Differential H2O2 Metabolism among Glioblastoma Subtypes Confers Variable Responses to Pharmacological Ascorbate Therapy Combined with Chemoradiation
Abstract
:1. Introduction
2. Results
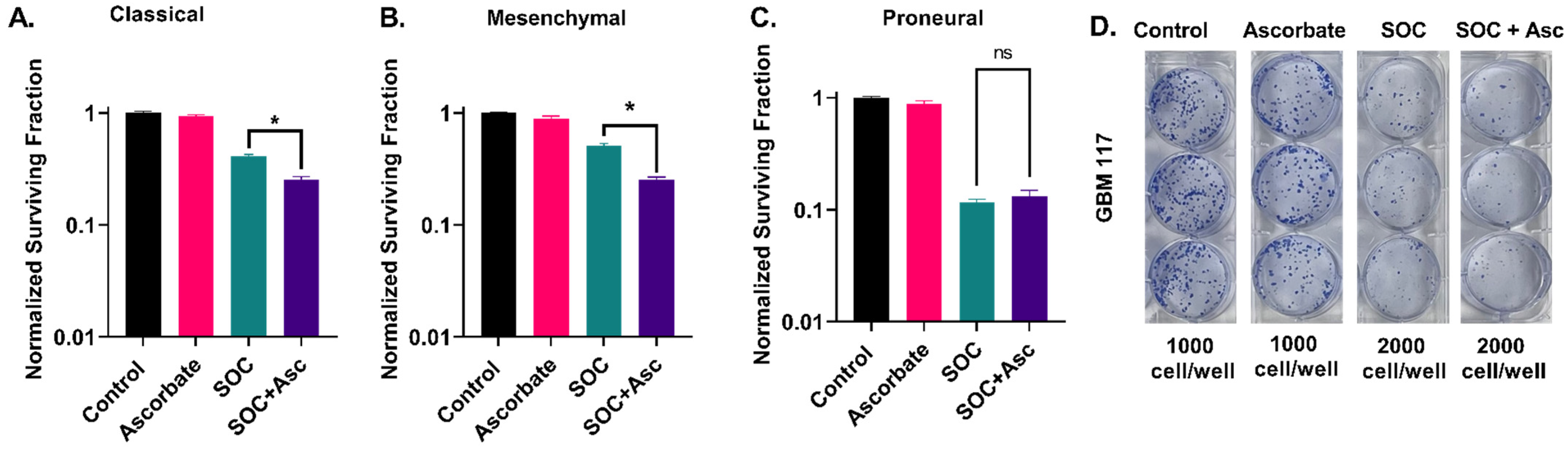
2.1. Glioblastoma Subtypes Exhibit a Differential Response to P-AscH− with SOC
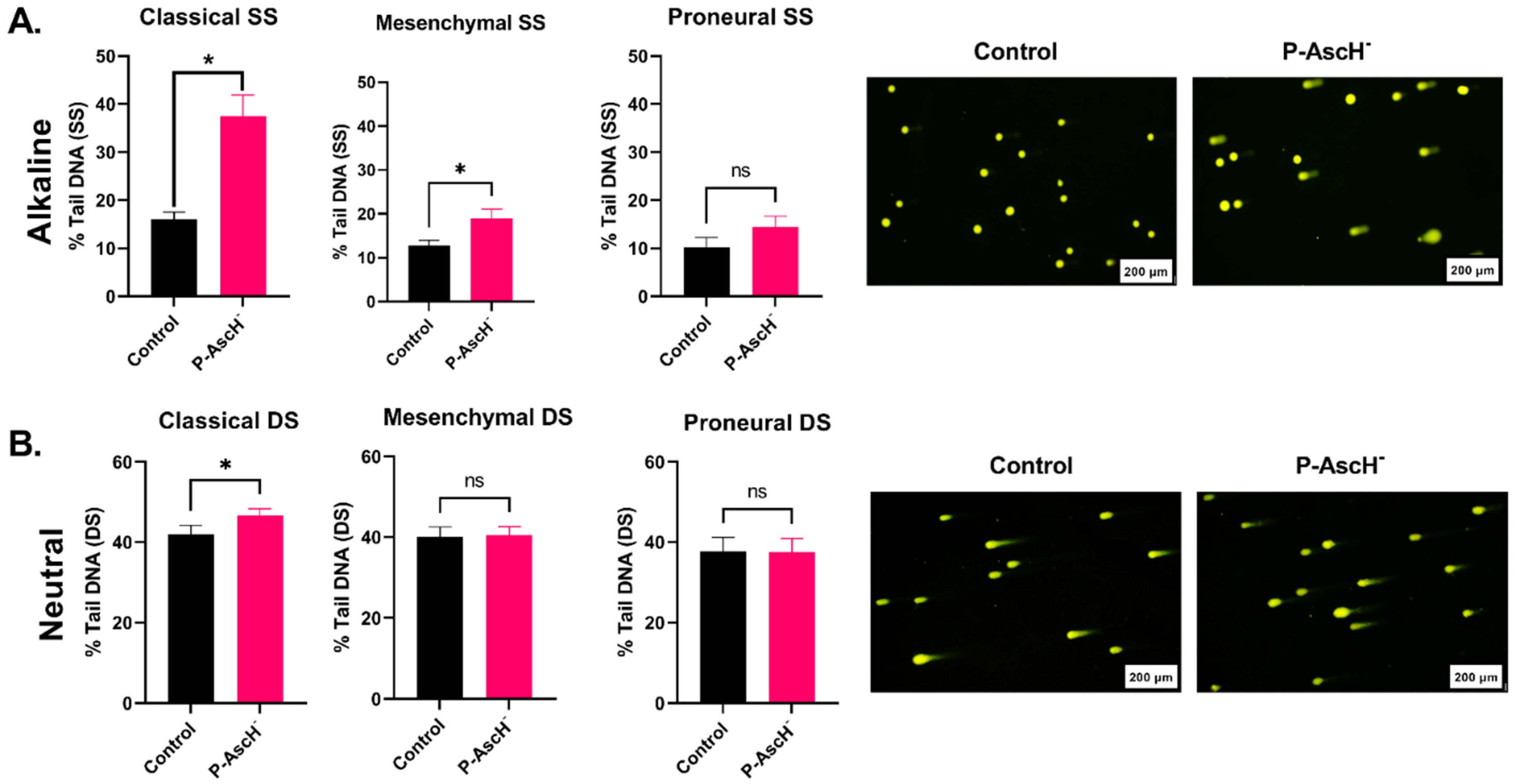
2.2. DNA Damage Responses following P-AscH− Treatments Are Subtype-Dependent
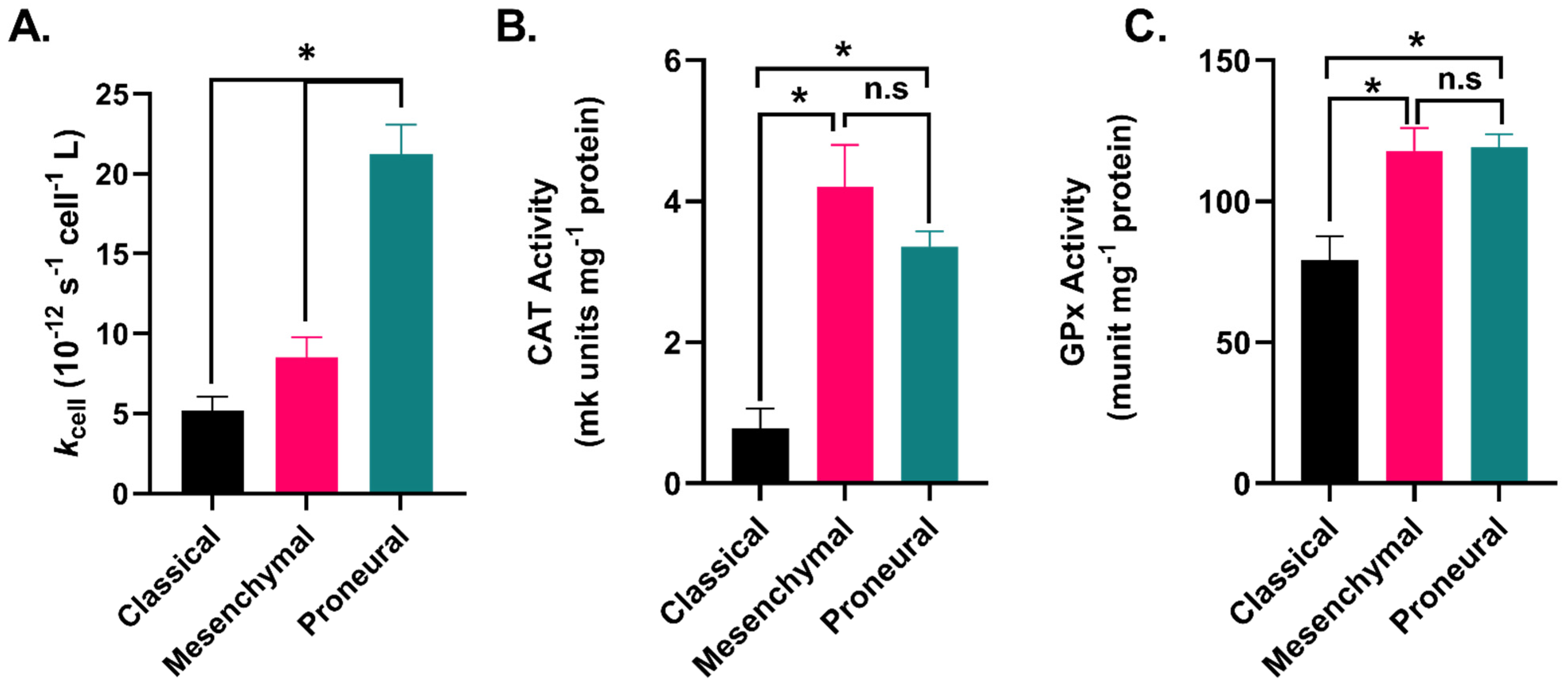
2.3. GBM Subtypes Exhibit Subtype-Dependent Differences in Hydrogen Peroxide Metabolism
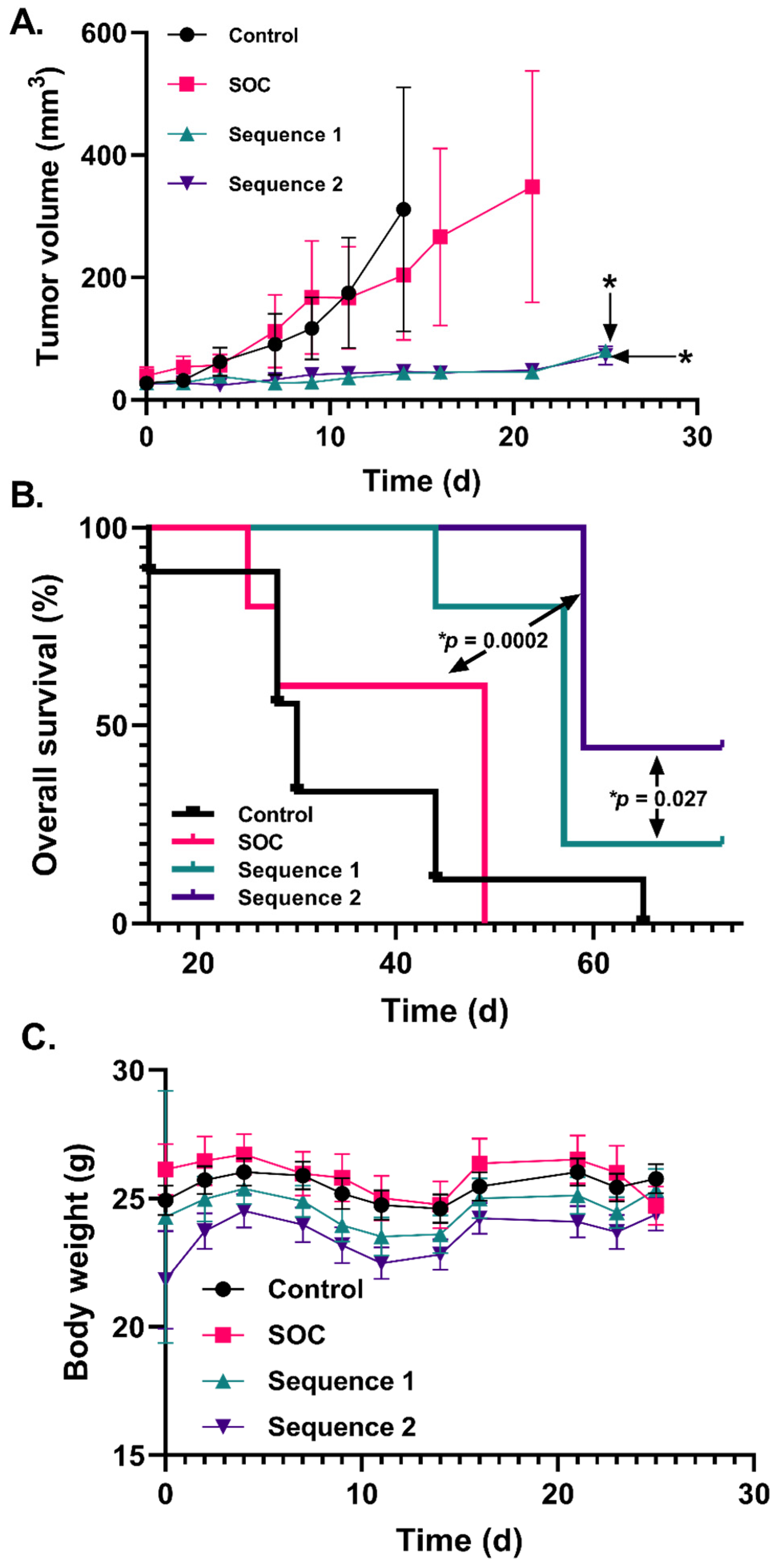
2.4. Increased P-AscH− Dosing Further Improves Overall Survival in a Murine Xenograft Model of Mesenchymal GBM
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Colony Formation Assay
4.3. P-AscH− Treatments
4.4. Neutral and Alkaline Comet Assays
4.5. Hydrogen Peroxide Removal Assay
4.6. Catalase Activity Assay
4.7. Glutathione Peroxidase Activity Assay
4.8. U87 Xenograft Murine Model
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro-Oncol. 2019, 21 (Suppl. S5), v1–v100. [Google Scholar] [CrossRef]
- Wen, P.Y.; Kesari, S. Malignant Gliomas in Adults. N. Engl. J. Med. 2008, 359, 492–507. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncology 2021, 23, iii1–iii105. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, H.; Liang, Q.; Chen, L.; Ren, J. Radiotherapy for glioblastoma: Clinical issues and nanotechnology strategies. Biomater. Sci. 2021, 10, 892–908. [Google Scholar] [CrossRef] [PubMed]
- Aiyappa-Maudsley, R.; Chalmers, A.J.; Parsons, J.L. Factors affecting the radiation response in glioblastoma. Neuro-Oncology Adv. 2022, 4, vdac156. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Quintana, R.; Walker, D.J.; Williams, K.J.; Forster, D.M.; Chalmers, A.J. Radiation-induced neuroinflammation: A potential protective role for poly(ADP-ribose) polymerase inhibitors? Neurooncol. Adv. 2022, 4, vdab190. [Google Scholar] [CrossRef] [PubMed]
- Turnquist, C.; Harris, B.T.; Harris, C.C. Radiation-induced brain injury: Current concepts and therapeutic strategies targeting neuroinflammation. Neuro-Oncology Adv. 2020, 2, vdaa057. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Canney, M.; Carpentier, A.; Idbaih, A. Overcoming the blood brain barrier in glioblastoma: Status and future perspective. Rev. Neurol. 2023, 179, 430–436. [Google Scholar] [CrossRef]
- Ou, A.; Yung, W.K.A.; Majd, N. Molecular Mechanisms of Treatment Resistance in Glioblastoma. Int. J. Mol. Sci. 2020, 22, 351. [Google Scholar] [CrossRef]
- Lam, M.S.; Aw, J.J.; Tan, D.; Vijayakumar, R.; Lim, H.Y.G.; Yada, S.; Pang, Q.Y.; Barker, N.; Tang, C.; Ang, B.T.; et al. Unveiling the Influence of Tumor Microenvironment and Spatial Heterogeneity on Temozolomide Resistance in Glioblastoma Using an Advanced Human In Vitro Model of the Blood-Brain Barrier and Glioblastoma. Small 2023, e2302280. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Pooput, C.; Kirk, K.L.; Krishna, M.C.; Khosh, D.B.; Drisko, J.; Levine, M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 11105–11109. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, J.D.; Alexander, M.S.; Waldron, T.J.; Sibenaller, Z.A.; Spitz, D.R.; Buettner, G.R.; Allen, B.G.; Cullen, J.J. Pharmacological Ascorbate as a Means of Sensitizing Cancer Cells to Radio-Chemotherapy While Protecting Normal Tissue. Semin. Radiat. Oncol. 2018, 29, 25–32. [Google Scholar] [CrossRef]
- Alexander, M.S.; Wilkes, J.G.; Schroeder, S.R.; Buettner, G.R.; Wagner, B.A.; Du, J.; Gibson-Corley, K.; O’leary, B.R.; Spitz, D.R.; Buatti, J.M.; et al. Pharmacologic Ascorbate Reduces Radiation-Induced Normal Tissue Toxicity and Enhances Tumor Radiosensitization in Pancreatic Cancer. Cancer Res. 2018, 78, 6838–6851. [Google Scholar] [CrossRef] [PubMed]
- Zaher, A.; Stephens, L.M.; Miller, A.M.; Hartwig, S.M.; Stolwijk, J.M.; Petronek, M.S.; Zacharias, Z.R.; Wadas, T.J.; Monga, V.; Cullen, J.J.; et al. Pharmacological ascorbate as a novel therapeutic strategy to enhance cancer immunotherapy. Front. Immunol. 2022, 13, 989000. [Google Scholar] [CrossRef]
- Schoenfeld, J.D.; Sibenaller, Z.A.; Mapuskar, K.A.; Wagner, B.A.; Cramer-Morales, K.L.; Furqan, M.; Sandhu, S.; Carlisle, T.L.; Smith, M.C.; Abu Hejleh, T.; et al. O2⋅− and H2O2-Mediated Disruption of Fe Metabolism Causes the Differential Susceptibility of NSCLC and GBM Cancer Cells to Pharmacological Ascorbate. Cancer Cell 2017, 31, 487–500.e8. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef]
- Petronek, M.; Stolwijk, J.; Murray, S.; Steinbach, E.; Zakharia, Y.; Buettner, G.; Spitz, D.; Allen, B. Utilization of redox modulating small molecules that selectively act as pro-oxidants in cancer cells to open a therapeutic window for improving cancer therapy. Redox Biol. 2021, 42, 101864. [Google Scholar] [CrossRef]
- Petronek, M.S.; Monga, V.; Bodeker, K.L.; Kwofie, M.; Lee, C.-Y.; Mapuskar, K.A.; Stolwijk, J.M.; Zaher, A.; Wagner, B.A.; Smith, M.C.; et al. Magnetic resonance imaging of iron metabolism with T2* mapping predicts an enhanced clinical response to pharmacological ascorbate in patients with GBM. Clin. Cancer Res. 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, R.G.W.; Hoadley, K.A.; Purdom, E.; Wang, V.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Jill, P.; Alexe, G.; et al. Integrated Genomic Analysis Identifies Clinically Relevant Subtypes of Glioblastoma Characterized by Abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, B.; Hu, X.; Kim, H.; Squatrito, M.; Scarpace, L.; deCarvalho, A.C.; Lyu, S.; Li, P.; Li, Y.; et al. Tumor Evolution of Glioma-Intrinsic Gene Expression Subtypes Associates with Immunological Changes in the Microenvironment. Cancer Cell 2017, 32, 42–56.e46. [Google Scholar] [CrossRef] [PubMed]
- Phillips, H.S.; Kharbanda, S.; Chen, R.; Forrest, W.F.; Soriano, R.H.; Wu, T.D.; Misra, A.; Nigro, J.M.; Colman, H.; Soroceanu, L.; et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006, 9, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Henle, E.S.; Linn, S. Formation, Prevention, and Repair of DNA Damage by Iron/Hydrogen Peroxide. J. Biol. Chem. 1997, 272, 19095–19098. [Google Scholar] [CrossRef] [PubMed]
- Duarte, T.L.; Almeida, G.M.; Jones, G.D. Investigation of the role of extracellular H2O2 and transition metal ions in the genotoxic action of ascorbic acid in cell culture models. Toxicol. Lett. 2007, 170, 57–65. [Google Scholar] [CrossRef]
- Du, J.; Cieslak, J.A., 3rd; Welsh, J.L.; Sibenaller, Z.A.; Allen, B.G.; Wagner, B.A.; Kalen, A.L.; Doskey, C.M.; Strother, R.K.; Button, A.M.; et al. Pharmacological Ascorbate Radiosensitizes Pancreatic Cancer. Cancer Res. 2015, 75, 3314–3326. [Google Scholar] [CrossRef] [PubMed]
- Herst, P.M.; Broadley, K.W.R.; Harper, J.L.; McConnell, M.J. Pharmacological concentrations of ascorbate radiosensitize glioblastoma multiforme primary cells by increasing oxidative DNA damage and inhibiting G2/M arrest. Free Radic. Biol. Med. 2012, 52, 1486–1493. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, L.; Wang, S.; Zhao, B.; Lv, H.; Shang, P. Labile iron affects pharmacological ascorbate-induced toxicity in osteosarcoma cell lines. Free Radic. Res. 2020, 54, 385–396. [Google Scholar] [CrossRef]
- Rivière, J.; Ravanat, J.-L.; Wagner, J.R. Ascorbate and H2O2 induced oxidative DNA damage in Jurkat cells. Free Radic. Biol. Med. 2006, 40, 2071–2079. [Google Scholar] [CrossRef]
- Ma, E.; Chen, P.; Wilkins, H.M.; Wang, T.; Swerdlow, R.H.; Chen, Q. Pharmacologic ascorbate induces neuroblastoma cell death by hydrogen peroxide mediated DNA damage and reduction in cancer cell glycolysis. Free Radic. Biol. Med. 2017, 113, 36–47. [Google Scholar] [CrossRef]
- Behnan, J.; Finocchiaro, G.; Hanna, G. The landscape of the mesenchymal signature in brain tumours. Brain 2019, 142, 847–866. [Google Scholar] [CrossRef]
- Fedele, M.; Cerchia, L.; Pegoraro, S.; Sgarra, R.; Manfioletti, G. Proneural-Mesenchymal Transition: Phenotypic Plasticity to Acquire Multitherapy Resistance in Glioblastoma. Int. J. Mol. Sci. 2019, 20, 2746. [Google Scholar] [CrossRef]
- Cannan, W.J.; Pederson, D.S. Mechanisms and Consequences of Double-Strand DNA Break Formation in Chromatin. J. Cell. Physiol. 2015, 231, 3–14. [Google Scholar] [CrossRef]
- Freifelder, D.; Trumbo, B. Matching of single-strand breaks to form double-strand breaks in DNA. Biopolymers 1972, 11, 1116. [Google Scholar] [CrossRef]
- Nordberg, J.; Arnér, E.S. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Petronek, M.; Teferi, N.; Caster, J.; Stolwijk, J.; Zaher, A.; Buatti, J.; Hasan, D.; Wafa, E.; Salem, A.; Gillan, E.; et al. Magnetite nanoparticles as a kinetically favorable source of iron to enhance GBM response to chemoradiosensitization with pharmacological ascorbate. Redox Biol. 2023, 62, 102651. [Google Scholar] [CrossRef]
- Reiber, H.; Ruff, M.; Uhr, M. Ascorbate concentration in human cerebrospinal fluid (CSF) and serum. Intrathecal accumulation and CSF flow rate. Clin. Chim. Acta 1993, 217, 163–173. [Google Scholar] [CrossRef]
- Turner, N.; Farrow, B.; Betrie, A.H.; Finnis, M.E.; Lankadeva, Y.R.; Sharman, J.; Tan, P.; Abdelhamid, Y.A.; Deane, A.M.; Plummer, M.P. Cerebrospinal fluid and plasma ascorbate concentrations following subarachnoid haemorrhage. Crit. Care Resusc. 2023. [Google Scholar] [CrossRef]
- Heinz-Erian, P.; Achmüller, M.; Berger, H.; Bonjour, J.P.; Gasser, R.; Hornig, D.; Nirk, S.; Schneeberger, H. Cerebrospinal fluid and plasma levels of vitamin C in children. Padiatr. Padol. 1985, 20, 49–54. [Google Scholar]
- Kaffes, I.; Szulzewsky, F.; Chen, Z.; Herting, C.J.; Gabanic, B.; Vega, J.E.V.; Shelton, J.; Switchenko, J.M.; Ross, J.L.; McSwain, L.F.; et al. Human Mesenchymal glioblastomas are characterized by an increased immune cell presence compared to Proneural and Classical tumors. OncoImmunology 2019, 8, e1655360. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; Van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Wagner, B.A.; Witmer, J.R.; Erve, T.J.V.; Buettner, G.R. An assay for the rate of removal of extracellular hydrogen peroxide by cells. Redox Biol. 2013, 1, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Doskey, C.M.; Buranasudja, V.; Wagner, B.A.; Wilkes, J.G.; Du, J.; Cullen, J.J.; Buettner, G.R. Tumor cells have decreased ability to metabolize H2O2: Implications for pharmacological ascorbate in cancer therapy. Redox Biol. 2016, 10, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzym. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef]
| Phase | Active Treatment (2 Weeks) | Adjuvant Phase (2 Weeks) | ||||
|---|---|---|---|---|---|---|
| Treatment | IR | TMZ | P-AscH− | IR | TMZ | P-AscH− |
| Control | - | - | - | - | - | - |
| SOC | 2 Gy × 3 weekly. | 2.5 mg kg−1 weekly | - | - | 2.5 mg kg−1 weekly | - |
| Sequence 1 | 2 Gy × 3 weekly. | 2.5 mg kg−1 weekly | 4 g kg−1 × 3 weekly | - | 2.5 mg kg−1 weekly | 4 g kg−1 weekly |
| Sequence 2 | 2 Gy × 3 weekly. | 2.5 mg kg−1 weekly | 4 g kg−1 × 5 weekly | - | 2.5 mg kg−1 weekly | 4 g kg−1 weekly |
| Cell Line | Source | Subtype | Sex | IDH Status | MGMT Methylation |
|---|---|---|---|---|---|
| U251 | Millipore Sigma | Mesenchymal | M | Wild-type | Unmethylated |
| U87 | ATCC | Mesenchymal | M | Wild-type | Unmethylated |
| * GBM06 | Primary tumor | Classical | M | Wild-type | Unmethylated |
| * GBM76 | Recurrent tumor | Classical | M | Wild-type | Methylated |
| * GBM39 | Primary tumor | Mesenchymal | M | Wild-type | Methylated |
| * GBM117 | Primary tumor | Proneural | M | Wild-type | Methylated |
| * GBM85 | Primary tumor | Proneural | M | Wild-type | Methylated |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaher, A.; Mapuskar, K.A.; Sarkaria, J.N.; Spitz, D.R.; Petronek, M.S.; Allen, B.G. Differential H2O2 Metabolism among Glioblastoma Subtypes Confers Variable Responses to Pharmacological Ascorbate Therapy Combined with Chemoradiation. Int. J. Mol. Sci. 2023, 24, 17158. https://doi.org/10.3390/ijms242417158
Zaher A, Mapuskar KA, Sarkaria JN, Spitz DR, Petronek MS, Allen BG. Differential H2O2 Metabolism among Glioblastoma Subtypes Confers Variable Responses to Pharmacological Ascorbate Therapy Combined with Chemoradiation. International Journal of Molecular Sciences. 2023; 24(24):17158. https://doi.org/10.3390/ijms242417158
Chicago/Turabian StyleZaher, Amira, Kranti A. Mapuskar, Jann N. Sarkaria, Douglas R. Spitz, Michael S. Petronek, and Bryan G. Allen. 2023. "Differential H2O2 Metabolism among Glioblastoma Subtypes Confers Variable Responses to Pharmacological Ascorbate Therapy Combined with Chemoradiation" International Journal of Molecular Sciences 24, no. 24: 17158. https://doi.org/10.3390/ijms242417158
APA StyleZaher, A., Mapuskar, K. A., Sarkaria, J. N., Spitz, D. R., Petronek, M. S., & Allen, B. G. (2023). Differential H2O2 Metabolism among Glioblastoma Subtypes Confers Variable Responses to Pharmacological Ascorbate Therapy Combined with Chemoradiation. International Journal of Molecular Sciences, 24(24), 17158. https://doi.org/10.3390/ijms242417158








