The Role of Cellular Senescence in Cyclophosphamide-Induced Primary Ovarian Insufficiency
Abstract
:1. Introduction
2. Results
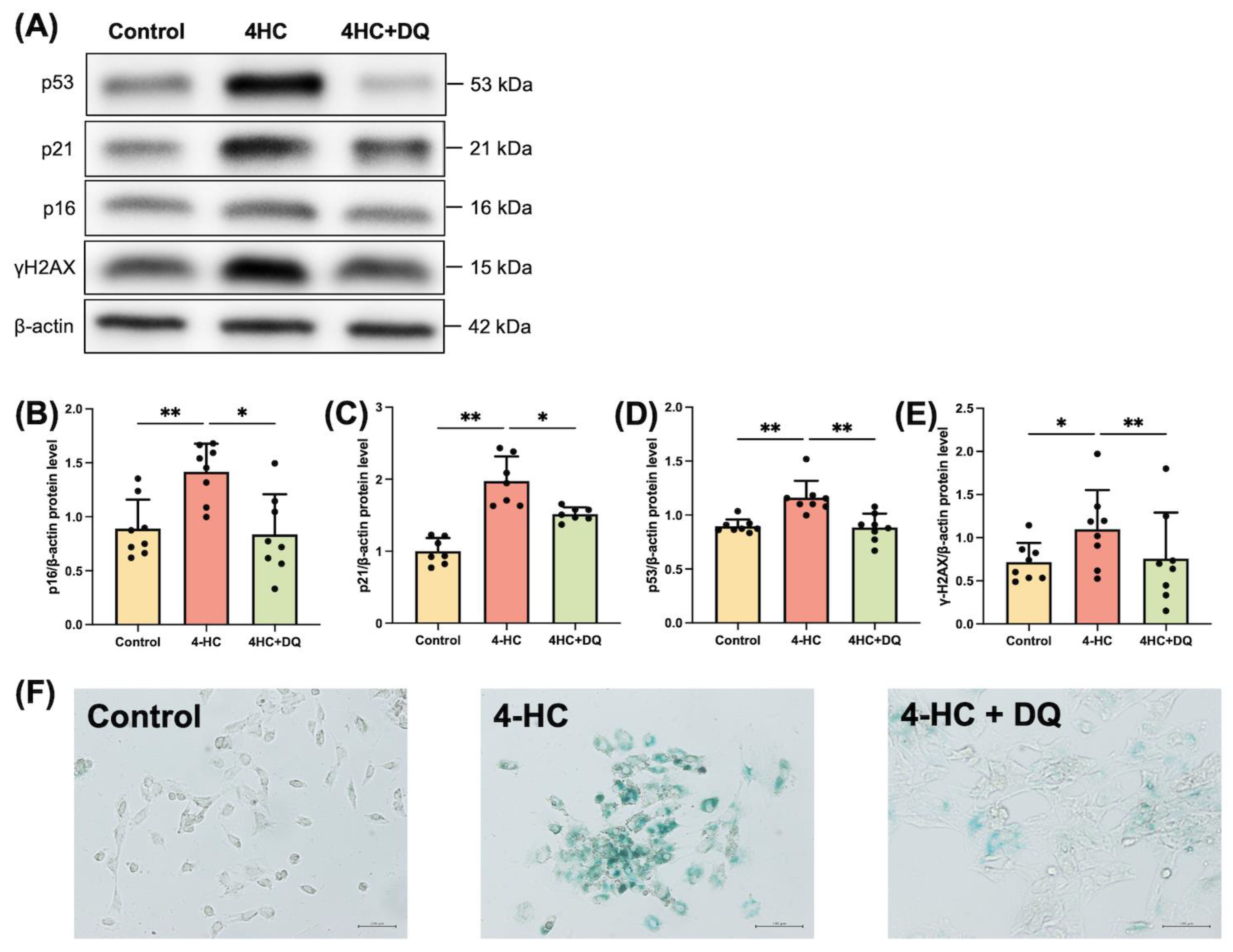
2.1. Cy induces Senescence of Human GLCs
2.2. DQ Alleviate Senescence of Granulosa Cells in POI Model Mice
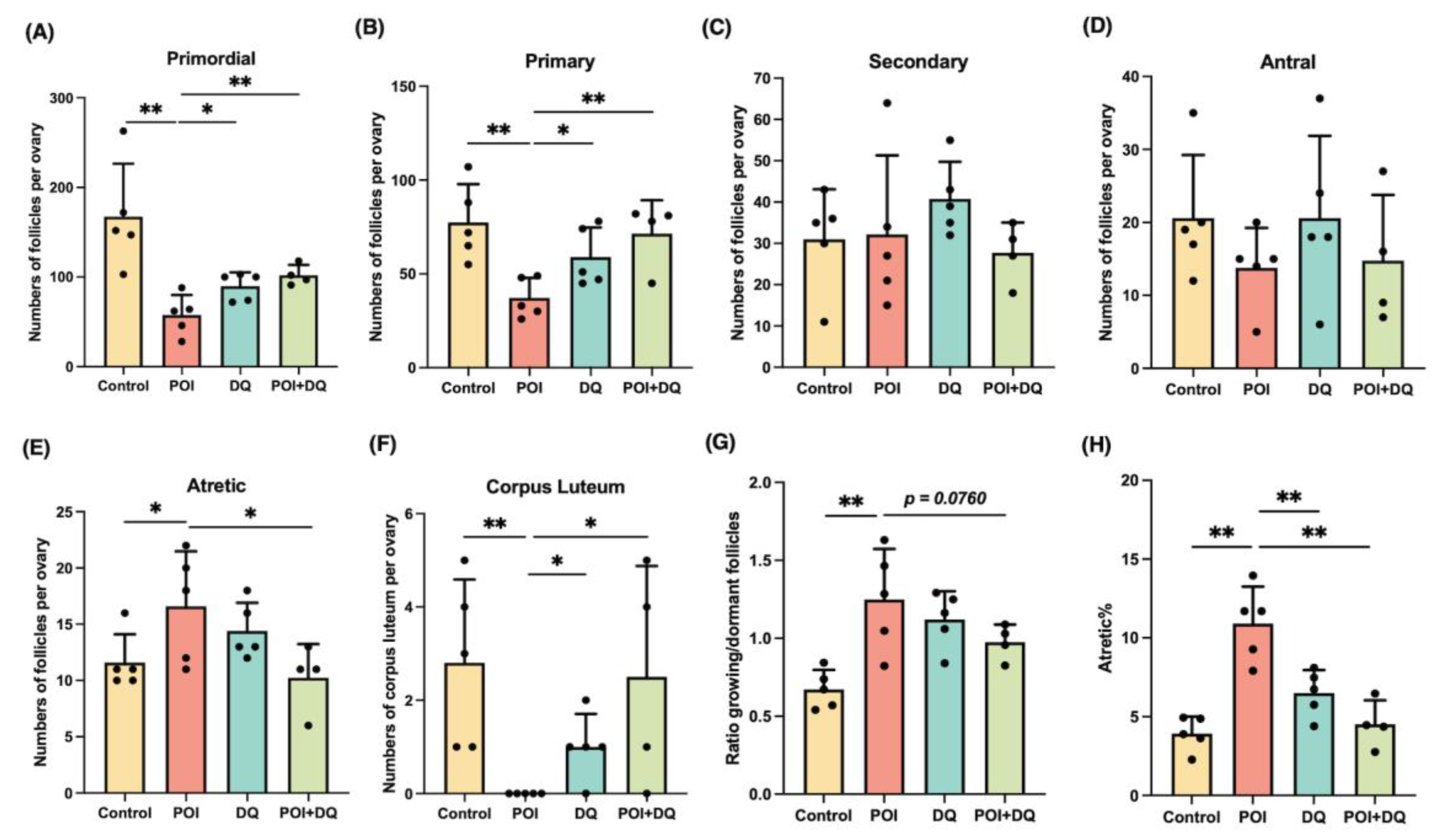
2.3. DQ Partially Recover Burn-Out in POI Model Mice
2.4. DQ Improve Fertility of POI Model Mice
3. Discussion
4. Materials and Methods
4.1. Human Specimens
4.2. Isolation and Culture of Human GLCs
4.3. Treatment of Human GLCs
4.4. POI Animal Model
4.4.1. Ovulation Induction
4.4.2. Mating Test
4.5. Western Blotting
4.6. Histology and Immunohistochemistry
4.7. SA-β-gal Staining
4.8. ELISA
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Cancer Society, Inc. Cancer Facts & Figures 2023; American Cancer Society, Inc.: Atlanta, GA, USA, 2022. [Google Scholar]
- Giaquinto, A.N.; Sung, H.; Miller, K.D.; Kramer, J.L.; Newman, L.A.; Minihan, A.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2022. CA Cancer J. Clin. 2022, 72, 524–541. [Google Scholar] [CrossRef]
- Wallace, W.H.; Kelsey, T.W. Human ovarian reserve from conception to the menopause. PLoS ONE 2010, 5, e8772. [Google Scholar] [CrossRef]
- Meirow, D. Reproduction post-chemotherapy in young cancer patients. Mol. Cell Endocrinol. 2000, 169, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Chon, S.J.; Umair, Z.; Yoon, M.S. Premature Ovarian Insufficiency: Past, Present, and Future. Front. Cell Dev. Biol. 2021, 9, 672890. [Google Scholar] [CrossRef] [PubMed]
- Morgan, S.; Anderson, R.A.; Gourley, C.; Wallace, W.H.; Spears, N. How do chemotherapeutic agents damage the ovary? Hum. Reprod. Update 2012, 18, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Tchkonia, T.; Zhu, Y.; van Deursen, J.; Campisi, J.; Kirkland, J.L. Cellular senescence and the senescent secretory phenotype: Therapeutic opportunities. J. Clin. Investig. 2013, 123, 966–972. [Google Scholar] [CrossRef]
- Khosla, S.; Farr, J.N.; Tchkonia, T.; Kirkland, J.L. The role of cellular senescence in ageing and endocrine disease. Nat. Rev. Endocrinol. 2020, 16, 263–275. [Google Scholar] [CrossRef]
- Davalos, A.R.; Kawahara, M.; Malhotra, G.K.; Schaum, N.; Huang, J.; Ved, U.; Beausejour, C.M.; Coppe, J.P.; Rodier, F.; Campisi, J. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J. Cell Biol. 2013, 201, 613–629. [Google Scholar] [CrossRef]
- Debacq-Chainiaux, F.; Erusalimsky, J.D.; Campisi, J.; Toussaint, O. Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat. Protoc. 2009, 4, 1798–1806. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Cellular Senescence: A Translational Perspective. EBioMedicine 2017, 21, 21–28. [Google Scholar] [CrossRef]
- Tchkonia, T.; Kirkland, J.L. Aging, Cell Senescence, and Chronic Disease: Emerging Therapeutic Strategies. JAMA 2018, 320, 1319–1320. [Google Scholar] [CrossRef] [PubMed]
- Justice, J.N.; Nambiar, A.M.; Tchkonia, T.; LeBrasseur, N.K.; Pascual, R.; Hashmi, S.K.; Prata, L.; Masternak, M.M.; Kritchevsky, S.B.; Musi, N.; et al. Senolytics in idiopathic pulmonary fibrosis: Results from a first-in-human, open-label, pilot study. EBioMedicine 2019, 40, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Wissler Gerdes, E.O.; Misra, A.; Netto, J.M.E.; Tchkonia, T.; Kirkland, J.L. Strategies for late phase preclinical and early clinical trials of senolytics. Mech. Ageing Dev. 2021, 200, 111591. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Tang, X.; Li, Y.; Gao, Y.; Chen, R.; Chen, Q.; Wen, J.; Wu, T.; Zhang, Y.; Lu, H.; et al. Senotherapy Protects against Cisplatin-Induced Ovarian Injury by Removing Senescent Cells and Alleviating DNA Damage. Oxid. Med. Cell Longev. 2022, 2022, 9144644. [Google Scholar] [CrossRef] [PubMed]
- Hense, J.D.; Garcia, D.N.; Isola, J.V.; Alvarado-Rincon, J.A.; Zanini, B.M.; Prosczek, J.B.; Stout, M.B.; Mason, J.B.; Walsh, P.T.; Brieno-Enriquez, M.A.; et al. Senolytic treatment reverses obesity-mediated senescent cell accumulation in the ovary. Geroscience 2022, 44, 1747–1759. [Google Scholar] [CrossRef]
- Palaniyappan, A. Cyclophosphamide induces premature senescence in normal human fibroblasts by activating MAP kinases. Biogerontology 2009, 10, 677–682. [Google Scholar] [CrossRef]
- Pascuali, N.; Scotti, L.; Di Pietro, M.; Oubina, G.; Bas, D.; May, M.; Gomez Munoz, A.; Cuasnicu, P.S.; Cohen, D.J.; Tesone, M.; et al. Ceramide-1-phosphate has protective properties against cyclophosphamide-induced ovarian damage in a mice model of premature ovarian failure. Hum. Reprod. 2018, 33, 844–859. [Google Scholar] [CrossRef]
- Kalich-Philosoph, L.; Roness, H.; Carmely, A.; Fishel-Bartal, M.; Ligumsky, H.; Paglin, S.; Wolf, I.; Kanety, H.; Sredni, B.; Meirow, D. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci. Transl. Med. 2013, 5, 185ra162. [Google Scholar] [CrossRef]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent cells: An emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16, 718–735. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, T.; Wang, S.; Chi, H.; Chen, C.; Zheng, J. Cyclophosphamide promotes the proliferation inhibition of mouse ovarian granulosa cells and premature ovarian failure by activating the lncRNA-Meg3-p53-p66Shc pathway. Gene 2017, 596, 1–8. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T.; Zhu, Y.; Niedernhofer, L.J.; Robbins, P.D. The Clinical Potential of Senolytic Drugs. J. Am. Geriatr. Soc. 2017, 65, 2297–2301. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wu, T.; Tang, X.; Wen, J.; Zhang, Y.; Zhang, J.; Wang, S. Increased cellular senescence in doxorubicin-induced murine ovarian injury: Effect of senolytics. Geroscience 2023, 45, 1775–1790. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, Y.; Shao, L.; Laberge, R.M.; Demaria, M.; Campisi, J.; Janakiraman, K.; Sharpless, N.E.; Ding, S.; Feng, W.; et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat. Med. 2016, 22, 78–83. [Google Scholar] [CrossRef]
- Camell, C.D.; Yousefzadeh, M.J.; Zhu, Y.; Prata, L.; Huggins, M.A.; Pierson, M.; Zhang, L.; O’Kelly, R.D.; Pirtskhalava, T.; Xun, P.; et al. Senolytics reduce coronavirus-related mortality in old mice. Science 2021, 373, eabe4832. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Tchkonia, T.; Pirtskhalava, T.; Gower, A.C.; Ding, H.; Giorgadze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Hickson, L.J.; Langhi Prata, L.G.P.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef]
- Szymanska, K.J.; Tan, X.; Oktay, K. Unraveling the mechanisms of chemotherapy-induced damage to human primordial follicle reserve: Road to developing therapeutics for fertility preservation and reversing ovarian aging. Mol. Hum. Reprod. 2020, 26, 553–566. [Google Scholar] [CrossRef]
- Bayrak, A.; Oktay, K. The expression of cyclin-dependent kinase inhibitors p15, p16, p21, and p27 during ovarian follicle growth initiation in the mouse. Reprod. Biol. Endocrinol. 2003, 1, 41. [Google Scholar] [CrossRef]
- Kusamoto, A.; Harada, M.; Azhary, J.M.K.; Kunitomi, C.; Nose, E.; Koike, H.; Xu, Z.; Urata, Y.; Kaku, T.; Takahashi, N.; et al. Temporal relationship between alterations in the gut microbiome and the development of polycystic ovary syndrome-like phenotypes in prenatally androgenized female mice. FASEB J. 2021, 35, e21971. [Google Scholar] [CrossRef]
- Takahashi, N.; Harada, M.; Hirota, Y.; Zhao, L.; Yoshino, O.; Urata, Y.; Izumi, G.; Takamura, M.; Hirata, T.; Koga, K.; et al. A potential role of endoplasmic reticulum stress in development of ovarian hyperstimulation syndrome. Mol. Cell Endocrinol. 2016, 428, 161–169. [Google Scholar] [CrossRef]
- de Jonge, M.E.; Huitema, A.D.; Rodenhuis, S.; Beijnen, J.H. Clinical pharmacokinetics of cyclophosphamide. Clin. Pharmacokinet. 2005, 44, 1135–1164. [Google Scholar] [CrossRef]
- Lau, S.; Rangarajan, R.; Philidet, C.; Kruger-Genge, A.; Braune, S.; Kammerer, S.; Kupper, J.H.; Lendlein, A.; Jung, F. Effects of acrolein in comparison to its prodrug cyclophosphamide on human primary endothelial cells in vitro. Toxicol. In Vitro 2020, 62, 104685. [Google Scholar] [CrossRef] [PubMed]
- Dynes, J.; Osz, K.; Hooper, A.; Petrik, J. Low-dose metronomic delivery of cyclophosphamide is less detrimental to granulosa cell viability, ovarian function, and fertility than maximum tolerated dose delivery in the mouse. Biol. Reprod. 2017, 97, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Edmonds, M.E.; Woodruff, T.K.; Kim, S.Y. Inhibitors of apoptosis protect the ovarian reserve from cyclophosphamide. J. Endocrinol. 2019, 240, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.; Zhang, L.; Chen, W.; Zhang, Y.; Hua, R.; Wang, W.; Zhang, T.; Wu, H. The protective effects of pretreatment with resveratrol in cyclophosphamide-induced rat ovarian granulosa cell injury: In vitro study. Reprod. Toxicol. 2020, 95, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Kovacovicova, K.; Skolnaja, M.; Heinmaa, M.; Mistrik, M.; Pata, P.; Pata, I.; Bartek, J.; Vinciguerra, M. Senolytic Cocktail Dasatinib+Quercetin (D+Q) Does Not Enhance the Efficacy of Senescence-Inducing Chemotherapy in Liver Cancer. Front. Oncol. 2018, 8, 459. [Google Scholar] [CrossRef]
- Iske, J.; Seyda, M.; Heinbokel, T.; Maenosono, R.; Minami, K.; Nian, Y.; Quante, M.; Falk, C.S.; Azuma, H.; Martin, F.; et al. Senolytics prevent mt-DNA-induced inflammation and promote the survival of aged organs following transplantation. Nat. Commun. 2020, 11, 4289. [Google Scholar] [CrossRef]
- Xu, M.; Pirtskhalava, T.; Farr, J.N.; Weigand, B.M.; Palmer, A.K.; Weivoda, M.M.; Inman, C.L.; Ogrodnik, M.B.; Hachfeld, C.M.; Fraser, D.G.; et al. Senolytics improve physical function and increase lifespan in old age. Nat. Med. 2018, 24, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Koike, H.; Harada, M.; Kusamoto, A.; Kunitomi, C.; Xu, Z.; Tanaka, T.; Urata, Y.; Nose, E.; Takahashi, N.; Wada-Hiraike, O.; et al. Notch Signaling Induced by Endoplasmic Reticulum Stress Regulates Cumulus-Oocyte Complex Expansion in Polycystic Ovary Syndrome. Biomolecules 2022, 12, 1037. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Myers, M.; Britt, K.L.; Wreford, N.G.; Ebling, F.J.; Kerr, J.B. Methods for quantifying follicular numbers within the mouse ovary. Reproduction 2004, 127, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Takahashi, N.; Harada, M.; Kunitomi, C.; Kusamoto, A.; Koike, H.; Tanaka, T.; Sakaguchi, N.; Urata, Y.; Wada-Hiraike, O.; et al. The Role of Cellular Senescence in Cyclophosphamide-Induced Primary Ovarian Insufficiency. Int. J. Mol. Sci. 2023, 24, 17193. https://doi.org/10.3390/ijms242417193
Xu Z, Takahashi N, Harada M, Kunitomi C, Kusamoto A, Koike H, Tanaka T, Sakaguchi N, Urata Y, Wada-Hiraike O, et al. The Role of Cellular Senescence in Cyclophosphamide-Induced Primary Ovarian Insufficiency. International Journal of Molecular Sciences. 2023; 24(24):17193. https://doi.org/10.3390/ijms242417193
Chicago/Turabian StyleXu, Zixin, Nozomi Takahashi, Miyuki Harada, Chisato Kunitomi, Akari Kusamoto, Hiroshi Koike, Tsurugi Tanaka, Nanoka Sakaguchi, Yoko Urata, Osamu Wada-Hiraike, and et al. 2023. "The Role of Cellular Senescence in Cyclophosphamide-Induced Primary Ovarian Insufficiency" International Journal of Molecular Sciences 24, no. 24: 17193. https://doi.org/10.3390/ijms242417193
APA StyleXu, Z., Takahashi, N., Harada, M., Kunitomi, C., Kusamoto, A., Koike, H., Tanaka, T., Sakaguchi, N., Urata, Y., Wada-Hiraike, O., Hirota, Y., & Osuga, Y. (2023). The Role of Cellular Senescence in Cyclophosphamide-Induced Primary Ovarian Insufficiency. International Journal of Molecular Sciences, 24(24), 17193. https://doi.org/10.3390/ijms242417193






