Combining Transcriptome and Whole Genome Re-Sequencing to Screen Disease Resistance Genes for Wheat Dwarf Bunt
Abstract
1. Introduction
2. Results
2.1. Whole Genome Re-Sequencing Analysis
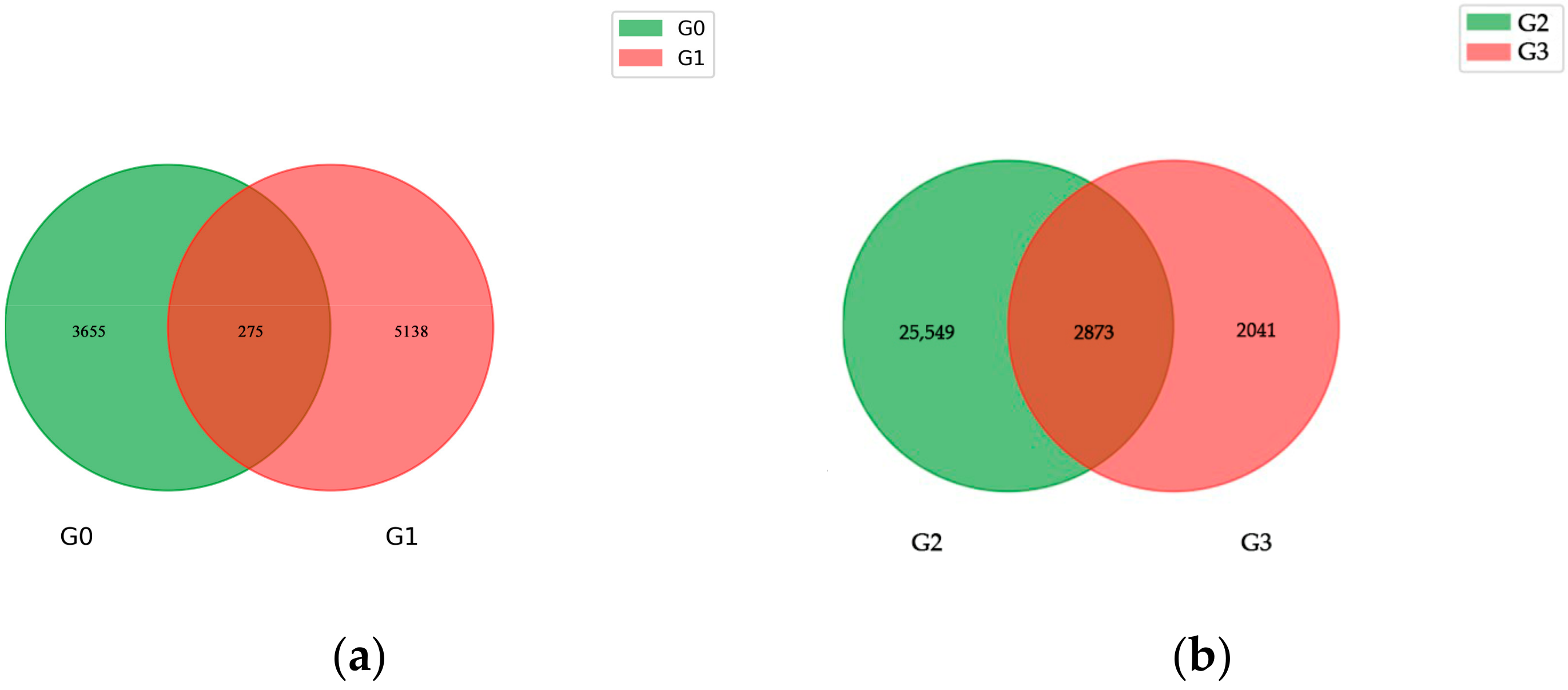
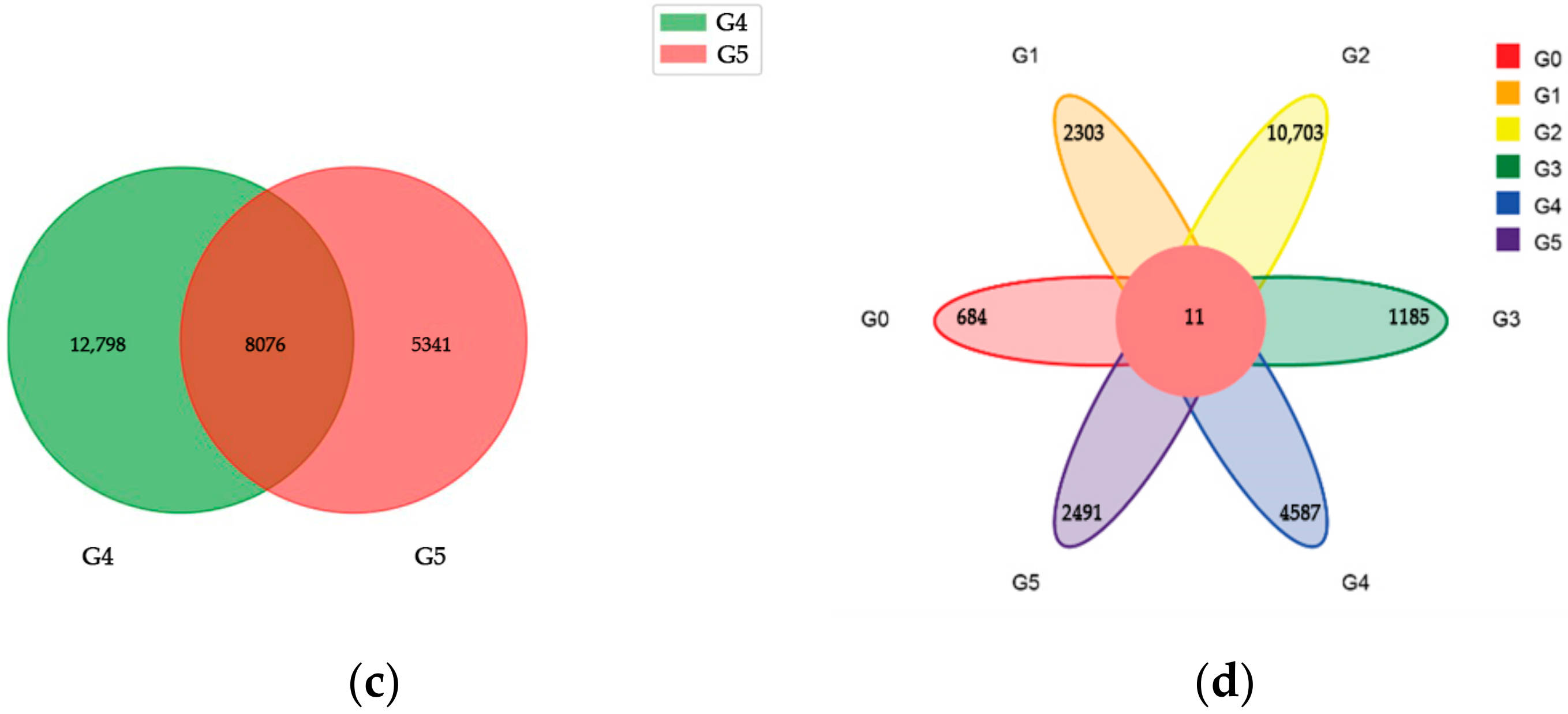
2.2. RNA Sequencing Analysis
2.3. Functional Annotation of DEGs
2.4. Transcription Factor Prediction
2.5. Candidate Resistance Gene Analysis by Integrating WGRS and RNA-Seq
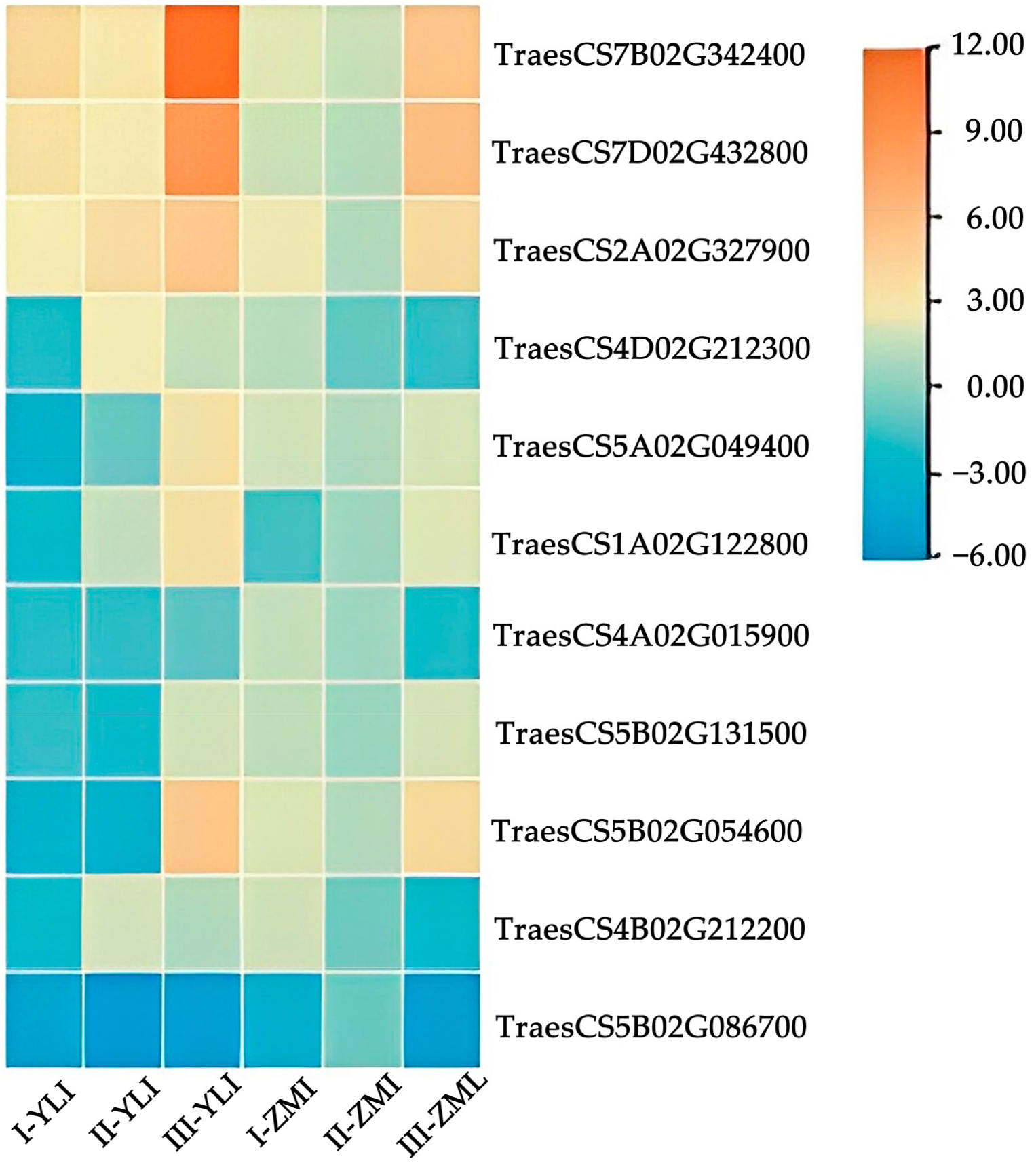
2.6. qRT-PCR Analysis of Candidate Resistance Genes
3. Discussion
3.1. Combined Analysis Based on WGRS and RNA-Seq
3.2. Tryptophan Metabolism and Plant Disease Resistance
3.3. Benzoxazinoid Biosynthesis and Plant Disease Resistance
4. Materials and Methods
4.1. Plant and Fungal Materials
4.2. Pathogen Inoculation
4.3. DNA Extraction, Library Construction and Whole Genome Re-Sequencing
4.4. Variant Detection and Annotation
4.5. RNA Extraction, cDNA Library Construction and Sequencing
4.6. Gene Expression and Annotation
4.7. Expression Profiles of Candidate Resistance Genes Using qRT-PCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patil, S.; Brennan, M.; Mason, S.; Brennan, C. The effects of fortification of legumes and extrusion on the protein digestibility of wheat based snack. Foods 2016, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Jiang, W.; Qin, D.; Liu, T.; Zhang, J.; Chen, W.; Gao, L. Characterization of the microbial communities in wheat tissues and rhizosphere soil caused by dwarf bunt of wheat. Sci. Rep. 2021, 11, 5773. [Google Scholar] [CrossRef] [PubMed]
- Muhae-Ud-Din, G.; Tarafder, E.; Nizamani, M.M.; Wang, Y. First report of wheat dwarf bunt caused by Tilletia controversa Kühn in Pakistan. Plant Dis. 2023, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Nian, S.J.; Yin, Y.P.; Li, M.H.; Cai, J.; Wang, Z.K. Development of a PCR based diagnostic tool specific to wheat dwarf bunt, caused by Tilletia controversa. Eur. J. Plant Pathol. 2009, 124, 585–594. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Duan, X.Y.; Jia, W.M. Advances in risk analyses of introduction and establishment of wheat dwarf bunt in China. Plant Prot. 2007, 2, 6–10. [Google Scholar]
- Wilcoxson, R.D.; Saari, E.E. Bunt and Smut Diseases of Wheat: Concepts and Methods of Disease Management; CIMMYT: México-Veracruz, Mexico, 1996; pp. 12–25. [Google Scholar]
- Zhu, H.Y.; Ma, Z.H.; Zhou, Y.L. A review of studies on wheat dwarf bunt (TCK). In Proceedings of the 6th Youth Symposium of Chinese Society for Plant Pathology—Research Progress Plant Pathology (Volume Five), 2003-08; Chinese Society for Plant Pathology: Shenyang, China, 2003; p. 4. [Google Scholar]
- Hong, X. Methods and Techniques of Plant Quarantine; Beijing Industrial Press: Beijing, China, 2006; p. 150. [Google Scholar]
- Clawson, J.; Nischwitz, C.; Krause, M.; Krause, W. Dwarf Bunt in Winter Wheat; Utah State University Extension: Logan, UT, USA, 2023; pp. 1–4. [Google Scholar]
- Goates, B.J. Identification of new pathogenic races of common bunt and dwarf bunt fungi, and evaluation of known races using an expanded set of differential wheat lines. Plant Dis. 2012, 96, 361–369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Muellner, A.E.; Eshonkulov, B.; Hagenguth, J.; Pachler, B.; Michel, S.; Buerstmayr, M.; Hole, D.; Buerstmayr, H. Genetic mapping of the common and dwarf bunt resistance gene Bt12 descending from the wheat landrace PI119333. Euphytica 2020, 216, 83. [Google Scholar] [CrossRef]
- Samal, P.; Molla, K.A.; Bal, A.; Ray, S.; Swain, H.; Khandual, A.; Sahoo, P.; Behera, M.; Jaiswal, S.; Iquebal, A.; et al. Comparative transcriptome profiling reveals the basis of differential sheath blight disease response in tolerant and susceptible rice genotypes. Protoplasma Int. J. Cell Biol. 2022, 259, 61–73. [Google Scholar] [CrossRef]
- Liao, Z.X.; Ni, Z.; Wei, X.L.; Chen, L.; Li, J.Y.; Yu, Y.H.; Jiang, W.; Jiang, B.L.; He, Y.Q.; Huang, S. Dual RNA-seq of Xanthomonas oryzae pv. oryzicola infecting rice reveals novel insights into bacterial-plant interaction. PLoS ONE 2019, 14, e0215039. [Google Scholar] [CrossRef]
- Li, Y.; Ruperao, P.; Batley, J.; Edwards, D.; Davidson, J.; Hobson, K.; Sutton, T. Genome analysis identified novel candidate genes for ascochyta blight resistance in chickpea using whole genome re-sequencing data. Front. Plant Sci. 2017, 8, 359. [Google Scholar] [CrossRef]
- Patil, G.B.; Lakhssassi, N.; Wan, J.; Song, L.; Zhou, Z.; Klepadlo, M.; Vuong, T.D.; Stec, A.O.; Kahil, S.S.; Colantonio, V.; et al. Whole genome re-sequencing reveals the impact of the interaction of copy number variants of the rhg1 and Rhg4 genes on broad-based resistance to soybean cyst nematode. Plant Biotechnol. J. 2019, 17, 1595–1611. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Han, X.; Zhu, Y.; Fang, Z.; Gao, D.; Ma, D. Transcriptome Profiles of Circular RNAs in Common Wheat during Fusarium Head Blight Disease. Data 2022, 7, 121. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Y.; Wang, C.; Liu, M.; Li, H.; Fu, Y.; Wang, Y.; Nie, Y.; Liu, X.; Ji, W. Large-scale transcriptome comparison reveals distinct gene activations in wheat responding to stripe rust and powdery mildew. BMC Genom. 2014, 15, 898. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Feng, Z.; Zhang, X.; Jiang, K.; Jin, X.; Hang, Y.; Chen, J.-Q.; Tian, D. Genome-wide investigation on the genetic variations of rice disease resistance genes. Plant Mol. Biol. 2006, 62, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.; Angelini, C.; De Feis, I.; Ciccodicola, A. Uncovering the complexity of transcriptomes with RNA-Seq. J. Biomed. Biotechnol. 2010, 1, 853–916. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Li, R.; Xu, X.; Jin, W.; Xu, M.; Zhao, H.; Xiang, Z.; Song, W.; Ying, K.; Zhang, M.; et al. Genome-wide patterns of genetic variation among elite maize inbred lines. Nat. Genet. 2010, 42, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Causse, M.; Desplat, N.; Pascual, L.; Le Paslier, M.-C.; Sauvage, C.; Bauchet, G.; Bérard, A.; Bounon, R.; Tchoumakov, M.; Brunel, D.; et al. Whole genome resequencing in tomato reveals variation associated with introgression and breeding events. BMC Genom. 2013, 14, 791. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Meng, X.; He, L.S.; He, P. Transcriptional regulation of pattern-triggered immunity in plants. Physiol. Behav. 2016, 176, 100–106. [Google Scholar] [CrossRef]
- Gordon, T.; Wang, R.; Hole, D.; Bockelman, H.; Bonman, J.M.; Chen, J. Genetic characterization and genome-wide association mapping for dwarf bunt resistance in bread wheat accessions from the USDA National Small Grains Collection. Theor. Appl. Genet. 2020, 133, 1069–1080. [Google Scholar] [CrossRef]
- Wang, R.; Gordon, T.; Hole, D.; Zhao, W.; Isham, K.; Bonman, J.M.; Goates, B.; Chen, J. Identification and assessment of two major QTLs for dwarf bunt resistance in winter wheat line ‘IDO835’. Theor. Appl. Genet. 2019, 132, 2755–2766. [Google Scholar] [CrossRef]
- Ley, T.J.; Mardis, E.R.; Ding, L.; Fulton, B.; McLellan, M.D.; Chen, K.; Dooling, D.; Dunford-Shore, B.H.; McGrath, S.; Hickenbotham, M.; et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature 2018, 456, 66–72. [Google Scholar] [CrossRef]
- Liu, Z.H. Genome-Wide Resequencing Reveals Microevolution of Chinese Short Fat-Tailed Sheep and Genetic Variation of Reproduction-Related Gene; Shandong Agricultural University: Tai’an, China, 2015. [Google Scholar]
- Su, W.J.; Zhao, N.; Lei, J.; Wang, L.J.; Chai, S.S.; Yang, X.S. SNP sites developed by specific length amplification fragment sequencing (SLAF-Seq) in sweetpotato. Sci. Agric. Sin. 2016, 49, 27–47. [Google Scholar]
- Wang, A.P.; Tu, M.; Xu, Y.; Zhan, X.F.; Cai, H.; Chen, R.H. Correlation analysis of whole genome re-sequencing and transcriptome of potato. Mol. Plant Breed. 2018, 16, 5161–5172. [Google Scholar]
- Yin, M.H.; Wu, P.H.; Liu, B.W.; Hu, G.M.; Wang, Q.; Zhang, H.L. Analysis of correlation between whole genome re-sequencing and transcriptome of shangrao early pear. Mol. Plant Breed. 2019, 17, 3490–3496. [Google Scholar]
- Shen, T.; Xiao, Y.; Su, S.Q.; Jia, Y.F.; Guo, Q.L.; Liu, Q.; Xiao, Y.G. Combined Analysis of Whole Genome Re-Sequencing and Transcriptome of Wheat under the Stress of Tilletia controversa Kühn. Mol. Plant Breed. 2022, 1–17. Available online: https://kns-cnki-net.webvpn.xjau.edu.cn/kcms/detail/46.1068.S.20211125.1939.008.html (accessed on 10 February 2023).
- Radwanski, E.R.; Last, R.L. Tryptophan biosynthesis and metabolism: Biochemical and molecular genetics. Plant Cell 1995, 7, 921–934. [Google Scholar] [PubMed]
- Niyogi, K.K.; Fink, G.R. Two anthranilate synthase genes in Arabidopsis: Defense-related regulation of the tryptophan pathway. Plant Cell 1992, 4, 721–733. [Google Scholar] [PubMed]
- Sonderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of glucosinolates—Gene discovery and beyond. Trends Plant Sci. 2010, 15, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, P.; Piślewska-Bednarek, M.; Ver Loren van Themaat, E.; Maddula, R.K.; Svatoš, A.; Schulze-Lefert, P. Conservation and clade-specific diversification of pathogen-inducible tryptophan and indole glucosinolate metabolism in Arabidopsis thaliana relatives. New Phytol. 2011, 192, 713–726. [Google Scholar] [CrossRef]
- Fujiwara, T.; Maisonneuve, S.; Isshiki, M.; Mizutani, M.; Chen, L.; Wong, H.L.; Kawasaki, T.; Shimamoto, K. Sekiguchi lesion gene encodes a cytochrome P450 monooxygenasethat catalyzes conversion of tryptamine to serotonin in rice. J. Biol. Chem. 2010, 285, 11308–11313. [Google Scholar] [CrossRef]
- Gish, L.A.; Clark, S.E. The RLK/Pelle family of kinases. Plant J. 2011, 66, 117–127. [Google Scholar] [CrossRef]
- Ishihara, A.; Hashimoto, Y.; Tanaka, C.; Dubouzet, J.G.; Nakao, T.; Matsuda, F.; Nishioka, T.; Miyagawa, H.; Wakasa, K. The tryptophan pathway is involved in the defense responses of rice against pathogenic infection via serotonin production. Plant J. 2008, 54, 481–495. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Liu, J.; Din, G.M.U.; Zhang, H.; Du, Z.; Chen, W.; Liu, T.; Zhang, J.; Zhao, S.; Gao, L.; et al. Transcriptome analysis of wheat spikes in response to Tilletia controversa Kühn which cause wheat dwarf bunt. Sci. Rep. 2020, 10, 21567. [Google Scholar] [CrossRef] [PubMed]
- Wahlroos, O.; Virtanen, A.I. The precursors of 6-methoxy-benzoxazolinone in maize and wheat plants. Their isolation and some of their properties. Acta Chem. Scand. 1959, 13, 1906–1908. [Google Scholar] [CrossRef]
- Sicker, D.; Frey, M.; Schulz, M.; Gierl, A. Role of natural benzoxazinonesin the survival strategy of plants. In International Review of Cytology, A Survey of Cell Biology; Jeon, K.W., Ed.; Academic Press: San Diego, CA, USA, 2000; Volume 198, pp. 319–346. [Google Scholar]
- Ishizaki, T.; Hashimoto, Y.; Shudo, K. Reaction of 4-acetoxy-1, 4-benzoxazin-3-one with amino acid derivatives. Heterocycles 1983, 20, 1481–1485. [Google Scholar]
- Akria, O.; Atsushi, I.; Morifumi, H. Induced accumulation of 2-hydroxy-4, 7-dimcthoxy-1, 4-benzoxazin-3-one gluciside (HDMBOA-Glc) in maize leaves. Phytochemistry 2001, 56, 669–675. [Google Scholar]
- Wang, J.H.; Wang, Q.M. Studies on the relation between resistance to Sphacelotheca reiliana (KIIHN) clint var. Zeae pass. and 1,4-dihydroxy-7-methoxy-2H1,4-benzoxazin-3(4H)-one in corn. Plant Prot. 1989, 16, 187–191. [Google Scholar]
- Bucker, C.; Grambow HJ, Z. Alterations in 1, 4-benzoxazinone levels following inoculation with stem rust in wheat leaves carrying various alleles for resistance and their possible role as phytoalexins in moderately resistant leaves. Naturforsch 1990, 45, 1151–1155. [Google Scholar] [CrossRef]
- Zong, Q.Q. Resistance evaluation of Xinjiang winter wheat varieties to dwarf bunt. Zhongguo Zhibao Daokan China Plant Prot. 2020, 40, 81–83. [Google Scholar]
- Su, S.; Zhang, Z.; Shen, T.; Chen, J.; Liu, Q. Kernel Transcriptome Profiles of Susceptible Wheat Genotypes in Response to Wheat Dwarf Bunt. Int. J. Mol. Sci. 2023, 24, 17281. [Google Scholar]
- Abd-Elsalam, K.; Bahkali, A.; Moslem, M.; Amin, O.E.; Niessen, L. An optimized protocol for DNA extraction from wheat seeds and loop-mediated isothermal amplification (LAMP) to detect Fusarium graminearum contamination of wheat grain. Int. J. Mol. Sci. 2011, 12, 3459–3472. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Picard. Available online: http://sourceforge.net/projects/picard/ (accessed on 10 February 2023).
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The fenome analysis toolkit: A mapreduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Florea, L.; Song, L.; Salzberg, S.L. Thousands of exon skipping events differential among splicing patterns in sixteen human tissues. F1000Research 2013, 2, 188. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. Gene Ontol. Consort. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Koonin, E.V. The Clusters of Orthologous Groups (COGs) Database: Phylogenetic classification of proteins from complete genomes. In The NCBI Handbook; McEntyre, J., Ostell, J., Eds.; Nature Portfolio: London, UK, 2002. [Google Scholar]
- Kanehisa, M.; Goto, S.; Kawashima, S.; Kawashima, S.; Okuno, Y.; Hattori, M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004, 32, D277–D280. [Google Scholar] [CrossRef]
- Pfaf, M.W. A new mathematical model for relative quantifcation in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
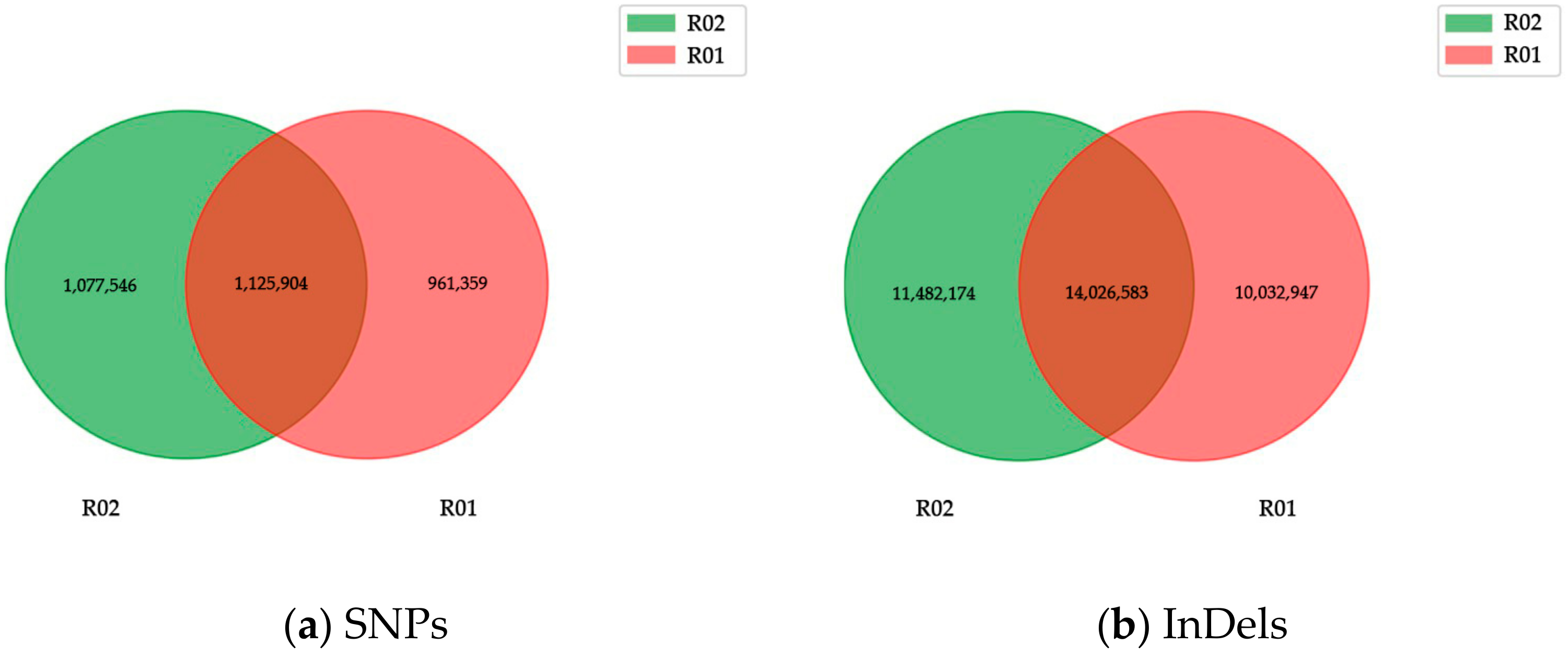
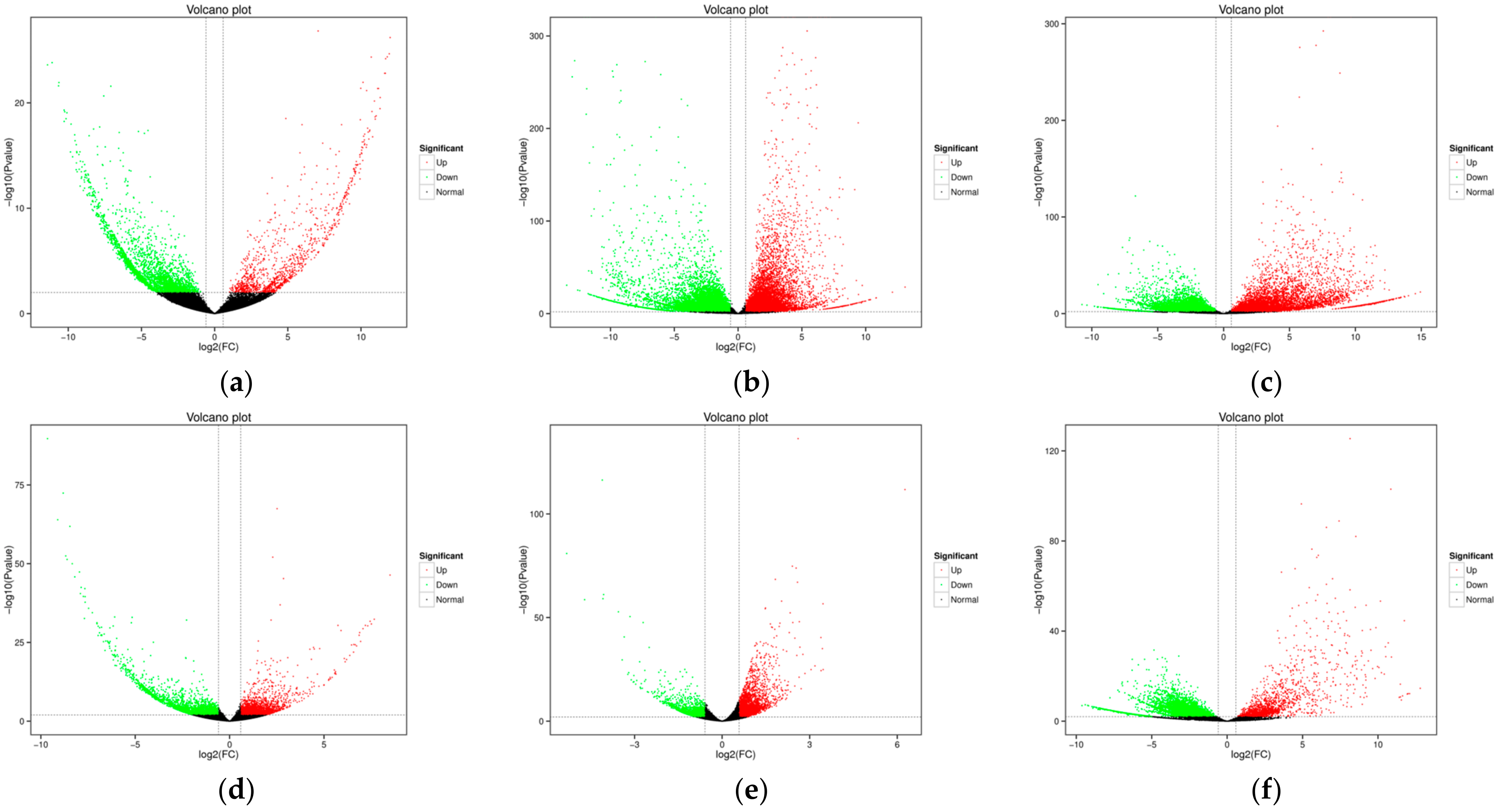
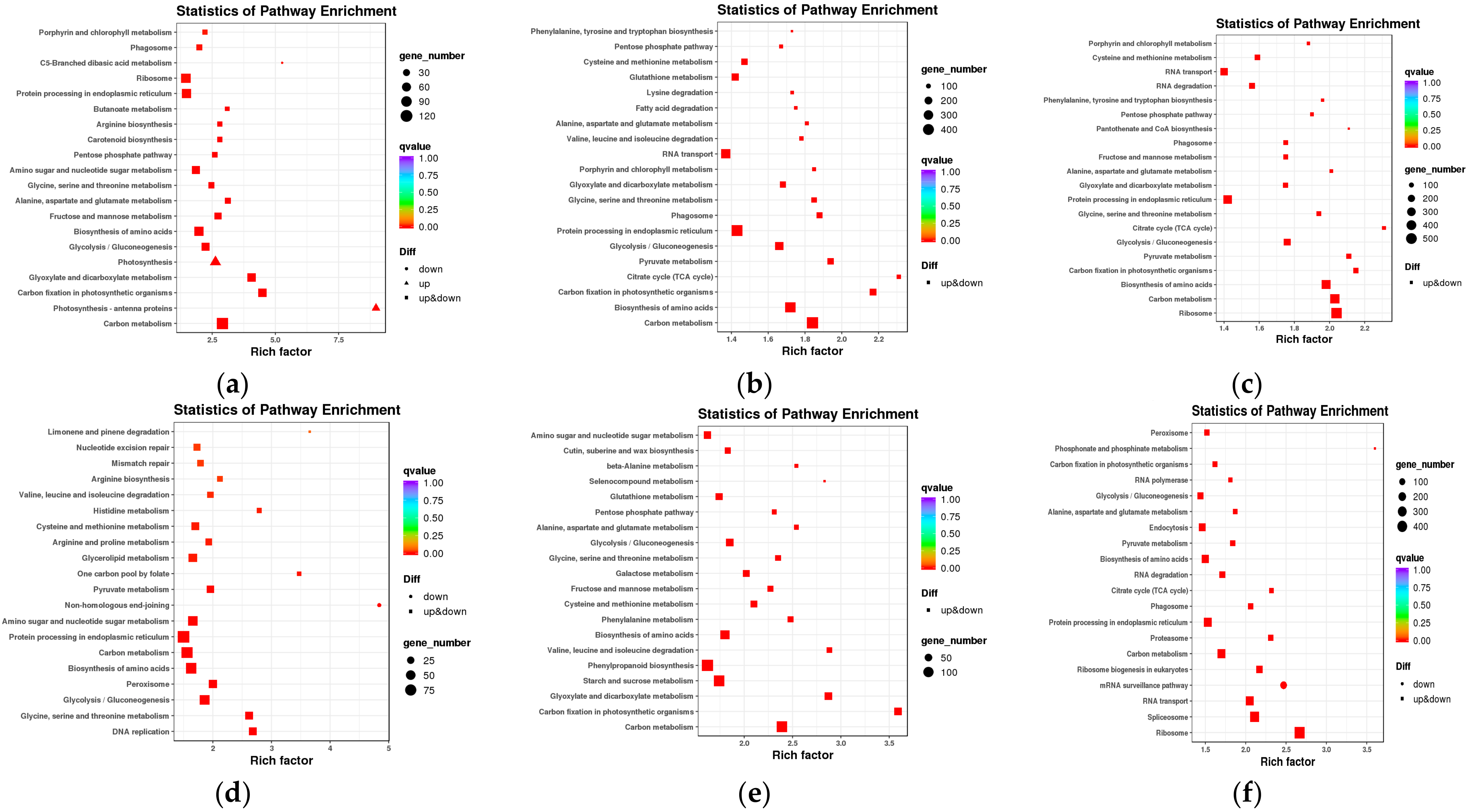
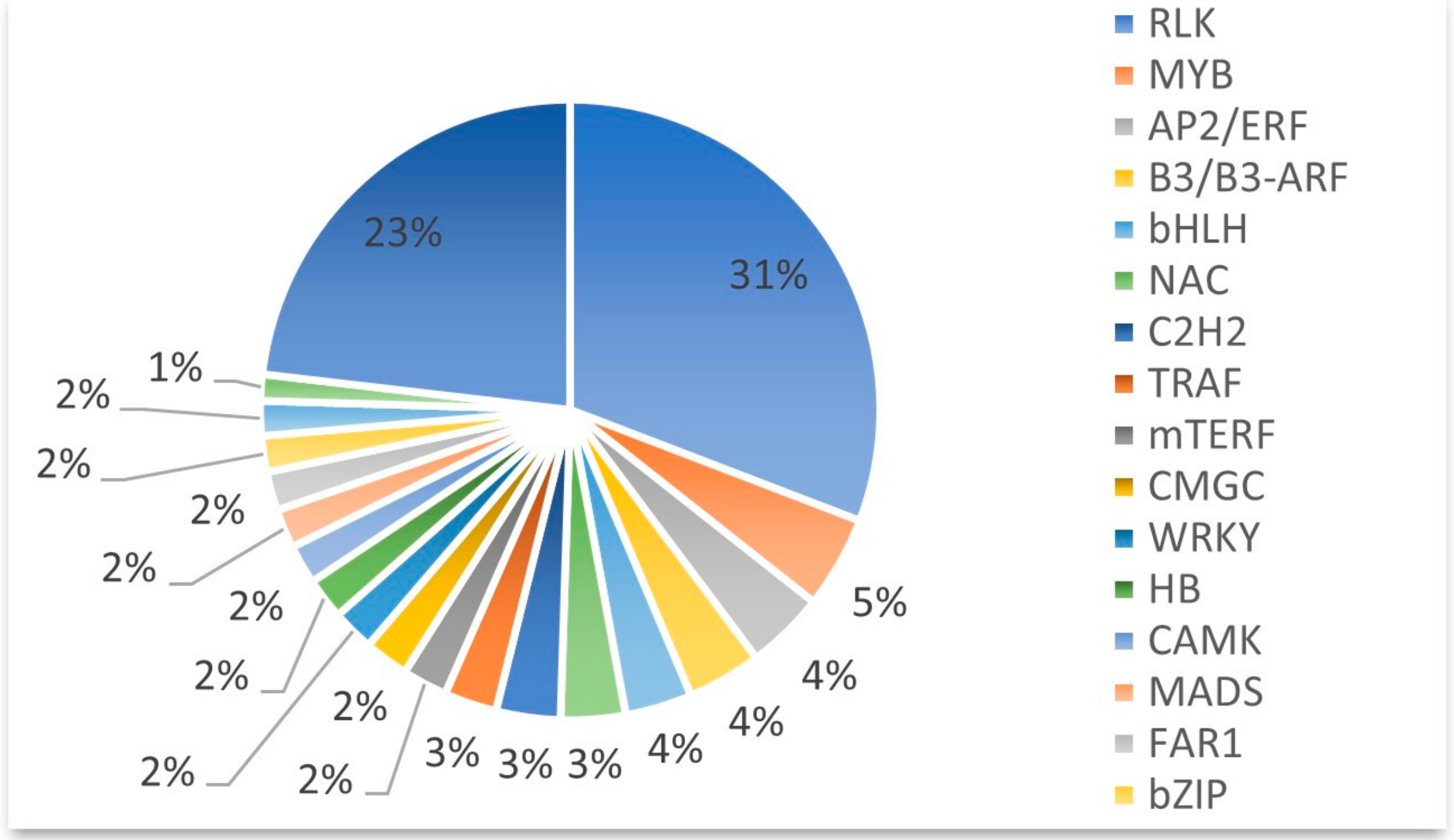
| Variety | Number of SNPs | Numberof InDels | Genes with Non-Synonymous SNPs | Genes with InDels | Total Genes with Variations |
|---|---|---|---|---|---|
| Zhongmai 175 | 24,085,517 | 2,203,974 | 30,742 | 9625 | 32,417 |
| Yili 053 | 25,534,555 | 2,318,474 | 31,512 | 9647 | 33,171 |
| Group | DEG Set | Total | GO | KEGG | COG |
|---|---|---|---|---|---|
| Group 1 | I-YLC vs. I-YLI | 3683 | 2941 | 2406 | 1383 |
| Group 2 | I-ZMC vs. I-ZMI | 15,525 | 11,858 | 10,029 | 5306 |
| Group 3 | II-YLC vs. II-YLI | 1117 | 730 | 606 | 303 |
| Group 4 | II-ZMC vs. II-ZMI | 4691 | 3688 | 3119 | 1799 |
| Group 5 | III-YLC vs. III-YLI | 24,877 | 19,451 | 16,697 | 8934 |
| Group 6 | III-ZMC vs. III-ZMI | 10,535 | 7992 | 6868 | 3668 |
| Gene ID | Regulation | KEGG Annotation | SNP Mutation | |||||
|---|---|---|---|---|---|---|---|---|
| I-YLC vs. I-YLI | II-YLC vs. II-YLI | III-YLC vs. III-YLI | I-ZMC vs. I-ZMI | II-ZMC vs. II-ZMI | III-ZMC vs. III-ZMI | |||
| TraesCS7B02G342400 | up | up | up | up | up | up | Benzoxazinoid biosynthesis (ko00402) | Yes |
| TraesCS7D02G432800 | up | up | up | up | up | up | Benzoxazinoid biosynthesis (ko00402) | Yes |
| TraesCS2A02G327900 | up | up | up | up | up | up | Tryptophan metabolism (ko00380) | Yes |
| TraesCS5A02G049400 | down | down | up | up | up | up | Protein processing in endoplasmic reticulum (ko04141) | No |
| TraesCS5B02G131500 | down | down | up | up | up | up | -- | Yes |
| TraesCS5B02G054600 | down | down | up | up | up | up | Protein processing in endoplasmic reticulum (ko04141) | Yes |
| TraesCS5B02G086700 | down | down | down | down | down | down | -- | Yes |
| TraesCS4A02G015900 | down | down | down | up | up | down | Alanine, aspartate and glutamate metabolism (ko00250) | Yes |
| TraesCS4B02G212200 | down | up | up | up | down | down | Glycine, serine and threonine metabolism (ko00260) | No |
| TraesCS4D02G212300 | down | up | up | up | down | down | Cysteine and methionine metabolism (ko00270) | Yes |
| TraesCS1A02G122800 | down | up | up | down | up | up | Valine, leucine and isoleucine degradation (ko00280) | Yes |
| Gene ID | ZM175 | YL053 | ||||
|---|---|---|---|---|---|---|
| qRT-PCR | FPKM | Validated | qRT-PCR | FPKM | Validated | |
| TraesCS7B02G342400 | 0.15 ± 0.21 c | 110.78 | + | 0.07 ± 0.06 ab | 175.8 | + |
| TraesCS7D02G432800 | 0.38 ± 0.06 bc | 78.11 | + | 0.61 ± 0.31 ab | 79.31 | + |
| TraesCS2A02G327900 | 0.31 ± 0.01 c | 24.2 | + | 0.31 ± 0.17 ab | 52.97 | + |
| TraesCS5A02G049400 | 0.85 ± 0.1 bc | 11.8 | + | 0.78 ± 0.19 ab | 39.71 | + |
| TraesCS5B02G131500 | 1.29 ± 0.11 bc | 241.8 | + | 1.1 ± 1.57 ab | 235.09 | + |
| TraesCS5B02G054600 | 1.03 ± 1.07 bc | 15.11 | + | 0.56 ± 0.45 ab | 15.42 | + |
| TraesCS5B02G086700 | 2.06 ± 0.03 ab | 10.41 | + | 0.22 ± 0.07 ab | 8.78 | + |
| TraesCS4A02G015900 | 0.72 ± 0.42 bc | 30.87 | + | 1.47 ± 0.98 ab | 48.21 | + |
| TraesCS4B02G212200 | 3.27 ± 2.46 a | 111.19 | + | 2.16 ± 2.39 ab | 329.83 | + |
| TraesCS4D02G212300 | 1.14 ± 0.68 bc | 38.7 | + | 2.33 ± 1.85 a | 147.36 | + |
| TraesCS1A02G122800 | 0.34 ± 0.02 c | 11.89 | + | 0.23 ± 0.14 ab | 12.52 | + |
| Sample Collection Time | Character | Name of Zhongmai 175 | Name of Yili 053 |
|---|---|---|---|
| Mid-filling stage | Diseased | Ⅰ-ZMI1 | Ⅰ-YLI1 |
| Ⅰ-ZMI2 | Ⅰ-YLI2 | ||
| Ⅰ-ZMI3 | Ⅰ-YLI3 | ||
| Healthy | Ⅰ-ZMC1 | Ⅰ-YLC1 | |
| Ⅰ-ZMC2 | Ⅰ-YLC2 | ||
| Ⅰ-ZMC3 | Ⅰ-YLC3 | ||
| Late-filling stage | Diseased | Ⅱ-ZMI1 | Ⅱ-YLI1 |
| Ⅱ-ZMI2 | Ⅱ-YLI2 | ||
| Ⅱ-ZMI3 | Ⅱ-YLI3 | ||
| Healthy | Ⅱ-ZMC1 | Ⅱ-YLC1 | |
| Ⅱ-ZMC2 | Ⅱ-YLC2 | ||
| Ⅱ-ZMC3 | Ⅱ-YLC3 | ||
| Maturity stage | Diseased | Ⅲ-ZMI1 | Ⅲ-YLI1 |
| Ⅲ-ZMI2 | Ⅲ-YLI2 | ||
| Ⅲ-ZMI3 | Ⅲ-YLI3 | ||
| Healthy | Ⅲ-ZMC1 | Ⅲ-YLC1 | |
| Ⅲ-ZMC2 | Ⅲ-YLC2 | ||
| Ⅲ-ZMC3 | Ⅲ-YLC3 |
| Gene ID | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| TraesCS7B02G342400 | GTCCAACGACAGGTTCCG | CACACCGTAGCTCCCTTG |
| TraesCS7D02G432800 | TTCCTCACCGTGCTGCTC | GTTCCCTTCGCCGTCCTC |
| TraesCS2A02G327900 | GGAGACCGTCAAGAGCTA | GTGTCCTGCGTGTAGTTG |
| TraesCS5A02G049400 | CAGCATCTGCCTGGAGAC | GACTTGGACCTGGAGGAG |
| TraesCS5B02G131500 | GACCTGCTGAAAGGATCT | TTGTTCTTGGGATTCTCGTA |
| TraesCS5B02G054600 | ATGACATCTTCGGAGTACC | GACTTGGACTTGGAGGAG |
| TraesCS5B02G086700 | CATCCGAAGCAGCAGTCT | GCTTGGAGACGATGGACT |
| TraesCS4A02G015900 | GAATCGGCAACGGTCTAC | CCAATCCACCAGCAGAAC |
| TraesCS4B02G212200 | CTGAAGAAGGAGGAGGTG | AGGAACTTACCACTGCTG |
| TraesCS4D02G212300 | AAGAAGGAGCAGGAGGAG | TCTGGATGGACTTGACCT |
| TraesCS1A02G122800 | ACATTAAACGCACCAACCT | ACCATCTTCCGAGTCTCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Shen, T.; Wen, Z.; Chen, J.; Liu, Q. Combining Transcriptome and Whole Genome Re-Sequencing to Screen Disease Resistance Genes for Wheat Dwarf Bunt. Int. J. Mol. Sci. 2023, 24, 17356. https://doi.org/10.3390/ijms242417356
Jia Y, Shen T, Wen Z, Chen J, Liu Q. Combining Transcriptome and Whole Genome Re-Sequencing to Screen Disease Resistance Genes for Wheat Dwarf Bunt. International Journal of Molecular Sciences. 2023; 24(24):17356. https://doi.org/10.3390/ijms242417356
Chicago/Turabian StyleJia, Yufeng, Tong Shen, Zhiwei Wen, Jing Chen, and Qi Liu. 2023. "Combining Transcriptome and Whole Genome Re-Sequencing to Screen Disease Resistance Genes for Wheat Dwarf Bunt" International Journal of Molecular Sciences 24, no. 24: 17356. https://doi.org/10.3390/ijms242417356
APA StyleJia, Y., Shen, T., Wen, Z., Chen, J., & Liu, Q. (2023). Combining Transcriptome and Whole Genome Re-Sequencing to Screen Disease Resistance Genes for Wheat Dwarf Bunt. International Journal of Molecular Sciences, 24(24), 17356. https://doi.org/10.3390/ijms242417356






