Rhamnogalacturonan I with β-(1,4)-Galactan Side Chains as an Ever-Present Component of Tertiary Cell Wall of Plant Fibers
Abstract
:1. Introduction
2. Results
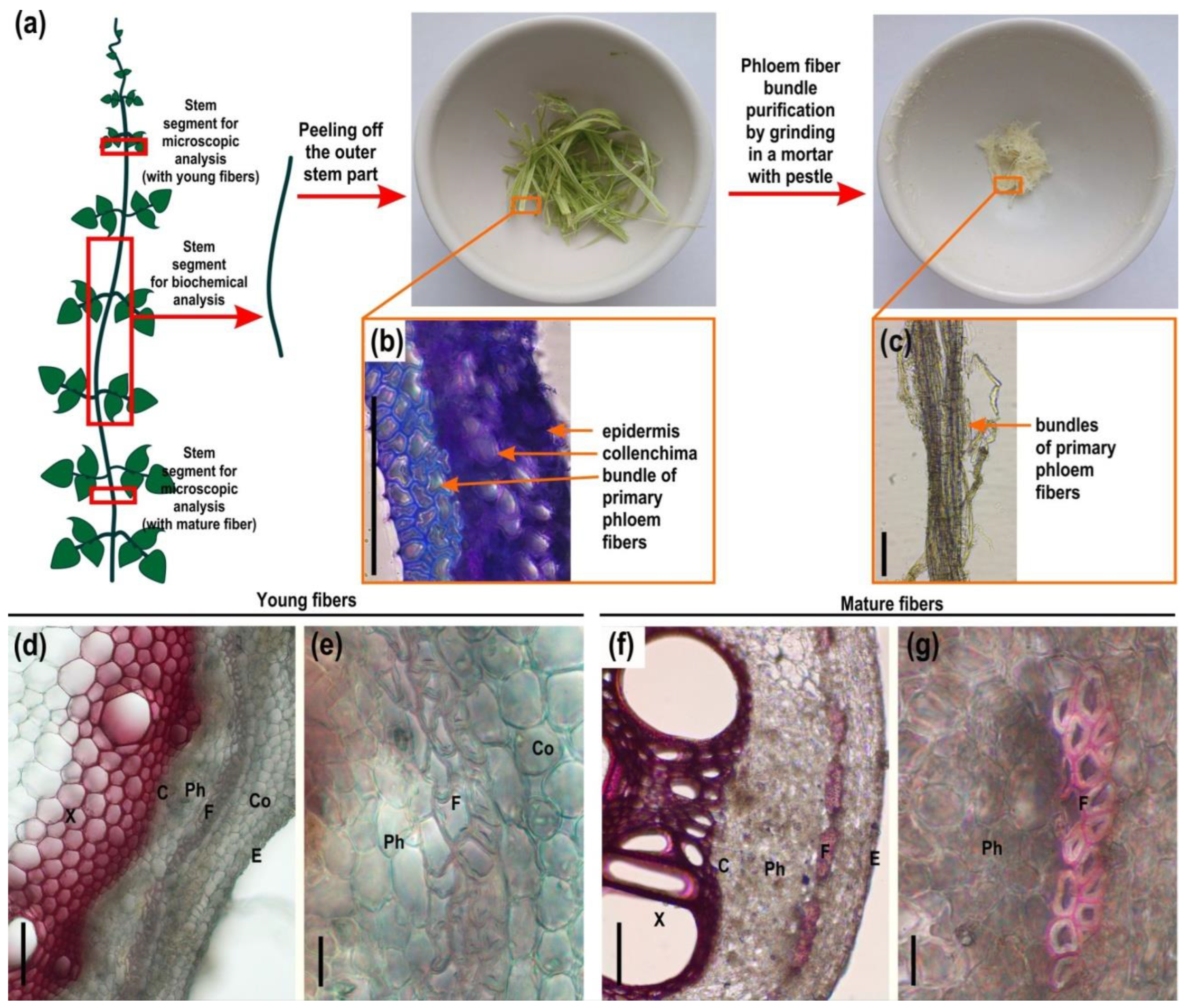
2.1. Microscopy and Immunohistochemistry of the Phloem Fiber Cell Wall in Phaseolus vulgaris Stem
2.2. Cell Wall Composition of Phaseolus vulgaris Fibers
2.3. Polymers Extracted with Buffer and Obtained after Complete Cellulose Destruction from the Cell Wall of Phaseolus vulgaris Fibers
2.4. Immunohistochemistry of Fiber Cell Walls in Other Species of Fabacea and in Species of Some Other Families
2.5. β-Galactosidase Activity in Tertiary Cell Walls of Common Bean and Some Other Plant Species
3. Discussion
3.1. Phloem Fibers of Phaseolus vulgaris Stem Deposit Tertiary Cell Wall
3.2. Structural Details of Rhamnogalacturonan I in Gelatinous Fibers of Various Species May Differ
3.3. Rhamnogalacturonan I with Side Chains of β-(1,4)-Galactan as an Ubiquitous Polymer of Tertiary Cell Wall in Various Species
4. Materials and Methods
4.1. Plant Material
4.2. Light Microscopy and Immunohistochemistry of Common Bean Stem
4.3. Confocal Microscopy and Immunohistochemistry of Fibers from Plants Belonging to Malpighiales, Fabales, and Rosales
4.4. In Situ β-Galactosidase Activity
4.5. Isolation of Polysaccharide Fractions
4.6. Size-Exclusion Chromatography
4.7. Monosaccharide Analysis
4.8. Immunodot Analysis
4.9. NMR Spectroscopy
4.10. Dynamic Light Scattering
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gorshkova, T.; Chernova, T.; Mokshina, N.; Ageeva, M.; Mikshina, P. Plant ‘muscles’: Fibers with a tertiary cell wall. New Phytol. 2018, 218, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Gorshkova, T.; Petrova, A.; Mikshina, P. Review: Tertiary cell wall of plant fibers as a source of inspiration in material design. Carbohydr. Polym. 2022, 295, 119849. [Google Scholar] [CrossRef] [PubMed]
- Chery, J.G.; Glos, R.A.E.; Anderson, C.T. Do woody vines use gelatinous fibers to climb? New Phytol. 2022, 233, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Baena, M.S.; Onyenedum, J.G. Bouncing back stronger: Diversity, structure, and molecular regulation of gelatinous fiber development. Curr. Opin. Plant Biol. 2022, 67, 102198. [Google Scholar] [CrossRef] [PubMed]
- Mellerowicz, E.J.; Baucher, M.; Sundberg, B.; Boerjan, W. Unravelling cell wall formation in the woody dicot stem. Plant Mol. Biol. 2001, 47, 239–274. [Google Scholar] [CrossRef] [PubMed]
- Clair, B.; Alméras, T.; Pilate, G.; Jullien, D.; Sugiyama, J.; Riekel, C. Maturation stress generation in Poplar tension wood studied by synchrotron radiation microdiffraction. Plant Physiol. 2010, 155, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Guedes, F.T.P.; Laurans, F.; Quemener, B.; Assor, C.; Lainé-Prade, V.; Boizot, N.; Vigouroux, J.; Lesage-Descauses, M.-C.; Leplé, J.-C.; Déjardin, A.; et al. Non-cellulosic polysaccharide distribution during G-layer formation in poplar tension wood fibers: Abundance of rhamnogalacturonan I and arabinogalactan proteins but no evidence of xyloglucan. Planta 2017, 246, 857–878. [Google Scholar] [CrossRef] [PubMed]
- Chernova, T.E.; Mikshina, P.V.; Salnikov, V.V.; Ibragimova, N.N.; Sautkina, O.V.; Gorshkova, T.A. Development of distinct cell wall layers both in primary and secondary phloem fibers of hemp (Cannabis sativa L.). Ind. Crop. Prod. 2018, 117, 97–109. [Google Scholar] [CrossRef]
- Goudenhooft, C.; Bourmaud, A.; Baley, C. Flax (Linum usitatissimum L.) fibers for composite reinforcement: Exploring the link between plant growth, cell walls development, and fiber properties. Front. Plant Sci. 2019, 10, 411. [Google Scholar] [CrossRef]
- Gorshkova, T.A.; Chemikosova, S.B.; Sal’nikov, V.V.; Pavlencheva, N.V.; Gur’janov, O.P.; Stolle-Smits, T.; van Dam, J.E.G. Occurrence of cell-specific galactan is coinciding with bast fiber developmental transition in flax. Ind. Crop. Prod. 2004, 19, 217–224. [Google Scholar] [CrossRef]
- Gurjanov, O.P.; Ibragimova, N.N.; Gnezdilov, O.I.; Gorshkova, T.A. Polysaccharides, tightly bound to cellulose in cell wall of flax bast fibre: Isolation and identification. Carbohydr. Polym. 2008, 72, 719–729. [Google Scholar] [CrossRef]
- Mikshina, P.V.; Gurjanov, O.P.; Mukhitova, F.K.; Petrova, A.A.; Shashkov, A.S.; Gorshkova, T.A. Structural details of pectic galactan from the secondary cell walls of flax (Linum usitatissimum L.) phloem fibres. Carbohydr. Polym. 2012, 87, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Roach, M.J.; Mokshina, N.Y.; Badhan, A.; Snegireva, A.V.; Hobson, N.; Deyholos, M.K.; Gorshkova, T.A. Development of cellulosic secondary walls in flax fibers requires beta-galactosidase. Plant Physiol. 2011, 156, 1351–1363. [Google Scholar] [CrossRef] [PubMed]
- Arnould, O.; Siniscalco, D.; Bourmaud, A.; Le Duigou, A.; Baley, C. Better insight into the nano-mechanical properties of flax fibre cell walls. Ind. Crop. Prod. 2017, 97, 224–228. [Google Scholar] [CrossRef]
- Arend, M. Immunolocalization of (1,4)-β-galactan in tension wood fibers of poplar. Tree Physiol. 2008, 28, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Bowling, A.J.; Vaughn, K.C. Immunocytochemical characterization of tension wood: Gelatinous fibers contain more than just cellulose. Am. J. Bot. 2008, 95, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Bowling, A.J.; Vaughn, K.C. Gelatinous fibers are widespread in coiling tendrils and twining vines. Am. J. Bot. 2009, 96, 719–727. [Google Scholar] [CrossRef]
- Gritsch, C.; Wan, Y.; Mitchell, R.A.; Shewry, P.R.; Hanley, S.J.; Karp, A. G-fibre cell wall development in willow stems during tension wood induction. J. Exp. Bot. 2015, 66, 6447–6459. [Google Scholar] [CrossRef]
- Mikshina, P.V.; Idiyatullin, B.Z.; Petrova, A.A.; Shashkov, A.S.; Zuev, Y.F.; Gorshkova, T.A. Physicochemical properties of complex rhamnogalacturonan I from gelatinous cell walls of flax fibers. Carbohydr. Polym. 2015, 117, 853–861. [Google Scholar] [CrossRef]
- Gorshkova, T.; Mokshina, N.; Chernova, T.; Ibragimova, N.; Salnikov, V.; Mikshina, P.; Tryfona, T.; Banasiak, A.; Immerzeel, P.; Dupree, P.; et al. Aspen tension wood fibers contain β-(1→4)-galactans and acidic arabinogalactans retained by cellulose microfibrils in gelatinous walls. Plant Physiol. 2015, 169, 2048–2063. [Google Scholar] [CrossRef]
- Piva, T.C.; Machado, S.R.; Scremin-Dias, E. Anatomical and ultrastructural studies on gelatinous fibers in the organs of non-woody xerophytic and hydrophytic species. Botany 2019, 97, 529–536. [Google Scholar] [CrossRef]
- Piva, T.C.; Fortuna-Perez, A.P.; Vargas, W.d.; Machado, S.R. Variations in the architecture and histochemistry of the gelatinous fibers in Eriosema (DC.) Desv. (Leguminosae) species from the Brazilian Cerrado. Flora 2020, 268, 151624. [Google Scholar] [CrossRef]
- Fisher, J.B. Anatomy of axis contraction in seedlings from a fire prone habitat. Am. J. Bot. 2008, 95, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, N.; Gierlinger, N.; Pütz, N.; Fratzl, P.; Neinhuis, C.; Burgert, I. G-fibres in storage roots of Trifolium pretense (Fabaceae): Tensile stress generators for contraction. Plant J. 2010, 61, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.B.; Blanco, M.A. Gelatinous fibers and variant secondary growth related to stem undulation and contraction in a monkey ladder vine, Bauhinia glabra (Fabaceae). Am. J. Bot. 2014, 101, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, W.D.; Nakaba, S.; Yamagishi, Y.; Begum, S.; Marsoem, S.N.; Ko, J.-H.; Jin, H.-O.; Funada, R. Gibberellin mediates the development of gelatinous fibres in the tension wood of inclined Acacia mangium seedlings. Ann. Bot. 2013, 112, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Ralet, M.C.; Tranquet, O.; Poulain, D.; Moïse, A.; Guillon, F. Monoclonal antibodies to rhamnogalacturonan I backbone. Planta 2010, 231, 1373–1383. [Google Scholar] [CrossRef]
- Jones, L.; Seymour, G.B.; Knox, J.P. Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1[→]4)-[beta]-D-Galactan. Plant Physiol. 1997, 113, 1405–1412. [Google Scholar] [CrossRef]
- Torode, T.A.; O’Neill, R.; Marcus, S.E.; Cornuault, V.; Pose, S.; Lauder, R.P.; Kračun, S.K.; Rydahl, M.G.; Andersen, M.C.F.; Willats, W.G.T.; et al. Branched pectic galactan in phloem-sieve-element cell walls: Implications for cell mechanics. Plant Physiol. 2018, 176, 1547–1558. [Google Scholar] [CrossRef]
- Willats, W.G.; Marcus, S.E.; Knox, J.P. Generation of monoclonal antibody specific to (1→5)-alpha-L-arabinan. Carbohydr. Res. 1998, 308, 149–152. [Google Scholar] [CrossRef]
- Verhertbruggen, Y.; Marcus, S.E.; Haeger, A.; Verhoef, R.; Schols, H.A.; McCleary, B.V.; McKee, L.; Gilbert, H.J.; Knox, J.P. Developmental complexity of arabinan polysaccharides and their processing in plant cell walls. Plant J. 2009, 59, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Moller, I.; Marcus, S.E.; Haeger, A.; Verhertbruggen, Y.; Verhoef, R.; Schols, H.; Ulvskov, P.; Mikkelsen, J.D.; Knox, J.P.; Willats, W. High-throughput screening of monoclonal antibodies against plant cell wall glycans by hierarchical clustering of their carbohydrate microarray binding profiles. Glycoconj. J. 2008, 25, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, M.; Yates, E.A.; Willats, W.G.T.; Martin, H.; Knox, J.P. Immunochemical comparison of membrane-associated and secreted arabinogalactan-proteins in rice and carrot. Planta 1996, 198, 452–459. [Google Scholar] [CrossRef]
- Yates, E.A.; Valdor, J.F.; Haslam, S.M.; Morris, H.R.; Dell, A.; Mackie, W.; Knox, J.P. Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology 1996, 6, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.P.; Linstead, P.J.; Cooper, J.P.C.; Roberts, K. Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. Plant J. 1991, 1, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Yates, E.A.; Knox, J.P. Investigations into the occurrence of plant cell surface epitopes in exudate gums. Carbohydr. Polym. 1994, 24, 281–286. [Google Scholar] [CrossRef]
- Verhertbruggen, Y.; Marcus, S.E.; Haeger, A.; Ordaz-Ortiz, J.J.; Knox, J.P. An extended set of monoclonal antibodies to pectic homogalacturonan. Carbohydr. Res. 2009, 344, 1858–1862. [Google Scholar] [CrossRef] [PubMed]
- McCartney, L.; Marcus, S.E.; Knox, J.P. Monoclonal antibodies to plant cell wall xylans and arabinoxylans. J. Histochem. Cytochem. 2005, 53, 543–546. [Google Scholar] [CrossRef]
- Marcus, S.E.; Blake, A.W.; Benians, T.A.S.; Lee, K.J.D.; Poyser, C.; Donaldson, L.; Leroux, O.; Rogowski, A.; Petersen, H.L.; Boraston, A.; et al. Restricted access of proteins to mannan polysaccharides in intact plant cell walls. Plant J. 2010, 64, 191–203. [Google Scholar] [CrossRef]
- Dagel, D.J.; Liu, Y.-S.; Zhong, L.; Luo, Y.; Himmel, M.E.; Xu, Q.; Zeng, Y.; Ding, S.-Y.; Smith, S. In situ imaging of single carbohydrate-binding modules on cellulose microfibrils. J. Phys. Chem. B 2011, 115, 635–641. [Google Scholar] [CrossRef]
- Davis, E.A.; Derouet, C.; Hervé du Penhoat, H.C.; Morvan, C. Isolation and an NMR study of pectins from flax (Linum usitatissimum L.). Carbohydr. Res. 1990, 197, 205–215. [Google Scholar] [CrossRef]
- Colquhoun, I.J.; de Ruiter, G.A.; Schols, H.A.; Voragen, A.G.J. Identification by NMR spectroscopy of oligosaccharides obtained by treatment of the hairy regions of apple pectin with rhamnogalacturonase. Carbohydr. Res. 1990, 206, 131–144. [Google Scholar] [CrossRef]
- Meloche, C.G.; Knox, J.P.; Vaughn, K.C. A cortical band of gelatinous fibers causes the coiling of redvine tendrils: A model based upon cytochemical and immunocytochemical studies. Planta 2007, 225, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Westphal, Y.; Kühnel, S.; de Waard, P.; Hinz, S.W.A.; Schols, H.A.; Voragen, A.G.J.; Gruppen, H. Branched arabino-oligosaccharides isolated from sugar beet arabinan. Carbohydr. Res. 2010, 345, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Wefers, D.; Tyl, C.E.; Bunzel, M. Neutral pectin side chains of Amaranth (Amaranthus hypochondriacus) contain long, partially branched arabinans and short galactans, both with terminal arabinopyranoses. J. Agric. Food Chem. 2015, 63, 707–715. [Google Scholar] [CrossRef]
- Guo, Q.; Shan, Z.; Shao, Y.; Wang, N.; Qian, K.; Goff, H.D.; Wang, Q.; Cui, S.W.; Ding, H.H. Conformational properties of flaxseed rhamnogalacturonan-I and correlation between primary structure and conformation. Polymers 2022, 14, 2667. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Q.; Cui, S.W.; Huang, X.; Kakuda, Y. Elimination of aggregates of (1→3) (1→4)-β-D-glucan in dilute solutions for light scattering and size exclusion chromatography study. Food Hydrocoll. 2006, 20, 361–368. [Google Scholar] [CrossRef]
- Hourdet, D.; Muller, G. Solution properties of pectin polysaccharides II. Conformation and molecular size of high galacturonic acid content isolated pectin chains. Carbohydr. Polym. 1991, 16, 113–135. [Google Scholar] [CrossRef]
- Ralet, M.-C.; Crépeau, M.-J.; Lefèbvre, J.; Mouille, G.; Höfte, H.; Thibault, J.-F. Reduced number of homogalacturonan domains in pectins of an Arabidopsis mutant enhances the flexibility of the polymer. Biomacromolecules 2008, 9, 1454–1460. [Google Scholar] [CrossRef]
- Morris, G.A.; Ralet, M.C.; Bonnin, E.; Thibault, J.F.; Harding, S.E. Physical characterisation of the rhamnogalacturonan and homogalacturonan fractions of sugar beet (Beta vulgaris) pectin. Carbohydr. Polym. 2010, 82, 1161–1167. [Google Scholar] [CrossRef]
- Ha, M.-A.; Viëtor, R.J.; Jardine, G.D.; Apperley, D.C.; Jarvis, M.C. Conformation and mobility of the arabinan and galactan side-chains of pectin. Phytochemistry 2005, 66, 1817–1824. [Google Scholar] [CrossRef]
- Yapo, B.M.; Koffi, K.L. The polysaccharide composition of yellow passion fruit rind cell wall: Chemical and macromolecular features of extracted pectins and hemicellulosic polysaccharides. J. Sci. Food Agric. 2008, 88, 2125–2133. [Google Scholar] [CrossRef]
- Girault, R.; Bert, F.; Rihouey, C.; Jauneau, A.; Morvan, C.; Jarvis, M. Galactans and cellulose in flax fibres: Putative contributions to the tensile strength. Int. J. Biol. Macromol. 1997, 21, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Blake, A.W.; Marcus, S.E.; Copeland, J.E.; Blackburn, R.S.; Knox, J.P. In situ analysis of cell wall polymers associated with phloem fibre cells in stems of hemp, Cannabis sativa L. Planta 2008, 228, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Behr, M.; Legay, S.; Žižková, E.; Motyka, V.; Dobrev, P.I.; Hausman, J.F.; Lutts, S.; Guerriero, G. Studying secondary growth and bast fiber development: The hemp hypocotyl peeks behind the wall. Front. Plant Sci. 2016, 7, 1733. [Google Scholar] [CrossRef]
- Faleri, C.; Xu, X.; Mareri, L.; Hausman, J.F.; Cai, G.; Guerriero, G. Immunohistochemical analyses on two distinct internodes of stinging nettle show different distribution of polysaccharides and proteins in the cell walls of bast fibers. Protoplasma 2022, 259, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.C.H.; Hilger, H.H.; Stevens, P. Angiosperm phylogeny poster (APP)—Flowering plant systematics. Peer J. 2019, 7, e2320v6. [Google Scholar] [CrossRef]
- Klimm, F.; Speck, T.; Thielen, M. Force generation in the coiling tendrils of Passiflora caerulea. Adv. Sci. 2023, 10, 2301496. [Google Scholar] [CrossRef] [PubMed]
- Speck, T.; Cheng, T.; Klimm, F.; Menges, A.; Poppinga, S.; Speck, O.; Tahouni, Y.; Tauber, F.; Thielen, M. Plants as inspiration for material-based sensing and actuation in soft robots and machines. MRS Bull. 2023, 48, 730–745. [Google Scholar] [CrossRef]
- Jensen, W.A. Botanical Histochemistry; Freeman: San Francisco, CA, USA, 1962; 408p. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]









| Antibody/CBM | Specificity | References |
|---|---|---|
| INRA-RU2 1 | Rhamnogalacturonan I backbone | [27] |
| LM5 2 | β-(1,4)-d-Galactan | [28] |
| LM26 2 | β-(1,6)-Galactosyl substitution of β-(1,4)-d-galactan | [29] |
| LM6 2 | Prefer α-(1,5)-l-arabinan, may bind to arabinogalactan protein | [30,31] |
| LM13 2 | α-(1,5)-l-Arabinan (linear) | [31,32] |
| LM2 2 | Arabinogalactan protein | [33,34] |
| JIM14 2 | Arabinogalactan, arabinogalactan protein | [34,35,36] |
| LM19 2 | Low-esterified homogalacturonan | [37] |
| LM20 2 | High-esterified homogalacturonan | [37] |
| LM11 2 | Xylan, arabinoxylan | [38] |
| LM21 2 | Heteromannan | [39] |
| CBM3a 2 | Planar surface of crystalline cellulose | [40] |
| Cell Wall Fractions, % of Dry Weight of Cell Wall 1 | ||||||
|---|---|---|---|---|---|---|
| Buffer-Extractable Polysaccharides 2 | AO-Extractable Polysaccharides 2 | KOH-Extractable Polysaccharides 2 | Poly-Saccharides Strongly Retained by Cellulose (without Glc) 2 | Lignin-Bound Poly-Saccharides 3 | Cellulose 4 | Lignin 5 |
| 4.2 ± 1.9 | 4.7 ± 1.8 | 6.0 ± 1.2 | 3.7 ± 0.7 | 0.3 ± 0.1 | 74.7 ± 2.8 | 6.4 ± 0.9 |
| Cell wall monosaccharide proportion, mol% 1 | ||||||
| Rha | Ara | Gal | Glc | Xyl + Man | GalA | GlcA |
| 4.2 ± 0.1 | 6.6 ± 1.4 | 4.3 ± 1.7 | 63.3 ± 7.7 | 11.2 ± 2.7 | 9.9 ± 5.7 | 0.5 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chernova, T.; Mikshina, P.; Petrova, A.; Ibragimova, N.; Ageeva, M.; Gorshkova, T. Rhamnogalacturonan I with β-(1,4)-Galactan Side Chains as an Ever-Present Component of Tertiary Cell Wall of Plant Fibers. Int. J. Mol. Sci. 2023, 24, 17253. https://doi.org/10.3390/ijms242417253
Chernova T, Mikshina P, Petrova A, Ibragimova N, Ageeva M, Gorshkova T. Rhamnogalacturonan I with β-(1,4)-Galactan Side Chains as an Ever-Present Component of Tertiary Cell Wall of Plant Fibers. International Journal of Molecular Sciences. 2023; 24(24):17253. https://doi.org/10.3390/ijms242417253
Chicago/Turabian StyleChernova, Tatyana, Polina Mikshina, Anna Petrova, Nadezhda Ibragimova, Marina Ageeva, and Tatyana Gorshkova. 2023. "Rhamnogalacturonan I with β-(1,4)-Galactan Side Chains as an Ever-Present Component of Tertiary Cell Wall of Plant Fibers" International Journal of Molecular Sciences 24, no. 24: 17253. https://doi.org/10.3390/ijms242417253
APA StyleChernova, T., Mikshina, P., Petrova, A., Ibragimova, N., Ageeva, M., & Gorshkova, T. (2023). Rhamnogalacturonan I with β-(1,4)-Galactan Side Chains as an Ever-Present Component of Tertiary Cell Wall of Plant Fibers. International Journal of Molecular Sciences, 24(24), 17253. https://doi.org/10.3390/ijms242417253






