Multiplicative Effects of Essential Oils and Other Active Components on Skin Tissue and Skin Cancers
Abstract
1. Introduction
2. Anti-Inflammatory Role of EOs
2.1. Citrus limetta Peel Essential Oil (Cl-EO)
2.2. Baccharis dracunculifolia Essential Oil (BD-EO)
2.3. Perilla frutescens L. Britt Essential oil (PF-EO)
2.4. Grapefruit Essential Oil (G-EO)
2.5. Oregano Essential Oil (O-EO)
2.6. Satureja sahendica Essential Oil (SS-EO)
2.7. Matricaria chamomilla Essential Oil (MC-EO)
2.8. Helianthus annuus Plant Oil (HA-PO)
2.9. Mentha arvensis Essential Oil (MA-EO)
2.10. Rosmarinus officinalis Essential Oil (RO-EO)
2.11. Curcuma longa Essential Oil (Cl-EO)
2.12. Artemisia argyi Essential Oil (AA-EO)
2.13. Lavender-Essential Oil (L-EO)
2.14. Zanthoxylum coreanum Essential Oil (ZC-EO)
3. Anti-Cancer Effect of EOs
3.1. Aloysia citrodora Essential Oil (AC-EO)
3.2. Origanum majorana Essential Oil (OM-EO)
3.3. Artemisia capillaris Grass Clumps Essential Oil (AC-EO)
3.4. Camellia japonica Seed Essential Oil (CJ-EO)
3.5. Origanum syriacum (OS) and Origanum Ehrenbergii (OE)
3.6. Calocedrus formosana Essential Oil (CF-EO)
3.7. Melaleuca quinquenervia Essential Oil (MQ-EO)
3.8. Cinnamomum cassia Essential Oil (CC-EO)
3.9. Leaf of Alpinia nantoensis Essential Oil (LAN-EO) and Rhizome of Alpinia nantoensis Essential Oil (RAN-EO)
3.10. Chrysanthemum boreale MAKINO Essential Oil (CB-EO)
3.11. Vitex negundo Essential Oil (VN-EO)
3.12. Achillea millefolium Essential Oil (AM-EO)
3.13. Artemisia argyi Essential Oil (AA-EO)
3.14. Vetiveria zizanioides Essential Oil (VZ-EO)
3.15. Eucalyptus camaldulensis Essential Oil (EC-EO)
3.16. Acorus macrospadiceus Essential Oil (AM-EO)
4. Modulation of Skin Proliferation
4.1. Artemisia montana Pampan Essential Oil (AM-EO)
4.2. Pistacia lentiscus L. Plant Oil (PL-PO)
4.3. Lavender-Essential Oil (L-EO)
4.4. Calophyllum inophyllum Plant Oil (CI-PO)
4.5. Parrotiopsis jacquemontiana Plant Oil (PJ-PO)
4.6. Chrysanthemum boreale Makino Essential Oil (CB-EO)
4.7. Salvia aurea L. Essential Oil (SA-EO)
4.8. Rose Plant Oil
4.9. Zanthoxylum bungeanum Maxim Essential Oil (ZB-EO)
4.10. Prunus armeniaca Essential Oil (PA-EO)
5. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Noorpisheh Ghadimi, S.; Sharifi, N.; Osanloo, M. The leishmanicidal activity of essential oils: A systematic review. J. Herbmed Pharmacol. 2020, 9, 300–308. [Google Scholar] [CrossRef]
- Osanloo, M.; Yousefpoor, Y.; Alipanah, H.; Ghanbariasad, A.; Jalilvand, M.; Amani, A. In-vitro Assessment of Essential Oils as Anticancer Therapeutic Agents: A Systematic Literature Review. Jordan J. Pharm. Sci. 2022, 15, 173–203. [Google Scholar] [CrossRef]
- Osanloo, M.; Ghaznavi, G.; Abdollahi, A. Surveying the chemical composition and antibacterial activity of essential oils from selected medicinal plants against human pathogens. Iran. J. Microbiol. 2020, 12, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Bharti, S.; Rani, N.; Krishnamurthy, B.; Arya, D.S. Preclinical evidence for the pharmacological actions of naringin: A review. Planta Med. 2014, 80, 437–451. [Google Scholar] [CrossRef]
- Mohanty, S.; Maurya, A.K.; Jyotshna; Saxena, A.; Shanker, K.; Pal, A.; Bawankule, D.U. Flavonoids rich fraction of Citrus limetta fruit peels reduces proinflammatory cytokine production and attenuates malaria pathogenesis. Curr. Pharm. Biotechnol. 2015, 16, 544–552. [Google Scholar] [CrossRef]
- Kondo, M.; Goto, M.; Kodama, A.; Hirose, T. Fractional extraction by supercritical carbon dioxide for the deterpenation of bergamot oil. Ind. Eng. Chem. Res. 2000, 39, 4745–4748. [Google Scholar] [CrossRef]
- Misharina, T.A.; Samusenko, A.L. Antioxidant properties of essential oils from lemon, grapefruit, coriander, clove, and their mixtures. Appl. Biochem. Microbiol. 2008, 44, 438–442. [Google Scholar] [CrossRef]
- Mitoshi, M.; Kuriyama, I.; Nakayama, H.; Miyazato, H.; Sugimoto, K.; Kobayashi, Y.; Jippo, T.; Kuramochi, K.; Yoshida, H.; Mizushina, Y. Suppression of allergic and inflammatory responses by essential oils derived from herbal plants and citrus fruits. Int. J. Mol. Med. 2014, 33, 1643–1651. [Google Scholar] [CrossRef]
- Maurya, A.K.; Mohanty, S.; Pal, A.; Chanotiya, C.S.; Bawankule, D.U. The essential oil from Citrus limetta Risso peels alleviates skin inflammation: In-vitro and in-vivo study. J. Ethnopharmacol. 2018, 212, 86–94. [Google Scholar] [CrossRef]
- Freires, I.A.; Denny, C.; Benso, B.; de Alencar, S.M.; Rosalen, P.L. Antibacterial Activity of Essential Oils and Their Isolated Constituents against Cariogenic Bacteria: A Systematic Review. Molecules 2015, 20, 7329–7358. [Google Scholar] [CrossRef]
- Trojan-Rodrigues, M.; Alves, T.L.S.; Soares, G.L.G.; Ritter, M.R. Plants used as antidiabetics in popular medicine in Rio Grande do Sul, southern Brazil. J. Ethnopharmacol. 2012, 139, 155–163. [Google Scholar] [CrossRef]
- Aga, H.; Shibuya, T.; Sugimoto, T.; Kurimoto, M.; Nakajima, S. Isolation and Identification of Antimicrobial Compounds in Brazilian Propolis. Biosci. Biotechnol. Biochem. 1994, 58, 945–946. [Google Scholar] [CrossRef]
- da Silva Filho, A.A.; de Sousa, J.P.; Soares, S.; Furtado, N.A.; Andrade e Silva, M.L.; Cunha, W.R.; Gregorio, L.E.; Nanayakkara, N.P.; Bastos, J.K. Antimicrobial activity of the extract and isolated compounds from Baccharis dracunculifolia D. C. (Asteraceae). Z. Naturforsch. C J. Biosci. 2008, 63, 40–46. [Google Scholar] [CrossRef]
- Barbosa, E.V.; Assumpçäo, Y.M.; Teixeira, I.M.; Pereira, R.F.A.; Ribeiro, V.P.; Bastos, J.K.; Cardoso, C.V.; Liberal, M.H.T.; Penna, B.A.; Rocha, L.M. In vitro comparison between antimicrobial and antibiofilm effects of Green Propolis and Baccharis dracunculifolia against Staphylococcus pseudintermedius isolate. Acad. Bras. Cienc. 2022, 94, e20211103. [Google Scholar] [CrossRef]
- dos Santos, D.A.; Fukui Mde, J.; Dhammika Nanayakkara, N.P.; Khan, S.I.; Sousa, J.P.; Bastos, J.K.; Andrade, S.F.; da Silva Filho, A.A.; Quintao, N.L. Anti-inflammatory and antinociceptive effects of Baccharis dracunculifolia DC (Asteraceae) in different experimental models. J. Ethnopharmacol. 2010, 127, 543–550. [Google Scholar] [CrossRef]
- Bachiega, T.F.; de Sousa, J.P.; Bastos, J.K.; Sforcin, J.M. Immunomodulatory/anti-inflammatory effects of Baccharis dracunculifolia leaves. Nat. Prod. Res. 2013, 27, 1646–1650. [Google Scholar] [CrossRef] [PubMed]
- Missima, F.; da Silva Filho, A.A.; Nunes, G.A.; Bueno, P.C.; de Sousa, J.P.; Bastos, J.K.; Sforcin, J.M. Effect of Baccharis dracunculifolia D.C. (Asteraceae) extracts and its isolated compounds on macrophage activation. J. Pharm. Pharmacol. 2007, 59, 463–468. [Google Scholar] [CrossRef]
- Brandenburg, M.M.; Rocha, F.G.; Pawloski, P.L.; Soley, B.D.S.; Rockenbach, A.; Scharf, D.R.; Heiden, G.; Ascari, J.; Cabrini, D.A.; Otuki, M.F. Baccharis dracunculifolia (Asteraceae) essential oil displays anti-inflammatory activity in models of skin inflammation. J. Ethnopharmacol. 2020, 259, 112840. [Google Scholar] [CrossRef] [PubMed]
- Seema Singh, S.S.; Verma, S.K. Biological activities and therapeutic potential of Perilla frutescens (purple mint): A review. Int. J. Pharm. Sci. Res. 2022, 13, 645–653. [Google Scholar] [CrossRef]
- Zi, Y.; Yao, M.; Lu, Z.; Lu, F.; Bie, X.; Zhang, C.; Zhao, H. Glycoglycerolipids from the leaves of Perilla frutescens (L.) Britton (Labiatae) and their anti-inflammatory activities in lipopolysaccharide-stimulated RAW264.7 cells. Phytochemistry 2021, 184, 112679. [Google Scholar] [CrossRef]
- Wang, H.S.; Guo, L.; Liu, L.; Han, B.Q.; Niu, X.D. Composite chitosan films prepared using nisin and Perilla frutescense essential oil and their use to extend strawberry shelf life. Food Biosci. 2021, 41, 101037. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, Y.; Huang, J.; Gu, H.; Li, C.; Zhang, L.; Liu, G.; Zhou, W.; Du, Z. The Essential Oil Derived from Perilla frutescens (L.) Britt. Attenuates Imiquimod-Induced Psoriasis-like Skin Lesions in BALB/c Mice. Molecules 2022, 27, 2996. [Google Scholar] [CrossRef] [PubMed]
- Dillmann, C.; Ringel, C.; Ringleb, J.; Mora, J.; Olesch, C.; Fink, A.F.; Roberts, E.; Brune, B.; Weigert, A. S1PR4 Signaling Attenuates ILT 7 Internalization To Limit IFN-alpha Production by Human Plasmacytoid Dendritic Cells. J. Immunol. 2016, 196, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Arakura, F.; Hida, S.; Ichikawa, E.; Yajima, C.; Nakajima, S.; Saida, T.; Taki, S. Genetic control directed toward spontaneous IFN-alpha/IFN-beta responses and downstream IFN-gamma expression influences the pathogenesis of a murine psoriasis-like skin disease. J. Immunol. 2007, 179, 3249–3257. [Google Scholar] [CrossRef] [PubMed]
- Works, M.G.; Yin, F.; Yin, C.C.; Yiu, Y.; Shew, K.; Tran, T.T.; Dunlap, N.; Lam, J.; Mitchell, T.; Reader, J.; et al. Inhibition of TYK2 and JAK1 ameliorates imiquimod-induced psoriasis-like dermatitis by inhibiting IL-22 and the IL-23/IL-17 axis. J. Immunol. 2014, 193, 3278–3287. [Google Scholar] [CrossRef]
- Ajayi-Moses, O.B.; Ogidi, C.O.; Akinyele, B.J. Bioactivity of Citrus essential oils (CEOs) against microorganisms associated with spoilage of some fruits. Chem. Biol. Technol. Ag. 2019, 6, 22. [Google Scholar] [CrossRef]
- Raspo, M.A.; Vignola, M.B.; Andreatta, A.E.; Juliani, H.R. Antioxidant and antimicrobial activities of citrus essential oils from Argentina and the United States. Food Biosci. 2020, 36, 100651. [Google Scholar] [CrossRef]
- Jing, L.; Lei, Z.; Li, L.; Xie, R.; Xi, W.; Guan, Y.; Sumner, L.W.; Zhou, Z. Antifungal Activity of Citrus Essential Oils. J. Agric. Food Chem. 2014, 62, 3011–3033. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.A.; Jeon, S.K.; Lee, E.J.; Shim, C.H.; Lee, I.S. Comparative study of the chemical composition and antioxidant activity of six essential oils and their components. Nat. Prod. Res. 2010, 24, 140–151. [Google Scholar] [CrossRef]
- Gonzalez-Mas, M.C.; Rambla, J.L.; Lopez-Gresa, M.P.; Blazquez, M.A.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef]
- Njoroge, S.M.; Koaze, H.; Karanja, P.N.; Sawamura, M. Volatile constituents of redblush grapefruit (Citrus paradisi) and pummelo (Citrus grandis) peel essential oils from Kenya. J. Agric. Food Chem. 2005, 53, 9790–9794. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.K.; Mottern, H.H. Florida grapefruit oil. J. Ind. Eng. Chem 1934, 26, 634–637. [Google Scholar] [CrossRef]
- Flamini, G.; Cioni, P.L. Odour gradients and patterns in volatile emission of different plant parts and developing fruits of grapefruit (Citrus paradisi L.). Food Chem. 2010, 120, 984–992. [Google Scholar] [CrossRef]
- Wang, J.; Rong, L.; Jiang, Z.T. Chemical Composition as Identified by GC-MS and Antioxidant Activity of Grapefruit Mint Essential Oil. Food Sci. 2013, 34, 91–94. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Neng, N.R.; Nogueira, J.M.F.; Saraiva, J.A.; Nunes, M.L. Chemical composition and antibacterial and antioxidant properties of commercial essential oils. Ind. Crop. Prod. 2013, 43, 587–595. [Google Scholar] [CrossRef]
- Ahmed, S.; Rattanpal, H.S.; Gul, K.; Dar, R.A.; Sharma, A. Chemical composition, antioxidant activity and GC-MS analysis of juice and peel oil of grapefruit varieties cultivated in India. J. Integr. Agric. 2019, 18, 1634–1642. [Google Scholar] [CrossRef]
- Sun, J.; Chu, Y.F.; Wu, X.; Liu, R.H. Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 2002, 50, 7449–7454. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, D.; Bosco, L.; Moschetti, M.; Tinnirello, V.; Pucci, M.; Corleone, V.; Raimondo, S.; Alessandro, R.; Fontana, S. Anti-inflammatory properties of an aldehydes-enriched fraction of grapefruit essential oil. J. Food Sci. 2023, 88, 1172–1187. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, W.; Jiménez-Belenguer, A.; Settanni, L.; Perdones, A.; Moschetti, M.; Palazzolo, E.; Guarrasi, V.; Vargas, M.; Germanà, M.A.; Moschetti, G. Antilisterial effect of citrus essential oils and their performance in edible film formulations. Food Control 2016, 59, 750–758. [Google Scholar] [CrossRef]
- Negi, P.; Jayaprakasha, G. Antibacterial activity of grapefruit (Citrus paradisi) peel extracts. Eur. Food Res. Technol. 2001, 213, 484–487. [Google Scholar]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Perez-Álvarez, J. Antibacterial activity of lemon (Citrus lemon L.), mandarin (Citrus reticulata L.), grapefruit (Citrus paradisi L.) and orange (Citrus sinensis L.) essential oils. J. Food Saf. 2008, 28, 567–576. [Google Scholar] [CrossRef]
- Uysal, B.; Sozmen, F.; Aktas, O.; Oksal, B.S.; Kose, E.O. Essential oil composition and antibacterial activity of the grapefruit (Citrus paradisi L.) peel essential oils obtained by solvent-free microwave extraction: Comparison with hydrodistillation. Int. J. Food Sci. Technol. 2011, 46, 1455–1461. [Google Scholar] [CrossRef]
- Okunowo, W.O.; Oyedeji, O.; Afolabi, L.O.; Matanmi, E. Essential Oil of Grape Fruit (Citrus paradisi) Peels and Its Antimicrobial Activities. Am. J. Plant Sci. 2013, 4, 1–9. [Google Scholar] [CrossRef]
- Deng, W.; Liu, K.; Cao, S.; Sun, J.; Zhong, B.; Chun, J. Chemical Composition, Antimicrobial, Antioxidant, and Antiproliferative Properties of Grapefruit Essential Oil Prepared by Molecular Distillation. Molecules 2020, 25, 217. [Google Scholar] [CrossRef] [PubMed]
- Stanly, C.; Alfieri, M.; Ambrosone, A.; Leone, A.; Fiume, I.; Pocsfalvi, G. Grapefruit-Derived Micro and Nanovesicles Show Distinct Metabolome Profiles and Anticancer Activities in the A375 Human Melanoma Cell Line. Cells 2020, 9, 2722. [Google Scholar] [CrossRef] [PubMed]
- Diab, K.A. In Vitro Studies on Phytochemical Content, Antioxidant, Anticancer, Immunomodulatory, and Antigenotoxic Activities of Lemon, Grapefruit, and Mandarin Citrus Peels. Asian Pac. J. Cancer Prev. 2016, 17, 3559–3567. [Google Scholar] [PubMed]
- Kawai, E.; Takeda, R.; Ota, A.; Morita, E.; Imai, D.; Suzuki, Y.; Yokoyama, H.; Ueda, S.; Nakahara, H.; Miyamoto, T.; et al. Increase in diastolic blood pressure induced by fragrance inhalation of grapefruit essential oil is positively correlated with muscle sympathetic nerve activity. J. Physiol. Sci. 2020, 70, 2. [Google Scholar] [CrossRef]
- Denkova-Kostova, R.; Teneva, D.; Tomova, T.; Goranov, B.; Denkova, Z.; Shopska, V.; Slavchev, A.; Hristova-Ivanova, Y. Chemical composition, antioxidant and antimicrobial activity of essential oils from tangerine (Citrus reticulata L.), grapefruit (Citrus paradisi L.), lemon (Citrus lemon L.) and cinnamon (Cinnamomum zeylanicum Blume). Z. Naturforsch. C J. Biosci. 2021, 76, 175–185. [Google Scholar] [CrossRef]
- d’alessio, P.A.; Mirshahi, M.; Bisson, J.F.; Bene, M.C. Skin repair properties of d-Limonene and perillyl alcohol in murine models. Antiinflamm. Antiallergy Agents Med. Chem. 2014, 13, 29–35. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Hua, J.; Zhu, Z.; Wang, M. Protective Effects of Grapefruit Essential Oil against Staphylococcus aureus-Induced Inflammation and Cell Damage in Human Epidermal Keratinocytes. Chem. Biodivers. 2022, 19, e202200205. [Google Scholar] [CrossRef]
- Avola, R.; Granata, G.; Geraci, C.; Napoli, E.; Graziano, A.C.E.; Cardile, V. Oregano (Origanum vulgare L.) essential oil provides anti-inflammatory activity and facilitates wound healing in a human keratinocytes cell model. Food Chem. Toxicol. 2020, 144, 111586. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.S.; Vattem, D.A.; Lin, Y.T.; Shetty, K. Phenolic antioxidants from clonal oregano (Origanum vulgare) with antimicrobial activity against Helicobacter pylori. Process Biochem. 2005, 40, 809–816. [Google Scholar] [CrossRef]
- Wojdylo, A.; Oszmianski, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Ocana-Fuentes, A.; Arranz-Gutierrez, E.; Senorans, F.J.; Reglero, G. Supercritical fluid extraction of oregano (Origanum vulgare) essentials oils: Anti-inflammatory properties based on cytokine response on THP-1 macrophages. Food Chem. Toxicol. 2010, 48, 1568–1575. [Google Scholar] [CrossRef]
- Sahin, F.; Güllüce, M.; Daferera, D.; Sökmen, A.; Sökmen, M.; Polissiou, M.; Agar, G.; Özer, H. Biological activities of the essential oils and methanol extract of Origanum vulgare ssp vulgare in the Eastern Anatolia region of Turkey. Food Control 2004, 15, 549–557. [Google Scholar] [CrossRef]
- Granata, G.; Stracquadanio, S.; Leonardi, M.; Napoli, E.; Consoli, G.M.L.; Cafiso, V.; Stefani, S.; Geraci, C. Essential oils encapsulated in polymer-based nanocapsules as potential candidates for application in food preservation. Food Chem. 2018, 269, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Menichini, F.; Conforti, F.; Tundis, R.; Bonesi, M.; Saab, A.M.; Statti, G.A.; Cindio, B.d.; Houghton, P.J.; Menichini, F.; et al. Chemical analysis, antioxidant, antiinflammatory and anticholinesterase activities of Origanum ehrenbergii Boiss and Origanum syriacum L. essential oils. Food Chem. 2009, 117, 174–180. [Google Scholar] [CrossRef]
- Bukovska, A.; Cikos, S.; Juhas, S.; Il’kova, G.; Rehak, P.; Koppel, J. Effects of a combination of thyme and oregano essential oils on TNBS-induced colitis in mice. Mediat. Inflamm. 2007, 2007, 23296. [Google Scholar] [CrossRef] [PubMed]
- Omarizadeh, K.; Farahpour, M.R.; Alipour, M. Topical Administration of an Ointment Prepared From Satureja sahendica Essential Oil Accelerated Infected Full-Thickness Wound Healing by Modulating Inflammatory Response in a Mouse Model. Wounds 2021, 33, 321–328. [Google Scholar] [CrossRef]
- Sefidkon, F.; Jamzad, Z.; Mirza, M. Chemical variation in the essential oil of Satureja sahendica from Iran. Food Chem. 2004, 88, 325–328. [Google Scholar] [CrossRef]
- Eftekhar, F.; Raei, F.; Yousefzadi, M.; Ebrahimi, S.N.; Hadian, J. Antibacterial activity and essential oil composition of Satureja spicigera from Iran. Z. Naturforsch. C J. Biosci. 2009, 64, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfari, G.; Minaie, B.; Yasa, N.; Nakhai, L.A.; Mohammadirad, A.; Nikfar, S.; Dehghan, G.; Boushehri, V.S.; Jamshidi, H.; Khorasani, R.; et al. Biochemical and histopathological evidences for beneficial effects of Satureja khuzestanica jamzad essential oil on the mouse model of inflammatory bowel diseases. Toxicol. Mech. Methods 2006, 16, 365–372. [Google Scholar] [CrossRef]
- Hadian, N.; Rezaei, M.; Mosayebi, Z.; Meshkani, F. CO2 reforming of methane over nickel catalysts supported on nanocrystalline MgAl2O4 with high surface area. J. Nat. Gas Chem. 2012, 21, 200–206. [Google Scholar] [CrossRef]
- Hajilou, H.; Farahpour, M.R.; Hamishehkar, H. Polycaprolactone nanofiber coated with chitosan and functionalized as a novel wound dressing for healing infected wounds. Int. J. Biol. Macromol. 2020, 164, 2358–2369. [Google Scholar] [CrossRef] [PubMed]
- Pakyari, M.; Farrokhi, A.; Maharlooei, M.K.; Ghahary, A. Critical Role of Transforming Growth Factor Beta in Different Phases of Wound Healing. Adv. Wound Care 2013, 2, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Haleagrahara, N.; Chakravarthi, S.; Mathews, L. Insulin like growth factor-1 (IGF-1) causes overproduction of IL-8, an angiogenic cytokine and stimulates neovascularization in isoproterenol-induced myocardial infarction in rats. Int. J. Mol. Sci. 2011, 12, 8562–8574. [Google Scholar] [CrossRef]
- Metcalfe, A.D.; Ferguson, M.W. Tissue engineering of replacement skin: The crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J. R. Soc. Interface 2007, 4, 413–437. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.T.; Lauritzen, H.P.; Hirshman, M.F.; Smyth, G.; Goodyear, L.J.; Kahn, C.R. Differential Role of Insulin/IGF-1 Receptor Signaling in Muscle Growth and Glucose Homeostasis. Cell Rep. 2015, 11, 1220–1235. [Google Scholar] [CrossRef]
- Ramirez, H.; Patel, S.B.; Pastar, I. The Role of TGFbeta Signaling in Wound Epithelialization. Adv. Wound Care 2014, 3, 482–491. [Google Scholar] [CrossRef]
- Chen, G.; Lv, C.; Nie, Q.; Li, X.; Lv, Y.; Liao, G.; Liu, S.; Ge, W.; Chen, J.; Du, Y. Essential Oil of Matricaria chamomilla Alleviate Psoriatic-Like Skin Inflammation by Inhibiting PI3K/Akt/mTOR and p38MAPK Signaling Pathway. Clin. Cosmet. Investig. Dermatol. 2024, 17, 59–77. [Google Scholar] [CrossRef]
- Kroll, U.; Cordes, C. Pharmaceutical prerequisites for a multi-target therapy. Phytomedicine 2006, 13 (Suppl. 5), 12–19. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, P.; Ercolano, G.; Sirignano, C.; Rubino, V.; Rigano, D.; Ianaro, A.; Formisano, C. Chamomile essential oils exert anti-inflammatory effects involving human and murine macrophages: Evidence to support a therapeutic action. J. Ethnopharmacol. 2023, 311, 116391. [Google Scholar] [CrossRef] [PubMed]
- Forster, H.B.; Niklas, H.; Lutz, S. Antispasmodic effects of some medicinal plants. Planta Med. 1980, 40, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.; Zou, J.; Jia, Y.; Wang, Y.; Li, J.; Wang, C.; Sun, J.; Guo, D.; Wang, F.; et al. The Mechanism Action of German Chamomile (Matricaria recutita L.) in the Treatment of Eczema: Based on Dose-Effect Weight Coefficient Network Pharmacology. Front. Pharmacol. 2021, 12, 706836. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Heo, Y.; Kim, Y.C. Effect of German chamomile oil application on alleviating atopic dermatitis-like immune alterations in mice. J. Vet. Sci. 2010, 11, 35–41. [Google Scholar] [CrossRef]
- Fecker, R.; Magyari-Pavel, I.Z.; Cocan, I.; Alexa, E.; Popescu, I.M.; Lombrea, A.; Bora, L.; Dehelean, C.A.; Buda, V.; Folescu, R.; et al. Oxidative Stability and Protective Effect of the Mixture between Helianthus annuus L. and Oenothera biennis L. Oils on 3D Tissue Models of Skin Irritation and Phototoxicity. Plants 2022, 11, 2977. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Lei, L.; Zeng, Q.; Yao, Y.; Wu, Y.; Li, Q.; Gao, L.; Du, H.; Xie, Y.; Huang, J.; et al. Ozone Therapy Attenuates NF-kappaB-Mediated Local Inflammatory Response and Activation of Th17 Cells in Treatment for Psoriasis. Int. J. Biol. Sci. 2020, 16, 1833–1845. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, J.O.; Lee, S.; Heo, J.; Cho, K.H.; Bahuguna, A.; Yoo, K.H.; Kim, B.J. Ozonated Sunflower Oil (OSO) Alleviates Inflammatory Responses in Oxazolone-Induced Atopic Dermatitis (AD)-Like Mice and LPS-Treated RAW 264.7 Cells. J. Microbiol. Biotechnol. 2024, 34, 765–773. [Google Scholar] [CrossRef]
- Kim, S.Y.; Han, S.D.; Kim, M.; Mony, T.J.; Lee, E.S.; Kim, K.M.; Choi, S.H.; Hong, S.H.; Choi, J.W.; Park, S.J. Mentha arvensis Essential Oil Exerts Anti-Inflammatory in LPS-Stimulated Inflammatory Responses via Inhibition of ERK/NF-kappaB Signaling Pathway and Anti-Atopic Dermatitis-like Effects in 2,4-Dinitrochlorobezene-Induced BALB/c Mice. Antioxidants 2021, 10, 1941. [Google Scholar] [CrossRef]
- Salehi, B.; Stojanovic-Radic, Z.; Matejic, J.; Sharopov, F.; Antolak, H.; Kregiel, D.; Sen, S.; Sharifi-Rad, M.; Acharya, K.; Sharifi-Rad, R.; et al. Plants of Genus Mentha: From Farm to Food Factory. Plants 2018, 7, 70. [Google Scholar] [CrossRef]
- Dong-Hun Choi, O.-T.S.; Lim, M.-H. Comparative study of the biological activities effect of Mentha arvensis L. extracts from water and 80% ethanol. J. Korean Appl. Sci. Technol. 2019, 36, 208–216. [Google Scholar] [CrossRef]
- Kim, S.Y.; Sapkota, A.; Bae, Y.J.; Choi, S.H.; Bae, H.J.; Kim, H.J.; Cho, Y.E.; Choi, Y.Y.; An, J.Y.; Cho, S.Y.; et al. The Anti-Atopic Dermatitis Effects of Mentha arvensis Essential Oil Are Involved in the Inhibition of the NLRP3 Inflammasome in DNCB-Challenged Atopic Dermatitis BALB/c Mice. Int. J. Mol. Sci. 2023, 24, 7720. [Google Scholar] [CrossRef] [PubMed]
- Li, J.K.; Duan, J.W.; Wang, Y.J.; Zhou, P.J.; Wang, X.; Xia, N.; Wang, J.; Li, J.; Wang, W.F.; Wang, X.; et al. The JAK/STAT/NF-κB signaling pathway can be regulated by rosemary essential oil, thereby providing a potential treatment for DNCB-induced in mice. Biomed. Pharmacother. 2023, 168, 115727. [Google Scholar] [CrossRef]
- Jamila, F.; Mostafa, E. Ethnobotanical survey of medicinal plants used by people in Oriental Morocco to manage various ailments. J. Ethnopharmacol. 2014, 154, 76–87. [Google Scholar] [CrossRef]
- Takaki, I.; Bersani-Amado, L.E.; Vendruscolo, A.; Sartoretto, S.M.; Diniz, S.P.; Bersani-Amado, C.A.; Cuman, R.K. Anti-inflammatory and antinociceptive effects of Rosmarinus officinalis L. essential oil in experimental animal models. J. Med. Food 2008, 11, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Sanjay Jain, S.S.; Satish Nayak, S. Sumbhate. Plant Review Recent trends in Curcuma Longa Linn. Pharmacogn. Rev. 2007, 1, 119–128. [Google Scholar]
- Srivilai, J.; Waranuch, N.; Tangsumranjit, A.; Khorana, N.; Ingkaninan, K. Germacrone and sesquiterpene-enriched extracts from Curcuma aeruginosa Roxb. increase skin penetration of minoxidil, a hair growth promoter. Drug Deliv. Transl. Res. 2018, 8, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Agarwal, K.; Singh, M.; Saxena, A.; Yadav, P.; Maurya, A.K.; Yadav, A.; Tandon, S.; Chanda, D.; Bawankule, D.U. Essential oil from waste leaves of Curcuma longa L. alleviates skin inflammation. Inflammopharmacology 2018, 26, 1245–1255. [Google Scholar] [CrossRef]
- Chen, L.L.; Zhang, H.J.; Chao, J.; Liu, J.F. Essential oil of Artemisia argyi suppresses inflammatory responses by inhibiting JAK/STATs activation. J. Ethnopharmacol. 2017, 204, 107–117. [Google Scholar] [CrossRef]
- Huang, H.C.; Wang, H.F.; Yih, K.H.; Chang, L.Z.; Chang, T.M. Dual Bioactivities of Essential Oil Extracted from the Leaves of Artemisia argyi as an Antimelanogenic versus Antioxidant Agent and Chemical Composition Analysis by GC/MS. Int. J. Mol. Sci. 2012, 13, 14679–14697. [Google Scholar] [CrossRef] [PubMed]
- Nakasugi, T.; Nakashima, M.; Komai, K. Antimutagens in gaiyou (Artemisia argyi levl. et vant.). J. Agric. Food Chem. 2000, 48, 3256–3266. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.; Efferth, T.; Bauer, R. Activity-guided isolation of scopoletin and isoscopoletin, the inhibitory active principles towards CCRF-CEM leukaemia cells and multi-drug resistant CEM/ADR5000 cells, from Artemisia argyi. Planta Med. 2006, 72, 862–864. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.A.; Lee, K.W.; Yoon, D.Y.; Lee, H.J. Jaceosidin, a pharmacologically active flavone derived from Artemisia argyi, inhibits phorbol-ester-induced upregulation of COX-2 and MMP-9 by blocking phosphorylation of ERK-1 and -2 in cultured human mammary epithelial cells. Ann. N. Y. Acad. Sci. 2007, 1095, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.M.; Kang, H.M.; Son, K.H.; Kim, J.H.; Lee, C.W.; Kim, H.M.; Chang, S.I.; Kwon, B.M. Antitumor activity of flavones isolated from Artemisia argyi. Planta Med. 2003, 69, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Yuan, H.; Wang, C.; Liu, J.; Lan, M. Antitumor and immunomodulatory activities of a polysaccharide from Artemisia argyi. Carbohydr. Polym. 2013, 98, 1236–1243. [Google Scholar] [CrossRef]
- Ge, Y.B.; Wang, Z.G.; Xiong, Y.; Huang, X.J.; Mei, Z.N.; Hong, Z.G. Anti-inflammatory and blood stasis activities of essential oil extracted from Artemisia argyi leaf in animals. J. Nat. Med. 2016, 70, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Bian, R.L. Pharmacological studies on the antiasthmatic principle of essential oil of Artemisia argyi--terpinenol-4 (author’s transl). Zhonghua Jie He He Hu Xi Xi Ji Bing Za Zhi 1981, 4, 203–206. [Google Scholar] [PubMed]
- Wenqiang, G.; Shufen, L.; Ruixiang, Y.; Yanfeng, H. Comparison of composition and antifungal activity of Artemisia argyi Levl. et Vant inflorescence essential oil extracted by hydrodistillation and supercritical carbon dioxide. Nat. Prod. Res. 2006, 20, 992–998. [Google Scholar] [CrossRef]
- Cavanagh, H.M.; Wilkinson, J.M. Biological activities of lavender essential oil. Phytother. Res. 2002, 16, 301–308. [Google Scholar] [CrossRef]
- Chioca, L.R.; Ferro, M.M.; Baretta, I.P.; Oliveira, S.M.; Silva, C.R.; Ferreira, J.; Losso, E.M.; Andreatini, R. Anxiolytic-like effect of lavender essential oil inhalation in mice: Participation of serotonergic but not GABAA/benzodiazepine neurotransmission. J. Ethnopharmacol. 2013, 147, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, K.; Lu, C.; Dong, L.; Gao, L.; Yan, M.; Aibai, S.; Liu, X. Protective effect of lavender oil on scopolamine induced cognitive deficits in mice and H(2)O(2) induced cytotoxicity in PC12 cells. J. Ethnopharmacol. 2016, 193, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.L.; Luft, C.; Lunardelli, A.; Amaral, R.H.; Melo, D.A.; Donadio, M.V.; Nunes, F.B.; de Azambuja, M.S.; Santana, J.C.; Moraes, C.M.; et al. Antioxidant, analgesic and anti-inflammatory effects of lavender essential oil. Acad. Bras. Cienc. 2015, 87, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Cardia, G.F.E.; Silva-Filho, S.E.; Silva, E.L.; Uchida, N.S.; Cavalcante, H.A.O.; Cassarotti, L.L.; Salvadego, V.E.C.; Spironello, R.A.; Bersani-Amado, C.A.; Cuman, R.K.N. Effect of Lavender (Lavandula angustifolia) Essential Oil on Acute Inflammatory Response. Evid. Based Complement. Altern. Med. 2018, 2018, 1413940. [Google Scholar] [CrossRef] [PubMed]
- Adaszynska-Skwirzynska, M.; Szczerbinska, D. The antimicrobial activity of lavender essential oil (Lavandula angustifolia) and its influence on the production performance of broiler chickens. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- de Rapper, S.; Kamatou, G.; Viljoen, A.; van Vuuren, S. The In Vitro Antimicrobial Activity of Lavandula angustifolia Essential Oil in Combination with Other Aroma-Therapeutic Oils. Evid. Based Complement. Altern. Med. 2013, 2013, 852049. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.M.; Kawanami, H.; Kawahata, H.; Aoki, M. Wound healing potential of lavender oil by acceleration of granulation and wound contraction through induction of TGF-beta in a rat model. BMC Complement. Altern. Med. 2016, 16, 144. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, A.; Mahmodi, M.A.; Nobakht, Z. Effect of aromatherapy massage with lavender essential oil on pain in patients with osteoarthritis of the knee: A randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2016, 25, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.K.; Sinha, P.; Yadav, K.S.; Shukla, A.; Saxena, A.; Bawankule, D.U.; Tandon, S.; Khan, F.; Chanotiya, C.S.; Yadav, N.P. Anti-psoriatic effect of Lavandula angustifolia essential oil and its major components linalool and linalyl acetate. J. Ethnopharmacol. 2020, 261, 113127. [Google Scholar] [CrossRef]
- Peana, A.T.; D’Aquila, P.S.; Panin, F.; Serra, G.; Pippia, P.; Moretti, M.D. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine 2002, 9, 721–726. [Google Scholar] [CrossRef]
- Shibata, C.; Sasaki, I.; Naito, H.; Ueno, T.; Matsuno, S. The herbal medicine Dai-Kenchu-Tou stimulates upper gut motility through cholinergic and 5-hydroxytryptamine 3 receptors in conscious dogs. Surgery 1999, 126, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.G.; Chang, C.S.; Chae, Y.A. Variation of volatile composition in the leaf of Zanthoxylum schinofolium siebold et zucc. and Zanthoxylum piperitum DC. Korean J. Med. Crop. Sci. 2002, 10, 162–166. [Google Scholar]
- Choi, H.J. Evaluation of Antiviral Activity of Zanthoxylum Species Against Picornaviruses. Osong. Public Health Res. Perspect. 2016, 7, 400–403. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.H.; Park, J.U.; Jo, S.J.; Ahn, J.H.; Park, J.H.; Yang, J.Y.; Lee, S.S.; Park, M.J.; Kim, Y.R. Anti-allergic Inflammatory Effects of the Essential Oil From Fruits of Zanthoxylum coreanum Nakai. Front. Pharmacol. 2018, 9, 1441. [Google Scholar] [CrossRef] [PubMed]
- Gautam, N.; Mantha, A.K.; Mittal, S. Essential Oils and Their Constituents as Anticancer Agents: A Mechanistic View. Biomed Res. Int. 2014, 2014, 154106. [Google Scholar] [CrossRef] [PubMed]
- Tayarani-Najaran, Z.; Akaberi, M.; Vatani, M.; Emami, S.A. Evaluation of antioxidant and anti-melanogenic activities of different extracts from aerial parts of Nepeta binaludensis Jamzad in murine melanoma B16F10 cells. Iran. J. Basic Med. Sci. 2016, 19, 662–669. [Google Scholar]
- Videira, I.F.; Moura, D.F.; Magina, S. Mechanisms regulating melanogenesis. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef]
- Kim, M.J.; Mohamed, E.A.; Kim, D.S.; Park, M.J.; Ahn, B.J.; Jeung, E.B.; An, B.S. Inhibitory effects and underlying mechanisms of Artemisia capillaris essential oil on melanogenesis in the B16F10 cell line. Mol. Med. Rep. 2022, 25, 1–12. [Google Scholar] [CrossRef]
- Munoz-Munoz, J.L.; Garcia-Molina, F.; Varon, R.; Tudela, J.; Garcia-Canovas, F.; Rodriguez-Lopez, J.N. Generation of hydrogen peroxide in the melanin biosynthesis pathway. Biochim. Biophys. Acta 2009, 1794, 1017–1029. [Google Scholar] [CrossRef]
- Perluigi, M.; De Marco, F.; Foppoli, C.; Coccia, R.; Blarzino, C.; Marcante, M.L.; Cini, C. Tyrosinase protects human melanocytes from ROS-generating compounds. Biochem. Biophys. Res. Commun. 2003, 305, 250–256. [Google Scholar] [CrossRef]
- Liu, G.S.; Peshavariya, H.; Higuchi, M.; Brewer, A.C.; Chang, C.W.; Chan, E.C.; Dusting, G.J. Microphthalmia-associated transcription factor modulates expression of NADPH oxidase type 4: A negative regulator of melanogenesis. Free Radic. Biol. Med. 2012, 52, 1835–1843. [Google Scholar] [CrossRef]
- Yasui, H.; Sakurai, H. Age-dependent generation of reactive oxygen species in the skin of live hairless rats exposed to UVA light. Exp. Dermatol. 2003, 12, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Funasaka, Y.; Komoto, M.; Ichihashi, M. Depigmenting effect of alpha-tocopheryl ferulate on normal human melanocytes. Pigment. Cell Res. 2000, 13 (Suppl. 8), 170–174. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Rostamiasrabadi, P.; Shahpiri, Z.; Marques, A.M.; Rahimi, R.; Farzaei, M.H. Aloysia citrodora Paláu (Lemon verbena): A review of phytochemistry and pharmacology. J. Ethnopharmacol. 2018, 222, 34–51. [Google Scholar] [CrossRef]
- Spyridopoulou, K.; Aravidou, T.; Lampri, E.; Effraimidou, E.; Pappa, A.; Chlichlia, K. Antitumor Potential of Lippia citriodora Essential Oil in Breast Tumor-Bearing Mice. Antioxidants 2021, 10, 875. [Google Scholar] [CrossRef]
- Karimivaselabadi, A.; Osanloo, M.; Ghanbariasad, A.; Zarenezhad, E.; Hosseini, H. Comparison of chitosan nanoparticles containing Lippia citriodora essential oil and citral on the induction of apoptosis in A375 melanoma cells. BMC Complement. Med. Ther. 2023, 23, 435. [Google Scholar] [CrossRef] [PubMed]
- Najar, B.; Shortrede, J.E.; Pistelli, L.; Buhagiar, J. Chemical Composition and in Vitro Cytotoxic Screening of Sixteen Commercial Essential Oils on Five Cancer Cell Lines. Chem. Biodivers. 2020, 17, e1900478. [Google Scholar] [CrossRef]
- Zeng, S.; Kapur, A.; Patankar, M.S.; Xiong, M.P. Formulation, Characterization, and Antitumor Properties of Trans- and Cis-Citral in the 4T1 Breast Cancer Xenograft Mouse Model. Pharm. Res. 2015, 32, 2548–2558. [Google Scholar] [CrossRef] [PubMed]
- Oukerrou, M.A.; Tilaoui, M.; Mouse, H.A.; Bouchmaa, N.; Zyad, A. Differential Cytotoxic Activity of Essential Oil of Lippia citriodora from Different Regions in Morocco. Chem. Biodivers. 2017, 14, 1–11. [Google Scholar] [CrossRef]
- Fitsiou, E.; Mitropoulou, G.; Spyridopoulou, K.; Vamvakias, M.; Bardouki, H.; Galanis, A.; Chlichlia, K.; Kourkoutas, Y.; Panayiotidis, M.; Pappa, A. Chemical Composition and Evaluation of the Biological Properties of the Essential Oil of the Dietary Phytochemical Lippia citriodora. Molecules 2018, 23, 123. [Google Scholar] [CrossRef]
- Lertsatitthanakorn, P.; Taweechaisupapong, S.; Aromdee, C.; Khunkitti, W. In vitro bioactivities of essential oils used for acne control. Int. J. Aromather. 2006, 16, 43–49. [Google Scholar] [CrossRef]
- Farahmandfar, R.; Asnaashari, M.; Pourshayegan, M.; Maghsoudi, S.; Moniri, H. Evaluation of antioxidant properties of lemon verbena (Lippia citriodora) essential oil and its capacity in sunflower oil stabilization during storage time. Food Sci. Nutr. 2018, 6, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Salama, Y.; Jaradat, N.; Hattori, K.; Heissig, B. Aloysia Citrodora Essential Oil Inhibits Melanoma Cell Growth and Migration by Targeting HB-EGF-EGFR Signaling. Int. J. Mol. Sci. 2021, 22, 8151. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Kawakami, T.; Watabe, H.; Itoh, F.; Mizoguchi, M.; Soma, Y. Expression of matrilysin (matrix metalloproteinase-7) in primary cutaneous and metastatic melanoma. Br. J. Dermatol. 2007, 156, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Zigrino, P.; Nischt, R.; Mauch, C. The Disintegrin-like and Cysteine-rich domains of ADAM-9 Mediate Interactions between Melanoma Cells and Fibroblasts. J. Biol. Chem. 2011, 286, 6801–6807. [Google Scholar] [CrossRef] [PubMed]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Heissig, B.; Hattori, K.; Friedrich, M.; Rafii, S.; Werb, Z. Angiogenesis: Vascular remodeling of the extracellular matrix involves metalloproteinases. Curr. Opin. Hematol. 2003, 10, 136–141. [Google Scholar] [CrossRef]
- Villanueva, J.; Herlyn, M. Melanoma and the tumor microenvironment. Curr. Oncol. Rep. 2008, 10, 439–446. [Google Scholar] [CrossRef]
- Winkler, J.; Abisoye-Ogunniyan, A.; Metcalf, K.J.; Werb, Z. Concepts of extracellular matrix remodelling in tumour progression and metastasis. Nat. Commun. 2020, 11, 5120. [Google Scholar] [CrossRef]
- Hudson, L.G.; Moss, N.M.; Stack, M.S. EGF-receptor regulation of matrix metalloproteinases in epithelial ovarian carcinoma. Future Oncol. 2009, 5, 323–338. [Google Scholar] [CrossRef]
- Mader, C.C.; Oser, M.; Magalhaes, M.A.; Bravo-Cordero, J.J.; Condeelis, J.; Koleske, A.J.; Gil-Henn, H. An EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Cancer Res. 2011, 71, 1730–1741. [Google Scholar] [CrossRef] [PubMed]
- Osanloo, M.; Alipanah, H.; Farjam, M.; Taheri, A.; Zarenezhad, E. Anticancer activity of Chitosan nanoparticles containing Satureja khuzistanica essential oil, and carvacrol against human Melanoma and Breast Cancer. Russ. J. Bioorg. Chem. 2023, 49, 594–601. [Google Scholar] [CrossRef]
- Alipanah, H.; Farjam, M.; Zarenezhad, E.; Roozitalab, G.; Osanloo, M. Chitosan nanoparticles containing limonene and limonene-rich essential oils: Potential phytotherapy agents for the treatment of melanoma and breast cancers. BMC Complement. Med. Ther. 2021, 21, 186. [Google Scholar] [CrossRef] [PubMed]
- Qasemi, H.; Fereidouni, Z.; Karimi, J.; Abdollahi, A.; Zarenezhad, E.; Rasti, F.; Osanloo, M. Promising antibacterial effect of impregnated nanofiber mats with a green nanogel against clinical and standard strains of Pseudomonas aeruginosa and Staphylococcus aureus. J. Drug Deliv. Sci. Technol. 2021, 66, 102844. [Google Scholar] [CrossRef]
- Rasti, F.; Yousefpoor, Y.; Abdollahi, A.; Safari, M.; Roozitalab, G.; Osanloo, M. Antioxidative, anticancer, and antibacterial activities of a nanogel containing Mentha spicata L. essential oil and electrospun nanofibers of polycaprolactone-hydroxypropyl methylcellulose. BMC Complement. Med. Ther. 2022, 22, 261. [Google Scholar] [CrossRef]
- Valizadeh, A.; Khaleghi, A.A.; Alipanah, H.; Zarenezhad, E.; Osanloo, M. Anticarcinogenic Effect of Chitosan Nanoparticles Containing Syzygium aromaticum Essential Oil or Eugenol Toward Breast and Skin Cancer Cell Lines. Bionanoscience 2021, 11, 678–686. [Google Scholar] [CrossRef]
- Rajivgandhi, G.; Saravanan, K.; Ramachandran, G.; Li, J.L.; Yin, L.Z.; Quero, F.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Manoharan, N.; et al. Enhanced anti-cancer activity of chitosan loaded Morinda citrifolia essential oil against A549 human lung cancer cells. Int. J. Biol. Macromol. 2020, 164, 4010–4021. [Google Scholar] [CrossRef]
- Hesami, S.; Safi, S.; Larijani, K.; Badi, H.N.; Abdossi, V.; Hadidi, M. Synthesis and characterization of chitosan nanoparticles loaded with greater celandine (Chelidonium majus L.) essential oil as an anticancer agent on MCF-7 cell line. Int. J. Biol. Macromol. 2022, 194, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Rasti, F.; Ahmadi, E.; Safari, M.; Abdollahi, A.; Satvati, S.; Ranjbar, R.; Osanloo, M. Anticancer, antioxidant, and antibacterial effects of nanoemulsion of Origanum majorana essential oil. Iran. J. Microbiol. 2023, 15, 565–573. [Google Scholar] [CrossRef]
- Prakash, B.; Singh, P.; Kedia, A.; Dubey, N.K. Assessment of some essential oils as food preservatives based on antifungal, antiaflatoxin, antioxidant activities and in vivo efficacy in food system. Food Res. Int. 2012, 49, 201–208. [Google Scholar] [CrossRef]
- Maleki, H.; Azadi, H.; Yousefpoor, Y.; Doostan, M.; Doostan, M.; Farzaei, M.H. Encapsulation of Ginger Extract in Nanoemulsions: Preparation, Characterization and in vivo Evaluation in Rheumatoid Arthritis. J. Pharm. Sci. 2023, 112, 1687–1697. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Alam, A.; Ansari, M.J.; Alqarni, M.H.; Salkini, M.A.; Raish, M. Antioxidant, Antibacterial, and Anticancer Activity of Ultrasonic Nanoemulsion of Cinnamomum cassia L. Essential Oil. Plants 2023, 12, 834. [Google Scholar] [CrossRef]
- Panyajai, P.; Chueahongthong, F.; Viriyaadhammaa, N.; Nirachonkul, W.; Tima, S.; Chiampanichayakul, S.; Anuchapreeda, S.; Okonogi, S. Anticancer activity of Zingiber ottensii essential oil and its nanoformulations. PLoS ONE 2022, 17, e0262335. [Google Scholar] [CrossRef]
- Navaei Shoorvarzi, S.; Shahraki, F.; Shafaei, N.; Karimi, E.; Oskoueian, E. Citrus aurantium L. bloom essential oil nanoemulsion: Synthesis, characterization, cytotoxicity, and its potential health impacts on mice. J. Food Biochem. 2020, 44, e13181. [Google Scholar] [CrossRef]
- Kim, J.; Jung, K.H.; Yan, H.H.; Cheon, M.J.; Kang, S.; Jin, X.; Park, S.; Oh, M.S.; Hong, S.S. Artemisia Capillaris leaves inhibit cell proliferation and induce apoptosis in hepatocellular carcinoma. BMC Complement. Altern. Med. 2018, 18, 147. [Google Scholar] [CrossRef]
- Hong, J.H.; Lee, J.W.; Park, J.H.; Lee, I.S. Antioxidative and cytoprotective effects of Artemisia capillaris fractions. Biofactors 2007, 31, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Baldea, I.; Mocan, T.; Cosgarea, R. The role of ultraviolet radiation and tyrosine stimulated melanogenesis in the induction of oxidative stress alterations in fair skin melanocytes. Exp. Oncol. 2009, 31, 200–208. [Google Scholar] [PubMed]
- Kim, E.S.; Park, S.J.; Goh, M.J.; Na, Y.J.; Jo, D.S.; Jo, Y.K.; Shin, J.H.; Choi, E.S.; Lee, H.K.; Kim, J.Y.; et al. Mitochondrial dynamics regulate melanogenesis through proteasomal degradation of MITF via ROS-ERK activation. Pigm. Cell Melanoma Res. 2014, 27, 1051–1062. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.-H.; Min, M.-J.; Oh, D.-S.; Shin, H.-J. Antimicrobial and Antioxidant Activity of Camellia japonica Extracts for Cosmetic Applications. KSBB J. 2013, 28, 99–105. [Google Scholar] [CrossRef]
- Lee, H.H.; Cho, J.Y.; Moon, J.H.; Park, K.H. Isolation and identification of antioxidative phenolic acids and flavonoid glycosides from Camellia japonica flowers. Hortic. Environ. Biotechnol. 2011, 52, 270–277. [Google Scholar] [CrossRef]
- Kim, K.Y.; Davidson, P.M.; Chung, H.J. Antibacterial activity in extracts of Camellia japonica L. petals and its application to a model food system. J. Food Prot. 2001, 64, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Wang, G.; Wang, X.; Wang, T.; Shen, J.; Zhang, A.; Zheng, L.; Zhang, Y. Essential Oils from The Dropped Flowers of Camellia japonica: Extraction Optimization, Chemical Profile and Antibacterial Property. Am. J. Biochem. Biotechnol. 2021, 17, 40–49. [Google Scholar] [CrossRef]
- Kim, S.; Jung, E.; Shin, S.; Kim, M.; Kim, Y.S.; Lee, J.; Park, D. Anti-inflammatory activity of Camellia japonica oil. BMB Rep. 2012, 45, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Lee, J.; Baek, J.; Jung, K.; Lee, J.; Huh, S.; Kim, S.; Koh, J.; Park, D. Effect of Camellia japonica oil on human type I procollagen production and skin barrier function. J. Ethnopharmacol. 2007, 112, 127–131. [Google Scholar] [CrossRef]
- Tahany, M.; Abdel-Rahman, N.M.K.; Abd El-Ghany, M.N.; Yosef, E. Purification, characterization and medicinal application of tyrosinase extracted from Saccharomyces cerevisiae. J. Innov. Pharm. Biol. Sci. 2019, 6, 1–11. [Google Scholar]
- Chou, S.T.; Chang, W.L.; Chang, C.T.; Hsu, S.L.; Lin, Y.C.; Shih, Y. Cinnamomum cassia essential oil inhibits alpha-MSH-induced melanin production and oxidative stress in murine B16 melanoma cells. Int. J. Mol. Sci. 2013, 14, 19186–19201. [Google Scholar] [CrossRef]
- Ha, S.Y.; Jung, J.Y.; Yang, J.K. Camellia japonica Essential Oil Inhibits alpha-MSH-Induced Melanin Production and Tyrosinase Activity in B16F10 Melanoma Cells. Evid. Based Complement. Altern. Med. 2021, 2021, 6328767. [Google Scholar] [CrossRef]
- Marc, E.B.; Nelly, A.; Annick, D.D.; Frederic, D. Plants used as remedies antirheumatic and antineuralgic in the traditional medicine of Lebanon. J. Ethnopharmacol. 2008, 120, 315–334. [Google Scholar] [CrossRef]
- El Khoury, R.; Michael Jubeli, R.; El Beyrouthy, M.; Baillet Guffroy, A.; Rizk, T.; Tfayli, A.; Lteif, R. Phytochemical screening and antityrosinase activity of carvacrol, thymoquinone, and four essential oils of Lebanese plants. J. Cosmet. Dermatol. 2019, 18, 944–952. [Google Scholar] [CrossRef]
- El Khoury, R.; Michael-Jubeli, R.; Bakar, J.; Dakroub, H.; Rizk, T.; Baillet-Guffroy, A.; Lteif, R.; Tfayli, A. Origanum essential oils reduce the level of melanin in B16-F1 melanocytes. Eur. J. Dermatol. 2019, 29, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, W.W.; Kumar, K.J.S.; Lee, H.J.; Tsao, N.W.; Wang, S.Y. Anti-Melanogenic Activity of Calocedrus formosana Wood Essential Oil and Its Chemical Composition Analysis. Plants 2021, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.Y.; Lin, C.C.; Hsu, S.L.; Cheng, Y.W.; Wu, J.H.; Cheng, C.L.; Yang, C.R. Leaf Extracts of Calocedrus formosana (Florin) Induce G2/M Cell Cycle Arrest and Apoptosis in Human Bladder Cancer Cells. Evid. Based Complement. Altern. Med. 2011, 2011, 380923. [Google Scholar] [CrossRef]
- Ho, C.L.; Tseng, Y.H.; Wang, E.I.; Liao, P.C.; Chou, J.C.; Lin, C.N.; Su, Y.C. Composition, antioxidant and antimicrobial activities of the seed essential oil of Calocedrus formosana from Taiwan. Nat. Prod. Commun. 2011, 6, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Wu, J.H.; Cheng, S.S.; Lo, C.P.; Chang, H.N.; Shyur, L.F.; Chang, S.T. Antioxidant activity of extracts from Calocedrus formosana leaf, bark, and heartwood. J. Wood Sci. 2004, 50, 422–426. [Google Scholar] [CrossRef]
- Chao, K.P.; Hua, K.F.; Hsu, H.Y.; Su, Y.C.; Chang, S.T. Anti-inflammatory activity of sugiol, a diterpene isolated from Calocedrus formosana bark. Planta Med. 2005, 71, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.S.; Wu, C.L.; Chang, H.T.; Kao, Y.T.; Chang, S.T. Antitermitic and antifungal activities of essential oil of Calocedrus formosana leaf and its composition. J. Chem. Ecol. 2004, 30, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Hellyer, R.O.; Lassak, E.V. The steam-volatile constituents of Melaleuca viridiflora sol. ex gaertn. Aust. J. Chem. 1968, 10, 2585–2587. [Google Scholar] [CrossRef][Green Version]
- Chao, W.W.; Su, C.C.; Peng, H.Y.; Chou, S.T. Melaleuca quinquenervia essential oil inhibits alpha-melanocyte-stimulating hormone-induced melanin production and oxidative stress in B16 melanoma cells. Phytomedicine 2017, 34, 191–201. [Google Scholar] [CrossRef]
- Mann, M.N.; Fisher, E.R. Investigation of Antibacterial 1,8-Cineole-Derived Thin Films Formed via Plasma-Enhanced Chemical Vapor Deposition. ACS Appl. Mater. Interfaces 2017, 9, 36548–36560. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Downregulation of melanogenesis: Drug discovery and therapeutic options. Drug Discov. Today 2017, 22, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Villarama, C.D.; Maibach, H.I. Glutathione as a depigmenting agent: An overview. Int. J. Cosmet. Sci. 2005, 27, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Ahn, Y.J. Growth-Inhibiting Effects of Cinnamomum cassia Bark-Derived Materials on Human Intestinal Bacteria. J. Agric. Food Chem. 1998, 46, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Kim, B.S.; Kim, M.K. Suppression effect of Cinnamomum cassia bark-derived component on nitric oxide synthase. J. Agric. Food Chem. 2002, 50, 7700–7703. [Google Scholar] [CrossRef] [PubMed]
- Verspohl, E.J.; Bauer, K.; Neddermann, E. Antidiabetic effect of Cinnamomum cassia and Cinnamomum zeylanicum in vivo and in vitro. Phytother. Res. 2005, 19, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fan, L.; Fan, S.; Wang, J.; Luo, T.; Tang, Y.; Chen, Z.; Yu, L. Cinnamomum cassia Presl: A Review of Its Traditional Uses, Phytochemistry, Pharmacology and Toxicology. Molecules 2019, 24, 3473. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, T.M.; Lee, I.; Ha, D.T.; Kim, H.; Min, B.; Bae, K. Tyrosinase-inhibitory constituents from the twigs of Cinnamomum cassia. J. Nat. Prod. 2009, 72, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhu, J.X.; Mo, S.F.; Pan, Y.; Kong, L.D. Effects of cassia oil on serum and hepatic uric acid levels in oxonate-induced mice and xanthine dehydrogenase and xanthine oxidase activities in mouse liver. J. Ethnopharmacol. 2006, 103, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Giordani, R.; Regli, P.; Kaloustian, J.; Portugal, H. Potentiation of antifungal activity of amphotericin B by essential oil from Cinnamomum cassia. Phytother. Res. 2006, 20, 58–61. [Google Scholar] [CrossRef]
- Padalia, R.C.; Verma, R.S.; Sundaresan, V.; Chanotiya, C.S. Chemical diversity in the genus Alpinia (Zingiberaceae): Comparative composition of four Alpinia species grown in Northern India. Chem. Biodivers. 2010, 7, 2076–2087. [Google Scholar] [CrossRef]
- Kumar, K.J.S.; Wang, S.H.; Tseng, Y.H.; Tsao, N.W.; Kuo, Y.H.; Wang, S.Y. trans-3-Methoxy-5-hydroxystilbene (MHS) from the rhizome of Alpinia nantonensis inhibits metastasis in human lung cancer cells. Phytomedicine 2018, 50, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.Y.; Weng, T.S.; Kumar, K.J.S.; Tseng, Y.H.; Tung, T.W.; Wang, S.Y.; Wang, H.C. Ethanol Extracts of Dietary Herb, Alpinia nantoensis, Exhibit Anticancer Potential in Human Breast Cancer Cells. Integr. Cancer Ther. 2019, 18, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.J.S.; Vani, M.G.; Wu, P.C.; Lee, H.J.; Tseng, Y.H.; Wang, S.Y. Essential Oils of Alpinia nantoensis Retard Forskolin-Induced Melanogenesis via ERK1/2-Mediated Proteasomal Degradation of MITF. Plants 2020, 9, 1672. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Kim, Y.H.; Yu, H.H.; Jeong, S.I.; Cha, J.D.; Kil, B.S.; You, Y.O. Antibacterial activity and chemical composition of essential oil of Chrysanthemum boreale. Planta Med. 2003, 69, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Won, K.J.; Yoon, M.S.; Yu, H.J.; Park, J.H.; Kim, B.; Lee, H.M. Chrysanthemum boreale flower floral water inhibits platelet-derived growth factor-stimulated migration and proliferation in vascular smooth muscle cells. Pharm. Biol. 2015, 53, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Won, K.J.; Yoon, M.S.; Hwang, D.I.; Yoon, S.W.; Park, J.H.; Kim, B.; Lee, H.M. Chrysanthemum boreale Makino essential oil induces keratinocyte proliferation and skin regeneration. Nat. Prod. Res. 2015, 29, 562–564. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Sung, J.; Sung, M.; Choi, Y.; Jeong, H.S.; Lee, J. Involvement of heme oxygenase-1 in the anti-inflammatory activity of Chrysanthemum boreale Makino extracts on the expression of inducible nitric oxide synthase in RAW264.7 macrophages. J. Ethnopharmacol. 2010, 131, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Won, K.J.; Hwang, D.I.; Park, S.M.; Kim, B.; Lee, H.M. Chemical Composition, Antioxidant and Anti melanogenic Activities of Essential Oils from Chrysanthemum boreale Makino at Different Harvesting Stages. Chem. Biodivers. 2018, 15, e1700506. [Google Scholar] [CrossRef]
- Huang, H.C.; Chang, T.Y.; Chang, L.Z.; Wang, H.F.; Yih, K.H.; Hsieh, W.Y.; Chang, T.M. Inhibition of melanogenesis versus antioxidant properties of essential oil extracted from leaves of Vitex negundo Linn and chemical composition analysis by GC-MS. Molecules 2012, 17, 3902–3916. [Google Scholar] [CrossRef]
- Gupta, R.K.; Tandon, V.R. Antinociceptive activity of Vitex-negundo Linn leaf extract. Indian J. Physiol. Pharmacol. 2005, 49, 163–170. [Google Scholar]
- Zheng, C.J.; Huang, B.K.; Han, T.; Zhang, Q.Y.; Zhang, H.; Rahman, K.; Qin, L.P. Antinociceptive activities of the liposoluble fraction from Vitex negundo seeds. Pharm. Biol. 2010, 48, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Tandon, V.R.; Gupta, R.K. An experimental evaluation of anticonvulsant activity of Vitex-negundo. Indian J. Physiol. Pharmacol. 2005, 49, 199–205. [Google Scholar] [PubMed]
- Dharmasiri, M.G.; Jayakody, J.R.; Galhena, G.; Liyanage, S.S.; Ratnasooriya, W.D. Anti-inflammatory and analgesic activities of mature fresh leaves of Vitex negundo. J. Ethnopharmacol. 2003, 87, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Haq, A.U.; Malik, A.; Khan, M.T.H.; Anwar-ul-Haq; Khan, S.B.; Ahmad, A.; Choudhary, M.I. Tyrosinase inhibitory lignans from the methanol extract of the roots of Vitex negundo Linn. and their structure-activity relationship. Phytomedicine 2006, 13, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Feizpour, A.; Boskabady, M.H.; Byrami, G.; Golamnezhad, Z.; Shafei, M.N. The effect of hydro-ethanolic extract of Achillea millefolium on muscarinic receptors of guinea pig tracheal smooth muscle. Indian J. Pharmacol. 2013, 45, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Mahady, G.B.; Pendland, S.L.; Stoia, A.; Hamill, F.A.; Fabricant, D.; Dietz, B.M.; Chadwick, L.R. In vitro susceptibility of Helicobacter pylori to botanical extracts used traditionally for the treatment of gastrointestinal disorders. Phytother. Res. 2005, 19, 988–991. [Google Scholar] [CrossRef]
- Benedek, B.; Geisz, N.; Jager, W.; Thalhammer, T.; Kopp, B. Choleretic effects of yarrow (Achillea millefolium s.l.) in the isolated perfused rat liver. Phytomedicine 2006, 13, 702–706. [Google Scholar] [CrossRef]
- Khan, A.U.; Gilani, A.H. Blood pressure lowering, cardiovascular inhibitory and bronchodilatory actions of Achillea millefolium. Phytother. Res. 2011, 25, 577–583. [Google Scholar] [CrossRef]
- Farasati Far, B.; Behzad, G.; Khalili, H. Achillea millefolium: Mechanism of action, pharmacokinetic, clinical drug-drug interactions and tolerability. Heliyon 2023, 9, e22841. [Google Scholar] [CrossRef]
- Chou, S.T.; Peng, H.Y.; Hsu, J.C.; Lin, C.C.; Shih, Y. Achillea millefolium L. essential oil inhibits LPS-induced oxidative stress and nitric oxide production in RAW 264.7 Macrophages. Int. J. Mol. Sci. 2013, 14, 12978–12993. [Google Scholar] [CrossRef]
- Peng, H.Y.; Lin, C.C.; Wang, H.Y.; Shih, Y.; Chou, S.T. The melanogenesis alteration effects of Achillea millefolium L. essential oil and linalyl acetate: Involvement of oxidative stress and the JNK and ERK signaling pathways in melanoma cells. PLoS ONE 2014, 9, e95186. [Google Scholar] [CrossRef]
- Aibibu, N.; Liu, Y.; Zeng, G.; Wang, X.; Chen, B.; Song, H.; Xu, L. Cadmium accumulation in vetiveria zizanioides and its effects on growth, physiological and biochemical characters. Bioresour. Technol. 2010, 101, 6297–6303. [Google Scholar] [CrossRef] [PubMed]
- Amarasiri, S.S.; Attanayake, A.P.; Arawwawala, L.; Jayatilaka, K.; Mudduwa, L.K.B. Nephroprotective activity of Vetiveria zizanioides (L.) Nash supplement in doxorubicin-induced nephrotoxicity model of Wistar rats. J. Food Biochem. 2021, 45, e13901. [Google Scholar] [CrossRef] [PubMed]
- Luqman, S.; Kumar, R.; Kaushik, S.; Srivastava, S.; Darokar, M.P.; Khanuja, S.P. Antioxidant potential of the root of Vetiveria zizanioides (L.) Nash. Indian J. Biochem. Biophys. 2009, 46, 122–125. [Google Scholar] [PubMed]
- David, A.; Wang, F.; Sun, X.; Li, H.; Lin, J.; Li, P.; Deng, G. Chemical Composition, Antioxidant, and Antimicrobial Activities of Vetiveria zizanioides (L.) Nash Essential Oil Extracted by Carbon Dioxide Expanded Ethanol. Molecules 2019, 24, 1897. [Google Scholar] [CrossRef]
- Chou, S.T.; Lai, C.P.; Lin, C.C.; Shih, Y. Study of the chemical composition, antioxidant activity and anti-inflammatory activity of essential oil from Vetiveria zizanioides. Food Chem. 2012, 134, 262–268. [Google Scholar] [CrossRef]
- Peng, H.Y.; Lai, C.C.; Lin, C.C.; Chou, S.T. Effect of Vetiveria zizanioides essential oil on melanogenesis in melanoma cells: Downregulation of tyrosinase expression and suppression of oxidative stress. Sci. World J. 2014, 2014, 213013. [Google Scholar] [CrossRef] [PubMed]
- Borzoui, E.; Khaghani, R.; Nouri-Ganbalani, G. Lethal and Sublethal Effects of Eucalyptus camaldulensis and Mentha piperita Essential Oils on the Khapra Beetle (Coleoptera: Dermestidae) in Terms of Feeding Inhibition, Oviposition, and Seed Damage. Environ. Entomol. 2021, 50, 692–698. [Google Scholar] [CrossRef]
- Butcher, P.A.; Otero, A.; McDonald, M.W.; Moran, G.F. Nuclear RFLP variation in Eucalyptus camaldulensis Dehnh. from northern Australia. Heredity 2002, 88, 402–412. [Google Scholar] [CrossRef]
- Cimanga, K.; Kambu, K.; Tona, L.; Apers, S.; De Bruyne, T.; Hermans, N.; Totté, J.; Pieters, L.; Vlietinck, A.J. Correlation between chemical composition and antibacterial activity of essential oils of some aromatic medicinal plants growing in the Democratic Republic of Congo. J. Ethnopharmacol. 2002, 79, 213–220. [Google Scholar] [CrossRef]
- Siramon, P.; Ohtani, Y.; Ichiura, H. Biological performance of Eucalyptus camaldulensis leaf oils from Thailand against the subterranean termite Coptotermes formosanus Shiraki. J. Wood Sci. 2009, 55, 41–46. [Google Scholar] [CrossRef]
- Siramon, P.; Ohtani, Y. Antioxidative and antiradical activities of Eucalyptus camaldulensis leaf oils from Thailand. J. Wood Sci. 2007, 53, 498–504. [Google Scholar] [CrossRef]
- Huang, H.C.; Ho, Y.C.; Lim, J.M.; Chang, T.Y.; Ho, C.L.; Chang, T.M. Investigation of the Anti-Melanogenic and Antioxidant Characteristics of Eucalyptus camaldulensis Flower Essential Oil and Determination of Its Chemical Composition. Int. J. Mol. Sci. 2015, 16, 10470–10490. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Cao, G.; Zhao, D.; Li, G.; Zhang, H.; Yan, M. Ethnic, Botanic, Phytochemistry and Pharmacology of the Acorus L. Genus: A Review. Molecules 2023, 28, 7117. [Google Scholar] [CrossRef]
- Kumar, R.; Prakash, O.; Pan, A.K.; Hore, S.K.; Chanotiya, C.S.; Mathela, C.S. Compositional variations and anthelmentic activity of essential oils from rhizomes of different wild populations of Acorus calamus L. and its major component, beta-Asarone. Nat. Prod. Commun. 2009, 4, 275–278. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. In vitro acetylcholinesterase inhibitory activity of the essential oil from Acorus calamus and its main constituents. Planta Med. 2007, 73, 283–285. [Google Scholar] [CrossRef]
- Cho, J.; Kong, J.Y.; Jeong, D.Y.; Lee, K.D.; Lee, D.U.; Kang, B.S. NMDA recepter-mediated neuroprotection by essential oils from the rhizomes of Acorus gramineus. Life Sci. 2001, 68, 1567–1573. [Google Scholar] [CrossRef]
- Huang, H.C.; Wang, H.F.; Yih, K.H.; Chang, L.Z.; Chang, T.M. The Dual Antimelanogenic and Antioxidant Activities of the Essential Oil Extracted from the Leaves of Acorus macrospadiceus (Yamamoto) F. N. Wei et Y. K. Li. Evid. Based Complement. Altern. Med. 2012, 2012, 781280. [Google Scholar] [CrossRef]
- Chereddy, K.K.; Coco, R.; Memvanga, P.B.; Ucakar, B.; des Rieux, A.; Vandermeulen, G.; Preat, V. Combined effect of PLGA and curcumin on wound healing activity. J. Control Release 2013, 171, 208–215. [Google Scholar] [CrossRef]
- Dwivedi, D.; Dwivedi, M.; Malviya, S.; Singh, V. Evaluation of wound healing, anti-microbial and antioxidant potential of Pongamia pinnata in wistar rats. J. Tradit. Complement. Med. 2017, 7, 79–85. [Google Scholar] [CrossRef]
- Nayak, B.S.; Sandiford, S.; Maxwell, A. Evaluation of the Wound-healing Activity of Ethanolic Extract of Morinda citrifolia L. Leaf. Evid. Based Complement. Altern. Med. 2009, 6, 351–356. [Google Scholar] [CrossRef]
- Wedler, J.; Rusanov, K.; Atanassov, I.; Butterweck, V. A Polyphenol-Enriched Fraction of Rose Oil Distillation Wastewater Inhibits Cell Proliferation, Migration and TNF-alpha-Induced VEGF Secretion in Human Immortalized Keratinocytes. Planta Med. 2016, 82, 1000–1008. [Google Scholar] [CrossRef]
- Mercurio, L.; Albanesi, C.; Madonna, S. Recent Updates on the Involvement of PI3K/AKT/mTOR Molecular Cascade in the Pathogenesis of Hyperproliferative Skin Disorders. Front. Med. 2021, 8, 665647. [Google Scholar] [CrossRef]
- Quadri, M.; Pellegrini, C.; Efimova, T.; Palazzo, E. Editorial: New tools and molecular advances in hyperproliferative skin disorders. Front. Med. 2022, 9, 1002872. [Google Scholar] [CrossRef]
- Choi, E.; Park, H.; Lee, J.; Kim, G. Anticancer, antiobesity, and anti-inflammatory activity of Artemisia species in vitro. J. Tradit. Chin. Med. 2013, 33, 92–97. [Google Scholar] [CrossRef]
- Elfawal, M.A.; Towler, M.J.; Reich, N.G.; Golenbock, D.; Weathers, P.J.; Rich, S.M. Dried Whole Plant Artemisia annua as an Antimalarial Therapy. PLoS ONE 2012, 7, e52746. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, P.; Hu, Y.; Liu, T.; Guo, Y.; Sun, P.; Zheng, J.; Ren, Z.; Wang, Y. Antiviral activities of Artemisia vulgaris L. extract against herpes simplex virus. Chin. Med. 2023, 18, 21. [Google Scholar] [CrossRef]
- Ali, M.Y.; Park, S.E.; Seong, S.H.; Zamponi, G.W.; Jung, H.A.; Choi, J.S. Ursonic acid from Artemisia montana exerts anti-diabetic effects through anti-glycating properties, and by inhibiting PTP1B and activating the PI3K/Akt signaling pathway in insulin-resistant C2C12 cells. Chem.-Biol. Interact. 2023, 376, 110452. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, J.; Min, H. In vitro anti-inflammatory activity of the Artemisia montana leaf ethanol extract in macrophage RAW 264.7 cells. Food Agric. Immunol. 2018, 29, 688–698. [Google Scholar] [CrossRef]
- Lee, G.-D.; Kim, J.-S.; Bae, J.-O.; Yoon, H.-S. Antioxidative Effectiveness of Water Extract and Ether Extract in Wormwood(Artemisia montana Pampan). J. Korean Soc. Food Sci. Nutr. 1992, 21, 17–22. [Google Scholar]
- Yoon, M.S.; Won, K.J.; Kim, D.Y.; Hwang, D.I.; Yoon, S.W.; Kim, B.; Lee, H.M. Skin regeneration effect and chemical composition of essential oil from Artemisia Montana. Nat. Prod. Commun. 2014, 9, 1619–1622. [Google Scholar] [CrossRef]
- Tang, L.; Sierra, J.O.; Kelly, R.; Kirsner, R.S.; Li, J. Wool-derived keratin stimulates human keratinocyte migration and types IV and VII collagen expression. Exp. Dermatol. 2012, 21, 458–460. [Google Scholar] [CrossRef]
- Pachi, V.K.; Mikropoulou, E.V.; Gkiouvetidis, P.; Siafakas, K.; Argyropoulou, A.; Angelis, A.; Mitakou, S.; Halabalaki, M. Traditional uses, phytochemistry and pharmacology of Chios mastic gum (Pistacia lentiscus var. Chia, Anacardiaceae): A review. J. Ethnopharmacol. 2020, 254, 112485. [Google Scholar] [CrossRef]
- Milia, E.; Bullitta, S.M.; Mastandrea, G.; Szotakova, B.; Schoubben, A.; Langhansova, L.; Quartu, M.; Bortone, A.; Eick, S. Leaves and Fruits Preparations of Pistacia lentiscus L.: A Review on the Ethnopharmacological Uses and Implications in Inflammation and Infection. Antibiotics 2021, 10, 425. [Google Scholar] [CrossRef]
- Mezni, F.; Khouja, M.L.; Gregoire, S.; Martine, L.; Khaldi, A.; Berdeaux, O. Effect of growing area on tocopherols, carotenoids and fatty acid composition of Pistacia lentiscus edible oil. Nat. Prod. Res. 2014, 28, 1225–1230. [Google Scholar] [CrossRef]
- Longo, L.; Scardino, A.; Vasapollo, G. Identification and quantification of anthocyanins in the berries of Pistacia lentiscus L., Phillyrea latifolia L. and Rubia peregrina L. Innov. Food Sci. Emerg. 2007, 8, 360–364. [Google Scholar] [CrossRef]
- Mharti, F.Z.; Lyoussi, B.; Abdellaoui, A. Antibacterial activity of the essential oils of Pistacia lentiscus used in Moroccan folkloric medicine. Nat. Prod. Commun. 2011, 6, 1505–1506. [Google Scholar] [CrossRef]
- Rahman, H.S. Phytochemical analysis and antioxidant and anticancer activities of mastic gum resin from Pistacia atlantica subspecies kurdica. Onco Targets Ther. 2018, 11, 4559–4572. [Google Scholar] [CrossRef]
- Siano, F.; Cutignano, A.; Moccia, S.; Russo, G.L.; Volpe, M.G.; Picariello, G. Phytochemical Characterization and Effects on Cell Proliferation of Lentisk (Pistacia lentiscus) Berry Oil: A Revalued Source of Phenolics. Plant Food Hum. Nutr. 2020, 75, 487–494. [Google Scholar] [CrossRef]
- Mezni, F.; Miled, K.; Khaldi, A.; Khouja, M.L.; Boubaker, S.; Maaroufi, A. Wound healing effect of Pistacia lentiscus L. seed oil: Confirmation of its uses in Mediterranean traditional medicine. Boletín Latinoam. Y Del Caribe De Plantas Med. Y Aromáticas 2020, 19, 314–320. [Google Scholar] [CrossRef]
- Allaw, M.; Manconi, M.; Caboni, P.; Bacchetta, G.; Escribano-Ferrer, E.; Peris, J.E.; Nacher, A.; Diez-Sales, O.; Manca, M.L. Formulation of liposomes loading lentisk oil to ameliorate topical delivery, attenuate oxidative stress damage and improve cell migration in scratch assay. Biomed. Pharmacother. 2021, 144, 112351. [Google Scholar] [CrossRef]
- Fadel, B.A.; Elwakil, B.H.; Fawzy, E.E.; Shaaban, M.M.; Olama, Z.A. Nanoemulsion of Lavandula angustifolia Essential Oil/Gold Nanoparticles: Antibacterial Effect against Multidrug-Resistant Wound-Causing Bacteria. Molecules 2023, 28, 6988. [Google Scholar] [CrossRef]
- Woollard, A.C.; Tatham, K.C.; Barker, S. The influence of essential oils on the process of wound healing: A review of the current evidence. J. Wound Care 2007, 16, 255–257. [Google Scholar] [CrossRef]
- Vakilian, K.; Atarha, M.; Bekhradi, R.; Chaman, R. Healing advantages of lavender essential oil during episiotomy recovery: A clinical trial. Complement. Ther. Clin. Pract. 2011, 17, 50–53. [Google Scholar] [CrossRef]
- Altaei, D.T. Topical lavender oil for the treatment of recurrent aphthous ulceration. Am. J. Dent. 2012, 25, 39–43. [Google Scholar]
- Koca Kutlu, A.; Cecen, D.; Gurgen, S.G.; Sayin, O.; Cetin, F. A Comparison Study of Growth Factor Expression following Treatment with Transcutaneous Electrical Nerve Stimulation, Saline Solution, Povidone-Iodine, and Lavender Oil in Wounds Healing. Evid. Based Complement. Altern. Med. 2013, 2013, 361832. [Google Scholar] [CrossRef]
- Clark, R.A.; Nielsen, L.D.; Welch, M.P.; McPherson, J.M. Collagen matrices attenuate the collagen-synthetic response of cultured fibroblasts to TGF-beta. J. Cell Sci. 1995, 108 Pt 3, 1251–1261. [Google Scholar] [CrossRef]
- Desmouliere, A.; Geinoz, A.; Gabbiani, F.; Gabbiani, G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 1993, 122, 103–111. [Google Scholar] [CrossRef]
- Crane, S.; Aurore, G.; Joseph, H.; Mouloungui, Z.; Bourgeois, P. Composition of fatty acids triacylglycerols and unsaponifiable matter in Calophyllum calaba L. oil from Guadeloupe. Phytochemistry 2005, 66, 1825–1831. [Google Scholar] [CrossRef]
- Ansel, J.L.; Lupo, E.; Mijouin, L.; Guillot, S.; Butaud, J.F.; Ho, R.; Lecellier, G.; Raharivelomanana, P.; Pichon, C. Biological Activity of Polynesian Calophyllum inophyllum Oil Extract on Human Skin Cells. Planta Med. 2016, 82, 961–966. [Google Scholar] [CrossRef]
- Bhalla, T.N.; Saxena, R.C.; Nigam, S.K.; Misra, G.; Bhargava, K.P. Calophyllolide--a new non-steroidal anti-inflammatory agent. Indian J. Med. Res. 1980, 72, 762–765. [Google Scholar]
- Yimdjo, M.C.; Azebaze, A.G.; Nkengfack, A.E.; Meyer, A.M.; Bodo, B.; Fomum, Z.T. Antimicrobial and cytotoxic agents from Calophyllum inophyllum. Phytochemistry 2004, 65, 2789–2795. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, R.; Dhachinamoorthi, D.; Senthilkumar, K.; Thamizhvanan, K. Antimicrobial activity of various extracts from various parts of Calophyllum inophyllum L. J. Appl. Pharm. Sci. 2011, 1, 1–12. [Google Scholar]
- Dweck, A.C.; Meadows, T. Tamanu (Calophyllum inophyllum)—The African, Asian, Polynesian and Pacific Panacea. Int. J. Cosmet. Sci. 2002, 24, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, S.S.; Gur, T.F.; Terzi, N.K.; Dogan, B. Evaluation of the cutaneous wound healing potential of tamanu oil in wounds induced in rats. J. Wound Care 2021, 30, Vi–Vx. [Google Scholar] [CrossRef]
- Ali, S.; Khan, M.R.; Irfanullah; Sajid, M.; Zahra, Z. Phytochemical investigation and antimicrobial appraisal of Parrotiopsis jacquemontiana (Decne) Rehder. BMC Complement. Altern. Med. 2018, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, M.R.; Iqbal, J.; Batool, R.; Naz, I.; Yaseen, T.; Abbasi, B.A.; Nasir, J.A.; El-Serehy, H.A. Chemical composition and pharmacological bio-efficacy of Parrotiopsis jacquemontiana (Decne) Rehder for anticancer activity. Saudi J. Biol. Sci. 2021, 28, 4969–4986. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Khan, M.R.; Batool, R.; Maryam, S.; Majid, M. Wound healing potential of oil extracted from Parrotiopsis jacquemontiana (Decne) Rehder. J. Ethnopharmacol. 2019, 236, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.B.; Sytsma, K.J. Staminal evolution in the genus Salvia (Lamiaceae): Molecular phylogenetic evidence for multiple origins of the staminal lever. Ann. Bot. 2007, 100, 375–391. [Google Scholar] [CrossRef]
- Badiee, P.; Nasirzadeh, A.R.; Motaffaf, M. Comparison of Salvia officinalis L. essential oil and antifungal agents against candida species. J. Pharm. Technol. Drug Res. 2012, 1, 7. [Google Scholar] [CrossRef]
- Miguel, G.; Cruz, C.; Faleiro, M.L.; Simoes, M.T.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Salvia officinalis L. essential oils: Effect of hydrodistillation time on the chemical composition, antioxidant and antimicrobial activities. Nat. Prod. Res. 2011, 25, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Dal Prá, V.; Bisol, L.B.; Detoni, S.; Denti, M.; Grando, J.; Pollo, C.; Pasquali, T.R.; Hofmann Júnio, A.E.; Mazzuti, M.A.; Macedo, S.M. Antiinflammatory activity of fracionated extracts of Salvia officinalis L. J. Appl. Pharm. Sci. 2011, 1, 67–71. [Google Scholar]
- Hamidpour, M.; Hamidpour, R.; Hamidpour, S.; Shahlari, M. Chemistry, Pharmacology, and Medicinal Property of Sage (Salvia) to Prevent and Cure Illnesses such as Obesity, Diabetes, Depression, Dementia, Lupus, Autism, Heart Disease, and Cancer. J. Tradit. Complement. Med. 2014, 4, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Medjahed, F.; Merouane, A.; Saadi, A.; Bader, A.; Cioni, P.L.; Flamini, G. Chemical profile and antifungal potential of essential oils from leaves and flowers of Salvia algeriensis (Desf.): A comparative study. Chil. J. Agric. Res. 2016, 76, 195–200. [Google Scholar] [CrossRef][Green Version]
- Cagno, V.; Sgorbini, B.; Sanna, C.; Cagliero, C.; Ballero, M.; Civra, A.; Donalisio, M.; Bicchi, C.; Lembo, D.; Rubiolo, P. In vitro anti-herpes simplex virus-2 activity of Salvia desoleana Atzei & V. Picci essential oil. PLoS ONE 2017, 12, e0172322. [Google Scholar] [CrossRef] [PubMed]
- Alves-Silva, J.M.; Maccioni, D.; Cocco, E.; Goncalves, M.J.; Porcedda, S.; Piras, A.; Cruz, M.T.; Salgueiro, L.; Maxia, A. Advances in the Phytochemical Characterisation and Bioactivities of Salvia aurea L. Essential Oil. Plants 2023, 12, 1247. [Google Scholar] [CrossRef]
- Boskabady, M.H.; Shafei, M.N.; Saberi, Z.; Amini, S. Pharmacological effects of rosa damascena. Iran. J. Basic Med. Sci. 2011, 14, 295–307. [Google Scholar] [PubMed]
- Fresco, P.; Borges, F.; Marques, M.P.; Diniz, C. The anticancer properties of dietary polyphenols and its relation with apoptosis. Curr. Pharm. Des. 2010, 16, 114–134. [Google Scholar] [CrossRef]
- Hisaka, T.; Sakai, H.; Sato, T.; Goto, Y.; Nomura, Y.; Fukutomi, S.; Fujita, F.; Mizobe, T.; Nakashima, O.; Tanigawa, M.; et al. Quercetin Suppresses Proliferation of Liver Cancer Cell Lines In Vitro. Anticancer. Res. 2020, 40, 4695–4700. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, H.S.; Wang, L.F.; Bai, M.H.; Wang, Y.C.; Jiang, X.F.; Liu, M. Ellagic acid exerts anti-proliferation effects via modulation of Tgf-beta/Smad3 signaling in MCF-7 breast cancer cells. Asian Pac. J. Cancer Prev. 2014, 15, 273–276. [Google Scholar] [CrossRef]
- Singh, P.; Arif, Y.; Bajguz, A.; Hayat, S. The role of quercetin in plants. Plant Physiol. Biochem. 2021, 166, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Lozoya, J.E.; Lombardini, L.; Cisneros-Zevallos, L. Phytochemical constituents and antioxidant capacity of different pecan [Carya illinoinensis (Wangenh.) K. Koch] cultivars. Food Chem 2007, 102, 1241–1249. [Google Scholar] [CrossRef]
- Daniel, E.M.; Krupnick, A.S.; Heur, Y.H.; Blinzler, J.A.; Nims, R.W.; Stoner, G.D. Extraction, stability, and quantitation of ellagic acid in various fruits and nuts. J. Food Compos. Anal. 1989, 2, 338–349. [Google Scholar] [CrossRef]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Torronen, A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1629. [Google Scholar] [CrossRef] [PubMed]
- Mertens-Talcott, S.U.; Talcott, S.T.; Percival, S.S. Low concentrations of quercetin and ellagic acid synergistically influence proliferation, cytotoxicity and apoptosis in MOLT-4 human leukemia cells. J. Nutr. 2003, 133, 2669–2674. [Google Scholar] [CrossRef]
- Li, K.; Zhou, R.; Wang Jia, W.; Li, Z.; Li, J.; Zhang, P.; Xiao, T. Zanthoxylum bungeanum essential oil induces apoptosis of HaCaT human keratinocytes. J. Ethnopharmacol. 2016, 186, 351–361. [Google Scholar] [CrossRef]
- Xia, L.A.; You, J.M.; Li, G.L.; Sun, Z.W.; Suo, Y.R. Compositional and Antioxidant Activity Analysis of Zanthoxylum bungeanum Seed Oil Obtained by Supercritical CO2 Fluid Extraction. J. Am. Oil Chem. Soc. 2011, 88, 23–32. [Google Scholar] [CrossRef]
- Li, Z.D.; Han, S.N.; Jiang, J.L.; Zhang, X.H.; Li, Y.; Chen, H.; Yuan, Y.J. Antitumor compound identification from Zanthoxylum bungeanum essential oil based on composition-activity relationship. Chem. Res. Chin. Univ. 2013, 29, 1068–1071. [Google Scholar] [CrossRef]
- Tezuka, Y.; Irikawa, S.; Kaneko, T.; Banskota, A.H.; Nagaoka, T.; Xiong, Q.; Hase, K.; Kadota, S. Screening of Chinese herbal drug extracts for inhibitory activity on nitric oxide production and identification of an active compound of Zanthoxylum bungeanum. J. Ethnopharmacol. 2001, 77, 209–217. [Google Scholar] [CrossRef]
- Zhu, R.X.; Zhong, K.; Zeng, W.C.; He, X.Y.; Gu, X.Q.; Zhao, Z.F.; Gao, H. Essential oil composition and antibacterial activity of Zanthoxylum bungeanum. Afr. J. Microbiol. Res. 2011, 5, 4631–4637. [Google Scholar] [CrossRef]
- Wang, C.F.; Yang, K.; Zhang, H.M.; Cao, J.; Fang, R.; Liu, Z.L.; Du, S.S.; Wang, Y.Y.; Deng, Z.W.; Zhou, L. Components and insecticidal activity against the maize weevils of Zanthoxylum schinifolium fruits and leaves. Molecules 2011, 16, 3077–3088. [Google Scholar] [CrossRef]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands; New York, NY, USA, 2012; pp. 442–450. [Google Scholar]
- Lee, H.H.; Ahn, J.H.; Kwon, A.R.; Lee, E.S.; Kwak, J.H.; Min, Y.H. Chemical composition and antimicrobial activity of the essential oil of apricot seed. Phytother. Res. 2014, 28, 1867–1872. [Google Scholar] [CrossRef]
- Gomaa, E.Z. In vitro antioxidant, antimicrobial, and antitumor activities of bitter almond and sweet apricot (Prunus armeniaca L.) kernels. Food Sci. Biotechnol. 2013, 22, 455–463. [Google Scholar] [CrossRef]
- Yigit, D.; Yigit, N.; Mavi, A. Antioxidant and antimicrobial activities of bitter and sweet apricot (Prunus armeniaca L.) kernels. Braz. J. Med. Biol. Res. 2009, 42, 346–352. [Google Scholar] [CrossRef]
- Korekar, G.; Stobdan, T.; Arora, R.; Yadav, A.; Singh, S.B. Antioxidant capacity and phenolics content of apricot (Prunus armeniaca L.) kernel as a function of genotype. Plant Foods Hum. Nutr. 2011, 66, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Abtahi, H.; Ghazavi, A.; Karimi, M.; Mollaghase, S.; Mosayebi, G. Antimicrobial Activities of Water and Methanol Extracts of Bitter Apricot Seeds. J. Med. Sci. 2008, 8, 433–436. [Google Scholar] [CrossRef]
- Chang, H.K.; Yang, H.Y.; Lee, T.H.; Shin, M.C.; Lee, M.H.; Shin, M.S.; Kim, C.J.; Kim, O.J.; Hong, S.P.; Cho, S. Armeniacae semen extract suppresses lipopolysaccharide-induced expressions of cyclooxygenase [correction of cycloosygenase]-2 and inducible nitric oxide synthase in mouse BV2 microglial cells. Biol. Pharm. Bull. 2005, 28, 449–454. [Google Scholar] [CrossRef]
- Do, J.S.; Hwang, J.K.; Seo, H.J.; Woo, W.H.; Nam, S.Y. Antiasthmatic activity and selective inhibition of type 2 helper T cell response by aqueous extract of semen armeniacae amarum. Immunopharmacol. Immunotoxicol. 2006, 28, 213–225. [Google Scholar] [CrossRef]
- Chang, H.K.; Shin, M.S.; Yang, H.Y.; Lee, J.W.; Kim, Y.S.; Lee, M.H.; Kim, J.; Kim, K.H.; Kim, C.J. Amygdalin induces apoptosis through regulation of Bax and Bcl-2 expressions in human DU145 and LNCaP prostate cancer cells. Biol. Pharm. Bull. 2006, 29, 1597–1602. [Google Scholar] [CrossRef]
- Li, K.; Yang, W.; Li, Z.; Jia, W.; Li, J.; Zhang, P.; Xiao, T. Bitter apricot essential oil induces apoptosis of human HaCaT keratinocytes. Int. Immunopharmacol. 2016, 34, 189–198. [Google Scholar] [CrossRef]
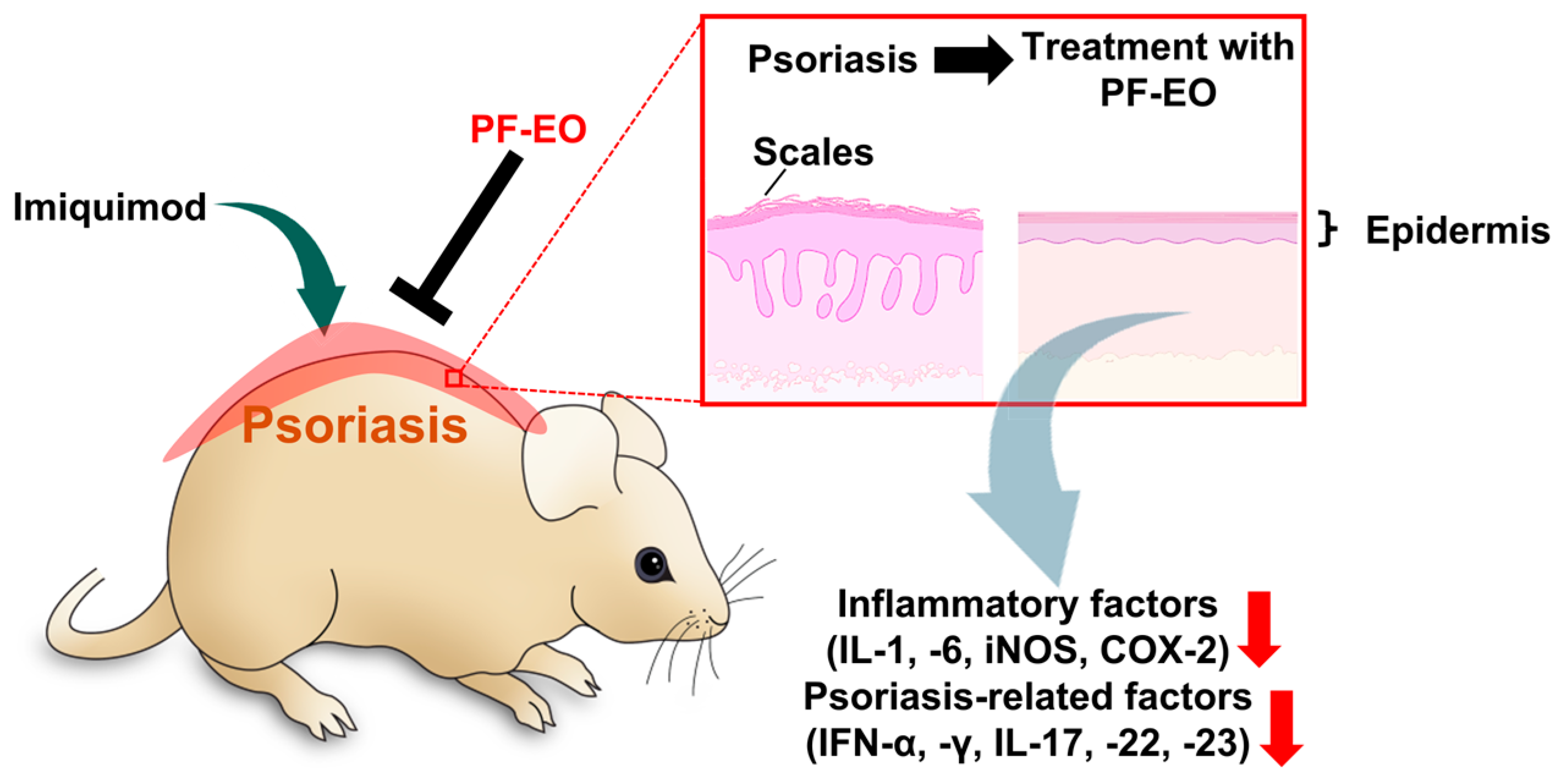
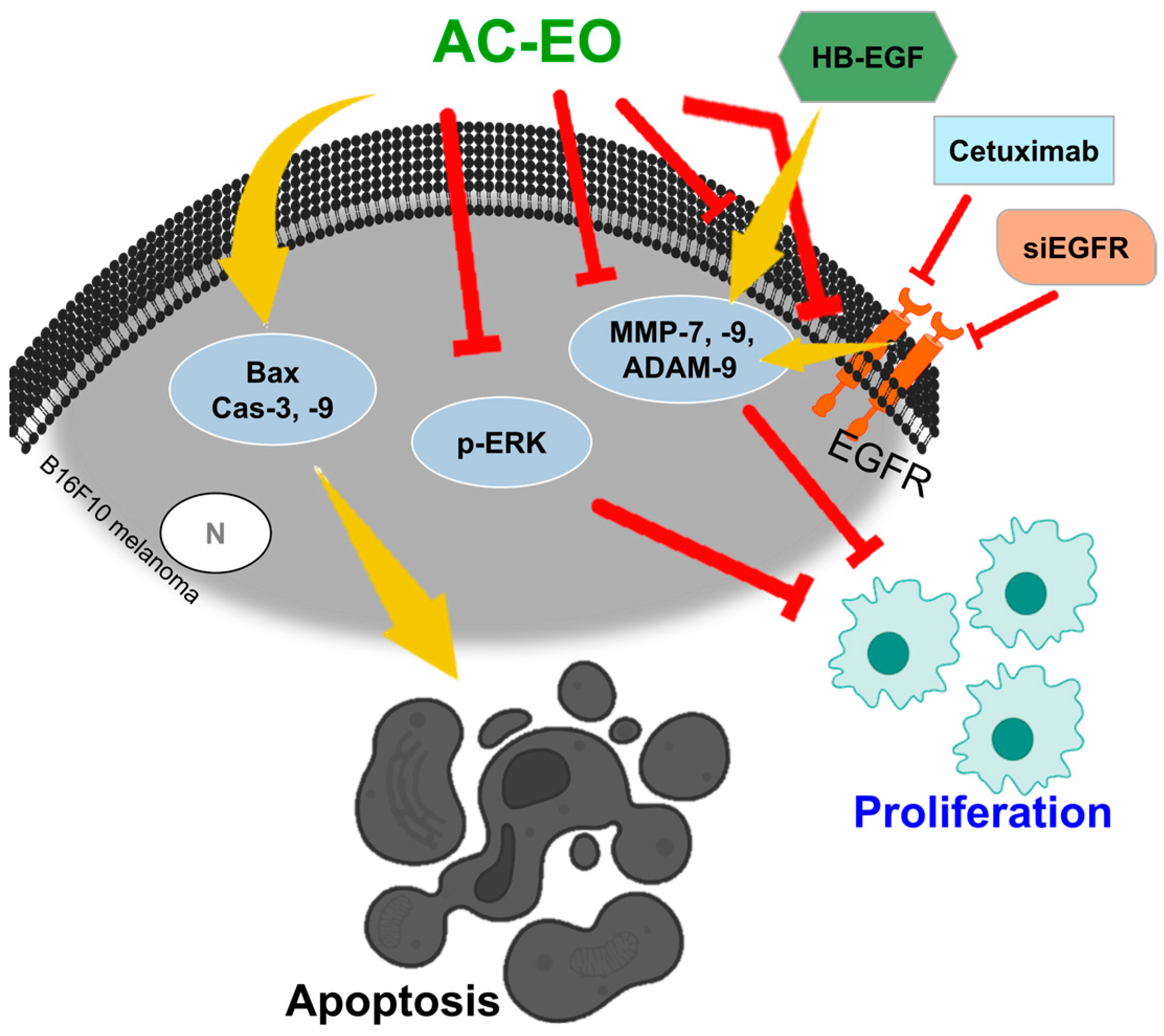
| Essential Oil (EO)/Plant Oil (PO) | Effects | Sources | Parts (Components) | Refs. |
|---|---|---|---|---|
| Citrus limetta peels EO | Anti-inflammatory and anti-oxidant | C. limetta Risso | Fruit peels (flavonoids) | [4,5,8,9] |
| Baccharis dracunculifolia EO | Anti-inflammatory, immunomodulator, anti-bacterial, anti-diabetic, and anti-microbial | B. dracunculifolia | Leaves | [10,11,12,13,14,15,16,17,18] |
| Perilla frutescens L. Britt EO | Anti-inflammatory, anti-bacterial, anti-oxidant, alleviation of cold, cough, nausea, vomiting, abdominal pain, constipation, asthma, and food poisoning | P. frutescens L. Britt | Stems and leaves | [19,20,21,22] |
| Grapefruit EO | Anti-inflammatory, anti-microbial, anti-oxidants, anti-bacterial, anti-proliferation, anti-cancer, and muscle sympathetic nerve activity regulator | C. maxima Burm. Merr | Grapefruit peel (terpenes and terpene oxides) | [29,30,31,34,35,36,37,38,39,40,41,42,43,44,45,46,47,50] |
| Oregano EO | Anti-inflammatory, prevention of neurodegenerative disorders, anti-oxidant, immunomodulatory, anti-cancer, anti-melanogenesis, and anti-microbial | O. vulgare | CRV, thymol | [51,52,53,54,57,58] |
| Satureja sahendica EO | Anti-inflammatory, anti-bacterial | S. sahendica | CRV, thymol, p-cymene, ß-caryophyllene, linalool, and other terpenoids | [59,60,64] |
| Matricaria chamomilla EO | Anti-inflammatory, alleviation of gastrointestinal conditions, anti-spasmodic, and wound healing | M. chamomilla | Azulene | [71,72,73,74] |
| Helianthus annuus PO | Anti-inflammatory, skin hydration | H. annuus | Oleic acid, linoleic acids | [77,79] |
| Mentha arvensis EO | Anti-inflammatory, anti-oxidant, | M. arvensis | Menthol, menthone, and piperitone | [80,82] |
| Rosmarinus officinalis EO | Anti-inflammatory, treat digestive problem, nervous system, and allergy | R. officinalis | Camphor, eucalyptol | [84,85,86] |
| Curcuma longa EO | Anti-inflammatory, skin penetration | C. longa | Rhizome (terpinolene and α-phellandrene) | [87,88,89] |
| Artemisia argyi EO | Anti-inflammatory, anti-mutagenic, anti-tumor, anti-oxidant, anti-asthmatic, anti-fungal, and anti-melanogenesis | A. argyi | Leaves (cineole, camphor, eucalyptol, and caryophyllene) | [90,91,92,93,94,95,96,97,98,99] |
| Lavender EO | Anti-inflammatory, skin protection and wound healing, anxiolytic, neuroprotective, anti-oxidant, analgesic, anti-microbial, and alleviation of joint pain | L. angustifolia | Blossoms (Linalool and linalyl acetate) | [100,101,102,103,104,105,106,107,108,109,110,252,253,254,255,256] |
| Zanthoxylum coreanum EO | Anti-inflammatory, anti-viral | Z. coreanum | β-Ocimene, α-pinene | [111,112,113,114] |
| Aloysia citrodora EO (Lippia citriodora EO) | Anti-melanoma, anti-cancer, anti-oxidant, and anti-bacterial | A. citrodora (L. citriodora) | Geranial, neral, flavonoid, phenol, 1,8-cineole, limonene, and citral | [124,125,126,127,128,129,130,131,132,133] |
| Origanum majorana EO | Anti-melanoma, anti-bacterial, anti-oxidant, and anti-fungal | O. majorana | Terpinen-4-o1, L-α-Terpineol | [149,150] |
| Artemisia capillaris EO | Anti-melanogenesis, anti-cancer, and anti-oxidant | A. capillaris | Grass clumps | [118,156,157] |
| Camellia japonica seed EO | Anti-melanogenesis, anti-oxidant, anti-bacterial, anti-inflammatory, and skin barrier function | C. japonica | Seed (Hexamethylcyclotrisiloxane and octamethylcyclotetrasiloxane) | [160,161,162,163,164,165,168] |
| Origanum syriacum EO and Origanum ehrenbergii EO | Anti-melanogenesis, rheumatism maceration, and neuralgic treatment | O. syriacum and O. ehrenbergii | Terpenoids, quinones, and CRV | [57,169,170,171] |
| Calocedrus formosana EO | Anti-melanogenesis, anti-oxidant, anti-fungal, anti-inflammatory, and anti-cancer | C. formosana | α-Terpineol, Terpinen-4-o1, and thymol | [172,173,174,175,176,177] |
| Melaleuca quinquenervia EO | Anti-melanogenesis, anti-bacterial | M. quinquenervia | 1,8-Cineole, α-Pinene, and α-Terpineol | [178,179,180] |
| Cinnamomum cassia EO | Anti-melanogenesis, anti-bacterial, anti-inflammatory, anti-diabetic, hypouricemic, and anti-fungal | C. cassia | Stem bark and twig (cis-2-methoxycinnamic acid and cinnamaldehyde) | [167,183,184,185,187,188,189] |
| Leaf of Alpinia nantoensis EO and rhizome of Alpinia nantoensis EO | Anti-melanogenesis, anti-cancer, | A. nantoensis | Leaf, rhizome, and stem (camphor, camphene, β-pinene, p-cymene, α-pinene, and D-limonene) | [191,192,193] |
| Chrysanthemum boreale MAKINO EO | Anti-melanogenesis, anti-inflammatory, anti-bacterial, skin regeneration, and wound healing | C. boreale | Flower | [194,196,197] |
| Vitex negundo EO | Anti-melanogenesis, anti-nociceptive, anti-convulsant, anti-inflammatory, and analgesic | V. negundo | leaves and roots (sesquiterpene and monoterpene) | [199,200,201,202,203,204] |
| Achillea millefolium EO | Anti-melanogenesis, alleviation of inflammatory, gastrointestinal disorders, hepatobiliary conditions, overactive cardiovascular, and respiratory infection | A. millefolium | Artemisia ketone, camphor, linalyl acetate, and 1,8-cineole | [206,207,208,209,210,211] |
| Vetiveria zizanioides EO | Anti-melanogenesis, anti-oxidants, antimicrobial, and anti-inflammatory | V. zizanioides | Roots (Cedr-8-en-13-ol and α-Amorphene) | [212,213,214,215,216,217] |
| Eucalyptus camaldulensis EO | Anti-melanogenesis, anti-bacterial, anti-termitic, and anti-oxidant | E. camaldulensis | Leaf (Eucalyptol and γ-Terpinene) | [220,221,222,223] |
| Acorus macrospadiceus EO | Anti-melanogenesis | A. macrospadiceus | Chavicol methyl ether and nootkatone | [228] |
| Artemisia montana Pampan EO | Wound healing, anti-diabetic, anti-inflammatory, and anti-oxidant | A. montana | β-caryophyllene, germacrene D, 1,8-cineole, and camphor | [238,239,240,241] |
| Pistacia lentiscus PO | Wound healing, anti-bacterial, anti-oxidants, and proliferation | P. lentiscus L. | Oleic, palmitic, linoleic, tocopherols, carotenoids, and anthocyanins | [243,244,245,246,247,248,249,250,251] |
| Calophyllum inophyllum PO | Anti-inflammatory, anti-microbial, anti-fungal, and wound healing | C. inophyllum | Calophyllolide, calophyllic acid, inophyllum, and polyphenols | [261,262,263,264,265] |
| Parrotiopsis jacquemontiana PO | Wound healing, anti-microbial, and anti-cancer | P. jacquemontiana | 2, 6-dimethyl-8-oxoocta-2, 6-dienoic acid, syringol, and catechol | [266,267,268] |
| Salvia aurea L. EO | Wound healing | S. aurea | 1,8-cineole, β-pinene, cis-thujone, and camphor | [276] |
| Polyphenol-enriched fraction of Rose plant oil distillation wastewater | Hyperproliferation inhibition, anti-HIV, anti-bacterial, anti-oxidant, anti-tussive, anti-diabetic, anti-inflammatory, and anti-plasmodial | R. damascena Mill. F. trigintipetala Dieck | Polyphenols, flavonoid | [232,277,278] |
| Zanthoxylum bungeanum Maxim EO | Hyperproliferation inhibition, anti-oxidants, anti-cancer, anti-inflammatory, anti-microbial, and anti-insecticidal | Z. bungeanum | D-Limonene, β-Myrcene | [286,287,288,289,290,291] |
| Prunus armeniaca EO | Hyperproliferation inhibition, anti-cancer, anti-oxidant, anti-microbial, anti-inflammatory, and anti-asthmatic, | P. armeniaca | Seeds (Benzaldehyde, benzoic acid, mandelonitrile) | [294,295,296,297,298,299,301] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Hong, J.H. Multiplicative Effects of Essential Oils and Other Active Components on Skin Tissue and Skin Cancers. Int. J. Mol. Sci. 2024, 25, 5397. https://doi.org/10.3390/ijms25105397
Kim HJ, Hong JH. Multiplicative Effects of Essential Oils and Other Active Components on Skin Tissue and Skin Cancers. International Journal of Molecular Sciences. 2024; 25(10):5397. https://doi.org/10.3390/ijms25105397
Chicago/Turabian StyleKim, Hyeong Jae, and Jeong Hee Hong. 2024. "Multiplicative Effects of Essential Oils and Other Active Components on Skin Tissue and Skin Cancers" International Journal of Molecular Sciences 25, no. 10: 5397. https://doi.org/10.3390/ijms25105397
APA StyleKim, H. J., & Hong, J. H. (2024). Multiplicative Effects of Essential Oils and Other Active Components on Skin Tissue and Skin Cancers. International Journal of Molecular Sciences, 25(10), 5397. https://doi.org/10.3390/ijms25105397







