.L. Complementary and alternative medicine use among adults and children: United States, 2007. Natl. Health. Stat. Report 2008, 12, 1–23. [Google Scholar]
- Smith, T.; May, G.; Eckl, V.; Reynolds, C.M. US sales of herbal supplements increase by 8.6% in 2019. Herb. Gram. 2020, 127, 54–69. Available online: https://www.herbalgram.org/news/press-releases/2020/us-herbal-supplement-sales-2019/ (accessed on 19 October 2023).
- Kang, D.H.; McArdle, T.; Suh, Y. Changes in complementary and alternative medicine use across cancer treatment and relationship to stress, mood, and quality of life. J. Altern. Complement. Med. 2014, 11, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Gafner, S. Botanical Adulterants Bulletin on Adulteration of Actaea racemosa. Botanical Adulterants Bulletin. United States. 2016. Available online: http://cms.herbalgram.org/BAP/pdf/BAP-BABs-BlackCohosh-FINAL.pdf (accessed on 19 October 2023).
- Guo, Y.; Yin, T.; Wang, X.; Zhang, F.; Pan, G.; Lv, H.; Wang, X.; Owoicho Orgah, J.; Zhu, Y.; Wu, H. Traditional uses, phytochemistry, pharmacology and toxicology of the genus Cimicifuga: A review. J. Ethnopharmacol. 2017, 209, 264–282. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Yu, Z.Y. Cimicifugae rhizoma: From origins, bioactive constituents to clinical outcomes. Curr. Med. Chem. 2006, 13, 2927–2951. [Google Scholar] [CrossRef] [PubMed]
- Setzer, W.N. The Phytochemistry of Cherokee Aromatic Medicinal Plants. Medicines 2018, 5, 121. [Google Scholar] [CrossRef]
- Kenda, M.; Glavač, N.K.; Nagy, M.; Sollner Dolenc, M.; On Behalf of The Oemonom. Herbal Products Used in Menopause and for Gynecological Disorders. Molecules 2021, 26, 7421. [Google Scholar] [CrossRef] [PubMed]
- Henneicke-von Zepelin, H.H. 60 years of Cimicifuga racemosa medicinal products: Clinical research milestones, current study findings and current development. Wien. Med. Wochenschr. 2017, 167, 147–159. [Google Scholar] [CrossRef]
- Predny, M.L.; De Angelis, P.; Chamberlain, J.L. Black Cohosh (Actaea racemosa): An Annotated Bibliography. U.S. Department of Agriculture Forest Service, Southern Research Station, General Technical Report SRS 97. United States. 2006. Available online: https://www.srs.fs.usda.gov/pubs/gtr/gtr_srs097.pdf (accessed on 28 October 2023).
- Salari, S.; Amiri, M.S.; Ramezani, M.; Moghadam, A.T.; Elyasi, S.; Sahebkar, A.; Emami, S.A. Ethnobotany, Phytochemistry, Traditional and Modern Uses of Actaea racemosa L. (Black cohosh): A Review. Adv. Exp. Med. Biol. 2021, 1308, 403–449. [Google Scholar] [CrossRef]
- Mohapatra, S.; Iqubal, A.; Ansari, M.J.; Jan, B.; Zahiruddin, S.; Mirza, M.A.; Ahmad, S.; Iqbal, Z. Benefits of Black Cohosh (Cimicifuga racemosa) for Women Health: An Up-Close and In-Depth Review. Pharmaceuticals 2022, 15, 278. [Google Scholar] [CrossRef]
- Black Cohosh Herbal Summary. European Medicines Agency. 2018. Available online: https://www.ema.europa.eu/en/documents/herbal-summary/black-cohosh-summary-public_en.pdf (accessed on 28 October 2023).
- Naser, B.; Schnitker, J.; Minkin, M.J.; de Arriba, S.G.; Nolte, K.U.; Osmers, R. Suspected black cohosh hepatotoxicity: No evidence by meta-analysis of randomized controlled clinical trials for isopropanolic black cohosh extract. Menopause 2011, 18, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Foster, S. Exploring the Peripatetic Maze of Black Cohosh Adulteration: A Review of the Nomenclature, Distribution, Chemistry, Market Status, Analytical Methods and Safety. Herbal. Gram 2013, 98, 32–51. Available online: http://cms.herbalgram.org/herbalgram/issue98/hg98feat-blackcohosh.html (accessed on 19 October 2023).
- Nikolić, D.; Lankin, D.C.; Cisowska, T.; Chen, S.N.; Pauli, G.F.; van Breemen, R.B. Nitrogen-Containing Constituents of Black Cohosh: Chemistry, Structure Elucidation, and Biological Activities. Recent. Adv. Phytochem. 2015, 45, 31–75. [Google Scholar] [CrossRef] [PubMed]
- Einbond, L.S.; Shimizu, M.; Xiao, D.; Nuntanakorn, P.; Lim, J.T.; Suzui, M.; Seter, C.; Pertel, T.; Kennelly, E.J.; Kronenberg, F.; et al. Growth inhibitory activity of extracts and purified components of black cohosh on human breast cancer cells. Breast Cancer Res. Treat. 2004, 83, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.; Amon, A.; Whitehead, S.A. Ethanolic extracts of black cohosh (Actaea racemosa) inhibit growth and oestradiol synthesis from oestrone sulphate in breast cancer cells. Maturitas 2007, 56, 359–367. [Google Scholar] [CrossRef]
- Hostanska, K.; Nisslein, T.; Freudenstein, J.; Reichling, J.; Saller, R. Evaluation of cell death caused by triterpene glycosides and phenolic substances from Cimicifuga racemosa extract in human MCF-7 breast cancer cells. Biol. Pharm. Bull. 2004, 27, 1970–1975. [Google Scholar] [CrossRef]
- Crone, M.; Hallman, K.; Lloyd, V.; Szmyd, M.; Badamo, B.; Morse, M.; Dinda, S. The antiestrogenic effects of black cohosh on BRCA1 and steroid receptors in breast cancer cells. Breast Cancer (Dove Med. Press) 2019, 11, 99–110. [Google Scholar] [CrossRef]
- Wu, X.X.; Yue, G.G.; Dong, J.R.; Lam, C.W.; Wong, C.K.; Qiu, M.H.; Lau, C.B. Actein Inhibits the Proliferation and Adhesion of Human Breast Cancer Cells and Suppresses Migration in vivo. Front. Pharmacol. 2018, 9, 1466. [Google Scholar] [CrossRef]
- Yue, G.G.; Xie, S.; Lee, J.K.; Kwok, H.F.; Gao, S.; Nian, Y.; Wu, X.X.; Wong, C.K.; Qiu, M.H.; Lau, C.B. New potential beneficial effects of actein, a triterpene glycoside isolated from Cimicifuga species, in breast cancer treatment. Sci. Rep. 2016, 6, 35263. [Google Scholar] [CrossRef]
- Rockwell, S.; Liu, Y.; Higgins, S.A. Alteration of the effects of cancer therapy agents on breast cancer cells by the herbal medicine black cohosh. Breast Cancer Res. Treat. 2005, 90, 233–239. [Google Scholar] [CrossRef]
- Evan, G.I.; Vousden, K.H. Proliferation, cell cycle and apoptosis in cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Slingerland, M.; Guchelaar, H.J.; Gelderblom, H. Liposomal drug formulations in cancer therapy: 15 years along the road. Drug Discov. Today. 2012, 17, 160–166. [Google Scholar] [CrossRef]
- Rivankar, S. An overview of doxorubicin formulations in cancer therapy. J. Cancer. Res. Ther. 2014, 10, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Kruger, A.; Kleschyov, A.L.; Kalinowski, L.; Daiber, A.; Wojnowski, L. Gp91phox-containing NAD(P)H oxidase increases superoxide formation by doxorubicin and NADPH. Free Radic. Biol. Med. 2007, 42, 466–473. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Hsieh, C.H.; Tsai, T.H. Concurrent administration of anticancer chemotherapy drug and herbal medicine on the perspective of pharmacokinetics. J. Food Drug. Anal. 2018, 26, S88–S95. [Google Scholar] [CrossRef] [PubMed]
- Seely, D.; Oneschuk, D. Interactions of natural health products with biomedical cancer treatments. Curr. Oncol. 2008, 15 (Suppl. 2), s109.es81–s110.es6. [Google Scholar]
- Pourroy, B.; Letellier, C.; Helvig, A.; Chanet, B.; De Crozals, F.; Alessandra, C. Development of a rapid risk evaluation tool for herbs/drugs interactions in cancer patients: A multicentric experience in south of France. Eur. J. Cancer Care 2017, 26, e12752. [Google Scholar] [CrossRef]
- Alsanad, S.M.; Howard, R.L.; Williamson, E.M. An assessment of the impact of herb-drug combinations used by cancer patients. BMC Complement. Altern. Med. 2016, 16, 393. [Google Scholar] [CrossRef]
- Wuttke, W.; Jarry, H.; Haunschild, J.; Stecher, G.; Schuh, M.; Seidlova-Wuttke, D. The non-estrogenic alternative for the treatment of climacteric complaints: Black cohosh (Cimicifuga or Actaea racemosa). J. Steroid. Biochem. Mol. Biol. 2014, 139, 302–310. [Google Scholar] [CrossRef]
- Arentz, S.; Abbott, J.A.; Smith, C.A.; Bensoussan, A. Herbal medicine for the management of polycystic ovary syndrome (PCOS) and associated oligo/amenorrhoea and hyperandrogenism; a review of the laboratory evidence for effects with corroborative clinical findings. BMC Complement. Altern. Med. 2014, 14, 511. [Google Scholar] [CrossRef]
- Gaube, F.; Wolfl, S.; Pusch, L.; Kroll, T.C.; Hamburger, M. Gene expression profiling reveals effects of Cimicifuga racemosa (L.) NUTT. (black cohosh) on the estrogen receptor positive human breast cancer cell line MCF-7. BMC Pharmacol. 2007, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Hostanska, K.; Nisslein, T.; Freudenstein, J.; Reichling, J.; Saller, R. Apoptosis of human prostate androgen-dependent and -independent carcinoma cells induced by an isopropanolic extract of black cohosh involves degradation of cytokeratin (CK) 18. Anticancer. Res. 2005, 25, 139–147. [Google Scholar] [PubMed]
- Chen, Z.; Wu, J.; Guo, Q. Actein Inhibits Cell Proliferation and Migration in Human Osteosarcoma. Med. Sci. Monit. 2016, 22, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Jöhrer, K.; Stuppner, H.; Greil, R.; Çiçek, S.S. Structure-Guided Identification of Black Cohosh (Actaea racemosa) Triterpenoids with In Vitro Activity against Multiple Myeloma. Molecules 2020, 25, 766. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Zhong, B.; Jiang, X.; Mao, F.; Liu, G.; Song, B.; Wang, C.Y.; Jiao, Y.; Wang, J.P.; Xu, Z.B.; et al. Actein induces autophagy and apoptosis in human bladder cancer by potentiating ROS/JNK and inhibiting AKT pathways. Oncotarget 2017, 8, 112498–112515. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.L.; Li, Y.J.; Yao, N.; Xu, J.; Chen, Z.S.; Yiu, A.; Zhang, C.X.; Ye, W.C.; Zhang, D.M. Acerinol, a cyclolanstane triterpenoid from Cimicifuga acerina, reverses ABCB1-mediated multidrug resistance in HepG2/ADM and MCF-7/ADR cells. Eur. J. Pharmacol. 2014, 733, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Sinreih, M.; Gregorič, K.; Gajser, K.; Rižner, T.L. Physiological Concentrations of Cimicifuga racemosa Extract Do Not Affect Expression of Genes Involved in Estrogen Biosynthesis and Action in Endometrial and Ovarian Cell Lines. Biomolecules 2022, 12, 545. [Google Scholar] [CrossRef]
- Pochet, S.; Lechon, A.S.; Lescrainier, C.; De Vriese, C.; Mathieu, V.; Hamdani, J.; Souard, F. Herb-anticancer drug interactions in real life based on VigiBase, the WHO global database. Sci. Rep. 2022, 12, 14178. [Google Scholar] [CrossRef]
- Masada, S. Authentication of the botanical origin of Western herbal products using Cimicifuga and Vitex products as examples. J. Nat. Med. 2016, 70, 361–375. [Google Scholar] [CrossRef]
- Yu, Y.; Tan, J.; Nie, J.; Lv, C.; Lu, J. Fibrous Roots of Cimicifuga Are at Risk of Hepatotoxicity. Molecules 2022, 27, 938. [Google Scholar] [CrossRef]
- Jiang, B.; Ma, C.; Motley, T.; Kronenberg, F.; Kennelly, E.J. Phytochemical fingerprinting to thwart black cohosh adulteration: A 15 Actaea species analysis. Phytochem. Anal. 2011, 22, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Hedhli, J.; Czerwinski, A.; Schuelke, M.; Płoska, A.; Sowinski, P.; Hood, L.; Mamer, S.B.; Cole, J.A.; Czaplewska, P.; Banach, M.; et al. Synthesis, Chemical Characterization and Multiscale Biological Evaluation of a Dimeric-cRGD Peptide for Targeted Imaging of α V β 3 Integrin Activity. Sci. Rep. 2017, 7, 3185. [Google Scholar] [CrossRef] [PubMed]
- Hedhli, J.; Slania, S.L.L.; Płoska, A.; Czerwinski, A.; Konopka, C.J.; Wozniak, M.; Banach, M.; Dobrucki, I.T.; Kalinowski, L.; Dobrucki, L.W. Evaluation of a dimeric-cRGD peptide for targeted PET-CT imaging of peripheral angiogenesis in diabetic mice. Sci. Rep. 2018, 8, 5401. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płoska, A.; Wozniak, M.; Hedhli, J.; Konopka, C.J.; Skondras, A.; Matatov, S.; Stawarz, A.; Schuh, S.; Czerwinski, A.; Dobrucki, L.W.; et al. In Vitro and In Vivo Imaging-Based Evaluation of Doxorubicin Anticancer Treatment in Combination with the Herbal Medicine Black Cohosh. Int. J. Mol. Sci. 2023, 24, 17506. https://doi.org/10.3390/ijms242417506
Płoska A, Wozniak M, Hedhli J, Konopka CJ, Skondras A, Matatov S, Stawarz A, Schuh S, Czerwinski A, Dobrucki LW, et al. In Vitro and In Vivo Imaging-Based Evaluation of Doxorubicin Anticancer Treatment in Combination with the Herbal Medicine Black Cohosh. International Journal of Molecular Sciences. 2023; 24(24):17506. https://doi.org/10.3390/ijms242417506
Chicago/Turabian StylePłoska, Agata, Marcin Wozniak, Jamila Hedhli, Christian J. Konopka, Antonios Skondras, Sarah Matatov, Andrew Stawarz, Sarah Schuh, Andrzej Czerwinski, Lawrence W. Dobrucki, and et al. 2023. "In Vitro and In Vivo Imaging-Based Evaluation of Doxorubicin Anticancer Treatment in Combination with the Herbal Medicine Black Cohosh" International Journal of Molecular Sciences 24, no. 24: 17506. https://doi.org/10.3390/ijms242417506
APA StylePłoska, A., Wozniak, M., Hedhli, J., Konopka, C. J., Skondras, A., Matatov, S., Stawarz, A., Schuh, S., Czerwinski, A., Dobrucki, L. W., Kalinowski, L., & Dobrucki, I. T. (2023). In Vitro and In Vivo Imaging-Based Evaluation of Doxorubicin Anticancer Treatment in Combination with the Herbal Medicine Black Cohosh. International Journal of Molecular Sciences, 24(24), 17506. https://doi.org/10.3390/ijms242417506






