New and Old Key Players in Liver Cancer
Abstract
1. Introduction
2. Liver Cancer Microenvironment
2.1. Fibrosis: Hepatic Stellate Cells and Cancer Associated Fibroblasts
2.2. Liver Endothelial Sinusoidal Cells
2.3. Liver Immune Cells
2.4. Platelets
2.5. Hepatic Stem/Progenitor Cells in Liver Cancer
3. Signaling Pathways in HCC
3.1. Tyrosine Kinase Receptors: MET and EGFR
3.2. TGF-β
| Trial ID | Study Title | Target | Intervention | Results | Phase | References |
|---|---|---|---|---|---|---|
| NCT01271504 | E7050 in combination with Sorafenib versus Sorafenib alone as first line therapy in participants with hepatocellular carcinoma (HCC) | MET | Golvatinib Sorafenib |
| 1–2 | [159] |
| NCT00107536 | Lapatinib ditosylate in treating patients with unresectable liver or biliary tract cancer | EGFR | Lapatinib |
| 2 | [191,220] |
| NCT00365391 | Bevacizumab and Erlotinib in treating patients with advanced liver cancer | EGFR | Erlotinib Bevacizumab |
| 2 | [187] |
| NCT00356889 | Bevacizumab and Erlotinib hydrochloride in treating patients with metastatic or unresectable biliary tumors | EGFR | Erlotinib Bevacizumab |
| 2 | [186] |
| NCT00753675 | Vandetanib, Gemcitabine or placebo plus Gemcitabine or Vandetanib monotherapy in advanced biliary tract cancer | EGFR | Vandetanib Gemcitabine |
| 2 | [221] |
| NCT00948935 | Study of Gemcitabine, Irinotecan and Panitumumab in patients with advanced and metastatic biliary tract adenocarcinoma | EGFR | Panitumumab Gemcitabine Irinotecan |
| 2 | [192,222] |
| NCT01093222 | Sorafenib Tosylate and Erlotinib hydrochloride in treating patients with locally advanced, unresectable, or metastatic gallbladder cancer or cholangiocarcinoma (CCA) | EGFR | Erlotinib Sorafenib |
| 2 | [190] |
| NCT02273362 | Erlotinib hydrochloride in preventing liver cancer in patients with cirrhosis of the liver | EGFR | Erlotinib |
| 1–2 | [188,189] |
| NCT01246986 | A Study of LY2157299 in participants with HCC | TGF-β | Galunisertib Sorafenib Ramucirumab |
| 2 | [218,219,223] |
| NCT02178358 | A Study of LY2157299 in participants with advanced HCC | TGF-β | Galunisertib Sorafenib |
| 2 | [216] |
| NCT02423343 | A Study of Galunisertib (LY2157299) in combination with Nivolumab in advanced refractory solid tumors and in recurrent or refractory NSCLC, or HCC | TGF-β | Galunisertib Nivolumab |
| 1–2 | [217] |
3.3. C3G, a New Signaling Player in HCC
4. Liver Cancer Mouse Models
| Genetically engineered mouse models | |||
| Model (Oncogene/TSG) | Altered Pathway | Type of Cancer | References |
| HBV, HCV | Viral model | HCC 13–24 months | [267,268] |
| WNT1, CTNNB1 | Wnt pathway | HCC | [240,248] |
| NOTCH1 | Notch pathway | CCA | [247] |
| P53, myc, E2F | Cell cycle | HCC | [237,245,246] |
| PTEN, PTEN/SMAD4 | PI3K/Akt pathway | HCC, CCA (SMAD4/PTEN) | [243,244] |
| IGF2 | Insulin growth factor pathway | HCC | [242] |
| EGFR, ERBB2 | EGF pathway | HCC, CCA | [172,241] |
| HGFR (met), HGF | HGF signaling | HCC (combination with DEN, b-catenin) | [149,150,239,240,269,270] |
| TGF-α (+Myc), TGF-α/TGF-β | EGFR signaling | HCC | [271,272,273,274] |
| KRAS/HRAS | Ras signaling | HCC, CCA (combination with PTEN) | [237] |
| Chemotoxic agents | |||
| Model (agent) | Mechanism of action | Type of cancer | References |
| DEN/DEN-CCL4 | Genotoxic hepatocarcinogen | 50–90 weeks 100% HCC | [252,257,275,276,277,278] |
| NMOR | Genotoxic | 12 weeks HCC with lung metastasis | [279] |
| DMN | Alkylate DNA and/or promote oxidative stress | Promote HCC | [280,281] |
| 2-AAF | Alkylate DNA and/or promote oxidative stress | Promote HCC | [282] |
| DMBA | Induces Ras mutation | Promote HCC | [253] |
| TAA | Genotoxic | Promote HCC and CCA | [251,252] |
| Furan | Genotoxic | Promote CCA | [256] |
| Dietary models | |||
| Model (diet) | Mechanism of action | Type of cancer | References |
| High nutrient | NASH/NAFLD | more than 80 weeks/20% HCC | [258] |
| MCD | Oxidative DNA damage and chromosomal instability | 30–35 weeks 25–100% HCC | [261,262] |
| CDE | Oxidative DNA damage and chromosomal instability | 30–35 weeks 25–100% HCC | [260] |
| CDAA | Oxidative DNA damage and chromosomal instability | 84 weeks 100% HCC | [259] |
| CDHFD | Oxidative DNA damage and chromosomal instability | 30–35 weeks 100% HCC | [258,264] |
| CDAHFD | Oxidative DNA damage and chromosomal instability | 30–35 weeks 100% HCC | [258,263] |
| Implantation models [264,265,266] | |||
| Model | Comments | Advantages | Disadvantages |
| Heterotopic | Subcutaneous inoculation of human cultured cells | Quick evaluation of tumor growth | No immune response |
| Orthotopic | Liver implantation of human cultured cells | Reproduce TME in immunodeficient mouse | Unable to trigger an immune response |
| Syngeneic | Heterotopic or orthotopic implantation of mouse tumor cells | Reproduce TME and mimic the metastatic behavior of HC in immunocompetent mouse | Differences among human and mouse disease |
| Humanized mouse models | Transplantation of cancer patient tissue directly into immunodeficient mice | Genetic and histological similarities. Identification of treatments | Reflect human disease and allow pharmacological testing |
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular Carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Zhu, Y.J.; Wang, H.Y.; Chen, L. Gender Disparity in Hepatocellular Carcinoma (HCC): Multiple Underlying Mechanisms. Sci. China Life Sci. 2017, 60, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Zucman-Rossi, J.; Pikarsky, E.; Sangro, B.; Schwartz, M.; Sherman, M.; Gores, G. Hepatocellular Carcinoma. Nat. Rev. Dis. Primers 2016, 2, 16018. [Google Scholar] [CrossRef] [PubMed]
- Philips, C.A.; Rajesh, S.; Nair, D.C.; Ahamed, R.; Abduljaleel, J.K.; Augustine, P. Hepatocellular Carcinoma in 2021: An Exhaustive Update. Cureus 2021, 13, e19274. [Google Scholar] [CrossRef] [PubMed]
- Montironi, C.; Castet, F.; Haber, P.K.; Pinyol, R.; Torres-Martin, M.; Torrens, L.; Mesropian, A.; Wang, H.; Puigvehi, M.; Maeda, M.; et al. Inflamed and Non-Inflamed Classes of HCC: A Revised Immunogenomic Classification. Gut 2023, 72, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Ogunwobi, O.O.; Harricharran, T.; Huaman, J.; Galuza, A.; Odumuwagun, O.; Tan, Y.; Ma, G.X.; Nguyen, M.T. Mechanisms of Hepatocellular Carcinoma Progression. World J. Gastroenterol. 2019, 25, 2279–2293. [Google Scholar] [CrossRef]
- Suresh, D.; Srinivas, A.N.; Kumar, D.P. Etiology of Hepatocellular Carcinoma: Special Focus on Fatty Liver Disease. Front. Oncol. 2020, 10, 601710. [Google Scholar] [CrossRef]
- Vetrano, E.; Rinaldi, L.; Mormone, A.; Giorgione, C.; Galiero, R.; Caturano, A.; Nevola, R.; Marfella, R.; Sasso, F.C. Non-Alcoholic Fatty Liver Disease (NAFLD), Type 2 Diabetes, and Non-Viral Hepatocarcinoma: Pathophysiological Mechanisms and New Therapeutic Strategies. Biomedicines 2023, 11, 468. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current Concepts and Future Challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef]
- Eslam, M.; Sanyal, A.J.; George, J.; Sanyal, A.; Neuschwander-Tetri, B.; Tiribelli, C.; Kleiner, D.E.; Brunt, E.; Bugianesi, E.; Yki-Järvinen, H.; et al. MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 2020, 158, 1999–2014.e1. [Google Scholar] [CrossRef]
- Craig, A.J.; von Felden, J.; Garcia-Lezana, T.; Sarcognato, S.; Villanueva, A. Tumour Evolution in Hepatocellular Carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Montal, R.; Sia, D.; Finn, R.S. Molecular Therapies and Precision Medicine for Hepatocellular Carcinoma. Nat. Rev. Clin. Oncol. 2018, 15, 599–616. [Google Scholar] [CrossRef] [PubMed]
- Schulze, K.; Nault, J.C.; Villanueva, A. Genetic Profiling of Hepatocellular Carcinoma Using Next-Generation Sequencing. J. Hepatol. 2016, 65, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Zucman-Rossi, J.; Villanueva, A.; Nault, J.C.; Llovet, J.M. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology 2015, 149, 1226–1239.e4. [Google Scholar] [CrossRef]
- Dimitroulis, D.; Damaskos, C.; Valsami, S.; Davakis, S.; Garmpis, N.; Spartalis, E.; Athanasiou, A.; Moris, D.; Sakellariou, S.; Kykalos, S.; et al. From Diagnosis to Treatment of Hepatocellular Carcinoma: An Epidemic Problem for Both Developed and Developing World. World J. Gastroenterol. 2017, 23, 5282–5294. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, B. Hepatocellular Carcinoma: Molecular Mechanism, Targeted Therapy, and Biomarkers. Cancer Metastasis Rev. 2023, 42, 629–652. [Google Scholar] [CrossRef]
- Santhakumar, C.; Gane, E.J.; Liu, K.; McCaughan, G.W. Current Perspectives on the Tumor Microenvironment in Hepatocellular Carcinoma. Hepatol. Int. 2020, 14, 947–957. [Google Scholar] [CrossRef]
- Gu, Y.; Guo, Y.; Gao, N.; Fang, Y.; Xu, C.; Hu, G.; Guo, M.; Ma, Y.; Zhang, Y.; Zhou, J.; et al. The Proteomic Characterization of the Peritumor Microenvironment in Human Hepatocellular Carcinoma. Oncogene 2022, 41, 2480–2491. [Google Scholar] [CrossRef] [PubMed]
- Dhar, D.; Baglieri, J.; Kisseleva, T.; Brenner, D.A. Mechanisms of Liver Fibrosis and Its Role in Liver Cancer. Exp. Biol. Med. 2020, 245, 96–108. [Google Scholar] [CrossRef]
- Yavuz, B.G.; Pestana, R.C.; Abugabal, Y.I.; Krishnan, S.; Chen, J.; Hassan, M.M.; Wolff, R.A.; Rashid, A.; Amin, H.M.; Kaseb, A.O. Origin and Role of Hepatic Myofibroblasts in Hepatocellular Carcinoma. Oncotarget 2020, 11, 1186–1201. [Google Scholar] [CrossRef] [PubMed]
- Acharya, P.; Chouhan, K.; Weiskirchen, S.; Weiskirchen, R. Cellular Mechanisms of Liver Fibrosis. Front. Pharmacol. 2021, 12, 671640. [Google Scholar] [CrossRef] [PubMed]
- Caja, L.; Dituri, F.; Mancarella, S.; Caballero-Diaz, D.; Moustakas, A.; Giannelli, G.; Fabregat, I. TGF-β and the Tissue Microenvironment: Relevance in Fibrosis and Cancer. Int. J. Mol. Sci. 2018, 19, 1294. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, P.S.; Rigual, M.d.M.; Djouder, N. Inflammatory and Non-Inflammatory Mechanisms Controlling Cirrhosis Development. Cancers 2021, 13, 5045. [Google Scholar] [CrossRef] [PubMed]
- Mederacke, I.; Hsu, C.C.; Troeger, J.S.; Huebener, P.; Mu, X.; Dapito, D.H.; Pradere, J.-P.; Schwabe, R.F. Fate Tracing Reveals Hepatic Stellate Cells as Dominant Contributors to Liver Fibrosis Independent of Its Aetiology. Nat. Commun. 2013, 4, 2823. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Tacke, F. Roles for Chemokines in Liver Disease. Gastroenterology 2014, 147, 577–594.e1. [Google Scholar] [CrossRef]
- Yang, W.; He, H.; Wang, T.; Su, N.; Zhang, F.; Jiang, K.; Zhu, J.; Zhang, C.; Niu, K.; Wang, L.; et al. Single-Cell Transcriptomic Analysis Reveals a Hepatic Stellate Cell–Activation Roadmap and Myofibroblast Origin During Liver Fibrosis in Mice. Hepatology 2021, 74, 2774–2790. [Google Scholar] [CrossRef]
- Affo, S.; Yu, L.-X.; Schwabe, R.F. The Role of Cancer-Associated Fibroblasts and Fibrosis in Liver Cancer. Annu. Rev. Pathol. Mech. Dis. 2017, 12, 153–186. [Google Scholar] [CrossRef]
- Lujambio, A.; Akkari, L.; Simon, J.; Grace, D.; Tschaharganeh, D.F.; Bolden, J.E.; Zhao, Z.; Thapar, V.; Joyce, J.A.; Krizhanovsky, V.; et al. Non-Cell-Autonomous Tumor Suppression by P53. Cell 2013, 153, 449–460. [Google Scholar] [CrossRef]
- Duran, A.; Hernandez, E.D.; Reina-Campos, M.; Castilla, E.A.; Subramaniam, S.; Raghunandan, S.; Roberts, L.R.; Kisseleva, T.; Karin, M.; Diaz-Meco, M.T.; et al. P62/SQSTM1 by Binding to Vitamin D Receptor Inhibits Hepatic Stellate Cell Activity, Fibrosis, and Liver Cancer. Cancer Cell 2016, 30, 595–609. [Google Scholar] [CrossRef]
- Kocabayoglu, P.; Lade, A.; Lee, Y.A.; Dragomir, A.-C.; Sun, X.; Fiel, M.I.; Thung, S.; Aloman, C.; Soriano, P.; Hoshida, Y.; et al. β-PDGF Receptor Expressed by Hepatic Stellate Cells Regulates Fibrosis in Murine Liver Injury, but Not Carcinogenesis. J. Hepatol. 2015, 63, 141–147. [Google Scholar] [CrossRef]
- Filliol, A.; Saito, Y.; Nair, A.; Dapito, D.H.; Yu, L.X.; Ravichandra, A.; Bhattacharjee, S.; Affo, S.; Fujiwara, N.; Su, H.; et al. Opposing Roles of Hepatic Stellate Cell Subpopulations in Hepatocarcinogenesis. Nature 2022, 610, 356–365. [Google Scholar] [CrossRef]
- Wei, J.; Yao, J.; Yang, C.; Mao, Y.; Zhu, D.; Xie, Y.; Liu, P.; Yan, M.; Ren, L.; Lin, Y.; et al. Heterogeneous Matrix Stiffness Regulates the Cancer Stem-like Cell Phenotype in Hepatocellular Carcinoma. J. Transl. Med. 2022, 20, 555. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Mo, H.; Liu, R.; Chen, T.; Yang, N.; Liu, Z. Matrix Stiffness-Induced Upregulation of Histone Acetyltransferase KAT6A Promotes Hepatocellular Carcinoma Progression through Regulating SOX2 Expression. Br. J. Cancer 2022, 127, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Cogliati, B.; Yashaswini, C.N.; Wang, S.; Sia, D.; Friedman, S.L. Friend or Foe? The Elusive Role of Hepatic Stellate Cells in Liver Cancer. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Ezhilarasan, D. Hepatic Stellate Cells in the Injured Liver: Perspectives beyond Hepatic Fibrosis. J. Cell Physiol. 2022, 237, 436–449. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.-C.; Chen, C.-H.; Liang, X.; Wang, L.; Gandhi, C.R.; Fung, J.J.; Lu, L.; Qian, S. Inhibition of T-Cell Responses by Hepatic Stellate Cells via B7-H1-Mediated T-Cell Apoptosis in Mice. Hepatology 2004, 40, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Chinnadurai, R.; Grakoui, A. B7-H4 Mediates Inhibition of T Cell Responses by Activated Murine Hepatic Stellate Cells. Hepatology 2010, 52, 2177–2185. [Google Scholar] [CrossRef]
- Karlmark, K.R.; Zimmermann, H.W.; Roderburg, C.; Gassler, N.; Wasmuth, H.E.; Luedde, T.; Trautwein, C.; Tacke, F. The Fractalkine Receptor CX3CR1 Protects against Liver Fibrosis by Controlling Differentiation and Survival of Infiltrating Hepatic Monocytes. Hepatology 2010, 52, 1769–1782. [Google Scholar] [CrossRef]
- Aoyama, T.; Inokuchi, S.; Brenner, D.A.; Seki, E. CX3CL1-CX3CR1 Interaction Prevents Carbon Tetrachloride-Induced Liver Inflammation and Fibrosis in Mice. Hepatology 2010, 52, 1390–1400. [Google Scholar] [CrossRef]
- Ramachandran, P.; Dobie, R.; Wilson-Kanamori, J.R.; Dora, E.F.; Henderson, B.E.P.; Luu, N.T.; Portman, J.R.; Matchett, K.P.; Brice, M.; Marwick, J.A.; et al. Resolving the Fibrotic Niche of Human Liver Cirrhosis at Single-Cell Level. Nature 2019, 575, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Oura, K.; Morishita, A.; Tani, J.; Masaki, T. Tumor Immune Microenvironment and Immunosuppressive Therapy in Hepatocellular Carcinoma: A Review. Int. J. Mol. Sci. 2021, 22, 5801. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Sun, G.; Zhang, Y.; Kong, X.; Rong, D.; Song, J.; Tang, W.; Wang, X. Targeting Immune Cells in the Tumor Microenvironment of HCC: New Opportunities and Challenges. Front. Cell Dev. Biol. 2021, 9, 775462. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Xiong, S.; Wang, X.; Zhao, Z.; Bai, S.; Wang, Y.; Zhao, Y.; Cheng, B. Cancer-Associated Fibroblasts Promote the Stemness of CD24+ Liver Cells via Paracrine Signaling. J. Mol. Med. 2019, 97, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Wang, R.; Chen, Q.; Luo, J.; Wang, J.; Zhao, Z.; Li, Y.; Wang, Y.; Wang, X.; Cheng, B. Cancer-Associated Fibroblasts Promote Stem Cell-like Properties of Hepatocellular Carcinoma Cells through IL-6/STAT3/Notch Signaling. Am. J. Cancer Res. 2018, 8, 302–316. [Google Scholar] [PubMed]
- Bhattacharjee, S.; Hamberger, F.; Ravichandra, A.; Miller, M.; Nair, A.; Affo, S.; Filliol, A.; Chin, L.; Savage, T.M.; Yin, D.; et al. Tumor Restriction by Type I Collagen Opposes Tumor-Promoting Effects of Cancer-Associated Fibroblasts. J. Clin. Investig. 2021, 131, e146987. [Google Scholar] [CrossRef] [PubMed]
- Wohlleber, D.; Knolle, P.A. The Role of Liver Sinusoidal Cells in Local Hepatic Immune Surveillance. Clin. Transl. Immunol. 2016, 5, e117. [Google Scholar] [CrossRef]
- Crispe, I.N. The Liver as a Lymphoid Organ. Annu. Rev. Immunol. 2009, 27, 147–163. [Google Scholar] [CrossRef]
- Ringelhan, M.; Pfister, D.; O’Connor, T.; Pikarsky, E.; Heikenwalder, M. The Immunology of Hepatocellular Carcinoma. Nat. Immunol. 2018, 19, 222–232. [Google Scholar] [CrossRef]
- Li, X.; Ramadori, P.; Pfister, D.; Seehawer, M.; Zender, L.; Heikenwalder, M. The Immunological and Metabolic Landscape in Primary and Metastatic Liver Cancer. Nat. Rev. Cancer 2021, 21, 541–557. [Google Scholar] [CrossRef]
- Triantafyllou, E.; Woollard, K.J.; McPhail, M.J.W.; Antoniades, C.G.; Possamai, L.A. The Role of Monocytes and Macrophages in Acute and Acute-on-Chronic Liver Failure. Front. Immunol. 2018, 9, 2948. [Google Scholar] [CrossRef] [PubMed]
- Ramadori, P.; Kam, S.; Heikenwalder, M. T Cells: Friends and Foes in NASH Pathogenesis and Hepatocarcinogenesis. Hepatology 2022, 75, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Malehmir, M.; Pfister, D.; Gallage, S.; Szydlowska, M.; Inverso, D.; Kotsiliti, E.; Leone, V.; Peiseler, M.; Surewaard, B.G.J.; Rath, D.; et al. Platelet GPIbα Is a Mediator and Potential Interventional Target for NASH and Subsequent Liver Cancer. Nat. Med. 2019, 25, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Chen, Y.; Gao, B. Natural Killer Cells in Liver Disease. Hepatology 2013, 57, 1654–1662. [Google Scholar] [CrossRef] [PubMed]
- Park, O.; Jeong, W.-I.; Wang, L.; Wang, H.; Lian, Z.-X.; Gershwin, M.E.; Gao, B. Diverse Roles of Invariant Natural Killer T Cells in Liver Injury and Fibrosis Induced by Carbon Tetrachloride. Hepatology 2009, 49, 1683–1694. [Google Scholar] [CrossRef]
- Talamantes, S.; Lisjak, M.; Gilglioni, E.H.; Llamoza-Torres, C.J.; Ramos-Molina, B.; Gurzov, E.N. Non-Alcoholic Fatty Liver Disease and Diabetes Mellitus as Growing Aetiologies of Hepatocellular Carcinoma. JHEP Rep. 2023, 5, 100811. [Google Scholar] [CrossRef]
- Tarantino, G.; Citro, V.; Balsano, C.; Capone, D. Could SCGF-Beta Levels Be Associated with Inflammation Markers and Insulin Resistance in Male Patients Suffering from Obesity-Related NAFLD? Diagnostics 2020, 10, 395. [Google Scholar] [CrossRef]
- Wolf, M.J.; Adili, A.; Piotrowitz, K.; Abdullah, Z.; Boege, Y.; Stemmer, K.; Ringelhan, M.; Simonavicius, N.; Egger, M.; Wohlleber, D.; et al. Metabolic Activation of Intrahepatic CD8+ T Cells and NKT Cells Causes Nonalcoholic Steatohepatitis and Liver Cancer via Cross-Talk with Hepatocytes. Cancer Cell 2014, 26, 549–564. [Google Scholar] [CrossRef]
- Yahoo, N.; Dudek, M.; Knolle, P.; Heikenwälder, M. Role of Immune Responses in the Development of NAFLD-Associated Liver Cancer and Prospects for Therapeutic Modulation. J Hepatol 2023, 79, 538–551. [Google Scholar] [CrossRef]
- Dudek, M.; Pfister, D.; Donakonda, S.; Filpe, P.; Schneider, A.; Laschinger, M.; Hartmann, D.; Hüser, N.; Meiser, P.; Bayerl, F.; et al. Auto-Aggressive CXCR6+ CD8 T Cells Cause Liver Immune Pathology in NASH. Nature 2021, 592, 444–449. [Google Scholar] [CrossRef]
- Lassen, M.G.; Lukens, J.R.; Dolina, J.S.; Brown, M.G.; Hahn, Y.S. Intrahepatic IL-10 Maintains NKG2A+Ly49− Liver NK Cells in a Functionally Hyporesponsive State. J. Immunol. 2010, 184, 2693–2701. [Google Scholar] [CrossRef]
- Varol, C.; Yona, S.; Jung, S. Origins and Tissue-context-dependent Fates of Blood Monocytes. Immunol. Cell Biol. 2009, 87, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Dal-Secco, D.; Wang, J.; Zeng, Z.; Kolaczkowska, E.; Wong, C.H.Y.; Petri, B.; Ransohoff, R.M.; Charo, I.F.; Jenne, C.N.; Kubes, P. A Dynamic Spectrum of Monocytes Arising from the In Situ Reprogramming of CCR2+ Monocytes at a Site of Sterile Injury. J. Exp. Med. 2015, 212, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Karlmark, K.R.; Weiskirchen, R.; Zimmermann, H.W.; Gassler, N.; Ginhoux, F.; Weber, C.; Merad, M.; Luedde, T.; Trautwein, C.; Tacke, F. Hepatic Recruitment of the Inflammatory Gr1+ Monocyte Subset upon Liver Injury Promotes Hepatic Fibrosis. Hepatology 2009, 50, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, W.; Yuan, Y.; Chen, P.; Li, B.; Li, J.; Chu, R.; Song, H.; Xie, D.; Jiang, X.; et al. Targeting of Tumour-Infiltrating Macrophages via CCL2/CCR2 Signalling as a Therapeutic Strategy against Hepatocellular Carcinoma. Gut 2017, 66, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Kuang, D.-M.; Peng, C.; Zhao, Q.; Wu, Y.; Chen, M.-S.; Zheng, L. Activated Monocytes in Peritumoral Stroma of Hepatocellular Carcinoma Promote Expansion of Memory T Helper 17 Cells. Hepatology 2010, 51, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Noy, R.; Pollard, J.W. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity 2014, 41, 49–61. [Google Scholar] [CrossRef]
- Yang, Y.; Ye, Y.-C.; Chen, Y.; Zhao, J.-L.; Gao, C.-C.; Han, H.; Liu, W.-C.; Qin, H.-Y. Crosstalk between Hepatic Tumor Cells and Macrophages via Wnt/β-Catenin Signaling Promotes M2-like Macrophage Polarization and Reinforces Tumor Malignant Behaviors. Cell Death Dis. 2018, 9, 793. [Google Scholar] [CrossRef]
- Samstein, R.M.; Arvey, A.; Josefowicz, S.Z.; Peng, X.; Reynolds, A.; Sandstrom, R.; Neph, S.; Sabo, P.; Kim, J.M.; Liao, W.; et al. Foxp3 Exploits a Pre-Existent Enhancer Landscape for Regulatory T Cell Lineage Specification. Cell 2012, 151, 153–166. [Google Scholar] [CrossRef]
- Chen, K.-J.; Lin, S.-Z.; Zhou, L.; Xie, H.-Y.; Zhou, W.-H.; Taki-Eldin, A.; Zheng, S.-S. Selective Recruitment of Regulatory T Cell through CCR6-CCL20 in Hepatocellular Carcinoma Fosters Tumor Progression and Predicts Poor Prognosis. PLoS ONE 2011, 6, e24671. [Google Scholar] [CrossRef]
- Garnelo, M.; Tan, A.; Her, Z.; Yeong, J.; Lim, C.J.; Chen, J.; Lim, K.H.; Weber, A.; Chow, P.; Chung, A.; et al. Interaction between Tumour-Infiltrating B Cells and T Cells Controls the Progression of Hepatocellular Carcinoma. Gut 2017, 66, 342–351. [Google Scholar] [CrossRef]
- Fu, J.; Xu, D.; Liu, Z.; Shi, M.; Zhao, P.; Fu, B.; Zhang, Z.; Yang, H.; Zhang, H.; Zhou, C.; et al. Increased Regulatory T Cells Correlate with CD8 T-Cell Impairment and Poor Survival in Hepatocellular Carcinoma Patients. Gastroenterology 2007, 132, 2328–2339. [Google Scholar] [CrossRef]
- Langhans, B.; Nischalke, H.D.; Krämer, B.; Dold, L.; Lutz, P.; Mohr, R.; Vogt, A.; Toma, M.; Eis-Hübinger, A.M.; Nattermann, J.; et al. Role of Regulatory T Cells and Checkpoint Inhibition in Hepatocellular Carcinoma. Cancer Immunol. Immunother. 2019, 68, 2055–2066. [Google Scholar] [CrossRef]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-Derived Suppressor Cells Coming of Age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef]
- Kapanadze, T.; Gamrekelashvili, J.; Ma, C.; Chan, C.; Zhao, F.; Hewitt, S.; Zender, L.; Kapoor, V.; Felsher, D.W.; Manns, M.P.; et al. Regulation of Accumulation and Function of Myeloid Derived Suppressor Cells in Different Murine Models of Hepatocellular Carcinoma. J. Hepatol. 2013, 59, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Hoechst, B.; Voigtlaender, T.; Ormandy, L.; Gamrekelashvili, J.; Zhao, F.; Wedemeyer, H.; Lehner, F.; Manns, M.P.; Greten, T.F.; Korangy, F. Myeloid Derived Suppressor Cells Inhibit Natural Killer Cells in Patients with Hepatocellular Carcinoma via the NKp30 Receptor. Hepatology 2009, 50, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in Cancer: Neutral No More. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef]
- Liu, K.; Wang, F.-S.; Xu, R. Neutrophils in Liver Diseases: Pathogenesis and Therapeutic Targets. Cell Mol. Immunol. 2021, 18, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gu, J.; Qian, Y.; Li, M.; Qian, Y.; Xu, M.; Li, J.; Wen, Y.; Xia, L.; Li, J.; et al. Deletion of C-C Motif Chemokine Ligand 5 Worsens Invariant Natural Killer T-Cell–Mediated Hepatitis via Compensatory Up-Regulation of CXCR2–Related Chemokine Activity. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-L.; Zhou, Z.-J.; Hu, Z.-Q.; Huang, X.-W.; Wang, Z.; Chen, E.-B.; Fan, J.; Cao, Y.; Dai, Z.; Zhou, J. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology 2016, 150, 1646–1658.e17. [Google Scholar] [CrossRef]
- Li, L.; Xu, L.; Yan, J.; Zhen, Z.-J.; Ji, Y.; Liu, C.-Q.; Lau, W.Y.; Zheng, L.; Xu, J. CXCR2–CXCL1 Axis Is Correlated with Neutrophil Infiltration and Predicts a Poor Prognosis in Hepatocellular Carcinoma. J. Exp. Clin. Cancer Res. 2015, 34, 129. [Google Scholar] [CrossRef]
- Zhou, S.; Yin, D.; Hu, Z.; Luo, C.; Zhou, Z.; Xin, H.; Yang, X.; Shi, Y.; Wang, Z.; Huang, X.; et al. A Positive Feedback Loop Between Cancer Stem-Like Cells and Tumor-Associated Neutrophils Controls Hepatocellular Carcinoma Progression. Hepatology 2019, 70, 1214–1230. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, A.; Brooks, M.W.; Houshyar, S.; Reinhardt, F.; Ardolino, M.; Fessler, E.; Chen, M.B.; Krall, J.A.; DeCock, J.; Zervantonakis, I.K.; et al. Neutrophils Suppress Intraluminal NK Cell–Mediated Tumor Cell Clearance and Enhance Extravasation of Disseminated Carcinoma Cells. Cancer Discov. 2016, 6, 630–649. [Google Scholar] [CrossRef] [PubMed]
- Labelle, M.; Begum, S.; Hynes, R.O. Platelets Guide the Formation of Early Metastatic Niches. Proc. Natl. Acad. Sci. USA 2014, 111, E3053–E3061. [Google Scholar] [CrossRef] [PubMed]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils Escort Circulating Tumour Cells to Enable Cell Cycle Progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Kubes, P. Neutrophils and Neutrophil Extracellular Traps in the Liver and Gastrointestinal System. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 206–221. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Q.; Zhang, X.; Liu, X.; Zhou, B.; Chen, J.; Huang, D.; Li, J.; Li, H.; Chen, F.; et al. DNA of Neutrophil Extracellular Traps Promotes Cancer Metastasis via CCDC25. Nature 2020, 583, 133–138. [Google Scholar] [CrossRef]
- Schneider, C.; Teufel, A.; Yevsa, T.; Staib, F.; Hohmeyer, A.; Walenda, G.; Zimmermann, H.W.; Vucur, M.; Huss, S.; Gassler, N.; et al. Adaptive Immunity Suppresses Formation and Progression of Diethylnitrosamine-Induced Liver Cancer. Gut 2012, 61, 1733–1743. [Google Scholar] [CrossRef]
- Mussbacher, M.; Brunnthaler, L.; Panhuber, A.; Starlinger, P.; Assinger, A. Till Death Do Us Part—The Multifaceted Role of Platelets in Liver Diseases. Int. J. Mol. Sci. 2021, 22, 3113. [Google Scholar] [CrossRef]
- Liu, P.; Hsu, C.; Su, C.; Huang, Y.; Hou, M.; Rich, N.E.; Fujiwara, N.; Hoshida, Y.; Singal, A.G.; Huo, T. Thrombocytosis Is Associated with Worse Survival in Patients with Hepatocellular Carcinoma. Liver Int. 2020, 40, 2522–2534. [Google Scholar] [CrossRef]
- Scheiner, B.; Kirstein, M.; Popp, S.; Hucke, F.; Bota, S.; Rohr-Udilova, N.; Reiberger, T.; Müller, C.; Trauner, M.; Peck-Radosavljevic, M.; et al. Association of Platelet Count and Mean Platelet Volume with Overall Survival in Patients with Cirrhosis and Unresectable Hepatocellular Carcinoma. Liver Cancer 2019, 8, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Poujol-Robert, A.; Boëlle, P.-Y.; Conti, F.; Durand, F.; Duvoux, C.; Wendum, D.; Paradis, V.; Mackiewicz, V.; Chazouillères, O.; Corpechot, C.; et al. Aspirin May Reduce Liver Fibrosis Progression: Evidence from a Multicenter Retrospective Study of Recurrent Hepatitis C after Liver Transplantation. Clin. Res. Hepatol. Gastroenterol. 2014, 38, 570–576. [Google Scholar] [CrossRef]
- Lee, M.; Chung, G.E.; Lee, J.; Oh, S.; Nam, J.Y.; Chang, Y.; Cho, H.; Ahn, H.; Cho, Y.Y.; Yoo, J.; et al. Antiplatelet Therapy and the Risk of Hepatocellular Carcinoma in Chronic Hepatitis B Patients on Antiviral Treatment. Hepatology 2017, 66, 1556–1569. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Song, M.J.; Kim, S.H.; Park, M. Comparing Various Scoring System for Predicting Overall Survival According to Treatment Modalities in Hepatocellular Carcinoma Focused on Platelet-Albumin-Bilirubin (PALBI) and Albumin-Bilirubin (ALBI) Grade: A Nationwide Cohort Study. PLoS ONE 2019, 14, e0216173. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.G.; Duberg, A.-S.; Aleman, S.; Chung, R.T.; Chan, A.T.; Ludvigsson, J.F. Association of Aspirin with Hepatocellular Carcinoma and Liver-Related Mortality. N. Engl. J. Med. 2020, 382, 1018–1028. [Google Scholar] [CrossRef]
- Zaldivar, M.M.; Pauels, K.; von Hundelshausen, P.; Berres, M.-L.; Schmitz, P.; Bornemann, J.; Kowalska, M.A.; Gassler, N.; Streetz, K.L.; Weiskirchen, R.; et al. CXC Chemokine Ligand 4 (Cxcl4) Is a Platelet-Derived Mediator of Experimental Liver Fibrosis. Hepatology 2010, 51, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Carr, B.I.; Guerra, V. Thrombocytosis and Hepatocellular Carcinoma. Dig. Dis. Sci. 2013, 58, 1790–1796. [Google Scholar] [CrossRef]
- Carr, B.I.; Pancoska, P.; Giannini, E.G.; Farinati, F.; Ciccarese, F.; Ludovico Rapaccini, G.; Di Marco, M.; Benvegnù, L.; Zoli, M.; Borzio, F.; et al. Identification of Two Clinical Hepatocellular Carcinoma Patient Phenotypes from Results of Standard Screening Parameters. Semin. Oncol. 2014, 41, 406–414. [Google Scholar] [CrossRef]
- Pang, Q.; Qu, K.; Zhang, J.-Y.; Song, S.-D.; Liu, S.-S.; Tai, M.-H.; Liu, H.-C.; Liu, C. The Prognostic Value of Platelet Count in Patients with Hepatocellular Carcinoma. Medicine 2015, 94, e1431. [Google Scholar] [CrossRef]
- Maini, M.K.; Schurich, A. Platelets Harness the Immune Response to Drive Liver Cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 12840–12841. [Google Scholar] [CrossRef]
- Pavlovic, N.; Rani, B.; Gerwins, P.; Heindryckx, F. Platelets as Key Factors in Hepatocellular Carcinoma. Cancers 2019, 11, 1022. [Google Scholar] [CrossRef] [PubMed]
- Iannacone, M.; Sitia, G.; Isogawa, M.; Marchese, P.; Castro, M.G.; Lowenstein, P.R.; Chisari, F.V.; Ruggeri, Z.M.; Guidotti, L.G. Platelets Mediate Cytotoxic T Lymphocyte–Induced Liver Damage. Nat. Med. 2005, 11, 1167–1169. [Google Scholar] [CrossRef] [PubMed]
- Sitia, G.; Aiolfi, R.; Di Lucia, P.; Mainetti, M.; Fiocchi, A.; Mingozzi, F.; Esposito, A.; Ruggeri, Z.M.; Chisari, F.V.; Iannacone, M.; et al. Antiplatelet Therapy Prevents Hepatocellular Carcinoma and Improves Survival in a Mouse Model of Chronic Hepatitis B. Proc. Natl. Acad. Sci. USA 2012, 109, E2165–E2172. [Google Scholar] [CrossRef]
- Yoshida, S.; Ikenaga, N.; Liu, S.B.; Peng, Z.-W.; Chung, J.; Sverdlov, D.Y.; Miyamoto, M.; Kim, Y.O.; Ogawa, S.; Arch, R.H.; et al. Extrahepatic Platelet-Derived Growth Factor-β, Delivered by Platelets, Promotes Activation of Hepatic Stellate Cells and Biliary Fibrosis in Mice. Gastroenterology 2014, 147, 1378–1392. [Google Scholar] [CrossRef]
- Gutiérrez-Herrero, S.; Maia, V.; Gutiérrez-Berzal, J.; Calzada, N.; Sanz, M.; González-Manchón, C.; Pericacho, M.; Ortiz-Rivero, S.; González-Porras, J.R.; Arechederra, M.; et al. C3G Transgenic Mouse Models with Specific Expression in Platelets Reveal a New Role for C3G in Platelet Clotting through Its GEF Activity. Biochim. Biophys. Acta Mol. Cell Res. 2012, 1823, 1366–1377. [Google Scholar] [CrossRef]
- Gutiérrez-Herrero, S.; Fernández-Infante, C.; Hernández-Cano, L.; Ortiz-Rivero, S.; Guijas, C.; Martín-Granado, V.; Ramón González-Porras, J.; Balsinde, J.; Porras, A.; Guerrero, C. C3G Contributes to Platelet Activation and Aggregation by Regulating Major Signaling Pathways. Signal Transduct. Target. Ther. 2020, 5, 29. [Google Scholar] [CrossRef] [PubMed]
- Lesurtel, M.; Graf, R.; Aleil, B.; Walther, D.J.; Tian, Y.; Jochum, W.; Gachet, C.; Bader, M.; Clavien, P.-A. Platelet-Derived Serotonin Mediates Liver Regeneration. Science 2006, 312, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Murata, S.; Hashimoto, I.; Nakano, Y.; Myronovych, A.; Watanabe, M.; Ohkohchi, N. Single Administration of Thrombopoietin Prevents Progression of Liver Fibrosis and Promotes Liver Regeneration After Partial Hepatectomy in Cirrhotic Rats. Ann. Surg. 2008, 248, 821–828. [Google Scholar] [CrossRef]
- Watanabe, M.; Murata, S.; Hashimoto, I.; Nakano, Y.; Ikeda, O.; Aoyagi, Y.; Matsuo, R.; Fukunaga, K.; Yasue, H.; Ohkohchi, N. Platelets Contribute to the Reduction of Liver Fibrosis in Mice. J. Gastroenterol. Hepatol. 2009, 24, 78–89. [Google Scholar] [CrossRef]
- Takahashi, K. Human Platelets Inhibit Liver Fibrosis in Severe Combined Immunodeficiency Mice. World J. Gastroenterol. 2013, 19, 5250. [Google Scholar] [CrossRef]
- Ma, C.; Fu, Q.; Diggs, L.P.; McVey, J.C.; McCallen, J.; Wabitsch, S.; Ruf, B.; Brown, Z.; Heinrich, B.; Zhang, Q.; et al. Platelets Control Liver Tumor Growth through P2Y12-Dependent CD40L Release in NAFLD. Cancer Cell 2022, 40, 986–998.e5. [Google Scholar] [CrossRef]
- Kodama, T.; Takehara, T.; Hikita, H.; Shimizu, S.; Li, W.; Miyagi, T.; Hosui, A.; Tatsumi, T.; Ishida, H.; Tadokoro, S.; et al. Thrombocytopenia Exacerbates Cholestasis-Induced Liver Fibrosis in Mice. Gastroenterology 2010, 138, 2487–2498.e7. [Google Scholar] [CrossRef]
- Kuwahara, R.; Kofman, A.V.; Landis, C.S.; Swenson, E.S.; Barendswaard, E.; Theise, N.D. The Hepatic Stem Cell Niche: Identification by Label-Retaining Cell Assay. Hepatology 2008, 47, 1994–2002. [Google Scholar] [CrossRef]
- Wright, N.; Samuelson, L.; Walkup, M.H.; Chandrasekaran, P.; Gerber, D.A. Enrichment of a Bipotent Hepatic Progenitor Cell from Naïve Adult Liver Tissue. Biochem. Biophys. Res. Commun. 2008, 366, 367–372. [Google Scholar] [CrossRef]
- Zhang, L.; Theise, N.; Chua, M.; Reid, L.M. The Stem Cell Niche of Human Livers: Symmetry between Development and Regeneration. Hepatology 2008, 48, 1598–1607. [Google Scholar] [CrossRef]
- Rumgay, H.; Arnold, M.; Ferlay, J.; Lesi, O.; Cabasag, C.J.; Vignat, J.; Laversanne, M.; McGlynn, K.A.; Soerjomataram, I. Global Burden of Primary Liver Cancer in 2020 and Predictions to 2040. J. Hepatol. 2022, 77, 1598–1606. [Google Scholar] [CrossRef]
- Dasgupta, P.; Henshaw, C.; Youlden, D.R.; Clark, P.J.; Aitken, J.F.; Baade, P.D. Global Trends in Incidence Rates of Primary Adult Liver Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Farazi, P.A.; De Pinho, R.A. Hepatocellular Carcinoma Pathogenesis: From Genes to Environment. Nat. Rev. Cancer 2006, 6, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Schneller, D.; Angel, P. Cellular Origin of Hepatocellular Carcinoma. In Hepatocellular Carcinoma; Codon Publications: Brisbane, Australia, 2019; pp. 1–28. [Google Scholar] [CrossRef][Green Version]
- Holczbauer, Á.; Wangensteen, K.J.; Shin, S. Cellular Origins of Regenerating Liver and Hepatocellular Carcinoma. JHEP Rep. 2022, 4, 100416. [Google Scholar] [CrossRef] [PubMed]
- Gromowski, T.; Lukacs-Kornek, V.; Cisowski, J. Current View of Liver Cancer Cell-of-Origin and Proposed Mechanisms Precluding Its Proper Determination. Cancer Cell Int. 2023, 23, 3. [Google Scholar] [CrossRef] [PubMed]
- Braun, L.; Goyette, M.; Yaswen, P.; Thompson, N.L.; Fausto4, N. Growth in Culture and Tumorigenicity after Transfection with the Ras Oncogene of Liver Epithelial Cells from Carcinogen-Treated Rats. Cancer Res. 1987, 47, 4116–4124. Available online: http://aacrjournals.org/cancerres/article-pdf/47/15/4116/2429364/cr0470154116.pdf (accessed on 29 September 2023). [PubMed]
- Strauss, R.P.; Audsley, K.M.; Passman, A.M.; van Vuuren, J.H.; Finch-Edmondson, M.L.; Callus, B.A.; Yeoh, G.C. Loss of ARF/INK4A Promotes Liver Progenitor Cell Transformation Toward Tumorigenicity Supporting Their Role in Hepatocarcinogenesis. Gene Expr. 2020, 20, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Salazar, R.; García-Arranz, M.; Sánchez, A.; Olmedillas-López, S.; Vega-Clemente, L.; Serrano, L.J.; Herrera, B.; García-Olmo, D. Oncological Transformation in Vitro of Hepatic Progenitor Cell Lines Isolated from Adult Mice. Sci. Rep. 2022, 12, 3149. [Google Scholar] [CrossRef]
- Zender, L.; Spector, M.S.; Xue, W.; Flemming, P.; Cordon-Cardo, C.; Silke, J.; Fan, S.-T.; Luk, J.M.; Wigler, M.; Hannon, G.J.; et al. Identification and Validation of Oncogenes in Liver Cancer Using an Integrative Oncogenomic Approach. Cell 2006, 125, 1253–1267. [Google Scholar] [CrossRef]
- Tang, D.; Chen, Y.; Fu, G.-B.; Yuan, T.-J.; Huang, W.-J.; Wang, Z.-Y.; Li, W.-J.; Jiao, Y.-F.; Yu, W.-F.; Yan, H.-X. EpCAM Inhibits Differentiation of Human Liver Progenitor Cells into Hepatocytes in Vitro by Activating Notch1 Signaling. Biochem. Biophys. Res. Commun. 2020, 525, 238–243. [Google Scholar] [CrossRef]
- Wu, K.; Ding, J.; Chen, C.; Sun, W.; Ning, B.-F.; Wen, W.; Huang, L.; Han, T.; Yang, W.; Wang, C.; et al. Hepatic Transforming Growth Factor Beta Gives Rise to Tumor-Initiating Cells and Promotes Liver Cancer Development. Hepatology 2012, 56, 2255–2267. [Google Scholar] [CrossRef] [PubMed]
- Almalé, L.; García-Álvaro, M.; Martínez-Palacián, A.; García-Bravo, M.; Lazcanoiturburu, N.; Addante, A.; Roncero, C.; Sanz, J.; López, M.; Bragado, P.; et al. C-Met Signaling Is Essential for Mouse Adult Liver Progenitor Cells Expansion After Transforming Growth Factor-β-Induced Epithelial–Mesenchymal Transition and Regulates Cell Phenotypic Switch. Stem Cells 2019, 37, 1108–1118. [Google Scholar] [CrossRef]
- Lu, L.; Li, Y.; Kim, S.M.; Bossuyt, W.; Liu, P.; Qiu, Q.; Wang, Y.; Halder, G.; Finegold, M.J.; Lee, J.-S.; et al. Hippo Signaling Is a Potent in Vivo Growth and Tumor Suppressor Pathway in the Mammalian Liver. Proc. Natl. Acad. Sci. USA 2010, 107, 1437–1442. [Google Scholar] [CrossRef]
- Lee, J.-S.; Heo, J.; Libbrecht, L.; Chu, I.-S.; Kaposi-Novak, P.; Calvisi, D.F.; Mikaelyan, A.; Roberts, L.R.; Demetris, A.J.; Sun, Z.; et al. A Novel Prognostic Subtype of Human Hepatocellular Carcinoma Derived from Hepatic Progenitor Cells. Nat. Med. 2006, 12, 410–416. [Google Scholar] [CrossRef]
- Yamashita, T.; Forgues, M.; Wang, W.; Jin, W.K.; Ye, Q.; Jia, H.; Budhu, A.; Zanetti, K.A.; Chen, Y.; Qin, L.X.; et al. EpCAM and α-Fetoprotein Expression Defines Novel Prognostic Subtypes of Hepatocellular Carcinoma. Cancer Res. 2008, 68, 1451–1461. [Google Scholar] [CrossRef]
- Barthet, V.J.A.; Brucoli, M.; Ladds, M.J.G.W.; Nössing, C.; Kiourtis, C.; Baudot, A.D.; O’Prey, J.; Zunino, B.; Müller, M.; May, S.; et al. Autophagy Suppresses the Formation of Hepatocyte-Derived Cancer-Initiating Ductular Progenitor Cells in the Liver. Sci. Adv. 2021, 7, eabf9141. [Google Scholar] [CrossRef]
- Shin, S.; Wangensteen, K.J.; Teta-Bissett, M.; Wang, Y.J.; Mosleh-Shirazi, E.; Buza, E.L.; Greenbaum, L.E.; Kaestner, K.H. Genetic Lineage Tracing Analysis of the Cell of Origin of Hepatotoxin-induced Liver Tumors in Mice. Hepatology 2016, 64, 1163–1177. [Google Scholar] [CrossRef] [PubMed]
- Tummala, K.S.; Brandt, M.; Teijeiro, A.; Graña, O.; Schwabe, R.F.; Perna, C.; Djouder, N. Hepatocellular Carcinomas Originate Predominantly from Hepatocytes and Benign Lesions from Hepatic Progenitor Cells. Cell Rep. 2017, 19, 584–600. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, N.; Van Haele, M.; Lanton, T.; Brashi, N.; Bromberg, Z.; Adler, H.; Giladi, H.; Peled, A.; Goldenberg, D.S.; Axelrod, J.H.; et al. Combined Hepatocellular-Cholangiocarcinoma Derives from Liver Progenitor Cells and Depends on Senescence and IL-6 Trans-Signaling. J. Hepatol. 2022, 77, 1631–1641. [Google Scholar] [CrossRef]
- Nakamura, T.; Sakai, K.; Nakamura, T.; Matsumoto, K. Hepatocyte Growth Factor Twenty Years on: Much More than a Growth Factor. J. Gastroenterol. Hepatol. 2011, 26, 188–202. [Google Scholar] [CrossRef]
- Bladt, F.; Riethmacher, D.; Isenmann, S.; Aguzzi, A.; Birchmeier, C. Essential Role for the C-Met Receptor in the Migration of Myogenic Precursor Cells into the Limb Bud. Nature 1995, 376, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ye, W.; Wang, Y.D.; Chen, W.D. HGF/c-Met: A Key Promoter in Liver Regeneration. Front. Pharmacol. 2022, 13, 808855. [Google Scholar] [CrossRef]
- Factor, V.M.; Seo, D.; Ishikawa, T.; Kaposi-Novak, P.; Marquardt, J.U.; Andersen, J.B.; Conner, E.A.; Thorgeirsson, S.S. Loss of C-Met Disrupts Gene Expression Program Required for G2/M Progression during Liver Regeneration in Mice. PLoS ONE 2010, 5, e12739. [Google Scholar] [CrossRef]
- Huh, C.-G.; Factor, V.M.; Sánchez, A.; Uchida, K.; Conner, E.A.; Thorgeirsson, S.S. Hepatocyte Growth Factorc-Met Signaling Pathway Is Required for Efficient Liver Regeneration and Repair. Proc. Natl. Acad. Sci. USA 2004, 101, 4477–4482. [Google Scholar] [CrossRef]
- Shiota, G.; Kunisada, T.; Oyama, K.; Udagawa, A.; Nomi, T.; Tanaka, K.; Tsutsumi, A.; Isono, M.; Nakamura, T.; Hamada, H.; et al. In Vivo Transfer of Hepatocyte Growth Factor Gene Accelerates Proliferation of Hepatic Oval Cells in a 2-acetylaminofluorene/Partial Hepatectomy Model in Rats. FEBS Lett. 2000, 470, 325–330. [Google Scholar] [CrossRef]
- Ishikawa, T.; Factor, V.M.; Marquardt, J.U.; Raggi, C.; Seo, D.; Kitade, M.; Conner, E.A.; Thorgeirsson, S.S. Hepatocyte Growth Factor/c-Met Signaling Is Required for Stem-Cell-Mediated Liver Regeneration in Mice. Hepatology 2012, 55, 1215–1226. [Google Scholar] [CrossRef]
- Giordano, S.; Columbano, A. Met as a Therapeutic Target in HCC: Facts and Hopes. J. Hepatol. 2014, 60, 442–452. [Google Scholar] [CrossRef]
- Salvi, A.; Arici, B.; Portolani, N.; Giulini, S.M.; De Petro, G.; Barlati, S. In Vitro C-Met Inhibition by Antisense RNA and Plasmid-Based RNAi down-Modulates Migration and Invasion of Hepatocellular Carcinoma Cells. Int. J. Oncol. 2007, 31, 451–460. [Google Scholar] [CrossRef]
- Xie, B.; Xing, R.; Chen, P.; Gou, Y.; Li, S.; Xiao, J.; Dong, J. Down-Regulation of c-Met Expression Inhibits Human HCC Cells Growth and Invasion by RNA Interference. J. Surg. Res. 2010, 162, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Ogunwobi, O.O.; Liu, C. Hepatocyte Growth Factor Upregulation Promotes Carcinogenesis and Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma via Akt and COX-2 Pathways. Clin. Exp. Metastasis 2011, 28, 721–731. [Google Scholar] [CrossRef] [PubMed]
- You, H.; Ding, W.; Dang, H.; Jiang, Y.; Rountree, C.B. C-Met Represents a Potential Therapeutic Target for Personalized Treatment in Hepatocellular Carcinoma. Hepatology 2011, 54, 879–889. [Google Scholar] [CrossRef]
- Fan, Y.; Arechederra, M.; Richelme, S.; Daian, F.; Novello, C.; Calderaro, J.; Di Tommaso, L.; Morcrette, G.; Rebouissou, S.; Donadon, M.; et al. A Phosphokinome-based Screen Uncovers New Drug Synergies for Cancer Driven by Liver-specific Gain of Nononcogenic Receptor Tyrosine Kinases. Hepatology 2017, 66, 1644–1661. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ferrell, L.D.; Faouzi, S.; Maher, J.J.; Bishop, J.M. Activation of the Met Receptor by Cell Attachment Induces and Sustains Hepatocellular Carcinomas in Transgenic Mice. J. Cell Biol. 2001, 153, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Takami, T.; Kaposi-Novak, P.; Uchida, K.; Gomez-Quiroz, L.E.; Conner, E.A.; Factor, V.M.; Thorgeirsson, S.S. Loss of Hepatocyte Growth Factor/c-Met Signaling Pathway Accelerates Early Stages of N-nitrosodiethylamine–Induced Hepatocarcinogenesis. Cancer Res. 2007, 67, 9844–9851. [Google Scholar] [CrossRef]
- Marx-Stoelting, P.; Borowiak, M.; Knorpp, T.; Birchmeier, C.; Buchmann, A.; Schwarz, M. Hepatocarcinogenesis in Mice with a Conditional Knockout of the Hepatocyte Growth Factor Receptor C-Met. Int. J. Cancer 2009, 124, 1767–1772. [Google Scholar] [CrossRef]
- Kaposi-Novak, P. Met-Regulated Expression Signature Defines a Subset of Human Hepatocellular Carcinomas with Poor Prognosis and Aggressive Phenotype. J. Clin. Investig. 2006, 116, 1582–1595. [Google Scholar] [CrossRef]
- Yu, J.; Chen, G.G.; Lai, P.B.S. Targeting Hepatocyte Growth Factor/C-mesenchymal–Epithelial Transition Factor Axis in Hepatocellular Carcinoma: Rationale and Therapeutic Strategies. Med. Res. Rev. 2021, 41, 507–524. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, L.; Wu, Q.; Zhang, M.; Zhang, J. Association between the Risk of Hepatitis Virus-Related Hepatocellular Carcinoma and EGF Polymorphism: A PRISMA-Compliant Updated Meta-Analysis. Medicine 2022, 101, e31280. [Google Scholar] [CrossRef] [PubMed]
- Arechederra, M.; Daian, F.; Yim, A.; Bazai, S.K.; Richelme, S.; Dono, R.; Saurin, A.J.; Habermann, B.H.; Maina, F. Hypermethylation of Gene Body CpG Islands Predicts High Dosage of Functional Oncogenes in Liver Cancer. Nat. Commun. 2018, 9, 3164. [Google Scholar] [CrossRef] [PubMed]
- Bouattour, M.; Raymond, E.; Qin, S.; Cheng, A.; Stammberger, U.; Locatelli, G.; Faivre, S. Recent Developments of C-Met as a Therapeutic Target in Hepatocellular Carcinoma. Hepatology 2018, 67, 1132–1149. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Chan, S.L.; Sukeepaisarnjaroen, W.; Han, G.; Choo, S.P.; Sriuranpong, V.; Pan, H.; Yau, T.; Guo, Y.; Chen, M.; et al. A Phase II Study of the Efficacy and Safety of the MET Inhibitor Capmatinib (INC280) in Patients with Advanced Hepatocellular Carcinoma. Ther. Adv. Med. Oncol. 2019, 11, 175883591988900. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wu, W.; Jiang, H.; Ma, L.; Pan, C.; Jin, C.; Mo, J.; Wang, L.; Wang, K. Selective Inhibitor of the C-Met Receptor Tyrosine Kinase in Advanced Hepatocellular Carcinoma: No Beneficial Effect with the Use of Tivantinib? Front. Immunol. 2021, 12, 731527. [Google Scholar] [CrossRef] [PubMed]
- Eisai Inc. E7050 in Combination with Sorafenib Versus Sorafenib Alone as First Line Therapy in Participants with Hepatocellular Carcinoma. NIH, US National library of Medicine. Available online: https://clinicaltrials.gov/study/NCT01271504 (accessed on 28 September 2023).
- Vogel, A.; Martinelli, E.; Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.-C.; Neumann, U.; et al. Updated Treatment Recommendations for Hepatocellular Carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann. Oncol. 2021, 32, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Chen, J.; Jin, R.; Zhao, J.; Liu, J.; Ying, H.; Yan, H.; Zhou, S.; Liang, Y.; Huang, D.; Liang, X.; et al. Potential Molecular, Cellular and Microenvironmental Mechanism of Sorafenib Resistance in Hepatocellular Carcinoma. Cancer Lett. 2015, 367, 1–11. [Google Scholar] [CrossRef]
- Sabbah, D.A.; Hajjo, R.; Sweidan, K. Review on Epidermal Growth Factor Receptor (EGFR) Structure, Signaling Pathways, Interactions, and Recent Updates of EGFR Inhibitors. Curr. Top. Med. Chem. 2020, 20, 815–834. [Google Scholar] [CrossRef]
- Burgess, A.W. Regulation of Signaling from the Epidermal Growth Factor Family. J. Phys. Chem. B 2022, 126, 7475–7485. [Google Scholar] [CrossRef]
- Bhushan, B.; Michalopoulos, G.K. Role of Epidermal Growth Factor Receptor in Liver Injury and Lipid Metabolism: Emerging New Roles for an Old Receptor. Chem. Biol. Interact. 2020, 324, 109090. [Google Scholar] [CrossRef]
- Xu, H.; Liu, L.; Cong, M.; Liu, T.; Sun, S.; Ma, H.; You, H.; Jia, J.; Wang, P. EGF Neutralization Antibodies Attenuate Liver Fibrosis by Inhibiting Myofibroblast Proliferation in Bile Duct Ligation Mice. Histochem. Cell Biol. 2020, 154, 107–116. [Google Scholar] [CrossRef]
- Perugorria, M.J.; Latasa, M.U.; Nicou, A.; Cartagena-Lirola, H.; Castillo, J.; Goñi, S.; Vespasiani-Gentilucci, U.; Zagami, M.G.; Lotersztajn, S.; Prieto, J.; et al. The Epidermal Growth Factor Receptor Ligand Amphiregulin Participates in the Development of Mouse Liver Fibrosis. Hepatology 2008, 48, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Scheving, L.A.; Zhang, X.; Threadgill, D.W.; Russell, W.E. Hepatocyte ERBB3 and EGFR Are Required for Maximal CCl4-Induced Liver Fibrosis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2016, 311, G807–G816. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.; Sigala, B.; Soeda, J.; Mouralidarane, A.; Morgan, M.; Mazzoccoli, G.; Rappa, F.; Cappello, F.; Cabibi, D.; Pazienza, V.; et al. Amphiregulin Activates Human Hepatic Stellate Cells and Is Upregulated in Non Alcoholic Steatohepatitis. Sci. Rep. 2015, 5, 8812. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, E.; Housset, C.; Cacheux, W.; Wendum, D.; Desbois-Mouthon, C.; Rey, C.; Clergue, F.; Poupon, R.; Barbu, V.; Rosmorduc, O. Gefitinib, an EGFR Inhibitor, Prevents Hepatocellular Carcinoma Development in the Rat Liver with Cirrhosis. Hepatology 2005, 41, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, B.C.; Hoshida, Y.; Fujii, T.; Wei, L.; Yamada, S.; Lauwers, G.Y.; McGinn, C.M.; DePeralta, D.K.; Chen, X.; Kuroda, T.; et al. Epidermal Growth Factor Receptor Inhibition Attenuates Liver Fibrosis and Development of Hepatocellular Carcinoma. Hepatology 2014, 59, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- López-Luque, J.; Caballero-Díaz, D.; Martinez-Palacián, A.; Roncero, C.; Moreno-Càceres, J.; García-Bravo, M.; Grueso, E.; Fernández, A.; Crosas-Molist, E.; García-Álvaro, M.; et al. Dissecting the Role of Epidermal Growth Factor Receptor Catalytic Activity during Liver Regeneration and Hepatocarcinogenesis. Hepatology 2016, 63, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Lanaya, H.; Natarajan, A.; Komposch, K.; Li, L.; Amberg, N.; Chen, L.; Wculek, S.K.; Hammer, M.; Zenz, R.; Peck-Radosavljevic, M.; et al. EGFR Has a Tumour-Promoting Role in Liver Macrophages during Hepatocellular Carcinoma Formation. Nat. Cell Biol. 2014, 16, 972–981. [Google Scholar] [CrossRef]
- Choung, S.; Kim, J.M.; Joung, K.H.; Lee, E.S.; Kim, H.J.; Ku, B.J. Epidermal Growth Factor Receptor Inhibition Attenuates Non-Alcoholic Fatty Liver Disease in Diet-Induced Obese Mice. PLoS ONE 2019, 14, e0210828. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Han, J.; Zhou, L.; Yu, Q.; Xu, J.; Jin, Z.; Yang, Y.; Jiang, L.; Lou, D. Inhibition of Epidermal Growth Factor Receptor (EGFR) Reduces Lipopolysaccharide (LPS)-Induced Activation and Inflammatory Cytokines in Hepatic Stellate Cells In Vitro. Med. Sci. Monit. 2018, 24, 5533–5541. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A.; Toffanin, S.; Llovet, J.M. Linking Molecular Classification of Hepatocellular Carcinoma and Personalized Medicine: Preliminary Steps. Curr. Opin. Oncol. 2008, 20, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Takeda, T.; Sakon, M.; Tsujimoto, M.; Higashiyama, S.; Noda, K.; Miyoshi, E.; Monden, M.; Matsuura, N. Expression and Clinical Significance of Erb-B Receptor Family in Hepatocellular Carcinoma. Br. J. Cancer 2001, 84, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Furuse, J. Growth Factors as Therapeutic Targets in HCC. Crit. Rev. Oncol. Hematol. 2008, 67, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Su, M.-C.; Lien, H.-C.; Jeng, Y.-M. Absence of Epidermal Growth Factor Receptor Exon 18–21 Mutation in Hepatocellular Carcinoma. Cancer Lett. 2005, 224, 117–121. [Google Scholar] [CrossRef]
- Ally, A.; Balasundaram, M.; Carlsen, R.; Chuah, E.; Clarke, A.; Dhalla, N.; Holt, R.A.; Jones, S.J.M.; Lee, D.; Ma, Y.; et al. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell 2017, 169, 1327–1341.e23. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, D.; Ning, F.; Du, J.; Wang, H. EGF Is Highly Expressed in Hepatocellular Carcinoma (HCC) and Promotes Motility of HCC Cells via Fibronectin. J. Cell Biochem. 2018, 119, 4170–4183. [Google Scholar] [CrossRef]
- Berasain, C.; Avila, M.A. The EGFR Signalling System in the Liver: From Hepatoprotection to Hepatocarcinogenesis. J. Gastroenterol. 2014, 49, 9–23. [Google Scholar] [CrossRef]
- Qu, X.; Zhang, L.; Li, S.; Li, T.; Zhao, X.; Wang, N.; Shi, Y. M6A-Related Angiogenic Genes to Construct Prognostic Signature, Reveal Immune and Oxidative Stress Landscape, and Screen Drugs in Hepatocellular Carcinoma. Oxid. Med. Cell Longev. 2022, 2022, 8301888. [Google Scholar] [CrossRef]
- Breuhahn, K.; Longerich, T.; Schirmacher, P. Dysregulation of Growth Factor Signaling in Human Hepatocellular Carcinoma. Oncogene 2006, 25, 3787–3800. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, K.; Dobashi, Y.; Suzuki, S.; Fujii, H.; Takeda, Y.; Ooi, A. Amplification and Overexpression of C-ErbB-2,Epidermal Growth Factor Receptor, and c-Met in Biliary Tract Cancers. J. Pathol. 2005, 206, 356–365. [Google Scholar] [CrossRef]
- National Cancer Institute (NCI). Bevacizumab and Erlotinib Hydrochloride in Treating Patients with Metastatic or Unresectable Biliary Tumors. Available online: https://clinicaltrials.gov/study/NCT00356889 (accessed on 28 September 2023).
- National Cancer Institute (NCI). Bevacizumab and Erlotinib in Treating Patients with Advanced Liver Cancer. Available online: https://clinicaltrials.gov/study/NCT00365391 (accessed on 28 September 2023).
- National Cancer Institute (NCI). Erlotinib Hydrochloride in Preventing Liver Cancer in Patients with Cirrhosis of the Liver. Available online: https://clinicaltrials.gov/study/NCT02273362 (accessed on 28 September 2023).
- Philip, P.A.; Mahoney, M.R.; Holen, K.D.; Northfelt, D.W.; Pitot, H.C.; Picus, J.; Flynn, P.J.; Erlichman, C. Phase 2 Study of Bevacizumab plus Erlotinib in Patients with Advanced Hepatocellular Cancer. Cancer 2012, 118, 2424–2430. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute (NCI). Sorafenib Tosylate and Erlotinib Hydrochloride in Treating Patients with Locally Advanced, Unresectable, or Metastatic Gallbladder Cancer or Cholangiocarcinoma. Available online: https://clinicaltrials.gov/study/NCT01093222 (accessed on 28 September 2023).
- Bekaii-Saab, T.; Markowitz, J.; Prescott, N.; Sadee, W.; Heerema, N.; Wei, L.; Dai, Z.; Papp, A.; Campbell, A.; Culler, K.; et al. A Multi-Institutional Phase II Study of the Efficacy and Tolerability of Lapatinib in Patients with Advanced Hepatocellular Carcinomas. Clin. Cancer Res. 2009, 15, 5895–5901. [Google Scholar] [CrossRef] [PubMed]
- Sohal, D.P.S.; Mykulowycz, K.; Uehara, T.; Teitelbaum, U.R.; Damjanov, N.; Giantonio, B.J.; Carberry, M.; Wissel, P.; Jacobs-Small, M.; O’Dwyer, P.J.; et al. A Phase II Trial of Gemcitabine, Irinotecan and Panitumumab in Advanced Cholangiocarcinoma. Ann. Oncol. 2013, 24, 3061–3065. [Google Scholar] [CrossRef] [PubMed]
- Berasain, C.; Perugorria, M.J.; Latasa, M.U.; Castillo, J.; Goñi, S.; Santamaría, M.; Prieto, J.; Avila, M.A. The Epidermal Growth Factor Receptor: A Link Between Inflammation and Liver Cancer. Exp. Biol. Med. 2009, 234, 713–725. [Google Scholar] [CrossRef]
- Shaker, M.E.; Gomaa, H.A.M.; Abdelgawad, M.A.; El-Mesery, M.; Shaaban, A.A.; Hazem, S.H. Emerging Roles of Tyrosine Kinases in Hepatic Inflammatory Diseases and Therapeutic Opportunities. Int. Immunopharmacol. 2023, 120, 110373. [Google Scholar] [CrossRef] [PubMed]
- David, C.J.; Massagué, J. Contextual Determinants of TGFβ Action in Development, Immunity and Cancer. Nat. Rev. Mol. Cell Biol. 2018, 19, 419–435. [Google Scholar] [CrossRef]
- Coulouarn, C.; Factor, V.M.; Thorgeirsson, S.S. Transforming Growth Factor-β Gene Expression Signature in Mouse Hepatocytes Predicts Clinical Outcome in Human Cancer. Hepatology 2008, 47, 2059–2067. [Google Scholar] [CrossRef]
- Dooley, S.; ten Dijke, P. TGF-β in Progression of Liver Disease. Cell Tissue Res. 2012, 347, 245–256. [Google Scholar] [CrossRef]
- Fabregat, I.; Moreno-Càceres, J.; Sánchez, A.; Dooley, S.; Dewidar, B.; Giannelli, G.; ten Dijke, P. TGF-β Signalling and Liver Disease. FEBS J. 2016, 283, 2219–2232. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.-Z.; Yao, D.-F.; Yao, M.; Qiu, L.-W.; Zong, L.; Wu, W.; Wu, X.-H.; Yao, D.-B.; Meng, X.-Y. Clinical Impact of Plasma TGF-Beta1 and Circulating TGF-Beta1 MRNA in Diagnosis of Hepatocellular Carcinoma. Hepatobiliary Pancreat. Dis. Int. 2008, 7, 288–295. [Google Scholar]
- Devan, A.R.; Pavithran, K.; Nair, B.; Murali, M.; Nath, L.R. Deciphering the Role of Transforming Growth Factor-Beta 1 as a Diagnostic-Prognostic-Therapeutic Candidate against Hepatocellular Carcinoma. World J. Gastroenterol. 2022, 28, 5250–5264. [Google Scholar] [CrossRef]
- Chen, J.; Zaidi, S.; Rao, S.; Chen, J.-S.; Phan, L.; Farci, P.; Su, X.; Shetty, K.; White, J.; Zamboni, F.; et al. Analysis of Genomes and Transcriptomes of Hepatocellular Carcinomas Identifies Mutations and Gene Expression Changes in the Transforming Growth Factor-β Pathway. Gastroenterology 2018, 154, 195–210. [Google Scholar] [CrossRef]
- Meyer, C.; Liu, Y.; Dooley, S. Caveolin and TGF-β Entanglements. J. Cell Physiol. 2013, 228, 2097–2102. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Càceres, J.; Caballero-Díaz, D.; Nwosu, Z.C.; Meyer, C.; López-Luque, J.; Malfettone, A.; Lastra, R.; Serrano, T.; Ramos, E.; Dooley, S.; et al. The Level of Caveolin-1 Expression Determines Response to TGF-β as a Tumour Suppressor in Hepatocellular Carcinoma Cells. Cell Death Dis. 2017, 8, e3098. [Google Scholar] [CrossRef] [PubMed]
- Gungor, M.Z.; Uysal, M.; Senturk, S. The Bright and the Dark Side of TGF-β Signaling in Hepatocellular Carcinoma: Mechanisms, Dysregulation, and Therapeutic Implications. Cancers 2022, 14, 940. [Google Scholar] [CrossRef]
- Liu, A.M.; Xu, Z.; Shek, F.H.; Wong, K.F.; Lee, N.P.; Poon, R.T.; Chen, J.; Luk, J.M. MiR-122 Targets Pyruvate Kinase M2 and Affects Metabolism of Hepatocellular Carcinoma. PLoS ONE 2014, 9, e86872. [Google Scholar] [CrossRef]
- Huang, S.; He, X.; Ding, J.; Liang, L.; Zhao, Y.; Zhang, Z.; Yao, X.; Pan, Z.; Zhang, P.; Li, J.; et al. Upregulation of MiR-23a∼27a∼24 Decreases Transforming Growth Factor-Beta-Induced Tumor-Suppressive Activities in Human Hepatocellular Carcinoma Cells. Int. J. Cancer 2008, 123, 972–978. [Google Scholar] [CrossRef]
- Murillo, M.M.; del Castillo, G.; Sánchez, A.; Fernández, M.; Fabregat, I. Involvement of EGF Receptor and C-Src in the Survival Signals Induced by TGF-Β1 in Hepatocytes. Oncogene 2005, 24, 4580–4587. [Google Scholar] [CrossRef]
- Caja, L.; Bertran, E.; Campbell, J.; Fausto, N.; Fabregat, I. The Transforming Growth Factor-Beta (TGF-β) Mediates Acquisition of a Mesenchymal Stem Cell-like Phenotype in Human Liver Cells. J. Cell Physiol. 2011, 226, 1214–1223. [Google Scholar] [CrossRef]
- Fischer, A.N.M.; Fuchs, E.; Mikula, M.; Huber, H.; Beug, H.; Mikulits, W. PDGF Essentially Links TGF-β Signaling to Nuclear β-Catenin Accumulation in Hepatocellular Carcinoma Progression. Oncogene 2007, 26, 3395–3405. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sanchez, E.; Vaquero, J.; Férnandez-Barrena, M.G.; Lasarte, J.J.; Avila, M.A.; Sarobe, P.; Reig, M.; Calvo, M.; Fabregat, I. The TGF-β Pathway: A Pharmacological Target in Hepatocellular Carcinoma? Cancers 2021, 13, 3248. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; He, J.; Pan, Q.; Yang, J.; Zhao, J.; Zhang, Y.; Huang, Y.; Tang, Y.; Wang, Q.; He, J.; et al. Cancer-Associated Fibroblast-Mediated Cellular Crosstalk Supports Hepatocellular Carcinoma Progression. Hepatology 2021, 73, 1717–1735. [Google Scholar] [CrossRef] [PubMed]
- Benetti, A.; Berenzi, A.; Gambarotti, M.; Garrafa, E.; Gelati, M.; Dessy, E.; Portolani, N.; Piardi, T.; Giulini, S.M.; Caruso, A.; et al. Transforming Growth Factor-Β1 and CD105 Promote the Migration of Hepatocellular Carcinoma–Derived Endothelium. Cancer Res. 2008, 68, 8626–8634. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Wang, X.; Liu, Q.; Shen, J.; Li, Z.; Li, Y.; Zhang, J. Inhibition of TGF-β/SMAD3/NF-ΚB Signaling by MicroRNA-491 Is Involved in Arsenic Trioxide-Induced Anti-Angiogenesis in Hepatocellular Carcinoma Cells. Toxicol. Lett. 2014, 231, 55–61. [Google Scholar] [CrossRef]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef] [PubMed]
- Holmgaard, R.B.; Schaer, D.A.; Li, Y.; Castaneda, S.P.; Murphy, M.Y.; Xu, X.; Inigo, I.; Dobkin, J.; Manro, J.R.; Iversen, P.W.; et al. Targeting the TGFβ Pathway with Galunisertib, a TGFβRI Small Molecule Inhibitor, Promotes Anti-Tumor Immunity Leading to Durable, Complete Responses, as Monotherapy and in Combination with Checkpoint Blockade. J. Immunother. Cancer 2018, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Eli Lilly and Company. A Study of LY2157299 in Participants with Advanced Hepatocellular Carcinoma. Available online: https://clinicaltrials.gov/study/NCT02178358 (accessed on 28 September 2023).
- Eli Lilly and Company. A Study of Galunisertib (LY2157299) in Combination with Nivolumab in Advanced Refractory Solid Tumors and in Recurrent or Refractory NSCLC, or Hepatocellular Carcinoma. Available online: https://clinicaltrials.gov/study/NCT02423343 (accessed on 28 September 2023).
- Eli Lilly and Company. A Study of LY2157299 in Participants with Hepatocellular Carcinoma. Available online: https://clinicaltrials.gov/study/NCT01246986 (accessed on 28 September 2023).
- Giannelli, G.; Santoro, A.; Kelley, R.K.; Gane, E.; Paradis, V.; Cleverly, A.; Smith, C.; Estrem, S.T.; Man, M.; Wang, S.; et al. Biomarkers and Overall Survival in Patients with Advanced Hepatocellular Carcinoma Treated with TGF-ΒRI Inhibitor Galunisertib. PLoS ONE 2020, 15, e0222259. [Google Scholar] [CrossRef]
- National Cancer Institute (NCI). Lapatinib Ditosylate in Treating Patients with Unresectable Liver or Biliary Tract Cancer. Available online: https://clinicaltrials.gov/study/NCT00107536 (accessed on 30 September 2023).
- Genzyme, a Sanofi Company. Vandetanib Gemcitabine or Placebo Plus Gemcitabine or Vandetanib Monotherapy in Advanced Biliary Tract Cancer (VANGOGH). Available online: https://clinicaltrials.gov/study/NCT00753675 (accessed on 29 September 2023).
- Abramson Cancer Center at Penn Medicine. Study of Gemcitabine, Irinotecan and Panitumumab in Patients with Advanced and Metastatic Biliary Tract Adenocarcinoma. Available online: https://clinicaltrials.gov/study/NCT00948935 (accessed on 29 September 2023).
- Faivre, S.; Santoro, A.; Kelley, R.K.; Gane, E.; Costentin, C.E.; Gueorguieva, I.; Smith, C.; Cleverly, A.; Lahn, M.M.; Raymond, E.; et al. Novel Transforming Growth Factor Beta Receptor I Kinase Inhibitor Galunisertib (LY2157299) in Advanced Hepatocellular Carcinoma. Liver Int. 2019, 39, 1468–1477. [Google Scholar] [CrossRef]
- Gotoh, T.; Hattori, S.; Nakamura, S.; Kitayama, H.; Noda, M.; Takai, Y.; Kaibuchi, K.; Matsui, H.; Hatase, O.; Takahashi, H.; et al. Identification of Rap1 as a Target for the Crk SH3 Domain-Binding Guanine Nucleotide-Releasing Factor C3G. Mol. Cell. Biol. 1995, 15, 6746–6753. Available online: http://mcb.asm.org/ (accessed on 29 September 2023). [CrossRef] [PubMed]
- Guerrero, C.; Fernandez-Medarde, A.; Rojas, J.; Font de Mora, J.; Esteban, L.; Santos, E. Transformation Suppressor Activity of C3G Is Independent of Its CDC25-Homology Domain. Oncogene 1998, 16, 613–624. [Google Scholar] [CrossRef]
- Priego, N.; Arechederra, M.; Sequera, C.; Bragado, P.; Vázquez-Carballo, A.; Gutiérrez-Uzquiza, Á.; Martín-Granado, V.; Ventura, J.J.; Kazanietz, M.G.; Guerrero, C.; et al. C3G Knock-down Enhances Migration and Invasion by Increasing Rap1-Mediated P38α Activation, While It Impairs Tumor Growth through P38α-Independent Mechanisms. Oncotarget 2016, 7, 45060–45078. [Google Scholar] [CrossRef] [PubMed]
- Sequera, C.; Bragado, P.; Manzano, S.; Arechederra, M.; Richelme, S.; Gutiérrez-Uzquiza, A.; Sánchez, A.; Maina, F.; Guerrero, C.; Porras, A. C3G Is Upregulated in Hepatocarcinoma, Contributing to Tumor Growth and Progression and to HGF/MET Pathway Activation. Cancers 2020, 12, 2282. [Google Scholar] [CrossRef] [PubMed]
- Manzano, S.; Gutierrez-uzquiza, A.; Bragado, P.; Cuesta, A.M.; Guerrero, C.; Porras, A. C3G Protein, a New Player in Glioblastoma. Int. J. Mol. Sci. 2021, 22, 10018. [Google Scholar] [CrossRef]
- Manzano, S.; Gutierrez-Uzquiza, A.; Bragado, P.; Sequera, C.; Herranz, Ó.; Rodrigo-Faus, M.; Jauregui, P.; Morgner, S.; Rubio, I.; Guerrero, C.; et al. C3G Downregulation Induces the Acquisition of a Mesenchymal Phenotype That Enhances Aggressiveness of Glioblastoma Cells. Cell Death Dis. 2021, 12, 348. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the CBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The CBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Sequera, C.; Manzano, S.; Guerrero, C.; Porras, A. How Rap and Its GEFs Control Liver Physiology and Cancer Development. C3G Alterations in Human Hepatocarcinoma. Hepatic Oncol. 2018, 5, HEP05. [Google Scholar] [CrossRef]
- The Results Published Here Are in Whole or Part Based Upon Data Generated by the TCGA Research Network. Available online: https://www.cancer.gov/tcga (accessed on 25 October 2023).
- Yang, J.; Guo, Z.; Song, M.; Pan, Q.; Zhao, J.; Huang, Y.; Han, Y.; Ouyang, D.; Yang, C.; Chen, H.; et al. Lenvatinib Improves Anti-PD-1 Therapeutic Efficacy by Promoting Vascular Normalization via the NRP-1-PDGFRβ Complex in Hepatocellular Carcinoma. Front. Immunol. 2023, 14, 1212577. [Google Scholar] [CrossRef] [PubMed]
- Palao, N.; Sequera, C.; Cuesta, Á.M.; Baquero, C.; Bragado, P.; Gutierrez-Uzquiza, A.; Sánchez, A.; Guerrero, C.; Porras, A. C3G Down-Regulation Enhances pro-Migratory and Stemness Properties of Oval Cells by Promoting an Epithelial-Mesenchymal-like Process. Int. J. Biol. Sci. 2022, 18, 5873–5884. [Google Scholar] [CrossRef] [PubMed]
- Brown, Z.J.; Heinrich, B.; Greten, T.F. Mouse Models of Hepatocellular Carcinoma: An Overview and Highlights for Immunotherapy Research. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 536–554. [Google Scholar] [CrossRef] [PubMed]
- Sandgren, E.P.; Quaife, C.J.; Pinkert, C.A.; Palmiter, R.D.; Brinster, R.L. Oncogene-Induced Liver Neoplasia in Transgenic Mice. Oncogene 1989, 4, 715–724. [Google Scholar]
- Arechederra, M.; Bazai, S.K.; Abdouni, A.; Sequera, C.; Mead, T.J.; Richelme, S.; Daian, F.; Audebert, S.; Dono, R.; Lozano, A.; et al. ADAMTSL5 Is an Epigenetically Activated Gene Underlying Tumorigenesis and Drug Resistance in Hepatocellular Carcinoma. J. Hepatol. 2021, 74, 893–906. [Google Scholar] [CrossRef]
- Sakata, H.; Takayama, H.; Sharp, R.; Rubin, J.S.; Merlino, G.; LaRochelle, W.J. Hepatocyte Growth Factor/Scatter Factor Overexpression Induces Growth, Abnormal Development, and Tumor Formation in Transgenic Mouse Livers. Cell Growth Differ. 1996, 7, 1513–1523. [Google Scholar] [PubMed]
- Tward, A.D.; Jones, K.D.; Yant, S.; Cheung, S.T.; Fan, S.T.; Chen, X.; Kay, M.A.; Wang, R.; Bishop, J.M. Distinct Pathways of Genomic Progression to Benign and Malignant Tumors of the Liver. Proc. Natl. Acad. Sci. USA 2007, 104, 14771–14776. [Google Scholar] [CrossRef]
- Kiguchi, K.; Carbajal, S.; Chan, K.; Beltrán, L.; Ruffino, L.; Shen, J.; Matsumoto, T.; Yoshimi, N.; DiGiovanni, J. Constitutive Expression of ErbB-2 in Gallbladder Epithelium Results in Development of Adenocarcinoma. Cancer Res. 2001, 61, 6971–6976. [Google Scholar]
- Rogler, C.E.; Yang, D.; Rossetti, L.; Donohoe, J.; Alt, E.; Chang, C.J.; Rosenfeld, R.; Neely, K.; Hintz, R. Altered Body Composition and Increased Frequency of Diverse Malignancies in Insulin-like Growth Factor-II Transgenic Mice. J. Biol. Chem. 1994, 269, 13779–13784. [Google Scholar] [CrossRef]
- Xu, X.; Kobayashi, S.; Qiao, W.; Li, C.; Xiao, C.; Radaeva, S.; Stiles, B.; Wang, R.-H.; Ohara, N.; Yoshino, T.; et al. Induction of Intrahepatic Cholangiocellular Carcinoma by Liver-Specific Disruption of Smad4 and Pten in Mice. J. Clin. Investig. 2006, 116, 1843–1852. [Google Scholar] [CrossRef]
- Horie, Y.; Suzuki, A.; Kataoka, E.; Sasaki, T.; Hamada, K.; Sasaki, J.; Mizuno, K.; Hasegawa, G.; Kishimoto, H.; Iizuka, M.; et al. Hepatocyte-Specific Pten Deficiency Results in Steatohepatitis and Hepatocellular Carcinomas. J. Clin. Investig. 2004, 113, 1774–1783. [Google Scholar] [CrossRef]
- Conner, E.A.; Lemmer, E.R.; Sánchez, A.; Factor, V.M.; Thorgeirsson, S.S. E2F1 Blocks and C-Myc Accelerates Hepatic Ploidy in Transgenic Mouse Models. Biochem. Biophys. Res. Commun. 2003, 302, 114–120. [Google Scholar] [CrossRef]
- Lewis, B.C.; Klimstra, D.S.; Socci, N.D.; Xu, S.; Koutcher, J.A.; Varmus, H.E. The Absence of P53 Promotes Metastasis in a Novel Somatic Mouse Model for Hepatocellular Carcinoma. Mol. Cell. Biol. 2005, 25, 1228–1237. [Google Scholar] [CrossRef]
- Zender, S.; Nickeleit, I.; Wuestefeld, T.; Sörensen, I.; Dauch, D.; Bozko, P.; El-Khatib, M.; Geffers, R.; Bektas, H.; Manns, M.P.; et al. A Critical Role for Notch Signaling in the Formation of Cholangiocellular Carcinomas. Cancer Cell 2013, 23, 784–795. [Google Scholar] [CrossRef]
- Cadoret, A.; Ovejero, C.; Saadi-Kheddouci, S.; Souil, E.; Fabre, M.; Romagnolo, B.; Kahn, A.; Perret, C. Hepatomegaly in Transgenic Mice Expressing an Oncogenic Form of Beta-Catenin. Cancer Res. 2001, 61, 3245–3249. [Google Scholar]
- Cho, K.; Ro, S.W.; Seo, S.H.; Jeon, Y.; Moon, H.; Kim, D.Y.; Kim, S.U. Genetically Engineered Mouse Models for Liver Cancer. Cancers 2020, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Loeuillard, E.; Fischbach, S.R.; Gores, G.J.; Rizvi, S. Animal Models of Cholangiocarcinoma. Biochim. Biophys. Acta-Mol. Basis Dis. 2019, 1865, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Benjamin, I.S.; Alexander, B. Reproducible Production of Thioacetamide-Induced Macronodular Cirrhosis in the Rat with No Mortality. J. Hepatol. 2002, 36, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Praet, M.M.; Roels, H.J. Histogenesis of Cholangiomas and Cholangiocarcinomas in Thioacetamide Fed Rats. Exp. Pathol. 1984, 26, 3–14. [Google Scholar] [CrossRef]
- Yoshimoto, S.; Loo, T.M.; Atarashi, K.; Kanda, H.; Sato, S.; Oyadomari, S.; Iwakura, Y.; Oshima, K.; Morita, H.; Hattori, M.; et al. Obesity-Induced Gut Microbial Metabolite Promotes Liver Cancer through Senescence Secretome. Nature 2013, 499, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Koen, H.; Pugh, T.D.; Goldfarb, S. Centrilobular Distribution of Diethylnitrosamine-Induced Hepatocellular Foci in the Mouse. Lab. Investig. 1983, 49, 78–81. [Google Scholar]
- Newell, P.; Villanueva, A.; Friedman, S.L.; Koike, K.; Llovet, J.M. Experimental Models of Hepatocellular Carcinoma. J. Hepatol. 2008, 48, 858–879. [Google Scholar] [CrossRef]
- Groos, J.; Bannasch, P.; Schwarz, M.; Kopp-Schneider, A. Comparison of Mode of Action of Four Hepatocarcinogens: A Model-Based Approach. Toxicol. Sci. 2007, 99, 446–454. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. Toxicology and Carcinogenesis Studies of Furan (CAS No. 110-00-9) in F344 Rats and B6C3F1 Mice(Gavage Studies). Natl. Toxicol. Program Tech. Rep. Ser. 1993, 402, 1–286. [Google Scholar]
- Gallage, S.; Avila, J.E.B.; Ramadori, P.; Focaccia, E.; Rahbari, M.; Ali, A.; Malek, N.P.; Anstee, Q.M.; Heikenwalder, M. A Researcher’s Guide to Preclinical Mouse NASH Models. Nat. Metab. 2022, 4, 1632–1649. [Google Scholar] [CrossRef] [PubMed]
- Denda, A.; Kitayama, W.; Kishida, H.; Murata, N.; Tsutsumi, M.; Tsujiuchi, T.; Nakae, D.; Konishi, Y. Development of Hepatocellular Adenomas and Carcinomas Associated with Fibrosis in C57BL/6J Male Mice given a Choline-Deficient, L-Amino Acid-Defined Diet. Jpn. J. Cancer Res. 2002, 93, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Denda, A.; Tang, Q.; Endoh, T.; Tsujiuchi, T.; Horiguchi, K.; Noguchi, O.; Mizumoto, Y.; Nakae, D.; Konishi, Y. Prevention by Acetylsalicylic Acid of Liver Cirrhosis and Carcinogenesis as Well as Generations of 8-Hydroxydeoxyguanosine and Thiobarbituric Acid-Reactive Substances Caused by a Choline-Deficient, L-Amino Acid-Defined Diet in Rats. Carcinogenesis 1994, 15, 1279–1283. [Google Scholar] [CrossRef] [PubMed]
- Itagaki, H.; Shimizu, K.; Morikawa, S.; Ogawa, K.; Ezaki, T. Morphological and Functional Characterization of Non-Alcoholic Fatty Liver Disease Induced by a Methionine-Choline-Deficient Diet in C57BL/6 Mice. Int. J. Clin. Exp. Pathol. 2013, 6, 2683–2696. [Google Scholar] [PubMed]
- Rizki, G.; Arnaboldi, L.; Gabrielli, B.; Yan, J.; Lee, G.S.; Ng, R.K.; Turner, S.M.; Badger, T.M.; Pitas, R.E.; Maher, J.J. Mice Fed a Lipogenic Methionine-Choline-Deficient Diet Develop Hypermetabolism Coincident with Hepatic Suppression of SCD-1. J. Lipid Res. 2006, 47, 2280–2290. [Google Scholar] [CrossRef] [PubMed]
- Ikawa-Yoshida, A.; Matsuo, S.; Kato, A.; Ohmori, Y.; Higashida, A.; Kaneko, E.; Matsumoto, M. Hepatocellular Carcinoma in a Mouse Model Fed a Choline-Deficient, L-Amino Acid-Defined, High-Fat Diet. Int. J. Exp. Pathol. 2017, 98, 221–233. [Google Scholar] [CrossRef]
- Kishida, N.; Matsuda, S.; Itano, O.; Shinoda, M.; Kitago, M.; Yagi, H.; Abe, Y.; Hibi, T.; Masugi, Y.; Aiura, K.; et al. Development of a Novel Mouse Model of Hepatocellular Carcinoma with Nonalcoholic Steatohepatitis Using a High-Fat, Choline-Deficient Diet and Intraperitoneal Injection of Diethylnitrosamine. BMC Gastroenterol. 2016, 16, 61. [Google Scholar] [CrossRef]
- He, L.; Tian, D.-A.; Li, P.-Y.; He, X.-X. Mouse Models of Liver Cancer: Progress and Recommendations. Oncotarget 2015, 6, 23306–23322. [Google Scholar] [CrossRef]
- Yang, H.; Li, T.W.H.; Peng, J.; Tang, X.; Ko, K.S.; Xia, M.; Aller, M.-A. A Mouse Model of Cholestasis-Associated Cholangiocarcinoma and Transcription Factors Involved in Progression. Gastroenterology 2011, 141, 378–388. [Google Scholar] [CrossRef]
- Kamegaya, Y.; Hiasa, Y.; Zukerberg, L.; Fowler, N.; Blackard, J.T.; Lin, W.; Choe, W.H.; Schmidt, E.V.; Chung, R.T. Hepatitis C Virus Acts as a Tumor Accelerator by Blocking Apoptosis in a Mouse Model of Hepatocarcinogenesis. Hepatology 2005, 41, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Koike, K.; Moriya, K.; Iino, S.; Yotsuyanagi, H.; Endo, Y.; Miyamura, T.; Kurokawa, K. High-Level Expression of Hepatitis B Virus HBx Gene and Hepatocarcinogenesis in Transgenic Mice. Hepatology 1994, 19, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, N.; Takayama, H.; Toyoda, M.; Otsuka, T.; Fukusato, T.; Merlino, G.; Takagi, H.; Mori, M. Hepatocyte Growth Factor Promotes Hepatocarcinogenesis through C-Met Autocrine Activation and Enhanced Angiogenesis in Transgenic Mice Treated with Diethylnitrosamine. Oncogene 2002, 21, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Arechederra, M.; Recalde, M.; Gárate-rascón, M.; Fernández-barrena, M.G.; Ávila, M.A.; Berasain, C. Epigenetic Biomarkers for the Diagnosis and Treatment of Liver Disease. Cancers 2021, 13, 1265. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.Y.; Morris, S.M.; Campbell, J.; Fausto, N.; Yeh, M.M.; Grady, W.M. TGF-Beta Inactivation and TGF-Alpha Overexpression Cooperate in an in Vivo Mouse Model to Induce Hepatocellular Carcinoma That Recapitulates Molecular Features of Human Liver Cancer. Int. J. Cancer 2010, 127, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Jhappan, C.; Stahle, C.; Harkins, R.N.; Fausto, N.; Smith, G.H.; Merlino, G.T. TGF Alpha Overexpression in Transgenic Mice Induces Liver Neoplasia and Abnormal Development of the Mammary Gland and Pancreas. Cell 1990, 61, 1137–1146. [Google Scholar] [CrossRef]
- Santoni-Rugiu, E.; Nagy, P.; Jensen, M.R.; Factor, V.M.; Thorgeirsson, S.S. Evolution of Neoplastic Development in the Liver of Transgenic Mice Co-Expressing c-Myc and Transforming Growth Factor-Alpha. Am. J. Pathol. 1996, 149, 407–428. [Google Scholar]
- Murakami, H.; Sanderson, N.D.; Nagy, P.; Marino, P.A.; Merlino, G.; Thorgeirsson, S.S. Transgenic Mouse Model for Synergistic Effects of Nuclear Oncogenes and Growth Factors in Tumorigenesis: Interaction of c-Myc and Transforming Growth Factor Alpha in Hepatic Oncogenesis. Cancer Res. 1993, 53, 1719–1723. [Google Scholar]
- Uehara, T.; Pogribny, I.P.; Rusyn, I. The DEN and CCl4 -Induced Mouse Model of Fibrosis and Inflammation-Associated Hepatocellular Carcinoma. Curr. Protoc. Pharmacol. 2014, 66, 14.30.1–14.30.10. [Google Scholar] [CrossRef]
- Poirier, L.A. Hepatocarcinogenesis by Diethylnitrosamine in Rats Fed High Dietary Levels of Lipotropes. J. Natl. Cancer Inst. 1975, 54, 137–140. [Google Scholar] [CrossRef]
- Pascale, R.M.; Simile, M.M.; Feo, F. Genomic Abnormalities in Hepatocarcinogenesis. Implications for a Chemopreventive Strategy. Anticancer Res. 1993, 13, 1341–1356. [Google Scholar] [PubMed]
- Solt, D.B.; Medline, A.; Farber, E. Rapid Emergence of Carcinogen-Induced Hyperplastic Lesions in a New Model for the Sequential Analysis of Liver Carcinogenesis. Am. J. Pathol. 1977, 88, 595–618. [Google Scholar] [PubMed]
- Shariff, M.I.F.; Tognarelli, J.M.; Lewis, M.R.; Want, E.J.; Mohamed, F.E.Z.; Ladep, N.G.; Crossey, M.M.E.; Khan, S.A.; Jalan, R.; Holmes, E.; et al. Plasma Lipid Profiling in a Rat Model of Hepatocellular Carcinoma: Potential Modulation Through Quinolone Administration. J. Clin. Exp. Hepatol. 2015, 5, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.M.; Magee, P.N. Some Toxic Properties of Dimethylnitrosamine. Br. J. Ind. Med. 1954, 11, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Encell, L.; Foiles, P.G.; Gold, B. The Relationship between N-nitrosodimethylamine Metabolism and DNA Methylation in Isolated Rat Hepatocytes. Carcinogenesis 1996, 17, 1127–1134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weisburger, E.K.; Ulland, B.M.; Nam, J.; Gart, J.J.; Weisburger, J.H. Carcinogenicity Tests of Certain Environmental and Industrial Chemicals. J. Natl. Cancer Inst. 1981, 67, 75–88. [Google Scholar] [PubMed]
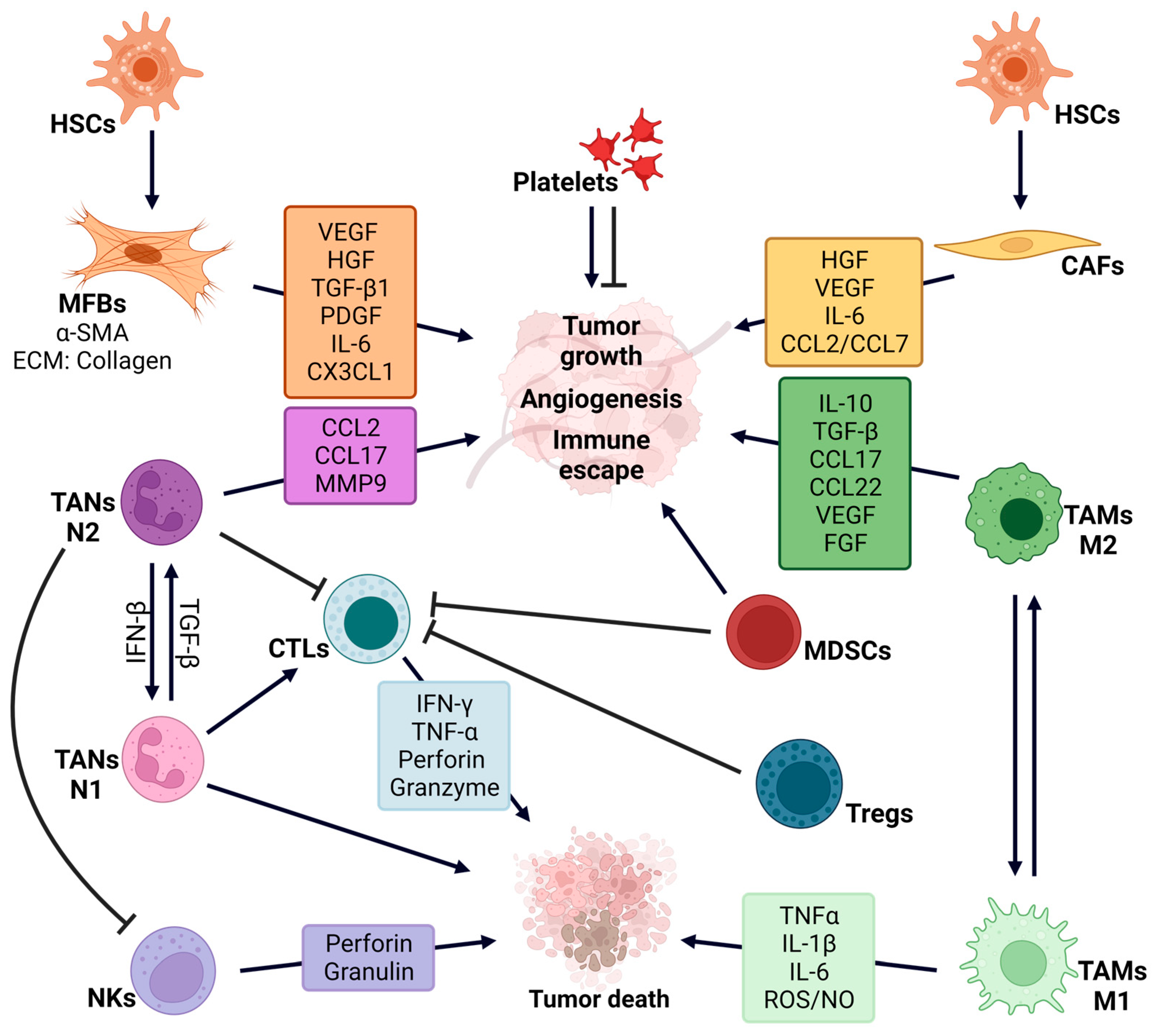
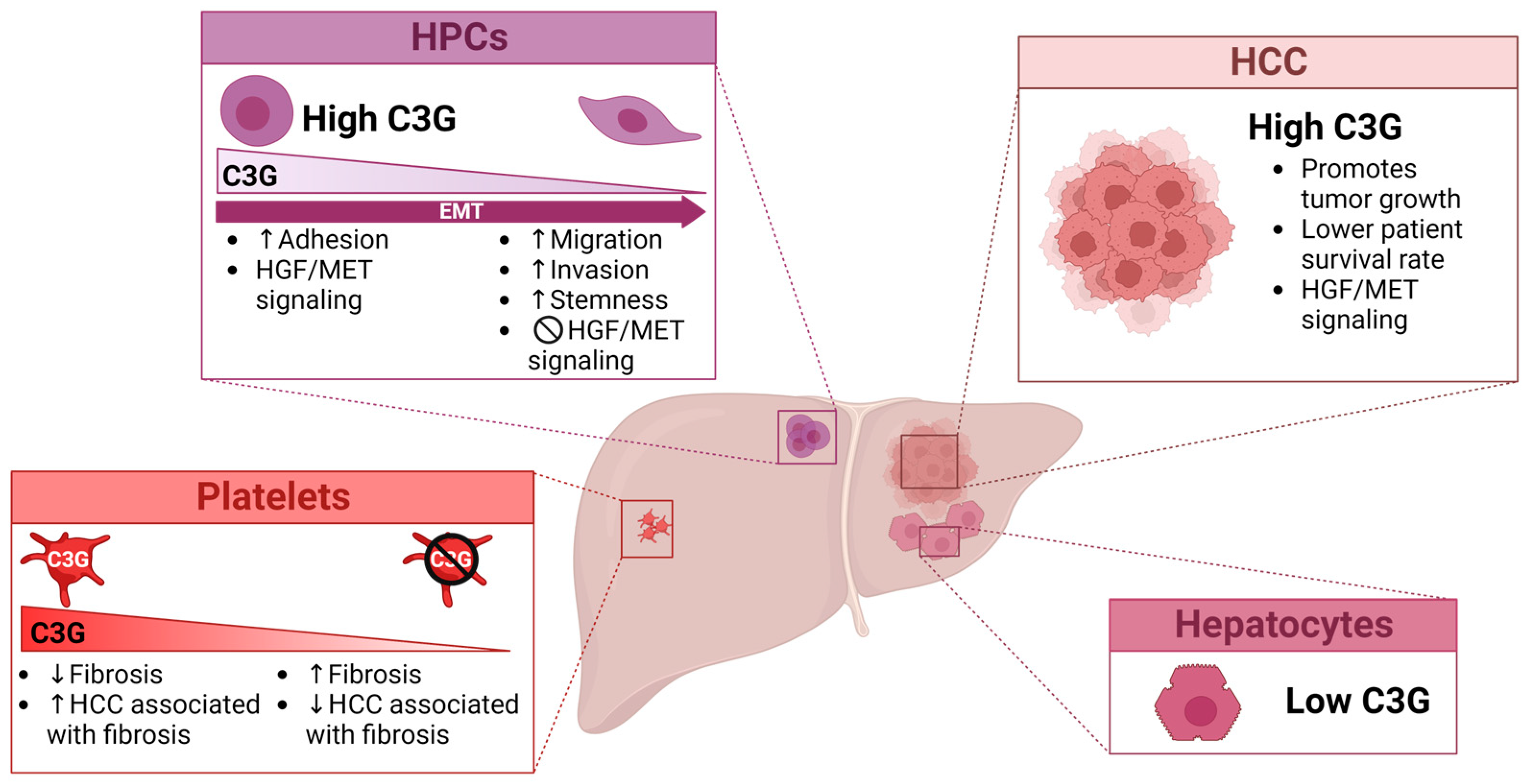
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuesta, Á.M.; Palao, N.; Bragado, P.; Gutierrez-Uzquiza, A.; Herrera, B.; Sánchez, A.; Porras, A. New and Old Key Players in Liver Cancer. Int. J. Mol. Sci. 2023, 24, 17152. https://doi.org/10.3390/ijms242417152
Cuesta ÁM, Palao N, Bragado P, Gutierrez-Uzquiza A, Herrera B, Sánchez A, Porras A. New and Old Key Players in Liver Cancer. International Journal of Molecular Sciences. 2023; 24(24):17152. https://doi.org/10.3390/ijms242417152
Chicago/Turabian StyleCuesta, Ángel M., Nerea Palao, Paloma Bragado, Alvaro Gutierrez-Uzquiza, Blanca Herrera, Aránzazu Sánchez, and Almudena Porras. 2023. "New and Old Key Players in Liver Cancer" International Journal of Molecular Sciences 24, no. 24: 17152. https://doi.org/10.3390/ijms242417152
APA StyleCuesta, Á. M., Palao, N., Bragado, P., Gutierrez-Uzquiza, A., Herrera, B., Sánchez, A., & Porras, A. (2023). New and Old Key Players in Liver Cancer. International Journal of Molecular Sciences, 24(24), 17152. https://doi.org/10.3390/ijms242417152










