Evaluation of the Antiviral Activity of Tabamide A and Its Structural Derivatives against Influenza Virus
Abstract
:1. Introduction
2. Results
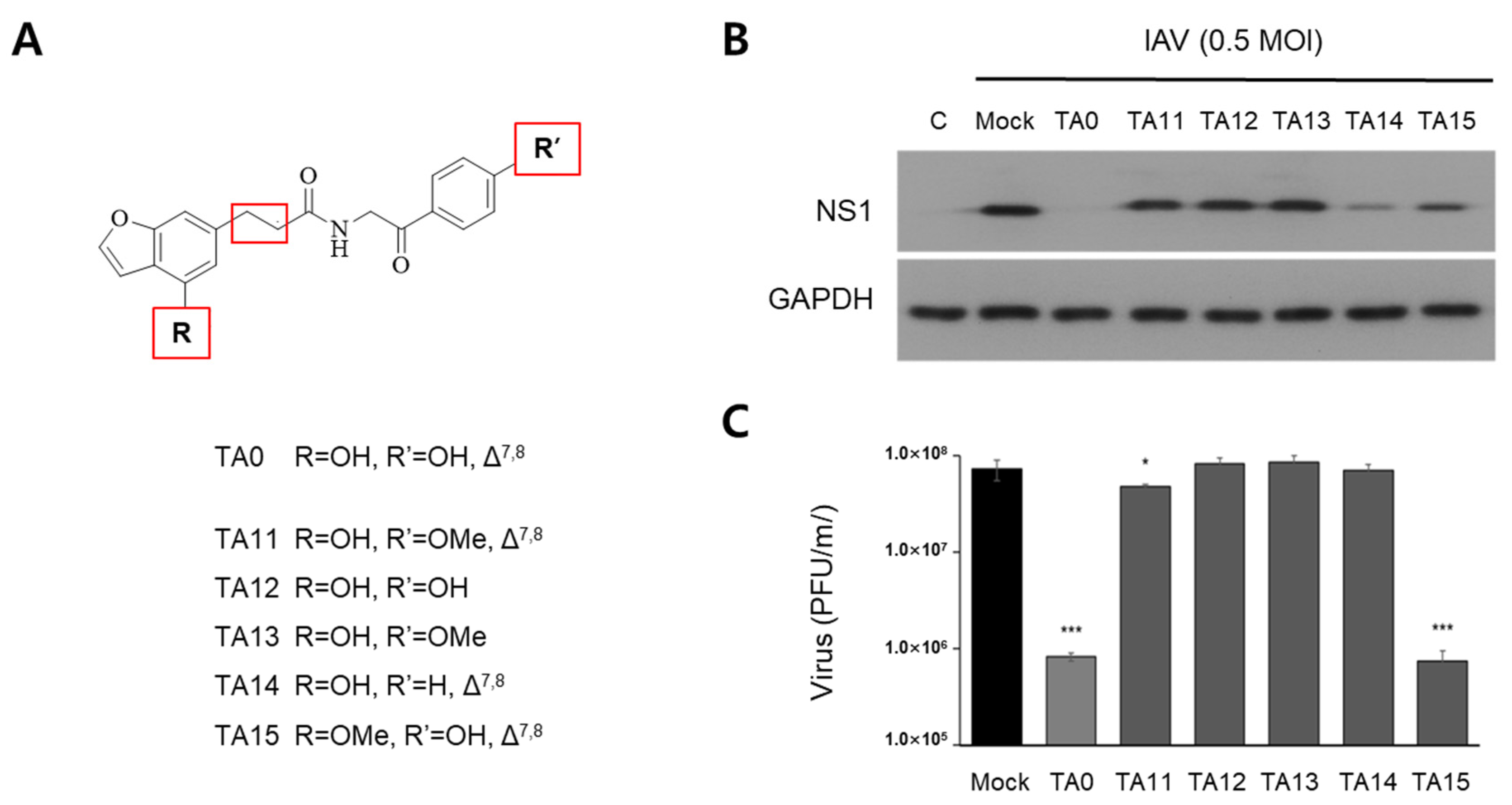
2.1. Anti-Influenza Activity of Synthetic TA0 and Its Derivatives
2.2. Synthesis of Secondary Derivatives Based on the Relationship between the Structure of Tabamide A and Its Antiviral Function
2.3. Secondary Derivatives of TA0 Effectively Inhibit IAV Infection
2.4. Inhibitory Role of TA25 on Viral Infection and Amplification
3. Discussion
4. Materials and Methods
4.1. TA0 (Tabamide A) and Its Derivatives
4.2. Cell Culture
4.3. Virus and Viral Infection
4.4. Determination of Virus-Induced Cytopathic Effects
4.5. Plaque Assay
4.6. Reverse Transcription and Real-Time PCR Analysis
4.7. Immunoblot Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, Y.; Ou, J.; Zhao, S.; Ma, K.; Lan, W.; Guan, W.; Wu, X.; Zhang, J.; Zhang, B.; Zhao, W.; et al. Characterization of Influenza A and B Viruses Circulating in Southern China During the 2017–2018 Season. Front. Microbiol. 2020, 11, 1079. [Google Scholar] [CrossRef]
- Ludwig, S.; Pleschka, S.; Planz, O.; Wolff, T. Ringing the alarm bells: Signalling and apoptosis in influenza virus infected cells. Cell Microbiol. 2006, 8, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Planz, O. Development of cellular signaling pathway inhibitors as new antivirals against influenza. Antivir. Res. 2013, 98, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, C.; Ludwig, S. A new player in a deadly game: Influenza viruses and the PI3K/Akt signalling pathway. Cell Microbiol. 2009, 11, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Staller, E.; Barclay, W.S. Host Cell Factors That Interact with Influenza Virus Ribonucleoproteins. Cold Spring Harb. Perspect. Med. 2021, 11, a038307. [Google Scholar] [CrossRef]
- Hirata, N.; Suizu, F.; Matsuda-Lennikov, M.; Edamura, T.; Bala, J.; Noguchi, M. Inhibition of Akt kinase activity suppresses entry and replication of influenza virus. Biochem. Biophys. Res. Commun. 2014, 450, 891–898. [Google Scholar] [CrossRef]
- Han, C.W.; Jeong, M.S.; Jang, S.B. Structure and Function of the Influenza A Virus Non-Structural Protein 1. J. Microbiol. Biotechnol. 2019, 29, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, C.; Wolff, T.; Pleschka, S.; Planz, O.; Beermann, W.; Bode, J.G.; Schmolke, M.; Ludwig, S. Influenza A virus NS1 protein activates the PI3K/Akt pathway to mediate antiapoptotic signaling responses. J. Virol. 2007, 81, 3058–3067. [Google Scholar] [CrossRef]
- Zhirnov, O.P.; Klenk, H.D. Influenza A virus proteins NS1 and hemagglutinin along with M2 are involved in stimulation of autophagy in infected cells. J. Virol. 2013, 87, 13107–13114. [Google Scholar] [CrossRef]
- Malik, G.; Zhou, Y. Innate Immune Sensing of Influenza A Virus. Viruses 2020, 12, 755. [Google Scholar] [CrossRef]
- Shie, J.J.; Fang, J.M. Development of effective anti-influenza drugs: Congeners and conjugates—A review. J. Biomed. Sci. 2019, 26, 84. [Google Scholar] [CrossRef]
- Davis, A.M.; Chabolla, B.J.; Newcomb, L.L. Emerging antiviral resistant strains of influenza A and the potential therapeutic targets within the viral ribonucleoprotein (vRNP) complex. Virol. J. 2014, 11, 167. [Google Scholar] [CrossRef] [PubMed]
- Gubareva, L.V.; Kaiser, L.; Hayden, F.G. Influenza virus neuraminidase inhibitors. Lancet 2000, 355, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, D.; Palombo, E.A.; Chia Yeo, T.; Lim Siok Ley, D.; Lee Tu, C.; Malherbe, F.; Grollo, L. Identification of traditional medicinal plant extracts with novel anti-influenza activity. PLoS ONE 2013, 8, e79293. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Jin, J.; Dou, J.; Guo, Q.; Ke, X.; Zhou, C.; Guo, M. Inhibitory Activity of Honeysuckle Extracts against Influenza A Virus In Vitro and In Vivo. Virol. Sin. 2021, 36, 490–500. [Google Scholar] [CrossRef]
- Wang, W.; Snooks, H.D.; Sang, S. The Chemistry and Health Benefits of Dietary Phenolamides. J. Agric. Food Chem. 2020, 68, 6248–6267. [Google Scholar] [CrossRef] [PubMed]
- Roumani, M.; Besseau, S.; Gagneul, D.; Robin, C.; Larbat, R. Phenolamides in plants: An update on their function, regulation, and origin of their biosynthetic enzymes. J. Exp. Bot. 2021, 72, 2334–2355. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.W. China at the crossroads: The economics of tobacco and health. Tob. Control 2006, 15 (Suppl. 1), i37–i41. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Zare, S.; Asadollahi, M.; Schuman, M.C. Ecological Roles and Biological Activities of Specialized Metabolites from the Genus Nicotiana. Chem. Rev. 2017, 117, 12227–12280. [Google Scholar] [CrossRef]
- Shang, S.-Z.; Duan, Y.-X.; Zhang, X.; Pu, J.-X.; Sun, H.-D.; Chen, Z.-Y.; Miao, M.-M.; Yang, G.-Y.; Chen, Y.-K. Phenolic amides from the leaves of Nicotiana tabacum and their anti-tobacco mosaic virus activities. Phytochem. Lett. 2014, 9, 184–187. [Google Scholar] [CrossRef]
- Chen, Y.-K.; Li, X.-S.; Yang, G.-Y.; Chen, Z.-Y.; Hu, Q.-F.; Miao, M.-M. Phenolic compounds from Nicotiana tabacum and their biological activities. J. Asian Nat. Prod. Res. 2012, 14, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Damodar, K.; Shin, S.; Jeon, S.H.; Lee, J.T. First synthesis of tabamides A–C and their derivatives: In vitro nitric oxide inhibitory activity. Tetrahedron Lett. 2021, 85, 153482. [Google Scholar] [CrossRef]
- Li, Y.; Anderson, D.H.; Liu, Q.; Zhou, Y. Mechanism of influenza A virus NS1 protein interaction with the p85beta, but not the p85alpha, subunit of phosphatidylinositol 3-kinase (PI3K) and up-regulation of PI3K activity. J. Biol. Chem. 2008, 283, 23397–23409. [Google Scholar] [CrossRef] [PubMed]
- Soares, J.A.; Leite, F.G.; Andrade, L.G.; Torres, A.A.; De Sousa, L.P.; Barcelos, L.S.; Teixeira, M.M.; Ferreira, P.C.; Kroon, E.G.; Souto-Padron, T.; et al. Activation of the PI3K/Akt pathway early during vaccinia and cowpox virus infections is required for both host survival and viral replication. J. Virol. 2009, 83, 6883–6899. [Google Scholar] [CrossRef]
- Ferraris, O.; Kessler, N.; Lina, B. Sensitivity of influenza viruses to zanamivir and oseltamivir: A study performed on viruses circulating in France prior to the introduction of neuraminidase inhibitors in clinical practice. Antivir. Res. 2005, 68, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Morokutti-Kurz, M.; König-Schuster, M.; Koller, C.; Graf, C.; Graf, P.; Kirchoff, N.; Reutterer, B.; Seifert, J.-M.; Unger, H.; Grassauer, A.; et al. The Intranasal Application of Zanamivir and Carrageenan Is Synergistically Active against Influenza A Virus in the Murine Model. PLoS ONE 2015, 10, e0128794. [Google Scholar] [CrossRef]
- Chai, N.; Swem, L.R.; Reichelt, M.; Chen-Harris, H.; Luis, E.; Park, S.; Fouts, A.; Lupardus, P.; Wu, T.D.; Li, O.; et al. Two Escape Mechanisms of Influenza A Virus to a Broadly Neutralizing Stalk-Binding Antibody. PLOS Pathog. 2016, 12, e1005702. [Google Scholar] [CrossRef]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef]
- Musarra-Pizzo, M.; Pennisi, R.; Ben-Amor, I.; Mandalari, G.; Sciortino, M.T. Antiviral Activity Exerted by Natural Products against Human Viruses. Viruses 2021, 13, 828. [Google Scholar] [CrossRef]
- Wu, W.; Li, R.; Li, X.; He, J.; Jiang, S.; Liu, S.; Yang, J. Quercetin as an Antiviral Agent Inhibits Influenza A Virus (IAV) Entry. Viruses 2015, 8, 6. [Google Scholar] [CrossRef]
- Jain, A.S.; Sushma, P.; Dharmashekar, C.; Beelagi, M.S.; Prasad, S.K.; Shivamallu, C.; Prasad, A.; Syed, A.; Marraiki, N.; Prasad, K.S. In silico evaluation of flavonoids as effective antiviral agents on the spike glycoprotein of SARS-CoV-2. Saudi J. Biol. Sci. 2021, 28, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Fanunza, E.; Iampietro, M.; Distinto, S.; Corona, A.; Quartu, M.; Maccioni, E.; Horvat, B.; Tramontano, E. Quercetin Blocks Ebola Virus Infection by Counteracting the VP24 Interferon-Inhibitory Function. Antimicrob. Agents Chemother. 2020, 64, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Takahashi, K.; Shiraki, K.; Sakamoto, K.; Smee, D.F.; Barnard, D.L.; Gowen, B.B.; Julander, J.G.; Morrey, J.D. T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections. Antiviral Res. 2009, 82, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Yang, T. Baloxavir Marboxil: The First Cap-Dependent Endonuclease Inhibitor for the Treatment of Influenza. Ann. Pharmacother. 2019, 53, 754–759. [Google Scholar] [CrossRef]
- Furuta, Y.; Gowen, B.B.; Takahashi, K.; Shiraki, K.; Smee, D.F.; Barnard, D.L. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res. 2013, 100, 446–454. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.Y.; Lee, J.H.; Kim, J.W.; Im, W.R.; Damodar, K.; Woo, H.R.; Kim, W.-K.; Lee, J.T.; Jeon, S.H. Evaluation of the Antiviral Activity of Tabamide A and Its Structural Derivatives against Influenza Virus. Int. J. Mol. Sci. 2023, 24, 17296. https://doi.org/10.3390/ijms242417296
Shin SY, Lee JH, Kim JW, Im WR, Damodar K, Woo HR, Kim W-K, Lee JT, Jeon SH. Evaluation of the Antiviral Activity of Tabamide A and Its Structural Derivatives against Influenza Virus. International Journal of Molecular Sciences. 2023; 24(24):17296. https://doi.org/10.3390/ijms242417296
Chicago/Turabian StyleShin, Soo Yong, Joo Hee Lee, Jin Woo Kim, Wonkyun Ronny Im, Kongara Damodar, Hyung Ryeol Woo, Won-Keun Kim, Jeong Tae Lee, and Sung Ho Jeon. 2023. "Evaluation of the Antiviral Activity of Tabamide A and Its Structural Derivatives against Influenza Virus" International Journal of Molecular Sciences 24, no. 24: 17296. https://doi.org/10.3390/ijms242417296
APA StyleShin, S. Y., Lee, J. H., Kim, J. W., Im, W. R., Damodar, K., Woo, H. R., Kim, W.-K., Lee, J. T., & Jeon, S. H. (2023). Evaluation of the Antiviral Activity of Tabamide A and Its Structural Derivatives against Influenza Virus. International Journal of Molecular Sciences, 24(24), 17296. https://doi.org/10.3390/ijms242417296






