Interplay between the Glymphatic System and the Endocannabinoid System: Implications for Brain Health and Disease
Abstract
:1. Introduction
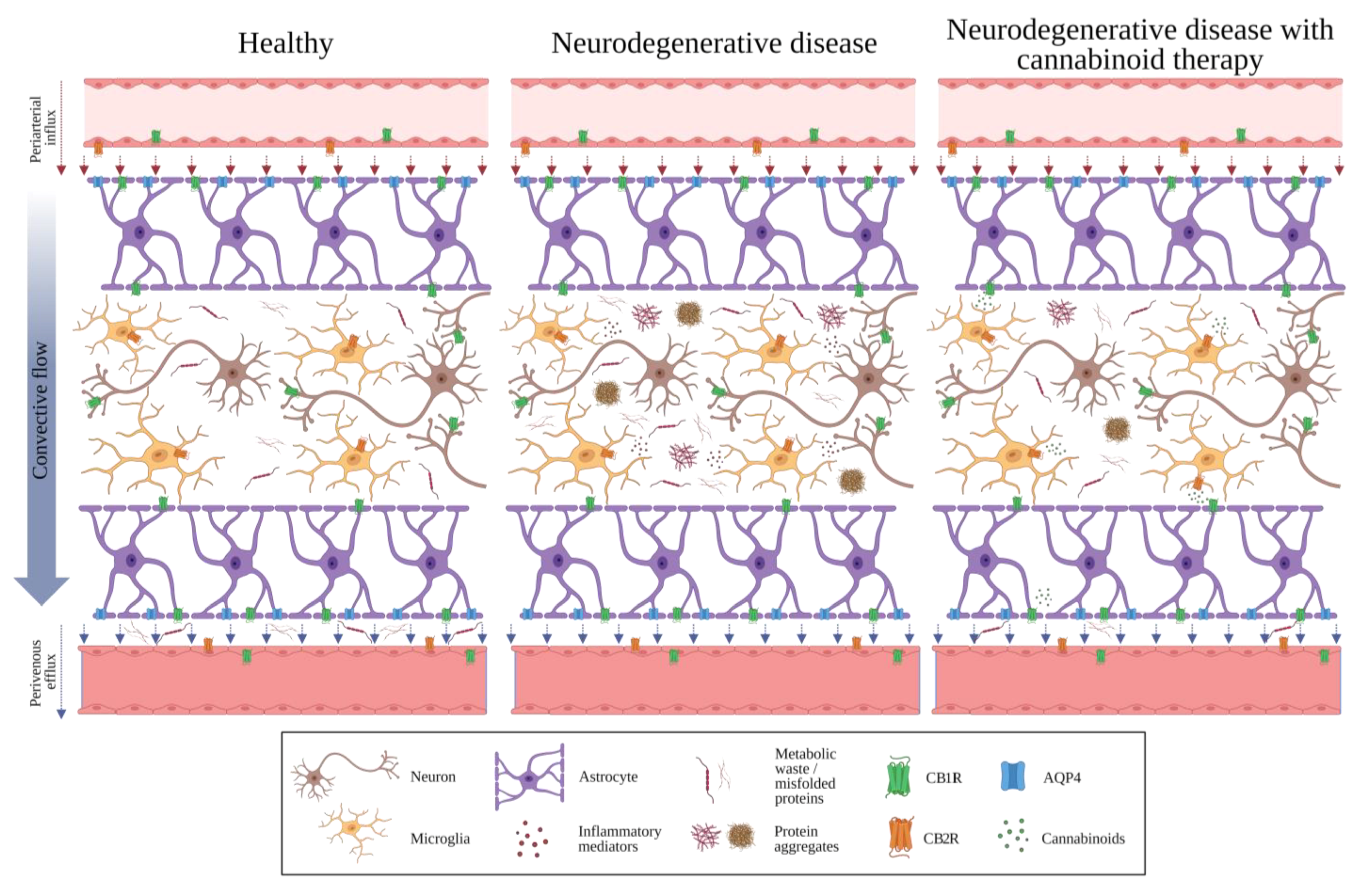
2. The Glymphatic System
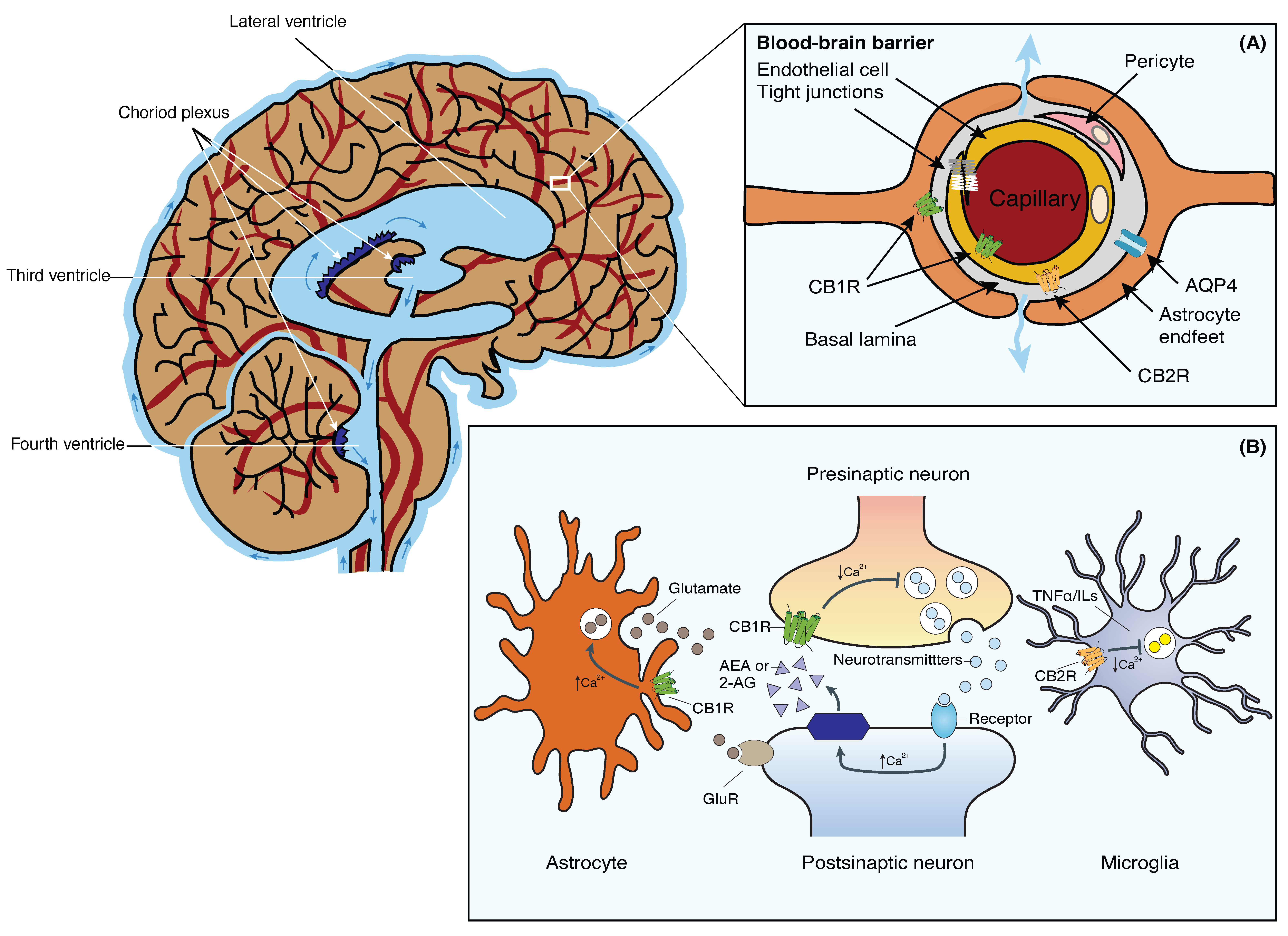
3. The Blood-Brain Barrier
4. The Endocannabinoid System
5. The Endocannabinoid System and the Blood-Brain Barrier
6. A Note about Sleep, the Glymphatic System, and Endocannabinoids
7. Potential Interactions between the GS, the BBB, and the ECS
7.1. Alzheimer’s Disease
7.2. Multiple Sclerosis
7.3. Huntington’s Disease
7.4. Parkinson’s Disease
7.5. Amyotrophic Lateral Sclerosis
8. Future Directions and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef] [PubMed]
- Naganawa, S.; Taoka, T. The Glymphatic System: A Review of the Challenges in Visualizing its Structure and Function with MR Imaging. Magn. Reson. Med. Sci. 2022, 21, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Jessen, N.A.; Munk, A.S.F.; Lundgaard, I.; Nedergaard, M. The Glymphatic System: A Beginner’s Guide. Neurochem. Res. 2015, 40, 2583–2599. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhao, M.; Chen, Y.; Yang, M.; Wang, Y. The Underlying Role of the Glymphatic System and Meningeal Lymphatic Vessels in Cerebral Small Vessel Disease. Biomolecules 2022, 12, 748. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Pizzo, M.E.; Preston, J.E.; Janigro, D.; Thorne, R.G. The role of brain barriers in fluid movement in the CNS: Is there a ‘glymphatic’ system? Acta Neuropathol. 2018, 135, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Chong, P.L.; Garic, D.; Shen, M.D.; Lundgaard, I.; Schwichtenberg, A.J. Sleep, cerebrospinal fluid, and the glymphatic system: A systematic review. Sleep Med. Rev. 2021, 61, 101572. [Google Scholar] [CrossRef]
- Eide, P.K.; Vinje, V.; Pripp, A.H.; Mardal, K.-A.; Ringstad, G. Sleep deprivation impairs molecular clearance from the human brain. Brain 2021, 144, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Demiral, Ş.B.; Tomasi, D.; Sarlls, J.; Lee, H.; Wiers, C.E.; Zehra, A.; Srivastava, T.; Ke, K.; Shokri-Kojori, E.; Freeman, C.R.; et al. Apparent diffusion coefficient changes in human brain during sleep—Does it inform on the existence of a glymphatic system? NeuroImage 2018, 185, 263–273. [Google Scholar] [CrossRef]
- Sun, B.-L.; Wang, L.-H.; Yang, T.; Sun, J.-Y.; Mao, L.-L.; Yang, M.-F.; Yuan, H.; Colvin, R.A.; Yang, X.-Y. Lymphatic drainage system of the brain: A novel target for intervention of neurological diseases. Prog. Neurobiol. 2018, 163–164, 118–143. [Google Scholar] [CrossRef]
- Taoka, T.; Naganawa, S. Neurofluid Dynamics and the Glymphatic System: A Neuroimaging Perspective. Korean J. Radiol. 2020, 21, 1199–1209. [Google Scholar] [CrossRef]
- Wu, C.; Lirng, J.; Ling, Y.; Wang, Y.; Wu, H.; Fuh, J.; Lin, P.; Wang, S.; Chen, S. Noninvasive Characterization of Human Glymphatics and Meningeal Lymphatics in an in vivo Model of Blood–Brain Barrier Leakage. Ann. Neurol. 2020, 89, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Saade, C.; Bou-Fakhredin, R.; Yousem, D.M.; Asmar, K.; Naffaa, L.; El-Merhi, F. Gadolinium and Multiple Sclerosis: Vessels, Barriers of the Brain, and Glymphatics. Am. J. Neuroradiol. 2018, 39, 2168–2176. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Cho, S.J.; Bae, Y.J.; Kim, J.-M. MRI-Based Demonstration of the Normal Glymphatic System in a Human Population: A Systematic Review. Front. Neurol. 2022, 13, 827398. [Google Scholar] [CrossRef]
- Lee, D.A.; Lee, H.; Park, K.M. Glymphatic dysfunction in isolated REM sleep behavior disorder. Acta Neurol. Scand. 2021, 145, 464–470. [Google Scholar] [CrossRef]
- Liang, T.; Chang, F.; Huang, Z.; Peng, D.; Zhou, X.; Liu, W. Evaluation of glymphatic system activity by diffusion tensor image analysis along the perivascular space (DTI-ALPS) in dementia patients. Br. J. Radiol. 2023, 96, 20220315. [Google Scholar] [CrossRef]
- Park, C.J.; Kim, S.-Y.; Kim, J.H.; Son, N.-H.; Park, J.Y.; Jeong, Y.H.; Kim, H.J.; Park, J.; Kim, W.J. Evaluation of glymphatic system activity using diffusion tensor image analysis along the perivascular space and amyloid PET in older adults with objectively normal cognition: A preliminary study. Front. Aging Neurosci. 2023, 15, 1221667. [Google Scholar] [CrossRef]
- Thomas, J.H. Fluid dynamics of cerebrospinal fluid flow in perivascular spaces. J. R. Soc. Interface 2019, 16, 20190572. [Google Scholar] [CrossRef]
- Stanton, E.H.; Persson, N.D.; Gomolka, R.S.; Lilius, T.; Sigurðsson, B.; Lee, H.; Xavier, A.L.R.; Benveniste, H.; Nedergaard, M.; Mori, Y. Mapping of CSF transport using high spatiotemporal resolution dynamic contrast-enhanced MRI in mice: Effect of anesthesia. Magn. Reson. Med. 2021, 85, 3326–3342. [Google Scholar] [CrossRef]
- Wang, M.; Ding, F.; Deng, S.; Guo, X.; Wang, W.; Iliff, J.J.; Nedergaard, M. Focal Solute Trapping and Global Glymphatic Pathway Impairment in a Murine Model of Multiple Microinfarcts. J. Neurosci. 2017, 37, 2870–2877. [Google Scholar] [CrossRef]
- Jiang, Q. MRI and glymphatic system. Stroke Vasc. Neurol. 2019, 4, 75–77. [Google Scholar] [CrossRef]
- Ahn, J.H.; Cho, H.; Kim, J.-H.; Kim, S.H.; Ham, J.-S.; Park, I.; Suh, S.H.; Hong, S.P.; Song, J.-H.; Hong, Y.-K.; et al. Meningeal lymphatic vessels at the skull base drain cerebrospinal fluid. Nature 2019, 572, 62–66. [Google Scholar] [CrossRef]
- Iliff, J.J.; Lee, H.; Yu, M.; Feng, T.; Logan, J.; Nedergaard, M.; Benveniste, H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J. Clin. Investig. 2013, 123, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Bohr, T.; Hjorth, P.G.; Holst, S.C.; Hrabětová, S.; Kiviniemi, V.; Lilius, T.; Lundgaard, I.; Mardal, K.-A.; Martens, E.A.; Mori, Y.; et al. The glymphatic system: Current understanding and modeling. iScience 2022, 25, 104987. [Google Scholar] [CrossRef] [PubMed]
- Turtzo, L.C.; Jikaria, N.; Cota, M.R.; Williford, J.P.; Uche, V.; Davis, T.; MacLaren, J.; Moses, A.D.; Parikh, G.; Castro, M.A.; et al. Meningeal blood–brain barrier disruption in acute traumatic brain injury. Brain Commun. 2020, 2, fcaa143. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Langen, U.H.; Ayloo, S.; Gu, C. Development and Cell Biology of the Blood-Brain Barrier. Annu. Rev. Cell Dev. Biol. 2019, 35, 591–613. [Google Scholar] [CrossRef]
- Zhao, Z.; Nelson, A.R.; Betsholtz, C.; Zlokovic, B.V. Establishment and Dysfunction of the Blood-Brain Barrier. Cell 2015, 163, 1064–1078. [Google Scholar] [CrossRef]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’driscoll, C.M. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef]
- Huang, X.; Hussain, B.; Chang, J. Peripheral inflammation and blood–brain barrier disruption: Effects and mechanisms. CNS Neurosci. Ther. 2020, 27, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Benveniste, H.; Liu, X.; Koundal, S.; Sanggaard, S.; Lee, H.; Wardlaw, J. The Glymphatic System and Waste Clearance with Brain Aging: A Review. Gerontology 2018, 65, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Crocq, M.-A. History of cannabis and the endocannabinoid system. Dialogues Clin. Neurosci. 2020, 22, 223–228. [Google Scholar] [CrossRef]
- Lowe, H.; Toyang, N.; Steele, B.; Bryant, J.; Ngwa, W. The Endocannabinoid System: A Potential Target for the Treatment of Various Diseases. Int. J. Mol. Sci. 2021, 22, 9472. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.-C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615. [Google Scholar] [CrossRef]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef]
- Joshi, N.; Onaivi, E.S. Endocannabinoid System Components: Overview and Tissue Distribution. In Recent Advances in Cannabinoid Physiology and Pathology; Advances in Experimental Medicine and Biology Book Series; Springer: Berlin/Heidelberg, Germany, 2019; Volume 1162, pp. 1–12. [Google Scholar] [CrossRef]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Fride, E.; Mechoulam, R. Pharmacological activity of the cannabinoid receptor agonist, anandamide, a brain constituent. Eur. J. Pharmacol. 1993, 231, 313–314. [Google Scholar] [CrossRef]
- Di Iorio, G.; Lupi, M.; Sarchione, F.; Matarazzo, I.; Santacroce, R.; Petruccelli, F.; Martinotti, G.; Di Giannantonio, M. The endocannabinoid system: A putative role in neurodegenerative diseases. Int. J. High Risk Behav. Addict. 2013, 2, 100–106. [Google Scholar] [CrossRef]
- Haspula, D.; Clark, M.A. Cannabinoid Receptors: An Update on Cell Signaling, Pathophysiological Roles and Therapeutic Opportunities in Neurological, Cardiovascular, and Inflammatory Diseases. Int. J. Mol. Sci. 2020, 21, 7693. [Google Scholar] [CrossRef]
- Kasatkina, L.A.; Rittchen, S.; Sturm, E.M. Neuroprotective and Immunomodulatory Action of the Endocannabinoid System under Neuroinflammation. Int. J. Mol. Sci. 2021, 22, 5431. [Google Scholar] [CrossRef]
- Golech, S.A.; McCarron, R.M.; Chen, Y.; Bembry, J.; Lenz, F.; Mechoulam, R.; Shohami, E.; Spatz, M. Human brain endothelium: Coexpression and function of vanilloid and endocannabinoid receptors. Mol. Brain Res. 2004, 132, 87–92. [Google Scholar] [CrossRef]
- Papa, A.; Pasquini, S.; Contri, C.; Gemma, S.; Campiani, G.; Butini, S.; Varani, K.; Vincenzi, F. Polypharmacological Approaches for CNS Diseases: Focus on Endocannabinoid Degradation Inhibition. Cells 2022, 11, 471. [Google Scholar] [CrossRef]
- Patel, D.C.; Wallis, G.; Fujinami, R.S.; Wilcox, K.S.; Smith, M.D. Cannabidiol reduces seizures following CNS infection with Theiler’s murine encephalomyelitis virus. Epilepsia Open 2019, 4, 431–442. [Google Scholar] [CrossRef]
- Jiang, H.; Li, H.; Cao, Y.; Zhang, R.; Zhou, L.; Zhou, Y.; Zeng, X.; Wu, J.; Wu, D.; Wu, D.; et al. Effects of cannabinoid (CBD) on blood brain barrier permeability after brain injury in rats. Brain Res. 2021, 1768, 147586. [Google Scholar] [CrossRef]
- Ramirez, S.H.; Haskó, J.; Skuba, A.; Fan, S.; Dykstra, H.; McCormick, R.; Reichenbach, N.; Krizbai, I.; Mahadevan, A.; Zhang, M.; et al. Activation of Cannabinoid Receptor 2 Attenuates Leukocyte–Endothelial Cell Interactions and Blood–Brain Barrier Dysfunction under Inflammatory Conditions. J. Neurosci. 2012, 32, 4004–4016. [Google Scholar] [CrossRef] [PubMed]
- Amenta, P.S.; Jallo, J.I.; Tuma, R.F.; Elliott, M.B. A cannabinoid type 2 receptor agonist attenuates blood–brain barrier damage and neurodegeneration in a murine model of traumatic brain injury. J. Neurosci. Res. 2012, 90, 2293–2305. [Google Scholar] [CrossRef] [PubMed]
- Calapai, F.; Cardia, L.; Sorbara, E.E.; Navarra, M.; Gangemi, S.; Calapai, G.; Mannucci, C. Cannabinoids, Blood–Brain Barrier, and Brain Disposition. Pharmaceutics 2020, 12, 265. [Google Scholar] [CrossRef]
- Salman, M.M.; Kitchen, P.; Iliff, J.J.; Bill, R.M. Aquaporin 4 and glymphatic flow have central roles in brain fluid homeostasis. Nat. Rev. Neurosci. 2021, 22, 650–651. [Google Scholar] [CrossRef]
- Holth, J.K.; Fritschi, S.K.; Wang, C.; Pedersen, N.P.; Cirrito, J.R.; Mahan, T.E.; Finn, M.B.; Manis, M.; Geerling, J.C.; Fuller, P.M.; et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 2019, 363, 880–884. [Google Scholar] [CrossRef]
- Shokri-Kojori, E.; Wang, G.-J.; Wiers, C.E.; Demiral, S.B.; Guo, M.; Kim, S.W.; Lindgren, E.; Ramirez, V.; Zehra, A.; Freeman, C.; et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl. Acad. Sci. USA 2018, 115, 4483–4488. [Google Scholar] [CrossRef] [PubMed]
- Keir, L.H.M.; Breen, D.P. New awakenings: Current understanding of sleep dysfunction and its treatment in Parkinson’s disease. J. Neurol. 2019, 267, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Gulyássy, P.; Todorov-Völgyi, K.; Tóth, V.; Györffy, B.A.; Puska, G.; Simor, A.; Juhász, G.; Drahos, L.; Kékesi, K.A. The Effect of Sleep Deprivation and Subsequent Recovery Period on the Synaptic Proteome of Rat Cerebral Cortex. Mol. Neurobiol. 2022, 59, 1301–1319. [Google Scholar] [CrossRef] [PubMed]
- Arighi, A.; Di Cristofori, A.; Fenoglio, C.; Borsa, S.; D’anca, M.; Fumagalli, G.G.; Locatelli, M.; Carrabba, G.; Pietroboni, A.M.; Ghezzi, L.; et al. Cerebrospinal Fluid Level of Aquaporin4: A New Window on Glymphatic System Involvement in Neurodegenerative Disease? J. Alzheimer’s Dis. 2019, 69, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Wang, W.; Zheng, X.; Xia, D.; Liu, H.; Qin, C.; Tian, H.; Teng, J. Decreased AQP4 Expression Aggravates α-Synuclein Pathology in Parkinson’s Disease Mice, Possibly via Impaired Glymphatic Clearance. J. Mol. Neurosci. 2021, 71, 2500–2513. [Google Scholar] [CrossRef] [PubMed]
- Matei, D.; Trofin, D.; Iordan, D.A.; Onu, I.; Condurache, I.; Ionite, C.; Buculei, I. The Endocannabinoid System and Physical Exercise. Int. J. Mol. Sci. 2023, 24, 1989. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Prim. 2021, 7, 33. [Google Scholar] [CrossRef]
- Aso, E.; Palomer, E.; Juvés, S.; Maldonado, R.; Muñoz, F.J.; Ferrer, I. CB1 Agonist ACEA Protects Neurons and Reduces the Cognitive Impairment of AβPP/PS1 Mice. J. Alzheimer’s Dis. 2012, 30, 439–459. [Google Scholar] [CrossRef]
- Manuel, I.; González de San Román, E.; Giralt, M.T.; Ferrer, I.; Rodríguez-Puertas, R. Type-1 Cannabinoid Receptor Activity During Alzheimer’s Disease Progression. J. Alzheimer’s Dis. 2014, 42, 761–766. [Google Scholar] [CrossRef]
- Kalifa, S.; Polston, E.K.; Allard, J.S.; Manaye, K.F. Distribution patterns of cannabinoid CB1 receptors in the hippocampus of APPswe/PS1ΔE9 double transgenic mice. Brain Res. 2011, 1376, 94–100. [Google Scholar] [CrossRef]
- Martín-Moreno, A.M.; Brera, B.; Spuch, C.; Carro, E.; García-García, L.; Delgado, M.; Pozo, M.A.; Innamorato, N.G.; Cuadrado, A.; de Ceballos, M.L. Prolonged oral cannabinoid administration prevents neuroinflammation, lowers β-amyloid levels and improves cognitive performance in Tg APP 2576 mice. J. Neuroinflammation 2012, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, C.; Beaulieu-Abdelahad, D.; Mullan, M.; Paris, D. Role of the cannabinoid system in the transit of beta-amyloid across the blood–brain barrier. Mol. Cell. Neurosci. 2013, 56, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Timler, A.; Bulsara, C.; Bulsara, M.; Vickery, A.; Smith, J.; Codde, J. Use of cannabinoid-based medicine among older residential care recipients diagnosed with dementia: Study protocol for a double-blind randomised crossover trial. Trials 2020, 21, 188. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, M.; Goldman, S.A. Glymphatic failure as a final common pathway to dementia. Science 2020, 370, 50–56. [Google Scholar] [CrossRef] [PubMed]
- DuBose, N.G.; DeJonge, S.R.; Jeng, B.; Motl, R.W. Vascular dysfunction in multiple sclerosis: Scoping review of current evidence for informing future research directions. Mult. Scler. Relat. Disord. 2023, 78, 104936. [Google Scholar] [CrossRef] [PubMed]
- Abbaoui, A.; Fatoba, O.; Yamashita, T. Meningeal T cells function in the central nervous system homeostasis and neurodegenerative diseases. Front. Cell. Neurosci. 2023, 17, 1181071. [Google Scholar] [CrossRef]
- Pop, E. Dexanabinol Pharmos. Curr. Opin. Investig. Drugs 2000, 1, 494–503. [Google Scholar]
- Lacroix, C.; Alleman-Brimault, I.; Zalta, A.; Rouby, F.; Cassé-Perrot, C.; Jouve, E.; Attolini, L.; Guilhaumou, R.; Micallef, J.; Blin, O. What Do We Know About Medical Cannabis in Neurological Disorders and What Are the Next Steps? Front. Pharmacol. 2022, 13, 883987. [Google Scholar] [CrossRef]
- Estrada, J.A.; Contreras, I. Endocannabinoid receptors in the CNS: Potential drug targets for the prevention and treatment of neurologic and psychiatric disorders. Curr. Neuropharmacol. 2020, 18, 769–787. [Google Scholar] [CrossRef]
- Richfield, E.K.; Herkenham, M. Selective vulnerability in Huntington’s disease: Preferential loss of cannabinoid receptors in lateral globus pallidus. Ann. Neurol. 1994, 36, 577–584. [Google Scholar] [CrossRef]
- Glass, M.; Dragunow, M.; Faull, R. The pattern of neurodegeneration in Huntington’s disease: A comparative study of cannabinoid, dopamine, adenosine and GABAA receptor alterations in the human basal ganglia in Huntington’s disease. Neuroscience 2000, 97, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Glass, M.; Faull, R.; Dragunow, M. Loss of cannabinoid receptors in the substantia nigra in huntington’s disease. Neuroscience 1993, 56, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Lastres-Becker, I.; Fezza, F.; Cebeira, M.; Bisogno, T.; Ramos, J.A.; Milone, A.; Fernández-Ruiz, J.; Di Marzo, V. Changes in endocannabinoid transmission in the basal ganglia in a rat model of Huntington’s disease. NeuroReport 2001, 12, 2125–2129. [Google Scholar] [CrossRef] [PubMed]
- Lastres-Becker, I.; Berrendero, F.; Lucas, J.; Martín-Aparicio, E.; Yamamoto, A.; Ramos, J.; Fernández-Ruiz, J. Loss of mRNA levels, binding and activation of GTP-binding proteins for cannabinoid CB1 receptors in the basal ganglia of a transgenic model of Huntington’s disease. Brain Res. 2002, 929, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Palazuelos, J.; Aguado, T.; Pazos, M.R.; Julien, B.; Carrasco, C.; Resel, E.; Sagredo, O.; Benito, C.; Romero, J.; Azcoitia, I.; et al. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington’s disease excitotoxicity. Brain 2009, 132, 3152–3164. [Google Scholar] [CrossRef]
- Battista, N.; Bari, M.; Tarditi, A.; Mariotti, C.; Bachoud-Lévi, A.-C.; Zuccato, C.; Finazzi-Agrò, A.; Genitrini, S.; Peschanski, M.; Di Donato, S.; et al. Severe deficiency of the fatty acid amide hydrolase (FAAH) activity segregates with the Huntington’s disease mutation in peripheral lymphocytes. Neurobiol. Dis. 2007, 27, 108–116. [Google Scholar] [CrossRef]
- Aarsland, D.; Creese, B.; Politis, M.; Chaudhuri, K.R.; Ffytche, D.H.; Weintraub, D.; Ballard, C. Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 2017, 13, 217–231. [Google Scholar] [CrossRef]
- Romanelli, R.J.; Williams, J.T.; Neve, K.A. Dopamine Receptor Signaling: Intracellular Pathways to Behavior; Humana Press EBooks: Totowa, NJ, USA, 2009; pp. 137–173. [Google Scholar] [CrossRef]
- Solinas, M.; Justinova, Z.; Goldberg, S.R.; Tanda, G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J. Neurochem. 2006, 98, 408–419. [Google Scholar] [CrossRef]
- Mathur, B.N.; Lovinger, D.M. Endocannabinoid–Dopamine Interactions in Striatal Synaptic Plasticity. Front. Pharmacol. 2012, 3, 66. [Google Scholar] [CrossRef]
- Beltramo, M.; de Fonseca, F.R.; Navarro, M.; Calignano, A.; Gorriti, M.A.; Grammatikopoulos, G.; Sadile, A.G.; Giuffrida, A.; Piomelli, D. Reversal of Dopamine D2 Receptor Responses by an Anandamide Transport Inhibitor. J. Neurosci. 2000, 20, 3401–3407. [Google Scholar] [CrossRef]
- Koethe, D.; Giuffrida, A.; Schreiber, D.; Hellmich, M.; Schultze-Lutter, F.; Ruhrmann, S.; Klosterkötter, J.; Piomelli, D.; Leweke, F.M. Anandamide elevation in cerebrospinal fluid in initial prodromal states of psychosis. Br. J. Psychiatry 2009, 194, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Pisani, V.; Moschella, V.; Bari, M.; Fezza, F.; Galati, S.; Bernardi, G.; Stanzione, P.; Pisani, A.; Maccarrone, M. Dynamic changes of anandamide in the cerebrospinal fluid of Parkinson’s disease patients. Mov. Disord. 2010, 25, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wan, H.; Zhang, M.; Wardlaw, J.M.; Feng, T.; Wang, Y. Perivascular space in Parkinson’s disease: Association with CSF amyloid/tau and cognitive decline. Park. Relat. Disord. 2022, 95, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Patricio, F.; Morales-Andrade, A.A.; Patricio-Martínez, A.; Limón, I.D. Cannabidiol as a Therapeutic Target: Evidence of its Neuroprotective and Neuromodulatory Function in Parkinson’s Disease. Front. Pharmacol. 2020, 11, 595635. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, S.; Li, C.; Wang, R.; Chen, M.; Chen, H.; Su, W. Diffusion Tensor Imaging Along the Perivascular Space Index in Different Stages of Parkinson’s Disease. Front. Aging Neurosci. 2021, 13, 773951. [Google Scholar] [CrossRef] [PubMed]
- McKnight, C.D.; Trujillo, P.; Lopez, A.M.; Petersen, K.; Considine, C.; Lin, Y.-C.; Yan, Y.; Kang, H.; Donahue, M.J.; Claassen, D.O. Diffusion along perivascular spaces reveals evidence supportive of glymphatic function impairment in Parkinson disease. Park. Relat. Disord. 2021, 89, 98–104. [Google Scholar] [CrossRef]
- Eisen, A. The Dying Forward Hypothesis of ALS: Tracing Its History. Brain Sci. 2021, 11, 300. [Google Scholar] [CrossRef]
- Alami, N.O.; Tang, L.; Wiesner, D.; Commisso, B.; Bayer, D.; Weishaupt, J.; Dupuis, L.; Wong, P.; Baumann, B.; Wirth, T.; et al. Multiplexed chemogenetics in astrocytes and motoneurons restore blood–spinal cord barrier in ALS. Life Sci. Alliance 2020, 3, e201900571. [Google Scholar] [CrossRef]
- Waters, S.; Swanson, M.E.V.; Dieriks, B.V.; Zhang, Y.B.; Grimsey, N.L.; Murray, H.C.; Turner, C.; Waldvogel, H.J.; Faull, R.L.M.; An, J.; et al. Blood-spinal cord barrier leakage is independent of motor neuron pathology in ALS. Acta Neuropathol. Commun. 2021, 9, 144. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, X.; Li, X.; Wang, H.; Wang, T. Elevated cerebrospinal fluid homocysteine is associated with blood-brain barrier disruption in amyotrophic lateral sclerosis patients. Neurol. Sci. 2020, 41, 1865–1872. [Google Scholar] [CrossRef]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2019, 16, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cueto, C.; García-Toscano, L.; Santos-García, I.; Gómez-Almería, M.; Gonzalo-Consuegra, C.; Espejo-Porras, F.; Fernández-Ruiz, J.; de Lago, E. Targeting the CB2 receptor and other endocannabinoid elements to delay disease progression in amyotrophic lateral sclerosis. Br. J. Pharmacol. 2021, 178, 1373–1387. [Google Scholar] [CrossRef]
- Espejo-Porras, F.; Fernández-Ruiz, J.; de Lago, E. Analysis of endocannabinoid receptors and enzymes in the post-mortem motor cortex and spinal cord of amyotrophic lateral sclerosis patients. Amyotroph. Lateral Scler. Front. Degener. 2018, 19, 377–386. [Google Scholar] [CrossRef]
- Mirian, A.; Moszczynski, A.; Soleimani, S.; Aubert, I.; Zinman, L.; Abrahao, A. Breached Barriers: A Scoping Review of Blood-Central Nervous System Barrier Pathology in Amyotrophic Lateral Sclerosis. Front. Cell. Neurosci. 2022, 16, 851563. [Google Scholar] [CrossRef] [PubMed]
- Watanabe-Matsumoto, S.; Moriwaki, Y.; Okuda, T.; Ohara, S.; Yamanaka, K.; Abe, Y.; Yasui, M.; Misawa, H. Dissociation of blood-brain barrier disruption and disease manifestation in an aquaporin-4-deficient mouse model of amyotrophic lateral sclerosis. Neurosci. Res. 2017, 133, 48–57. [Google Scholar] [CrossRef]
- Urbi, B.; Owusu, M.A.; Hughes, I.; Katz, M.; Broadley, S.; Sabet, A. Effects of cannabinoids in Amyotrophic Lateral Sclerosis (ALS) murine models: A systematic review and meta-analysis. J. Neurochem. 2018, 149, 284–297. [Google Scholar] [CrossRef]
- Buonincontri, V.; Viggiano, D.; Capasso, G. MO216. Preliminary study of the glymphatic system in CKD. Nephrol. Dial. Transplant. 2021, 36 (Suppl. 1), gfab092.0094. [Google Scholar] [CrossRef]
- Camberos-Barraza, J.; Osuna-Ramos, J.F.; Rábago-Monzón, Á.R.; Quiñonez-Angulo, L.F.; González-Peña, H.R.; Pérez-Ramos, A.A.; Camacho-Zamora, A.; López-Lazcano, H.; Valdez-Flores, M.A.; Angulo-Rojo, C.E.; et al. Scientific facts improve cannabis perception and public opinion: Results from Sinaloa, México. Sci. Rep. 2023, 13, 17318. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osuna-Ramos, J.F.; Camberos-Barraza, J.; Torres-Mondragón, L.E.; Rábago-Monzón, Á.R.; Camacho-Zamora, A.; Valdez-Flores, M.A.; Angulo-Rojo, C.E.; Guadrón-Llanos, A.M.; Picos-Cárdenas, V.J.; Calderón-Zamora, L.; et al. Interplay between the Glymphatic System and the Endocannabinoid System: Implications for Brain Health and Disease. Int. J. Mol. Sci. 2023, 24, 17458. https://doi.org/10.3390/ijms242417458
Osuna-Ramos JF, Camberos-Barraza J, Torres-Mondragón LE, Rábago-Monzón ÁR, Camacho-Zamora A, Valdez-Flores MA, Angulo-Rojo CE, Guadrón-Llanos AM, Picos-Cárdenas VJ, Calderón-Zamora L, et al. Interplay between the Glymphatic System and the Endocannabinoid System: Implications for Brain Health and Disease. International Journal of Molecular Sciences. 2023; 24(24):17458. https://doi.org/10.3390/ijms242417458
Chicago/Turabian StyleOsuna-Ramos, Juan F., Josué Camberos-Barraza, Laura E. Torres-Mondragón, Ángel R. Rábago-Monzón, Alejandro Camacho-Zamora, Marco A. Valdez-Flores, Carla E. Angulo-Rojo, Alma M. Guadrón-Llanos, Verónica J. Picos-Cárdenas, Loranda Calderón-Zamora, and et al. 2023. "Interplay between the Glymphatic System and the Endocannabinoid System: Implications for Brain Health and Disease" International Journal of Molecular Sciences 24, no. 24: 17458. https://doi.org/10.3390/ijms242417458
APA StyleOsuna-Ramos, J. F., Camberos-Barraza, J., Torres-Mondragón, L. E., Rábago-Monzón, Á. R., Camacho-Zamora, A., Valdez-Flores, M. A., Angulo-Rojo, C. E., Guadrón-Llanos, A. M., Picos-Cárdenas, V. J., Calderón-Zamora, L., Magaña-Gómez, J. A., Norzagaray-Valenzuela, C. D., Cárdenas-Torres, F. I., & De la Herrán-Arita, A. K. (2023). Interplay between the Glymphatic System and the Endocannabinoid System: Implications for Brain Health and Disease. International Journal of Molecular Sciences, 24(24), 17458. https://doi.org/10.3390/ijms242417458




