Abstract
Glaucoma is similar to a neurodegenerative disorder and leads to global irreversible loss of vision. Despite extensive research, the pathophysiological mechanisms of glaucoma remain unclear, and no complete cure has yet been identified for glaucoma. Recent studies have shown that microRNAs can serve as diagnostic biomarkers or therapeutic targets for glaucoma; however, there are few bibliometric studies that focus on using microRNAs in glaucoma research. Here, we have adopted a bibliometric analysis in the field of microRNAs in glaucoma research to manifest the current tendencies and research hotspots and to present a visual map of the past and emerging tendencies in this field. In this study, we retrieved publications in the Web of Science database that centered on this field between 2007 and 2022. Next, we used VOSviewer, CiteSpace, Scimago Graphica, and Microsoft Excel to present visual representations of a co-occurrence analysis, co-citation analysis, tendencies, hotspots, and the contributions of authors, institutions, journals, and countries/regions. The United States was the main contributor. Investigative Ophthalmology and Visual Science has published the most articles in this field. Over the past 15 years, there has been exponential growth in the number of publications and citations in this field across various countries, organizations, and authors. Thus, this study illustrates the current trends, hotspots, and emerging frontiers and provides new insight and guidance for searching for new diagnostic biomarkers and clinical trials for glaucoma in the future. Furthermore, international collaborations can also be used to broaden and deepen the field of microRNAs in glaucoma research.
1. Introduction
Glaucoma is a neurodegenerative disorder that affects the visual system and eventually leads to a global and irreversible loss of vision [1]. Glaucoma currently affects millions of people worldwide and is expected to affect 111.8 million people by 2040 [2]. The slow loss of retinal ganglion cells (RGCs) and their axons is a characteristic symptom of glaucoma [3]. Moreover, despite considerable research, the mechanisms underlying the pathogenesis of glaucoma currently remain unclear, although an increase in intraocular pressure (IOP) is known to be the main risk factor for glaucoma [4]. Presently, a reduction in the intraocular pressure in glaucoma patients is the only verified treatment approach, including for normal IOP glaucoma patients [5,6]. Glaucoma can be classified into either open-angle glaucoma (OAG) or closed-angle glaucoma (CAG), which are further divided into primary and secondary [7], with primary open-angle glaucoma (POAG) being the most common type of glaucoma. Currently, there is no complete cure for glaucoma, with current treatments concentrating on delaying the progression of the disease, meaning that early diagnosis and treatment are essential [8,9]; however, the early detection of POAG remains unsatisfactory, which exacerbates the encumbrance of worldwide glaucoma.
Numerous research studies have shown that microRNAs (miRNAs) play an essential role in the pathogenesis of POAG [4]. Moreover, some studies have identified miRNAs as both diagnostic and prospective biomarkers for various diseases, such as cardiovascular diseases and neurodegenerative diseases, including retinal disorders and cancers [10,11,12]. miRNAs, which belong to endogenous non-coding RNA, are formed from small single-stranded nucleotides (~22 bp in length) [13], and recognize and bind to specific target mRNA sequences to either induce their degradation [14] or suppress their translation [15], thereby modulating the expressions of target genes through post-transcriptional regulation [16]. Previous research has shown that miRNAs are dysregulated in multiple diseases and may be involved in the pathogenesis of glaucoma [17]. Thus, various miRNAs were found to serve as biomarkers of POAG and aid in its early diagnosis [18]. For instance, it has been shown that miR-24, miR-29b, miR-200c, and miR-204 are potential diagnostic biomarkers or therapy targets for glaucoma [17,19]. Additionally, several miRNAs (e.g., miR-143-3p, miR-125b-5p, and miR-1260b) found in aqueous humor (AH) may serve as targets for therapeutic intervention [4]. Further, a gene mutation in miR-182 caused an increase in IOP and was found to be upregulated in the AH of POAG patients [20]. Overall, miRNAs play an important role in the diagnosis and treatment of glaucoma [21], and while there is a lot of potential for miRNAs in this area, challenges remain.
Bibliometric analysis is a commonly used scientific and quantitative research approach for publications, which includes collecting, processing, and managing the data from previous scientific publications to summarize the advancements in research topics and analyze the contributions of authors, institutions, journals, and countries or regions [22,23]. In addition, bibliometric analysis can identify hotspots, emerging tendencies, and knowledge networks in a particular field [24]. However, there are minimal studies available that have applied a bibliometric analysis to the field of miRNAs in glaucoma.
Therefore, in this study, we performed a systematic bibliometric analysis of the literature for research including miRNAs and glaucoma between 2007 and 2022. Here, we analyze the number of annual publications, the contributions of the authors, institutions, journals, and countries or regions, the publishing trends, the international collaborations, the co-occurrence visualization analysis of keywords, and the references. Moreover, we provide an outlook on the recent progress in research over the past 15 years and identify the research hotspots and trends in this field. Taken together, we aimed to summarize the past research and provide a foundation of research or novel frontier to enable further research to be performed on miRNAs in glaucoma.
2. Results
2.1. General Data
We initially retrieved a total of 209 publications from the Web of Science Core Collection (WoSCC) database, covering the period from 1 January 2007 to 28 December 2022. Next, the publication types were limited to original and review articles, and the publication language was limited to English. Finally, 184 publications were included in this analysis. The analysis process and items are shown in Figure 1. In accordance with the annual number of publications, the search period was divided into two stages (Figure 2A). In the first stage (2007–2014), the number of annual publications was less than 10, while in the second stage (2014–2022), the number of annual publications was greater than 10. The peak year was 2021. A total of 132 articles were published in the previous five years, which accounted for approximately 71.74% of the included publications. There were 152 (82.61%) original articles and 32 (17.40%) review articles included in our analysis. The overall number of citations was 4014, with an average of 21.96 citations per paper. Moreover, the following bibliometric parameters were determined: 29 countries or regions, 276 organizations, 96 journals, 1467 co-cited journals, 860 authors, 6646 co-cited authors, 1040 keywords, and 8104 references. The countries that published the most articles included China, the United States, and Iran (Figure 2B). Since 2017, the number of annual publications in China has grown rapidly, and this indicator in the United States and Iran has maintained a relatively stable growth.
Figure 1.
Data screening flow chart and steps of bibliometric analysis. “*” indicates the truncated version of the term (microRNA* represents microRNA, microRNAs) recognized by the search algorithm.
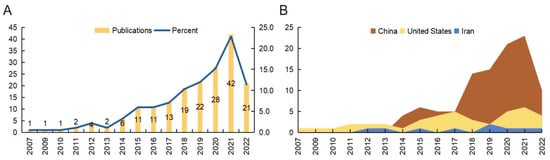
Figure 2.
The analysis of general data. (A) The number of papers published each year. (B) The top three active countries’ annual publications.
2.2. Active Countries or Regions
There was a total of 29 countries/regions that had published in this research field; however, when the minimum number of documents was set to five, there were only six countries/regions that met the threshold. As shown in Figure 3A, we summarized the number of publications, total citations, and average citations in the top 10 prolific countries/regions. The most productive country was China (n = 104; 1199 citations; average of 11.5 citations), which accounted for 56.52% of the total included papers, followed by the United States (n = 42; 1590 citations; average of 37.9 citations). Iran was ranked third (n = 9; 1110 citations; average of 12.3 citations). However, it is worth noting that Germany, which ranked 6th in the number of publications among the top 10 prolific countries/regions, had the highest number of average citations. In this field, 27 countries had cooperative relations (Figure 3B). The United States was the most active country in the field and cooperated with nine countries.
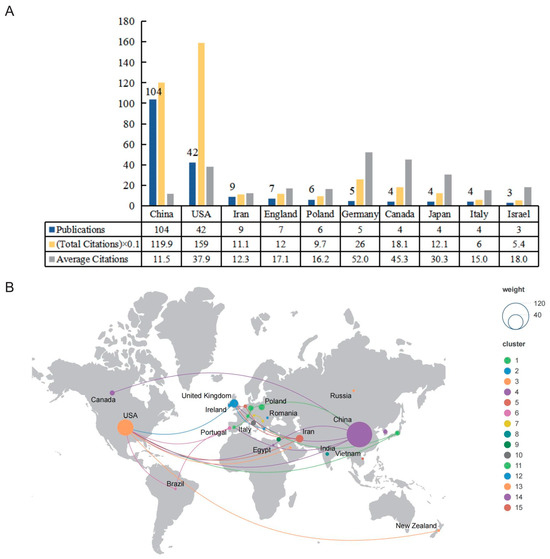
Figure 3.
The top 10 active countries/regions and cooperative relationships in this field. (A) The number of publications, total citations, and average citations in the top 10 countries/regions. (B) The cooperative relationships of countries/regions.
2.3. Active Organizations
There were 276 organizations, of which seven organizations published more than five papers. Figure 4A shows the top 10 productive institutions, while their publications accounted for 38.04% of the included data. Duke University was the most prominent contributor in this field, with the highest number of publications and total citations. Fudan University ranked second. Of the top 10 productive institutions, six were based in China, three were in the United States, and the remaining one was located in Iran. A total of 57 organizations were connected, and we have constructed a map of their cooperative relationships (Figure 4B). The link strength between Duke University and Augusta University was the highest. In terms of average publication years, Harvard University had the earliest average publication year, with an index of 2013.67, while Dalian Medical University and Zhejiang University were the most recently active institutes in this domain with indexes of 2022 and 2021, respectively.
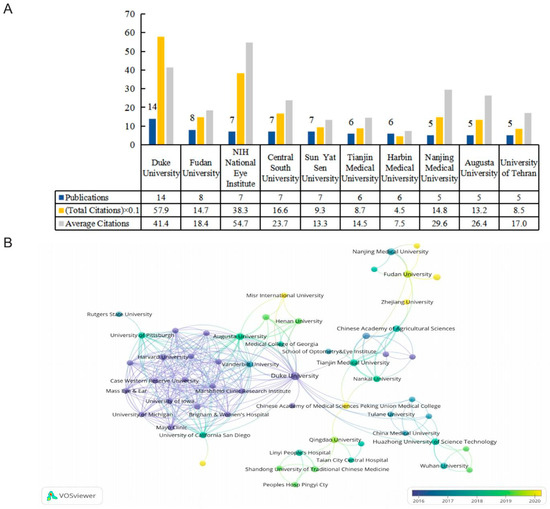
Figure 4.
(A) The numbers of publications, citations, and average citations in the top 10 active institutions. (B) The cooperative network overlay visualization map of institutions.
2.4. Top 10 Prolific Journals and Co-Cited Journals
We identified 96 journals using VOSviewer, while 18 journals met the threshold of a minimum of three publications. Table 1 summarizes the top 10 productive journals and co-cited journals and lists their relevant information. After sorting the number of publications, the Investigative Ophthalmology and Visual Science journal topped the list with 23 publications, while Experimental Eye Research (n = 10) and Scientific Reports (n = 6) ranked second and third, respectively. In terms of citations, Investigative Ophthalmology and Visual Science (750 citations) also ranked first, followed by Experimental Eye Research (211 citations) and Molecular Vision (157 citations). Notably, Biomedicine and Pharmacotherapy had the highest impact factor. In terms of co-citations, Investigative Ophthalmology and Visual Science (1107 co-citations), Experimental Eye Research (414 co-citations), and PloS One (332 co-citations) were the top three co-cited journals.

Table 1.
Top 10 prolific journals and co-cited journals on the application of miRNAs in glaucoma research.
2.5. Active Authors
In this study, we analyzed the top 10 prolific authors and co-cited authors, as shown in Table 2. Sorted by the number of publications, Pedro Gonzalez, Coralia Luna, and Xinghuai Sun had all published six articles in this field. However, David Lee Epstein’s citations and average citations were the highest among the top 10 prolific authors. In terms of co-citations, Coralia Luna, Harry A. Quigley, and Ben Mead formed the top three (Table 2). Among the 36 authors who had published more than three articles, 17 authors had collaborated with others. Initially, we visually analyzed the cooperative relationships between these 17 authors according to cluster (Figure 5A). There were four clusters in total. William Daniel Stamer and Xinghuai Sun frequently co-operated with other authors, while Xinghuai Sun had the biggest total link strength. Based on the average publication years, we created an overlay map (Figure 5B). David Lee Epstein authored the earliest publications, whereas Junyi Chen, Yuan Lei, and Chen Tan authored the most recent publications.

Table 2.
Top 10 prolific authors and co-cited authors on the application of miRNAs in glaucoma research.
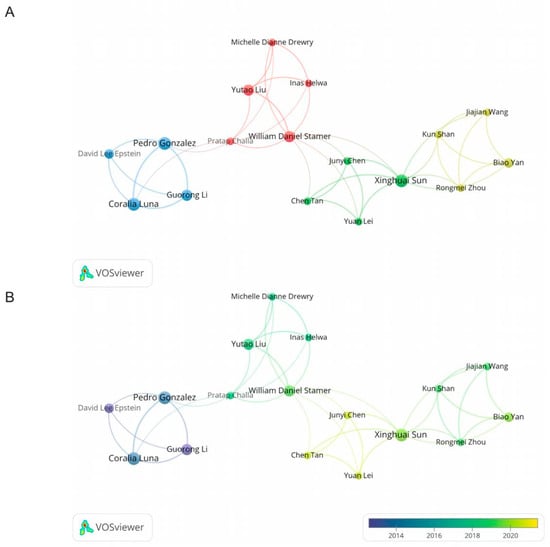
Figure 5.
The cooperative relationships between authors. (A) The co-authorship network visualization map of authors related to this field. (B) The overlay visualization map between authors.
2.6. Analysis of Keywords
Next, we obtained the research hotpots and trends by analyzing the co-occurrence of keywords [24]. This bibliometric information was analyzed by VOSviewer as follows: there were 1040 keywords in total, of which 59 appeared a minimum of five times, with a total of five clusters (Figure 6A). Next, we initially analyzed the frequently used keywords and found they mainly included “glaucoma” (n = 105), “microRNA” (n = 74), “expression” (n = 57), “open-angle glaucoma” (n = 44), “apoptosis” (n = 39), “aqueous humor” (n = 33), and “retinal ganglion cell” (n = 32). The keywords above also reflected the major themes associated with the investigators. In our overlay visualization map (Figure 6B), the light shade represents the most recent phase, and the dark shade represents the early phase. The following keywords reflected the recent attention of scholars in this field: “circular RNA” and “autophagy”. In addition, CiteSpace was also used to identify the major topics by detecting burst keywords during a particular period. Figure 6C exhibits the top 10 keywords alongside the strongest citation bursts, of which “open-angle glaucoma” was the keyword with the longest burst duration, and the burst strength of “genome wide association” was the highest.
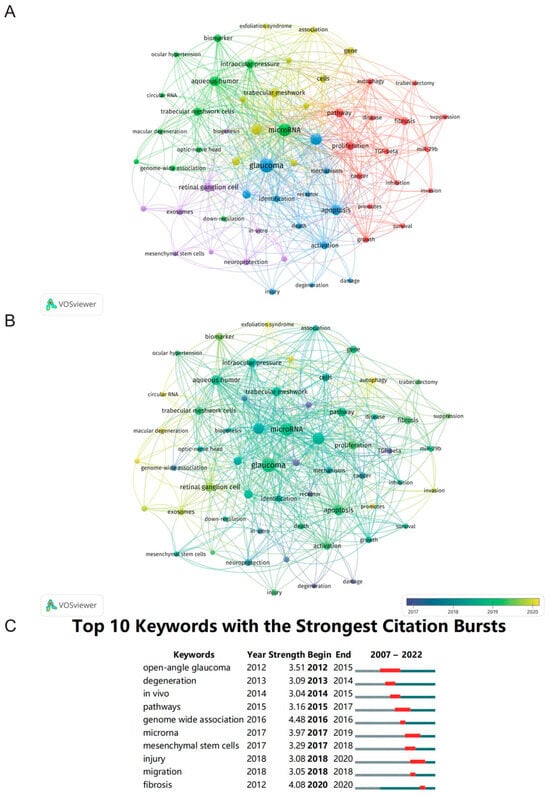
Figure 6.
Analysis of keywords. (A) The co-occurrence network visualization map of keywords. (B) The overlay map of keywords. (C) The top 10 keywords with the strongest citation bursts. The red segment of the blue line represents the burst duration of a keyword.
2.7. Cited Publications and References
Table 3 lists the top 10 cited publications; among these articles, eight are original research and two are reviews. Jonathan D. Ashwell et al. published a research article in Current Biology in 2007, which had the highest number of citations. It is entitled “Optineurin negatively regulates TNF alpha-induced NF-kappa B activation by competing with NEMO for ubiquitinated RIP” [25]. There is a review article entitled “MicroRNA dysregulation in neurodegenerative diseases: A systematic review” [26], and although it was published in 2019, it ranked 4th in terms of citations. Furthermore, 2 of the top 10 cited articles were authored by Pedro Gonzalez. In addition, two articles were published in Investigative Ophthalmology and Visual Science. Based on CiteSpace, we obtained a graph spectrum of the highest cited references (Figure 7A) and the references with the strongest citation bursts (Figure 7B). In Figure 7A, the red nodes represent highly cited references, and the top five references are “Drewry MD (2018)” [27], “Hindle AG (2019)” [28], “Tanaka Y (2014)” [29], “Guo RR (2017)” [21], and “Dismuke WM (2015)” [18]. The first two references depict those with the longest burst durations (Figure 7B). Yuji Tanaka et al. published an original article titled “Profiles of Extracellular miRNAs in the Aqueous Humor of Glaucoma Patients Assessed with a Microarray System”, which had the highest burst strength [29]. There were three articles with citation bursts ending in 2022, meaning that these articles had received continuous focus in recent years.

Table 3.
Top 10 most cited papers on the application of miRNAs in glaucoma research.
Table 3.
Top 10 most cited papers on the application of miRNAs in glaucoma research.
| Rank | Title | Type | Citations | Journal | IF (2022) | Corresponding Author | Affiliation | Year |
|---|---|---|---|---|---|---|---|---|
| 1 | Optineurin negatively regulates TNF alpha-induced NF-kappa B activation by competing with NEMO for ubiquitinated RIP [25] | Article | 212 | Current Biology | 9.2 | Jonathan D. Ashwell | NIH National Cancer Institute | 2007 |
| 2 | Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms [30] | Article | 206 | Stem Cells Translational Medicine | 6 | Ben Mead | NIH National Eye Institute | 2017 |
| 3 | The role of TGF-beta in the pathogenesis of primary open-angle glaucoma [31] | Review | 190 | Cell and Tissue Research | 3.6 | Ernst R. Tamm | University of Regensburg | 2012 |
| 4 | MicroRNA dysregulation in neurodegenerative diseases: A systematic review [26] | Review | 174 | Progress in Neurobiology | 6.7 | Alyson E. Fournier | McGill University | 2019 |
| 5 | Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress [32] | Article | 132 | Molecular Vision | 2.2 | Pedro Gonzalez | Duke University | 2009 |
| 6 | Human aqueous humor exosomes [18] | Article | 79 | Experimental Eye Research | 3.4 | Yutao Liu | University System of Georgia | 2015 |
| 7 | Coordinated Regulation of Extracellular Matrix Synthesis by the MicroRNA-29 Family in the Trabecular Meshwork [33] | Article | 78 | Investigative Ophthalmology and Visual Science | 4.4 | Douglas J. Rhee | Harvard University | 2011 |
| 8 | Profiles of Extracellular miRNAs in the Aqueous Humor of Glaucoma Patients Assessed with a Microarray System [29] | Article | 72 | Scientific Reports | 4.6 | Toru Nakazawa | Tohoku University | 2014 |
| 9 | MicroRNA-24 Regulates the Processing of Latent TGF beta 1 During Cyclic Mechanical Stress in Human Trabecular Meshwork Cells Through Direct Targeting of FURIN [34] | Article | 70 | Journal of Cellular Physiology | 5.6 | Pedro Gonzalez | Duke University | 2011 |
| 10 | Suppression of Type I Collagen Expression by miR-29b via PI3K, Akt, and Sp1 Pathway in Human Tenon’s Fibroblasts [35] | Article | 67 | Investigative Ophthalmology and Visual Science | 4.4 | Xuanchu Duan | Central South University | 2012 |
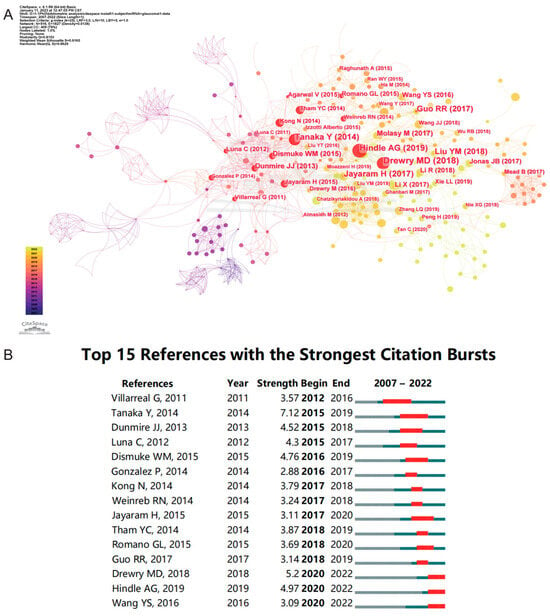
Figure 7.
Analysis of references. (A) The highly cited references. (B) The top 15 references with the strongest citation bursts. The blue line represents the time from its first appearance to 2022; the red line represents the burst time [1,2,18,19,21,27,28,29,33,36,37,38,39,40,41].
3. Discussion
The number of annual publications can reflect the development of a particular field [42]. Thus, we identified an overall upward trend in this research area from 2007 to 2021; however, there was a decrease in the number of new publications in 2022. We speculate that this occurred for two reasons: firstly, it could be because the field has developed in other directions, or it could be due to a delay in the WoSCC collection. Our analysis included 184 articles, which had been published between 2007 and 2022, from a total of 29 countries/regions, 276 institutions, 96 journals, 1467 co-cited journals, 860 authors, 6646 co-cited authors, 1040 keywords, and 8104 references. These numbers indicate that this research field has received significant global attention. Indeed, the top three countries with the highest numbers of publications were China (n = 104), the United States (n = 42), and Iran (n = 9). In particular, China published a large number of articles after 2017, which may be linked to the intensification of the problem of population aging in China [43]. Of the top 10 prolific institutions, six were located in China, three were in the United States, and the remaining one was found in Iran. Duke University was the most prominent contributor and had the highest number of publications and total citations, while Fudan University ranked second. Investigative Ophthalmology and Visual Science (n = 23; 750 citations), Experimental Eye Research (n = 10; 211 citations), and Scientific Reports (n = 6, 115 citations) were the top three journals with the most publications in this field.
3.1. MicroRNAs
MicroRNAs are a class of endogenous single-stranded non-coding RNAs (ncRNAs) that are approximately 22 nucleotides in length [13] and regulate the expressions of over 60% of human protein-coding genes [44]. They were originally identified in Caenorhabditis elegans in 1993, while Lin-4 was the first miRNA to be identified by Ambros and Ruvkun [45,46]. Thereafter, vast volumes of miRNAs have been found in viruses, vegetation, and animals [47,48], with more than 2500 miRNAs having currently been determined in the human genome [49].
The biogenesis of miRNAs comprises the following steps [13]: (1) the microprocessor complex, which consists of DGCR8 recognizing pri-miRNAs, which are hundreds to thousands of nucleotides in length, and cleaves them into pre-miRNAs. (2) Ran-GTP and exportin-5 transport the pre-miRNA from the nucleus. (3) Dicer cleaves the pre-miRNAs into mature miRNAs. (4) Argonaute (AGO) proteins and mature miRNA form an RNA-induced silencing complex (RISC). (5) The target mRNA is specifically recognized and bound to by RISC before being cleaved.
While a few miRNAs function by recognizing individual targets, most of them act by incompletely binding to several targets, then inhibiting the translation of targets or inducing target degradation at the post-transcriptional level [50]. One target mRNA can be modulated by several miRNAs, and one miRNA can modulate several targets [51]. It is well known that miRNAs can command cell proliferation, differentiation, apoptosis, and metastasis [52,53,54], and can serve as regulators of multiple biological pathways [55,56]. Furthermore, miRNAs are also known to play key roles in a variety of physiological and disease processes by modulating whole signaling pathways. The deregulation of miRNAs in diseases can also facilitate the development of biomarkers [57] and provide some insight into potential disease pathogeneses [58].
3.2. MicroRNAs in Neurodegenerative Diseases (NDs)
miRNAs have been found to regulate gene expressions at the post-transcriptional level. Moreover, gene regulation has been found to be dysfunctional in multiple NDs and their animal models [59,60,61,62,63]. NDs are synonymous with the progressive deterioration of neuron structures and functions, ultimately resulting in neuronal loss, which is considered to be the basis of most neurological impairments [64]. Presently, NDs form one of the most severe health issues owing to the increase in aging populations. Unfortunately, they remain incurable and non-reversible even after many years of study. NDs, including Alzheimer’s disease, Parkinson’s disease, glaucoma, age-related macular degeneration, amyotrophic lateral sclerosis, Huntington’s disease, etc., usually have shared cellular mechanisms and histopathological characteristics beyond neuronal cell dysfunction or death [65]. Although the underlying causes of individual NDs are different, many common pathobiological characteristics and mechanisms are consistent in neurodegeneration across diseases. Recent studies have revealed that miRNA expressions are significantly altered in the pathogenesis of NDs and contribute to the physiology of abnormal neurons [60,66]. However, most of the literature reviews that depict the changes in miRNA expressions in specific NDs and the regulation of miRNAs across different NDs have not been systematically overviewed [67,68,69]. Thus, the identification of a common miRNA dysregulation processes across NDs may help us to better understand the conserved molecular pathways that are impacted in these diseases and highlight some new targets for treatment. Camille A et al. [26] determined the commonly dysregulated miRNAs across different NDs. Specifically, miR-9-5p, miR-21-5p, the miR-29 family, miR-124-3p, miR-132-3p, miR-146a-5p, miR-155-5p, and miR-223-3p are predominantly dysregulated in 12 classes of NDs and their representative animal models. Among them, miR-146a-5p, miR-155-5p, and miR-223-3p are mainly upregulated across different NDs. They also summarized the pathways that are targeted by the usually dysregulated miRNAs in NDs—for instance, Aβ genesis, autophagy, homeostasis, apoptosis, NF-κB signaling, etc. Moreover, they noted the functional overlap by these miRNAs and suggested that miRNAs may act synergistically to directly or indirectly act upon their targets.
3.3. MicroRNAs in Glaucoma
3.3.1. Glaucoma
Glaucoma is a neurodegenerative disease, whereby a slow loss of RGCs and their axons will cause severe vision loss. As glaucoma progresses, the retinal nerve fiber layer and the optic nerve head suffer irreversible damage due to optic neuropathy [70]. To the best of our knowledge, the pathogenesis of glaucoma is still unknown; however, some risk factors of glaucoma have been identified, including advanced age, nearsightedness, race, IOP, reduced corneal thickness, positive family history of glaucoma, and potential vessel disease [71,72,73,74,75]. Some other factors, such as autoimmunity, defects in the autoregulation of ocular blood circulation, low intracranial pressure, aberrant structural sensitivity of the lamina cribrosa, and mitochondrial function disorders may also be engaged. Among these, IOP, which is caused by the blocked outflow of AH, forms the main risk factor [4]. Although the majority of patients possess an elevated IOP (>21 mmHg), there are also patients with low IOP values. The normal IOP values in healthy individuals range from 10 to 12 mmHg [76].
Glaucoma is categorized into OAG and CAG. OAG is the most frequent type of the disease, accounting for about 90% of all glaucoma patients [4,77], with POAG and exfoliation glaucoma, a kind of secondary glaucoma, representing the most common forms [78,79]. Among the many types of glaucoma, POAG is the most common, especially in people of European and African descent [80,81]. It is usually bilateral, although the severity is often asymmetric [2]. CAG is less common and is constituted in under 20% of patients in America. Primary angle closure glaucoma (PACG) is the most serious phase of closed-angle disorders [82].
Glaucoma is usually asymptomatic in its early stages of development and reducing the intraocular pressure is the only confirmed way to prevent or delay it. This kind of reduction can be achieved using non-surgical treatments, including eye drops and IOP-lowering medication, or surgical treatments, including laser trabeculoplasty, laser peripheral iridotomy, and incisional glaucoma surgery [7]. Although intraocular hypertension is frequently observed in patients with OAGs, only a small percentage of patients with ocular hypertension will develop this disease [83]. Despite the improved results from IOP-lowering treatments, a large number of glaucoma patients continue to lose their vision [84]. Predominantly, glaucoma is a chronic disease that requires lifelong therapy.
Numerous miRNAs are dysregulated in glaucoma and might play a vital role in the underlying pathogenic mechanisms of POAG [4]. Moreover, miRNAs can serve as diagnostic and prognostic biomarkers for glaucoma. For example, various miRNAs have been found in the patients-sourced samples with POAG [85], while they also fulfill a role in the trabecular meshwork (TM) [86], retina [87], and AH [29].
3.3.2. MicroRNAs in Aqueous Humor (AH)
The main and sole known changeable risk factor of glaucoma is IOP, which is sustained through balancing AH production and the rate of AH discharge through the TM [88]. miRNAs derived from AH might play a critical role in the pathologic status associated with glaucoma, although full miRNA profiles have not yet been identified in the AH from glaucoma patients. The AH is an appealing source of neoteric miRNAs, which can serve as biomarkers or therapy targets in glaucoma, whereby its reachability, specificity, and separation is different from other organs [29]. We eagerly anticipate the identification of novel miRNAs that can diagnose the varying subtypes, pathologic conditions, and pharmacologic effects of glaucoma, which might be significantly expedited by the application of new analytical instruments. Overall, miR-143-3p, miR-125b-5p, and miR-1260b found in the AH might serve as promising therapeutic targets for glaucoma [4].
3.3.3. MicroRNAs in Trabecular Meshwork (TM)
The damage associated with increased IOP is primarily characterized by the occurrence of TM degeneration [89]. TM is a vital constituent in the outflow pathway for AH and comprises the majority of the outflow resistance [90]. In POAG, a range of pathologic alterations is observed in the TM, especially in the elevation of extracellular matrix (ECM) molecules, such as collagens and fibronectins [91,92], which raise the outflow resistance and IOP [93]. Thus, it is necessary and urgent for us to clarify the pathologic mechanisms associated with the overproduction of ECM in the TM of glaucoma patients. To our knowledge, the synthesis of ECM is modulated by the transforming growth factor-β (TGF-β) family [94]. Additionally, studies have shown that the TGF-β/SMAD pathway can modulate ECM genes, which are related to increased IOP in glaucomatous optic neuropathy [95,96,97].
The diversely expressed miRNAs in the AH and blood of patients with glaucoma have previously been identified [27,98]. Recently, vast volumes of research have concentrated on the roles of miRNAs in regulating TM cell function during diverse pathologic status to attempt to identify a wider range of potential biomarkers for glaucoma therapy [99,100]. For example, the miR-29 family, which includes miR-29a, miR-29b-1, miR-29b-2, and miR-29c, plays an essential role in the development of fibrosis in POAG, owing to their antifibrotic effects on the TGF-β signaling pathway and the production and deposition of ECM [88]. Moreover, the miR-200 family, which includes miR-200a, miR-200b, miR-200c, miR-141, and miR-429, is a potential regulator of TM cell contractions. Among these, miR-200c has been shown to be the direct post-transcriptional inhibitor of genes associated with TM cell contraction, modulated trabecular contraction, and intraocular pressure in vivo [37]. Therefore, miR-200c is a valuable candidate for future therapeutic approaches in glaucoma research by regulating TM cell contraction. In addition, miR-486-5p can reduce the production of ECM and oxidative damage in human TM cells by targeting the TGF-β/SMAD2 pathway [101]. Overall, future miRNA-based therapies that focus on controlling the production of ECM, regulating TM cell contraction, or targeting the TGF-β may provide novel therapeutic approaches for glaucoma.
3.3.4. Manipulating microRNA Expressions
A better comprehension of the effects of miRNAs in normal and abnormal eyes can help dissect the physiologic and pathologic mechanisms of glaucoma. The possibilities for miRNA-based therapies are enhanced by the capacities of miRNA manipulations to alter gene expressions both in vitro and in vivo [102,103]. miRNA-based therapies can be achieved as follows: (1) the application of miRNA mimetics. miRNA mimetics, which resemble miRNA precursors, can be used to downregulate certain target proteins; however, this approach may be accompanied by off-target effects [104]. (2) The application of anti-miRNAs. Anti-miRNAs can be used to promote cell survival and suppress cell apoptosis, which can inhibit endogenous miRNAs [105]. (3) The application of targeting the miRNAs’ processing mechanisms [17]. (4) Other applications, such as target site blockers and miRNA sponges [103].
3.3.5. MicroRNAs in Mouse Models of Glaucoma
It is possible to explore the pathogenesis of human diseases using mouse models, which provide powerful research tools [106]. Regarding the significance of glaucoma mouse models for providing important mechanistic ideas to glaucoma pathogenesis, we analyzed and summarized the included articles that used glaucoma mouse models. In research that used acute glaucoma mouse models, in which the anterior chamber was cannulated with a needle, intravitreal infusion of polydopamine–polyethylenimine nanoparticles carried miR-21-5p [107], and mesenchymal stem cells (MSCs)-exosomes including miR-21a-5p [108,109] were used to provide new therapeutic pathways for neuroprotection against glaucoma. Moreover, there are some chronic glaucoma mouse models which were established by episcleral venous occlusion with cauterization [110], fixing a plastic ring to the ocular equator [111], infusing microbeads into the anterior chamber [112,113], laser photocoagulation [114], or directly using a genetic DBA/2J mouse model of glaucoma [115]. In these studies, the administration of microRNA mimic/inhibitor [110,111,112,114], application of microRNA knockout mice [113], and injection of bone marrow mesenchymal stem cell (BMSC)-derived exosomes including microRNAs [115] were used to develop microRNA-based advanced therapies. Furthermore, some other glaucoma models, such as optic nerve crush [116] and the injection of N-methyl-D-aspartic acid into the vitreous cavity [117], have also been applied in glaucoma research.
3.4. Other Non-Coding RNAs
miRNAs belong to endogenous ncRNAs, which form the greatest subset of RNA transcripts, which form 90% of the human genome. The ncRNAs do not translate into proteins and are involved in the regulation of diverse biological pathological processes [118,119]. Further, ncRNAs include miRNAs, circular RNAs (circRNAs), and long non-coding RNAs (lncRNAs).
3.4.1. Circular RNAs
CircRNAs are a special class of ncRNAs that are highly expressed in mammalian cells and exhibit tissue specificity [120]. Moreover, they have been shown to be ideal biomarkers for human diseases [121,122].
CircRNAs can act as miRNA sponges, regulating downstream mRNA expressions, thus modulating cellular functions; they have been extensively accepted [123,124]. Some circRNAs can serve as gene regulators and engage in the regulation of a variety of biological processes [125]. The biofunction of circRNAs acts in three main ways: (1) circRNAs function as miRNA sponges; (2) circRNAs function as transcriptional regulators; (3) circRNAs function as biomarkers of disease progression [126]. There are various research studies that suggest that circRNAs are highly associated with the progress of diverse eye diseases [127,128], including glaucoma, age-related macular degeneration, retinal detachment, and diabetic retinopathy, and play a role in retinal dysfunction [129,130,131] by functioning mainly as sponges for microRNAs. For instance, it has been verified that hsa_circ_0023826 was downregulated in patients with glaucoma and can be used as a biomarker for glaucoma [132]. Furthermore, circZRANB1 is a possible candidate for glaucoma treatment due to its association with glaucoma-induced retinal neurodegeneration [133]. Currently, some candidate circRNAs have been screened by high-throughput sequencing technology, whereas the role of most circRNAs has not been completely elucidated in glaucoma. Yanxi Wang et al. [126] revealed that circ_0080940 might facilitate the advancement of glaucoma by sponging miR-139-5p; thus, the inhibition of circ_0080940 might be an attractive therapy for treating glaucoma. A single circRNA can act as a “sponge of miRNA” to disrupt the binding sites of several miRNAs and suppress the activation of one or more miRNAs [134]. The sponging of miRNAs by circRNAs in most disorders highlights their potential as therapy candidates or biomarkers. For example, silencing circZYG11B could suppress I/R-induced RGC injury and attenuate I/R-induced retinal reactive gliosis through circZYG11B/microRNA-620/PTEN signaling; thus, circZYG11B might be a candidate for the identification and therapy of retinal ischemic diseases [135]. Chen et al. [136] revealed that circHipk2 and circTulp4 can serve as sponges of miR-124-3p and miR-204-5p/miR-26a-5p, respectively, and the interruption of circTulp4 expression induced impaired retinal function. Additionally, circZNF609 can regulate retinal neurodegeneration by sponging miR-615, thereby silencing the circZNF609-induced suppression of retinal reactive gliosis and the activation of neurogliocytes to promote RGCs’ survival [131]. It is worth noting that the dysregulation of circRNAs in sponging miRNAs is what usually induces disease and not the circRNAs themselves. Moreover, evidence indicates that the dysregulation of the circRNAs might promote NDs, cardiovascular disorders, vascular diseases, and cancers [137,138].
3.4.2. Long Non-Coding RNAs
The lncRNAs are a typical class of ncRNAs, are over 200 nucleotides in length, and are characterized by fewer exons and tissue or cell specificities [139]. LncRNAs are located mainly in the cell nucleus and can modify gene expressions at both the translational and transcriptional levels [140]. Moreover, lncRNAs can be subclassified based on the linkage to miRNAs and transcriptional direction [140]. Recent research has revealed that lncRNAs have multiple functions and play a modulating function in many ocular diseases, including glaucoma, diabetic retinopathy, age-related cataracts, and age-related macular degeneration [140]. In addition, some studies have shown that lncRNAs are closely related to the development of POAG [141,142]. Lili Xie et al. [142] suggested that lncRNAs T267384, ENST00000607393, and T342877 might be potential therapy biomarkers for POAG. Additionally, lncRNAs are prospective treatment candidates for preventing fibrosis after glaucoma filtration surgery [143]. However, the functions and associated mechanisms of the role most lncRNAs play in diseases have not been completely clarified.
3.4.3. Competing Endogenous RNAs
Interestingly, lncRNAs may emerge as competing endogenous RNAs (ceRNAs) and can potentially correspond to specific messenger RNAs (mRNAs) through sponging to their target miRNAs [144,145,146]. Moreover, miRNAs can bind to their specific mRNAs and repress their expressions in the ceRNA network. Furthermore, lncRNAs can competitively bind to miRNA response elements (MREs) with mRNA and attenuate miRNA-mediated inhibition, while also mediating the post-transcriptional modulation of target genes to regulate cellular activities [147,148,149]. For instance, lncRNA TGFβ2-AS1 might facilitate ECM generation, which is associated with the development of POAG, by targeting TGF-β2 in human TM cells [150]. Similar to lncRNAs, circRNAs can also function as ceRNAs for miRNAs, whereby miRNAs play certain modulatory effects on mRNAs. For example, Zhichao Yan et al. [146] suggested that circXPO5 and GRIN2A can act as ceRNAs and compete with miR-330-5p to reduce circXPO5, which will have beneficial effects for glaucoma patients. CeRNAs are related to the molecular mechanisms in ocular disease, such as age-related macular degeneration [151]; thus, a ceRNAs network analysis can efficiently identify molecules related to their regulation. It has been revealed that the network of ceRNAs can contribute to the understanding of the potential molecular mechanisms involved in POAG progression. Additionally, some potential POAG biomarkers and bio-target molecules were identified using a ceRNA analysis [152,153]. The construction and identification of the lncRNA or circRNA–miRNA–gene ceRNA network may contribute to illustrating the pathogenesis of diseases and identifying prospective biomarkers for disorders [154].
3.5. MicroRNAs in Exosomes
Exosomes, which are a kind of MSCs-derived factor that contribute to the parasecretory effects of MSCs [155], can be used as a cell-free therapy with lower immunogenicity for treating retinal diseases [156] and inflammatory disorders [157]. Exosomes are part of the extracellular vesicles (EVs), which are secreted by viable cells and range from 30 nm to 150 nm in diameter [158]. Moreover, they include mRNAs, ncRNAs (miRNAs, circRNAs, and lncRNAs), proteins, transcription factors, and other biofactors, and have been verified as promising potential therapy options and drug deliverers [159]. It has been demonstrated that exosomes are involved in intercellular communication [160], and MSC-derived exosomes have been applied in various diseases, such as apoplexy [161], corneal diseases [162], glaucoma [115,163], and hepatopathy [164]. Growing evidence has shown that the biomarkers derived from exosomes may be helpful for diagnosing various disorders, including Parkinson’s disease [165] and diabetes mellitus [166].
Mead et al. demonstrated that the therapy effects of exosomes were at least partially attributable to their miRNAs in both the glaucoma rodent models [163] and genetic DBA/2J mouse models [115]. In the former study [163], they used two glaucoma rodent models: one with the induction of ocular hypertension with intracameral microbeads and the other with induction of ocular hypertension with laser photocoagulation. BMSC-derived exosomes were injected into the vitreous every week or every month, and the results show that the BMSC-derived exosomes provided significant neuroprotection to the RGCs while preserving the retinal nerve fiber layer thickness and positive scotopic threshold response amplitude. However, BMSC-derived exosomes with knockdown of Argonaute2, a protein critical for miRNAs function, markedly alleviated the effects described above. This implies that BMSC-derived exosomes may exert their neuroprotective effects through miRNA-dependent mechanisms. Finally, they identified 43 miRNAs upregulated in BMSC-derived exosomes in comparison to fibroblast-derived exosomes by using RNA sequencing. In the latter study [115], they chose the genetic DBA/2J mouse as a chronic glaucoma model, and BMSC-derived exosomes were injected into the vitreous of 3-month-old DBA/2J mice once a month for 9 months. Consistent with the previous study, the delivery of BMSC-derived exosomes also exhibited significant neuroprotection for RGCs while reducing axonal damage in the optic nerve. Particularly noteworthy is that the BMSC-derived exosomes only retained the function of RGCs in 6-month-old DBA/2J mice rather than in 9 and 12 months. Further, miRNAs in exosomes are involved in a variety of pathologies and physiologies [167,168]. It has been proven that the exosomes originating from distinct cell types can be delivered intracellularly, while all cells exhibit an ability to functionally utilize the delivered miRNAs [169].
The exosomes secreted by BMSC comprise more than 150 distinct miRNAs, which can be delivered into the target cells [170]. Previous studies have shown that both the proteins and miRNAs found in exosomes can play a therapeutic role [171], while Mead et al. [30] determined that treating RGC using exosomes in a rat optic nerve crush model relies more on miRNAs than proteins. For the first time, they delivered BMSC-derived exosomes into the eye, and the cargos delivered by the exosomes successfully reached the inner layers of the retina, including the RGC, which then elicited therapeutic benefits via the miRNA-dependent mechanisms [30]. In addition, the delivery of exosomes enriched with miR-21-5p contributed to the neuroprotection against retinal ischemia-reperfusion injuries (IRIs) and was helpful in promoting the development of cell-free treatments for glaucoma [108]. Furthermore, BMSC-derived exosomes promoted axonal growth in primary adult rat cortical neurons, which mainly relied on the action of the miRNAs in the exosomes [172]. Thus, evaluating whether exosomes can provide long-term neuroprotection in glaucoma models will be essential to apply exosomes in the future clinically.
3.6. Research Frontiers
In this study, there were a total of 59 keywords with a minimum of five occurrences that were identified and divided into five clusters (Figure 6A). The results reveal that some terms, such as glaucoma, microRNA, expression, open-angle glaucoma, apoptosis, aqueous humor, and retinal ganglion cell, were more frequently used terminologies in the literature (Figure 6B) and could provide some clues for the future direction of miRNAs in glaucoma research. Special attention should be paid to circRNAs, exosomes, EVs, and autophagy since they were shown as emerging frontiers in this field over the past 15 years. The following topics might merit more in-depth study in the future: (1) the roles of circRNAs in retinal neurodegeneration. (2) The neuroprotective function of exosome-derived or EVs-derived miRNAs in eye diseases. (3) Autophagy engaging in the pathogenic mechanisms of glaucoma.
3.7. Limitations
We used bibliometric and visualized analyses to formulate the research actuality in the field of miRNAs in glaucoma research, which enabled this research to be comparatively exhaustive and objective. Nevertheless, this research still contains some inevitable limitations. Firstly, the literature data in this research were all collected from the WoSCC database, which is the most widely used and authoritative database; however, there are still some publications that are not incorporated into the WoSCC database. Secondly, publications not written in English and papers published before 2007 and in 2023 were not adopted in this research. In addition, only original articles and reviews were analyzed in this research. Thirdly, we ignored the value of the publications, meaning that high-quality publications and low-quality publications were of similar weight. Finally, by only considering the institution of the first author, contributions from other countries in the worldwide research network may have been overlooked, especially where the main authors were from other countries.
4. Materials and Methods
4.1. Search Strategy and Data Collection
We conducted an advanced search of the WoSCC database on 28 December 2022, and used the following search terms to identify publications primarily concerning miRNAs: TS = (“microRNA*” OR “miRNA*” OR “miR”) AND TS = (“glaucoma”). We limited the search period to between 1 January 2007 and 28 December 2022. Then, the document type was limited to original and review articles, while the publication language was limited to English. The exclusion criteria are as follows. The types of publications are meeting abstract, editorial material, correction, book chapter, letter, and non-English articles. Firstly, R.Z. and Y.T. searched and screened the publications separately; however, any appearance of a problem was discussed, and a consensus was reached. The identified publications that met the inclusion criteria were exported as plain text files in the format of “Full Record and Cited References”.
4.2. Data Analysis
The publications were imported into VOSviewer (version 1.6.11; Leiden University, Leiden, Netherlands) and CiteSpace (Version 6.2.R4, Drexel University, Philadelphia, PA, USA) to retrieve the title, keywords, authors, institutions, countries or regions, journals, publication year, citations, average citations, and cited references. The corresponding bibliometric parameters were exported to Microsoft Excel 2010 (Redmond, Washington, WA, USA) to identify the publication trend, the distribution of document types, and the largest contributors, including prolific authors, institutions, countries or regions, and journals. VOSviewer was used to illustrate the map and depict the strength of the collaborations between authors, institutions, countries, and journals to demonstrate their scientific influence in this field. Lastly, the co-occurrence of author keywords in VOSviewer, keywords with the strongest citation bursts, and co-cited references in CiteSpace were utilized to visualize the knowledge evolution, hot topics, and potential research frontiers in this field. For the network visualization maps generated by VOSviewer, the color of the node represents clustering, the size of the node shows the number of publications or the frequency of keywords, the link between the nodes indicates the cooperative or co-occurrence relationship, and the thickness of the link suggests the strength. In the overlay visualization map produced by VOSviewer, the difference is that the color of the node implies the average publication year, purple means early, and yellow means recent [173]. For the maps generated maps in CiteSpace, there is a blue line that indicates the period, and a red line that represents the period of the bursts [174].
5. Conclusions
In conclusion, the number of annual publications on miRNAs in glaucoma research exhibited a continuous upward trend during the past two decades. China is a pioneering country in this field and has contributed to the development of miRNAs in glaucoma research. Of course, institutional and individual cooperation is critical to the productivity of research involving miRNAs in glaucoma and will form the backbone of any future research. The results of this research can provide the foundations and new frontiers for future research studies on miRNAs in glaucoma by summarizing and visualizing the publication trends, research hotspots, collaboration relationships, research frontiers, etc., which will enable readers to rapidly and efficiently access the useful information in this field. These findings will help the research community to explore the emerging topics and mechanisms, and provide guidance for clinical trials on glaucoma in the future.
Author Contributions
Conceptualization, J.H., R.Z. and Y.T.; methodology, R.Z. and Y.T.; resource, J.H.; software, Y.T.; validation, R.Z.; visualization, Y.T.; writing—original draft, R.Z. and Y.T.; writing—review and editing, J.H., R.Z. and Y.T.; supervision, J.H.; funding acquisition, J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81671225) and the Enterprise Joint Innovation Project of Central South University (1053320220052, 1053320220057, 2023XQLH043).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used in this manuscript are available on the Web of Science.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
Retinal ganglion cells: RGCs; intraocular pressure: IOP; open-angle glaucoma: OAG; closed-angle glaucoma: CAG; primary open-angle glaucoma: POAG; primary angle closure glaucoma: PACG; microRNAs: miRNAs or miRs; neurodegenerative diseases: NDs; aqueous humor: AH; trabecular meshwork: TM; extracellular matrix: ECM; non-coding RNAs: ncRNAs; circular RNAs: circRNAs; long non-coding RNAs: lncRNAs; competing endogenous RNAs: ceRNAs; messenger RNAs: mRNAs; mesenchymal stem cells: MSCs; extracellular vesicles: EVs.
References
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Sharoukhov, D.; Bucinca-Cupallari, F.; Lim, H. Microtubule Imaging Reveals Cytoskeletal Deficit Predisposing the Retinal Ganglion Cell Axons to Atrophy in DBA/2J. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5292–5300. [Google Scholar] [CrossRef]
- Martinez, B.; Peplow, P.V. MicroRNAs as biomarkers in glaucoma and potential therapeutic targets. Neural Regen. Res. 2022, 17, 2368–2375. [Google Scholar] [CrossRef]
- Lusthaus, J.; Goldberg, I. Current management of glaucoma. Med. J. Aust. 2019, 210, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Kass, M.A.; Heuer, D.K.; Higginbotham, E.J.; Johnson, C.A.; Keltner, J.L.; Miller, J.P.; Parrish, R.K., 2nd; Wilson, M.R.; Gordon, M.O. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002, 120, 701–713. [Google Scholar] [CrossRef]
- Kang, J.M.; Tanna, A.P. Glaucoma. Med. Clin. N. Am. 2021, 105, 493–510. [Google Scholar] [CrossRef]
- Kubelick, K.P.; Snider, E.J.; Ethier, C.R.; Emelianov, S. Development of a stem cell tracking platform for ophthalmic applications using ultrasound and photoacoustic imaging. Theranostics 2019, 9, 3812–3824. [Google Scholar] [CrossRef]
- Gao, X.R.; Huang, H.; Kim, H. Polygenic Risk Score Is Associated with Intraocular Pressure and Improves Glaucoma Prediction in the UK Biobank Cohort. Transl. Vis. Sci. Technol. 2019, 8, 10. [Google Scholar] [CrossRef]
- Backes, C.; Meese, E.; Keller, A. Specific miRNA Disease Biomarkers in Blood, Serum and Plasma: Challenges and Prospects. Mol. Diagn. Ther. 2016, 20, 509–518. [Google Scholar] [CrossRef]
- Vishnoi, A.; Rani, S. MiRNA Biogenesis and Regulation of Diseases: An Overview. Methods Mol. Biol. 2017, 1509, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, A.; Braniewska, A.; Kozar-Kaminska, K. MicroRNA in cardiovascular biology and disease. Adv. Clin. Exp. Med. 2017, 26, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Niu, L.; Zhao, J.; Wang, M.; Li, K.; Zheng, Y. An update: Mechanisms of microRNA in primary open-angle glaucoma. Brief. Funct. Genom. 2021, 20, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Bagga, S.; Bracht, J.; Hunter, S.; Massirer, K.; Holtz, J.; Eachus, R.; Pasquinelli, A.E. Regulation by let-7 and lin-4 miRNAs results in target mRNA degradation. Cell 2005, 122, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Meister, G. miRNAs get an early start on translational silencing. Cell 2007, 131, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, A.; Pirtoli, L.; Giordano, A.; Dotta, F. Crosstalk between MicroRNA and Oxidative Stress in Physiology and Pathology. Int. J. Mol. Sci. 2020, 21, 1270. [Google Scholar] [CrossRef] [PubMed]
- Molasy, M.; Walczak, A.; Szaflik, J.; Szaflik, J.P.; Majsterek, I. MicroRNAs in glaucoma and neurodegenerative diseases. J. Hum. Genet. 2017, 62, 105–112. [Google Scholar] [CrossRef]
- Dismuke, W.M.; Challa, P.; Navarro, I.; Stamer, W.D.; Liu, Y. Human aqueous humor exosomes. Exp. Eye Res. 2015, 132, 73–77. [Google Scholar] [CrossRef]
- Gonzalez, P.; Li, G.; Qiu, J.; Wu, J.; Luna, C. Role of microRNAs in the trabecular meshwork. J. Ocul. Pharmacol. Ther. 2014, 30, 128–137. [Google Scholar] [CrossRef]
- Liu, Y.; Bailey, J.C.; Helwa, I.; Dismuke, W.M.; Cai, J.; Drewry, M.; Brilliant, M.H.; Budenz, D.L.; Christen, W.G.; Chasman, D.I.; et al. A Common Variant in MIR182 Is Associated with Primary Open-Angle Glaucoma in the NEIGHBORHOOD Consortium. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4528–4535. [Google Scholar] [CrossRef]
- Guo, R.; Shen, W.; Su, C.; Jiang, S.; Wang, J. Relationship between the Pathogenesis of Glaucoma and miRNA. Ophthalmic Res. 2017, 57, 194–199. [Google Scholar] [CrossRef]
- Agarwal, A.; Durairajanayagam, D.; Tatagari, S.; Esteves, S.C.; Harlev, A.; Henkel, R.; Roychoudhury, S.; Homa, S.; Puchalt, N.G.; Ramasamy, R.; et al. Bibliometrics: Tracking research impact by selecting the appropriate metrics. Asian J. Androl. 2016, 18, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.C.; Wang, H.S.; Yang, X.; Xu, C.Q.; Wang, T.; Yan, Y.J.; Fan, Z.X.; Ma, J.M.; Ye, J.; Mo, W. A Bibliometric Analysis and Visualization of Current Research Trends in Chinese Medicine for Osteosarcoma. Chin. J. Integr. Med. 2022, 28, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Zhang, Q.; Meng, M.; Huang, J. A bibliometric analysis of the application of stem cells in glaucoma research from 1999 to 2022. Front. Cell Dev. Biol. 2023, 11, 1081898. [Google Scholar] [CrossRef]
- Zhu, G.; Wu, C.J.; Zhao, Y.; Ashwell, J.D. Optineurin negatively regulates TNFalpha- induced NF-kappaB activation by competing with NEMO for ubiquitinated RIP. Curr. Biol. 2007, 17, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Juzwik, C.A.; Drake, S.S.; Zhang, Y.; Paradis-Isler, N.; Sylvester, A.; Amar-Zifkin, A.; Douglas, C.; Morquette, B.; Moore, C.S.; Fournier, A.E. microRNA dysregulation in neurodegenerative diseases: A systematic review. Prog. Neurobiol. 2019, 182, 101664. [Google Scholar] [CrossRef]
- Drewry, M.D.; Challa, P.; Kuchtey, J.G.; Navarro, I.; Helwa, I.; Hu, Y.; Mu, H.; Stamer, W.D.; Kuchtey, R.W.; Liu, Y. Differentially expressed microRNAs in the aqueous humor of patients with exfoliation glaucoma or primary open-angle glaucoma. Hum. Mol. Genet. 2018, 27, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Hindle, A.G.; Thoonen, R.; Jasien, J.V.; Grange, R.M.H.; Amin, K.; Wise, J.; Ozaki, M.; Ritch, R.; Malhotra, R.; Buys, E.S. Identification of Candidate miRNA Biomarkers for Glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 134–146. [Google Scholar] [CrossRef]
- Tanaka, Y.; Tsuda, S.; Kunikata, H.; Sato, J.; Kokubun, T.; Yasuda, M.; Nishiguchi, K.M.; Inada, T.; Nakazawa, T. Profiles of extracellular miRNAs in the aqueous humor of glaucoma patients assessed with a microarray system. Sci. Rep. 2014, 4, 5089. [Google Scholar] [CrossRef]
- Mead, B.; Tomarev, S. Bone Marrow-Derived Mesenchymal Stem Cells-Derived Exosomes Promote Survival of Retinal Ganglion Cells Through miRNA-Dependent Mechanisms. Stem Cells Transl. Med. 2017, 6, 1273–1285. [Google Scholar] [CrossRef]
- Fuchshofer, R.; Tamm, E.R. The role of TGF-beta in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012, 347, 279–290. [Google Scholar] [CrossRef]
- Luna, C.; Li, G.; Qiu, J.; Epstein, D.L.; Gonzalez, P. Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress. Mol. Vis. 2009, 15, 2488–2497. [Google Scholar]
- Villarreal, G., Jr.; Oh, D.J.; Kang, M.H.; Rhee, D.J. Coordinated regulation of extracellular matrix synthesis by the microRNA-29 family in the trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 2011, 52, 3391–3397. [Google Scholar] [CrossRef]
- Luna, C.; Li, G.; Qiu, J.; Epstein, D.L.; Gonzalez, P. MicroRNA-24 regulates the processing of latent TGFbeta1 during cyclic mechanical stress in human trabecular meshwork cells through direct targeting of FURIN. J. Cell Physiol. 2011, 226, 1407–1414. [Google Scholar] [CrossRef]
- Li, N.; Cui, J.; Duan, X.; Chen, H.; Fan, F. Suppression of type I collagen expression by miR-29b via PI3K, Akt, and Sp1 pathway in human Tenon’s fibroblasts. Invest. Ophthalmol. Vis. Sci. 2012, 53, 1670–1678. [Google Scholar] [CrossRef]
- Dunmire, J.J.; Lagouros, E.; Bouhenni, R.A.; Jones, M.; Edward, D.P. MicroRNA in aqueous humor from patients with cataract. Exp. Eye Res. 2013, 108, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Luna, C.; Li, G.; Huang, J.; Qiu, J.; Wu, J.; Yuan, F.; Epstein, D.L.; Gonzalez, P. Regulation of trabecular meshwork cell contraction and intraocular pressure by miR-200c. PLoS ONE 2012, 7, e51688. [Google Scholar] [CrossRef] [PubMed]
- Kong, N.; Lu, X.; Li, B. Downregulation of microRNA-100 protects apoptosis and promotes neuronal growth in retinal ganglion cells. BMC Mol. Biol. 2014, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, H.; Cepurna, W.O.; Johnson, E.C.; Morrison, J.C. MicroRNA Expression in the Glaucomatous Retina. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7971–7982. [Google Scholar] [CrossRef]
- Romano, G.L.; Platania, C.B.; Forte, S.; Salomone, S.; Drago, F.; Bucolo, C. MicroRNA target prediction in glaucoma. Prog. Brain Res. 2015, 220, 217–240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, F.; Wang, S. MicroRNA-93 is overexpressed and induces apoptosis in glaucoma trabecular meshwork cells. Mol. Med. Rep. 2016, 14, 5746–5750. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; He, M.; Cutrona, S.L.; Kiefe, C.I.; Liu, F.; Wang, Z. Theme Trends and Knowledge Structure on Mobile Health Apps: Bibliometric Analysis. JMIR Mhealth Uhealth 2020, 8, e18212. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Wang, J.; Bucan, K.; Theodoratou, E.; Rudan, I.; Chan, K.Y. National and subnational prevalence and burden of glaucoma in China: A systematic analysis. J. Glob. Health 2017, 7, 020705. [Google Scholar] [CrossRef] [PubMed]
- Friedman, R.C.; Farh, K.K.; Burge, C.B.; Bartel, D.P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862. [Google Scholar] [CrossRef]
- Li, L.; Xu, J.; Yang, D.; Tan, X.; Wang, H. Computational approaches for microRNA studies: A review. Mamm. Genome 2010, 21, 1–12. [Google Scholar] [CrossRef]
- Etheridge, A.; Lee, I.; Hood, L.; Galas, D.; Wang, K. Extracellular microRNA: A new source of biomarkers. Mutat. Res. 2011, 717, 85–90. [Google Scholar] [CrossRef]
- Bentwich, I.; Avniel, A.; Karov, Y.; Aharonov, R.; Gilad, S.; Barad, O.; Barzilai, A.; Einat, P.; Einav, U.; Meiri, E.; et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat. Genet. 2005, 37, 766–770. [Google Scholar] [CrossRef]
- Baek, D.; Villen, J.; Shin, C.; Camargo, F.D.; Gygi, S.P.; Bartel, D.P. The impact of microRNAs on protein output. Nature 2008, 455, 64–71. [Google Scholar] [CrossRef]
- Esquela-Kerscher, A.; Slack, F.J. Oncomirs—microRNAs with a role in cancer. Nat. Rev. Cancer 2006, 6, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Vernooy, S.Y.; Guo, M.; Hay, B.A. The Drosophila microRNA Mir-14 suppresses cell death and is required for normal fat metabolism. Curr. Biol. 2003, 13, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C. OncomiRs: The discovery and progress of microRNAs in cancers. Mol. Cancer 2007, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Brennecke, J.; Hipfner, D.R.; Stark, A.; Russell, R.B.; Cohen, S.M. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell 2003, 113, 25–36. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Chen, C.Z.; Li, L.; Lodish, H.F.; Bartel, D.P. MicroRNAs modulate hematopoietic lineage differentiation. Science 2004, 303, 83–86. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Sen, S. MicroRNA as Biomarkers and Diagnostics. J. Cell Physiol. 2016, 231, 25–30. [Google Scholar] [CrossRef]
- Ardekani, A.M.; Naeini, M.M. The Role of MicroRNAs in Human Diseases. Avicenna J. Med. Biotechnol. 2010, 2, 161–179. [Google Scholar]
- Shalgi, R.; Lieber, D.; Oren, M.; Pilpel, Y. Global and local architecture of the mammalian microRNA-transcription factor regulatory network. PLoS Comput. Biol. 2007, 3, e131. [Google Scholar] [CrossRef]
- Nelson, P.T.; Wang, W.X.; Rajeev, B.W. MicroRNAs (miRNAs) in neurodegenerative diseases. Brain Pathol. 2008, 18, 130–138. [Google Scholar] [CrossRef]
- Liang, H.; Li, W.H. MicroRNA regulation of human protein protein interaction network. RNA 2007, 13, 1402–1408. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.P.; Lau, N.C.; Garrett-Engele, P.; Grimson, A.; Schelter, J.M.; Castle, J.; Bartel, D.P.; Linsley, P.S.; Johnson, J.M. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005, 433, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Juzwik, C.A.; Drake, S.; Lecuyer, M.A.; Johnson, R.M.; Morquette, B.; Zhang, Y.; Charabati, M.; Sagan, S.M.; Bar-Or, A.; Prat, A.; et al. Neuronal microRNA regulation in Experimental Autoimmune Encephalomyelitis. Sci. Rep. 2018, 8, 13437. [Google Scholar] [CrossRef] [PubMed]
- Bredesen, D.E.; Rao, R.V.; Mehlen, P. Cell death in the nervous system. Nature 2006, 443, 796–802. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Maciotta, S.; Meregalli, M.; Torrente, Y. The involvement of microRNAs in neurodegenerative diseases. Front. Cell Neurosci. 2013, 7, 265. [Google Scholar] [CrossRef]
- Henshall, D.C.; Hamer, H.M.; Pasterkamp, R.J.; Goldstein, D.B.; Kjems, J.; Prehn, J.H.M.; Schorge, S.; Lamottke, K.; Rosenow, F. MicroRNAs in epilepsy: Pathophysiology and clinical utility. Lancet Neurol. 2016, 15, 1368–1376. [Google Scholar] [CrossRef]
- Swarbrick, S.; Wragg, N.; Ghosh, S.; Stolzing, A. Systematic Review of miRNA as Biomarkers in Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 6156–6167. [Google Scholar] [CrossRef]
- Dolati, S.; Marofi, F.; Babaloo, Z.; Aghebati-Maleki, L.; Roshangar, L.; Ahmadi, M.; Rikhtegar, R.; Yousefi, M. Dysregulated Network of miRNAs Involved in the Pathogenesis of Multiple Sclerosis. Biomed. Pharmacother. 2018, 104, 280–290. [Google Scholar] [CrossRef]
- Perdicchi, A.; Iester, M.; Iacovello, D.; Cutini, A.; Balestrieri, M.; Mutolo, M.G.; Ferreras, A.; Contestabile, M.T.; Recupero, S.M. Evaluation of Agreement between HRT III and iVue OCT in Glaucoma and Ocular Hypertension Patients. J. Ophthalmol. 2015, 2015, 691031. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Fingert, J.H.; Kuehn, M.H.; Alward, W.L. Primary open-angle glaucoma. N. Engl. J. Med. 2009, 360, 1113–1124. [Google Scholar] [CrossRef]
- Sommer, A. Intraocular pressure and glaucoma. Am. J. Ophthalmol. 1989, 107, 186–188. [Google Scholar] [CrossRef]
- Tielsch, J.M.; Sommer, A.; Katz, J.; Royall, R.M.; Quigley, H.A.; Javitt, J. Racial variations in the prevalence of primary open-angle glaucoma. The Baltimore Eye Survey. JAMA 1991, 266, 369–374. [Google Scholar] [CrossRef]
- Klein, B.E.; Klein, R.; Sponsel, W.E.; Franke, T.; Cantor, L.B.; Martone, J.; Menage, M.J. Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology 1992, 99, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Smith, W.; Attebo, K.; Healey, P.R. Prevalence of open-angle glaucoma in Australia. The Blue Mountains Eye Study. Ophthalmology 1996, 103, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Kovalyk, O.; Morales-Sanchez, J.; Verdu-Monedero, R.; Selles-Navarro, I.; Palazon-Cabanes, A.; Sancho-Gomez, J.L. PAPILA: Dataset with fundus images and clinical data of both eyes of the same patient for glaucoma assessment. Sci. Data 2022, 9, 291. [Google Scholar] [CrossRef]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef]
- Ritch, R. Exfoliation syndrome-the most common identifiable cause of open-angle glaucoma. J. Glaucoma 1994, 3, 176–177. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, B.; Tielsch, J.M.; Katz, J.; Gottsch, J.; Quigley, H.; Javitt, J.; Sommer, A. The cause-specific prevalence of visual impairment in an urban population. The Baltimore Eye Survey. Ophthalmology 1996, 103, 1721–1726. [Google Scholar] [CrossRef]
- Quigley, H.A.; Vitale, S. Models of open-angle glaucoma prevalence and incidence in the United States. Investig. Ophthalmol. Vis. Sci. 1997, 38, 83–91. [Google Scholar]
- Xu, B.Y.; Liang, S.; Pardeshi, A.A.; Lifton, J.; Moghimi, S.; Lewinger, J.P.; Varma, R. Differences in Ocular Biometric Measurements among Subtypes of Primary Angle Closure Disease: The Chinese American Eye Study. Ophthalmol. Glaucoma 2021, 4, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.S.; Wilson, M.R.; Liebmann, J.M.; Fechtner, R.D.; Weinreb, R.N. An evidence-based assessment of risk factors for the progression of ocular hypertension and glaucoma. Am. J. Ophthalmol. 2004, 138, S19–S31. [Google Scholar] [CrossRef] [PubMed]
- Leske, M.C.; Heijl, A.; Hussein, M.; Bengtsson, B.; Hyman, L.; Komaroff, E.; Early Manifest Glaucoma Trial, G. Factors for glaucoma progression and the effect of treatment: The early manifest glaucoma trial. Arch. Ophthalmol. 2003, 121, 48–56. [Google Scholar] [CrossRef]
- Lobo, J.; Gillis, A.J.M.; van den Berg, A.; Dorssers, L.C.J.; Belge, G.; Dieckmann, K.P.; Roest, H.P.; van der Laan, L.J.W.; Gietema, J.; Hamilton, R.J.; et al. Identification and Validation Model for Informative Liquid Biopsy-Based microRNA Biomarkers: Insights from Germ Cell Tumor In Vitro, In Vivo and Patient-Derived Data. Cells 2019, 8, 1637. [Google Scholar] [CrossRef]
- Callaghan, B.; Lester, K.; Lane, B.; Fan, X.; Goljanek-Whysall, K.; Simpson, D.A.; Sheridan, C.; Willoughby, C.E. Genome-wide transcriptome profiling of human trabecular meshwork cells treated with TGF-beta2. Sci. Rep. 2022, 12, 9564. [Google Scholar] [CrossRef]
- Seong, H.; Cho, H.K.; Kee, C.; Song, D.H.; Cho, M.C.; Kang, S.S. Profiles of microRNA in aqueous humor of normal tension glaucoma patients using RNA sequencing. Sci. Rep. 2021, 11, 19024. [Google Scholar] [CrossRef]
- Smyth, A.; Callaghan, B.; Willoughby, C.E.; O’Brien, C. The Role of miR-29 Family in TGF-beta Driven Fibrosis in Glaucomatous Optic Neuropathy. Int. J. Mol. Sci. 2022, 23, 10216. [Google Scholar] [CrossRef]
- Tektas, O.Y.; Lutjen-Drecoll, E. Structural changes of the trabecular meshwork in different kinds of glaucoma. Exp. Eye Res. 2009, 88, 769–775. [Google Scholar] [CrossRef]
- Tabak, S.; Schreiber-Avissar, S.; Beit-Yannai, E. Crosstalk between MicroRNA and Oxidative Stress in Primary Open-Angle Glaucoma. Int. J. Mol. Sci. 2021, 22, 2421. [Google Scholar] [CrossRef]
- Vranka, J.A.; Kelley, M.J.; Acott, T.S.; Keller, K.E. Extracellular matrix in the trabecular meshwork: Intraocular pressure regulation and dysregulation in glaucoma. Exp. Eye Res. 2015, 133, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Medina-Ortiz, W.E.; Belmares, R.; Neubauer, S.; Wordinger, R.J.; Clark, A.F. Cellular fibronectin expression in human trabecular meshwork and induction by transforming growth factor-beta2. Investig. Ophthalmol. Vis. Sci. 2013, 54, 6779–6788. [Google Scholar] [CrossRef] [PubMed]
- Mao, W.; Millar, J.C.; Wang, W.H.; Silverman, S.M.; Liu, Y.; Wordinger, R.J.; Rubin, J.S.; Pang, I.H.; Clark, A.F. Existence of the canonical Wnt signaling pathway in the human trabecular meshwork. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7043–7051. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Tang, P.M.; Li, J.; Lan, H.Y. TGF-beta/Smad signaling in renal fibrosis. Front. Physiol. 2015, 6, 82. [Google Scholar] [CrossRef] [PubMed]
- Acott, T.S.; Kelley, M.J. Extracellular matrix in the trabecular meshwork. Exp. Eye Res. 2008, 86, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Takai, Y.; Tanito, M.; Ohira, A. Multiplex cytokine analysis of aqueous humor in eyes with primary open-angle glaucoma, exfoliation glaucoma, and cataract. Investig. Ophthalmol. Vis. Sci. 2012, 53, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Pervan, C.L. Smad-independent TGF-beta2 signaling pathways in human trabecular meshwork cells. Exp. Eye Res. 2017, 158, 137–145. [Google Scholar] [CrossRef]
- Li, X.; Wang, J. Comparison of MicroRNA Expression in Aqueous Humor of Normal and Primary Open-Angle Glaucoma Patients Using PCR Arrays: A Pilot Study. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4988. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Tian, Y.; Cao, Y.; Ma, Q.; Zhao, S. MiR-137 promotes cell growth and inhibits extracellular matrix protein expression in H2O2-induced human trabecular meshwork cells by targeting Src. Neurosci. Lett. 2021, 755, 135902. [Google Scholar] [CrossRef]
- Yin, R.; Chen, X. Regulatory effect of miR-144-3p on the function of human trabecular meshwork cells and fibronectin-1. Exp. Ther. Med. 2019, 18, 647–653. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.; Long, H.; Zhou, B.; Jiang, H. miR-486-5p Restrains Extracellular Matrix Production and Oxidative Damage in Human Trabecular Meshwork Cells by Targeting TGF-beta/SMAD2 Pathway. J. Ophthalmol. 2022, 2022, 3584192. [Google Scholar] [CrossRef] [PubMed]
- Maurer, B.; Stanczyk, J.; Jungel, A.; Akhmetshina, A.; Trenkmann, M.; Brock, M.; Kowal-Bielecka, O.; Gay, R.E.; Michel, B.A.; Distler, J.H.; et al. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum. 2010, 62, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Gallant-Behm, C.L.; Piper, J.; Lynch, J.M.; Seto, A.G.; Hong, S.J.; Mustoe, T.A.; Maari, C.; Pestano, L.A.; Dalby, C.M.; Jackson, A.L.; et al. A MicroRNA-29 Mimic (Remlarsen) Represses Extracellular Matrix Expression and Fibroplasia in the Skin. J. Investig. Dermatol. 2019, 139, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Junn, E.; Mouradian, M.M. MicroRNAs in neurodegenerative diseases and their therapeutic potential. Pharmacol. Ther. 2012, 133, 142–150. [Google Scholar] [CrossRef]
- Orom, U.A.; Kauppinen, S.; Lund, A.H. LNA-modified oligonucleotides mediate specific inhibition of microRNA function. Gene 2006, 372, 137–141. [Google Scholar] [CrossRef]
- Yue, X.; Yu, X.; Petersen, F.; Riemekasten, G. Recent advances in mouse models for systemic sclerosis. Autoimmun. Rev. 2018, 17, 1225–1234. [Google Scholar] [CrossRef]
- Tan, C.; Jia, F.; Zhang, P.; Sun, X.; Qiao, Y.; Chen, X.; Wang, Y.; Chen, J.; Lei, Y. A miRNA stabilizing polydopamine nano-platform for intraocular delivery of miR-21-5p in glaucoma therapy. J. Mater. Chem. B 2021, 9, 3335–3345. [Google Scholar] [CrossRef]
- Yu, Z.; Wen, Y.; Jiang, N.; Li, Z.; Guan, J.; Zhang, Y.; Deng, C.; Zhao, L.; Zheng, S.G.; Zhu, Y.; et al. TNF-alpha stimulation enhances the neuroprotective effects of gingival MSCs derived exosomes in retinal ischemia-reperfusion injury via the MEG3/miR-21a-5p axis. Biomaterials 2022, 284, 121484. [Google Scholar] [CrossRef]
- Su, W.; Li, Z.; Jia, Y.; Zhu, Y.; Cai, W.; Wan, P.; Zhang, Y.; Zheng, S.G.; Zhuo, Y. microRNA-21a-5p/PDCD4 axis regulates mesenchymal stem cell-induced neuroprotection in acute glaucoma. J. Mol. Cell Biol. 2017, 9, 289–301. [Google Scholar] [CrossRef]
- Nie, X.G.; Fan, D.S.; Huang, Y.X.; He, Y.Y.; Dong, B.L.; Gao, F. Downregulation of microRNA-149 in retinal ganglion cells suppresses apoptosis through activation of the PI3K/Akt signaling pathway in mice with glaucoma. Am. J. Physiol. Cell Physiol. 2018, 315, C839–C849. [Google Scholar] [CrossRef]
- Guo, J.; Liu, H.; Fu, L. MicroRNA-124 ameliorates autophagic dysregulation in glaucoma via regulation of P2X7-mediated Akt/mTOR signaling. Cutan. Ocul. Toxicol. 2022, 41, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Sun, Y.B.; Hao, J.L.; Lu, C.W.; Bi, M.C.; Song, E. Neuroprotective effects of overexpressed microRNA-200a on activation of glaucoma-related retinal glial cells and apoptosis of ganglion cells via downregulating FGF7-mediated MAPK signaling pathway. Cell Signal 2019, 54, 179–190. [Google Scholar] [CrossRef]
- Li, X.; Zhao, F.; Xin, M.; Li, G.; Luna, C.; Li, G.; Zhou, Q.; He, Y.; Yu, B.; Olson, E.; et al. Regulation of intraocular pressure by microRNA cluster miR-143/145. Sci. Rep. 2017, 7, 915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; He, C.; Li, R.; Ke, Y.; Sun, K.; Wang, J. miR-708 and miR-335-3p Inhibit the Apoptosis of Retinal Ganglion Cells Through Suppressing Autophagy. J. Mol. Neurosci. 2021, 71, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Ahmed, Z.; Tomarev, S. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Neuroprotection in a Genetic DBA/2J Mouse Model of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5473–5480. [Google Scholar] [CrossRef]
- Mak, H.K.; Yung, J.S.Y.; Weinreb, R.N.; Ng, S.H.; Cao, X.; Ho, T.Y.C.; Ng, T.K.; Chu, W.K.; Yung, W.H.; Choy, K.W.; et al. MicroRNA-19a-PTEN Axis Is Involved in the Developmental Decline of Axon Regenerative Capacity in Retinal Ganglion Cells. Mol. Ther. Nucleic Acids 2020, 21, 251–263. [Google Scholar] [CrossRef]
- Zhang, L.Q.; Cui, H.; Yu, Y.B.; Shi, H.Q.; Zhou, Y.; Liu, M.J. MicroRNA-141-3p inhibits retinal neovascularization and retinal ganglion cell apoptosis in glaucoma mice through the inactivation of Docking protein 5-dependent mitogen-activated protein kinase signaling pathway. J. Cell Physiol. 2019, 234, 8873–8887. [Google Scholar] [CrossRef]
- Palazzo, A.F.; Lee, E.S. Non-coding RNA: What is functional and what is junk? Front. Genet. 2015, 6, 2. [Google Scholar] [CrossRef]
- Hombach, S.; Kretz, M. Non-coding RNAs: Classification, Biology and Functioning. Adv. Exp. Med. Biol. 2016, 937, 3–17. [Google Scholar] [CrossRef]
- Yu, C.Y.; Kuo, H.C. The emerging roles and functions of circular RNAs and their generation. J. Biomed. Sci. 2019, 26, 29. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, T.; Xiao, J. Circular RNAs: Promising Biomarkers for Human Diseases. eBioMedicine 2018, 34, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Verduci, L.; Strano, S.; Yarden, Y.; Blandino, G. The circRNA-microRNA code: Emerging implications for cancer diagnosis and treatment. Mol. Oncol. 2019, 13, 669–680. [Google Scholar] [CrossRef]
- Yang, L.; Han, B.; Zhang, Z.; Wang, S.; Bai, Y.; Zhang, Y.; Tang, Y.; Du, L.; Xu, L.; Wu, F.; et al. Extracellular Vesicle-Mediated Delivery of Circular RNA SCMH1 Promotes Functional Recovery in Rodent and Nonhuman Primate Ischemic Stroke Models. Circulation 2020, 142, 556–574. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Xu, M.; Zhou, J.; Kang, G.; Li, K. Circ_0080940 Regulates miR-139-5p/CTGF Pathway to Promote the Proliferation, Migration, Extracellular Matrix Deposition of Human Tenon’s Capsule Fibroblasts. Curr. Eye Res. 2023, 48, 34–43. [Google Scholar] [CrossRef]
- Moazzeni, H.; Khani, M.; Elahi, E. Insights into the regulatory molecules involved in glaucoma pathogenesis. Am. J. Med. Genet. C Semin. Med. Genet. 2020, 184, 782–827. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, J.; Yu, Y. CircRNA Is a Rising Star in Researches of Ocular Diseases. Front. Cell Dev. Biol. 2020, 8, 850. [Google Scholar] [CrossRef]
- Charteris, D.G.; Sethi, C.S.; Lewis, G.P.; Fisher, S.K. Proliferative vitreoretinopathy-developments in adjunctive treatment and retinal pathology. Eye 2002, 16, 369–374. [Google Scholar] [CrossRef]
- Liu, C.; Yao, M.D.; Li, C.P.; Shan, K.; Yang, H.; Wang, J.J.; Liu, B.; Li, X.M.; Yao, J.; Jiang, Q.; et al. Silencing of Circular RNA-ZNF609 Ameliorates Vascular Endothelial Dysfunction. Theranostics 2017, 7, 2863–2877. [Google Scholar] [CrossRef]
- Wang, J.J.; Liu, C.; Shan, K.; Liu, B.H.; Li, X.M.; Zhang, S.J.; Zhou, R.M.; Dong, R.; Yan, B.; Sun, X.H. Circular RNA-ZNF609 regulates retinal neurodegeneration by acting as miR-615 sponge. Theranostics 2018, 8, 3408–3415. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, R.; Shan, K.; Sun, Y.; Yan, B.; Sun, X.; Wang, J. Circular RNA Expression Profiling Identifies Glaucoma-Related Circular RNAs in Various Chronic Ocular Hypertension Rat Models. Front. Genet. 2020, 11, 556712. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Shan, K.; Liu, B.H.; Liu, C.; Zhou, R.M.; Li, X.M.; Dong, R.; Zhang, S.J.; Zhang, S.H.; Wu, J.H.; et al. Targeting circular RNA-ZRANB1 for therapeutic intervention in retinal neurodegeneration. Cell Death Dis. 2018, 9, 540. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Lee, Y.C.; Chang, K.L.; Hsiao, K.Y. Analysis of common targets for circular RNAs. BMC Bioinform. 2019, 20, 372. [Google Scholar] [CrossRef]
- Ma, C.; Yao, M.D.; Han, X.Y.; Shi, Z.H.; Yan, B.; Du, J.L. Silencing of circular RNA-ZYG11B exerts a neuroprotective effect against retinal neurodegeneration. Int. J. Mol. Med. 2022, 50, 106. [Google Scholar] [CrossRef]
- Chen, L.L. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat. Rev. Mol. Cell Biol. 2020, 21, 475–490. [Google Scholar] [CrossRef]
- Han, B.; Chao, J.; Yao, H. Circular RNA and its mechanisms in disease: From the bench to the clinic. Pharmacol. Ther. 2018, 187, 31–44. [Google Scholar] [CrossRef]
- Lee, E.C.S.; Elhassan, S.A.M.; Lim, G.P.L.; Kok, W.H.; Tan, S.W.; Leong, E.N.; Tan, S.H.; Chan, E.W.L.; Bhattamisra, S.K.; Rajendran, R.; et al. The roles of circular RNAs in human development and diseases. Biomed. Pharmacother. 2019, 111, 198–208. [Google Scholar] [CrossRef]
- Silva, A.; Bullock, M.; Calin, G. The Clinical Relevance of Long Non-Coding RNAs in Cancer. Cancers 2015, 7, 2169–2182. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y.; Wang, Y.; Gao, J.; Lv, J.; Sun, J.; Li, M.; Wang, M.; Zhao, Z.; Wang, J.; et al. Long non-coding RNAs in ocular diseases: New and potential therapeutic targets. FEBS J. 2019, 286, 2261–2272. [Google Scholar] [CrossRef]
- Xie, L.; Mao, M.; Wang, C.; Zhang, L.; Pan, Z.; Shi, J.; Duan, X.; Jia, S.; Jiang, B. Potential Biomarkers for Primary Open-Angle Glaucoma Identified by Long Noncoding RNA Profiling in the Aqueous Humor. Am. J. Pathol. 2019, 189, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, L.R.; Loomis, S.J.; Kang, J.H.; Yaspan, B.L.; Abdrabou, W.; Budenz, D.L.; Chen, T.C.; Delbono, E.; Friedman, D.S.; Gaasterland, D.; et al. CDKN2B-AS1 genotype-glaucoma feature correlations in primary open-angle glaucoma patients from the United States. Am. J. Ophthalmol. 2013, 155, 342–353 e345. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Tam, A.L.C.; Campbell, R.; Renwick, N. Emerging Evidence of Noncoding RNAs in Bleb Scarring after Glaucoma Filtration Surgery. Cells 2022, 11, 1301. [Google Scholar] [CrossRef]
- Militello, G.; Weirick, T.; John, D.; Doring, C.; Dimmeler, S.; Uchida, S. Screening and validation of lncRNAs and circRNAs as miRNA sponges. Brief. Bioinform. 2017, 18, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Smillie, C.L.; Sirey, T.; Ponting, C.P. Complexities of post-transcriptional regulation and the modeling of ceRNA crosstalk. Crit. Rev. Biochem. Mol. Biol. 2018, 53, 231–245. [Google Scholar] [CrossRef]
- Yan, Z.; Lai, M.; Jia, Y.; Deng, C.; Zhuo, Y. CircXPO5 Plays a Neuroprotective Function in the Lateral Geniculate Nucleus of Glaucoma by Regulating GRIN2A. Brain Sci. 2022, 12, 780. [Google Scholar] [CrossRef]
- Li, F.; Huang, C.; Li, Q.; Wu, X. Construction and Comprehensive Analysis for Dysregulated Long Non-Coding RNA (lncRNA)-Associated Competing Endogenous RNA (ceRNA) Network in Gastric Cancer. Med. Sci. Monit. 2018, 24, 37–49. [Google Scholar] [CrossRef]
- Huang, Y.; Xiang, B.; Liu, Y.; Wang, Y.; Kan, H. LncRNA CDKN2B-AS1 promotes tumor growth and metastasis of human hepatocellular carcinoma by targeting let-7c-5p/NAP1L1 axis. Cancer Lett. 2018, 437, 56–66. [Google Scholar] [CrossRef]
- Paraskevopoulou, M.D.; Hatzigeorgiou, A.G. Analyzing MiRNA-LncRNA Interactions. Methods Mol. Biol. 2016, 1402, 271–286. [Google Scholar] [CrossRef]
- Lv, Y.; Zhang, Z.; Xing, X.; Liu, A. lncRNA TGFbeta2-AS1 promotes ECM production via TGF-beta2 in human trabecular meshwork cells. Biochem. Biophys. Res. Commun. 2020, 527, 881–888. [Google Scholar] [CrossRef]
- Su, Y.; Yi, Y.; Li, L.; Chen, C. circRNA-miRNA-mRNA network in age-related macular degeneration: From construction to identification. Exp. Eye Res. 2021, 203, 108427. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, T.; Zhang, X.; Cai, X.; Sun, H. Network Integration Analysis and Immune Infiltration Analysis Reveal Potential Biomarkers for Primary Open-Angle Glaucoma. Front. Cell Dev. Biol. 2021, 9, 793638. [Google Scholar] [CrossRef]
- Li, H.; Ye, Z.; Li, Z. Identification of the potential biological target molecules related to primary open-angle glaucoma. BMC Ophthalmol. 2022, 22, 188. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Wang, T.; Zhou, F.; Yao, Y.; He, J.; Xu, D. Bioinformatics-based identification of miRNA-, lncRNA-, and mRNA-associated ceRNA networks and potential biomarkers for preeclampsia. Medicine 2020, 99, e22985. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Wan, Q.; Huang, J.; Han, L.; Chen, X.; Chen, G.; Olsen, N.; Zheng, S.G.; Liang, D. Culture medium from TNF-alpha-stimulated mesenchymal stem cells attenuates allergic conjunctivitis through multiple antiallergic mechanisms. J. Allergy Clin. Immunol. 2015, 136, 423–432 e428. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Tomarev, S. Extracellular vesicle therapy for retinal diseases. Prog. Retin. Eye Res. 2020, 79, 100849. [Google Scholar] [CrossRef]
- Harrell, C.R.; Jankovic, M.G.; Fellabaum, C.; Volarevic, A.; Djonov, V.; Arsenijevic, A.; Volarevic, V. Molecular Mechanisms Responsible for Anti-inflammatory and Immunosuppressive Effects of Mesenchymal Stem Cell-Derived Factors. Adv. Exp. Med. Biol. 2019, 1084, 187–206. [Google Scholar] [CrossRef]
- Pegtel, D.M.; Gould, S.J. Exosomes. Annu. Rev. Biochem. 2019, 88, 487–514. [Google Scholar] [CrossRef]
- Elahi, F.M.; Farwell, D.G.; Nolta, J.A.; Anderson, J.D. Preclinical translation of exosomes derived from mesenchymal stem/stromal cells. Stem Cells 2020, 38, 15–21. [Google Scholar] [CrossRef]
- Camussi, G.; Deregibus, M.C.; Bruno, S.; Cantaluppi, V.; Biancone, L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010, 78, 838–848. [Google Scholar] [CrossRef]
- Otero-Ortega, L.; Laso-Garcia, F.; Gomez-de Frutos, M.; Fuentes, B.; Diekhorst, L.; Diez-Tejedor, E.; Gutierrez-Fernandez, M. Role of Exosomes as a Treatment and Potential Biomarker for Stroke. Transl. Stroke Res. 2019, 10, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, H.; Ong, H.S.; Riau, A.K.; Stanzel, T.P.; Mehta, J.S.; Yam, G.H. Current Trends and Future Perspective of Mesenchymal Stem Cells and Exosomes in Corneal Diseases. Int. J. Mol. Sci. 2019, 20, 2853. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Amaral, J.; Tomarev, S. Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Neuroprotection in Rodent Models of Glaucoma. Investig. Ophthalmol. Vis. Sci. 2018, 59, 702–714. [Google Scholar] [CrossRef] [PubMed]
- Lou, G.; Chen, Z.; Zheng, M.; Liu, Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp. Mol. Med. 2017, 49, e346. [Google Scholar] [CrossRef]
- Shi, M.; Liu, C.; Cook, T.J.; Bullock, K.M.; Zhao, Y.; Ginghina, C.; Li, Y.; Aro, P.; Dator, R.; He, C.; et al. Plasma exosomal alpha-synuclein is likely CNS-derived and increased in Parkinson’s disease. Acta Neuropathol. 2014, 128, 639–650. [Google Scholar] [CrossRef]
- Garcia-Contreras, M.; Brooks, R.W.; Boccuzzi, L.; Robbins, P.D.; Ricordi, C. Exosomes as biomarkers and therapeutic tools for type 1 diabetes mellitus. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2940–2956. [Google Scholar]
- Zhang, Q.; Sun, J.; Huang, Y.; Bu, S.; Guo, Y.; Gu, T.; Li, B.; Wang, C.; Lai, D. Human Amniotic Epithelial Cell-Derived Exosomes Restore Ovarian Function by Transferring MicroRNAs against Apoptosis. Mol. Ther. Nucleic Acids 2019, 16, 407–418. [Google Scholar] [CrossRef]
- Chen, Q.L.; Xie, C.F.; Feng, K.L.; Cui, D.Y.; Sun, S.L.; Zhang, J.C.; Xiong, C.M.; Huang, J.H.; Chong, Z. microRNAs carried by exosomes promote epithelial-mesenchymal transition and metastasis of liver cancer cells. Am. J. Transl. Res. 2020, 12, 6811–6826. [Google Scholar]
- Kosaka, N.; Yoshioka, Y.; Fujita, Y.; Ochiya, T. Versatile roles of extracellular vesicles in cancer. J. Clin. Investig. 2016, 126, 1163–1172. [Google Scholar] [CrossRef]
- Chen, T.S.; Lai, R.C.; Lee, M.M.; Choo, A.B.; Lee, C.N.; Lim, S.K. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010, 38, 215–224. [Google Scholar] [CrossRef]
- Katsuda, T.; Ochiya, T. Molecular signatures of mesenchymal stem cell-derived extracellular vesicle-mediated tissue repair. Stem Cell Res. Ther. 2015, 6, 212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chopp, M.; Liu, X.S.; Katakowski, M.; Wang, X.; Tian, X.; Wu, D.; Zhang, Z.G. Exosomes Derived from Mesenchymal Stromal Cells Promote Axonal Growth of Cortical Neurons. Mol. Neurobiol. 2017, 54, 2659–2673. [Google Scholar] [CrossRef] [PubMed]
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Synnestvedt, M.B.; Chen, C.; Holmes, J.H. CiteSpace II: Visualization and knowledge discovery in bibliographic databases. AMIA Annu. Symp. Proc. 2005, 2005, 724–728. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).