Impact of a High-Fat Diet at a Young Age on Wound Healing in Mice
Abstract
:1. Introduction
2. Results
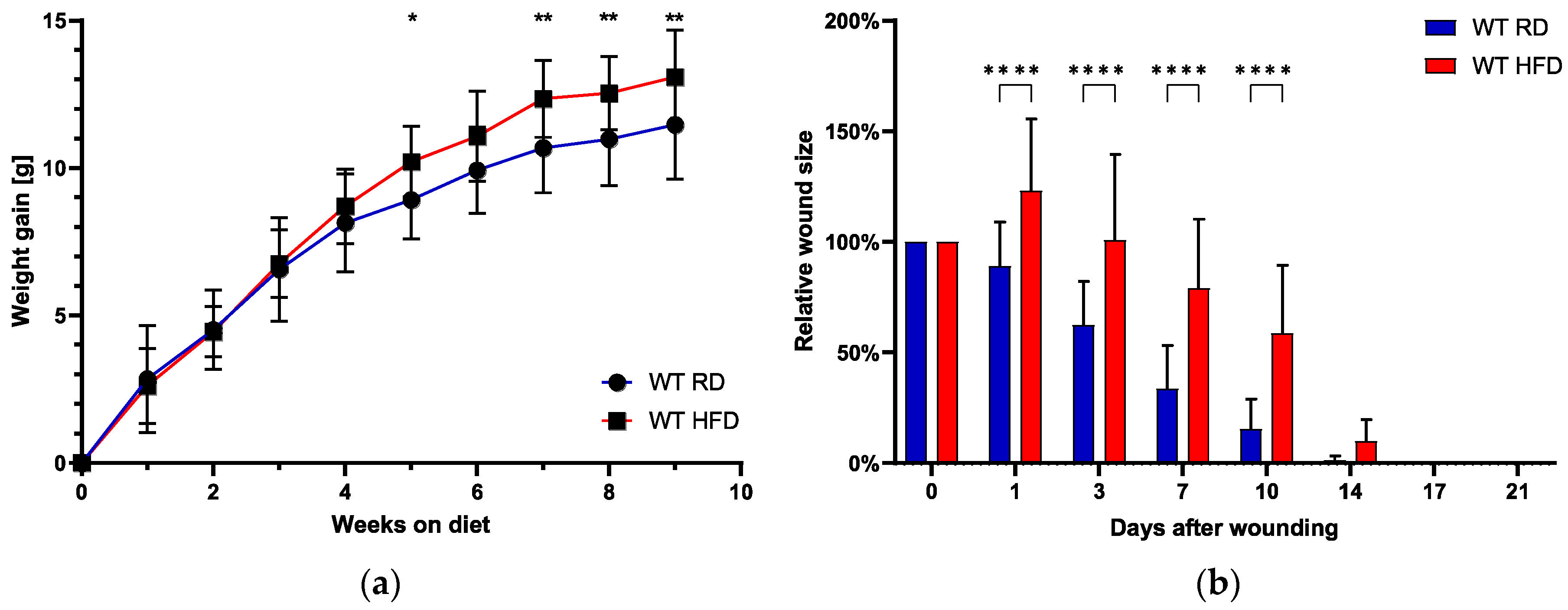
2.1. Early High-Fat Diet Induces Obese Phenotype and Delays Normal Wound Healing
2.2. Granulation Tissue Quality and Thickness Impaired with an Early High-Fat Diet
2.3. Delayed Myofibroblast Appearance in HFD-Fed Mice
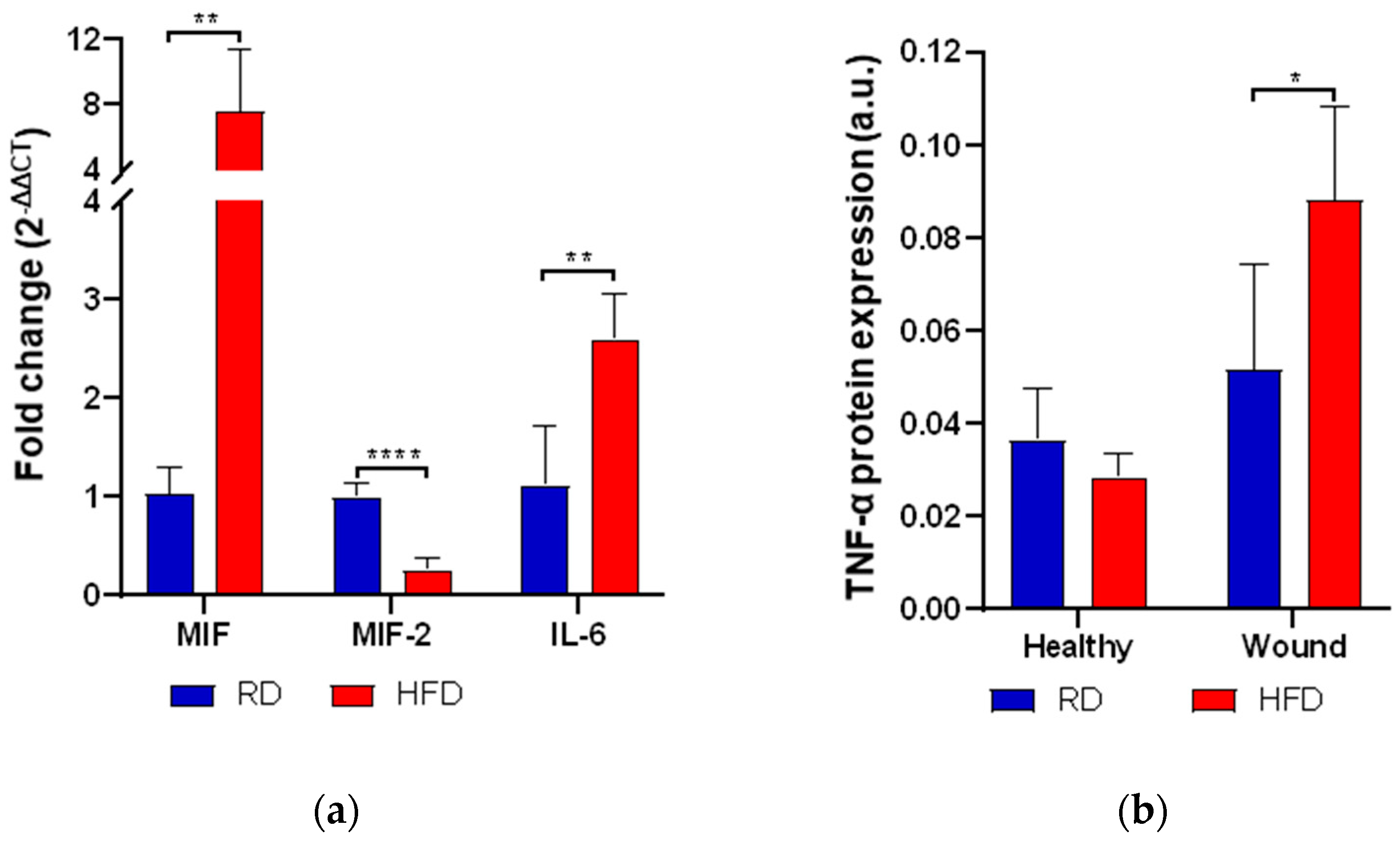
2.4. Early High-Fat Diet Increases Mediators of Inflammation
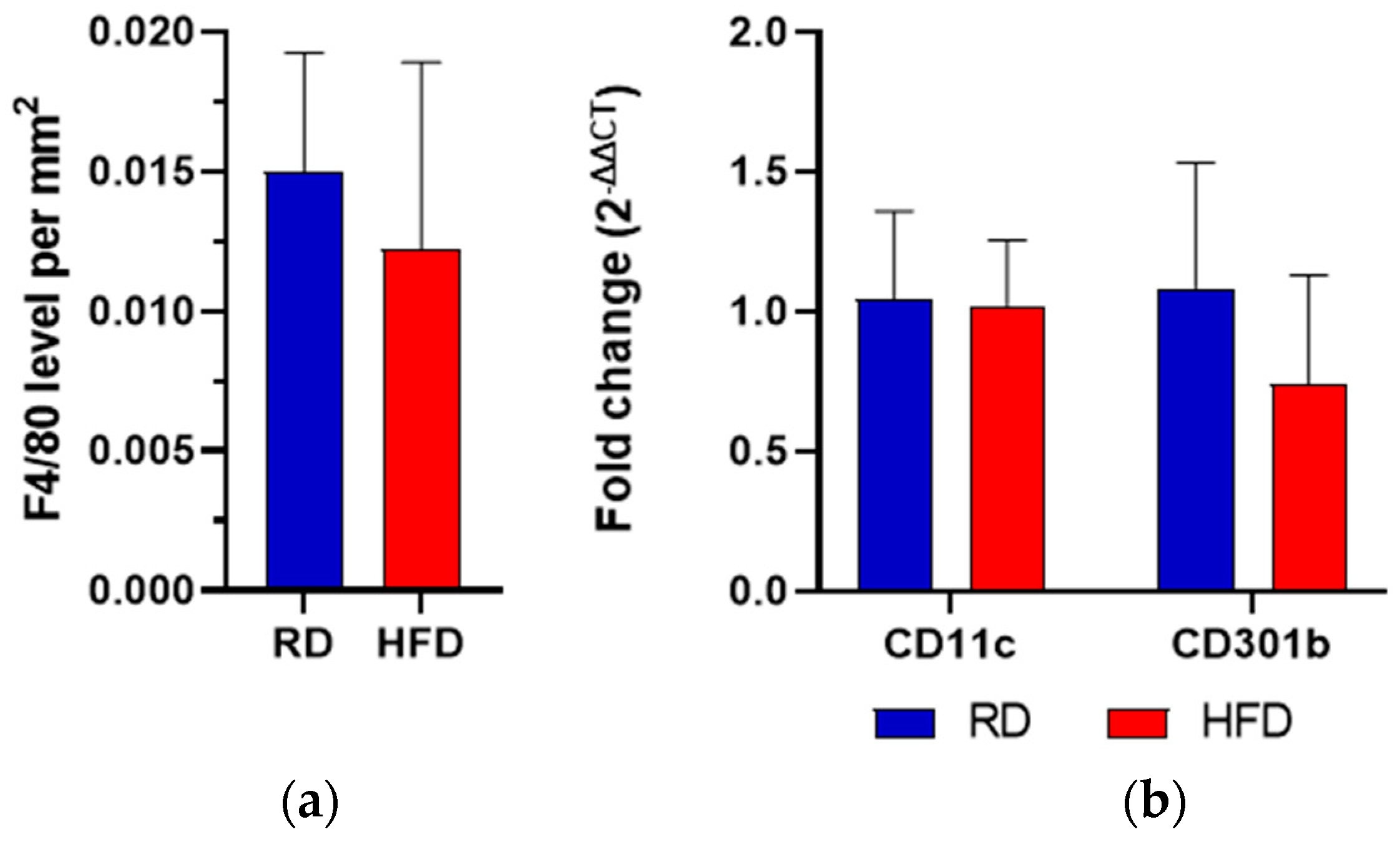
2.5. Macrophage Quantification and M1/M2 Phenotype Assessment
2.6. Early Vascularization Is Decreased after Wounding in Early HFD-Fed Animals
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
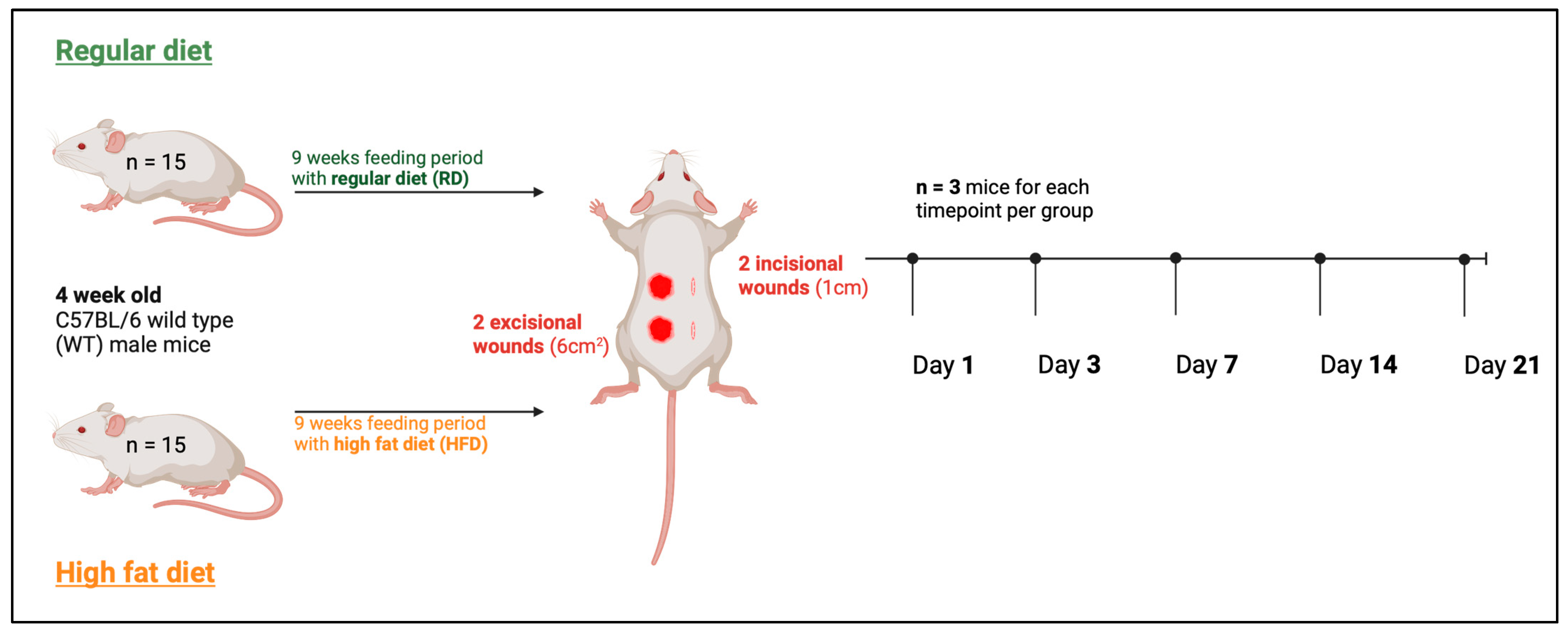
4.2. Animals
4.3. Diets
4.4. Wounding of Mice
4.5. Real-Time Quantitative PCR (RT-qPCR)
4.6. Histology: Masson–Goldner-Trichrome Staining and Immunohistochemistry
4.7. Granulation Tissue Quality Assessment
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metab. Clin. Exp. Metab. 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Pierpont, Y.N.; Dinh, T.P.; Salas, R.E.; Johnson, E.L.; Wright, T.G.; Robson, M.C.; Payne, W.G. Obesity and surgical wound healing: A current review. ISRN Obes. 2014, 2014, 638936. [Google Scholar] [CrossRef] [PubMed]
- Sanyaolu, A.; Okorie, C.; Qi, X.; Locke, J.; Rehman, S. Childhood and Adolescent Obesity in the United States: A Public Health Concern. Glob. Pediatr. Health 2019, 6, 2333794X19891305. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.; Llewellyn, A.; Owen, C.G.; Woolacott, N. Predicting adult obesity from childhood obesity: A systematic review and meta-analysis. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2016, 17, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Knoedler, S.; Broichhausen, S.; Guo, R.; Dai, R.; Knoedler, L.; Kauke-Navarro, M.; Diatta, F.; Pomahac, B.; Machens, H.-G.; Jiang, D.; et al. Fibroblasts–The cellular choreographers of wound healing. Front. Immunol. 2023, 14, 1233800. [Google Scholar] [CrossRef]
- Martin, P. Wound healing—Aiming for perfect skin regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Hinz, B. The role of myofibroblasts in wound healing. Curr. Res. Transl. Med. 2016, 64, 171–177. [Google Scholar] [CrossRef]
- Dong, J.; Chen, L.; Zhang, Y.; Jayaswal, N.; Mezghani, I.; Zhang, W.; Veves, A. Mast Cells in Diabetes and Diabetic Wound Healing. Adv. Ther. 2020, 37, 4519–4537. [Google Scholar] [CrossRef]
- Chmelař, J.; Chatzigeorgiou, A.; Chung, K.-J.; Prucnal, M.; Voehringer, D.; Roers, A.; Chavakis, T. No Role for Mast Cells in Obesity-Related Metabolic Dysregulation. Front. Immunol. 2016, 7, 524. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; María Aguilera, C.; Gil-Campos, M.; Cañete, R. Altered signalling and gene expression associated with the immune system and the inflammatory response in obesity. Br. J. Nutr. 2007, 98 (Suppl. S1), S121–S126. [Google Scholar] [CrossRef] [PubMed]
- Goren, I.; Müller, E.; Schiefelbein, D.; Christen, U.; Pfeilschifter, J.; Mühl, H.; Frank, S. Systemic anti-TNFalpha treatment restores diabetes-impaired skin repair in ob/ob mice by inactivation of macrophages. J. Investig. Dermatol. 2007, 127, 2259–2267. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Pallua, N.; Bernhagen, J.; Bucala, R. The macrophage migration inhibitory factor protein superfamily in obesity and wound repair. Exp. Mol. Med. 2015, 47, e161. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.Z.; Stevenson, A.W.; Prêle, C.M.; Fear, M.W.; Wood, F.M. The Role of IL-6 in Skin Fibrosis and Cutaneous Wound Healing. Biomedicines 2020, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-S.; Breuer, B.; Arnke, K.; Ruhl, T.; Hofer, T.; Simons, D.; Knobe, M.; Ganse, B.; Guidi, M.; Beier, J.P.; et al. The effect of the macrophage migration inhibitory factor (MIF) on excisional wound healing in vivo. J. Plast. Surg. Hand Surg. 2020, 54, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef]
- Schanuel, F.S.; Romana-Souza, B.; Monte-Alto-Costa, A. Short-Term Administration of a High-Fat Diet Impairs Wound Repair in Mice. Lipids 2020, 55, 23–33. [Google Scholar] [CrossRef]
- Kopcewicz, M.; Walendzik, K.; Bukowska, J.; Kur-Piotrowska, A.; Machcinska, S.; Gimble, J.M.; Gawronska-Kozak, B. Cutaneous wound healing in aged, high fat diet-induced obese female or male C57BL/6 mice. Aging 2020, 12, 7066–7111. [Google Scholar] [CrossRef]
- Suriano, F.; Vieira-Silva, S.; Falony, G.; Roumain, M.; Paquot, A.; Pelicaen, R.; Régnier, M.; Delzenne, N.M.; Raes, J.; Muccioli, G.G.; et al. Novel insights into the genetically obese (ob/ob) and diabetic (db/db) mice: Two sides of the same coin. MicroMicrobiome 2021, 9, 147. [Google Scholar] [CrossRef]
- Brun, P.; Castagliuolo, I.; Di Leo, V.; Buda, A.; Pinzani, M.; Palù, G.; Martines, D. Increased intestinal permeability in obese mice: New evidence in the pathogenesis of nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G518–G525. [Google Scholar] [CrossRef] [PubMed]
- Giesbertz, P.; Padberg, I.; Rein, D.; Ecker, J.; Höfle, A.S.; Spanier, B.; Daniel, H. Metabolite profiling in plasma and tissues of ob/ob and db/db mice identifies novel markers of obesity and type 2 diabetes. Diabetologia 2015, 58, 2133–2143. [Google Scholar] [CrossRef] [PubMed]
- Goodson, W.H., III; Hunt, T.K. Wound collagen accumulation in obese hyperglycemic mice. Diabetes 1986, 35, 491–495. [Google Scholar] [CrossRef]
- Greenhalgh, D.G.; Sprugel, K.H.; Murray, M.J.; Ross, R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am. J. Pathol. 1990, 136, 1235–1246. [Google Scholar]
- Dutta, S.; Sengupta, P. Men and mice: Relating their ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B. Formation and function of the myofibroblast during tissue repair. J. Investig. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef]
- Hantash, B.M.; Zhao, L.; Knowles, J.A.; Lorenz, H.P. Adult and fetal wound healing. Front. Biosci. 2008, 13, 51–61. [Google Scholar] [CrossRef]
- Dos Anjos Cassado, A. F4/80 as a Major Macrophage Marker: The Case of the Peritoneum and Spleen. Results Probl. Cell Differ. 2017, 62, 161–179. [Google Scholar] [CrossRef]
- Seitz, O.; Schürmann, C.; Hermes, N.; Müller, E.; Pfeilschifter, J.; Frank, S.; Goren, I. Wound healing in mice with high-fat diet- or ob gene-induced diabetes-obesity syndromes: A comparative study. Exp. Diabetes Res. 2010, 2010, 476969. [Google Scholar] [CrossRef]
- Li, J.; Wu, H.; Liu, Y.; Yang, L. High fat diet induced obesity model using four strainsof mice: Kunming, C57BL/6, BALB/c and ICR. Exp. Anim. 2020, 69, 326–335. [Google Scholar] [CrossRef]
- Pence, B.D.; DiPietro, L.A.; Woods, J.A. Exercise speeds cutaneous wound healing in high-fat diet-induced obese mice. Med. Sci. Sports Exerc. 2012, 44, 1846–1854. [Google Scholar] [CrossRef]
- Chu, D.T.; Malinowska, E.; Jura, M.; Kozak, L.P. C57BL/6J mice as a polygenic developmental model of diet-induced obesity. Physiol. Rep. 2017, 5, e13093. [Google Scholar] [CrossRef] [PubMed]
- Pierce, G.F.; Mustoe, T.A.; Altrock, B.W.; Deuel, T.F.; Thomason, A. Role of platelet-derived growth factor in wound healing. J. Cell. Biochem. 1991, 45, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Stoffels, J.M.; Zhao, C.; Baron, W. Fibronectin in tissue regeneration: Timely disassembly of the scaffold is necessary to complete the build. Cell. Mol. Life Sci. 2013, 70, 4243–4253. [Google Scholar] [CrossRef]
- Grinnell, F.; Billingham, R.E.; Burgess, L. Distribution of fibronectin during wound healing in vivo. J. Investig. Dermatol. 1981, 76, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Lenselink, E.A. Role of fibronectin in normal wound healing. Int. Wound J. 2015, 12, 313–316. [Google Scholar] [CrossRef]
- Blechman, J.M.; Lev, S.; Barg, J.; Eisenstein, M.; Vaks, B.; Vogel, Z.; Givol, D.; Yarden, Y. The fourth immunoglobulin domain of the stem cell factor receptor couples ligand binding to signal transduction. Cell 1995, 80, 103–113. [Google Scholar] [CrossRef]
- Heldin, C.H.; Westermark, B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol. Rev. 1999, 79, 1283–1316. [Google Scholar] [CrossRef]
- Lindley, L.E.; Stojadinovic, O.; Pastar, I.; Tomic-Canic, M. Biology and Biomarkers for Wound Healing. Plast. Reconstr. Surg. 2016, 138, 18S–28S. [Google Scholar] [CrossRef]
- Wang, P.H.; Huang, B.S.; Horng, H.C.; Yeh, C.C.; Chen, Y.J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Rosado Jde, D.; Rodriguez-Sosa, M. Macrophage migration inhibitory factor (MIF): A key player in protozoan infections. Int. J. Biol. Sci. 2011, 7, 1239–1256. [Google Scholar] [CrossRef]
- Hardman, M.J.; Waite, A.; Zeef, L.; Burow, M.; Nakayama, T.; Ashcroft, G.S. Macrophage migration inhibitory factor: A central regulator of wound healing. Am. J. Pathol. 2005, 167, 1561–1574. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, G.S.; Mills, S.J.; Lei, K.; Gibbons, L.; Jeong, M.-J.; Taniguchi, M.; Burow, M.; Horan, M.A.; Wahl, S.M.; Nakayama, T. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J. Clin. Investig. 2003, 111, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Tilstam, P.V.; Hwang, S.S.; Simons, D.; Schulte, W.; Leng, L.; Sauler, M.; Ganse, B.; Averdunk, L.; Kopp, R.; et al. D-dopachrome tautomerase in adipose tissue inflammation and wound repair. J. Cell. Mol. Med. 2017, 21, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, N.H.; Lee, C.H. Identity Crisis: CD301b+ Mononuclear Phagocytes Blur the M1-M2 Macrophage Line. Immunity 2016, 45, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Tilstam, P.V.; Schulte, W.; Holowka, T.; Kim, B.-S.; Nouws, J.; Sauler, M.; Piecychna, M.; Pantouris, G.; Lolis, E.; Leng, L.; et al. MIF but not MIF-2 recruits inflammatory macrophages in an experimental polymicrobial sepsis model. J. Clin. Investig. 2021, 131, jci127171. [Google Scholar] [CrossRef]
- Kumamoto, Y.; Camporez, J.P.G.; Jurczak, M.J.; Shanabrough, M.; Horvath, T.; Shulman, G.I.; Iwasaki, A. CD301b+ Mononuclear Phagocytes Maintain Positive Energy Balance through Secretion of Resistin-like Molecule Alpha. Immunity 2016, 45, 583–596. [Google Scholar] [CrossRef]
- Louiselle, A.E.; Niemiec, S.M.; Zgheib, C.; Liechty, K.W. Macrophage polarization and diabetic wound healing. Transl. Res. 2021, 236, 109–116. [Google Scholar] [CrossRef]
- Monika, P.; Waiker, P.V.; Chandraprabha, M.N.; Rangarajan, A.; Murthy, K.N.C. Myofibroblast progeny in wound biology and wound healing studies. Wound Repair Regen. 2021, 29, 531–547. [Google Scholar] [CrossRef] [PubMed]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Temporal control of PDGFRα regulates the fibroblast-to-myofibroblast transition in wound healing. Cell Rep. 2022, 40, 111192. [Google Scholar] [CrossRef]
- Driskell, R.R.; Lichtenberger, B.M.; Hoste, E.; Kretzschmar, K.; Simons, B.D.; Charalambous, M.; Ferron, S.R.; Herault, Y.; Pavlovic, G.; Ferguson-Smith, A.C.; et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 2013, 504, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Ansell, D.M.; Campbell, L.; Thomason, H.A.; Brass, A.; Hardman, M.J. A statistical analysis of murine incisional and excisional acute wound models. Wound Repair Regen. 2014, 22, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Masson-Meyers, D.S.; Andrade, T.A.M.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental models and methods for cutaneous wound healing assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Taylor, S.C.; Nadeau, K.; Abbasi, M.; Lachance, C.; Nguyen, M.; Fenrich, J. The Ultimate qPCR Experiment: Producing Publication Quality, Reproducible Data the First Time. Trends Biotechnol. 2019, 37, 761–774. [Google Scholar] [CrossRef]
- Goldner, J. A modification of the masson trichrome technique for routine laboratory purposes. Am. J. Pathol. 1938, 14, 237–243. [Google Scholar]
- Arda, O.; Göksügür, N.; Tüzün, Y. Basic histological structure and functions of facial skin. Clin. Dermatol. 2014, 32, 3–13. [Google Scholar] [CrossRef]




| Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| CD11c | TGC CAG GAT GAC CTT AGT GTC G | CAG AGT GAC TGT GGT TCC GTA G |
| CD301b | GAC TGA GTT CTC GCC TCT GG | CTG GGA AGG AAT TAG AGC AAA CT |
| Col I | CTG GCG GTT CAG GTC CAA TG | GAA GCC TCG GTG TCC CTT CA |
| Col III | GAC CAA AAG GTG ATG CTG GAC AG | CAA GAC CTC GTG CTC CAG TTA G |
| Col IV | GGT GTG CGG TTT GTG AAG CA | TGG CGT GGG CTT CTT GAA CA |
| Eln | TCC TGG GAT TGG AGG CAT TGC A | ACC AGG CAC TAA ACC TCC AGC A |
| Fn | CGG ACG CTG CGA AAA GAT GA | ACT TGG CTG GCA ACC CTT CT |
| Il-6 | TAC CAC TTC ACA AGT CGG AGG C | CTG CAA GTG CAT CAT CGT TGT TC |
| Mif | CGC TTT GTA CCG TCC T | CGT GCC GCT AAA AGT CA |
| Mif-2/D-Dt | CTC TTC TCC CGC TAA CAT GC | TCA TGC CAG GTC GTA TCG TA |
| Pdgf A | GCA AGA CCA GGA CGG TCA TTT AC | TGT TCA GGA ATG TCA CAC GCC |
| Tgf-β1 | TGA TAC GCC TGA GTG GCT GTC T | CAC AAG AGC AGT GAG CGC TGA A |
| Primary Antibody | Host Species | Used Dilution | Secondary Antibody-HRP |
|---|---|---|---|
| CD31 | rabbit (polyclonal) | 1:50 | anti-rabbit |
| F4/80 | rat IgG (monoclonal) | 1:400 | anti-rat IgG |
| F(ab) | goat IgG (polyclonal) | 1:50 | - |
| α-SMA | mouse (monoclonal) | 1:2 | anti-mouse |
| tumor necrosis factor-α | rabbit (monoclonal) | 1:100 | anti-rabbit |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnke, K.; Pfister, P.; Reid, G.; Vasella, M.; Ruhl, T.; Seitz, A.-K.; Lindenblatt, N.; Cinelli, P.; Kim, B.-S. Impact of a High-Fat Diet at a Young Age on Wound Healing in Mice. Int. J. Mol. Sci. 2023, 24, 17299. https://doi.org/10.3390/ijms242417299
Arnke K, Pfister P, Reid G, Vasella M, Ruhl T, Seitz A-K, Lindenblatt N, Cinelli P, Kim B-S. Impact of a High-Fat Diet at a Young Age on Wound Healing in Mice. International Journal of Molecular Sciences. 2023; 24(24):17299. https://doi.org/10.3390/ijms242417299
Chicago/Turabian StyleArnke, Kevin, Pablo Pfister, Gregory Reid, Mauro Vasella, Tim Ruhl, Ann-Kathrin Seitz, Nicole Lindenblatt, Paolo Cinelli, and Bong-Sung Kim. 2023. "Impact of a High-Fat Diet at a Young Age on Wound Healing in Mice" International Journal of Molecular Sciences 24, no. 24: 17299. https://doi.org/10.3390/ijms242417299
APA StyleArnke, K., Pfister, P., Reid, G., Vasella, M., Ruhl, T., Seitz, A.-K., Lindenblatt, N., Cinelli, P., & Kim, B.-S. (2023). Impact of a High-Fat Diet at a Young Age on Wound Healing in Mice. International Journal of Molecular Sciences, 24(24), 17299. https://doi.org/10.3390/ijms242417299








