Visualization of the Dynamics of Photoinduced Crawling Motion of 4-(Methylamino)Azobenzene Crystals via Diffracted X-ray Tracking
Abstract
:1. Introduction
2. Results
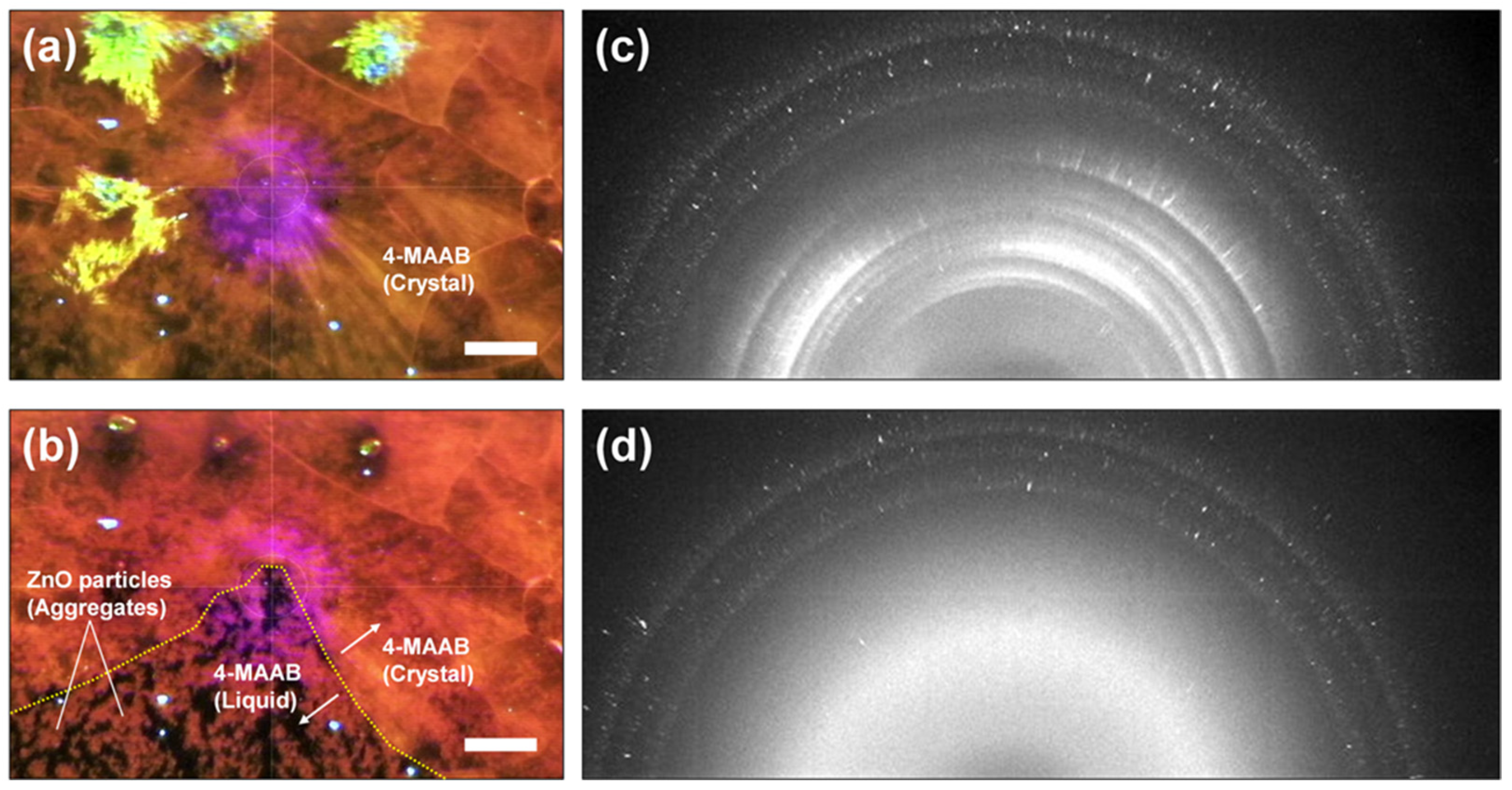
2.1. Time-Resolved XRD of 4-MAAB Crystal Containing ZnO Particles
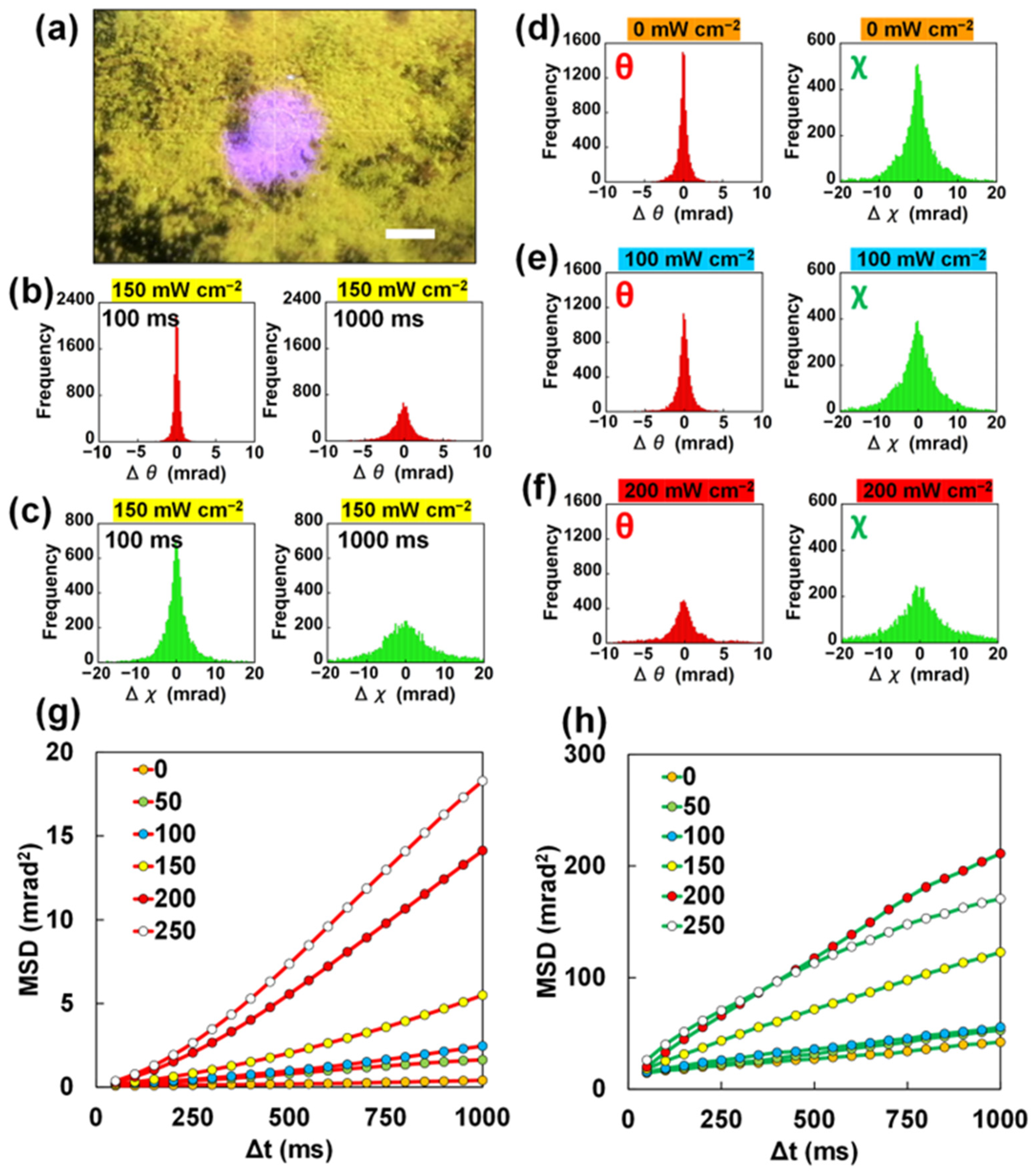
2.2. DXT Analysis for Polycrystalline 4-MAAB Powder
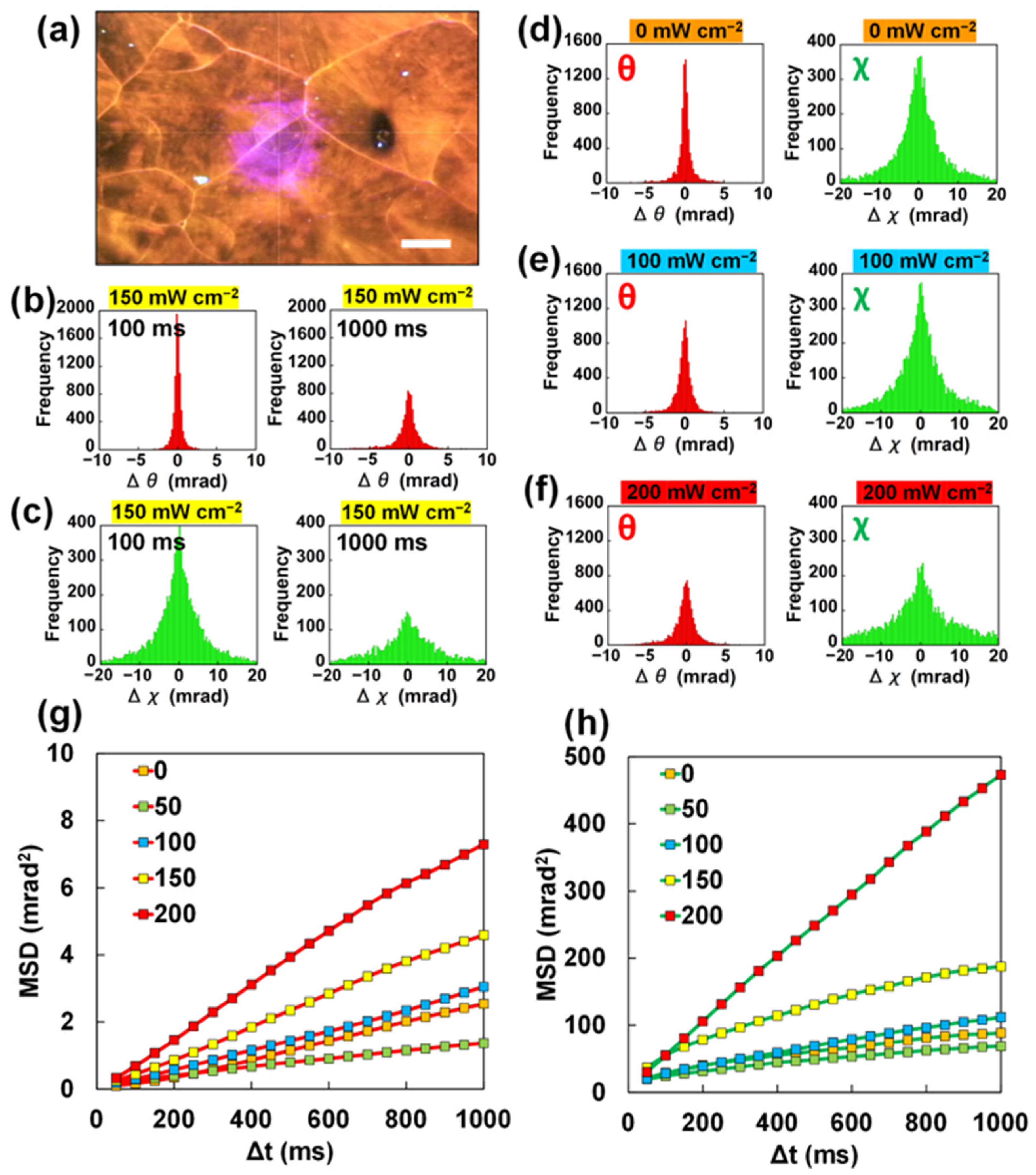
2.3. DXT Analysis for a 4-MAABcrystalline Film
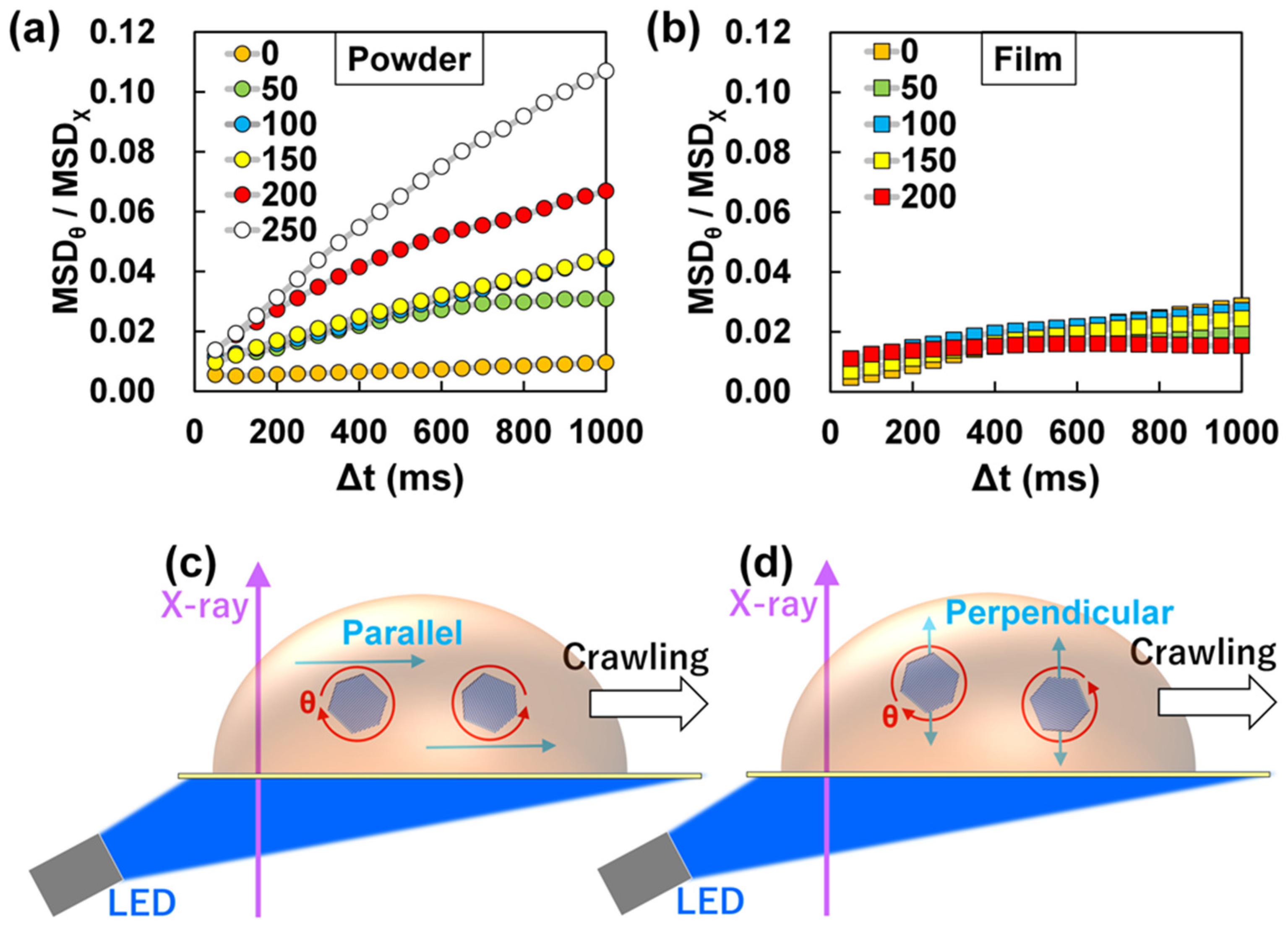
3. Discussion
4. Materials and Methods
4.1. Sample Preparation for Time-Resolved XRD Experiments
4.2. DXT Measurements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J. Can Man-Made Nanomachines Compete with Nature Biomotors? ACS Nano 2009, 3, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M. Electrochemically powered self-propelled electrophoretic nanosubmarines. Nanoscale 2010, 2, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Duan, W.; Ahmed, S.; Mallouk, T.E.; Sen, A. Small power: Autonomous nano- and micromotors propelled by self-generated gradients. Nano Today 2013, 8, 531–554. [Google Scholar] [CrossRef]
- Soler, L.; Magdanz, V.; Fomin, V.M.; Sanchez, S.; Schmidt, O.G. Self-Propelled Micromotors for Cleaning Polluted Water. ACS Nano 2013, 7, 9611–9620. [Google Scholar] [CrossRef] [PubMed]
- Guix, M.; Mayorga-Martinez, C.C.; Merkoçi, A. Nano/Micromotors in (Bio)chemical Science Applications. Chem. Rev. 2014, 114, 6285–6322. [Google Scholar] [CrossRef]
- Sánchez, S.; Soler, L.; Katuri, J. Chemically Powered Micro- and Nanomotors. Angew. Chem. Int. Ed. 2015, 54, 1414–1444. [Google Scholar] [CrossRef]
- Feng, W.; Li, L.; Du, X.; Welle, A.; Levkin, P.A. Single-Step Fabrication of High-Density Microdroplet Arrays of Low-Surface-Tension Liquids. Adv. Mater. 2016, 28, 3202–3208. [Google Scholar] [CrossRef]
- Huang, Z.D.; Yang, Q.; Su, M.; Li, Z.; Hu, X.T.; Li, Y.F.; Pan, Q.; Ren, W.J.; Li, F.Y.; Song, Y.L. A General Approach for Fluid Patterning and Application in Fabricating Microdevices. Adv. Mater. 2018, 30, 1802172. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Li, A.; Liu, Z.; Zhang, Z.; Li, K.; Qiao, Y.; Song, Y. Self-Driven Droplet Vehicle for Material Patterning. Adv. Mater. Interfaces 2021, 8, 2101309. [Google Scholar] [CrossRef]
- Song, K.M.; Kim, M.; Cho, H.; Shin, H.; Kim, G.Y.; Yim, S.; Nam, T.W.; Jung, Y.S. Noninvasive and Direct Patterning of High-Resolution Full-Color Quantum Dot Arrays by Programmed Microwetting. ACS Nano 2022, 16, 16598–16607. [Google Scholar] [CrossRef]
- Matsui, M.; Murasaki, N.; Fujibayashi, K.; Kishimoto, Y. Electrification of pure water flowing down a through set up with a resin sheet. J. Electrost. 1993, 31, 1–10. [Google Scholar] [CrossRef]
- Lee, J.K.; Walker, K.L.; Han, H.S.; Kang, J.; Prinz, F.B.; Waymouth, R.M.; Nam, H.G.; Zare, R.N. Spontaneous generation of hydrogen peroxide from aqueous microdroplets. Proc. Natl. Acad. Sci. USA 2019, 116, 19294–19298. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Torii, T.; Higuchi, T. Chemical reactions in microdroplets by electrostatic manipulation of droplets in liquid media. Lab Chip 2002, 2, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Millman, J.R.; Bhatt, K.H.; Prevo, B.G.; Velev, O.D. Anisotropic particle synthesis in dielectrophoretically controlled microdroplet reactors. Nat. Mater. 2004, 4, 98–102. [Google Scholar] [CrossRef]
- Lin, S.; Cao, L.N.Y.; Tang, Z.; Wang, Z.L. Size-dependent charge transfer between water microdroplets. Proc. Natl. Acad. Sci. USA 2023, 120, e2307977120. [Google Scholar] [CrossRef]
- Liu, W.W.; Zhu, Y.; Feng, Y.M.; Fang, J.; Fang, Q. Droplet-Based Multivolume Digital Polymerase Chain Reaction by a Surface-Assisted Multifactor Fluid Segmentation Approach. Anal. Chem. 2017, 89, 822–829. [Google Scholar] [CrossRef]
- Li, H.; Yang, Q.; Li, G.; Li, M.; Wang, S.; Song, Y. Splitting a Droplet for Femtoliter Liquid Patterns and Single Cell Isolation. ACS Appl. Mater. Interfaces 2015, 7, 9060–9065. [Google Scholar] [CrossRef]
- Guo, X.L.; Wei, Y.; Lou, Q.; Zhu, Y.; Fang, Q. Manipulating Femtoliter to Picoliter Droplets by Pins for Single Cell Analysis and Quantitative Biological Assay. Anal. Chem. 2018, 90, 5810–5817. [Google Scholar] [CrossRef]
- Wei, X.; Chen, K.; Cai, B.; Rao, L.; Wang, Z.; Sun, Y.; Yu, M.; Liu, W.; Guo, S.; Zhao, X.-Z. An Acoustic Droplet-Induced Enzyme Responsive Platform for the Capture and On-Demand Release of Single Circulating Tumor Cells. ACS Appl. Mater. Interfaces 2019, 11, 41118–41126. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Pan, Y.; Wang, X.; Ma, T.; Li, B.; Chu, J. A low-cost self-dispersing method of droplet array generation enabled by a simple reusable mask for bioanalysis and bioassays. Anal. Bioanal. Chem. 2022, 414, 1141–1149. [Google Scholar] [CrossRef]
- Ichimura, K.; Oh, S.-K.; Nakagawa, M. Light-driven motion of liquids on a photoresponsive surface. Science 2000, 288, 1624–1626. [Google Scholar] [CrossRef]
- Hwang, H.; Papadopoulos, P.; Fujii, S.; Wooh, S. Driving Droplets on Liquid Repellent Surfaces via Light-Driven Marangoni Propulsion. Adv. Funct. Mater. 2022, 32, 2111311. [Google Scholar] [CrossRef]
- Li, D.; Li, W.; Chen, R.; Zhu, X.; Ye, D.; Yang, Y.; Liao, Q. Light-Controlled Particle Enrichment Patterns in Droplets. Anal. Chem. 2023, 95, 5828–5837. [Google Scholar] [CrossRef]
- Gao, C.; Wang, L.; Lin, Y.; Li, J.; Liu, Y.; Li, X.; Feng, S.; Zheng, Y. Droplets Manipulated on Photothermal Organogel Surfaces. Adv. Funct. Mater. 2018, 28, 1803072. [Google Scholar] [CrossRef]
- Brzoska, J.B.; Brochard-Wyart, F.; Rondelez, F. Motions of Droplets on Hydrophobic Model Surfaces Induced by Thermal gradients. Langmuir 1993, 9, 2220–2224. [Google Scholar] [CrossRef]
- Linke, H.; Alemán, B.J.; Melling, L.D.; Taormina, M.J.; Francis, M.J.; Dow-Hygelund, C.C.; Narayanan, V.; Taylor, R.P.; Stout, A. Self-propelled Leidenfrost Droplets. Phys. Rev. Lett. 2006, 96, 154502. [Google Scholar] [CrossRef]
- Cira, N.J.; Benusiglio, A.; Prakash, M. Vapour-Mediated Sensing and Motility in Two Component Droplets. Nature 2015, 519, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Bouillant, A.; Mouterde, T.; Bourrianne, P.; Lagarde, A.; Clanet, C.; Quéré, D. Leidenfrost wheels. Nat. Phys. 2018, 14, 1188–1192. [Google Scholar] [CrossRef]
- Pollack, M.G.; Fair, R.B.; Shenderov, A.D. Electrowetting-based actuation of liquid droplets for microfluidic applications. Appl. Phys. Lett. 2000, 77, 1725–1726. [Google Scholar] [CrossRef]
- Hayes, R.A.; Feenstra, B.J. Video-speed electronic paper based on electrowetting. Nature 2003, 425, 383–385. [Google Scholar] [CrossRef]
- Li, J.; Ha, N.S.; Liu, T.; van Dam, R.M.; Kim, C.J. Ionic-surfactant-mediated electro-dewetting for digital microfluidics. Nature 2019, 572, 507–510. [Google Scholar] [CrossRef]
- Srinivasan, V.; Pamula, V.K.; Fair, R.B. An integrated digital microfluidic lab-on-a-chip for clinical diagnostics on human physiological fluids. Lab Chip 2004, 4, 310–315. [Google Scholar]
- Khalil, K.S.; Mahmoudi, S.R.; Abu-Dheir, N.; Varanasi, K.K. Active Surfaces: Ferrofluid-Impregnated Surfaces for Active Manipulation of Droplets. Appl. Phys. Lett. 2014, 105, 041604. [Google Scholar] [CrossRef]
- Timonen, J.V.I.; Latikka, M.; Leibler, L.; Ras, R.H.A.; Ikkala, O. Switchable Static and Dynamic Self-Assembly of Magnetic Droplets on Superhydrophobic Surfaces. Science 2013, 341, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Zhang, N.; Zheng, X.; Hou, G.; Tian, Y.; Du, Y.; Jiang, L.; Dou, S.X. Fast Responsive and Controllable Liquid Transport on a Magnetic Fluid/Nanoarray Composite Interface. ACS Nano 2016, 10, 6220–6226. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Kent, N.; Ceballos, A.; Streubel, R.; Jiang, Y.; Chai, Y.; Kim, P.Y.; Forth, J.; Hellman, F.; Shi, S.; et al. Reconfigurable ferromagnetic liquid droplets. Science 2019, 365, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Uchida, E.; Azumi, R.; Norikane, Y. Light-Induced Crawling of Crystals on a Glass Surface. Nat. Commun. 2015, 6, 7310. [Google Scholar] [CrossRef]
- Saito, K.; Ohnuma, M.; Norikane, Y. Negative Phototactic Behaviour of Crystals on a Glass Surface. Chem. Commun. 2019, 55, 9303–9306. [Google Scholar] [CrossRef]
- Saito, K.; Ichiyanagi, K.; Nozawa, S.; Haruki, R.; Fan, D.; Kanazawa, T.; Norikane, Y. Transportation of Nano/Microparticles via Photoinduced Crawling of Azobenzene Crystals. Adv. Mater. Interf. 2023, 10, 2202525. [Google Scholar] [CrossRef]
- Norikane, Y.; Hirai, Y.; Yoshida, M. Photoinduced Isothermal Phase Transitions of Liquid-Crystalline Macrocyclic Azobenzenes. Chem. Commun. 2011, 47, 1770–1772. [Google Scholar] [CrossRef]
- Qiu, Q.; Gerkman, M.A.; Shi, Y.; Han, G.G.D. Design of phase-transition molecular solar thermal energy storage compounds: Compact molecules with high energy densities. Chem. Commun. 2021, 57, 9458–9461. [Google Scholar] [CrossRef] [PubMed]
- Han, G.G.D.; Li, H.; Grossman, J.C. Optically-controlled long-term storage and release of thermal energy in phase-change materials. Nat. Commun. 2017, 8, 1446. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Halcovitch, N.R.; Griffin, J.M. Crystalline azobenzene composites as photochemical phase-change materials. New J. Chem. 2022, 46, 4057–4061. [Google Scholar] [CrossRef]
- Baroncini, M.; d’Agostino, S.; Bergamini, G.; Ceroni, P.; Comotti, A.; Sozzani, P.; Bassanetti, I.; Grepioni, F.; Hernandez, T.M.; Silvi, S.; et al. Photoinduced reversible switching of porosity in molecular crystals based on star-shaped azobenzene tetramers. Nat. Chem. 2015, 7, 634–640. [Google Scholar] [CrossRef]
- Ishiba, K.; Morikawa, M.; Chikara, C.; Yamada, T.; Iwase, K.; Kawakita, M.; Kimizuka, N. Photoliquefiable Ionic Crystals: A Phase Crossover Approach for Photon Energy Storage Materials with Functional Multiplicity. Angew. Chem. Int. Ed. 2015, 54, 1532–1536. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Shangguan, Z.; Zhang, Z.-Y.; Yu, C.; He, Y.; Fang, D.; Sun, W.; Li, Y.-C.; Yuan, C.; Wu, S.; et al. Visible-Light-Induced Reversible Photochemical Crystal–Liquid Transitions of Azo-Switches for Smart and Robust Adhesives. Chem. Mater. 2022, 34, 2636–2644. [Google Scholar] [CrossRef]
- Sun, S.; Liang, S.; Xu, W.-C.; Wang, M.; Gao, J.; Zhang, Q.; Wu, S. Photoswitches with different numbers of azo chromophores for molecular solar thermal storage. Soft Matter 2022, 18, 8840–8849. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Ni, Y.; Ma, S.; Yu, H. A Programmable and Biomimetic Photo-Actuator: A Composite of a Photo-Liquefiable Azobenzene Derivative and Commercial Plastic Film. J. Mater. Chem. C 2018, 6, 10815–10821. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, S.; Ma, Y.; Yi, J.; Jiang, Y.; Chang, X.; Li, Q. Liquid and photoliquefiable azobenzene derivatives for solvent-free molecular solar thermal fuels. ACS Appl. Mater. Interfaces 2022, 14, 35623–35634. [Google Scholar] [CrossRef]
- Norikane, Y.; Hayashino, M.; Ohnuma, M.; Abe, K.; Kikkawa, Y.; Saito, K.; Manabe, K.; Miyake, K.; Nakano, M.; Takada, N. Effect of Surface Properties on the Photo-Induced Crawling Motion of Azobenzene Crystals on Glass Surfaces. Front. Chem. 2021, 9, 684767. [Google Scholar] [CrossRef]
- Sasaki, Y.C.; Suzuki, Y.; Yagi, N.; Adachi, S.; Ishibashi, M.; Suda, H.; Toyota, K.; Yanagihara, M. Tracking of individual nanocrystals using diffracted x rays. Phys. Rev. E 2000, 62, 3843–3847. [Google Scholar] [CrossRef] [PubMed]
- Mio, K.; Ohkubo, T.; Sasaki, D.; Arai, T.; Sugiura, M.; Fujimura, S.; Nozawa, S.; Sekiguchi, H.; Kuramochi, M.; Sasaki, Y.C. Real-Time Observation of Capsaicin-Induced Intracellular Domain Dynamics of TRPV1 Using the Diffracted X-ray Tracking Method. Membranes 2023, 13, 708. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, Y.; Sekiguchi, H.; Wong, C.J.; Nishijima, M.; Ikezaki, K.; Hamada, D.; Goto, Y.; Sasaki, Y.C. Nanoscale Dynamics of Protein Assembly Networks in Supersaturated Solutions. Sci. Rep. 2017, 7, 13883. [Google Scholar] [CrossRef]
- Hemauer, J.; Qiu, M.; Feng, J.J.; Loudet, J.C. Particle rotation speeds up capillary interactions. Eur. Phys. J. E 2021, 44, 30. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, K.; Ichiyanagi, K.; Fukaya, R.; Haruki, R.; Nozawa, S.; Sasaki, D.; Arai, T.; Sasaki, Y.C.; McGehee, K.; Saikawa, M.; et al. Visualization of the Dynamics of Photoinduced Crawling Motion of 4-(Methylamino)Azobenzene Crystals via Diffracted X-ray Tracking. Int. J. Mol. Sci. 2023, 24, 17462. https://doi.org/10.3390/ijms242417462
Saito K, Ichiyanagi K, Fukaya R, Haruki R, Nozawa S, Sasaki D, Arai T, Sasaki YC, McGehee K, Saikawa M, et al. Visualization of the Dynamics of Photoinduced Crawling Motion of 4-(Methylamino)Azobenzene Crystals via Diffracted X-ray Tracking. International Journal of Molecular Sciences. 2023; 24(24):17462. https://doi.org/10.3390/ijms242417462
Chicago/Turabian StyleSaito, Koichiro, Kouhei Ichiyanagi, Ryo Fukaya, Rie Haruki, Shunsuke Nozawa, Daisuke Sasaki, Tatsuya Arai, Yuji C. Sasaki, Keegan McGehee, Makoto Saikawa, and et al. 2023. "Visualization of the Dynamics of Photoinduced Crawling Motion of 4-(Methylamino)Azobenzene Crystals via Diffracted X-ray Tracking" International Journal of Molecular Sciences 24, no. 24: 17462. https://doi.org/10.3390/ijms242417462
APA StyleSaito, K., Ichiyanagi, K., Fukaya, R., Haruki, R., Nozawa, S., Sasaki, D., Arai, T., Sasaki, Y. C., McGehee, K., Saikawa, M., Gao, M., Wei, Z., Kwaria, D., & Norikane, Y. (2023). Visualization of the Dynamics of Photoinduced Crawling Motion of 4-(Methylamino)Azobenzene Crystals via Diffracted X-ray Tracking. International Journal of Molecular Sciences, 24(24), 17462. https://doi.org/10.3390/ijms242417462






