Is Wheat Glutenin Extract Intrinsically Allergenic? Evaluation Using a Novel Adjuvant-Free Mouse Model of Systemic Anaphylaxis
Abstract
:1. Introduction
2. Results
2.1. Chronic Application of Wheat Glutenin (WG) onto Undamaged Skin Elicits Robust Specific IgE, and IgG1 Antibody Response in Balb/c Mice
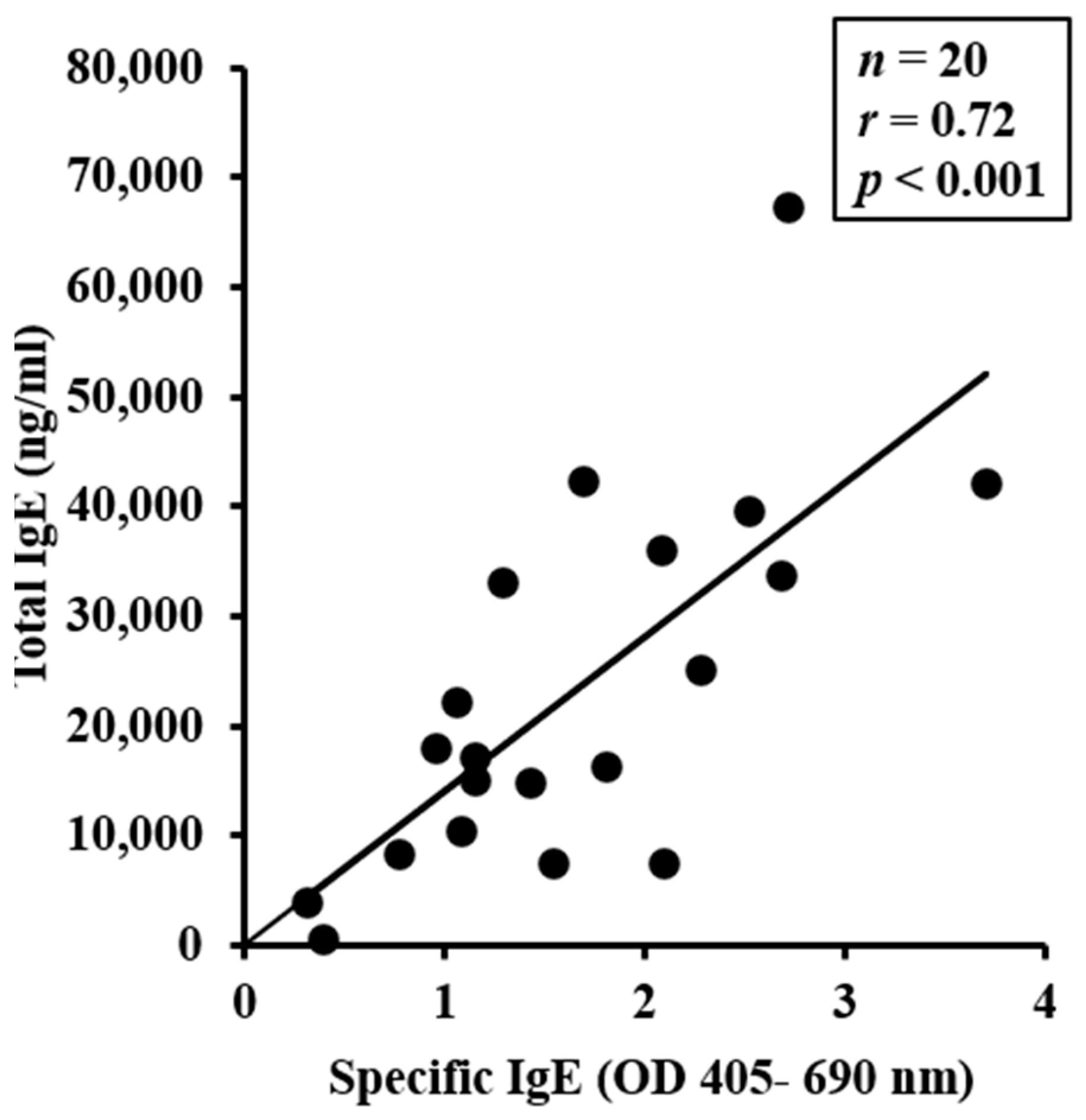
2.2. Chronic Application of Wheat Glutenin (WG) onto Undamaged Skin also Elevates Total IgE Levels, Which Correlate with sIgE Levels
2.3. WG-Sensitized, but Not Vehicle-Sensitized Mice, Exhibit Life-Threatening Symptoms of Anaphylaxis upon Systemic Challenge with WG
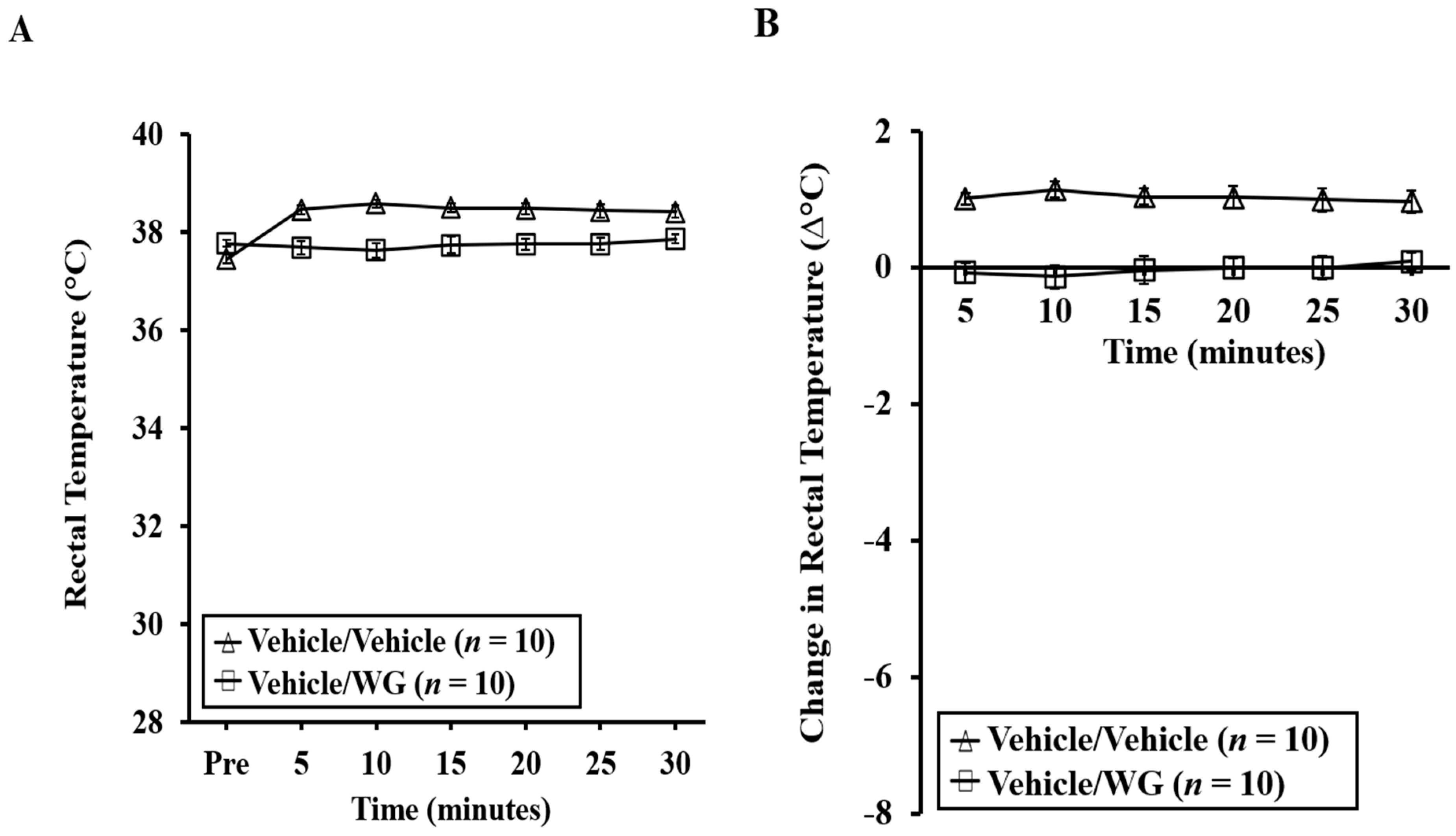
2.4. Mice Experiencing Systemic Anaphylaxis Symptoms Following a Systemic Challenge with WG Displayed Pronounced Hypothermic Shock Responses (HSR)
2.5. Systemic Anaphylaxis Is also Linked to Substantial Mucosal Mast Cell Degranulation in This Model
2.6. Proteomic Analysis and Identification of Differentially Expressed Immune Biomarkers in the Spleen of Mice Undergoing Systemic Anaphylaxis
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Mice Breeding and Establishment of a Plant-Protein-Free Mouse Colony
4.3. Preparation of Acid-Soluble Protein Extract from Wheat Flour
4.4. Skin Sensitization, Bleeding, and Plasma Sample Preparation
4.5. Elicitation of Systemic Anaphylaxis and Clinical Symptom Scoring
4.6. Determination of Hypothermic Shock Responses
4.7. Measurement of Specific IgE Antibody Levels
4.8. Measurement of Total Plasma IgE Concentration
4.9. Quantification of Mucosal Mast Cell Protease-1 (MMCP-1) Level
4.10. Spleen Extract Preparation and Proteomic Analysis of Immune Biomarkers
4.11. IgG1
4.12. IgE Cross-Reactivity
4.13. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Venter, C.; Pereira, B.; Grundy, J.; Clayton, C.B.; Arshad, S.H.; Dean, T. Prevalence of Sensitization Reported and Objectively Assessed Food Hypersensitivity amongst Six-Year-Old Children: A Population-Based Study. Pediatr. Allergy Immunol. 2006, 17, 356–363. [Google Scholar] [CrossRef]
- Venter, C.; Pereira, B.; Voigt, K.; Grundy, J.; Clayton, C.B.; Higgins, B.; Arshad, S.H.; Dean, T. Prevalence and Cumulative Incidence of Food Hypersensitivity in the First 3 Years of Life. Allergy 2008, 63, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Vierk, K.A.; Koehler, K.M.; Fein, S.B.; Street, D.A. Prevalence of Self-Reported Food Allergy in American Adults and Use of Food Labels. J. Allergy Clin. Immunol. 2007, 119, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, A.; Khullar, K.; Saltzman, R.; Fiedler, J.; Garrett, J.P.; Naimi, D.R.; Spergel, J.M. Oral Food Challenge to Wheat: A Near-Fatal Anaphylaxis and Review of 93 Food Challenges in Children. World Allergy Organ. J. 2013, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, A.; Muraro, A. Food-Induced Anaphylaxis. Immunol. Allergy Clin. N. Am. 2012, 32, 165–195. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, G.; Quirce, S.; Diaz-Perales, A. Wheat Allergens Associated with Baker’s Asthma. J. Investig. Allergol. Clin. Immunol. 2011, 21, 81–92. [Google Scholar] [PubMed]
- Cianferoni, A. Wheat Allergy: Diagnosis and Management. J. Asthma Allergy 2016, 9, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Sampson, H.A. Food Allergy: A Review and Update on Epidemiology, Pathogenesis, Diagnosis, Prevention, and Management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Jiang, J.; Blumenstock, J.A.; Davis, M.M.; Schleimer, R.P.; Nadeau, K.C. Prevalence and Severity of Food Allergies Among US Adults. JAMA Netw. Open 2019, 2, e185630. [Google Scholar] [CrossRef]
- De Sousa, T.; Ribeiro, M.; Sabença, C.; Igrejas, G. The 10,000-Year Success Story of Wheat! Foods 2021, 10, 2124. [Google Scholar] [CrossRef]
- Cabanillas, B. Gluten-Related Disorders: Celiac Disease, Wheat Allergy, and Nonceliac Gluten Sensitivity. Crit. Rev. Food Sci. Nutr. 2020, 60, 2606–2621. [Google Scholar] [CrossRef] [PubMed]
- Ricci, G.; Andreozzi, L.; Cipriani, F.; Giannetti, A.; Gallucci, M.; Caffarelli, C. Wheat Allergy in Children: A Comprehensive Update. Medicina 2019, 55, 400. [Google Scholar] [CrossRef]
- Pastorello, E.A.; Farioli, L.; Conti, A.; Pravettoni, V.; Bonomi, S.; Iametti, S.; Fortunato, D.; Scibilia, J.; Bindslev-Jensen, C.; Ballmer-Weber, B.; et al. Wheat IgE-Mediated Food Allergy in European Patients: α-Amylase Inhibitors, Lipid Transfer Proteins and Low-Molecular-Weight Glutenins—Allergenic Molecules Recognized by Double-Blind, Placebo-Controlled Food Challenge. Int. Arch. Allergy Immunol. 2007, 144, 10–22. [Google Scholar] [CrossRef]
- Gao, H.; Jin, Y.; Jian, D.I.; Olson, E.; Ng, P.K.W.; Gangur, V. Development and Validation of a Mouse-Based Primary Screening Method for Testing Relative Allergenicity of Proteins from Different Wheat Genotypes. J. Immunol. Methods 2019, 464, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Platts-Mills, T.A.E. The Allergy Epidemics: 1870–2010. J. Allergy Clin. Immunol. 2015, 136, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Renz, H.; Allen, K.J.; Sicherer, S.H.; Sampson, H.A.; Lack, G.; Beyer, K.; Oettgen, H.C. Food Allergy. Nat. Rev. Dis. Prim. 2018, 4, 17098. [Google Scholar] [CrossRef] [PubMed]
- Kroghsbo, S.; Rigby, N.M.; Johnson, P.E.; Adel-Patient, K.; Bøgh, K.L.; Salt, L.J.; Mills, E.N.C.; Madsen, C.B. Assessment of the Sensitizing Potential of Processed Peanut Proteins in Brown Norway Rats: Roasting Does Not Enhance Allergenicity. PLoS ONE 2014, 9, e96475. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Adamidi, C.; Lozano, R.M.; Yee, B.C.; Momma, M.; Kobrehel, K.; Ermel, R.; Frick, O.L. Thioredoxin-Linked Mitigation of Allergic Responses to Wheat. Proc. Natl. Acad. Sci. USA 1997, 94, 5372–5377. [Google Scholar] [CrossRef]
- Kozai, H.; Yano, H.; Matsuda, T.; Kato, Y. Wheat-Dependent Exercise-Induced Anaphylaxis in Mice Is Caused by Gliadin and Glutenin Treatments. Immunol. Lett. 2006, 102, 83–90. [Google Scholar] [CrossRef]
- Bodinier, M.; Leroy, M.; Ah-Leung, S.; Blanc, F.; Tranquet, O.; Denery-Papini, S.; Wal, J.M.; Adel-Patient, K. Sensitization and Elicitation of an Allergic Reaction to Wheat Gliadins in Mice. J. Agric. Food Chem. 2009, 57, 1219–1225. [Google Scholar] [CrossRef]
- Adachi, R.; Nakamura, R.; Sakai, S.; Fukutomi, Y.; Teshima, R. Sensitization to Acid-Hydrolyzed Wheat Protein by Transdermal Administration to BALB/c Mice, and Comparison with Gluten. Allergy Eur. J. Allergy Clin. Immunol. 2012, 67, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, R.; Gao, H.; Chandra, S.; Sundar, V.; Loy, J.; Van Antwerp, C.; Ng, P.K.W.; Gangur, V. Chronic Application of Alcohol-Soluble Gluten Extract over Undamaged Skin Causes Clinical Sensitization for Life-Threatening Anaphylaxis via Activation of Systemic Th2 Immune Responses in Mice. Front. Allergy 2023, 4, 1214051. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Jorgensen, R.; Raghunath, R.; Chandra, S.; Othman, A.; Olson, E.; Ng, P.K.W.; Gangur, V. Intrinsic Allergenicity Potential of Salt-Soluble Protein Extracts from the Diploid, Tetraploid and Hexaploid Wheats: Validation Using an Adjuvant-Free Mouse Model. Int. J. Mol. Sci. 2023, 24, 5453. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Nagano, T.; Yano, H.; Matsuda, T.; Ikeda, T.M.; Haruma, K.; Kato, Y. Impact of (0-5 Gliadin on Wheat-Dependent Exercise-Induced Anaphylaxis in Mice. Biosci. Biotechnol. Biochem. 2011, 75, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Gourbeyre, P.; Denery-Papini, S.; Larré, C.; Gaudin, J.C.; Brossard, C.; Bodinier, M. Wheat Gliadins Modified by Deamidation Are More Efficient than Native Gliadins in Inducing a Th2 Response in Balb/c Mice Experimentally Sensitized to Wheat Allergens. Mol. Nutr. Food Res. 2012, 56, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Wu, S.; Dong, L.; Hu, Y.; Wang, J.; Zhang, Y.; Wang, S. Methylglyoxal Decoration of Glutenin during Heat Processing Could Alleviate the Resulting Allergic Reaction in Mice. Nutrients 2020, 12, 2844. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; O’Mahony, L.; Burks, A.W.; Plaut, M.; Lack, G.; Akdis, C.A. Mechanisms of Food Allergy. J. Allergy Clin. Immunol. 2018, 141, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Wärnberg Gerdin, S.; Lie, A.; Asarnoj, A.; Borres, M.P.; Lødrup Carlsen, K.C.; Färdig, M.; Konradsen, J.R.; Monceyron Jonassen, C.; Olsson Mägi, C.A.; Rehbinder, E.M.; et al. Impaired Skin Barrier and Allergic Sensitization in Early Infancy. Allergy Eur. J. Allergy Clin. Immunol. 2022, 77, 1464–1476. [Google Scholar] [CrossRef]
- Brough, H.A.; Nadeau, K.C.; Sindher, S.B.; Alkotob, S.S.; Chan, S.; Bahnson, H.T.; Leung, D.Y.M.; Lack, G. Epicutaneous Sensitization in the Development of Food Allergy: What Is the Evidence and How Can This Be Prevented? Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 2185–2205. [Google Scholar] [CrossRef]
- Fukutomi, Y.; Itagaki, Y.; Taniguchi, M.; Saito, A.; Yasueda, H.; Nakazawa, T.; Hasegawa, M.; Nakamura, H.; Akiyama, K. Rhinoconjunctival Sensitization to Hydrolyzed Wheat Protein in Facial Soap Can Induce Wheat-Dependent Exercise-Induced Anaphylaxis. J. Allergy Clin. Immunol. 2011, 127, 531–533.e3. [Google Scholar] [CrossRef]
- Chinuki, Y.; Morita, E. Wheat-Dependent Exercise-Induced Anaphylaxis Sensitized with Hydrolyzed Wheat Protein in Soap. Allergol. Int. 2012, 61, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Laurière, M.; Pecquet, C.; Bouchez-Mahiout, I.; Snégaroff, J.; Bayrou, O.; Raison-Peyron, N.; Vigan, M. Hydrolysed Wheat Proteins Present in Cosmetics Can Induce Immediate Hypersensitivities. Contact Dermat. 2006, 54, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Hiragun, M.; Ishii, K.; Hiragun, T.; Shindo, H.; Mihara, S.; Matsuo, H.; Hide, M. The Sensitivity and Clinical Course of Patients with Wheat-Dependent Exercise-Induced Anaphylaxis Sensitized to Hydrolyzed Wheat Protein in Facial Soap—Secondary Publication. Allergol. Int. 2013, 62, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Ito, T.; Kawakami, H.; Fuzishiro, K.; Hirano, H.; Okubo, Y.; Tsuboi, R. Eighteen Cases of Wheat Allergy and Wheat-Dependent Exercise-Induced Urticaria/Anaphylaxis Sensitized by Hydrolyzed Wheat Protein in Soap. Int. J. Dermatol. 2015, 54, e302–e305. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Acharya, H.G.; Acharya, D.; Jorgensen, R.; Gao, H.; Secord, J.; Ng, P.K.W.; Gangur, V. Advances in Molecular Mechanisms of Wheat Allergenicity in Animal Models: A Comprehensive Review. Molecules 2019, 24, 1142. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, N.P.; Parvataneni, S.; Hassan, H.M.A.; Harkema, J.; Samineni, S.; Navuluri, L.; Kelly, C.J.; Gangur, V. An Adjuvant-Free Mouse Model of Tree Nut Allergy Using Hazelnut as a Model Tree Nut. Int. Arch. Allergy Immunol. 2007, 144, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Khodoun, M.V.; Strait, R.; Armstrong, L.; Yanase, N.; Finkelman, F.D. Identification of Markers That Distinguish IgE- from IgG-Mediated Anaphylaxis. Proc. Natl. Acad. Sci. USA 2011, 108, 12413–12418. [Google Scholar] [CrossRef] [PubMed]
- Hosoki, K.; Aguilera-Aguirre, L.; Brasier, A.R.; Kurosky, A.; Boldogh, I.; Sur, S. Facilitation of Allergic Sensitization and Allergic Airway Inflammation by Pollen-Induced Innate Neutrophil Recruitment. Am. J. Respir. Cell Mol. Biol. 2016, 54, 81–90. [Google Scholar] [CrossRef]
- Gilles, S.; Traidl-Hoffmann, C. CD27 Expression on Allergen-Specific T Cells: A New Surrogate for Successful Allergen-Specific Immunotherapy? J. Allergy Clin. Immunol. 2012, 129, 552–554. [Google Scholar] [CrossRef]
- Feng, H.; Xiong, X.; Chen, Z.; Luo, N.; Wu, Y. MALAT1 Induces Food Allergy by Promoting Release of IL-6 from Dendritic Cells and Suppressing the Immunomodulatory Function of Tregs. J. Asthma Allergy 2022, 15, 529–544. [Google Scholar] [CrossRef]
- Shik, D.; Tomar, S.; Lee, J.B.; Chen, C.Y.; Smith, A.; Wang, Y.H. IL-9-Producing Cells in the Development of IgE-Mediated Food Allergy. Semin. Immunopathol. 2017, 39, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Herberth, G.; Daegelmann, C.; Röder, S.; Behrendt, H.; Krämer, U.; Borte, M.; Heinrich, J.; Herbarth, O.; Lehmann, I. IL-17E but Not IL-17A Is Associated with Allergic Sensitization: Results from the LISA Study. Pediatr. Allergy Immunol. 2010, 21, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.M.; Fasano, A.; Mann, D.L. Monocytes Differentiated with IL-15 Support Th17 and Th1 Responses to Wheat Gliadin: Implications for Celiac Disease. Clin. Immunol. 2010, 135, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, H.W. IL-23 Plays a Significant Role in the Augmentation of Particulate Matter-Mediated Allergic Airway Inflammation. J. Cell. Mol. Med. 2022, 26, 4506–4519. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chehade, M.; Liu, W.; Xiong, H.; Mayer, L.; Berin, M.C. Allergen-IgE Complexes Trigger CD23-Dependent CCL20 Release from Human Intestinal Epithelial Cells. Gastroenterology 2007, 133, 1905–1915. [Google Scholar] [CrossRef] [PubMed]
- Khodoun, M.V.; Tomar, S.; Tocker, J.E.; Wang, Y.H.; Finkelman, F.D. Prevention of Food Allergy Development and Suppression of Established Food Allergy by Neutralization of Thymic Stromal Lymphopoietin, IL-25, and IL-33. J. Allergy Clin. Immunol. 2018, 141, 171–179.e1. [Google Scholar] [CrossRef] [PubMed]
- Osterfeld, H.; Ahrens, R.; Strait, R.; Finkelman, F.D.; Renauld, J.C.; Hogan, S.P. Differential Roles for the IL-9/IL-9 Receptor α-Chain Pathway in Systemic and Oral Antigen-Induced Anaphylaxis. J. Allergy Clin. Immunol. 2010, 125, 469–476. [Google Scholar] [CrossRef]
- Ramalho, R.; Almeida, J.; Beltrão, M.; Pirraco, A.; Costa, R.; Sokhatska, O.; Guardão, L.; Palmares, C.; Guimarães, J.T.; Delgado, L.; et al. Substance P Antagonist Improves Both Obesity and Asthma in a Mouse Model. Allergy Eur. J. Allergy Clin. Immunol. 2013, 68, 48–54. [Google Scholar] [CrossRef]
- Heinolainen, K.; Karaman, S.; D’Amico, G.; Tammela, T.; Sormunen, R.; Eklund, L.; Alitalo, K.; Zarkada, G. VEGFR3 Modulates Vascular Permeability by Controlling VEGF/VEGFR2 Signaling. Circ. Res. 2017, 120, 1414–1425. [Google Scholar] [CrossRef]
- Rissanen, T.T.; Markkanen, J.E.; Gruchala, M.; Heikura, T.; Puranen, A.; Kettunen, M.I.; Kholová, I.; Kauppinen, R.A.; Achen, M.G.; Stacker, S.A.; et al. VEGF-D Is the Strongest Angiogenic and Lymphangiogenic Effector among VEGFs Delivered into Skeletal Muscle via Adenoviruses. Circ. Res. 2003, 92, 1098–1106. [Google Scholar] [CrossRef]
- Koussih, L.; Atoui, S.; Tliba, O.; Gounni, A.S. New Insights on the Role of Pentraxin-3 in Allergic Asthma. Front. Allergy 2021, 2, 678023. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Tashimo, H.; Matsuo, Y.; Ishida, H.; Yoshiura, K.; Sato, K.; Yamashita, N.; Kakiuchi, T.; Ohta, K. Role of CCL21 and CCL19 in Allergic Inflammation in the Ovalbumin-Specific Murine Asthmatic Model. J. Allergy Clin. Immunol. 2006, 117, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J. The TWEAK/Fn14 Axis in Anaphylactic Shock. J. Allergy Clin. Immunol. 2020, 145, 491–493. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Barbero, N.; Yuste-Montalvo, A.; Nuñez-Borque, E.; Jensen, B.M.; Gutiérrez-Muñoz, C.; Tome-Amat, J.; Garrido-Arandia, M.; Díaz-Perales, A.; Ballesteros-Martinez, C.; Laguna, J.J.; et al. The TNF-like Weak Inducer of the Apoptosis/Fibroblast Growth Factor–Inducible Molecule 14 Axis Mediates Histamine and Platelet-Activating Factor–Induced Subcutaneous Vascular Leakage and Anaphylactic Shock. J. Allergy Clin. Immunol. 2020, 145, 583–596.e6. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, Y.; Obata, K.; Mukai, K.; Shindou, H.; Yoshida, M.; Nishikado, H.; Kawano, Y.; Minegishi, Y.; Shimizu, T.; Karasuyama, H. Basophils Play a Pivotal Role in Immunoglobulin-G-Mediated but Not Immunoglobulin-E-Mediated Systemic Anaphylaxis. Immunity 2008, 28, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, I.; Dombrowicz, D.; Martin, T.R.; Ravetch, J.V.; Kinet, J.P.; Galli, S.J. Systemic Anaphylaxis in the Mouse Can Be Mediated Largely through IgG1 and FcyRIII. J. Clin. Investig. 1997, 99, 901–914. [Google Scholar] [CrossRef] [PubMed]
- Ning, J. Biotechnology Notification File No. 000170 CVM Note to the File. 2022. Available online: https://www.fda.gov/search?s=000170 (accessed on 2 October 2023).
- Boehm, T. GM Wheat Contamination Incident a Reminder of Need for Better Regulation. Available online: https://www.nfu.ca/gm-wheat-contamination-incident-a-reminder-of-need-for-better-regulation/ (accessed on 2 October 2023).
- FAO. FAO/WHO Decison Tree. Available online: https://www.fao.org/3/y0820e/y0820e00.htm (accessed on 4 February 2023).
- Gao, H.; Jorgensen, R.; Raghunath, R.; Nagisetty, S.; Ng, P.K.W.; Gangur, V. Creating Hypo-/Nonallergenic Wheat Products Using Processing Methods: Fact or Fiction? Compr. Rev. Food Sci. Food Saf. 2021, 20, 6089–6115. [Google Scholar] [CrossRef]
- Chen, C.H.; Bushuk, W. Nature of Proteins in Triticale and Its Parental Species. C. J. Plant Sci. 1969, 50, 9–14. [Google Scholar] [CrossRef]
- Jin, Y.; Gao, H.; Jorgensen, R.; Salloum, J.; Jian, D.I.; Ng, P.K.W.; Gangur, V. Mechanisms of Wheat Allergenicity in Mice: Comparison of Adjuvant-Free vs. Alum-Adjuvant Models. Int. J. Mol. Sci. 2020, 21, 3205. [Google Scholar] [CrossRef]
- Jin, Y.; Ebaugh, S.; Martens, A.; Gao, H.; Olson, E.; Ng, P.K.W.; Gangur, V. A Mouse Model of Anaphylaxis and Atopic Dermatitis to Salt-Soluble Wheat Protein Extract. Int. Arch. Allergy Immunol. 2017, 174, 7–16. [Google Scholar] [CrossRef]
- Birmingham, N.; Payankaulam, S.; Thanesvorakul, S.; Stefura, B.; HayGlass, K.; Gangur, V. An ELISA-Based Method for Measurement of Food-Specific IgE Antibody in Mouse Serum: An Alternative to the Passive Cutaneous Anaphylaxis Assay. J. Immunol. Methods 2003, 275, 89–98. [Google Scholar] [CrossRef]




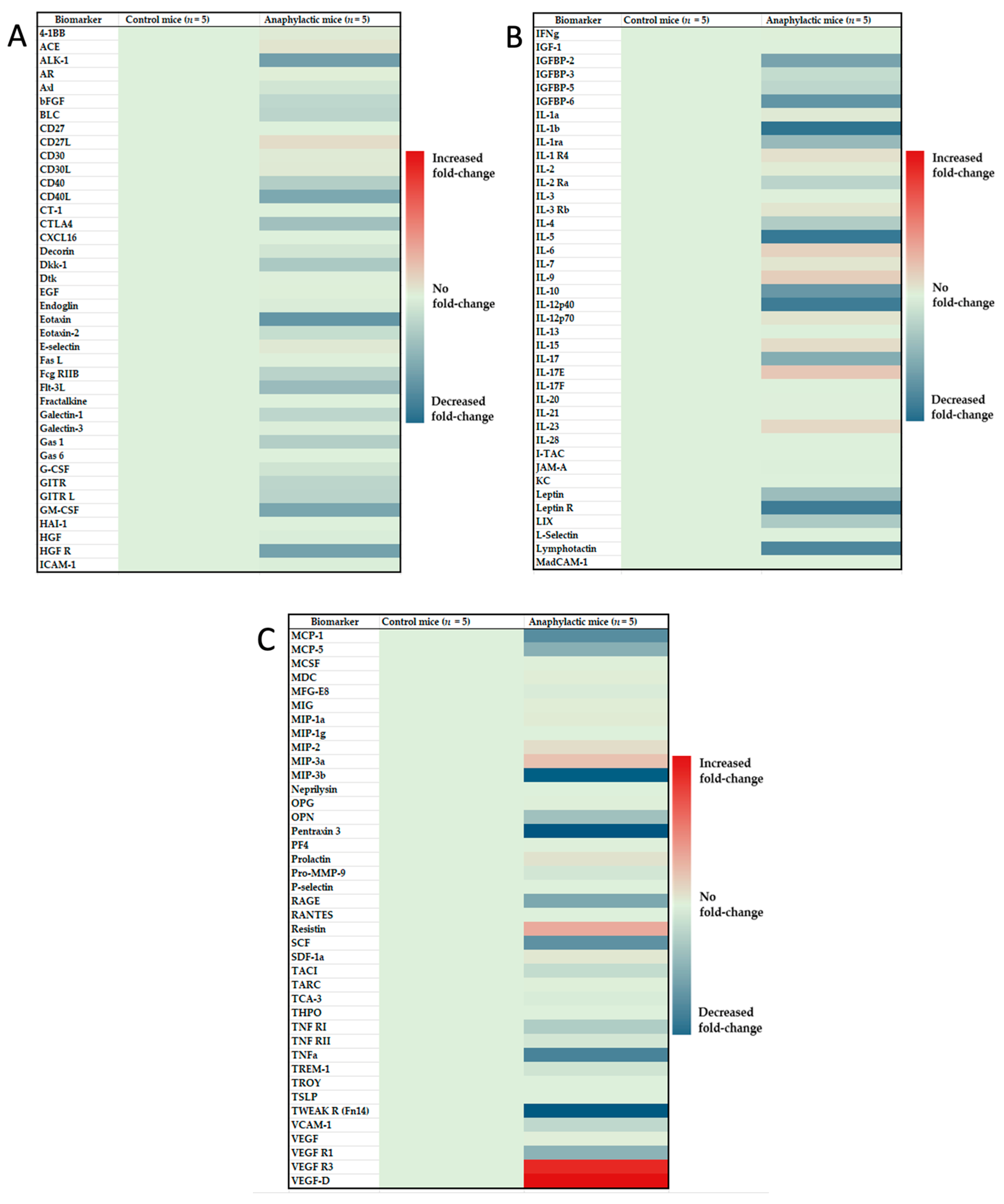
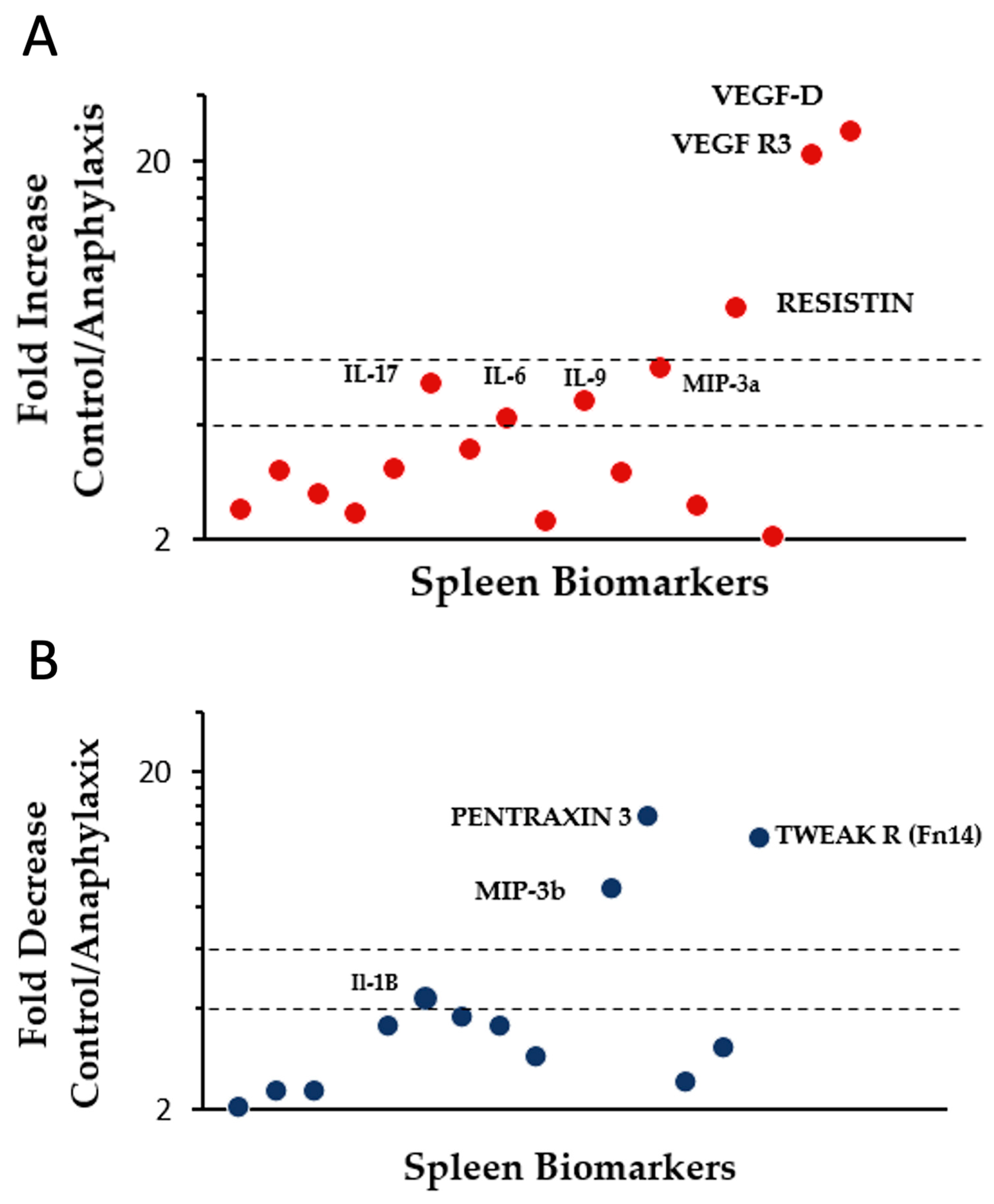
| Biomarker | Control Mice (n = 5) | Anaphylactic Mice (n = 5) | Student’s t-Test p < |
|---|---|---|---|
| ACE | 38,316.3 ± 1853.51 | 91,252.75 ± 2471.8 | 0.001 |
| CD27L | <40 (LOD) | 121.18 ± 18.33 | 0.005 |
| CD30L | 7.57 ± 0.44 | 13.68 ± 0.67 | 0.001 |
| Dtk | 1117.62 ± 26.36 | 1348.25 ± 46.15 | 0.005 |
| IL-1a | 14.09 ± 2.34 | 25.88 ± 2.26 | 0.05 |
| IL-1 R4 | 391.66 ± 114.88 | 1022.84 ± 20.13 | 0.005 |
| IL-2 | 45.09 ± 1.4 | 75.5 ± 9.72 | 0.05 |
| IL-6 | 59.98 ± 8.13 | 247.23 ± 26.76 | 0.001 |
| IL-9 | 27.2 ± 4.86 | 124.92 ± 14.87 | 0.001 |
| IL-12p70 | 33.78 ± 9.04 | 78.5 ± 11.13 | 0.05 |
| IL-15 | 8185.98 ± 1532.53 | 25,061.06 ± 3151.03 | 0.005 |
| IL-17E | 44.87 ± 19.9 | 191.72 ± 89.92 | 0.05 |
| IL-23 | 273.89 ± 96.98 | 939.23 ± 164.11 | 0.05 |
| MCSF | 80.09 ± 2.31 | 102.92 ± 3.43 | 0.001 |
| MDC | 132.25 ± 5.29 | 198.63 ± 8.4 | 0.001 |
| MIG | 280.49 ± 12.63 | 419.55 ± 6.13 | 0.001 |
| MIP-1a | 78.61 ± 7.59 | 132.23 ± 2.6 | 0.001 |
| MIP-1g | 943.39 ± 9.41 | 1001.9 ± 10.58 | 0.01 |
| MIP-2 | 1.56 ± 0.76 | 4.65 ± 0.29 | 0.01 |
| OPG | 299.86 ± 5.99 | 373.37 ± 22.5 | 0.05 |
| PF4 | 27,562.44 ± 363.93 | 31,638.78 ± 629.49 | 0.001 |
| Prolactin | 6.8 ± 1.27 | 16.57 ± 3.65 | 0.05 |
| Resistin | 152.79 ± 11.04 | 1237.69 ± 38.04 | 0.001 |
| SDF-1a | 161.28 ± 5.99 | 325.65 ± 14.87 | 0.001 |
| VEGF | 253.17 ± 6.92 | 332.56 ± 6.53 | 0.001 |
| VEGF R3 | <20 (LOD) | 410.16 ± 105.46 | 0.01 |
| VEGF-D | 1.67 ± 0.57 | 39.8 ± 3.04 | 0.001 |
| Biomarker | Control Mice (n = 5) | Anaphylactic Mice (n = 5) | Student’s t-Test p < |
|---|---|---|---|
| ALK-1 | 270.55 ± 10.48 | 134.18 ± 9.6 | 0.001 |
| bFGF | 3828.16 ± 43.65 | 3255.51 ± 24.39 | 0.001 |
| BLC | 5639.31 ± 124.83 | 4678.57 ± 44.06 | 0.001 |
| CD40 | 4686.52 ± 208.06 | 3714.48 ± 242.08 | 0.05 |
| CD40L | 3709.09 ± 69.08 | 2050.96 ± 74.63 | 0.001 |
| CTLA4 | 887.48 ± 10.02 | 628.25 ± 9.38 | 0.001 |
| Decorin | 14,941.56 ± 302.52 | 13,997.32 ± 180.47 | 0.05 |
| Dkk-1 | 684.12 ± 27.13 | 521.43 ± 47.91 | 0.05 |
| Eotaxin | 417.45 ± 3.96 | 184.63 ± 3.05 | 0.001 |
| Fcg RIIB | 6800.55 ± 101.37 | 5598.51 ± 122.2 | 0.001 |
| Flt-3L | 828.49 ± 8.84 | 559.04 ± 7.96 | 0.001 |
| Galectin-1 | 6404.2 ± 235.85 | 5393.21 ± 164.45 | 0.05 |
| Gas 1 | 1232.06 ± 66.89 | 974.73 ± 30.9 | 0.05 |
| GITR | 9266.56 ± 301.11 | 7734.18 ± 336.51 | 0.05 |
| HGF R | 768.41 ± 125.47 | 394.09 ± 80.25 | 0.05 |
| IGFBP-2 | 408.43 ± 23.57 | 215.84 ± 36.66 | 0.005 |
| IGFBP-3 | 2167.55 ± 64.32 | 1898.07 ± 38.67 | 0.05 |
| IGFBP-6 | 1228.83 ± 77.13 | 541.13 ± 23.11 | 0.001 |
| IL-1b | 14.15 ± 0.7 | 3.29 ± 0.63 | 0.001 |
| IL-1ra | 618.95 ± 24.81 | 410.55 ± 14.05 | 0.001 |
| IL-2 Ra | 810.79 ± 36.47 | 672 ± 26.15 | 0.05 |
| IL-5 | 25.43 ± 3.36 | <6.8 (LOD) | 0.001 |
| IL-12p40 | 12.32 ± 1.19 | 3.51 ± 0.91 | 0.001 |
| IL-17 | 4.14 ± 0.67 | <2.4 (LOD) | 0.05 |
| Leptin | 911.32 ± 76.19 | 626.32 ± 34.19 | 0.05 |
| Leptin R | 136.2 ± 28.35 | 38.75 ± 6.82 | 0.05 |
| LIX | 193.96 ± 2.38 | 149.09 ± 4.5 | 0.001 |
| Lymphotactin | 9155.09 ± 515.92 | 3209.5 ± 663.09 | 0.001 |
| MCP-5 | 144.37 ± 11.22 | 86.37 ± 11.55 | 0.05 |
| MIP-3b | 23.78 ± 1.6 | 2.67 ± 0.78 | 0.001 |
| OPN | 1870.22 ± 44.85 | 1329.38 ± 67.48 | 0.001 |
| Pentraxin 3 | 376.37 ± 66.35 | 25.64 ± 4.36 | 0.005 |
| SCF | 140.35 ± 3.85 | 58.14 ± 5.73 | 0.001 |
| TNF RI | 919.47 ± 30.65 | 720.22 ± 21.12 | 0.005 |
| TNFα | 53.92 ± 7.94 | 17.62 ± 5.1 | 0.01 |
| TWEAK R | 2839.09 ± 90.38 | 224.95 ± 82.17 | 0.001 |
| VEGF R1 | 436 ± 43.67 | 269.85 ± 13.02 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jorgensen, R.; Gao, H.; Arul Arasan, T.S.; Van Antwerp, C.; Sundar, V.; Ng, P.K.W.; Gangur, V. Is Wheat Glutenin Extract Intrinsically Allergenic? Evaluation Using a Novel Adjuvant-Free Mouse Model of Systemic Anaphylaxis. Int. J. Mol. Sci. 2023, 24, 17247. https://doi.org/10.3390/ijms242417247
Jorgensen R, Gao H, Arul Arasan TS, Van Antwerp C, Sundar V, Ng PKW, Gangur V. Is Wheat Glutenin Extract Intrinsically Allergenic? Evaluation Using a Novel Adjuvant-Free Mouse Model of Systemic Anaphylaxis. International Journal of Molecular Sciences. 2023; 24(24):17247. https://doi.org/10.3390/ijms242417247
Chicago/Turabian StyleJorgensen, Rick, Haoran Gao, Tamil Selvan Arul Arasan, Chris Van Antwerp, Vaisheswini Sundar, Perry K. W. Ng, and Venu Gangur. 2023. "Is Wheat Glutenin Extract Intrinsically Allergenic? Evaluation Using a Novel Adjuvant-Free Mouse Model of Systemic Anaphylaxis" International Journal of Molecular Sciences 24, no. 24: 17247. https://doi.org/10.3390/ijms242417247
APA StyleJorgensen, R., Gao, H., Arul Arasan, T. S., Van Antwerp, C., Sundar, V., Ng, P. K. W., & Gangur, V. (2023). Is Wheat Glutenin Extract Intrinsically Allergenic? Evaluation Using a Novel Adjuvant-Free Mouse Model of Systemic Anaphylaxis. International Journal of Molecular Sciences, 24(24), 17247. https://doi.org/10.3390/ijms242417247






