Physiological Characteristics and Transcriptome Analysis of Exogenous Brassinosteroid-Treated Kiwifruit
Abstract
:1. Introduction
2. Results
2.1. Physiological and Biochemical Results
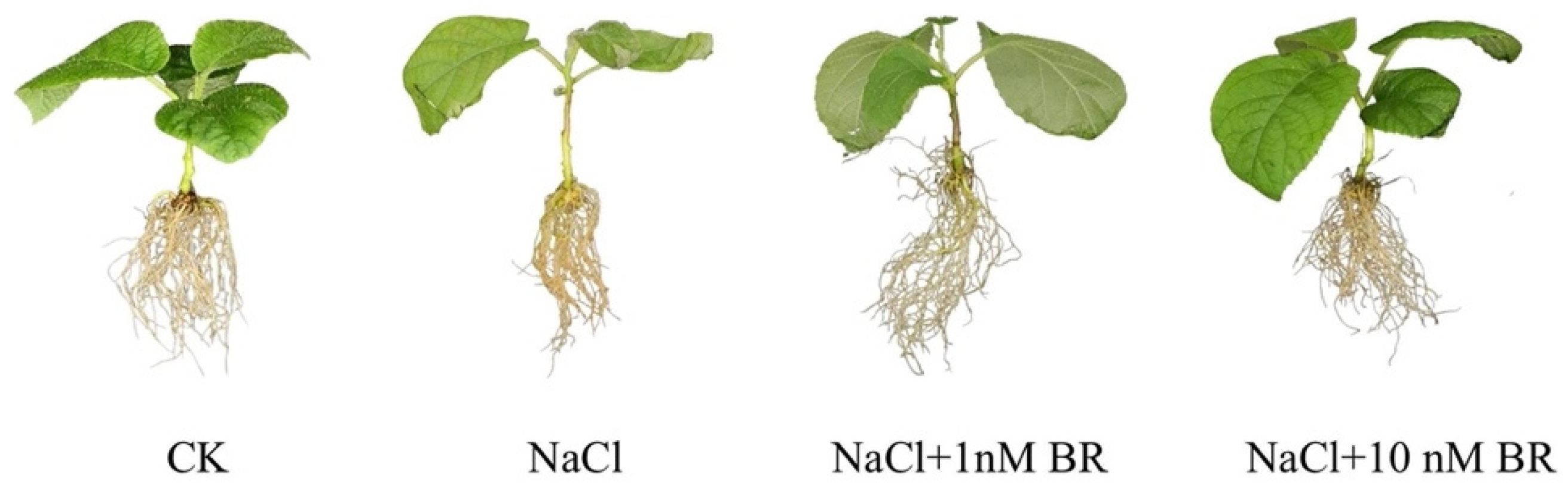
2.1.1. Effect of BRs on the Morphology of Kiwifruit Seedlings under Salt Stress
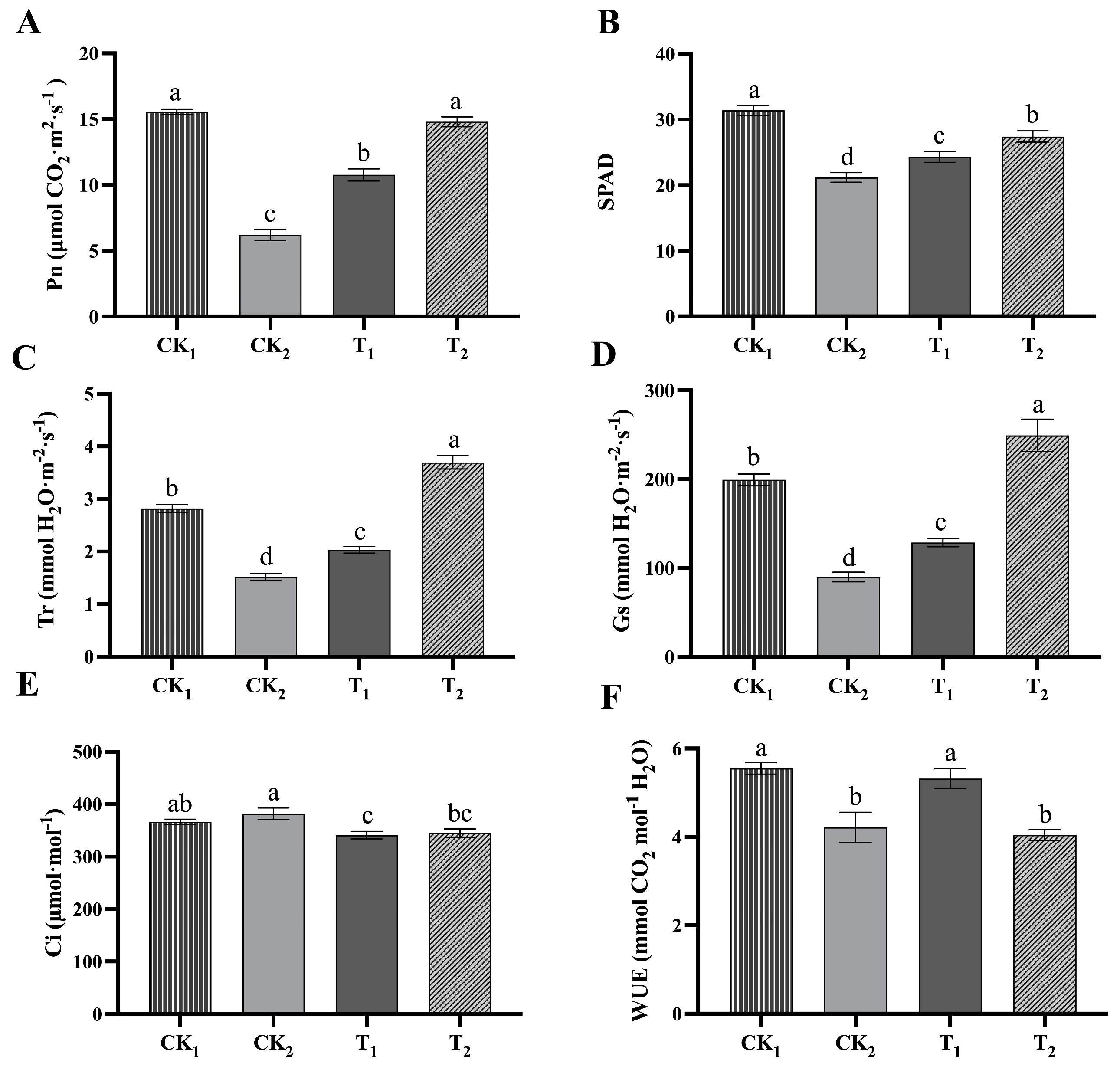
2.1.2. Effect of BRs on Photosynthesis in Kiwifruit Seedlings under Salt Stress
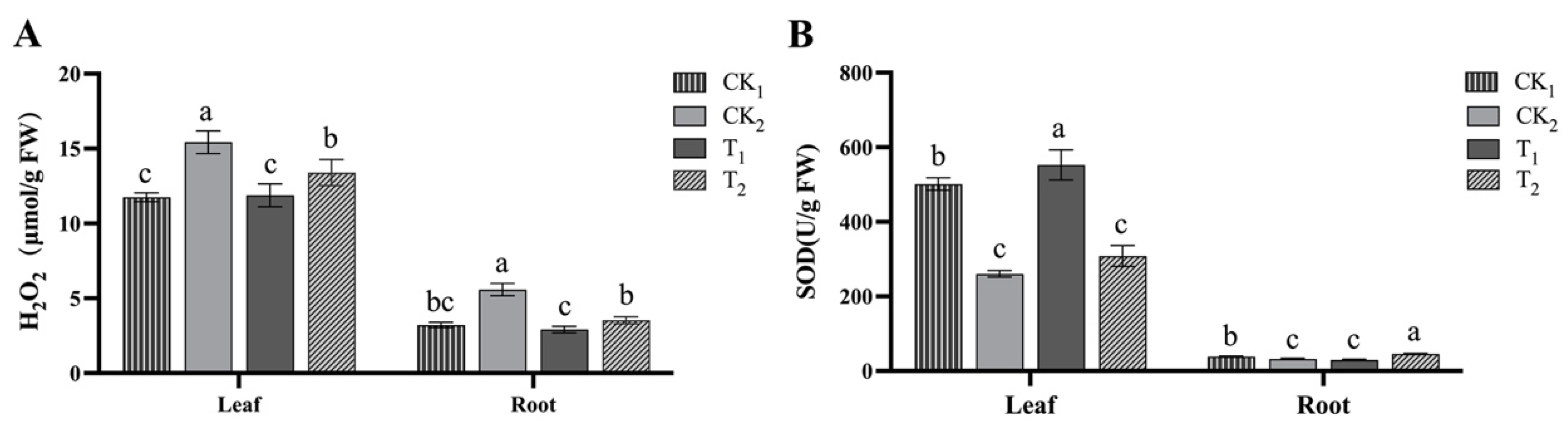
2.1.3. Effect of BR on Antioxidant Capacity of Kiwifruit Seedlings under Salt Stress
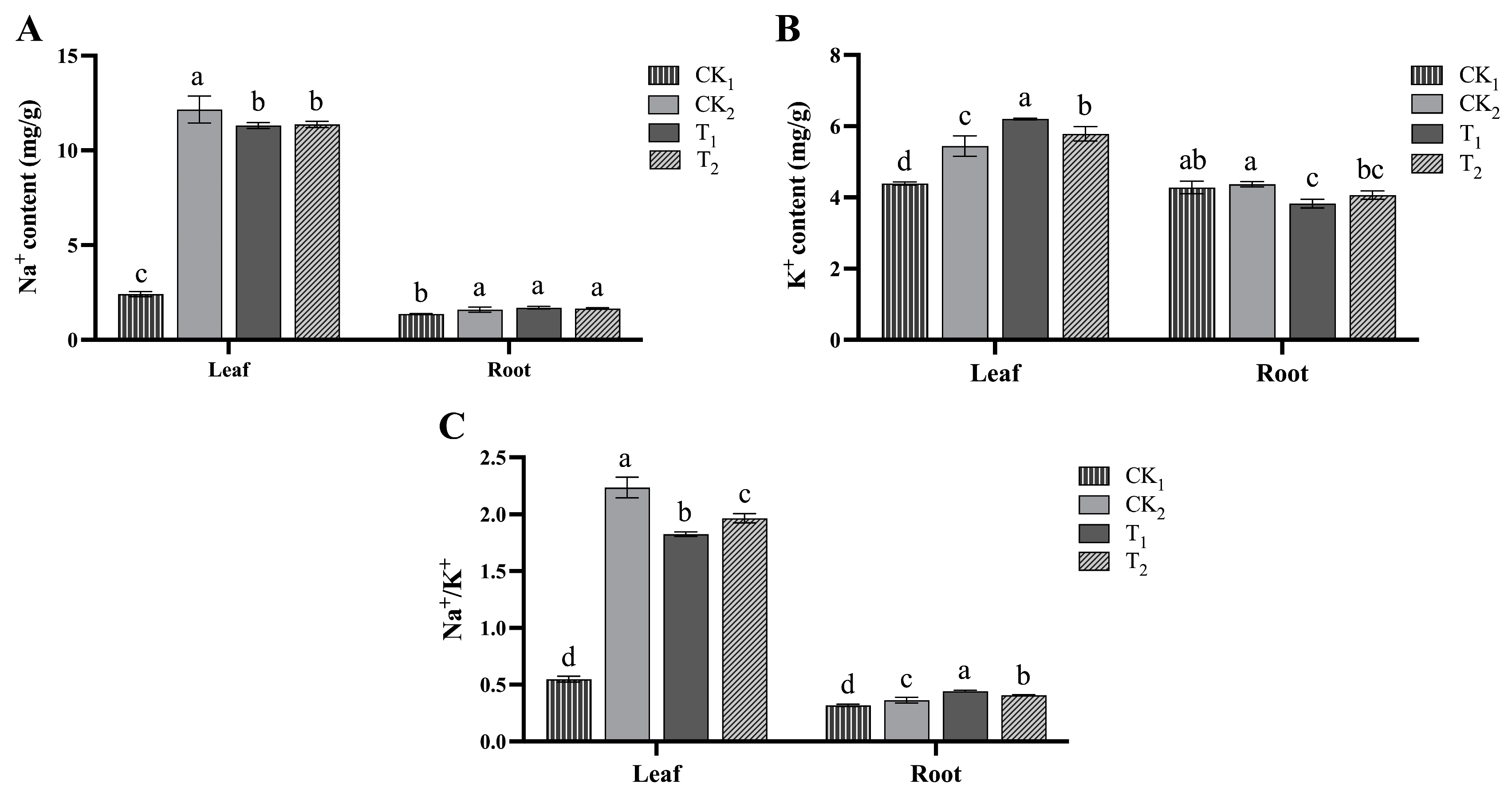
2.1.4. Effects of BRs on Ion Regulation of Kiwifruit Seedlings under Salt Stress
2.2. Comprehensive Analysis of Physiological Indicators in Kiwifruit Seedlings
2.3. Transcriptome Results
2.3.1. Sequencing Data Assembly and Mapping
2.3.2. Expression of Differential Genes
2.4. DEGs Induced by BR under Salt Stress
2.4.1. DEGs Related to Plant Hormone Transduction under BR Treatment and Salt Stress
2.4.2. Differential Genes Involved in BR-Induced Ion Transport under Salt Stress
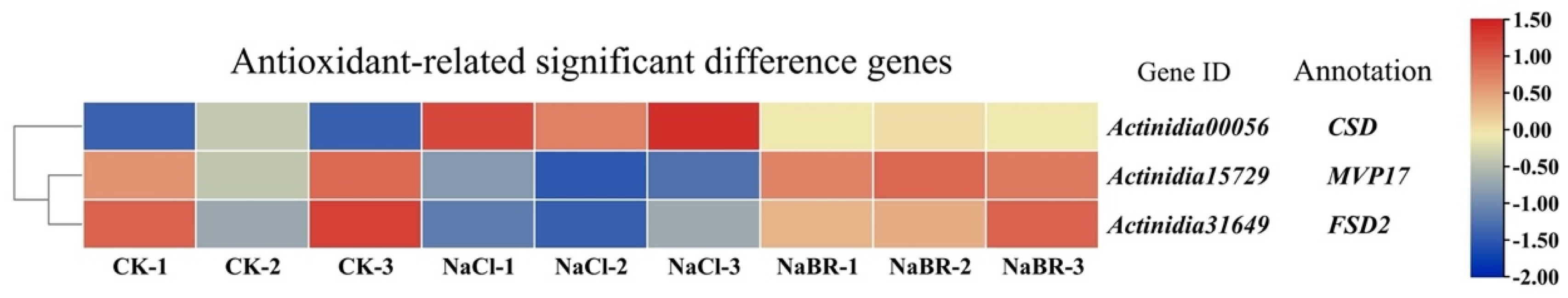
2.4.3. Differential Genes for BR-Induced Antioxidants under Salt Stress
2.5. Validation of RNA-Seq Results by qRT-PCR
3. Discussion
3.1. Effects of BR on the Physiology of Kiwifruit Seedlings under Salt Stress
3.2. Effect of BRs on Differential Gene Expression in Kiwifruit Seedlings under Salt Stress
4. Materials and Methods
4.1. Plant Materials and Experiments
4.2. Measurement of Plant Photosynthesis-Related Parameters
4.3. Measurement of SPAD Values
4.4. Measurement of Relevant Biochemical Indicators
4.5. Statistical Analyses
4.6. Transcriptome Sequencing
4.6.1. RNA Extraction and RNA-Seq
4.6.2. Sequence Assembly and Analysis
4.6.3. Functional Annotation and Enrichment Analysis of Differentially Expressed Genes
4.7. Quantitative Real-Time PCR (RT-qPCR)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omar, M.M.; Shitindi, M.J.; Massawe, B.J.; Fue, K.G.; Meliyo, J.L.; Pedersen, O. Salt-affected soils in Tanzanian agricultural lands: Type of soils and extent of the problem. Sustain. Environ. 2023, 9, 2205731. [Google Scholar] [CrossRef]
- Van Zelm, E.; Zhang, Y.; Testerink, C. Salt Tolerance Mechanisms of Plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.P.; Ansell, J.; Drummond, L.N. The nutritional and health attributes of kiwifruit: A review. Eur. J. Nutr. 2018, 57, 2659–2676. [Google Scholar] [CrossRef]
- Li, M.; Wu, Z.; Gu, H.; Cheng, D.; Guo, X.; Li, L.; Shi, C.Y.; Xu, G.Y.; Gu, S.C.; Abid, M.; et al. AvNAC030, a NAC Domain Transcription Factor, Enhances Salt Stress Tolerance in Kiwifruit. Int. J. Mol. Sci. 2021, 22, 11897. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Guo, D.; Wang, R.; Zhong, Y.; Fang, J. Development status and suggestions on Chinese kiwifruit industry. J. Fruit Sci. 2020, 37, 754–763. [Google Scholar]
- Mu, R.; Liu, Y. Study on Salt-alkali Tolerant and Waterlogging Tolerance of Kiwifruit. J. Anhui Agric. Sci. 2020, 48, 52–54+79. [Google Scholar]
- Sharma, I.; Ching, E.; Saini, S.; Bhardwaj, R.; Pati, P.K. Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol. Biochem. 2013, 69, 17–26. [Google Scholar] [CrossRef]
- Hoque, M.A.; Okuma, E.; Banu MN, A.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Exogenous proline mitigates the detrimental effects of salt stress more than exogenous betaine by increasing antioxidant enzyme activities. J. Plant Physiol. 2007, 164, 553–561. [Google Scholar] [CrossRef]
- Ogweno, J.O.; Song, X.S.; Shi, K.; Hu, W.H.; Mao, W.H.; Zhou, Y.H.; Yu, J.Q.; Nogues, S. Brassinosteroids alleviate heat-induced inhibition of photosynthesis by increasing carboxylation efficiency and enhancing antioxidant systems in Lycopersicon esculentum. J. Plant Growth Regul. 2008, 27, 49–57. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Guo, Y. Unraveling salt stress signaling in plants. J. Integr. Plant Biol. 2018, 60, 796–804. [Google Scholar] [CrossRef]
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.J.; Ma, X.L.; Wan, P.; Liu, L.Y. Plant salt-tolerance mechanism: A review. Biochem. Biophys. Res. Commun. 2018, 495, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Shi, H.Z. Physiological and molecular mechanisms of plant salt tolerance. Photosynth. Res. 2013, 115, 1–22. [Google Scholar] [CrossRef]
- Kaleem, F.; Shabir, G.; Aslam, K.; Rasul, S.; Manzoor, H.; Shah, S.M.; Khan, A.R. An Overview of the Genetics of Plant Response to Salt Stress: Present Status and the Way Forward. Appl. Biochem. Biotech. 2018, 186, 306–334. [Google Scholar] [CrossRef]
- Grove, M.D.; Spencer, G.F.; Rohwedder, W.K.; Mandava, N.; Worley, J.F.; Warthen, J.D., Jr.; Steffens, G.L.; Flippen-Anderson, J.L.; Cook, J.C. Brassinolide, a plant growth-promoting steroid isolated from Brassica napus pollen. Nature 1979, 281, 216–217. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Gu, S.C.; Gu, H.; Cheng, D.W.; Li, L.; Guo, X.Z.; Wang, M.; He, S.S.; Li, M.; Chen, J.Y. Physiological and transcriptomic analyses of brassinosteroid function in kiwifruit root. Environ. Exp. Bot. 2022, 194, 104685. [Google Scholar] [CrossRef]
- Wani, A.S.; Ahmad, A.; Hayat, S.; Tahir, I. Epibrassinolide and proline alleviate the photosynthetic and yield inhibition under salt stress by acting on antioxidant system in mustard. Plant Physiol. Biochem. 2019, 135, 385–394. [Google Scholar] [CrossRef]
- Rattan, A.; Kapoor, D.; Kapoor, N.; Bhardwaj, R.; Sharma, A. Brassinosteroids Regulate Functional Components of Antioxidative Defense System in Salt Stressed Maize Seedlings. J. Plant Growth Regul. 2020, 39, 1465–1475. [Google Scholar] [CrossRef]
- Gupta, P.; Srivastava, S.; Seth, C.S. 24-Epibrassinolide and Sodium Nitroprusside alleviate the salinity stress in Brassica juncea L. cv. Varuna through cross talk among proline, nitrogen metabolism and abscisic acid. Plant Soil 2017, 411, 483–498. [Google Scholar] [CrossRef]
- Shahid, M.A.; Pervez, M.A.; Balal, R.M.; Mattson, N.S.; Rashid, A.; Ahmad, R.; Ayyub, C.M.; Abbas, T. Brassinosteroid (24-epibrassinolide) enhances growth and alleviates the deleterious effects induced by salt stress in pea (Pisum sativum L.). Aust. J. Crop Sci. 2011, 5, 500–510. [Google Scholar]
- Liu, M.L.; Wang, C.C.; Xu, Q.; Jiang, B.L.; Zhang, L.T.; Zhang, Y.; Tian, Z.B.; Chang, C.; Zhang, H.P. Genome-wide identification of the CPK gene family in wheat (Triticum aestivum L.) and characterization of TaCPK40 associated with seed dormancy and germination. Plant Physiol. Biochem. 2023, 196, 608–623. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Maggio, A.; Bressan, R.A.; Yun, D.J. Role and Functional Differences of HKT1-Type Transporters in Plants under Salt Stress. Int. J. Mol. Sci. 2019, 20, 1059. [Google Scholar] [CrossRef] [PubMed]
- Myouga, F.; Hosoda, C.; Umezawa, T.; Iizumi, H.; Kuromori, T.; Motohashi, R.; Shono, Y.; Nagata, N.; Ikeuchi, M.; Shinozaki, K. A Heterocomplex of Iron Superoxide Dismutases Defends Chloroplast Nucleoids against Oxidative Stress and Is Essential for Chloroplast Development in Arabidopsis. Plant Cell 2008, 20, 3148–3162. [Google Scholar] [CrossRef]
- Tanveer, M.; Shahzad, B.; Sharma, A.; Biju, S.; Bhardwaj, R. 24-Epibrassinolide; an active brassinolide and its role in salt stress tolerance in plants: A review. Plant Physiol. Biochem. 2018, 130, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Yemets, O.; Gauslaa, Y.; Solhaug, K.A. Monitoring with lichens—Conductivity methods assess salt and heavy metal damage more efficiently than chlorophyll fluorescence. Ecol. Indic. 2015, 55, 59–64. [Google Scholar] [CrossRef]
- Kostopoulou, Z.; Therios, I.; Roumeliotis, E.; Kanellis, A.K.; Molassiotis, A. Melatonin combined with ascorbic acid provides salt adaptation in Citrus aurantium L. seedlings. Plant Physiol. Biochem. 2015, 86, 155–165. [Google Scholar] [CrossRef]
- Sudhir, P.; Murthy, S.D.S. Effects of salt stress on basic processes of photosynthesis. Photosynthetica 2004, 42, 481–486. [Google Scholar] [CrossRef]
- Hayat, S.; Hasan, S.A.; Yusuf, M.; Hayat, Q.; Ahmad, A. Effect of 28-homobrassinolide on photosynthesis, fluorescence and antioxidant system in the presence or absence of salinity and temperature in Vigna radiata. Environ. Exp. Bot. 2010, 69, 105–112. [Google Scholar] [CrossRef]
- Hu, W.H.; Yan, X.H.; Xiao, Y.; Zeng, J.J.; Qi, H.J.; Ogweno, J.O. 24-Epibrassinosteroid alleviate drought-induced inhibition of photosynthesis in Capsicum annuum. Sci. Hortic. 2013, 150, 232–237. [Google Scholar] [CrossRef]
- Wu, W.L.; Zhang, Q.; Ervin, E.H.; Yang, Z.P.; Zhang, X.Z. Physiological Mechanism of Enhancing Salt Stress Tolerance of Perennial Ryegrass by 24-Epibrassinolide. Front. Plant Sci. 2017, 8, 1017. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; Sharkey, T.D. Stomatal Conductance and Photosynthesis. Annu. Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Kou, J.T. Effects of Exogenous 2, 4-epibrassinolide on Photosynthetic Characteristics of Oat Seedlings under NaCl Stress. Acta Agric. Boreali Sin. 2020, 35, 79–87. [Google Scholar]
- Wu, X.; Ding, H.; Zhu, Z.; Yang, S.; Zha, D. Effects of 24-epibrassinolide on photosynthesis of eggplant (Solanum melongena L.) seedlings under salt stress. Afr. J. Biotechnol. 2012, 11, 8665–8671. [Google Scholar]
- Zhang, Y.P.; Yang, S.J.; Chen, Y.Y. Effects of 2, 4-Epibrassinolide on Antioxidant Enzyme Activities and Photosynthesis in Melon Seedlings under High Temperature Stress. Acta Bot. Boreali Occident. Sin. 2011, 31, 1347–1354. [Google Scholar]
- Li, N.; Wang, M.; Sun, J.; Shu, S.; Guo, S.; Wang, J.; Sun, H. Effects of Exogenous 24-Epibrassinolide on Growth and Photosynthesis of Tomato Seedlings under Low Light Stress. Acta Bot. Boreali Occident. Sin. 2013, 33, 1395–1402. [Google Scholar]
- Li, M.; Fan, G.; Zhang, Y.; Liu, S.; Zhang, Y. Effects of exogenous 2, 4-epibrassinolide (EBR) on growth and carbohydrate metabolism of Brassica pekinensis Lour. Rupr. under weak light stress. J. South. Agric. 2019, 50, 1028–1034. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS Metabolism in Plants under Environmental Stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Wu, X.X.; Cha, D.S.; Zhu, Z.W.; Li, X. Effects of Exogenous 24-Epibrassinolide on Seed Germination, Physiological Characteristics of Eggplant Seedlings under NaCl Stress. Acta Phytophysiol. Sin. 2011, 47, 607–612. [Google Scholar]
- Kou, J.T.; Shi, S.L. Effect of 2,4-epibrassinolide on seed germination and seedling growth of Medicago sativaunder salt stress. Grassl. Turf 2015, 35, 1–8, 19. [Google Scholar]
- An, H.; Sheng, W.; Yu, Y.; Zhang, L.; Zeng, H.; Chen, G. Effects of Exogenous 2, 4-Epibrassinolide on Physiological Characteristics of Rice Seedlings Under Salt Stress. Mol. Plant Breed. 2021, 19, 2740–2746. [Google Scholar]
- Hao, S.H.; Wang, Y.R.; Yan, Y.X.; Liu, Y.H.; Wang, J.Y.; Chen, S. A Review on Plant Responses to Salt Stress and Their Mechanisms of Salt Resistance. Horticulturae 2021, 7, 132. [Google Scholar] [CrossRef]
- Xu, W.W.; Huang, W.C. Calcium-Dependent Protein Kinases in Phytohormone Signaling Pathways. Int. J. Mol. Sci. 2017, 18, 2436. [Google Scholar] [CrossRef]
- Sathyanarayanan, P.V.; Poovaiah, B.W. Decoding Ca2+ signals in plants. Crit. Rev. Plant Sci. 2004, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Hakata, M.; Nakamura, H.; Aoki, N.; Komatsu, S.; Ichikawa, H.; Hirochika, H.; Ohsugi, R. Functional characterisation of OsCPK21, a calcium-dependent protein kinase that confers salt tolerance in rice. Plant Mol. Biol. 2011, 75, 179–191. [Google Scholar] [CrossRef]
- Dong, H.; Wu, C.; Luo, C.; Wei, M.; Qu, S.; Wang, S. Overexpression of MdCPK1a gene, a calcium dependent protein kinase in apple, increase tobacco cold tolerance via scavenging ROS accumulation. PLoS ONE 2020, 15, e0242139. [Google Scholar] [CrossRef]
- Pehlivan, N.; Sun, L.; Mishra, N. Defensive manoeuvres of NHX1 and SOS1 co/overexpression in plant salt tolerance. Turk. J. Bot. 2020, 44, 367–376. [Google Scholar] [CrossRef]
- Zhang, W.D.; Wang, P.; Bao, Z.L.T.; Ma, Q.; Duan, L.J.; Bao, A.K.; Zhang, J.L.; Wang, S.M. SOS1, HKT1;5, and NHX1 Synergistically Modulate Na+ Homeostasis in the Halophytic Grass Puccinellia tenuiflora. Front. Plant Sci. 2017, 8, 576. [Google Scholar] [CrossRef]
- Horie, T.; Yoshida, K.; Nakayama, H.; Yamada, K.; Oiki, S.; Shinmyo, A. Two types of HKT transporters with different properties of Na+ and K+ transport in Oryza sativa. Plant J. 2001, 27, 129–138. [Google Scholar] [CrossRef]
- Sunarpi, X.; Horie, T.; Motoda, J.; Kubo, M.; Yang, H.; Yoda, K.; Horie, R.; Chan, W.Y.; Leung, H.Y.; Hattori, K.; et al. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant J. 2005, 44, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Davenport, R.J.; Munoz-Mayor, A.; Jha, D.; Essah, P.A.; Rus, A.; Tester, M. The Na+ transporter AtHKT1;1 controls retrieval of Na+ from the xylem in Arabidopsis. Plant Cell Environ. 2007, 30, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Rus, A.; Lee, B.H.; Munoz-Mayor, A.; Sharkhuu, A.; Miura, K.; Zhu, J.K.; Bressan, R.A.; Hasegawa, P.M. AtHKT1 facilitates Na+ homeostasis and K+ nutrition in planta. Plant Physiol. 2004, 136, 2500–2511. [Google Scholar] [CrossRef] [PubMed]
- Kader, M.A.; Seidel, T.; Golldack, D.; Lindberg, S. Expressions of OsHKT1, OsHKT2, and OsVHA are differentially regulated under NaCl stress in salt-sensitive and salt-tolerant rice (Oryza sativa L.) cultivars. J. Exp. Bot. 2006, 57, 4257–4268. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.S.; Sintaha, M.; Cheung, M.Y.; Lam, H.M. Plant Hormone Signaling Crosstalks between Biotic and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 86. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.P.; Duan, X.B.; Luo, L.; Dai, S.J.; Ding, Z.J.; Xia, G.M. How Plant Hormones Mediate Salt Stress Responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Nanda, S.; Ashraf, M.; SiddiquI, A.R.; Masood, S.; Khaskheli, M.A.; Suleman, M.; Zhu, L.F.; Zhu, C.Q.; Cao, X.C.; et al. Interplay Impact of Exogenous Application of Abscisic Acid (ABA) and Brassinosteroids (BRs) in Rice Growth, Physiology, and Resistance under Sodium Chloride Stress. Life 2023, 13, 498. [Google Scholar] [CrossRef]
- Wang, X.X.; Chen, X.D.; Wang, Q.J.; Chen, M.; Liu, X.; Gao, D.S.; Li, D.M.; Li, L. MdBZR1 and MdBZR1-2like Transcription Factors Improves Salt Tolerance by Regulating Gibberellin Biosynthesis in Apple. Front. Plant Sci. 2019, 10, 1473. [Google Scholar] [CrossRef]
- Niu, M.; Xie, J.; Chen, C.; Cao, H.; Sun, J.; Kong, Q.; Shabala, S.; Shabala, L.; Huang, Y.; Bie, Z. An early ABA-induced stomatal closure, Na+ sequestration in leaf vein and K+ retention in mesophyll confer salt tissue tolerance in Cucurbita species. J. Exp. Bot. 2018, 69, 4945–4960. [Google Scholar] [CrossRef]
- Thalmann, M.; Pazmino, D.; Seung, D.; Horrer, D.; Nigro, A.; Meier, T.; Koelling, K.; Pfeifhofer, H.W.; Zeeman, S.C.; Santelia, D. Regulation of Leaf Starch Degradation by Abscisic Acid Is Important for Osmotic Stress Tolerance in Plants. Plant Cell 2016, 28, 1860–1878. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.-J.; Bressan, R.A.; Song, C.-P.; Zhu, J.-K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Edel, K.H.; Kudla, J. Integration of calcium and ABA signaling. Curr. Opin. Plant Biol. 2016, 33, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.S.; Cai, Z.Y.; Wang, X.L. The primary signaling outputs of brassinosteroids are regulated by abscisic acid signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 4543–4548. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, F.F.; Xie, Q. Balancing growth and adaptation to stress: Crosstalk between brassinosteroid and abscisic acid signaling. Plant Cell Environ. 2020, 43, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Umezawa, T.; Sugiyama, N.; Mizoguchi, M.; Hayashi, S.; Myouga, F.; Yamaguchi-Shinozaki, K.; Ishihama, Y.; Hirayama, T.; Shinozaki, K. Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 17588–17593. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Szostkiewicz, I.; Korte, A.; Moes, D.; Yang, Y.; Christmann, A.; Grill, E. Regulators of PP2C Phosphatase Activity Function as Abscisic Acid Sensors. Science 2009, 324, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.W.; Luo, L.; Wan, Y.S.; Liu, F.Z. Genome-wide characterization of the PP2C gene family in peanut (Arachis hypogaea L.) and the identification of candidate genes involved in salinity-stress response. Front. Plant Sci. 2023, 14, 1093913. [Google Scholar] [CrossRef]
- Xue, T.T.; Wang, D.; Zhang, S.Z.; Ehlting, J.; Ni, F.; Jakab, S.; Zheng, C.C.; Zhong, Y. Genome-wide and expression analysis of protein phosphatase 2C in rice and Arabidopsis. BMC Genom. 2008, 9, 550. [Google Scholar] [CrossRef]
- Lan, H.; Carson, R.; Provart, N.J.; Bonner, A.J. Combining classifiers to predict gene function in Arabidopsis thaliana using large-scale gene expression measurements. BMC Bioinform. 2007, 8, 358. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.P.; Deng, F.N.; Yuan, R.; Shen, F.F. Genome-wide characterization and expression analyses of superoxide dismutase (SOD) genes in Gossypium hirsutum. BMC Genom. 2017, 18, 376. [Google Scholar] [CrossRef]
- Bowler, C.; Alliotte, T.; De Loose, M.; Van Montagu, M.; Inze, D. The induction of manganese superoxide dismutase in response to stress in Nicotiana plumbaginifolia. EMBO J. 1989, 8, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Ying, Y.; Chen, J.; Wang, X.C. Transgenic Arabidopsis overexpressing Mn-SOD enhanced salt-tolerance. Plant Sci. 2004, 167, 671–677. [Google Scholar] [CrossRef]
- Yan, H.; Li, Q.; Park, S.; Wang, X.; Liu, Y.J.; Zhang, Y.G.; Tang, W.; Kou, M.; Ma, D.F. Overexpression of CuZnSOD and APX enhance salt stress tolerance in sweet potato. Plant Physiol. Biochem. 2016, 109, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.; Chauhan, R.; Gill, T.; Swarnkar, M.K.; Sreenivasulu, Y.; Kumar, S.; Kumar, N.; Shankar, R.; Ahuja, P.S.; Singh, A.K. Expression of SOD and APX genes positively regulates secondary cell wall biosynthesis and promotes plant growth and yield in Arabidopsis under salt stress. Plant Mol. Biol. 2015, 87, 615–631. [Google Scholar] [CrossRef] [PubMed]
- Gallie, D.R.; Chen, Z. Chloroplast-localized iron superoxide dismutases FSD2 and FSD3 are functionally distinct in Arabidopsis. PLoS ONE 2019, 14, e0220078. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Wang, L.G.; Wang, S.Q.; Li, W. RSeQC: Quality control of RNA-seq experiments. Bioinformatics 2012, 28, 2184–2185. [Google Scholar] [CrossRef]
- Kim, D.; Landmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sun, S.H.; Lin, M.M.; Qi, X.J.; Chen, J.Y.; Gu, H.; Zhong, Y.P.; Sun, L.M.; Muhammad, A.B.; Bai, D.F.; Hu, C.G.; et al. Full-length transcriptome profiling reveals insight into the cold response of two kiwifruit genotypes (A. arguta) with contrasting freezing tolerances. BMC Plant Biol. 2021, 21, 365. [Google Scholar] [CrossRef]


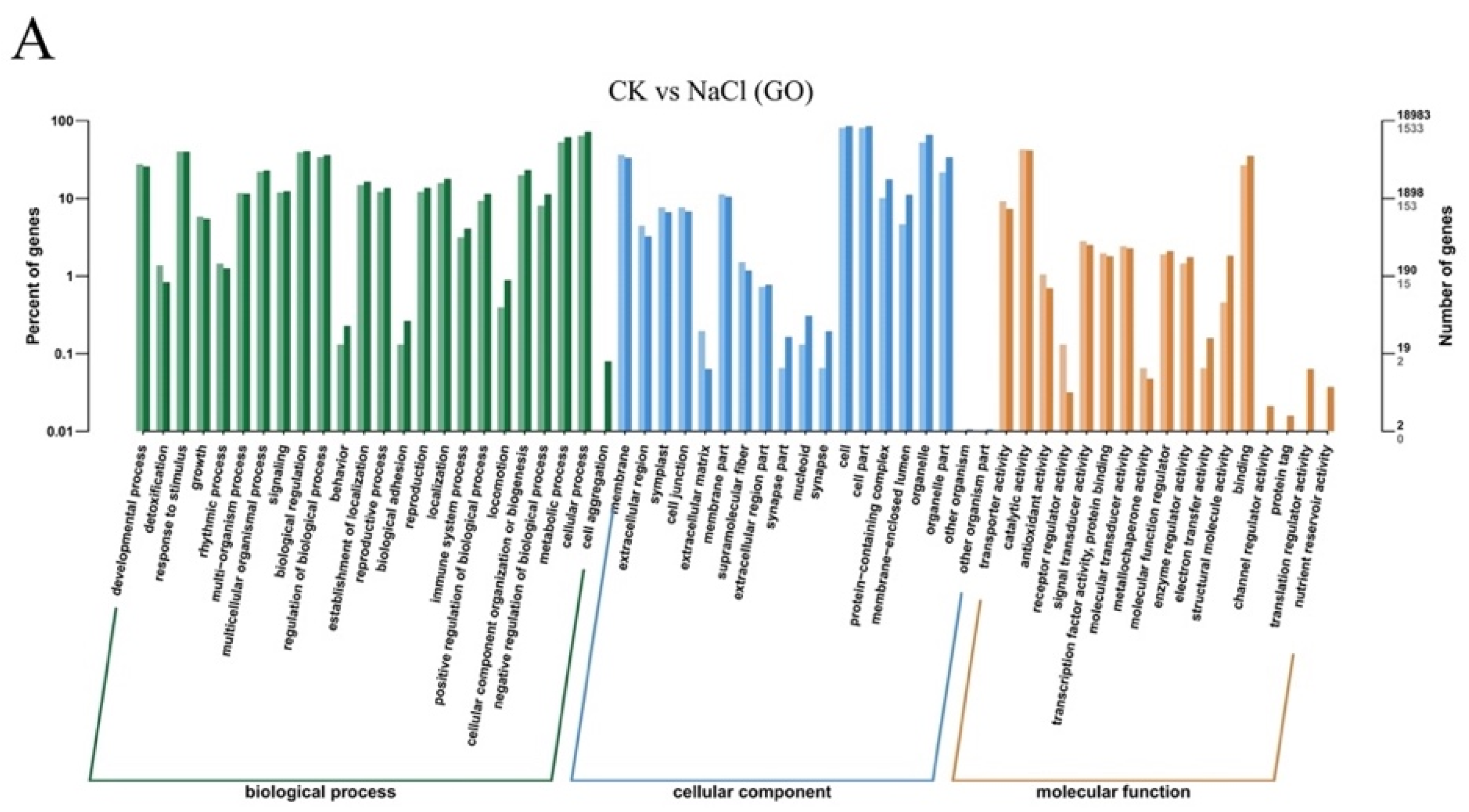
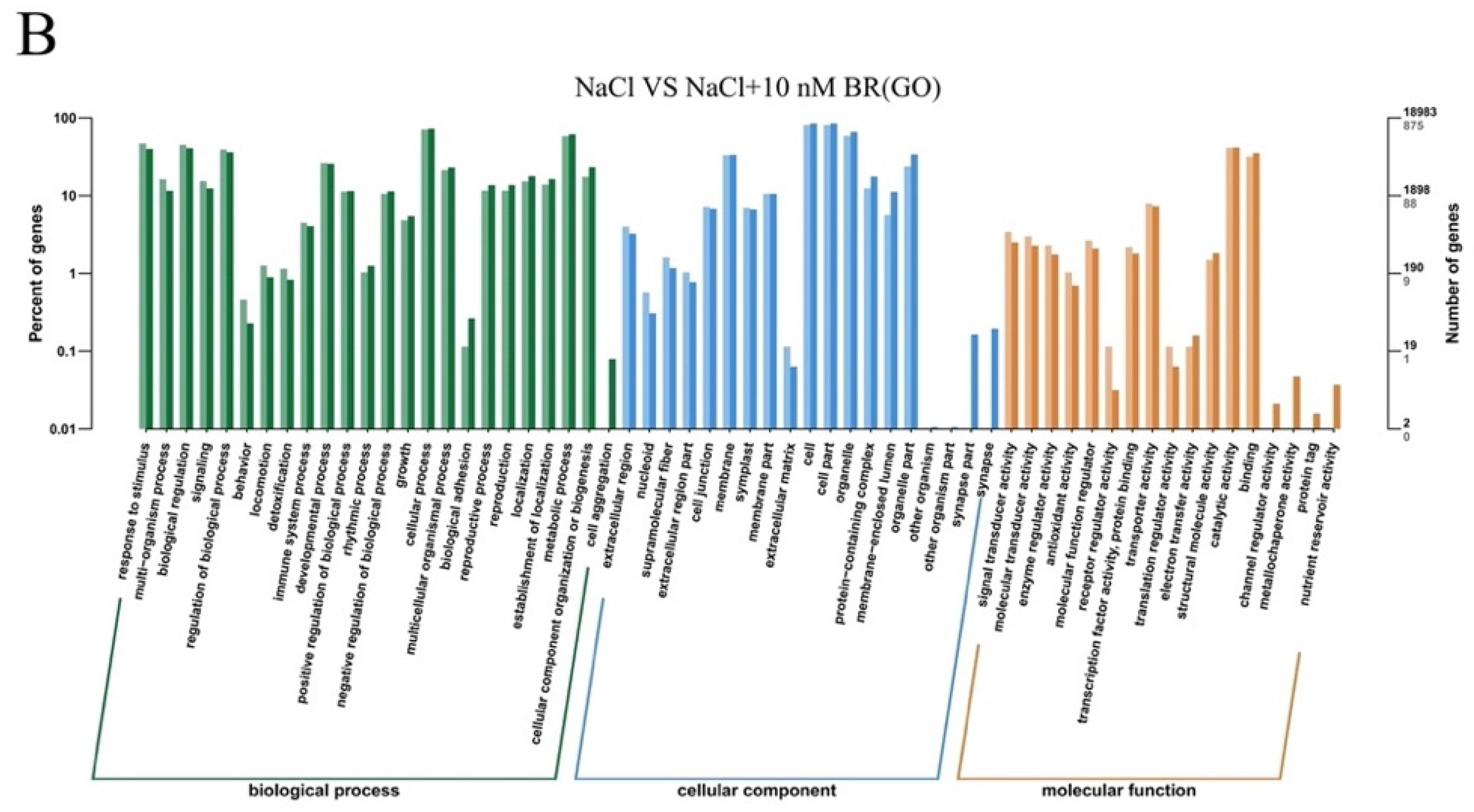

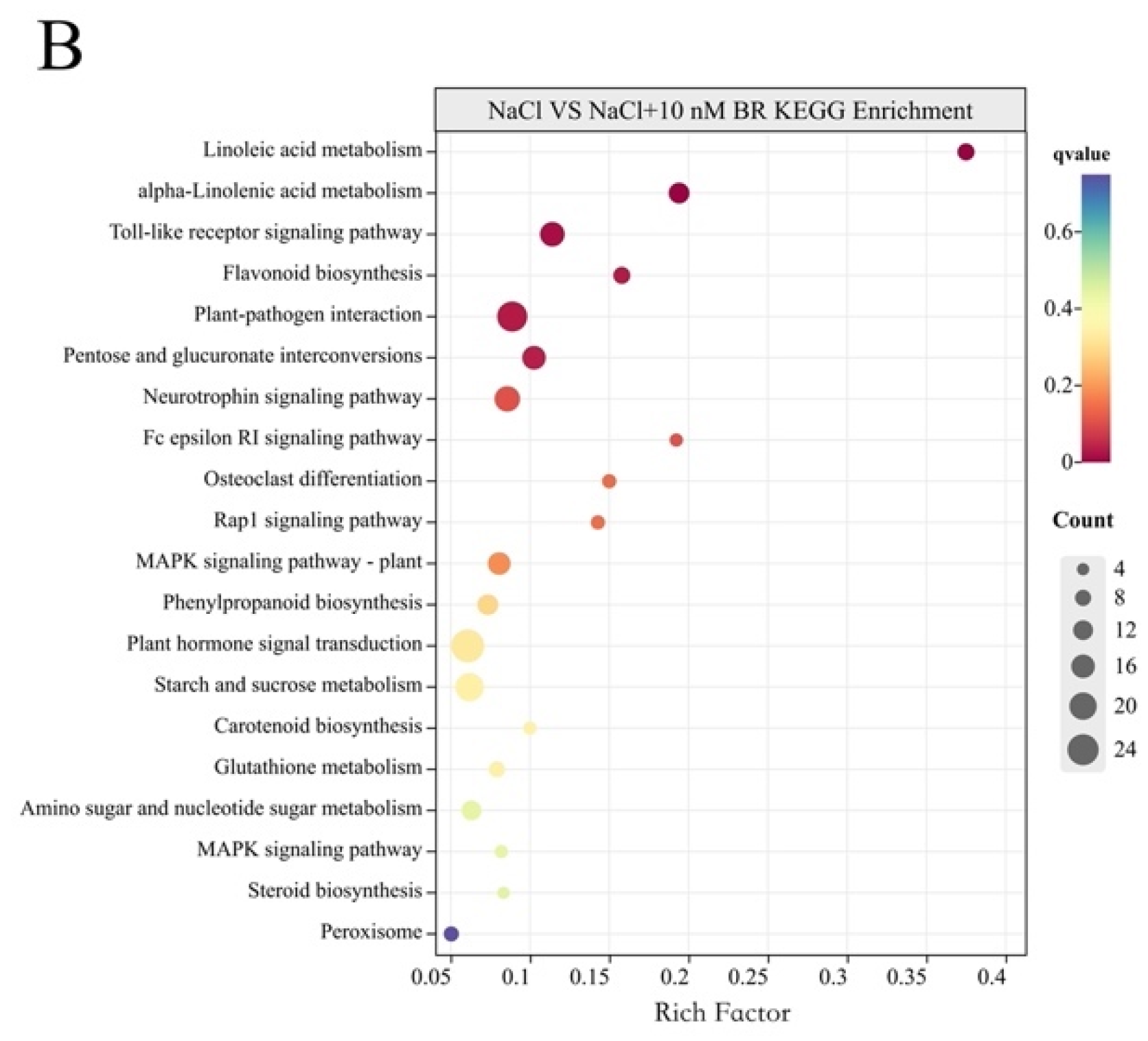
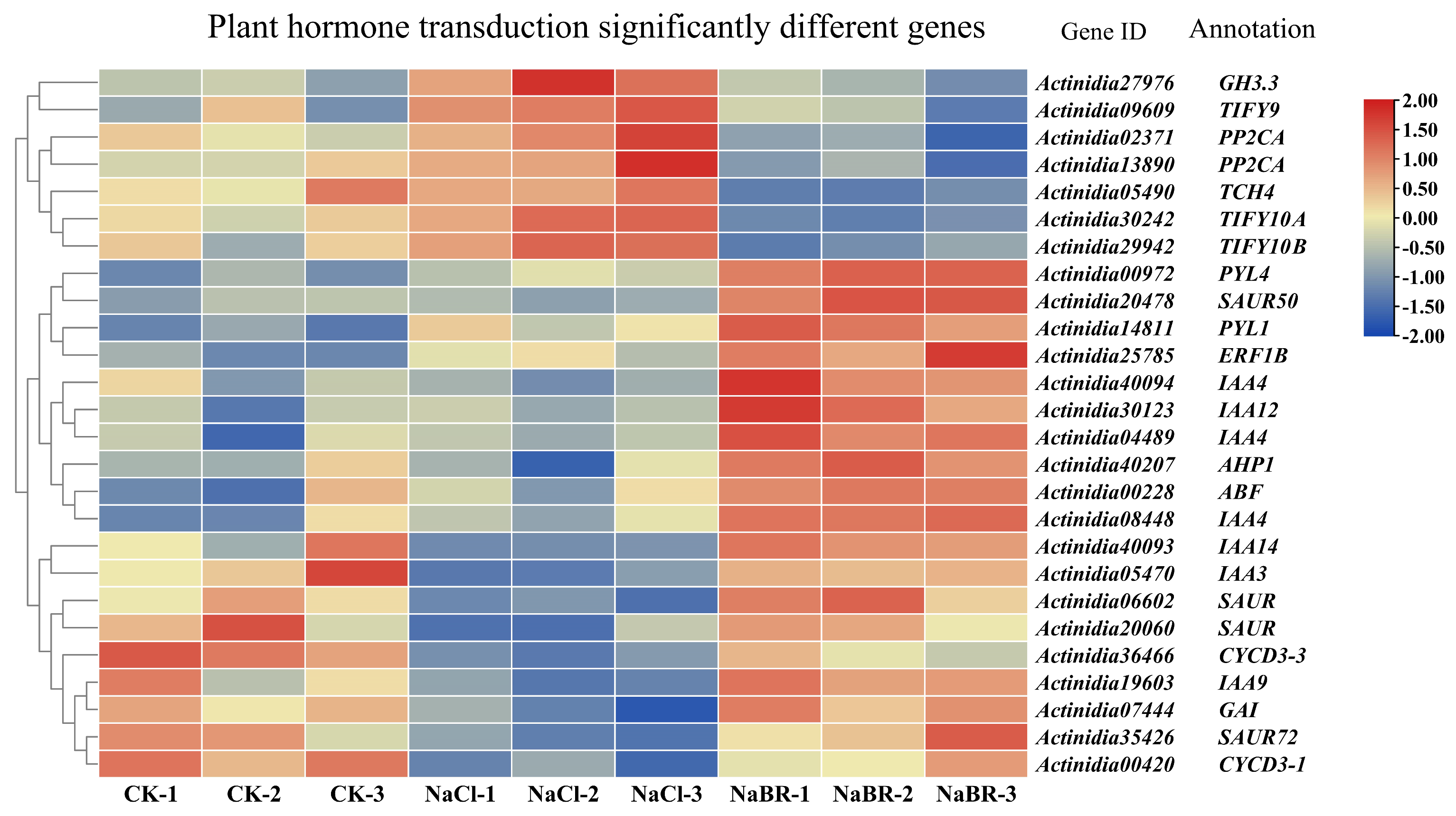



| Principal Component | PC1 | PC2 | PC3 |
|---|---|---|---|
| Cumulative variance explained rate (%) | 56.865 | 83.096 | 100 |
| Weight (%) | 56.865 | 26.231 | 16.904 |
| Pn | 0.195 | 0.121 | −0.286 |
| SPAD | 0.205 | 0.167 | −0.051 |
| L-H2O2 | 0.2 | −0.199 | 0.024 |
| L-SOD | 0.161 | −0.303 | 0.177 |
| R-H2O2 | 0.179 | −0.25 | −0.181 |
| R-SOD | 0.071 | 0.328 | −0.481 |
| L-Na+/K+ | 0.177 | 0.159 | 0.364 |
| R-Na+/K+ | 0.074 | 0.328 | 0.476 |
| Z Value | Sort | |
|---|---|---|
| CK (CK1) | 0.935 | 1 |
| NaCl (CK2) | −0.522 | 4 |
| NaCl + 1 nM BR (T1) | −0.332 | 3 |
| NaCl + 10 nM BR(T2) | −0.081 | 2 |
| Sample | Name | Total Raw Reads Count | Total Clean Reads Count | Clean Reads Ratios |
|---|---|---|---|---|
| Root | CK (CK1)-1 | 46,099,850 | 44,015,926 | 95.48% |
| CK (CK1)-2 | 62,790,266 | 59,672,992 | 95.04% | |
| CK (CK1)-3 | 37,868,464 | 36,313,204 | 95.89% | |
| NaCl (CK2)-1 | 45,737,256 | 43,448,892 | 95.00% | |
| NaCl (CK2)-2 | 38,979,938 | 37,349,052 | 95.82% | |
| NaCl (CK2)-3 | 43,867,674 | 41,751,154 | 95.18% | |
| NaCl + 10 nM BR(T2)-1 | 40,418,566 | 38,676,650 | 95.69% | |
| NaCl + 10 nM BR(T2)-2 | 39,910,140 | 38,213,008 | 95.75% | |
| NaCl + 10 nM BR(T2)-3 | 38,721,286 | 37,049,964 | 95.68% |
| Sample | Name | Total Reads | Total Mapped |
|---|---|---|---|
| Root | CK (CK1)-1 | 43,440,582 (100.00%) | 37,431,515 (86.17%) |
| CK (CK1)-2 | 58,776,918 (100.00%) | 50,181,238 (85.38%) | |
| CK (CK1)-3 | 33,194,052 (100.00%) | 28,456,592 (85.73%) | |
| NaCl (CK2)-1 | 41,902,434 (100.00%) | 34,880,169 (83.24%) | |
| NaCl (CK2)-2 | 36,117,688 (100.00%) | 30,721,327 (85.06%) | |
| NaCl (CK2)-3 | 33,398,428 (100.00%) | 28,175,797 (84.36%) | |
| NaCl + 10 nM BR(T2)-1 | 36,674,346 (100.00%) | 30,404,547 (82.90%) | |
| NaCl + 10 nM BR(T2)-2 | 31,311,032 (100.00%) | 26,544,735 (84.78%) | |
| NaCl + 10 nM BR(T2)-3 | 33,492,586 (100.00%) | 27,914,130 (83.34%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Cheng, D.; Li, L.; Sun, X.; He, S.; Li, M.; Chen, J. Physiological Characteristics and Transcriptome Analysis of Exogenous Brassinosteroid-Treated Kiwifruit. Int. J. Mol. Sci. 2023, 24, 17252. https://doi.org/10.3390/ijms242417252
Chen C, Cheng D, Li L, Sun X, He S, Li M, Chen J. Physiological Characteristics and Transcriptome Analysis of Exogenous Brassinosteroid-Treated Kiwifruit. International Journal of Molecular Sciences. 2023; 24(24):17252. https://doi.org/10.3390/ijms242417252
Chicago/Turabian StyleChen, Chen, Dawei Cheng, Lan Li, Xiaoxu Sun, Shasha He, Ming Li, and Jinyong Chen. 2023. "Physiological Characteristics and Transcriptome Analysis of Exogenous Brassinosteroid-Treated Kiwifruit" International Journal of Molecular Sciences 24, no. 24: 17252. https://doi.org/10.3390/ijms242417252
APA StyleChen, C., Cheng, D., Li, L., Sun, X., He, S., Li, M., & Chen, J. (2023). Physiological Characteristics and Transcriptome Analysis of Exogenous Brassinosteroid-Treated Kiwifruit. International Journal of Molecular Sciences, 24(24), 17252. https://doi.org/10.3390/ijms242417252





