Plant Secondary Compounds Promote White Adipose Tissue Browning via Modulation of the Gut Microbiota in Small Mammals
Abstract
:1. Introduction
2. Results
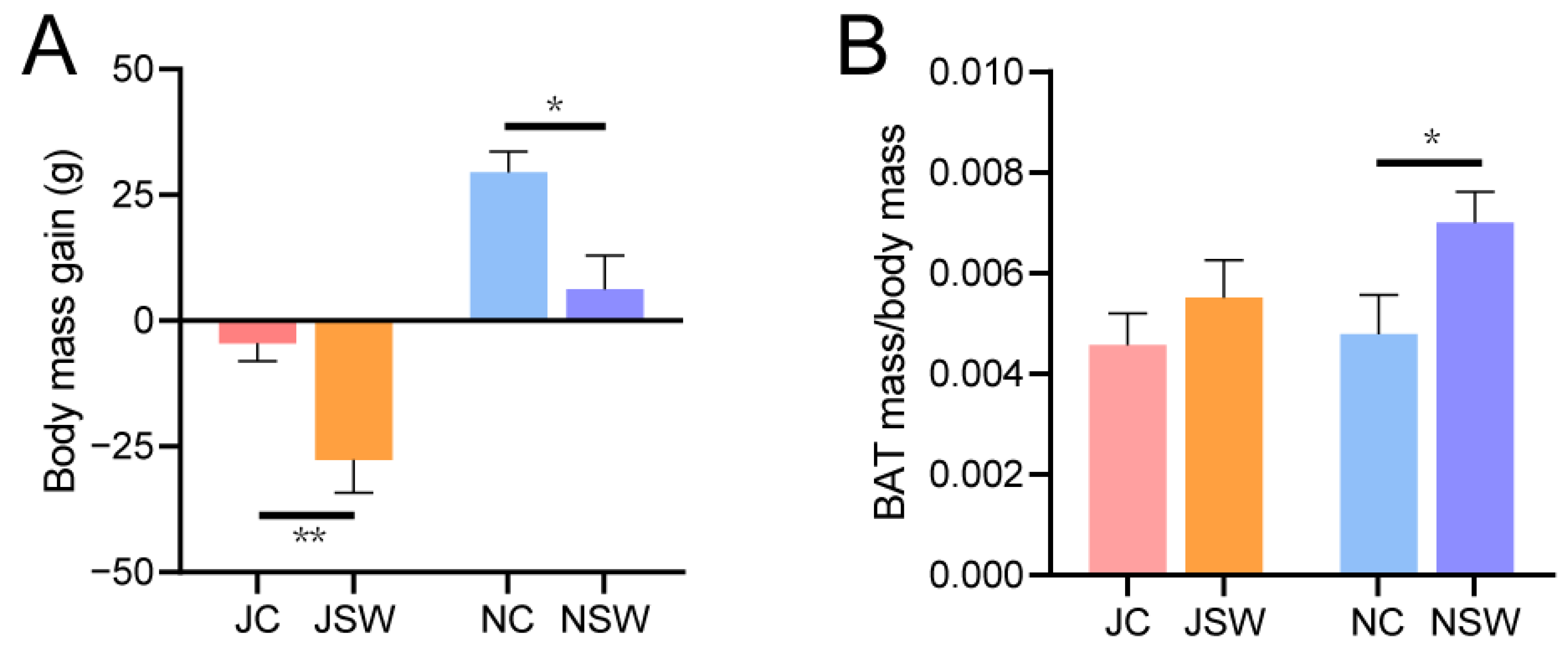
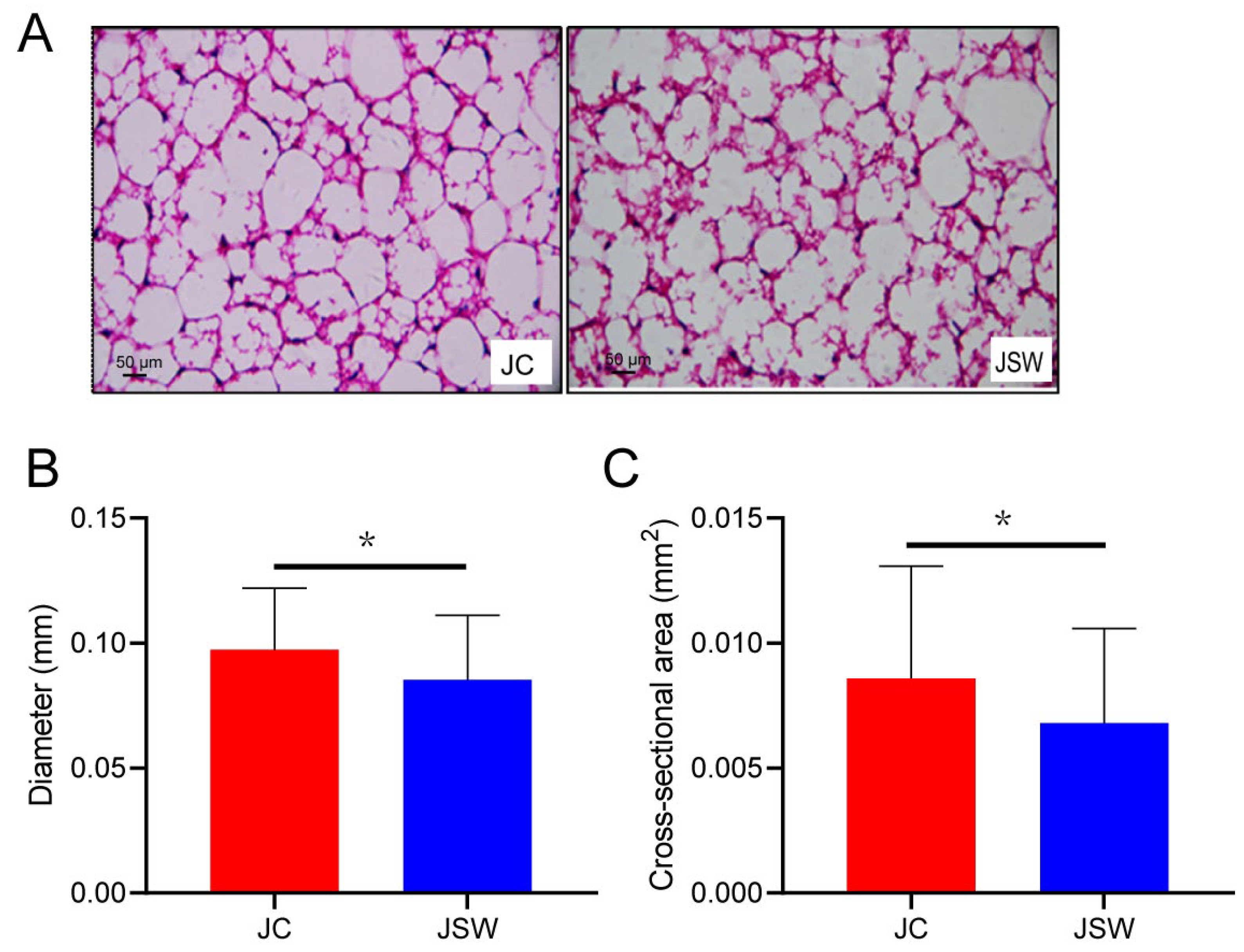
2.1. Changes in the Body Mass and BAT of Pikas
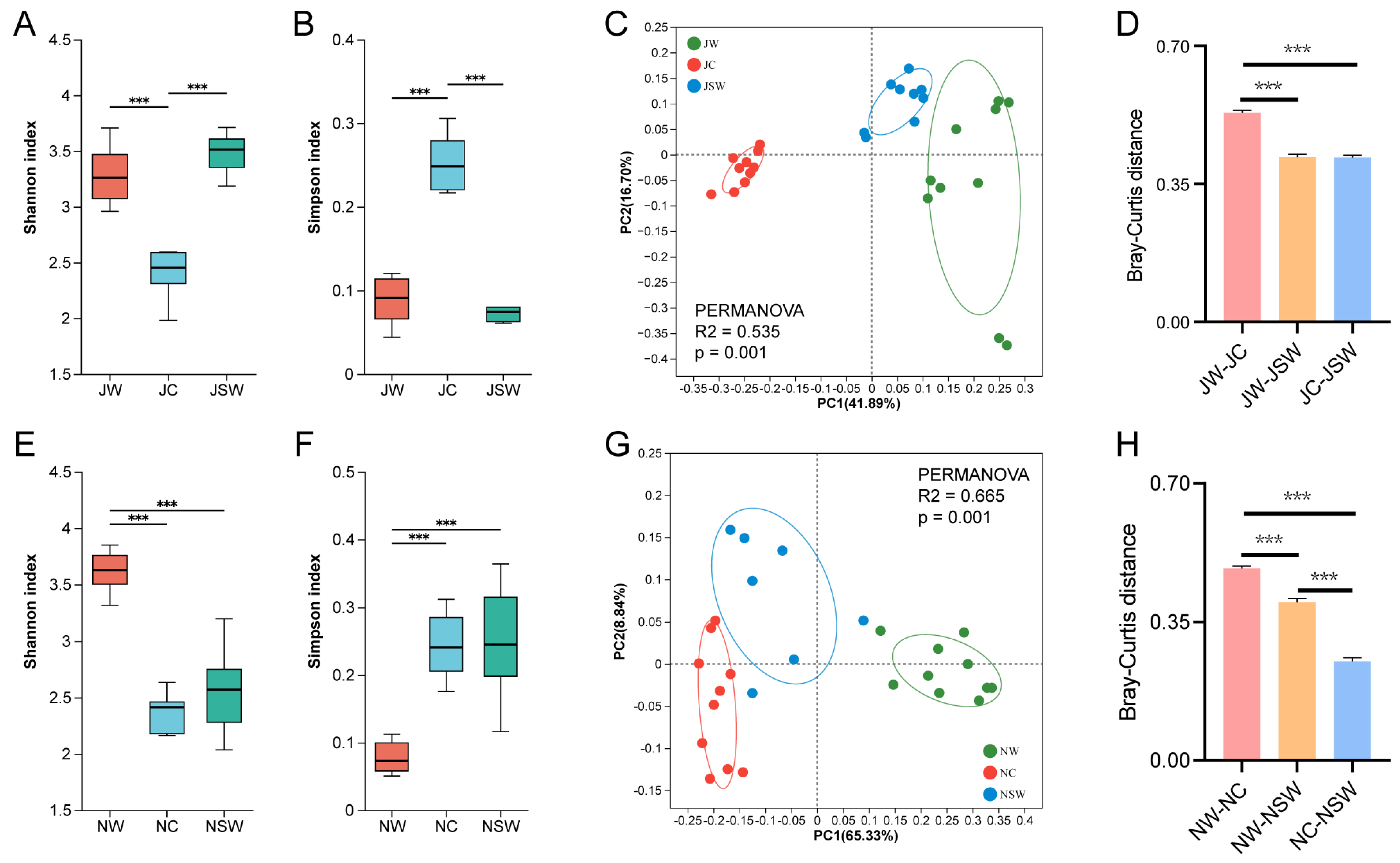
2.2. The Differences in the Gut Microbiota Diversity of Pikas
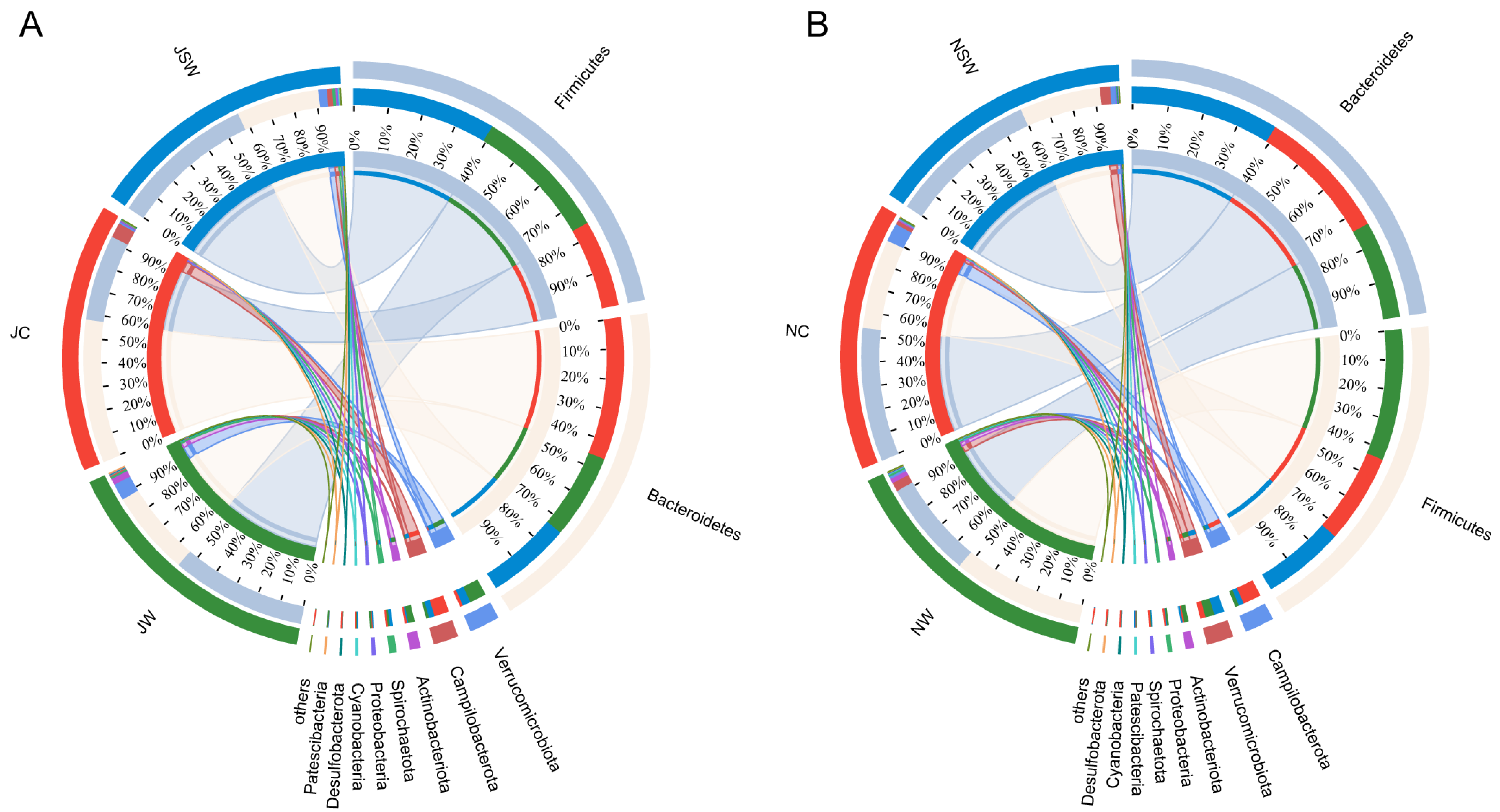
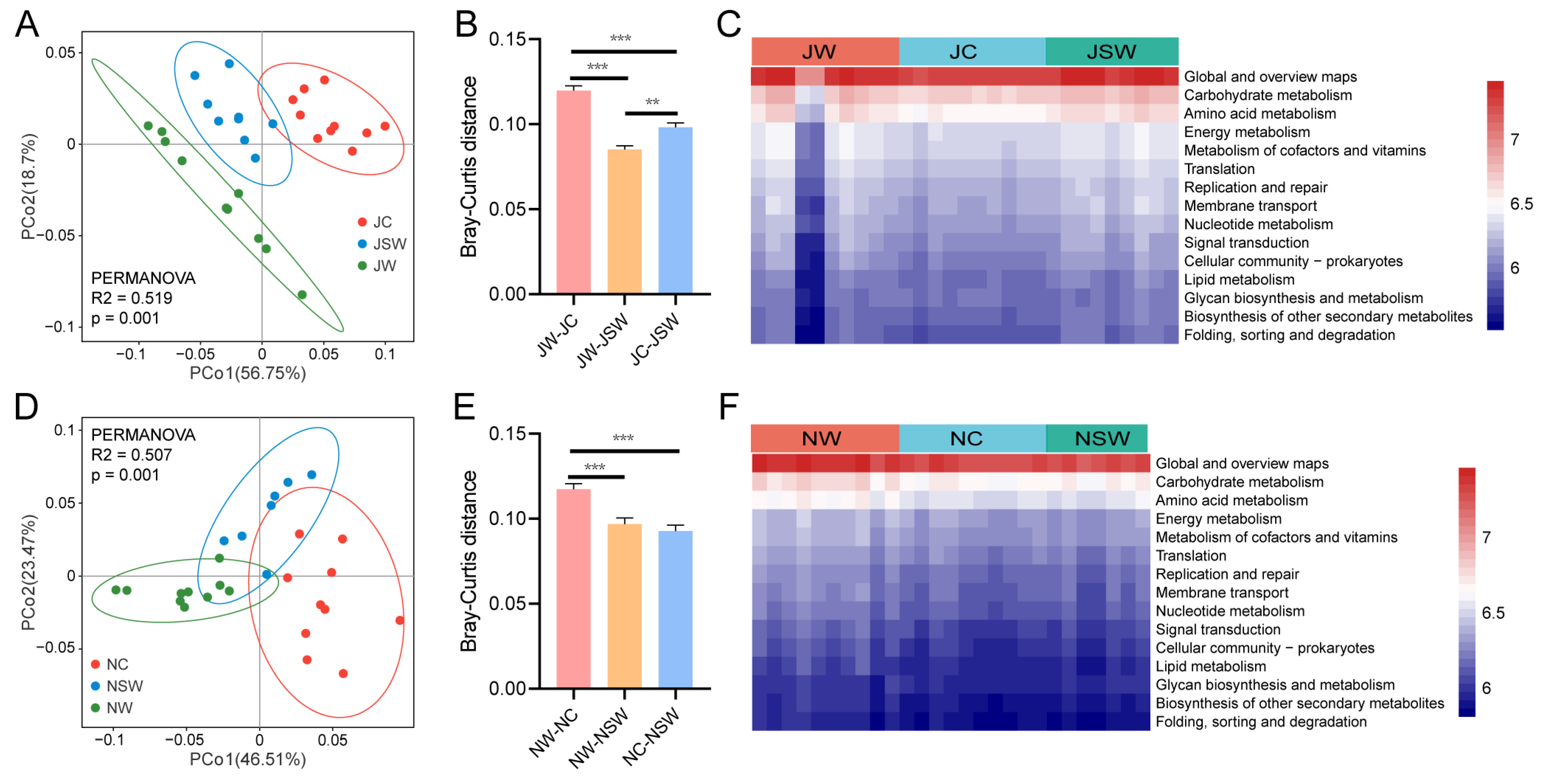
2.3. The Composition and Differences in the Gut Microbiota of Pikas
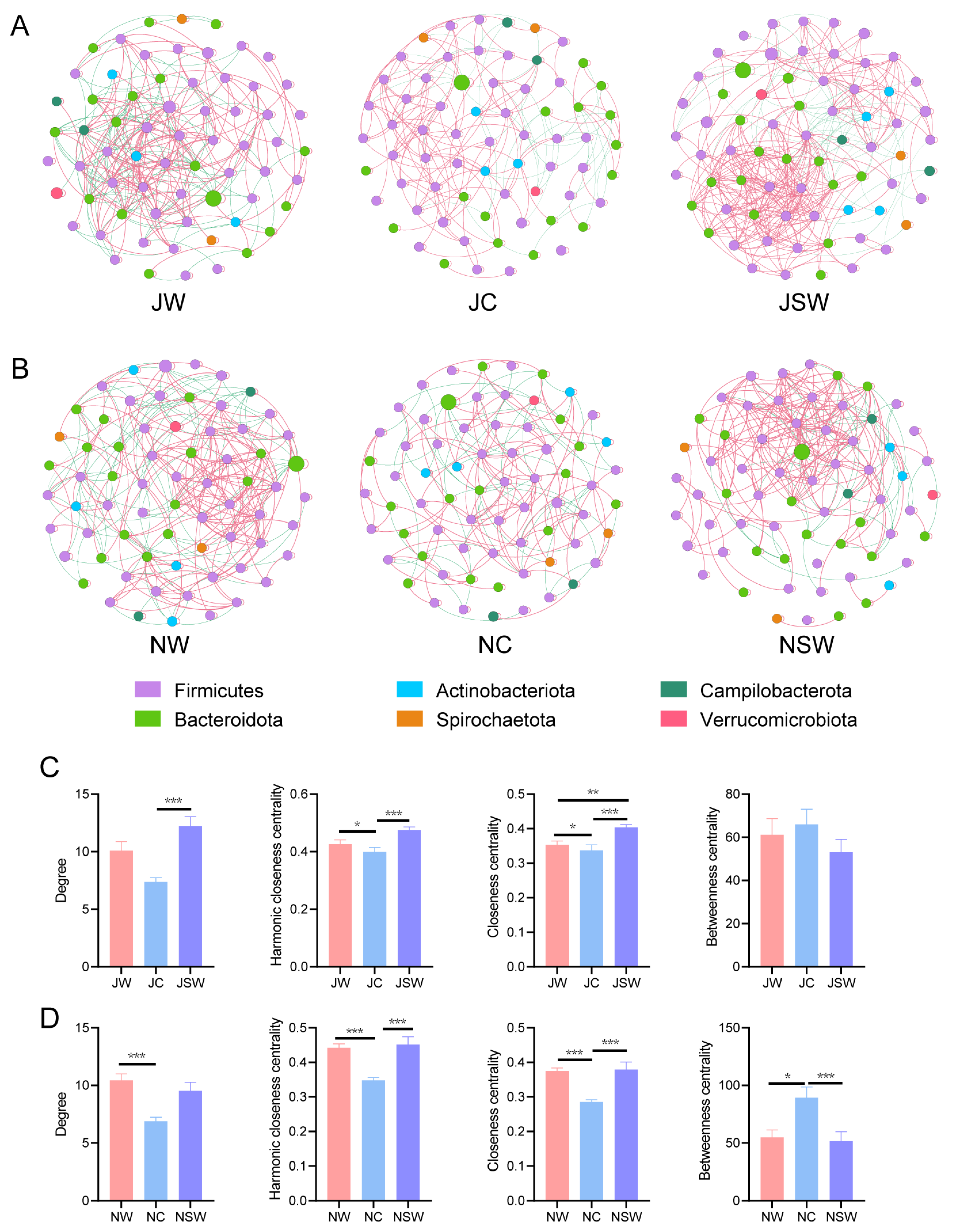
2.4. The Co-Occurrence Network of Gut Microbiota in Pikas
2.5. The Functional Profile of the Gut Microbiota in Pikas
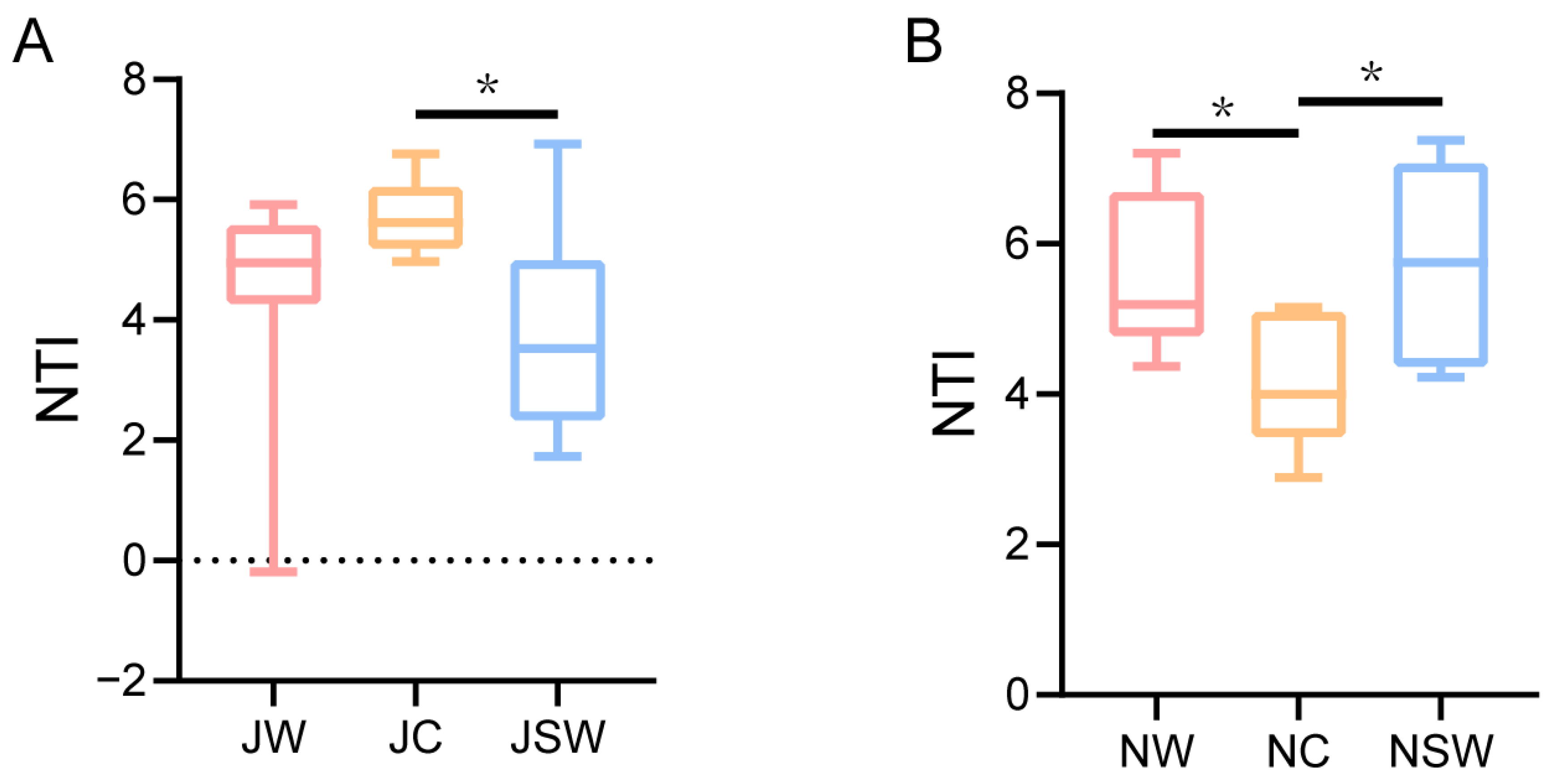
2.6. The Assembly of Gut Microbiota in Pikas
2.7. The Relationship between BAT and Gut Microbiota
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Experimental Design
4.3. SW Extraction
4.4. The Amplicon Sequencing Analysis of the 16S rRNA Gene
4.5. WAT Morphology
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanaka, M. Improving obesity and blood pressure. Hypertens. Res. 2020, 43, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.Q.; Jin, Y.X.; Li, D.X.; Zhang, J.X.; Han, J.Y.; Li, Y. Multidisciplinary Progress in Obesity Research. Genes 2022, 13, 1772. [Google Scholar] [CrossRef]
- Klein, S.; Gastaldelli, A.; Yki-Järvinen, H.; Scherer, P.E. Why does obesity cause diabetes? Cell Metab. 2022, 34, 11–20. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity and Overweight. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 9 June 2021).
- WHO. Facts in Pictures: Obesity. 2021. Available online: https://www.who.int/news-room/facts-in-pictures/detail/6-facts-on-obesity (accessed on 9 June 2021).
- Landovská, P.; Karbanová, M. Social costs of obesity in the Czech Republic. Eur. J. Health Econ. 2023, 24, 1321–1341. [Google Scholar] [CrossRef]
- Ataey, A.; Jafarvand, E.; Adham, D.; Moradi-Asl, E. The Relationship Between Obesity, Overweight, and the Human Development Index in World Health Organization Eastern Mediterranean Region Countries. J. Prev. Med. Public Health 2020, 53, 98–105. [Google Scholar] [CrossRef]
- Dobbs, R.; Sawers, C.; Thompson, F.; Manyika, J.; Woetzel, J.R.; Child, P.; McKenna, S.; Spatharou, A. Overcoming Obesity: An Initial Economic Analysis; McKinsey Global Institute: New York, NY, USA, 2014. [Google Scholar]
- Calderon-Dominguez, M.; Mir, J.F.; Fucho, R.; Weber, M.; Serra, D.; Herrero, L. Fatty acid metabolism and the basis of brown adipose tissue function. Adipocyte 2016, 5, 98–118. [Google Scholar] [CrossRef]
- Schrauwen, P.; van Marken Lichtenbelt, W.D.; Spiegelman, B.M. The future of brown adipose tissues in the treatment of type 2 diabetes. Diabetologia 2015, 58, 1704–1707. [Google Scholar] [CrossRef]
- Jeremic, N.; Chaturvedi, P.; Tyagi, S.C. Browning of White Fat: Novel Insight into Factors, Mechanisms, and Therapeutics. J. Cell. Physiol. 2017, 232, 61–68. [Google Scholar] [CrossRef]
- Bartelt, A.; Heeren, J. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 2013, 10, 24–36. [Google Scholar] [CrossRef]
- Xue, H.; Wang, Z.; Hua, Y.; Ke, S.; Wang, Y.; Zhang, J.; Pan, Y.-H.; Huang, W.; Irwin, D.M.; Zhang, S. Molecular signatures and functional analysis of beige adipocytes induced from in vivo intra-abdominal adipocytes. Sci. Adv. 2018, 4, eaar5319. [Google Scholar] [CrossRef]
- Bai, Z.Z.; Tana, W.R.; Liu, S.; Han, S.R.; Chen, L.; McClain, D.; Ge, R.L. Intermittent cold exposure results in visceral adipose tissue “browning” in the plateau pika (Ochotona curzoniae). Comp. Biochem. Physiol. Part A 2015, 184, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Qimuge, N.R.; Qin, J.; Cai, R.; Li, X.; Chu, G.Y.; Pang, W.J.; Yang, G.S. Acute and chronic cold exposure differentially affects the browning of porcine white adipose tissue. Animal 2018, 12, 1435–1441. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab. 2017, 26, 672–685.e674. [Google Scholar] [CrossRef] [PubMed]
- Lone, J.; Choi, J.H.; Kim, S.W.; Yun, J.W. Curcumin induces brown fat-like phenotype in 3T3-L1 and primary white adipocytes. J. Nutr. Biochem. 2016, 27, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Azhar, Y.; Parmar, A.; Miller, C.N.; Samuels, J.S.; Rayalam, S. Phytochemicals as novel agents for the induction of browning in white adipose tissue. Nutr. Metab. 2016, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Lei, X.; Wang, J.; Wang, L.; Zhong, Q.; Fang, X.; Li, P.; Du, B.; Wang, Y.; Liao, Z. Lipopolysaccharides derived from gram-negative bacterial pool of human gut microbiota promote inflammation and obesity development. Int. Rev. Immunol. 2021, 41, 45–56. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, L.; Yang, L.; Chu, H. The critical role of gut microbiota in obesity. Front. Endocrinol. 2022, 13, 1025706. [Google Scholar] [CrossRef]
- Zuojian, F.; Changlin, Z. Studies on the pikas (genus: Ochotona) of China—Taxonomic notes and distribution. Acta Theriol. Sin. 1985, 5, 269–289. [Google Scholar]
- An, Z.; Wei, L.; Xu, B.; Wang, Z.; Gao, C.; Li, J.; Wei, L.; Qi, D.; Shi, P.; Zhang, T.; et al. A homotetrameric hemoglobin expressed in alveolar epithelial cells increases blood oxygenation in high-altitude plateau pika (Ochotona curzoniae). Cell Rep. 2022, 41, 111446. [Google Scholar] [CrossRef]
- Speakman, J.R.; Chi, Q.; Ołdakowski, Ł.; Fu, H.; Fletcher, Q.E.; Hambly, C.; Togo, J.; Liu, X.; Piertney, S.B.; Wang, X.; et al. Surviving winter on the Qinghai-Tibetan Plateau: Pikas suppress energy demands and exploit yak feces to survive winter. Proc. Natl. Acad. Sci. USA 2021, 118, e2100707118. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-M.; Zhang, Y.-M.; Wang, D.-H. Seasonal thermogenesis and body mass regulation in plateau pikas (Ochotona curzoniae). Oecologia 2006, 149, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, S.S.; Wang, W.L.; Zhang, L.; Zhao, B.Y. Effect of swainsonine in Oxytropis kansuensis on Golgi alpha-mannosidase II expression in the brain tissues of Sprague-Dawley rats. J. Agric. Food Chem. 2014, 62, 7407–7412. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Xia, W. Utilization of the food resources by plateau pika. Acta Theriol. Sin. 1985, 5, 251–262. [Google Scholar]
- Fan, C.; Zhang, L.Z.; Fu, H.B.; Liu, C.F.; Li, W.J.; Cheng, Q.; Zhang, H.; Jia, S.A.; Zhang, Y.M. Enterotypes of the Gut Microbial Community and Their Response to Plant Secondary Compounds in Plateau Pikas. Microorganisms 2020, 8, 1311. [Google Scholar] [CrossRef]
- Ren, S.; Fan, C.; Zhang, L.; Tang, X.; Fu, H.; Liu, C.; Jia, S.; Zhang, Y. The plant secondary compound swainsonine reshapes gut microbiota in plateau pikas (Ochotona curzoniae). Appl. Microbiol. Biotechnol. 2021, 105, 6419–6433. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Yadav, P.; Vashishth, D.; Sharma, K.; Kumar, A.; Chahal, J.; Dalal, S.; Kataria, S.K. A review on obesity management through natural compounds and a green nanomedicine-based approach. Molecules 2021, 26, 3278. [Google Scholar] [CrossRef]
- Mir, S.A.; Shah, M.A.; Ganai, S.A.; Ahmad, T.; Gani, M. Understanding the role of active components from plant sources in obesity management. J. Saudi Soc. Agric. Sci. 2019, 18, 168–176. [Google Scholar] [CrossRef]
- Yang, X.D.; Ge, X.C.; Jiang, S.Y.; Yang, Y.Y. Potential lipolytic regulators derived from natural products as effective approaches to treat obesity. Front. Endocrinol. 2022, 13, 1000739. [Google Scholar] [CrossRef]
- Song, D.; Cheng, L.; Zhang, X.; Wu, Z.; Zheng, X. The modulatory effect and the mechanism of flavonoids on obesity. J. Food Biochem. 2019, 43, e12954. [Google Scholar] [CrossRef]
- Lipina, C.; Hundal, H.S. Carnosic acid stimulates glucose uptake in skeletal muscle cells via a PME-1/PP2A/PKB signalling axis. Cell Signal 2014, 26, 2343–2349. [Google Scholar] [CrossRef] [PubMed]
- Mougios, V.; Ring, S.; Petridou, A.; Nikolaidis, M.G. Duration of coffee- and exercise-induced changes in the fatty acid profile of human serum. J. Appl. Physiol. 2003, 94, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Diepvens, K.; Westerterp, K.R.; Westerterp-Plantenga, M.S. Obesity and thermogenesis related to the consumption of caffeine, ephedrine, capsaicin, and green tea. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R77–R85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Q.X.; Wang, X.; Zhang, L.; Qu, W.; Bao, B.; Liu, C.A.; Liu, J. Dietary luteolin activates browning and thermogenesis in mice through an AMPK/PGC1alpha pathway-mediated mechanism. Int. J. Obes. 2016, 40, 1841–1849. [Google Scholar] [CrossRef] [PubMed]
- Milton-Laskibar, I.; Gomez-Zorita, S.; Arias, N.; Romo-Miguel, N.; Gonzalez, M.; Fernandez-Quintela, A.; Portillo, M.P. Effects of resveratrol and its derivative pterostilbene on brown adipose tissue thermogenic activation and on white adipose tissue browning process. J. Physiol. Biochem. 2020, 76, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Hale, V.L.; Tan, C.L.; Niu, K.; Yang, Y.; Zhang, Q.; Knight, R.; Amato, K.R. Gut microbiota in wild and captive Guizhou snub-nosed monkeys, Rhinopithecus brelichi. Am. J. Primatol. 2019, 81, e22989. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Chi, X.; Qin, W.; Wang, L.; Song, P.; Cai, Z.; Zhang, J.; Zhang, T. Comparison of the gut microbiota composition between the wild and captive Tibetan wild ass (Equus kiang). J. Appl. Microbiol. 2019, 126, 1869–1878. [Google Scholar] [CrossRef]
- Li, Y.; Hu, X.; Yang, S.; Zhou, J.; Zhang, T.; Qi, L.; Sun, X.; Fan, M.; Xu, S.; Cha, M.; et al. Comparative Analysis of the Gut Microbiota Composition between Captive and Wild Forest Musk Deer. Front. Microbiol. 2017, 8, 1705. [Google Scholar] [CrossRef]
- Li, Y.; Yan, Y.; Fu, H.; Jin, S.; He, S.; Wang, Z.; Dong, G.; Li, B.; Guo, S. Does diet or macronutrients intake drive the structure and function of gut microbiota? Front. Microbiol. 2023, 14, 1126189. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, X.J. Effects of a high fat diet on intestinal microbiota and gastrointestinal diseases. World J. Gastroenterol. 2016, 22, 8905–8909. [Google Scholar] [CrossRef]
- Domínguez-Avila, J.A.; Villa-Rodriguez, J.A.; Montiel-Herrera, M.; Pacheco-Ordaz, R.; Roopchand, D.E.; Venema, K.; González-Aguilar, G.A. Phenolic Compounds Promote Diversity of Gut Microbiota and Maintain Colonic Health. Dig. Dis. Sci. 2021, 66, 3270–3289. [Google Scholar] [CrossRef]
- Kohl, K.D.; Dearing, M.D. Experience matters: Prior exposure to plant toxins enhances diversity of gut microbes in herbivores. Ecol. Lett. 2012, 15, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef]
- Liao, W.; Yin, X.; Li, Q.; Zhang, H.; Liu, Z.; Zheng, X.; Zheng, L.; Feng, X. Resveratrol-Induced White Adipose Tissue Browning in Obese Mice by Remodeling Fecal Microbiota. Molecules 2018, 23, 3356. [Google Scholar] [CrossRef]
- Han, X.; Guo, J.; Yin, M.; Liu, Y.; You, Y.; Zhan, J.; Huang, W. Grape Extract Activates Brown Adipose Tissue Through Pathway Involving the Regulation of Gut Microbiota and Bile Acid. Mol. Nutr. Food Res. 2020, 64, e2000149. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, L.; Li, Y.; Wu, Y.; Wu, T.; Feng, H.; Xu, Z.; Liu, Y.; Ruan, Z.; Zhou, S. Puerarin improves intestinal barrier function through enhancing goblet cells and mucus barrier. J. Funct. Foods 2020, 75, 104246. [Google Scholar] [CrossRef]
- Bi, Y.; Yang, C.; Diao, Q.; Tu, Y. Effects of dietary supplementation with two alternatives to antibiotics on intestinal microbiota of preweaned calves challenged with Escherichia coli K99. Sci. Rep. 2017, 7, 5439. [Google Scholar] [CrossRef]
- Joch, M.; Mrázek, J.; Skřivanová, E.; Čermák, L.; Marounek, M. Effects of pure plant secondary metabolites on methane production, rumen fermentation and rumen bacteria populations in vitro. J. Anim. Physiol. Anim. Nutr. 2018, 102, 869–881. [Google Scholar] [CrossRef]
- Li, G.L.; Li, J.; Kohl, K.D.; Yin, B.F.; Wei, W.H.; Wan, X.R.; Zhu, B.L.; Zhang, Z.B. Dietary shifts influenced by livestock grazing shape the gut microbiota composition and co-occurrence networks in a local rodent species. J. Anim. Ecol. 2019, 88, 302–314. [Google Scholar] [CrossRef]
- Zhang, S.; Shu, J.; Xue, H.; Zhang, W.; Zhang, Y.; Liu, Y.; Fang, L.; Wang, Y.; Wang, H.; Heck, M. The Gut Microbiota in Camellia Weevils Are Influenced by Plant Secondary Metabolites and Contribute to Saponin Degradation. mSystems 2020, 5, e00692-19. [Google Scholar] [CrossRef]
- García-Amado, M.; Michelangeli, F.; Gueneau, P.; Perez, M.; Domínguez-Bello, M. Bacterial detoxification of saponins in the crop of the avian foregut fermenter Opisthocomus hoazin. J. Anim. Feed Sci. 2007, 16 (Suppl. S2), 82–85. [Google Scholar] [CrossRef]
- Santolini, M.; Barabási, A.L. Predicting perturbation patterns from the topology of biological networks. Proc. Natl. Acad. Sci. USA 2018, 115, E6375–E6383. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Zhang, L.; Jia, S.; Tang, X.; Fu, H.; Li, W.; Liu, C.; Zhang, H.; Cheng, Q.; Zhang, Y. Seasonal variations in the composition and functional profiles of gut microbiota reflect dietary changes in plateau pikas. Integr. Zool. 2022, 17, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Mota, R.; Kohl, K.D.; Orr, T.J.; Dearing, M.D. Natural diets promote retention of the native gut microbiota in captive rodents. ISME J. 2020, 14, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gao, H.M.; Song, P.F.; Liang, C.B.; Jiang, F.; Xu, B.; Liu, D.X.; Zhang, T.Z. Captivity Shifts Gut Microbiota Communities in White-Lipped Deer (Cervus albirostris). Animals 2022, 12, 431. [Google Scholar] [CrossRef]
- Maurice, C.F.; Cl Knowles, S.; Ladau, J.; Pollard, K.S.; Fenton, A.; Pedersen, A.B.; Turnbaugh, P.J. Marked seasonal variation in the wild mouse gut microbiota. ISME J. 2015, 9, 2423–2434. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Yao, Y.; Wang, C.; Dong, M.; Wu, Y.; Li, D.; Xie, M.; Ni, Q.; Zhang, M.; Xu, H. Seasonal dynamics of gut microbiota in a cohort of wild Tibetan macaques (Macaca thibetana) in western China. Glob. Ecol. Conserv. 2021, 25, e01409. [Google Scholar] [CrossRef]
- Gacesa, R.; Kurilshikov, A.; Vich Vila, A.; Sinha, T.; Klaassen, M.A.Y.; Bolte, L.A.; Andreu-Sanchez, S.; Chen, L.; Collij, V.; Hu, S.; et al. Environmental factors shaping the gut microbiome in a Dutch population. Nature 2022, 604, 732–739. [Google Scholar] [CrossRef]
- Bajinka, O.; Darboe, A.; Tan, Y.; Abdelhalim, K.A.; Cham, L.B. Gut microbiota and the human gut physiological changes. Ann. Microbiol. 2020, 70, 62. [Google Scholar] [CrossRef]
- Esquivel-Elizondo, S.; Ilhan, Z.E.; Garcia-Pena, E.I.; Krajmalnik-Brown, R. Insights into Butyrate Production in a Controlled Fermentation System via Gene Predictions. mSystems 2017, 2, e00051-17. [Google Scholar] [CrossRef]
- Shah, H.N.; Collins, D.M. Prevotella, a new genus to include Bacteroides melaninogenicus and related species formerly classified in the genus Bacteroides. Int. J. Syst. Bacteriol. 1990, 40, 205–208. [Google Scholar] [CrossRef] [PubMed]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Kyrou, I.; Tan, B.K.; Dimitriadis, G.K.; Ramanjaneya, M.; Tripathi, G.; Patel, V.; James, S.; Kawan, M.; Chen, J.; et al. Short-Chain Fatty Acid Acetate Stimulates Adipogenesis and Mitochondrial Biogenesis via GPR43 in Brown Adipocytes. Endocrinology 2016, 157, 1881–1894. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.; Liu, Y.; Huang, L.; Zheng, L.; Zhou, M.; Lang, H.; Wang, X.; Yi, L.; Mi, M. Resveratrol enhances brown adipose tissue activity and white adipose tissue browning in part by regulating bile acid metabolism via gut microbiota remodeling. Int. J. Obes. 2020, 44, 1678–1690. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, X.; Yu, Y.; Wang, J. Preliminary study on improving the extraction technology of the Swainsonine from Oxytropis kansuensis. J. Northwest Sci-Tech Univ. Agric. For. (Nat. Sci. Ed.) 2006, 34, 97–99+104. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Revelle, W.R. Psych: Procedures for Personality and Psychological Research. 2017. Available online: https://CRAN.R-project.org/package=psych (accessed on 28 March 2021).
- Jacomy, M.; Venturini, T.; Heymann, S.; Bastian, M. ForceAtlas2, a continuous graph layout algorithm for handy network visualization designed for the Gephi software. PLoS ONE 2014, 9, e98679. [Google Scholar] [CrossRef]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, S.; Zhang, L.; Tang, X.; Fan, C.; Zhao, Y.; Cheng, Q.; Zhang, Y. Plant Secondary Compounds Promote White Adipose Tissue Browning via Modulation of the Gut Microbiota in Small Mammals. Int. J. Mol. Sci. 2023, 24, 17420. https://doi.org/10.3390/ijms242417420
Ren S, Zhang L, Tang X, Fan C, Zhao Y, Cheng Q, Zhang Y. Plant Secondary Compounds Promote White Adipose Tissue Browning via Modulation of the Gut Microbiota in Small Mammals. International Journal of Molecular Sciences. 2023; 24(24):17420. https://doi.org/10.3390/ijms242417420
Chicago/Turabian StyleRen, Shien, Liangzhi Zhang, Xianjiang Tang, Chao Fan, Yaqi Zhao, Qi Cheng, and Yanming Zhang. 2023. "Plant Secondary Compounds Promote White Adipose Tissue Browning via Modulation of the Gut Microbiota in Small Mammals" International Journal of Molecular Sciences 24, no. 24: 17420. https://doi.org/10.3390/ijms242417420
APA StyleRen, S., Zhang, L., Tang, X., Fan, C., Zhao, Y., Cheng, Q., & Zhang, Y. (2023). Plant Secondary Compounds Promote White Adipose Tissue Browning via Modulation of the Gut Microbiota in Small Mammals. International Journal of Molecular Sciences, 24(24), 17420. https://doi.org/10.3390/ijms242417420





