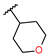The reagents and solvents for synthesis, analysis, and purification were purchased from commercial suppliers (Fisher Scientific (France), Sigma Aldrich (France), Key Organics (UK), and Fluorochem (UK and Ireland)) and used without further purification. The progress of all the reactions was routinely monitored by thin-layer chromatography (TLC) and/or by high-performance liquid chromatography–mass spectrometry (HPLC–MS). TLC was performed using Merck® commercial aluminum sheets coated with silica gel 60 F254. Visualization was achieved by fluorescence quenching under UV light at 254 nm and 215 nm or by staining with potassium permanganate. Purifications were performed by flash chromatography and reverse chromatography. Flash chromatography purifications were conducted in columns prepacked with Reveleris® flash cartridges, Buchi, France (40 μm, Büchi® FlashPure, Villebon sur Yvette, France or 15–40 μM, Macherey-Nagel® Chromabond®, Hoerdt, France) under pressure with an Interchim Puriflash® 430 instrument (Montluçon, France). The products were detected by UV absorption at 254 nm and by ELSD. Reverse chromatographies were conducted using Combiflash® C18 Rf200 in 4g, 12g, and C18 columns (Serlabo Technologies, EntraiguessurlaSorgues, France). The products were detected by UV absorption at 215 nm and 254 nm. UPLC–MS analysis was performed on an LC–MS Waters ACQUITY UPLC I-Class system (Guyancourt, France) equipped with a UPLC I BIN SOL MGR solvent manager, a UPLC I SMP MGR-FTN sample manager, an ACQUITY UPLC I-Class eλ PDA photodiode array detector (210–400 nm), and an ACQUITY QDa (Performance) mass detector (full scan ESI+/− in the range 30–1250). A Waters Acquity BEH C18 column (1.7 μm particle size; dimensions: 50 mm × 2.1 mm) was used for UPLC analysis (Guyancourt, France). The injection volume was 0.5 μL. For a 5 min analysis, the elution was carried out with a gradient starting at 98% H2O and 5 mM ammonium formate (pH 3.8) and reaching 98% CH3CN and 5 mM ammonium formate (pH 3.8; 5% aqueous) over 3.5 min at a flow rate of 600 µL/min. For a 30 min analysis, the elution was carried out with a gradient starting at 98% H2O and 5 mM ammonium formate (pH 3.8) and reaching 98% CH3CN and 5 mM ammonium formate (pH 3.8; 5% aqueous) over 25 min at a flow rate of 600 µL/min. Purity (%) was determined by reverse-phase UPLC, using UV detection (at 215 and 254 nm). HRMS analyses were performed on an LCT Premier XE Micromass (Guyancourt, France), using a Waters XBridge BEH C18 column with a 3.5 μm particle size and dimensions of 50 mm × 4.6 mm. A gradient starting from 98% H2O and 5 mM ammonium formate (pH 3.8) and reaching 100% CH3CN and 5 mM ammonium formate (pH 3.8; 5% aqueous) within 3 min at a flow rate of 2 mL/min was used. NMR spectra were recorded on a Bruker® Avance-300 spectrometer (Wissembourg, France). The results were calibrated to signals from the solvent as an internal reference (e.g., 7.26 (residual CDCl3) and 77.16 (CDCl3) ppm and 2.50 (residual DMSO d6) and 39.52 (DMSO d6) ppm for 1H and 13C NMR spectra, respectively.) Chemical shifts (δ) are in parts per million (ppm) downfield from tetramethylsilane (TMS). The assignments were made using one-dimensional (1D) 1H and 13C spectra and two-dimensional (2D) HSQC-DEPT, COSY, and HMBC spectra. NMR coupling constants (J) are reported in hertz (Hz), and splitting patterns are indicated as follows: s (singlet); brs (broad singlet); d (doublet); dd (doublet of doublet); ddd (double of doublet of doublet); dt (doublet of triplet); t (triplet); td (triplet of doublet); q (quartet); m (multiplet).
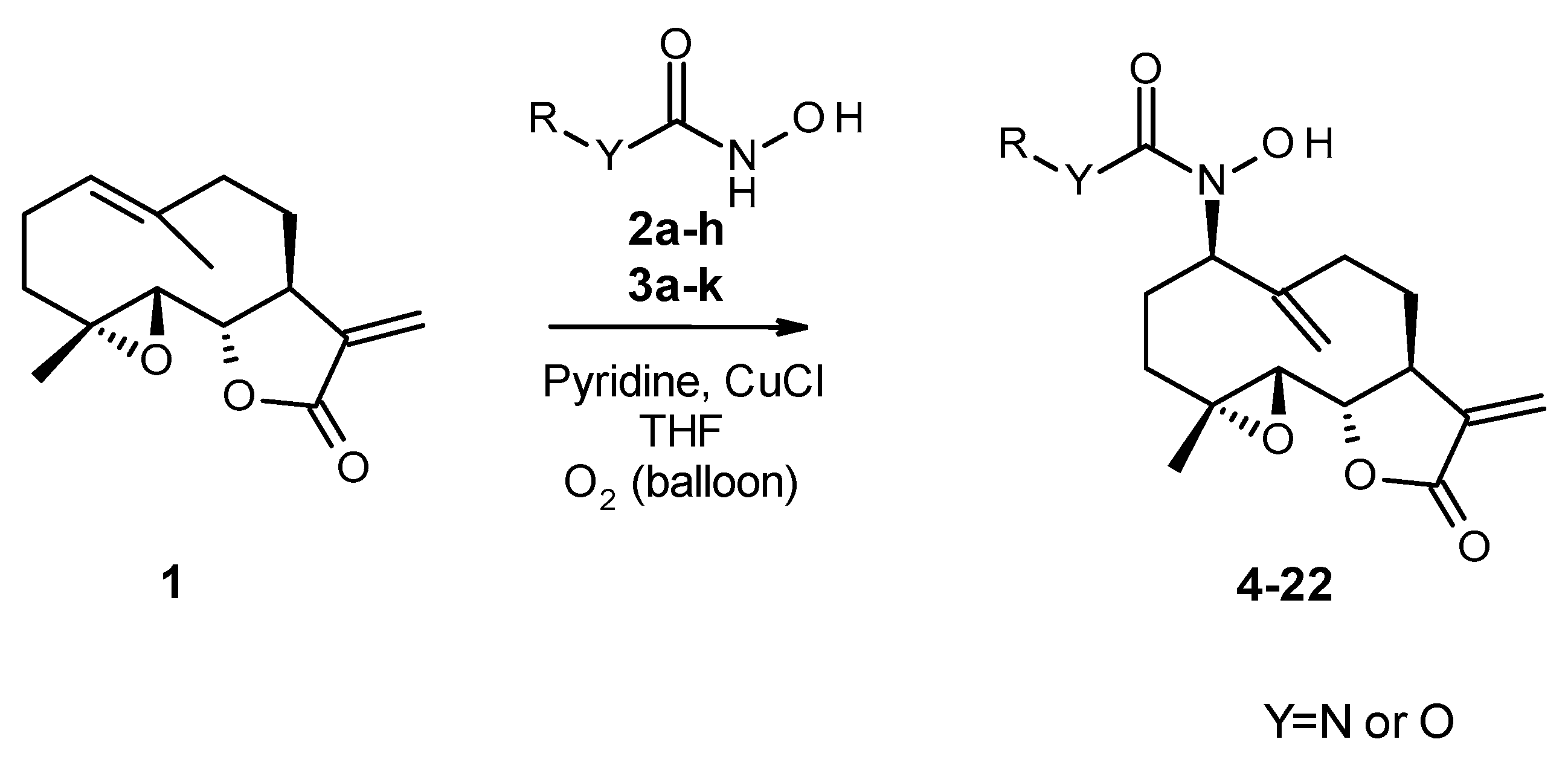
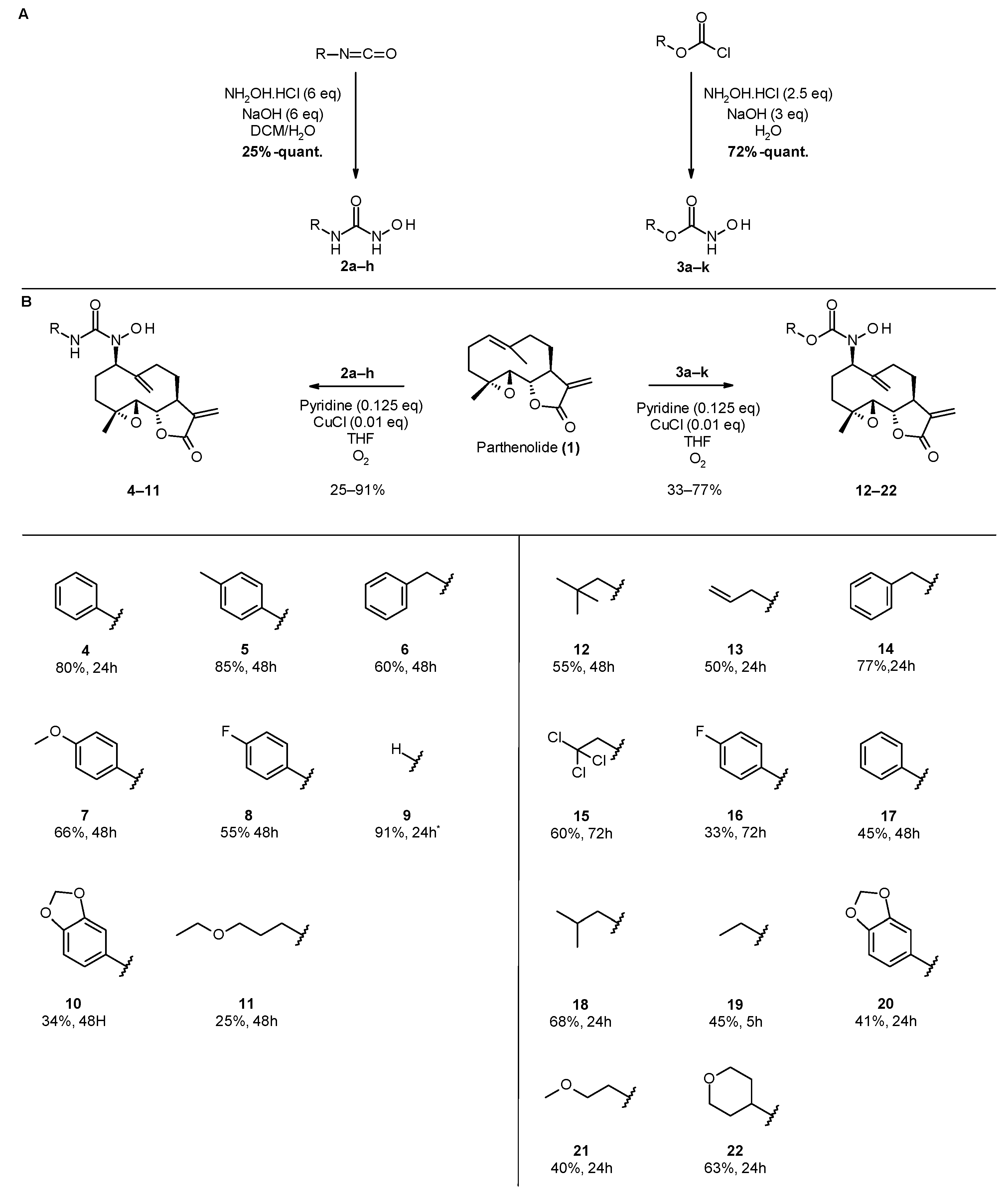
3.1.3. General Procedure for the Synthesis of Acylnitroso-Ene Derivatives (4–22)
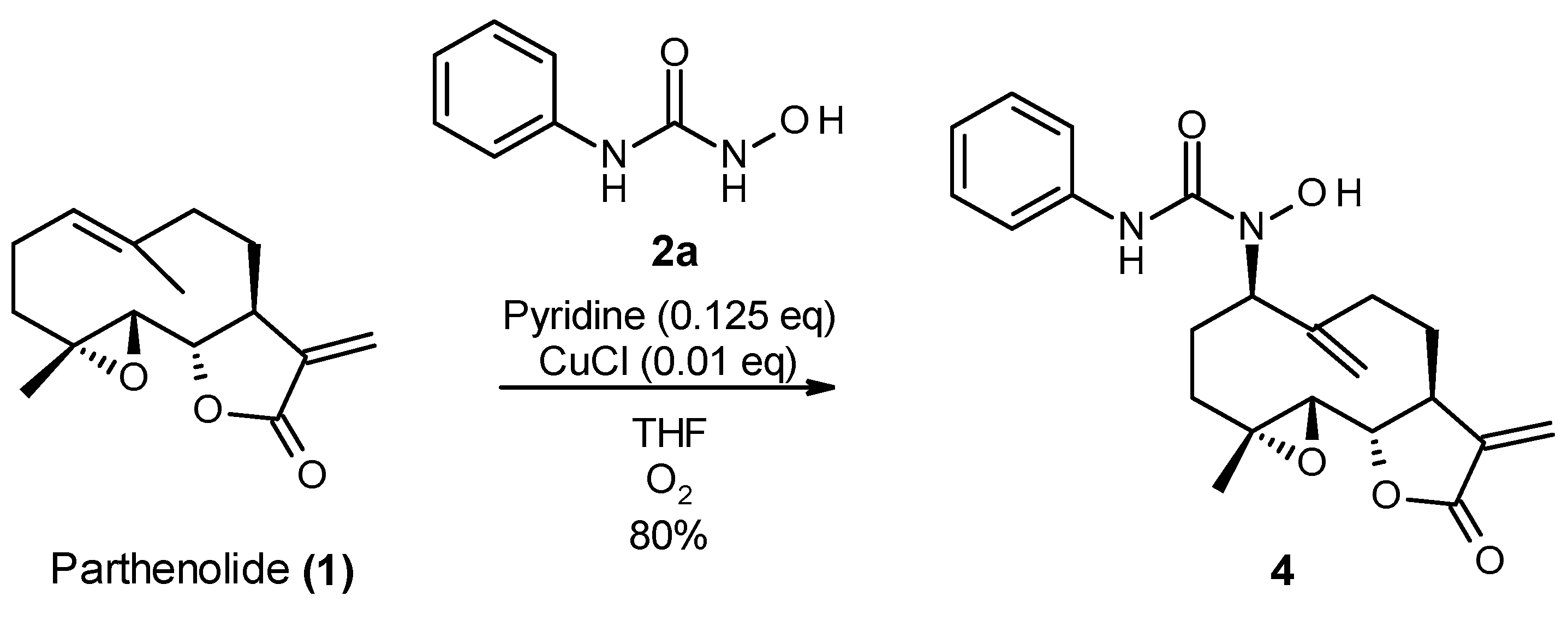
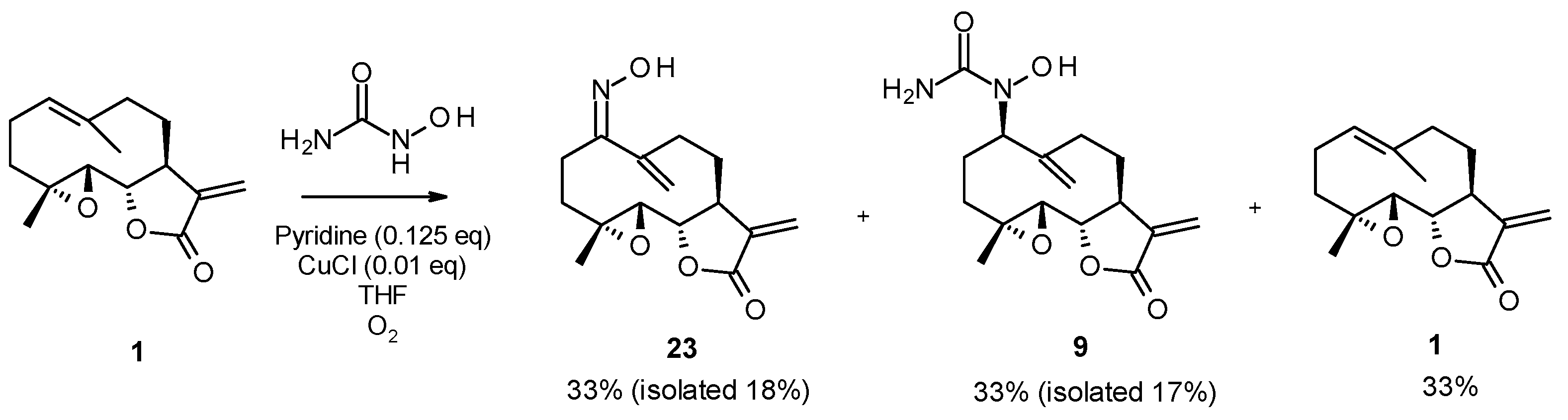
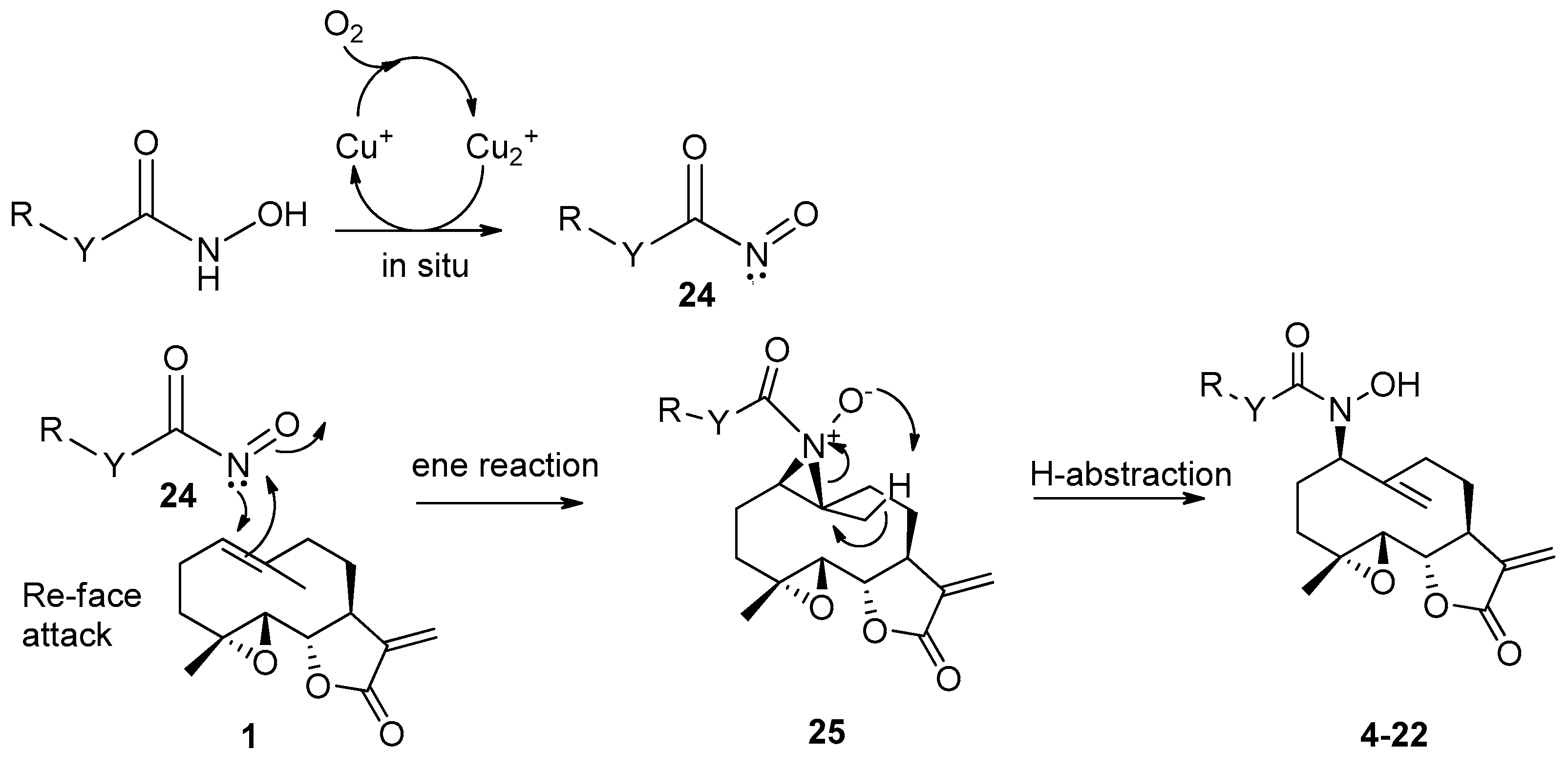
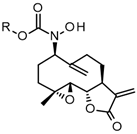
In a double-neck flask, CuCl (0.05 eq., 1 mg) and pyridine (0.125 eq., 2 µL) were added to the parthenolide (50 mg) and hydroxylamine derivative (1.1 eq.). Then, 2.2 mL of THF was added. The reaction mixture was stirred at room temperature under O2 (in a balloon). The reaction was monitored using TLC. After 24 h or 48 h, the reaction was stopped with a solution of EDTA (0.5 M, pH 7.0), diluted with ethyl acetate, and stirred until the color no longer persisted in the organic layer. Then, the water phase was extracted three times withe ethyl acetate. The organic phase was dried over MgSO4 and concentrated under reduced pressure to give a crude residue. Purification by flash chromatography afforded the desired compound.
1-hydroxy-1-[(1S,2S,4R,7R,11S)-4-methyl-8,12-dimethylene-13-oxo-3,14-dioxatricyclo[9.3.0.02,4] tetradecan-7-yl]-3-phenyl-urea (4) was obtained as a white powder after purification by flash chromatography in column 4g, with a gradient from 100% dichloromethane to 50/50 dichloromethane/ethyl acetate over 30 min (64 mg, 80%, purity at 254 nm: 100%). 1H NMR (300 MHz, DMSO-d6) δ = 9.42 (s, 1H, OH); 8.90 (s, 1H, NH); 7.59 (dd, J = 1.17, 8.76 Hz, 2H, 2 Ar-ortho); 7.23 (t, J = 7.47 Hz, 2H, 2 Ar-meta); 6.96 (m, 1H, Ar-para); 6.03 (d, J = 3.40 Hz, H-11); 5.64 (d, J = 3.12 Hz, 1H, H-11); 5.32 (s, 1H, H-14); 5.08 (s, 1H, H-14); 4.71 (dd, J = 2.84, 11.07 Hz, 1H, H-7); 3.97 (t, J = 9.20 Hz, 1H, H-2); 3.25 (m, 1H, H-1); 2.94 (d, J = 8.51 Hz, 1H, H-3); 2.14–2.38 (m, 5H, H-5, 6, 9, 10); 1.64–1.75 (m, 2H, H-10,6); 1.35 (s, 3H, H-15); 1.07 (t, 1H, H-5). 13C NMR (75 MHz, DMSO-d6) δ = 169.84 (Cq, C-13); 157.62 (Cq, C-16); 144.81 (Cq, C-12); 140.28 (Cq, C-8); 139.83 (Cq, C-17); 128.84 (CH, 2 C-Ar meta); 122.73 (CH, C-para Ar); 119.80 (CH, 2 C-Ar ortho); 119.61 (CH2, C-11); 116.22 (CH2, C-14); 80.18 (CH, C-2); 64.20 (CH, C-7); 63.18 (CH, C-3); 60.87 (Cq, C-4); 44.28 (CH, C-1); 36.46 (CH2, C-5); 29.82 (CH2, C-9); 26.12 (CH2, C-6); 25.09 (CH2, C-10); 18.15 (CH3, C-15). HRMS (TOF, ES+) m/z [M+H]+: calcd. for C22H27N2O5 399.1920; found 399.1940.
1-hydroxy-1-[(1S,2S,4R,7R,11S)-4-methyl-8,12-dimethylene-13-oxo-3,14-dioxatricyclo[9.3.0.02,4]tetradecan-7-yl]-3-(p-tolyl)urea (5) was obtained as a white powder after purification by flash chromatography in column 4g, with a gradient from 100% dichloromethane to 70/30 dichloromethane/ethyl acetate over 30 min (71 mg, 85%, purity at 254 nm: 96%). 1H NMR (300 MHz, DMSO-d6) δ = 9.36 (s, 1H, OH); 8.79 (s, 1H, NH); 7.46 (d, J = 8.32 Hz, 2H ortho); 7.03 (d, J = 8.13 Hz, 2H meta); 6.03 (d, J = 3.43 Hz, 1H, H-11′); 5.64 (d, J = 3.09 Hz, 1H, H-11); 5.32 (s, 1H, H-14′); 5.07 (s, 1H, H-14); 4.69 (dd, J = 2.83, 11.16 Hz, 1H, H-7); 3.97 (t, J = 9.27 Hz, 1H, H-2); 3.25 (m, 1H, H-1); 2.93 (d, J = 8.70 Hz, 1H, H-3); 2.13–2.37 (m, 8H, H-5′, H-6′, H10′, H-9, H-21); 1.64–1.72 (m, 2H, H-10, H-6); 1.35 (s, 3H, H-15); 1.06 (t, J = 12.11 Hz, 1H, H-5). 13C NMR (75 MHz, DMSO-d6) δ = 169.39 (Cq, C-13); 157.26 (Cq, C-16); 144.39 (Cq, C-8); 139.84 (Cq, C-12); 136.80 (Cq, C-17); 131.10 (Cq, C-20); 128.80 (CH, C-Ar meta); 119.42 (CH, C-Ar ortho); 119.17 (CH2, C-11); 115.73 (CH2, C-14); 79.74 (CH, C-2); 63.79 (CH, C-7); 62.74 (CH, C-3); 60.43 (Cq, C-4); 43.84 (CH, C-1); 36.02 (CH2, C-5); 29.40 (CH2, C-9); 25.66 (CH2, C-6); 24.67 (CH2, C-10); 20.36 (CH3, C-21); 17.70 (CH3, C-15). HRMS (TOF, ES+) m/z [M+NH4]+: calcd. for C23H29N2O5 413.2096; found 413.2076.
3-benzyl-1-hydroxy-1-[(1S,2S,4R,7R,11S)-4-methyl-8,12-dimethylene-13-oxo-3,14-dioxatricyclo [9.3.0.02,4]tetradecan-7-yl]urea (6) was obtained as a white powder after purification by flash chromatography in column 4g, with a gradient from 100% dichloromethane to 70/30 dichloromethane/ethyl acetate over 30 min (50 mg, 60%, purity at 254 nm: 100%). 1H NMR (300 MHz, DMSO-d6) δ = 9.01 (s, 1H, OH); 7.44 (t, J = 6.16 Hz, 1H, NH); 7.17–7.32 (m, 5H, H-Ar); 6.03 (d, J = 3.36 Hz, 1H, H-11); 5.65 (d, J = 3.17 Hz, 1H, H-11′); 5.27 (s, 1H, H-14); 5.04 (s, 1H, H-14′); 4.58 (dd, J = 3.17, 11.38 Hz, 1H, H-7); 4.22 (d, J = 6.34 Hz, 2H, H-17); 3.96 (t, J = 9.14 Hz, 1H, H-2); 3.24 (m, 1H, H-1); 2.90 (d, J = 8.77 Hz, 1H, H-3); 2.11–2.33 (m, 5H, H-5′, 6′, 9, 9′, 10′); 1.61–1.72 (m, 2H, H-6, 10); 1.33 (s, 3H, H-15); 1.02 (t, J = 13.07 Hz, 1H, H-5). 13C NMR (75 MHz, DMSO-d6) δ = 169.39 (Cq, C-13); 160.40 (Cq, C-16); 144.53 (Cq, C-8); 140.62 (Cq, C-18); 139.85 (Cq, C-12); 128.07 (CH, 2xC-Ar meta); 127.01 (CH, 2xC-Ar ortho); 126.48 (CH, C-Ar para); 119.16 (CH2, C-11); 115.48 (CH2, C-14); 79.75 (CH, C-2); 64.49 (CH, C-7); 62.74 (CH, C-3); 60.44 (Cq, C-4); 43.81 (CH, C-1); 42.84 (CH2, C-17); 36.05 (CH2, C-5); 29.47 (CH2, C-9); 25.50 (CH2, C-6); 24.67 (CH2, C-10); 17.69 (CH3, C-15). HRMS (TOF, ES+) m/z [M+H]+: calcd. for C23H29N2O5 413.2076; found 413.2081.
1-hydroxy-3-(4-methoxyphenyl)-1-[(1S,2S,4R,7R,11S)-4-methyl-8,12-dimethylene-13-oxo-3,14-dioxatricyclo[9.3.0.02,4]tetradecan-7-yl]urea (7) was obtained as a white powder after purification by flash chromatography in column 4g, with a gradient from 100% dichloromethane to 70/30 dichloromethane/ethyl acetate over 30 min (57 mg, 66%, purity at 254 nm: 99%). 1H NMR (300 MHz, DMSO-d6) δ = 9.33 (s, 1H, OH); 8.78 (s, 1H, NH); 7.47 (m, 2H, H-18); 6.81 (m, 2H, H-19); 6.03 (d, J = 3.38 Hz, 1H, H-11); 5.64 (d, J = 3.11 Hz, 1H, H-11); 5.31 (s, 1H, H-14); 5.07 (s, 1H, H-14); 4.48 (dd, J = 3.25, 11.13 Hz, 1H, H-7); 3.97 (t, J = 8.77 Hz, 1H, H-2); 3.69 (s, 3H, H-20); 3.24 (m, 1H, H-1); 2.87 (d, J = 8.80 Hz, 1H, H-3); 2.11–2.38 (m, 5H, H-9, H-10, H-6, H-5); 1.64–1.73 (m, 2H, H-10, H-6); 1.34 (s, 3H, H-15); 1.06 (t, J = 13.37 Hz, 1H, H-5). 13C NMR (75 MHz, CDCl3) δ = 169.44 (Cq, C-13); 157.54 (Cq, C-16); 154.77 (Cq, C-20); 144.44 (Cq, C-8); 139.85 (Cq, C-12); 132.44 (Cq, C-17); 121.12 (CH, C-18); 119.21 (CH2, C-11); 115.73 (CH2, C-14); 113.60 (CH, C-19); 79.78 (CH, C-2); 63.93 (CH, C-7); 62.76 (CH, C-3); 60.47 (Cq, C-4); 55.16 (CH3, C-20); 43.87 (CH, C-1); 36.06 (CH2, C-5); 29.45 (CH2, C-9); 25.68 (CH2, C-6); 24.70 (CH2, C-10); 17.72 (CH3, C-15). HRMS (TOF, ES+) m/z [M+H]+: calcd. for C23H29N2O6 429.2026; found 429.2025.
3-(4-fluorophenyl)-1-hydroxy-1-[(1S,2S,4R,7R,11S)-4-methyl-8,12-dimethylene-13-oxo-3,14-dioxatricyclo[9.3.0.02,4]tetradecan-7-yl]urea (8) was obtained as a white powder after purification by flash chromatography in column 4g, with a gradient from 100% dichloromethane to 70/30 dichloromethane/ethyl acetate over 30 min (46 mg, 55%, purity at 254 nm: 99%). 1H NMR (300 MHz, DMSO-d6) δ = 9.41 (s, 1H, OH); 9.01 (s, 1H, NH); 7.61 (m, 2H, H-18); 7.06 (m, 2H, H-19); 6.03 (d, J = 3.53 Hz, 1H, H-11′); 5.64 (d, J = 3.23 Hz, 1H, H-11); 5.32 (s, 1H, H-14′); 5.08 (s, 1H, H-14); 4.70 (dd, J = 2.60, 10.93 Hz, 1H, H-7); 3.97 (t, J = 9.22 Hz, 1H, H-2); 3.25 (m, 1H, H-1); 2.93 (d, J = 8.80 Hz, 1H, H-3); 2.14–2.38 (m, 5H, H-5′, H-6′, H-10′, 2xH-9); 1.64–1.76 (m, 2H, H-10, H-6); 1.35 (s, 3H, H-15); 1.06 (t, J = 13.27 Hz, 1H, H-5). 13C NMR (75 MHz, DMSO-d6) δ = 169.39 (Cq, C-13); 159.20 (d, J = 238.68 Hz, Cq, C-20); 157.27 (Cq, C-16); 144.35 (Cq, C-8); 139.83 (Cq, C-12); 135.82 (d, J = 2.42 Hz, Cq, C-17); 121.16 (d, J = 7.63 Hz, CH, C-18); 119.16 (CH2, C-11); 115.78 (CH2, C-14); 114.99 (d, J = 21.64 Hz, CH, C-19); 79.73 (CH, C-2); 63.81 (CH, C-7); 62.73 (CH, C-3); 60.42 (Cq, C-4); 43.81 (CH, C-1); 36.99 (CH2, C-5); 29.35 (CH2, C-9); 25.66 (CH2, C-6); 24.63 (CH2, C-10); 17.70 (CH3, C-15). HRMS (TOF, ES+) m/z [M+]+: calcd. for C22H26FN2O5 417.1826; found 417.1810.
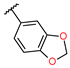
3-(1,3-benzodioxol-5-yl)-1-hydroxy-1-[(1S,2S,4R,7R,11S)-4-methyl-8,12-dimethylene-13-oxo-3,14-dioxatricyclo[9.3.0.02,4] tetradecan-7-yl]urea (10) was obtained as a white powder after purification by flash chromatography in column 4g, with a gradient from 100% dichloromethane to 70/30 dichloromethane/ethyl acetate over 30 min (30 mg, 34%, purity at 254 nm: 95%). 1H NMR (300 MHz, DMSO-d6) δ = 9.36 (s, 1H, OH); 8.83 (s, 1H, NH); 7.27 (d, J = 2.05 Hz, 1H, H-18); 7.02 (dd, J = 2.03, 8.29 Hz, 1H, H-23); 6.77 (d, J = 8.39 Hz, 1H, H-22); 6.03 (d, J = 3.41 Hz, 1H, H-11′); 5.93 (s, 2H, H-20); 5.64 (d, J = 3.05 Hz, 1H, H-11); 5.31 (s, 1H, H-14′); 5.07 (s, 1H, H-14); 4.67 (dd, J = 3.00, 11.57 Hz, 1H, H-7); 3.97 (t, J = 9.06 Hz, 1H, H-2); 3.23 (m, 1H, H-1); 2.93 (d, J = 8.72 Hz, 1H, H-3); 2.11–2.35 (m, 5H, H-5′, H-6′, H-10′, 2xH-9); 1.65–1.72 (m, 2H, H-10, H-6); 1.35 (s, 3H, H-15); 1.06 (t, J = 12.08 Hz, 1H, H-5). 13C NMR (75 MHz, DMSO-d6) δ = 169.35 (Cq, C-13); 157.28 (Cq, C-16); 146.85 (Cq, C-19); 144.32 (Cq, C-8); 142.25 (Cq, C-21); 139.80 (Cq, C-12); 133.77, (Cq, C-17); 119.12 (CH2, C-11); 115.70 (CH2, C-14); 112.14 (CH, C-23); 107.70 (CH, C-22); 101.72 (CH, C-18); 100.71 (CH2, C-20); 79.72 (CH, C-2); 63.83 (CH, C-7); 62.70 (CH, C-3); 60.39 (Cq, C-4); 43.85 (CH, C-1); 36.01 (CH2, C-5); 29.41 (CH2, C-9); 25.63 (CH2, C-6); 24.66 (CH2, C-10); 17.67 (CH3, C-15). HRMS (TOF, ES+) m/z [M+H]+: calcd. for C23H27N2O7 443.1818; found 443.1812.
3-(3-ethoxypropyl)-1-hydroxy-1-[(1S,2S,4R,7R,11S)-4-methyl-8,12-dimethylene-13-oxo-3,14-dioxatricyclo[9.3.0.02,4]tetradecan-7-yl]urea (11) was obtained as a white powder after purification by flash chromatography in column 4g, with a gradient from 100% dichloromethane to 40/50/10 dichloromethane/ethyl acetate/methanol over 30 min (20 mg, 24%, purity at 254 nm: 100%). 1H NMR (300 MHz, DMSO-d6) δ = 8.90 (s, 1H, OH); 6.89 (t, J = 5.85 Hz, 1H, NH); 6.02 (d, J = 3.30 Hz, 1H, H-11′); 5.64 (d, J = 3.15 Hz, 1H, H-11); 5.24 (s, 1H, H-14′); 5.03 (s, 1H, H-14); 4.54 (dd, J = 2.36, 10.60 Hz, 1H, H-7); 3.95 (t, J = 9.20 Hz, 1H, H-2); 3.34–3.41 (m, 4H, H-20,H-19); 3.24 (m, 1H, H-1); 3.08 (q, J = 6.71 Hz, 2H, H-17); 2.89 (d, J = 8.57 Hz, 1H, H-3); 2.08–2.37 (m, 5H, H-5′, H-6′, H-10′, 2xH-9); 1.56–1.65 (m, 4H, H-10, H-6, 2xH-18); 1.32 (s, 3H, H-15); 1.07 (m, 4H, H-5, H-21). 13C NMR (75 MHz, DMSO-d6) δ = 169.35 (Cq, C-13); 160.32 (Cq, C-16); 144.54 (Cq, C-8); 139.80 (Cq, C-12); 119.12 (CH2, C-11); 115.70 (CH2, C-14); 79.72 (CH, C-2); 67.98 (CH2, C-19); 65.29 (CH2, C-20); 64.37 (CH, C-7); 62.70 (CH, C-3); 60.40 (Cq, C-4); 43.86 (CH, C-1); 37.20 (CH2, C-17) 36.08 (CH2, C-5); 29.94 (2xCH2, C-9, C-18); 25.43 (CH2, C-6); 24.75 (CH2, C-10); 17.65 (CH3, C-15); 15.10 (CH3, C-21). HRMS (TOF, ES+) m/z [M+H]+: calcd. for C21H33N2O6 409.2339; found 409.2329.
tert-butyl-N-hydroxy-N-[(1S,2S,4R,7R,11S)-4-methyl-8,12-dimethylene-13-oxo-3,14-dioxatricyclo[9.3.0.02,4]tetradecan-7-yl]carbamate (12) was obtained as a white powder after purification by flash chromatography in column 4g, with a gradient from 100% dichloromethane to 70/30 dichloromethane/ethyl acetate over 30 min (42 mg, 55%, purity at 254 nm: 96%). 1H NMR (300 MHz, DMSO-d6) δ = 8.95 (s, 1H, OH); 6.03 (d, J = 3.36 Hz, 1H, H-11); 5.65 (d, J = 3.07 Hz, 1H, H-11′); 5.27 (s, 1H, H-14); 5.04 (s, 1H, H-14′); 4.46 (dd, J = 3.07, 10.98 Hz, 1H, H-7); 3.96 (t, J = 9.19 Hz, 1H, H-2); 3.23 (m, 1H, H-3); 2.88 (d, J = 8.77 Hz, 1H, H-1); 2.26–2.39 (m, 2H, H-9, H-10); 2.10–2.20 (m, 3H, H-9′, H-6, H-5′); 1.61–1.68 (m, 2H, H-6′, H-10′); 1.40 (s, 9H, H-Boc); 1.33 (s, 3H, H-15) 1.02 (t, J = 12.541 Hz, 1H, H-5). 13C NMR (75 MHz, DMSO-d6) δ = 169.34 (Cq, C-13); 155.40 (Cq, C-16); 144.50 (Cq, C-8); 139.77 (Cq, C-12); 119.18 (CH2-11); 115.55 (CH2-14); 79.66 (CH, C-2); 64.56 (CH, C-7); 62.88 (CH, C-1); 60.35 (Cq, C-4); 43.51 (CH, C-3); 35.69 (CH2, C-5); 28.67 (CH2, C-9); 28.08 (CH3-Boc); 25.65 (CH2, C-6); 24.53 (CH2, C-10); 17.70 (CH3-15). Quaternary carbon C-18 is not shown. HRMS (TOF, ES+) m/z [M+NH4]+: calcd. for C20H33N2O6 397.2339; found 397.2343.
Allyl-N-hydroxy-N-[(1S,2S,4R,7R,11S)-4-methyl-8,12-dimethylene-13-oxo-3,14-dioxatricyclo [9.3.0.02,4]tetradecan-7-yl]carbamate (13) was obtained as a white powder after purification by flash chromatography in column 4g, with a gradient from 100% dichloromethane to 70/30 dichloromethane/ethyl acetate over 30 min (37 mg, 50%, purity at 254 nm: 100%). 1H NMR (300 MHz, DMSO-d6) δ = 9.25 (s, 1H, OH); 6.03 (d, 1H, J = 3.41 Hz, H-11); 5.92 (m, 1H, H-18); 5.62 (d, 1H, J = 3.12 Hz, H-11′); 5.26–5.33 (m, 3H, H-14, 2xH-19); 5.06 (s, 1H, H-14′); 4.52–4.55 (m, 3H, 2xH-17, H-7); 3.96 (t, J = 9.23 Hz, 1H, H-2); 3.24 (m, 1H, H-1); 2.90 (d, J = 8.98 Hz, 1H, H-3); 2.29–2.36 (m, 2H, H-9, H-10); 2.11–2.21 (m, 3H, H-9′, H-6, H-5′); 1.64–1.77 (m, 2H, H-6′, H-10′); 1.35 (s, 3H, H-15); 1.05 (t, J = 13.08 Hz, 1H H-5). 13C NMR (75 MHz, DMSO-d6) δ = 169.37 (Cq, C-13); 155.80 (Cq, C-16); 144.23 (Cq, C-8); 139.79 (Cq, C-12); 133.23 (CH, C-18); 119.15 (CH2, C-11); 117.45 (CH2, C-19); 115.98 (CH2, C-14); 79.64 (CH, C-2); 65.48 (CH, C-17); 64.67 (Cq, C-7); 62.82 (CH, C-3); 60.34 (Cq, C-4); 43.49 (CH, C-1); 35.58 (CH2, C-5); 28.60 (CH2, C-9); 25.59 (CH2, C-6); 24.35 (CH2, C-10); 17.71 (CH3, C-15). HRMS (TOF, ES+) m/z [M+H]+: calcd. for C19H26NO6 364.1760; found 364.1773.
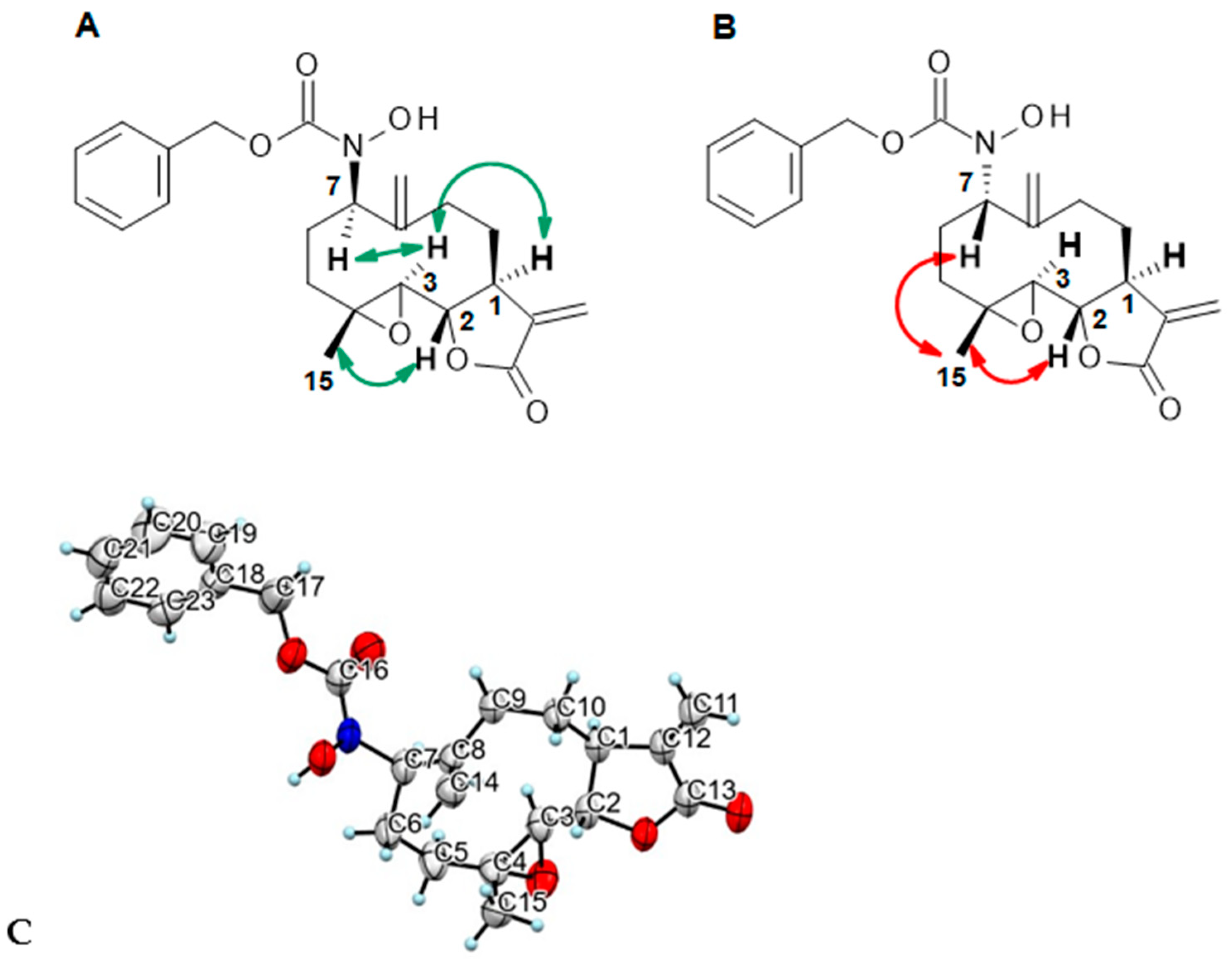
Benzyl-N-hydroxy-N-[(1S,2S,4R,7R,11S)-4-methyl-8,12-dimethylene-13-oxo-3,14-dioxatricyclo[9.3.0.02,4]tetradecan-7-yl]carbamate (14) was obtained as a white powder after purification by flash chromatography in column 4g, with a gradient from 100% dichloromethane to 70/30 dichloromethane/ethyl acetate over 30 min (64 mg, 77%, purity at 254 nm: 98%). 1H NMR (300 MHz, DMSO-d6) δ = 9.27 (s, 1H, OH); 7.31–7.38 (m, 5H-Ar); 6.03 (d, J = 3.43 Hz, 1H, H-11); 5.64 (d, J = 3.05 Hz, 1H, H-11); 5.29 (s, 1H, H-14); 5.09 (d, J = 3.15 Hz, 2H, H-17); 5.06 (s, 1H, H-14); 4.56 (dd, J = 3.05, 10.88 Hz, H-7); 3.96 (t, J = 9.24 Hz, 1H, H-2); 3.22 (m, 1H, H-1); 2.89 (d, J = 8.87 Hz, 1H, H-3); 2.29–2.36 (m, 2H, H-9, H-10); 2.11–2.20 (m, 3H, H-6, H-5, H-9); 1.63–1.71 (m, 2H, H-6, H-10); 1.34 (s, 3H, H-15); 1.05 (t, J = 12.57 Hz, 1H, H-5). 13C NMR (75 MHz, DMSO-d6) δ = 169.36 (Cq, C-13); 155.95 (Cq, C-16); 144.21 (Cq, C-8); 139.78 (Cq, C-12); 136.62 (Cq, C-18); 128.39 (CH, 2 C-Ar); 127.98 (CH, C-para Ar); 127.89 (CH, 2 C-Ar); 119.17 (CH2, C-11); 116.03 (CH2, C-14); 79.64 (CH, C-2); 66.46 (CH2, C-17); 64.66 (CH, C-7); 62.82 (CH, C-3); 60.33 (Cq, C-4); 43.44 (CH, C-1); 35.57 (CH2, C-5); 28.59 (CH2, C-9); 25.59 (CH2, C-6); 24.43 (CH2, C-10); 17.70 (CH3, C-15). HRMS (TOF, ES+) m/z [M+NH4]+: calcd. for C23H31N2O6 431.2182; found 431.2187.
2,2,2-trichloroethyl-N-hydroxy-N-[(1S,2S,4R,7R,11S)-4-methyl-8,12-dimethylene-13-oxo-3,14-dioxatricyclo[9.3.0.02,4]tetradecan-7-yl]carbamate (15) was obtained as a white powder after purification by flash chromatography in column 4g, with a gradient from 100% dichloromethane to 80/20 dichloromethane/ethyl acetate over 30 min (55 mg, 60%, purity at 215 nm: 100%). 1H NMR (300 MHz, DMSO-d6) δ = 9.60 (s, 1H, OH); 6.03 (d, J = 3.44 Hz, 1H, H-11); 5.66 (d, J = 3.17 Hz, 1H, H-11); 5.35 (s, 1H, H-14); 5.12 (s, 1H, H-14); 4.88 (m, 2H, H-17); 4.59 (dd, J = 3.07 Hz, 1H, H-7); 3.98 (t, J = 9.10 Hz, 1H, H-2); 3.22 (m, 1H, H-1); 2.90 (d, J = 8.91 Hz, 1H, H-3); 2.13–2.35 (m, 5H, H-5, H-6, H-9, H10); 1.65–1.76 (m, 2H, H-6, H-10); 1.34 (s, 3H, H-15); 1.05 (t, J = 12.63 Hz, 1H, H-5). 13C NMR (75 MHz, DMSO-d6) δ = 169.80 (Cq, C-13); 154.35 (Cq, C-16); 144.13 (Cq, C-12); 140.19 (Cq, C-8); 119.72 (CH2, C-11); 116.96 (CH2, C-14); 96.25 (Cq, C-16); 80.10 (CH, C-2); 74.60 (CH2, C-17); 65.52 (CH, C-7); 63.31 (CH, C-3); 60.75 (Cq, C-4); 44.08 (CH, C-1); 36.07 (CH2, C-5); 29.15 (CH2, C-9); 26.02 (CH2, C-6); 25.15 (CH2, C-10); 18.12 (CH3, C-15). HRMS (TOF, ES+) m/z [M+H]+: calcd. for C18H23Cl3NO6 454.0584; found 454.0591.
(4-fluorophenyl)-N-hydroxy-N-[(1S,2S,4R,7R,11S)-4-methyl-8,12-dimethylene-13-oxo-3,14-dioxatricyclo[9.3.0.02,4]tetradecan-7-yl]carbamate (16) was obtained as a slightly yellow powder after purification by flash chromatography in column 4g, with a gradient from 100% dichloromethane to 70/30 dichloromethane/ethyl acetate over 30 min (28 mg, 33%, purity at 254 nm: 98%). 1H NMR (300 MHz, DMSO-d6) δ = 9.67 (s, 1H); 7.11–7.25 (m, 4H, H-Ar); 6.04 (d, J = 3.50 Hz, 1H, H-11); 5.66 (d, J = 3.22 Hz, 1H, H-11′); 5.38 (s, 1H, H-14); 5.15 (s, 1H, H-14′); 4.63 (dd, J = 3.01, 11.46 Hz, 1H, H-7); 3.98 (t, J= 9.06 Hz, 1H, H-2); 3.25 (m, 1H, H-1); 2.93 (d, J = 8.93 Hz, 1H, H-3); 2.15–2.42 (m, 5H, H-5, H-6, H-10, H-9); 1.67–1.84 (m, 2H, H-6′, H-10′); 1.36 (s, 3H, H-15); 1.10 (t, J = 12.62 Hz, 1H, H-5′). 13C NMR (75 MHz, DMSO-d6) δ = 169.38 (Cq, C-13); 159.29 (d, J = 241.78 Hz, Cq, C-20); 147.06 (d, J = 2.99 Hz, Cq, C-17); 143.90 (Cq, C-8); 139.77 (Cq, C-12); 123.51 (d, J = 8.78 Hz, 2xCH, C-18); 119.17 (CH2, C-11); 116.39 (CH2, C-14); 115.95 (d, J = 23.10 Hz, 2xCH, C-19); 79.61 (CH, C-2); 65.04 (CH, C-7); 62.70 (CH, C-3); 60.34 (Cq, C-4); 43.64 (CH, C-1); 35.53 (CH2, C-5); 28.63 (CH2, C-9); 25.60 (CH2, C-6); 24.29 (CH2, C-10); 17.69 (CH3, C-15). HRMS (TOF, ES+) m/z [M+H]+: calcd. for C22H25FNO6 418.1654; found 418.1666.
Phenyl-N-hydroxy-N-[(1S,2S,4R,7R,11S)-4-methyl-8,12-dimethylene-13-oxo-3,14-dioxatricyclo[9.3.0.02,4]tetradecan-7-yl]carbamate (17) was obtained as a white powder after purification by flash chromatography in column 4g, with a gradient from 100% dichloromethane to 80/20 dichloromethane/ethyl acetate over 30 min (37 mg, 45%, purity at 254 nm: 99%). 1H NMR (300 MHz, DMSO-d6) δ = 9.64 (s, 1H, OH); 7.40 (m, 2H, H-18); 7.23 (m, 1H, H-20); 7.09 (m, 2H, H-19); 6.03 (d, J = 3.44 Hz, 1H, H-11′); 5.66 (d, J = 3.13 Hz, 1H, H-11); 5.38 (s, 1H, H-14′); 5.15 (s, 1H, H-14); 4.64 (dd, J = 3.06, 11.38 Hz, 1H, H-7); 3.98 (t, J = 9.08 Hz, 1H, H-2); 3.26 (m, 1H, H-1); 2.93 (d, J = 9.08 Hz, 1H, H-3); 2.15–2.43 (m, 5H, H-5′, H-6′, H-10′, H-9); 1.65–1.84 (m, 2H, H-10, H-6); 1.36 (s, 3H, H-15); 1.11 (t, J = 12.49 Hz, 1H, H-5). 13C NMR (75 MHz, DMSO-d6) δ = 169.37 (Cq, C-13); 154.06 (Cq, C-16); 151.00 (Cq, C-17) 143.97 (Cq, C-8); 139.78 (Cq, C-12); 129.40 (2xCH, C-18); 125.36 (CH, C-20); 121.70 (2xCH, C-19); 119.16 (CH2, C-11); 116.35 (CH2, C-14); 79.61 (CH, C-2); 65.01 (CH, C-7); 62.71 (CH, C-3); 60.34 (Cq, C-4); 43.60 (CH, C-1); 35.53 (CH2, C-5); 28.61 (CH2, C-9); 25.62 (CH2, C-6); 24.27 (CH2, C-10); 17.69 (CH3, C-15). HRMS (TOF, ES+) m/z [M+H]+: calcd. for C22H26NO 400.1760; found 400.1747.
Isobutyl-N-hydroxy-N-[(1S,2S,4R,7R,11S)-4-methyl-8,12-dimethylene-13-oxo-3,14-dioxatricyclo[9.3.0.02,4]tetradecan-7-yl]carbamate (18) was obtained as a white powder after purification by flash chromatography in column 4g, with a gradient from 100% dichloromethane to 70/30 dichloromethane/ethyl acetate over 30 min (50 mg, 65%, purity at 215 nm: 98%). 1H NMR (300 MHz, DMSO-d6) δ = 9.16 (s, 1H, OH); 6.03 (d, J = 3.39 Hz, 1H, H-11); 5.65 (d, J = 3.13 Hz, 1H, H-11); 5.28 (s, 1H, H-14); 5.06 (s, 1H, H-14); 4.51 (dd, J = 2.90, 10.90 Hz, H-7); 3.97 (t, J = 9.16 Hz, 1H, H-2); 3.79 (d, J = 6.03 Hz, 2H, H-17); 3.23 (m, 1H, H-1); 2.89 (d, J = 8.93 Hz, 1H, H-3); 2.29–2.38 (m, 2H, H-9, H-10); 2.11–2.23 (m, 3H, H-6, H-5, H-9); 1.85 (m, 1H, H-18); 1.64–1.71 (m, 2H, H-6, H-10); 1.34 (s, 3H, H-15); 1.04 (t, J = 12.32 Hz, 1H, H-5); 0.87 (d, J = 6.67 Hz, 6H, H-19, H-20). 13C NMR (75 MHz, DMSO-d6) δ = 169.83 (Cq, C-13); 156.72 (Cq, C-16); 144.75 (Cq, C-8); 140.22 (Cq, C-12); 119.64 (CH2, C-11); 116.34 (CH2, C-14); 80.10 (CH, C-2); 71.40 (CH2, C-17) 65.18 (CH, C-7); 63.32 (CH, C-3); 60.80 (Cq, C-4); 43.93 (CH, C-1); 36.06 (CH2, C-5); 29.05 (CH2, C-9); 28.03 (CH, C-18); 26.06 (CH2, C-6); 24.88 (CH2, C-10); 19.32 (CH3, C-19, C-20); 18.15 (CH3, C-15). HRMS (TOF, ES+) m/z [M+H]+: calcd. for C20H30NO6 380.2073; found 380.2045.
Ethyl-N-hydroxy-N-[(1S,2S,4R,7R,11S)-4-methyl-8,12-dimethylene-13-oxo-3,14-dioxatricyclo[9.3.0.02,4]tetradecan-7-yl]carbamate (19) was obtained as a white powder after purification by flash chromatography in column 4g, with a gradient from 100% dichloromethane to 60/40 dichloromethane/ethyl acetate over 30 min (30 mg, 42%, purity at 254 nm: 98%). 1H NMR (300 MHz, DMSO-d6) δ = 9.17 (s, 1H, OH); 6.02 (d, J = 3.40 Hz, 1H, H-11); 5.64 (d, J = 3.14 Hz, 1H, H-11′); 5.28 (s, 1H, H-14); 5.05 (s, 1H, H-14′); 4.51 (dd, J = 2.99, 10.85 Hz, 1H, H-7); 3.93–4.08 (m, 3H, H-2, 2xH-17); 3.23 (m, 1H, H-1); 2.89 (d, J = 8.89 Hz, H-3); 2.28–2.38 (m, 2H, H-9, H-10); 2.08–2.21 (m, 3H, H-6, H-5, H-9′); 1.63–1.70 (m, 2H, H-6′, H-10′); 1.33 (s, 3H, H-15); 1.17 (t, J = 7.09 Hz, 3H, H-18); 1.04 (t, J = 12.66 Hz, 1H, H-5′). 13C NMR (75 MHz, DMSO-d6) δ = 169.47 (Cq, C-13); 156.30 (Cq, C-16); 144.35 (Cq, C-8); 139.83 (Cq, C-12); 119.25 (CH2, C-11); 115.95 (CH2, C-14); 79.73 (CH, C-2); 64.64 (CH, C-7); 62.90 (CH, C-3); 61.10 (CH2-C-17); 60.43 (Cq, C-4); 43.53 (CH, C-1); 35.65 (CH2, C-5); 28.66 (CH2, C-9); 25.64 (CH2, C-6); 24.42 (CH2, C-10); 17.76 (CH3, C-15); 14.65 (CH3, C-18). HRMS (TOF, ES+) m/z [M+H]+: calcd. for C18H26NO6 352.1760; found 352.1764.
1,3-benzodioxol-5-yl-N-hydroxy-N-[(1S,2S,4R,7R,11S)-4-methyl-8,12-dimethylene-13-oxo-3,14-dioxatricyclo[9.3.0.02,4]tetradecan-7-yl]carbamate (20) was obtained as a white powder after purification by flash chromatography in column 4g, with a gradient from 100% dichloromethane to 80/20 dichloromethane/ethyl acetate over 30 min (37 mg, 41%, purity at 254 nm: 96%). 1H NMR (300 MHz, DMSO-d6) δ = 9.61 (s, 1H, OH); 6.88 (d, 1H, J = 8.46 Hz, H-22); 6.73 (d, 1H, J = 2.37 Hz, H-18); 6.53 (dd, 1H, J = 2.37, 8.46 Hz, H-21); 6.02–6.05 (m, 3 H, H-20, H-11); 5.66 (d, 1H, J = 3.47 Hz, H-11′); 5.37 (s, 1H, H-14); 5.14 (s, 1H, 14′); 4.60 (dd, 1H, J = 10.94, 2.43 Hz, H-7); 3.97 (t, 1H, J = 8.99 Hz, H-2); 3.25 (m, 1H, H-1); 2.90 (d, 1H, J = 8.76 Hz, H-3); 2.13–2.43 (m, 5H, H-9, H-9′, H-10, H-6, H-5); 1.65–1.85 (m, 2H, H-10′, H-6′); 1.36 (s, 3H, H-15); 1.10 (m, 1H, H-5′). 13C NMR (75 MHz, DMSO-d6) δ = 169.38 (Cq, C-13); 154.27 (Cq, C-16); 147.49 (Cq, C-19); 145.29 (Cq, C-17); 144.61 (Cq, C-21);143.93 (Cq, C-8); 139.78 (Cq, C-12); 119.17 (CH2, C-11); 116.34 (CH2, C-14); 114.09 (CH, C-23); 107.82 (CH, C-22); 103.94 (CH, C-18); 101.66 (CH2, C-20); 79.02 (CH, C-2); 65.01 (CH, C-7); 62.70 (CH, C-3); 60.35 (Cq, C-4); 43.62 (CH, C-1); 35.53 (CH2, C-5); 28.63 (CH2, C-9); 25.60 (CH2, C-6); 24.28 (CH2, C-10); 17.69 (CH3, C-15). HRMS (TOF, ES+) m/z [M+H]+: calcd. for C23H26NO8 444.1658; found 444.1629.
2-methoxyethyl-N-hydroxy-N-[(1S,2S,4R,7R,11S)-4-methyl-8,12-dimethylene-13-oxo-3,14-dioxatricyclo[9.3.0.02,4]tetradecan-7-yl]carbamate (21) was obtained as a white powder after purification by flash chromatography in column 4g, with a gradient from 100% dichloromethane to 70/30 dichloromethane/ethyl acetate over 30 min (30 mg, 40%, purity at 254 nm: 99%). 1H NMR (300 MHz, DMSO-d6) δ = 9.23 (s, 1H, OH); 6.03 (d, J = 3.45 Hz, 1H, H-11); 5.65 (d, J = 3.06 Hz, 1H, H-11′); 5.29 (s, 1H, H-14); 5.06 (s, 1H, H-14′); 4.51 (dd, J = 2.87, 10.52 Hz, 1H, H-7); 4.11 (m, 2H, H-17) 3.97 (t, J = 9.18 Hz, H-2); 3.50 (t, J = 4.78 Hz, 2H, H-18); 3.22–3.26 (m, 4H, H-1, H-19); 2.89 (d, J = 8.99 Hz, H-3); 2.28–2.38 (m, 2H, H-9, H-10); 2.08–2.21 (m, 3H, H-6, H-5, H-9′); 1.63–1.73 (m, 2H, H-6′, H-10′); 1.34 (s, 3H, H-15); 1.04 (t, J = 12.63 Hz, 1H, H-5′). 13C NMR (75 MHz, DMSO-d6) δ = 169.83 (Cq, C-13); 156.55 (Cq, C-16); 144.76 (Cq, C-8); 140.24 (Cq, C-12); 119.66 (CH2, C-11); 116.36 (CH2, C-14); 80.16 (CH, C-2); 70.55 (CH2, C-18); 64.96 (CH, C-7); 64.65 (CH2, C-17); 63.34 (CH, C-3); 60.79 (Cq, C-4); 58.46 (CH3, C-19); 43.87 (CH, C-1); 36.06 (CH2, C-5); 29.06 (CH2, C-9); 26.01 (CH2, C-6); 25.06 (CH2, C-10); 18.17 (CH3, C-15). HRMS (TOF, ES+) m/z [M+H]+: calcd. for C19H28NO7 382.1866; found 382.1875.
Tetrahydropyran-4-yl-N-hydroxy-N-[(1S,2S,4R,7R,11S)-4-methyl-8,12-dimethylene-13-oxo-3,14-dioxatricyclo[9.3.0.02,4]tetradecan-7-yl]carbamate (22) was obtained as a white powder after purification by flash chromatography in column 4g, with a gradient from 100% dichloromethane to 70/30 dichloromethane/ethyl acetate over 30 min (52 mg, 63%, purity at 254 nm: 95%). 1H NMR (300 MHz, DMSO-d6) δ = 9.18 (s, 1H, OH); 6.03 (d, J = 3.47 Hz, 1H, H-11); 5.65 (d, J = 3.16 Hz, 1H, H-11′); 5.28 (s, 1H, H-14); 5.05 (s, 1H, H-14′); 4.73 (m, 1H, H-17); 4.52 (dd, J = 3.00, 10.75 Hz, 1H, H-7); 3.97 (t, J = 9.17 Hz, 1H, H-2); 3.79 (m, 2H, H-19); 3.42 (m, 2H, H-20 under water peak); 3.23 (m, 1H, H-1); 2.89 (d, J = 9.01 Hz, H-3); 2.11–2.38 (m, 5H, H-6, H-5, 2xH-9, H-10); 1.84 (m, 2H, H-18); 1.63–1.70 (m, 2H, H-6′, H-10′); 1.52 (m, 2H, H-21); 1.33 (s, 3H, H-15); 1.04 (t, J = 14.57 Hz, 1H, H-5′). 13C NMR (75 MHz, DMSO-d6) δ = 169.83 (Cq, C-13); 155.92 (Cq, C-16); 144.73 (Cq, C-8); 140.23 (Cq, C-12); 119.65 (CH2, C-11); 116.33 (CH2, C-14); 80.10 (CH, C-2); 70.62 (CH, C-17); 65.08 (CH, C-7); 64.99 (2xCH2, C-19, C-20); 63.32, (CH, C-3); 60.81 (Cq, C-4); 43.96 (CH, C-1); 36.05 (CH2, C-5); 32.36 (2xCH2, C-18, C-21); 29.08 (CH2, C-9); 26.08 (CH2, C-6); 24.90 (CH2, C-10); 18.16 (CH3, C-15). HRMS (TOF, ES+) m/z [M+NH4]+: calcd. for C21H33N2O7 425.2288; found 425.2279.