Brassinosteroid Signaling Pathways: Insights into Plant Responses under Abiotic Stress
Abstract
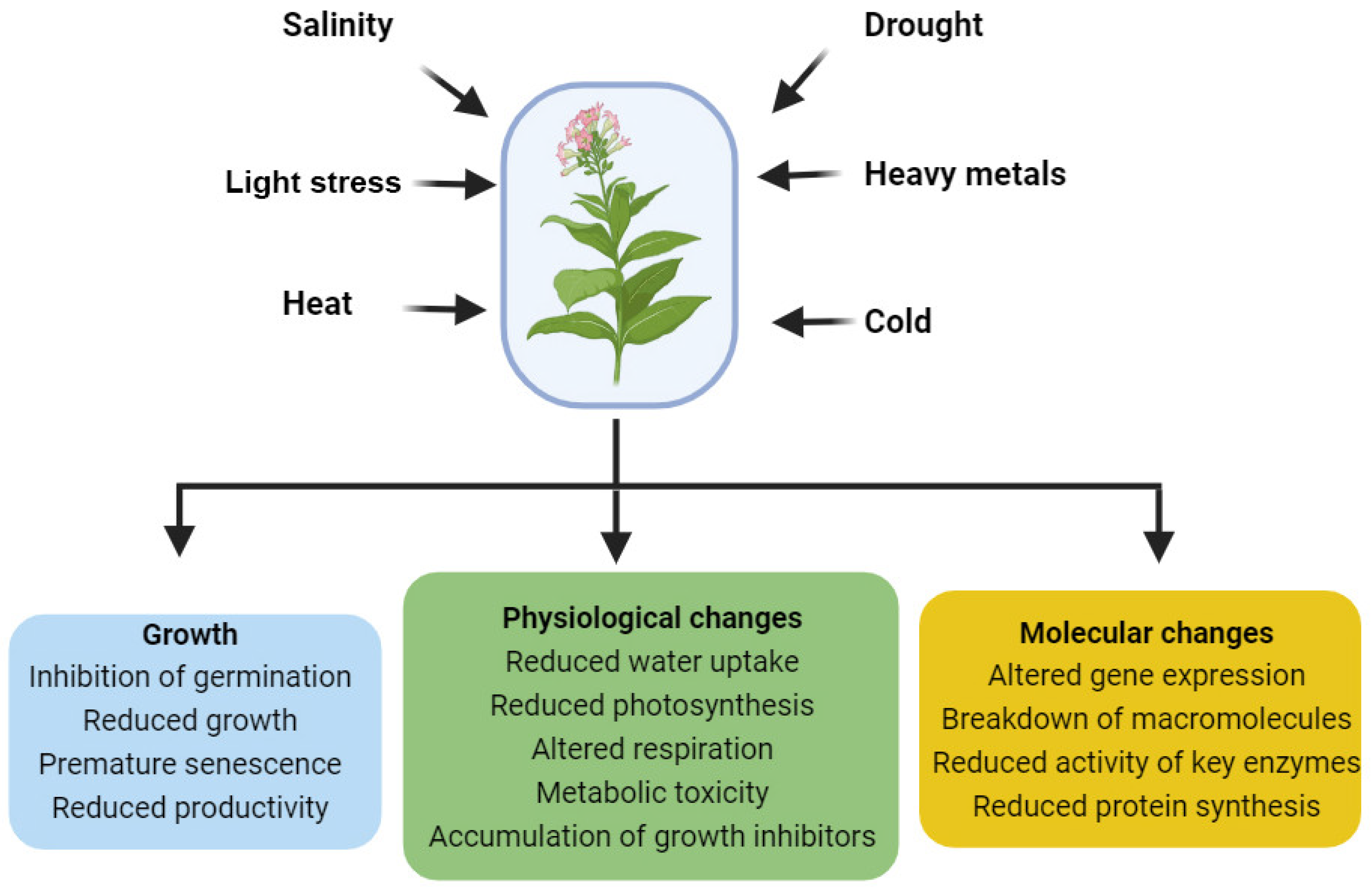
:1. Introduction
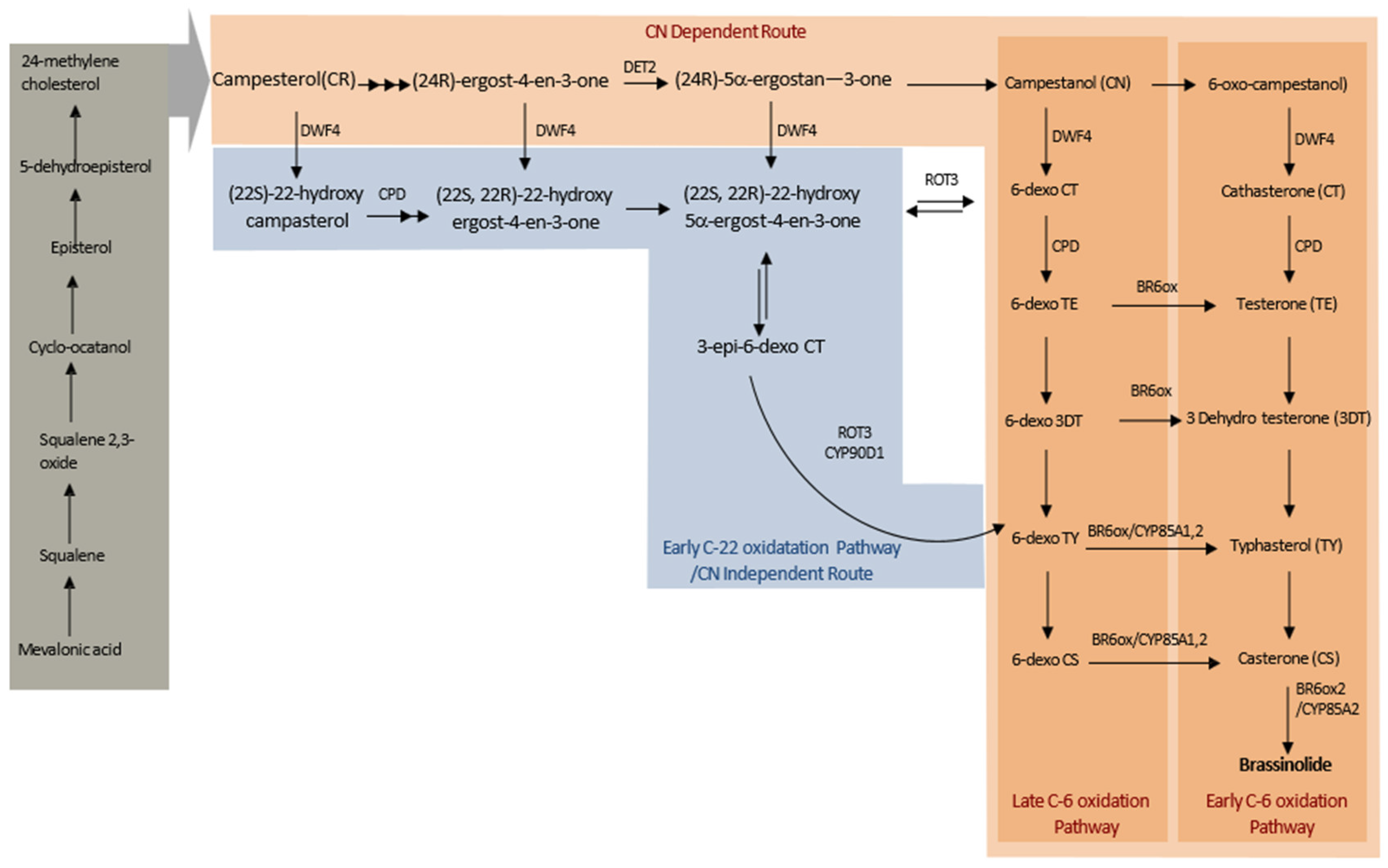
2. Structure and Biosynthesis of BRs
3. Role of BRs in Plant Growth and Development
4. BRs and Redox Homeostasis
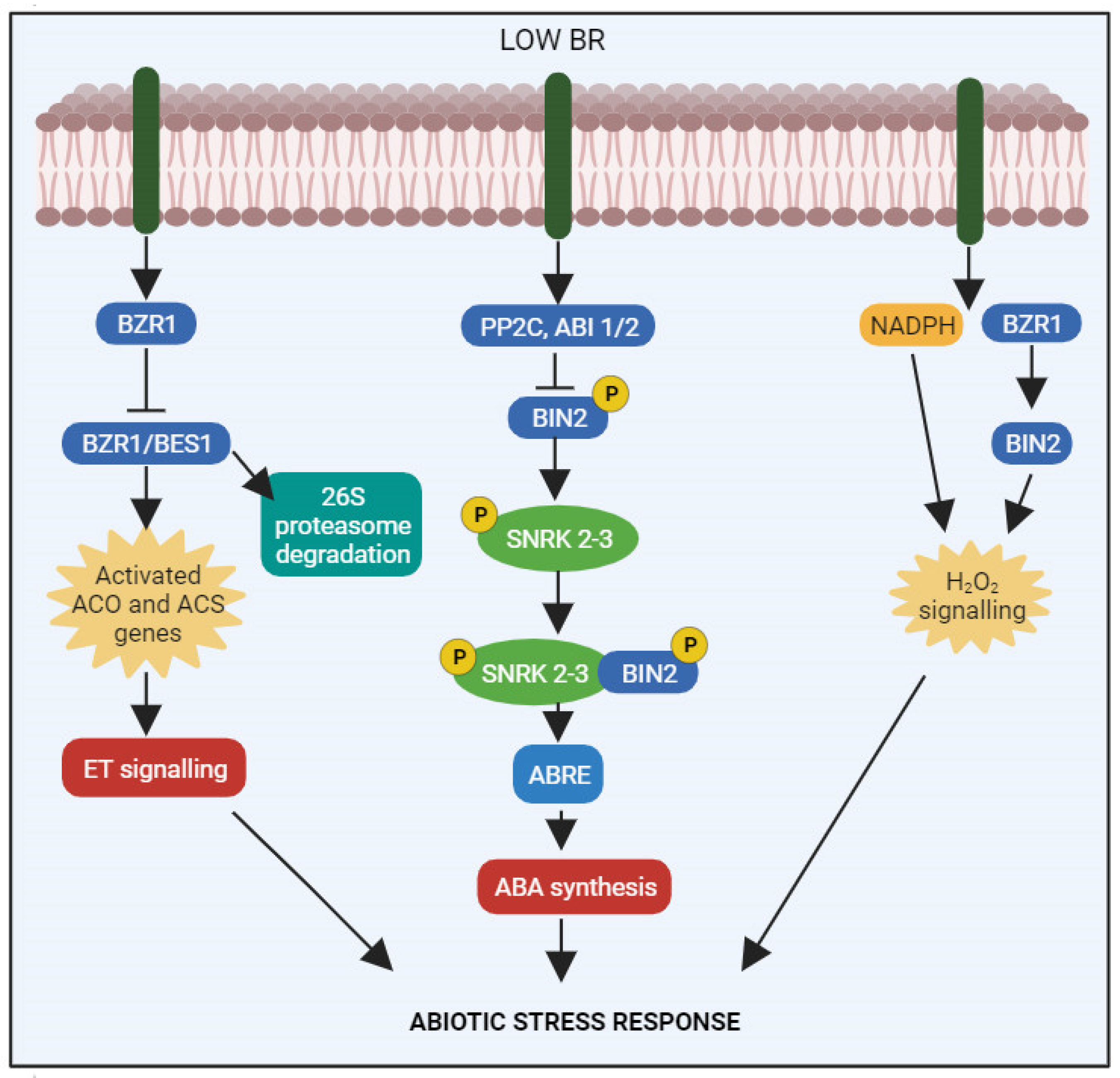
5. BRs as Regulators of Abiotic Stress Responses
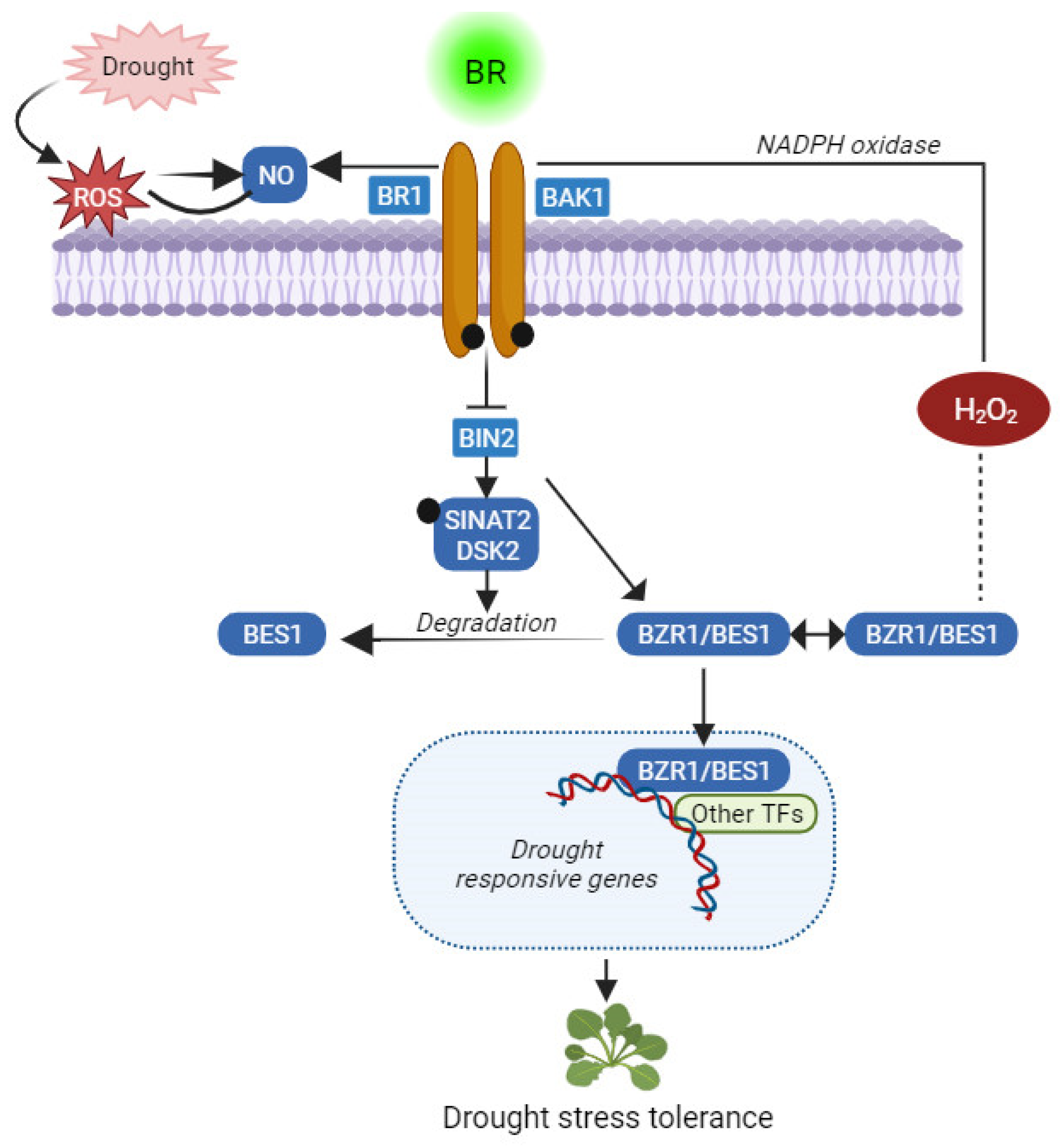
6. BRs and Drought
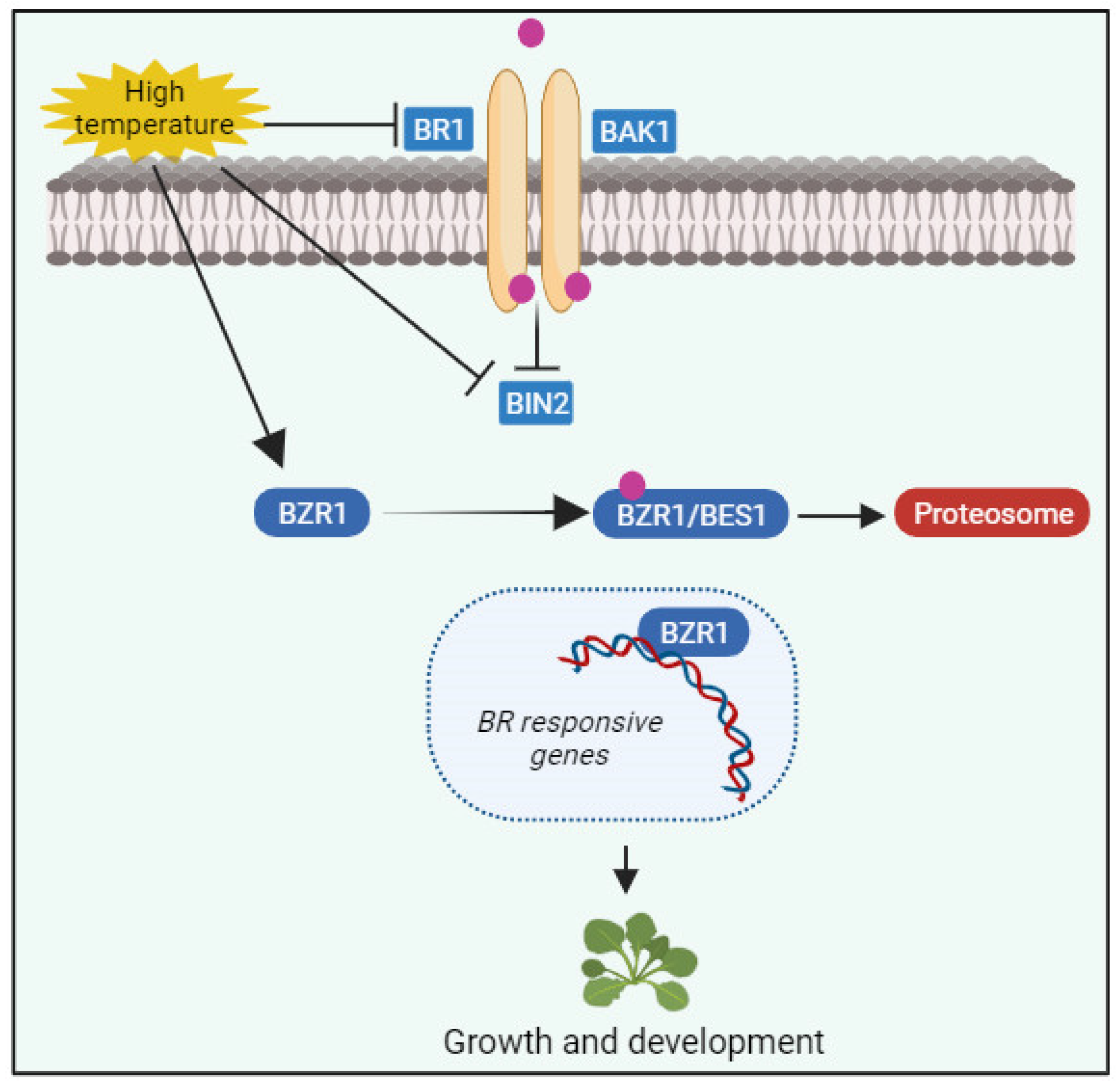
7. BRs in Plant Response to Extreme Temperature Stresses
8. Interaction of BRs with Other Hormonal Pathways
8.1. BRs and Ethylene
8.2. BRs and Hydrogen Peroxide
8.3. BRs and Abscisic Acid
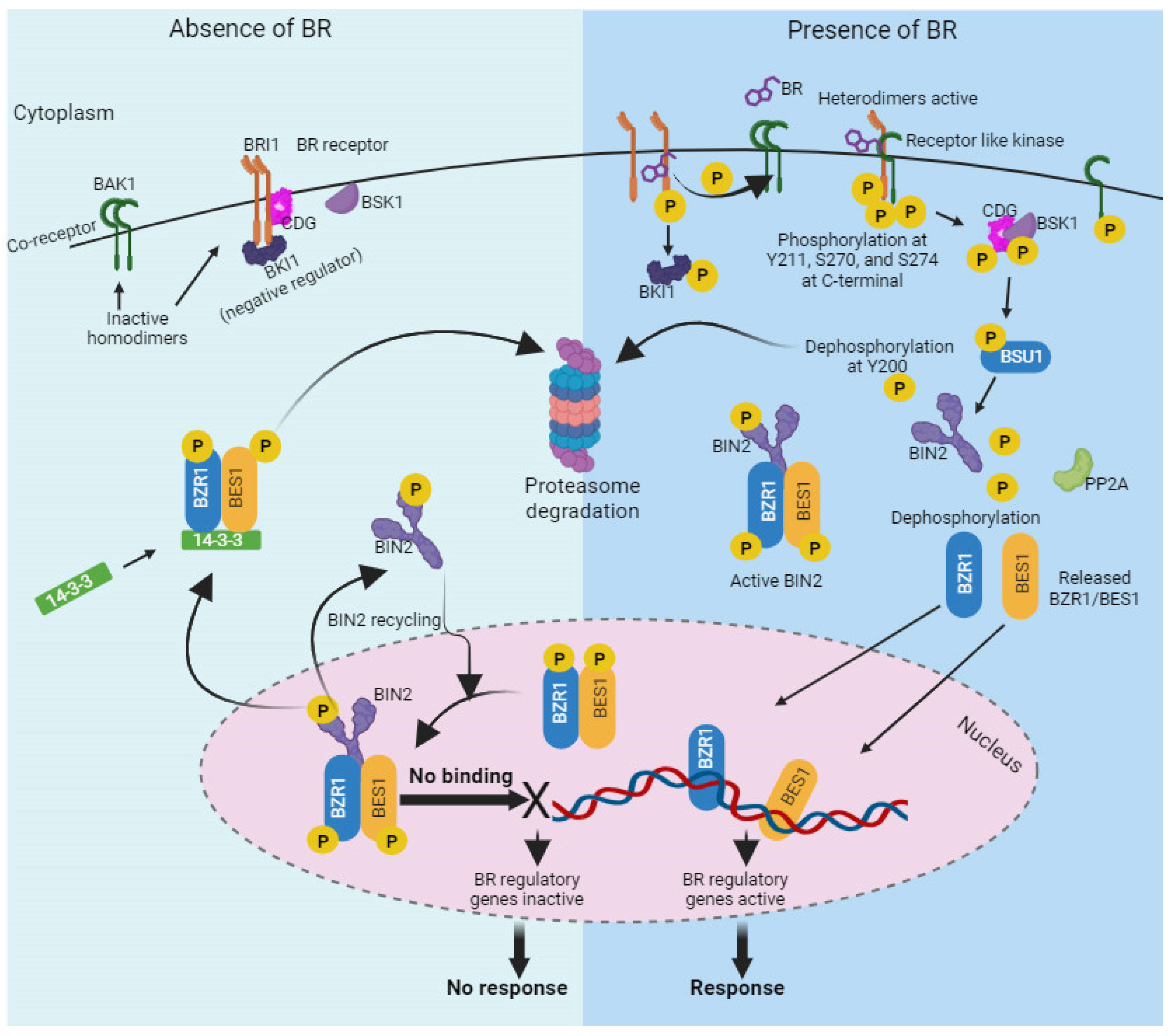
9. BR Signaling Pathway
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kanwar, M.K.; Bhardwaj, R.; Arora, P.; Chowdhary, S.P.; Sharma, P.; Kumar, S. Plant Steroid Hormones Produced under Ni Stress Are Involved in the Regulation of Metal Uptake and Oxidative Stress in Brassica juncea L. Chemosphere 2012, 86, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, B.; Tanveer, M.; Che, Z.; Rehman, A.; Cheema, S.A.; Sharma, A.; Song, H.; ur Rehman, S.; Zhaorong, D. Role of 24-Epibrassinolide (EBL) in Mediating Heavy Metal and Pesticide Induced Oxidative Stress in Plants: A Review. Ecotoxicol. Environ. Saf. 2018, 147, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed Priming with Phytohormones: An Effective Approach for the Mitigation of Abiotic Stress. Plants 2021, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, H.M.D.; Abbas, A.; Ahmad, R. Fascinating Role of Silicon Nanoparticles to Mitigate Adverse Effects of Salinity in Fruit Trees: A Mechanistic Approach. Silicon 2022, 14, 8319–8326. [Google Scholar] [CrossRef]
- Bajguz, A. Brassinosteroids—Occurence and Chemical Structures in Plants. Brassinosteroids A Class Plant Horm. 2011, 1–27. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Choudhary, S.P.; Chen, S.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Role of Brassinosteroids in Alleviation of Phenanthrene–Cadmium Co-Contamination-Induced Photosynthetic Inhibition and Oxidative Stress in Tomato. J. Exp. Bot. 2013, 64, 199–233. [Google Scholar] [CrossRef]
- Basit, F.; Bhat, J.A.; Dong, Z.; Mou, Q.; Zhu, X.; Wang, Y.; Hu, J.; Jan, B.L.; Shakoor, A.; Guan, Y.; et al. Chromium Toxicity Induced Oxidative Damage in Two Rice Cultivars and Its Mitigation through External Supplementation of Brassinosteroids and Spermine. Chemosphere 2022, 302, 134423. [Google Scholar] [CrossRef]
- Yusuf, M.; Saeed, T.; Almenhali, H.A.; Azzam, F.; Hamzah, A.I.A.H.; Khan, T.A. Melatonin Improved Efficiency of 24-Epibrassinolide to Counter the Collective Stress of Drought and Salt through Osmoprotectant and Antioxidant System in Pea Plants. Sci. Hortic. 2023, 323, 112453. [Google Scholar] [CrossRef]
- Anwar, A.; Liu, Y.; Dong, R.; Bai, L.; Yu, X.; Li, Y. The Physiological and Molecular Mechanism of Brassinosteroid in Response to Stress: A Review. Biol. Res. 2018, 51, 46. [Google Scholar] [CrossRef]
- Kapoor, D.; Rattan, A.; Gautam, V.; Kapoor, N.; Bhardwaj, R. 24-Epibrassinolide Mediated Changes in Photosynthetic Pigments and Antioxidative Defence System of Radish Seedlings under Cadmium and Mercury Stress. J. Stress Physiol. Biochem. 2014, 10, 110–121. [Google Scholar]
- Khan, T.A.; Fariduddin, Q.; Yusuf, M. Low-Temperature Stress: Is Phytohormones Application a Remedy? Environ. Sci. Pollut. Res. 2017, 24, 21574–21590. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.A.; Yusuf, M.; Ahmad, A.; Bashir, Z.; Saeed, T.; Fariduddin, Q.; Hayat, S.; Mock, H.-P.; Wu, T. Proteomic and Physiological Assessment of Stress Sensitive and Tolerant Variety of Tomato Treated with Brassinosteroids and Hydrogen Peroxide under Low-Temperature Stress. Food Chem. 2019, 289, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Khan, T.A.; Yusuf, M.; Fariduddin, Q. Silicon-Mediated Role of 24-Epibrassinolide in Wheat under High-Temperature Stress. Environ. Sci. Pollut. Res. 2019, 26, 17163–17172. [Google Scholar] [CrossRef] [PubMed]
- Nazir, F.; Fariduddin, Q.; Hussain, A.; Khan, T.A. Brassinosteroid and Hydrogen Peroxide Improve Photosynthetic Machinery, Stomatal Movement, Root Morphology and Cell Viability and Reduce Cu- Triggered Oxidative Burst in Tomato. Ecotoxicol. Environ. Saf. 2021, 207, 111081. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, S.; Yokota, T. Biosynthesis and Metabolism of Brassinosteroids. Annu. Rev. Plant Biol. 2003, 54, 137–164. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Fujioka, S.; Takatsuto, S.; Tsujimoto, M.; Choe, S. Castasterone Is a Likely End Product of Brassinosteroid Biosynthetic Pathway in Rice. Biochem. Biophys. Res. Commun. 2008, 374, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, J. Regulation of Brassinosteroid Biosynthesis and Inactivation. J. Integr. Plant Biol. 2012, 54, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, S.; Noguchi, T.; Yokota, T.; Takatsuto, S.; Yoshida, S. Brassinosteroids in Arabidopsis Thaliana. Phytochemistry 1998, 48, 595–599. [Google Scholar] [CrossRef]
- Fang, P.; Yan, M.; Chi, C.; Wang, M.; Zhou, Y.; Zhou, J.; Shi, K.; Xia, X.; Foyer, C.H.; Yu, J. Brassinosteroids Act as a Positive Regulator of Photoprotection in Response to Chilling Stress. Plant Physiol. 2019, 180, 2061–2076. [Google Scholar] [CrossRef]
- Lin, W.H. Designed Manipulation of the Brassinosteroid Signal to Enhance Crop Yield. Front. Plant Sci. 2020, 11, 854. [Google Scholar] [CrossRef]
- Lv, B.; Tian, H.; Zhang, F.; Liu, J.; Lu, S.; Bai, M.; Li, C.; Ding, Z. Brassinosteroids Regulate Root Growth by Controlling Reactive Oxygen Species Homeostasis and Dual Effect on Ethylene Synthesis in Arabidopsis. PLoS Genet. 2018, 14, e1007144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, F.; Xie, Q. Balancing Growth and Adaptation to Stress: Crosstalk between Brassinosteroid and Abscisic Acid Signaling. Plant Cell Environ. 2020, 43, 2325–2335. [Google Scholar] [CrossRef] [PubMed]
- Ackerman-Lavert, M.; Savaldi-Goldstein, S. Growth Models from a Brassinosteroid Perspective. Curr. Opin. Plant Biol. 2020, 53, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Makarevitch, I.; Thompson, A.; Muehlbauer, G.J.; Springer, N.M. Brd1 Gene in Maize Encodes a Brassinosteroid C-6 Oxidase. PLoS ONE 2012, 7, e30798. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, Y. Roles of Brassinosteroids in Plant Reproduction. Int. J. Mol. Sci. 2020, 21, 872. [Google Scholar] [CrossRef] [PubMed]
- Jaillais, Y.; Vert, G. Brassinosteroids, Gibberellins and Light-Mediated Signalling Are the Three-Way Controls of Plant Sprouting. Nat. Cell Biol. 2012, 14, 788–790. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liang, W.; Cui, X.; Chen, M.; Yin, C.; Luo, Z.; Zhu, J.; Lucas, W.J.; Wang, Z.; Zhang, D. Brassinosteroids Promote Development of Rice Pollen Grains and Seeds by Triggering Expression of Carbon Starved Anther, a MYB Domain Protein. Plant J. Cell Mol. Biol. 2015, 82, 570–581. [Google Scholar] [CrossRef]
- Jaiswal, S.; Båga, M.; Chibbar, R.N. Brassinosteroid Receptor Mutation Influences Starch Granule Size Distribution in Barley Grains. Plant Physiol. Biochem. 2020, 154, 369–378. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Xie, Z.; Liu, S.; Nolan, T.; Ye, H.; Zhang, M.; Guo, H.; Schnable, P.S.; Li, Z.; et al. Histone Lysine Methyltransferase SDG8 Is Involved in Brassinosteroid-Regulated Gene Expression in Arabidopsis Thaliana. Mol. Plant 2014, 7, 1303–1315. [Google Scholar] [CrossRef]
- Huang, H.Y.; Jiang, W.B.; Hu, Y.W.; Wu, P.; Zhu, J.Y.; Liang, W.Q.; Wang, Z.Y.; Lin, W.H. BR Signal Influences Arabidopsis Ovule and Seed Number through Regulating Related Genes Expression by BZR1. Mol. Plant 2013, 6, 456–469. [Google Scholar] [CrossRef]
- Jia, Z.; Giehl, R.F.H.; Meyer, R.C.; Altmann, T.; von Wirén, N. Natural Variation of BSK3 Tunes Brassinosteroid Signaling to Regulate Root Foraging under Low Nitrogen. Nat. Commun. 2019, 10, 2378. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Ueguchi-Tanaka, M.; Fujioka, S.; Takatsuto, S.; Yoshida, S.; Hasegawa, Y.; Ashikari, M.; Kitano, H.; Matsuoka, M. The Rice Brassinosteroid-Deficient Dwarf2 Mutant, Defective in the Rice Homolog of Arabidopsis DIMINUTO/DWARF1, Is Rescued by the Endogenously Accumulated Alternative Bioactive Brassinosteroid, Dolichosterone. Plant Cell 2005, 17, 2243–2254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhu, K.; Wang, Z.; Zhang, H.; Gu, J.; Liu, L.; Yang, J.; Zhang, J. Brassinosteroids Function in Spikelet Differentiation and Degeneration in Rice. J. Integr. Plant Biol. 2019, 61, 943–963. [Google Scholar] [CrossRef]
- Janeczko, A.; Oklestkova, J.; Novak, O.; Śniegowska-Świerk, K.; Snaczke, Z.; Pociecha, E. Disturbances in Production of Progesterone and Their Implications in Plant Studies. Steroids 2015, 96, 153–163. [Google Scholar] [CrossRef]
- Trevisan, S.; Forestan, C.; Brojanigo, S.; Quaggiotti, S.; Varotto, S. Brassinosteroid Application Affects the Growth and Gravitropic Response of Maize by Regulating Gene Expression in the Roots, Shoots and Leaves. Plant Growth Regul. 2020, 92, 117–130. [Google Scholar] [CrossRef]
- Ye, Q.; Zhu, W.; Li, L.; Zhang, S.; Yin, Y.; Ma, H.; Wang, X. Brassinosteroids Control Male Fertility by Regulating the Expression of Key Genes Involved in Arabidopsis Anther and Pollen Development. Proc. Natl. Acad. Sci. USA 2010, 107, 6100–6105. [Google Scholar] [CrossRef] [PubMed]
- Gudesblat, G.E.; Schneider-Pizoń, J.; Betti, C.; Mayerhofer, J.; Vanhoutte, I.; Van Dongen, W.; Boeren, S.; Zhiponova, M.; De Vries, S.; Jonak, C.; et al. SPEECHLESS Integrates Brassinosteroid and Stomata Signalling Pathways. Nat. Cell Biol. 2012, 14, 548–554. [Google Scholar] [CrossRef]
- Zhu, J.-Y.; Sae-Seaw, J.; Wang, Z.-Y. Brassinosteroid Signalling. Development 2013, 140, 1615–1620. [Google Scholar] [CrossRef]
- Rozhon, W.; Akter, S.; Fernandez, A.; Poppenberger, B. Inhibitors of Brassinosteroid Biosynthesis and Signal Transduction. Molecules 2019, 24, 4372. [Google Scholar] [CrossRef]
- Jiang, Y.; Cheng, F.; Zhou, Y.; Xia, X.; Mao, W.; Shi, K.; Chen, Z.; Yu, J. Cellular Glutathione Redox Homeostasis Plays an Important Role in the Brassinosteroid-induced Increase in CO2 Assimilation in Cucumis Sativus. New Phytol. 2012, 194, 932–943. [Google Scholar] [CrossRef]
- Della Rovere, F.; Piacentini, D.; Fattorini, L.; Girardi, N.; Bellanima, D.; Falasca, G.; Altamura, M.M.; Betti, C. Brassinosteroids Mitigate Cadmium Effects in Arabidopsis Root System without Any Cooperation with Nitric Oxide. Int. J. Mol. Sci. 2022, 23, 825. [Google Scholar] [CrossRef] [PubMed]
- Saeed, T.; Khan, T.A.; Ahmad, A.; Yusuf, M.; Kappachery, S.; Fariduddin, Q.; Mudgal, G.; Gururani, M.A. Exploring the Effects of Selenium and Brassinosteroids on Photosynthesis and Protein Expression Patterns in Tomato Plants under Low Temperatures. Plants 2023, 12, 3351. [Google Scholar] [CrossRef] [PubMed]
- Gillani, S.F.A.; Zhuang, Z.; Rasheed, A.; Haq, I.U.; Abbasi, A.; Ahmed, S.; Wang, Y.; Khan, M.T.; Sardar, R.; Peng, Y. Brassinosteroids Induced Drought Resistance of Contrasting Drought-Responsive Genotypes of Maize at Physiological and Transcriptomic Levels. Front. Plant Sci. 2022, 13, 961680. [Google Scholar] [CrossRef] [PubMed]
- Mahesh, K.; Balaraju, P.; Ramakrishna, B.; Ram Rao, S.S. Effect of Brassinosteroids on Germination and Seedling Growth of Radish (Raphanus Sativus L.) under PEG-6000 Induced Water Stress. Am. J. Plant Sci. 2013, 04, 2305–2313. [Google Scholar] [CrossRef]
- Mumtaz, M.A.; Hao, Y.; Mehmood, S.; Shu, H.; Zhou, Y.; Jin, W.; Chen, C.; Li, L.; Altaf, M.A.; Wang, Z. Physiological and Transcriptomic Analysis Provide Molecular Insight into 24-Epibrassinolide Mediated Cr(VI)-Toxicity Tolerance in Pepper Plants. Environ. Pollut. 2022, 306, 119375. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, M.K.; Bhardwaj, R.; Chowdhary, S.P.; Arora, P.; Sharma, P.; Kumar, S. Isolation and Characterization of 24-Epibrassinolide from Brassica juncea L. and Its Effects on Growth, Ni Ion Uptake, Antioxidant Defense of Brassica Plants and in Vitro Cytotoxicity. Acta Physiol. Plant. 2013, 35, 1351–1362. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Khalil, R.R.A.E.; Mir, B.A.; Yusuf, M.; Ahmad, A. 24-Epibrassinolide Regulates Photosynthesis, Antioxidant Enzyme Activities and Proline Content of Cucumis Sativus under Salt and/or Copper Stress. Environ. Monit. Assess. 2013, 185, 7845–7856. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ashraf, M.; Wijaya, L.; Ahmad, P. The Putative Role of Endogenous Nitric Oxide in Brassinosteroid-Induced Antioxidant Defence System in Pepper (Capsicum annuum L.) Plants under Water Stress. Plant Physiol. Biochem. PPB 2019, 143, 119–128. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. The Role of Nitrate Reductase in Brassinosteroid-Induced Endogenous Nitric Oxide Generation to Improve Cadmium Stress Tolerance of Pepper Plants by Upregulating the Ascorbate-Glutathione Cycle. Ecotoxicol. Environ. Saf. 2020, 196, 110483. [Google Scholar] [CrossRef]
- Khamsuk, O.; Sonjaroon, W.; Suwanwong, S.; Jutamanee, K.; Suksamrarn, A. Effects of 24-Epibrassinolide and the Synthetic Brassinosteroid Mimic on Chili Pepper under Drought. Acta Physiol. Plant. 2018, 40, 106. [Google Scholar] [CrossRef]
- Nawaz, F.; Naeem, M.; Zulfiqar, B.; Akram, A.; Ashraf, M.Y.; Raheel, M.; Shabbir, R.N.; Hussain, R.A.; Anwar, I.; Aurangzaib, M. Understanding Brassinosteroid-Regulated Mechanisms to Improve Stress Tolerance in Plants: A Critical Review. Environ. Sci. Pollut. Res. Int. 2017, 24, 15959–15975. [Google Scholar] [CrossRef] [PubMed]
- Sirhindi, G.; Kaur, H.; Bhardwaj, R.; Sharma, P.; Mushtaq, R. 28-Homobrassinolide Potential for Oxidative Interface in Brassica Juncea under Temperature Stress. Acta Physiol. Plant. 2017, 39, 228. [Google Scholar] [CrossRef]
- Samancioglu, A.; Kocaçinar, F.; Demirkiran, A.R.; Korkmaz, A. Enhancing Water Stress Tolerance in Pepper at Seedling Stage by 24-Epibrassinolid (EBL) Applications. Acta Hortic. 2016, 1142, 409–416. [Google Scholar] [CrossRef]
- Lv, J.; Zong, X.; Shakeel Ahmad, A.; Wu, X.; Wu, C.; Li, Y.; Wang, S. Alteration in Morpho-Physiological Attributes of Leymus Chinensis (Trin.) Tzvelev by Exogenous Application of Brassinolide under Varying Levels of Drought Stress. Chil. J. Agric. Res. 2020, 80, 61–71. [Google Scholar] [CrossRef]
- Cai, Z.; Liu, J.; Wang, H.; Yang, C.; Chen, Y.; Li, Y.; Pan, S.; Dong, R.; Tang, G.; De Dios Barajas-Lopez, J.; et al. GSK3-like Kinases Positively Modulate Abscisic Acid Signaling through Phosphorylating Subgroup III SnRK2s in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 9651–9656. [Google Scholar] [CrossRef]
- Chung, Y.; Kwon, S.I.; Choe, S. Antagonistic Regulation of Arabidopsis Growth by Brassinosteroids and Abiotic Stresses. Mol. Cells 2014, 37, 795–803. [Google Scholar] [CrossRef]
- Ye, H.; Liu, S.; Tang, B.; Chen, J.; Xie, Z.; Nolan, T.M.; Jiang, H.; Guo, H.; Lin, H.Y.; Li, L.; et al. RD26 Mediates Crosstalk between Drought and Brassinosteroid Signalling Pathways. Nat. Commun. 2017, 8, 14573. [Google Scholar] [CrossRef]
- Nolan, T.M.; Brennan, B.; Yang, M.; Chen, J.; Zhang, M.; Li, Z.; Wang, X.; Bassham, D.C.; Walley, J.; Yin, Y. Selective Autophagy of BES1 Mediated by DSK2 Balances Plant Growth and Survival. Dev. Cell 2017, 41, 33–46.e7. [Google Scholar] [CrossRef]
- Dong, H.; Chen, Q.; Dai, Y.; Hu, W.; Zhang, S.; Huang, X. Genome-Wide Identification of PbrbHLH Family Genes, and Expression Analysis in Response to Drought and Cold Stresses in Pear (Pyrus Bretschneideri). BMC Plant Biol. 2021, 21, 86. [Google Scholar] [CrossRef]
- Ma, M.; Chen, Q.; Dong, H.; Zhang, S.; Huang, X. Genome-Wide Identification and Expression Analysis of the bZIP Transcription Factors, and Functional Analysis in Response to Drought and Cold Stresses in Pear (Pyrus Breschneideri). BMC Plant Biol. 2021, 21, 583. [Google Scholar] [CrossRef]
- Li, Y.H.; Liu, Y.J.; Xu, X.L.; Jin, M.; An, L.Z.; Zhang, H. Effect of 24-Epibrassinolide on Drought Stress-Induced Changes in Chorispora Bungeana. Biol. Plant. 2012, 56, 192–196. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Najafi-Kakavand, S.; Abbas, S.; Shoaib, Y.; Anwar, S.; Sharifi, S.; Lu, G.; Siddique, K.H.M. Role of Phytohormones in Regulating Cold Stress Tolerance: Physiological and Molecular Approaches for Developing Cold-Smart Crop Plants. Plant Stress 2023, 8, 100152. [Google Scholar] [CrossRef]
- Li, T.; Xu, X.; Li, Y.; Wang, H.; Li, Z.; Li, Z. Comparative Transcriptome Analysis Reveals Differential Transcription in Heat-Susceptible and Heat-Tolerant Pepper (Capsicum Annum L.) Cultivars under Heat Stress. J. Plant Biol. 2015, 58, 411–424. [Google Scholar] [CrossRef]
- Zhang, X.D.; Wang, R.P.; Zhang, F.J.; Tao, F.Q.; Li, W.Q. Lipid Profiling and Tolerance to Low-Temperature Stress in Thellungiella Salsuginea in Comparison with Arabidopsis Thaliana. Biol. Plant. 2013, 57, 149–153. [Google Scholar] [CrossRef]
- Neha; Twinkle; Mohapatra, S.; Sirhindi, G.; Dogra, V. Seed Priming with Brassinolides Improves Growth and Reinforces Antioxidative Defenses under Normal and Heat Stress Conditions in Seedlings of Brassica Juncea. Physiol. Plant. 2022, 174, e13814. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, C.; Delker, C.; Martinez, C.; Bürstenbinder, K.; Janitza, P.; Lippmann, R.; Ludwig, W.; Sun, H.; James, G.V.; Klecker, M.; et al. Brassinosteroids Dominate Hormonal Regulation of Plant Thermomorphogenesis via BZR1. Curr. Biol. CB 2018, 28, 303–310. [Google Scholar] [CrossRef]
- Martínez, C.; Espinosa-Ruíz, A.; de Lucas, M.; Bernardo-García, S.; Franco-Zorrilla, J.M.; Prat, S. PIF4-Induced BR Synthesis Is Critical to Diurnal and Thermomorphogenic Growth. EMBO J. 2018, 37, e99552. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Zhu, X.H.; Ding, H.D.; Yang, S.J.; Chen, Y.Y. Foliar Application of 24-Epibrassinolide Alleviates High-Temperature-Induced Inhibition of Photosynthesis in Seedlings of Two Melon Cultivars. Photosynthetica 2013, 51, 341–349. [Google Scholar] [CrossRef]
- Jiroutová, P.; Mikulík, J.; Novák, O.; Strnad, M.; Oklestkova, J. Brassinosteroids Induce Strong, Dose-Dependent Inhibition of Etiolated Pea Seedling Growth Correlated with Ethylene Production. Biomolecules 2019, 9, 849. [Google Scholar] [CrossRef]
- Hu, S.; Liu, L.; Li, S.; Shao, Z.; Meng, F.; Liu, H.; Duan, W.; Liang, D.; Zhu, C.; Xu, T.; et al. Regulation of Fruit Ripening by the Brassinosteroid Biosynthetic Gene SlCYP90B3 via an Ethylene-Dependent Pathway in Tomato. Hortic. Res. 2020, 7, 163. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, X.; Song, X.; Yang, J.; Pang, Y. Genome-Wide Identification and Characterization of APETALA2/Ethylene-Responsive Element Binding Factor Superfamily Genes in Soybean Seed Development. Front. Plant Sci. 2020, 11, 56647. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.G.; Zhu, T.; Zhang, D.W.; Lin, H.H. The Alternative Respiratory Pathway Is Involved in Brassinosteroid-Induced Environmental Stress Tolerance in Nicotiana Benthamiana. J. Exp. Bot. 2015, 66, 6219–6232. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.J.; Deng, X.G.; Zhu, T.; Zheng, T.; Li, P.X.; Wu, J.Q.; Zhang, D.W.; Lin, H.H. Ethylene Is Involved in Brassinosteroids Induced Alternative Respiratory Pathway in Cucumber (Cucumis Sativus L.) Seedlings Response to Abiotic Stress. Front. Plant Sci. 2015, 6, 982. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Deng, X.; Zhou, X.; Zhu, L.; Zou, L.; Li, P.; Zhang, D.; Lin, H. Ethylene and Hydrogen Peroxide Are Involved in Brassinosteroid-Induced Salt Tolerance in Tomato. Sci. Rep. 2016, 6, 35392. [Google Scholar] [CrossRef] [PubMed]
- Cui, F.; Liu, L.; Zhao, Q.; Zhang, Z.; Li, Q.; Lin, B.; Wu, Y.; Tang, S.; Xie, Q. Arabidopsis Ubiquitin Conjugase UBC32 Is an ERAD Component That Functions in Brassinosteroid-Mediated Salt Stress Tolerance. Plant Cell 2012, 24, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Tang, Q.; Hua, X. Arabidopsis Brassinosteroid Mutants Det2-1 and Bin2-1 Display Altered Salt Tolerance. J. Plant Growth Regul. 2010, 29, 44–52. [Google Scholar] [CrossRef]
- Geng, Y.; Wu, R.; Wee, C.W.; Xie, F.; Wei, X.; Chan, P.M.Y.; Tham, C.; Duan, L.; Dinneny, J.R. A Spatio-Temporal Understanding of Growth Regulation during the Salt Stress Response in Arabidopsis. Plant Cell 2013, 25, 2132–2154. [Google Scholar] [CrossRef]
- Hansen, M.; Chae, H.S.; Kieber, J.J. Regulation of ACS Protein Stability by Cytokinin and Brassinosteroid. Plant J. Cell Mol. Biol. 2009, 57, 606–614. [Google Scholar] [CrossRef]
- Guo, Y.F.; Shan, W.; Liang, S.M.; Wu, C.J.; Wei, W.; Chen, J.Y.; Lu, W.J.; Kuang, J.F. MaBZR1/2 Act as Transcriptional Repressors of Ethylene Biosynthetic Genes in Banana Fruit. Physiol. Plant. 2019, 165, 555–568. [Google Scholar] [CrossRef]
- Zhu, T.; Tan, W.R.; Deng, X.G.; Zheng, T.; Zhang, D.W.; Lin, H.H. Effects of Brassinosteroids on Quality Attributes and Ethylene Synthesis in Postharvest Tomato Fruit. Postharvest Biol. Technol. 2015, 100, 196–204. [Google Scholar] [CrossRef]
- Polko, J.K.; Pierik, R.; Van Zanten, M.; Tarkowská, D.; Strnad, M.; Voesenek, L.A.C.J.; Peeters, A.J.M. Ethylene Promotes Hyponastic Growth through Interaction with ROTUNDIFOLIA3/CYP90C1 in Arabidopsis. J. Exp. Bot. 2013, 64, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The Roles of Reactive Oxygen Metabolism in Drought: Not so Cut and Dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef] [PubMed]
- Abd Elhady, S.A.; El-Gawad, H.G.A.; Ibrahim, M.F.M.; Mukherjee, S.; Elkelish, A.; Azab, E.; Gobouri, A.A.; Farag, R.; Ibrahim, H.A.; El-Azm, N.A. Hydrogen Peroxide Supplementation in Irrigation Water Alleviates Drought Stress and Boosts Growth and Productivity of Potato Plants. Sustainability 2021, 13, 899. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Khan, T.A.; Yusuf, M. Hydrogen Peroxide Mediated Tolerance to Copper Stress in the Presence of 28-Homobrassinolide in Vigna Radiata. Acta Physiol. Plant. 2014, 36, 2767–2778. [Google Scholar] [CrossRef]
- Tian, Y.; Fan, M.; Qin, Z.; Lv, H.; Wang, M.; Zhang, Z.; Zhou, W.; Zhao, N.; Li, X.; Han, C.; et al. Hydrogen Peroxide Positively Regulates Brassinosteroid Signaling through Oxidation of the BRASSINAZOLE-RESISTANT1 Transcription Factor. Nat. Commun. 2018, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Guo, S.; Shu, S.; Sun, J.; Tezuka, T. Effects of Proline on Photosynthesis, Root Reactive Oxygen Species (ROS) Metabolism in Two Melon Cultivars (Cucumis Melo L.) under NaCl Stress. Afr. J. Biotechnol. 2011, 10, 18381–18390. [Google Scholar] [CrossRef]
- Fujii, H.; Zhu, J.K. Arabidopsis Mutant Deficient in 3 Abscisic Acid-Activated Protein Kinases Reveals Critical Roles in Growth, Reproduction, and Stress. Proc. Natl. Acad. Sci. USA 2009, 106, 8380–8385. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M.; et al. Abscisic Acid Signaling and Abiotic Stress Tolerance in Plants: A Review on Current Knowledge and Future Prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Kao, C.H. Role of Abscisic Acid in Cadmium Tolerance of Rice (Oryza sativa L.) Seedlings. Plant Cell Environ. 2003, 26, 867–874. [Google Scholar] [CrossRef]
- Fediuc, E.; Lips, S.H.; Erdei, L. O-Acetylserine (Thiol) Lyase Activity in Phragmites and Typha Plants under Cadmium and NaCl Stress Conditions and the Involvement of ABA in the Stress Response. J. Plant Physiol. 2005, 162, 865–872. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, V.P.; Kumar, D.; Chauhan, D.K. Rice Seedlings under Cadmium Stress: Effect of Silicon on Growth, Cadmium Uptake, Oxidative Stress, Antioxidant Capacity and Root and Leaf Structures. Chem. Ecol. 2012, 28, 281–291. [Google Scholar] [CrossRef]
- Zhang, D.P. (Ed.) Abscisic Acid: Metabolism, Transport and Signaling. In Abscisic Acid: Metabolism, Transportand Signaling; Springer: Dutch, The Netherlands, 2014; pp. 1–465. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Bai, M.-Y.; Wu, J.; Zhu, J.-Y.; Wang, H.; Zhang, Z.; Wang, W.; Sun, Y.; Zhao, J.; Sun, X.; et al. Antagonistic HLH/bHLH Transcription Factors Mediate Brassinosteroid Regulation of Cell Elongation and Plant Development in Rice and Arabidopsis. Plant Cell 2009, 21, 3767–3780. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Nanda, S.; Ashraf, M.; Siddiqui, A.R.; Masood, S.; Khaskheli, M.A.; Suleman, M.; Zhu, L.; Zhu, C.; Cao, X.; et al. Interplay Impact of Exogenous Application of Abscisic Acid (ABA) and Brassinosteroids (BRs) in Rice Growth, Physiology, and Resistance under Sodium Chloride Stress. Life 2023, 13, 498. [Google Scholar] [CrossRef] [PubMed]
- Belin, C.; De Franco, P.O.; Bourbousse, C.; Chaignepain, S.; Schmitter, J.M.; Vavasseur, A.; Giraudat, J.; Barbier-Brygoo, H.; Thomine, S. Identification of Features Regulating OST1 Kinase Activity and OST1 Function in Guard Cells. Plant Physiol. 2006, 141, 1316–1327. [Google Scholar] [CrossRef] [PubMed]
- Yunta, C.; Martínez-Ripoll, M.; Zhu, J.K.; Albert, A. The Structure of Arabidopsis Thaliana OST1 Provides Insights into the Kinase Regulation Mechanism in Response to Osmotic Stress. J. Mol. Biol. 2011, 414, 135–144. [Google Scholar] [CrossRef]
- Wolf, S.; van der Does, D.; Ladwig, F.; Sticht, C.; Kolbeck, A.; Schürholz, A.-K.; Augustin, S.; Keinath, N.; Rausch, T.; Greiner, S.; et al. A Receptor-like Protein Mediates the Response to Pectin Modification by Activating Brassinosteroid Signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 15261–15266. [Google Scholar] [CrossRef]
- Li, J.; Chory, J. A Putative Leucine-Rich Repeat Receptor Kinase Involved in Brassinosteroid Signal Transduction. Cell 1997, 90, 929–938. [Google Scholar] [CrossRef]
- Wang, X.; Chory, J. Brassinosteroids Regulate Dissociation of BKI1, a Negative Regulator of BRI1 Signaling, from the Plasma Membrane. Science 2006, 313, 1118–1122. [Google Scholar] [CrossRef]
- Jaillais, Y.; Hothorn, M.; Belkhadir, Y.; Dabi, T.; Nimchuk, Z.L.; Meyerowitz, E.M.; Chory, J. Tyrosine Phosphorylation Controls Brassinosteroid Receptor Activation by Triggering Membrane Release of Its Kinase Inhibitor. Genes Dev. 2011, 25, 232–237. [Google Scholar] [CrossRef]
- Choudhary, S.P.; Oral, H.V.; Bhardwaj, R.; Yu, J.-Q.; Tran, L.-S.P. Interaction of Brassinosteroids and Polyamines Enhances Copper Stress Tolerance in Raphanus Sativus. J. Exp. Bot. 2012, 63, 5659–5675. [Google Scholar] [CrossRef]
- Kim, T.-W.; Wang, Z.-Y. Brassinosteroid Signal Transduction from Receptor Kinases to Transcription Factors. Annu. Rev. Plant Biol. 2010, 61, 681–704. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-M.; Lin, W.-H.; Zhu, S.; Zhu, J.-Y.; Sun, Y.; Fan, X.-Y.; Cheng, M.; Hao, Y.; Oh, E.; Tian, M.; et al. Integration of Light- and Brassinosteroid-Signaling Pathways by a GATA Transcription Factor in Arabidopsis. Dev. Cell 2010, 19, 872–883. [Google Scholar] [CrossRef] [PubMed]
- Gudesblat, G.E.; Russinova, E. Plants Grow on Brassinosteroids. Curr. Opin. Plant Biol. 2011, 14, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Vert, G.; Chory, J. Downstream Nuclear Events in Brassinosteroid Signalling. Nature 2006, 441, 96–100. [Google Scholar] [CrossRef]
| Abiotic Stress | Plant Species | Responses | References |
|---|---|---|---|
| Cd | Arabidopsis thaliana L. | Arabidopsis root system is protected from Cd-induced stress by BRs, as they reverse its harmful morphogenic effects on apices of all root types | [41] |
| Low temperatures | Lycopersicon esculentum L. | BR-mediated enhancement of the photosynthetic apparatus and antioxidant system | [42] |
| Drought | Zea mays L. | BRs increase root and shoot growth as well as chlorophyll content, in addition to compensating for harmful drought-induced changes in maize genotypes | [43] |
| Water stress | Raphanus sativus L. | Enhanced levels of free proline, SOD, CAT, and APX, required to mitigate the repressive effects of water stress on seedlings | [44] |
| Drought and salinity | Pisum sativum L. | Increased CAT, POX, and SOD activity, leading to improved seedling growth | |
| Cu | Lycopersicon esculentum L. | Enhanced photosynthesis-related attributes and antioxidant capacity | |
| Cr | Capsicum annuum L. | EBL possesses distinct regulatory systems for mitigating Cr stress, including interactions between plant hormones, MAPK signaling, and ROS scavenging | [45] |
| High temperatures | Triticum aestivum L. | Increased CAT, POX, and SOD activity, resulting in enhanced seedling growth | [13] |
| Ni | Brassica juncea L. | Increased antioxidant enzyme activity, reducing Ni-related stress | [46] |
| Cu and NaCl | Cucumis sativus L. | Increased CAT, POX, and SOD activity, enhancing growth, carbonic anhydrase activity, and photosynthetic efficiency | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, T.A.; Kappachery, S.; Karumannil, S.; AlHosani, M.; Almansoori, N.; Almansoori, H.; Yusuf, M.; Tran, L.-S.P.; Gururani, M.A. Brassinosteroid Signaling Pathways: Insights into Plant Responses under Abiotic Stress. Int. J. Mol. Sci. 2023, 24, 17246. https://doi.org/10.3390/ijms242417246
Khan TA, Kappachery S, Karumannil S, AlHosani M, Almansoori N, Almansoori H, Yusuf M, Tran L-SP, Gururani MA. Brassinosteroid Signaling Pathways: Insights into Plant Responses under Abiotic Stress. International Journal of Molecular Sciences. 2023; 24(24):17246. https://doi.org/10.3390/ijms242417246
Chicago/Turabian StyleKhan, Tanveer Alam, Sajeesh Kappachery, Sameera Karumannil, Mohamed AlHosani, Nemah Almansoori, Hamda Almansoori, Mohammad Yusuf, Lam-Son Phan Tran, and Mayank Anand Gururani. 2023. "Brassinosteroid Signaling Pathways: Insights into Plant Responses under Abiotic Stress" International Journal of Molecular Sciences 24, no. 24: 17246. https://doi.org/10.3390/ijms242417246
APA StyleKhan, T. A., Kappachery, S., Karumannil, S., AlHosani, M., Almansoori, N., Almansoori, H., Yusuf, M., Tran, L.-S. P., & Gururani, M. A. (2023). Brassinosteroid Signaling Pathways: Insights into Plant Responses under Abiotic Stress. International Journal of Molecular Sciences, 24(24), 17246. https://doi.org/10.3390/ijms242417246








