Natural Functional Beverages as an Approach to Manage Diabetes
Abstract
:1. Introduction
2. Diabetes
2.1. Type 1 Diabetes
2.2. Type 2 Diabetes
2.3. Prevalence
2.4. Diagnosis and Treatment
3. Functional Beverages
3.1. Definition of Functional Beverages
3.2. Types of Functional Beverages
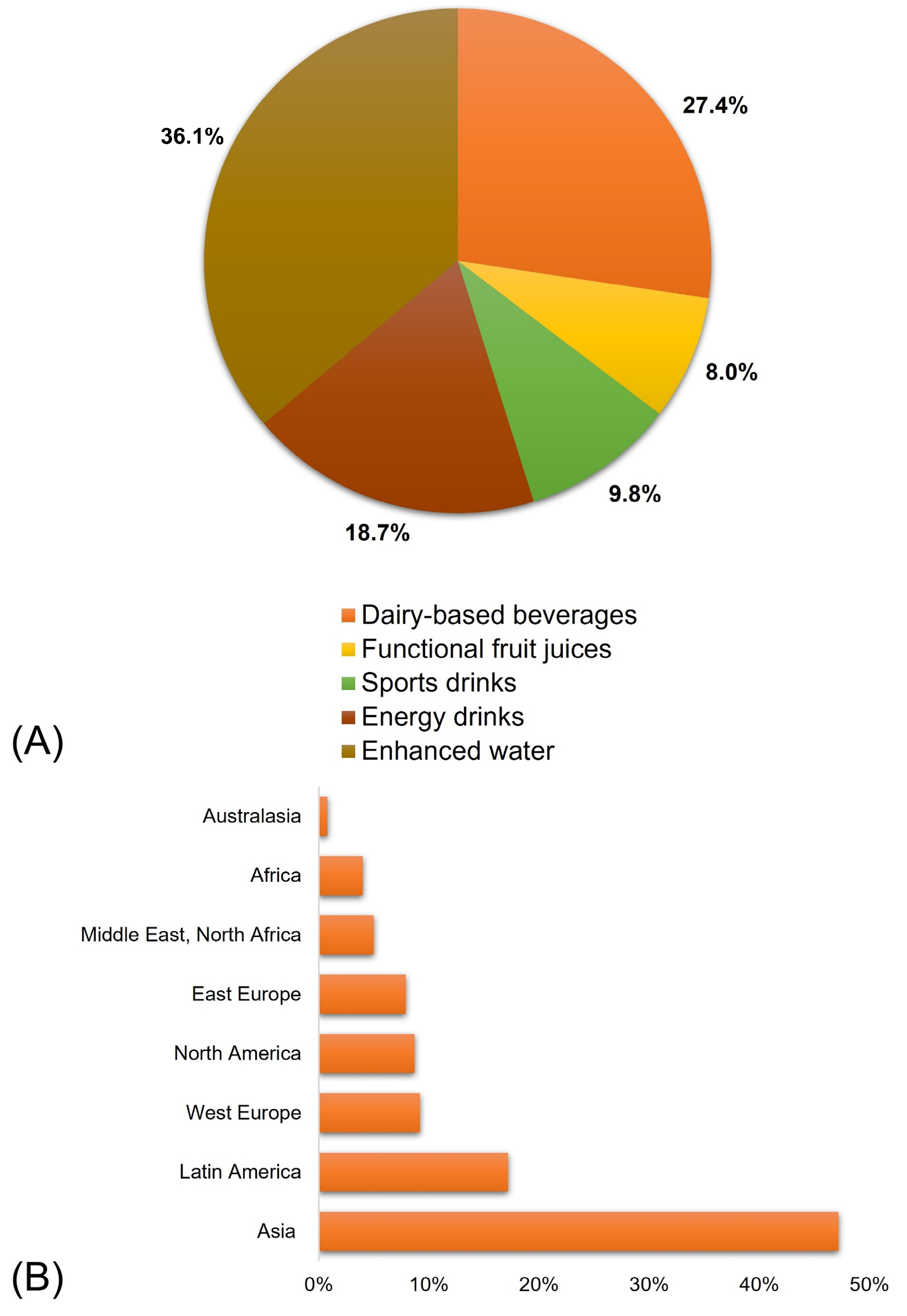
3.3. Market for Functional Foods and Beverages
4. The Role of Fruit- and Vegetable-Based Functional Foods and Beverages against Diabetes
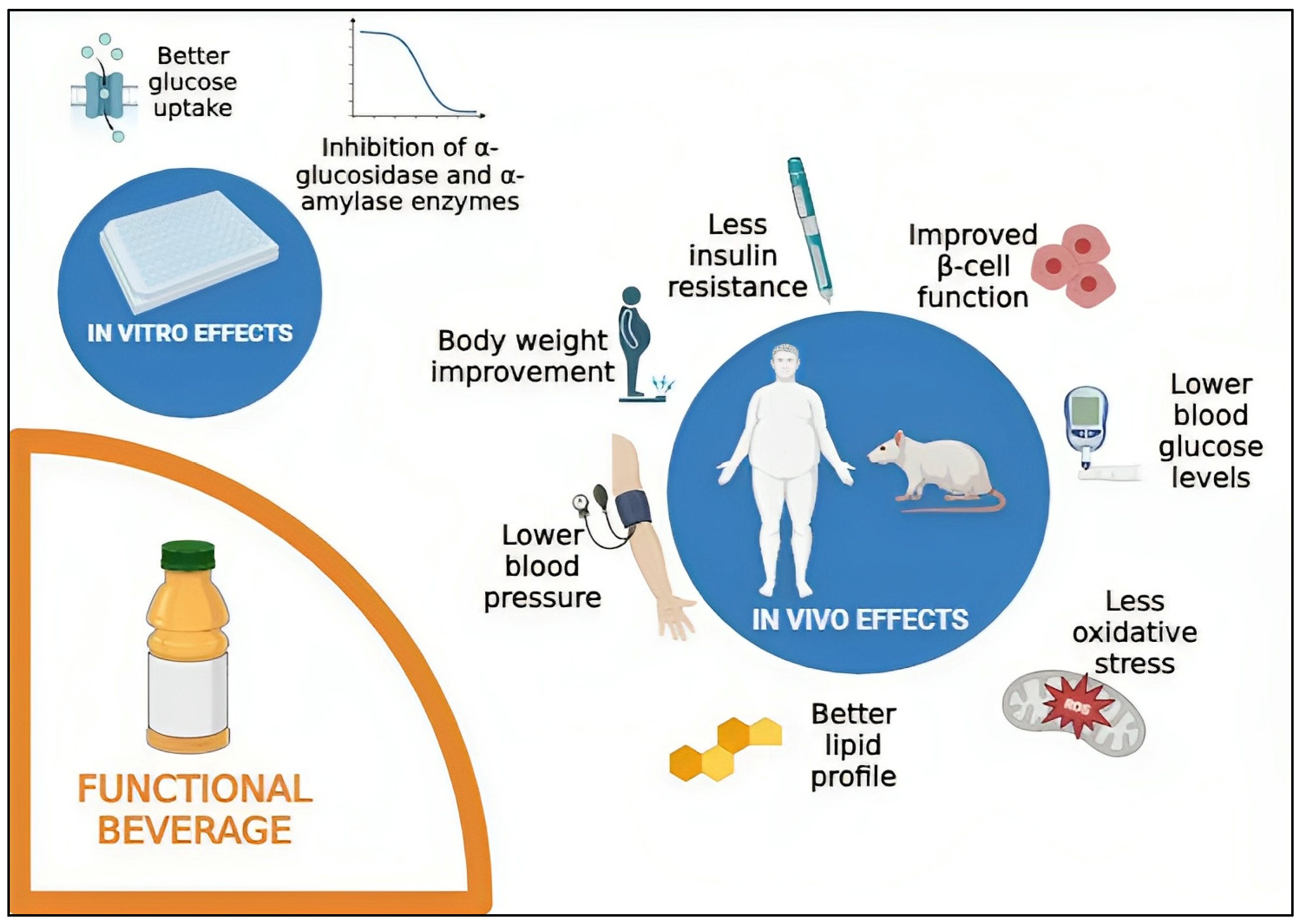
4.1. In Vitro Studies with Functional Beverages and Diabetes
4.1.1. α-Glucosidase and α-Amylase Inhibition
4.1.2. Glucose Uptake
| Beverage | Assays | Results | Reference |
|---|---|---|---|
| Fermented bitter gourd juice | α-glucosidase inhibition (measured as glucose production reduction) | ↓ glucose production = 14.5–19.2% | [69] |
| Prunus fruit smoothies | α-amylase and α-glucosidase inhibition | IC50 amy ≤ 1.00–8.03 mg/mL IC50 gluco = 1.20–6.94 mg/mL | [71] |
| Bitter gourd fruit juice | Glucose uptake by diaphragms from diabetic rats | Glucose uptake (absence of insulin): ↑ 1.40 mg/g tissue Glucose uptake (presence of insulin): ↑ 4.08 mg/g tissue | [81] |
| Apple and blackcurrant polyphenol-rich drinks | Glucose uptake by Caco-2 cells | ↓ glucose uptake (apple polyphenols) = 46–51% IC50 (blackcurrant polyphenols) = 0.51–0.63 mg/mL | [84] |
| Fermented sprouted quinoa yoghurt beverages | α-amylase inhibition | IC50 amy (100 µL) = 30.48–39.36 mg/mL IC50 amy (200 µL) = 39.44–51.57 mg/mL IC50 amy (400 µL) = 50.06–71.28 mg/mL | [72] |
| Tigernut beverages fortified with extracts of Vernonia amygdalina and bitter gourd | α-amylase and α-glucosidase inhibition | Inhibition amy = 20.59–60.14% Inhibition gluco = 38.82–75.54% | [70] |
| Probiotics-fermented blueberry juices | α-amylase and α-glucosidase inhibition Glucose uptake by HepG2 cells | IC50 amy = 0.25–2.67 mg/mL IC50 gluco = 1–40.68 mg/mL ↑ glucose uptake ≈ 1 mmol/L | [73] |
| Sea-buckthorn based smoothies | α-amylase, α-glucosidase and pancreatic lipase inhibition | Inhibition amy = 20.03–49.82% Inhibition gluco = 6.12–98.61% Inhibition lipase = 50.80–96.31% | [16] |
| Fruit/Vegetable | Main Bioactive Compounds | References |
|---|---|---|
| Bitter gourd (Momordica charantia) | Charantin, polypeptide-P, vicine | [85,86,87] |
| Sour cherry (Prunus cerasus) | Chlorogenic acid, rutin, diadzin, amygdalin, quercetin, naringenin, gallic acid | [88] |
| Peach (Prunus persica) | Protocatechuic, p-hydroxybenzoic, p-hydroxyphenylacetic, chlorogenic, p-coumaric and ferulic acids, catechin | [89] |
| Apricot (Prunus armeniaca) | Catechin, chlorogenic acid, rutin | [90] |
| Plum fruit (Prunus cv. ‘Promis) | n.f. | |
| Apple (Malus sylvestris) | Catechin, chlorogenic acid, epicatechin, hyperoside, quercitrin, phloridzin | [91] |
| Blackcurrant * | Neochlorogenic acid, chlorogenic acid, epigallocatechin, catechin, epicatechin, myricetin malonyl-glucoside, delphinidin 3-O-glucoside, delphinidin 3-O-rutinoside, cyanidin 3-O-glucoside, cyanidin 3-O-rutinoside | [92] |
| Quinoa * | Hydroxybenzoic acid, vanillic acid, syringic acid, coumaric acid, ferulic acid | [93] |
| Tigernut (Cyperus esculentus) | Oleuropin, pyrogallol, catechin, chlorogenic acid, calicylic acid | [94] |
| Blueberry (Vaccinium corymbosum) | Delphinidin-3-galactoside, malvidin-3-galactoside, malvidin-3-glucoside | [95] |
| Sea-buckthorn (Hippophaë rhamnoides) | Orhamnetin 3-O-rutinoside, isorhamnetin 3-O-glucoside, isorhamnetin 3-glucoside 7-rhamnoside, isorhamnetin 3-neohesperidoside, quercetin 3-rutinoside, quercetin 3-O-glucoside, kaempferol 3-sorphoroside-7-O-rhamnoside, isorhamnetin 3-O-sorphoroside-7-O-rhamnoside, rutin, free isorhamnetin | [96,97,98] |
| Indian Gooseberry (Emblica officinalis) | Myricetin, tannic acid, syringic acid, coumaric acid, caffeic acid, gallic acid | [99] |
| Noni (Morinda citrifolia) | Damnacanthal, morindone, morindin, caproic acid, caprylic acid, xeronine | [100] |
| Tomato (Solanum lycopersicum) | Lycopene, quercetin, kaempferol, naringenin, caffeic acid, lutein | [101] |
| Pomegranate (Punica granatum) | Punicic acid, punicalagin, ellagic acid, gallic acid, oleanolic acid, ursolic acid and uallic acids | [102] |
| Grapefruit * | Narirutin, naringin, naringenin | [103] |
| Palm fruit (Elaeis guineensis) | Protocatechuic acid, p-hydroxybenzoic acid, caffeic acid, p-coumaric acid, ferulic acid, 2,4-dimethoxybenzoic acid, cinnamic acid | [104] |
| Strawberry (Fragaria × ananassa) | Catechin, pelargonidin, quercetin glucuronides, delphinidin, kaempferol derivatives | [105] |
| Cranberry (Vaccinium macrocarpon) | Peonidin 3-O-galactoside, peonidin 3-O-arabinoside, cyanidin 3-O-galactoside, cyanidin 3-O-arabinoside, myricetin 3-galactoside, quercetin 3-galactoside, quercetin-3-α-L-arabinofuranoside, quercetin 3-rhamnoside, ursolic acid, oleanolic acid | [106] |
| Cowpea (Vigna Sinensis) | n.f. | |
| Açaí (Euterpe oleracea Mart.) | Vanillic acid, syringic acid, p-hydroxybenzoic acid, protocatechuic acid, ferulic acid, catechin, procyanidin oligomers | [107] |
| Clementine (Citrus clementina) | Narirutin, naringin, (neo)hesperidin | [108] |
| Gooseberry (Physalis angulata) | Cholesteryl acetate, Lupeol acetate, α-Tocopherol | [108] |
| Bengkuang * | n.f. | |
| Pigeon pea (Cajanus cajan) | Quercetin, quercetin 3-O-glucoside, quercetin 3-O-methylether, isoprenylated-genistein, cajanol, cajanin | [109] |
4.2. In Vivo Studies with Functional Beverages and Diabetes
4.2.1. Effects on Body Weight
4.2.2. Effects on Glucose and Insulin Metabolism
4.2.3. Effects on the Lipid Profile
4.2.4. Antioxidant Status
4.3. Clinical Trials with Functional Beverages and Diabetes
| Beverage | Administration | Relevant Results | Reference |
|---|---|---|---|
| Emblica officinalis fruit juice | 1 mL/kg, daily, 8 weeks in STZ-DR | ↓ serum glucose, FBG, TAG, TC, VLDL-C ↑ serum insulin, FBI, HDL-C, LDL-C | [157] |
| Fermented noni fruit juice | 0.0015 mL/kg, 2 × day, 12 weeks in HFD-OR | ↓ body weight, FBG, insulin resistance ↑ insulin, glucose tolerance | [112] |
| Processed tomato-vinegar beverage | 14 mL/kg, daily, 6 weeks in HFD-OR | ↓ TAG, body weight, insulin resistance ↑ glucose tolerance, HDL-C, GCK activity | [113] |
| Fresh pomegranate juice | 1.5 mL/kg, once, in T2D patients | ↑ β-cell function ↓ FPG, insulin resistance | [145] |
| Bitter gourd fermented beverage | 45 mL, daily, for 1 and 6 months in diabetic patients | ↓ FBG and PPBS (1 month) ↓ FBG and PPBS, = blood lipid profile, ↑ HbA1c (6 months) | [148] |
| Grapefruit sweetened juices | 2–3 mL, daily, for 2 weeks in HFD-OR | ↓ body weight, FBG, FSI, liver TAG | [114] |
| Vaccinium corymbosum infusion | Cup of juice, daily, for 2 years, in a pre-diabetic | ↓ serum glucose, HbA1c, insulin resistance | [151] |
| Palm fruit juice | 170–720 mg GAE/kg, daily, for 4–36 weeks in CS-DR | ↓ blood glucose, TAG, TC, liver lipids =body weight | [119] |
| Palm fruit juice | HC diet + 5400 mg GAE/kg, 4 weeks in DR | ↓ blood glucose, ↓ body weight, TAG | [138] |
| Apple and blackcurrant polyphenol-rich drinks | 200 g, once, in healthy patients | ↓ PPG, insulin, C-peptide, GIP =TAG | [84] |
| Bitter gourd fruit juice | 10 mL/kg, 14 days before diabetes and 21 days after, in STZ-DR | ↓ serum glucose, insulin resistance, serum TC, TAG, pancreatic MDA ↑ serum insulin, β-cell function, HDL-C, TAOC, pancreatic GSH | [81] |
| Strawberry and cranberry polyphenols beverage | 333 mg polyphenols, daily, for 6 weeks, in insulin-resistant patients | ↓ insulin resistance =TC, LDL-cholesterol, HDL-cholesterol, TAG | [149] |
| Cowpea juice, tomato juice and green apple juices combined | Combinations, daily, for 28 days in ALL-DR | ↓ FBG | [35] |
| Pigeon pea beverage dilluted in water | 2700 mg/kg, daily, for 2 weeks in DHR | ↓ plasma glucose, TC, MDA =body weight | [118] |
| Açaí beverage | 2 × 325 mL, daily, for 12 weeks, in patients with metabolic syndrome | ↓ IFN-γ plasma level, 8-isoprostane | [150] |
| Fermented bitter gourd juice | 10 mL/kg, daily, for 4 weeks in STZ-DR | ↓ body weight loss, blood glucose, FBG, serum insulin, insulin resistance, TC, LDL-C, TAG, MDA ↑ HDL-C | [120] |
| Emblica officinalis fruit juice | 2 mL/kg, daily, for 42 days in DR; for 4 weeks in HF-DR | ↓ body weight, FBG, insulin resistance, HbA1c, TAG, blood pressure; TC =HDL-C | [115] |
| Citrus concentrate enriched with b-cryptoxanthin, hesperidin and pectin | 2 mL, daily, for 8 weeks in HF-PDR | ↑ glucose tolerance ↓ plasma glucose, plasma insulin, TAG, LDL-C, VLDL-C, blood pressure =TC, HDL-C | [158] |
| Physalis angulata fruit juice | 1 and 2 mL/kg, daily, for 2 weeks in STZ-DR | ↓ FBG =body weight | [121] |
| Yogurt bengkuang tape ketan hitam | 200 mL, daily, for 2 weeks in T2D patients | =FBG ↓ plasma MDA | [152] |
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gayathry, K.S.; John, J.A. Functional Beverages: Special Focus on Anti-Diabetic Potential. J. Food Process. Preserv. 2021, 45, e15974. [Google Scholar] [CrossRef]
- World Health Organization. Noncommunicable Diseases. Available online: https://www.who.int/en/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 20 July 2023).
- Olaiya, C.O.; Soetan, K.O.; Esan, A.M. The Role of Nutraceuticals, Functional Foods and Value Added Food Products in the Prevention and Treatment of Chronic Diseases. Afr. J. Food Sci. 2016, 10, 185–193. [Google Scholar] [CrossRef]
- World Health Organization. WHO Global Report on Traditional and Complementary Medicine 2019; World Health Organization: Geneva, Switzerland, 2019; ISBN 9789241515436.
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. Available online: https://www.who.int/publications/i/item/9789241565257 (accessed on 29 May 2023).
- Ong, K.L.; Stafford, L.K.; McLaughlin, S.A.; Boyko, E.J.; Vollset, S.E.; Smith, A.E.; Dalton, B.E.; Duprey, J.; Cruz, J.A.; Hagins, H.; et al. Global, Regional, and National Burden of Diabetes from 1990 to 2021, with Projections of Prevalence to 2050: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Caswell, H. The Role of Fruit Juice in the Diet: An Overview. Nutr. Bull. 2009, 34, 273–288. [Google Scholar] [CrossRef]
- Beam, A.; Clinger, E.; Hao, L. Effect of Diet and Dietary Components on the Composition of the Gut Microbiota. Nutrients 2021, 13, 2795. [Google Scholar] [CrossRef]
- Kramer, H. Diet and Chronic Kidney Disease. Adv. Nutr. 2019, 10, S367–S379. [Google Scholar] [CrossRef]
- Ojo, O. Nutrition and Chronic Conditions. Nutrients 2019, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Corbo, M.R.; Bevilacqua, A.; Petruzzi, L.; Casanova, F.P.; Sinigaglia, M. Functional Beverages: The Emerging Side of Functional Foods Commercial Trends, Research, and Health Implications. Food Sci. Food Saf. 2014, 13, 1192–1206. [Google Scholar] [CrossRef]
- Selcuk, M.Y.; Aygen, B.; Dogukan, A.; Tuzcu, Z.; Akdemir, F.; Komorowski, J.R.; Atalay, M.; Sahin, K. Chromium Picolinate and Chromium Histidinate Protects against Renal Dysfunction by Modulation of NF-B Pathway in High-Fat Diet Fed and Streptozotocin-Induced Diabetic Rats. Nutr. Metab. 2012, 9, 30. [Google Scholar] [CrossRef]
- Rafighi, Z.; Shiva, A.; Arab, S.; Mohd Yousof, R. Association of Dietary Vitamin C and E Intake and Antioxidant Enzymes in Type 2 Diabetes Mellitus Patients. Glob. J. Health Sci. 2013, 5, 183–187. [Google Scholar] [CrossRef]
- Shoji, T.; Yamada, M.; Miura, T.; Nagashima, K.; Ogura, K.; Inagaki, N.; Maeda-Yamamoto, M. Chronic Administration of Apple Polyphenols Ameliorates Hyperglycaemia in High-Normal and Borderline Subjects: A Randomised, Placebo-Controlled Trial. Diabetes Res. Clin. Pract. 2017, 129, 43–51. [Google Scholar] [CrossRef]
- Kim, M. High-Methoxyl Pectin Has Greater Enhancing Effect on Glucose Uptake in Intestinal Perfused Rats. Nutrition 2005, 21, 372–377. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Nowicka, P. Anti-Diabetic, Anti-Cholinesterase, and Antioxidant Potential, Chemical Composition and Sensory Evaluation of Novel Sea Buckthorn-Based Smoothies. Food Chem. 2021, 338, 128105. [Google Scholar] [CrossRef] [PubMed]
- Dey, G.; Sireswar, S. Tailoring Functional Beverages from Fruits and Vegetables for Specific Disease Conditions-Are We There Yet? Crit. Rev. Food Sci. Nutr. 2021, 61, 2034–2046. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, Z.H.; Qiang, H.; Wu, J.; Shen, M.; Zhang, L.; Lyu, J. Trends in the Incidence of Diabetes Mellitus: Results from the Global Burden of Disease Study 2017 and Implications for Diabetes Mellitus Prevention. BMC Public Health 2020, 20, 1415. [Google Scholar] [CrossRef] [PubMed]
- United Nations. Political Declaration of the High-Level Meeting of the General Assemblyon the Prevention and Control of Noncommunicable Diseases; World Health Organization: Geneva, Switzerland, 2011. Available online: https://digitallibrary.un.org/record/710899/?ln=en (accessed on 26 July 2023).
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed.; International Diabetes Federation: Brussels, Belgium, 2013. [Google Scholar] [CrossRef]
- Wootton-Beard, P.C.; Ryan, L. Improving Public Health?: The Role of Antioxidant-Rich Fruit and Vegetable Beverages. Food Res. Int. 2011, 44, 3135–3148. [Google Scholar] [CrossRef]
- Zhou, B.; Lu, Y.; Hajifathalian, K.; Bentham, J.; Di Cesare, M.; Danaei, G.; Bixby, H.; Cowan, M.J.; Ali, M.K.; Taddei, C.; et al. Worldwide Trends in Diabetes Since 1980: A Pooled Analysis of 751 Population-Based Studies with 4.4 Million Participants. Lancet 2016, 387, 1513–1530. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 Diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- Deshpande, A.D.; Harris-Hayes, M.; Schootman, M. Epidemiology of Diabetes and Diabetes-Related Complications. Phys. Ther. 2008, 88, 1254–1264. [Google Scholar] [CrossRef]
- Patterson, C.C.; Harjutsalo, V.; Rosenbauer, J.; Neu, A.; Cinek, O.; Skrivarhaug, T.; Rami-Merhar, B.; Soltesz, G.; Svensson, J.; Parslow, R.C.; et al. Trends and Cyclical Variation in the Incidence of Childhood Type 1 Diabetes in 26 European Centres in the 25 Year Period 1989–2013: A Multicentre Prospective Registration Study. Diabetologia 2019, 62, 408–417. [Google Scholar] [CrossRef]
- Patterson, C.C.; Dahlquist, G.G.; Gyürüs, E.; Green, A.; Soltész, G.; Group, E.S. Incidence Trends for Childhood Type 1 Diabetes in Europe during 1989–2003 and Predicted New Cases 2005-20: A Multicentre Prospective Registration Study. Lancet 2009, 373, 2027–2033. [Google Scholar] [CrossRef]
- Venkatakrishnan, K.; Chiu, H.F.; Wang, C.K. Popular Functional Foods and Herbs for the Management of tyPe-2-Diabetes Mellitus: A Comprehensive Review with Special Reference to Clinical Trials and Its Proposed Mechanism. J. Funct. Foods 2019, 57, 425–438. [Google Scholar] [CrossRef]
- Li, P.; Tang, Y.; Liu, L.; Wang, D.; Zhang, L.; Piao, C. Therapeutic Potential of Buckwheat Hull Flavonoids in Db/Db Mice, a Model of Type 2 Diabetes. J. Funct. Foods 2019, 52, 284–290. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, Y.; Tanaka, Y.; Zhang, W. Risk Factors Contributing to Type 2 Diabetes and Recent Advances in the Treatment and Prevention. Int. J. Med. Sci. 2014, 11, 1185–1200. [Google Scholar] [CrossRef]
- Tinajero, M.G.; Malik, V.S. An Update on the Epidemiology of Type 2 Diabetes: A Global Perspective. Endocrinol. Metab. Clin. N. Am. 2021, 50, 337–355. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization Diabetes. Available online: https://www.who.int/europe/health-topics/diabetes#tab=tab_1 (accessed on 26 July 2023).
- Centers for Disease Control and Prevention. Diabetes Tests. Available online: https://www.cdc.gov/diabetes/basics/getting-tested.html (accessed on 7 July 2023).
- Saad Masood Butt Management and Treatment of Type 2 Diabetes. Int. J. Comput. Inf. Manuf. 2022, 2, 15–27. [CrossRef]
- Koh, S.P.; Maarof, S.; Sew, Y.S.; Sabidi, S.; Abdullah, R.; Mohd Danial, A.; Nur Diyana, A.; Mustaffa, R. Fermented Jackfruit Leaf Beverage Offers New Affordable and Effective Diabetes Therapy. Food Res. 2020, 4, 19–25. [Google Scholar] [CrossRef]
- Naim, A.; Anisa, L.; Marjoni, R. Antidiabetes Effects—Combination of Cowpea Juice (Vigna sinensis L.), Tomato Juice (Solanum lycopersicum L.), and Green Apple Juice (Malus sylvestris Mill.) in White Male Mice. Int. J. Green Pharm. 2018, 12, S633–S637. [Google Scholar]
- Gupta, A.; Sanwal, N.; Bareen, M.A.; Barua, S.; Sharma, N.; Joshua Olatunji, O.; Prakash Nirmal, N.; Sahu, J.K. Trends in Functional Beverages: Functional Ingredients, Processing Technologies, Stability, Health Benefits, and Consumer Perspective. Food Res. Int. 2023, 170, 113046. [Google Scholar] [CrossRef]
- Manousi, N.; Sarakatsianos, I.; Samanidou, V. Extraction Techniques of Phenolic Compounds and Other Bioactive Compounds From Medicinal and Aromatic Plants. In Engineering Tools in the Beverage Industry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 283–314. [Google Scholar] [CrossRef]
- Raman, M.; Ambalam, P.; Doble, M. Probiotics, Prebiotics, and Fibers in Nutritive and Functional Beverages. In Nutrients in Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 315–367. [Google Scholar] [CrossRef]
- European Parliament and the Council of the European Union. Regulation (EC) 1924/2006 on Nutrition and Health Claims Made on Foods; European Union: Strasbourg, France, 2006; pp. 1–15.
- Gonçalves, A.C.; Nunes, A.R.; Flores-Félix, J.D.; Alves, G.; Silva, L.R. Cherries and Blueberries-Based Beverages: Functional Foods with Antidiabetic and Immune Booster Properties. Molecules 2022, 27, 3294. [Google Scholar] [CrossRef]
- Cong, L.; Bremer, P.; Mirosa, M. Functional Beverages in Selected Countries of Asia Pacific Region: A Review. Beverages 2020, 6, 21. [Google Scholar] [CrossRef]
- Henry, C.J. Functional Foods. Eur. J. Clin. Nutr. 2010, 64, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Heckman, M.A.; Sherry, K.; de Mejia, E.G. Energy Drinks: An Assessment of Their Market Size, Consumer Demographics, Ingredient Profile, Functionality, and Regulations in the United States. Compr. Rev. Food Sci. Food Saf. 2010, 9, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Sugajski, M.; Buszewska-Forajta, M.; Buszewski, B. Functional Beverages in the 21st Century. Beverages 2023, 9, 27. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Kelleher, A.H.; Maykish, A.; Kristo, A.S. Non-Alcoholic Beverages, Old and Novel, and Their Potential Effects on Human Health, with a Focus on Hydration and Cardiometabolic Health. Medicina 2020, 56, 490. [Google Scholar] [CrossRef] [PubMed]
- Grumezescu, A.M.; Holban, A.M. Functional and Medicinal Beverages Volume 11: The Science of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; ISBN 9780128163979. [Google Scholar]
- Technavio. Functional Foods and Beverages Market by Product, Distribution Channel, and Geography—Forecast and Analysis 2023–2027. Available online: https://www.technavio.com/report/functional-foods-and-beverages-market-industry-analysis (accessed on 30 May 2023).
- DataIntelo. Global Functional Beverages Market by Types (Dairy Based Beverages, Enhanced Water, Energy Drinks, Sports Drinks, Functional Fruit Juices, and Others), Distribution Channels (Store Based, Online, Supermarkets & Hypermarkets, and Convenience Stores), and Regions (Asia Pacific, Europe, North America, Middle East & Africa, and Latin America)—Global Industry Analysis, Growth, Share, Size, Trends, and Forecast from 2022 to 2030; DataIntelo: Pune, India, 2019; Available online: https://dataintelo.com/report/functional-beverages-market/ (accessed on 21 November 2023).
- Arthur, R. Unprecedented Growth for Asia Beverage Market; Beverage Daily: West Sussex, UK, 2016; Available online: https://www.beveragedaily.com/Article/2016/05/20/Unprecedented-growth-for-Asia-beverage-market-global-consumption-data (accessed on 21 November 2023).
- Ashaolu, T.J.; Adeyeye, S.A.O. African Functional Foods and Beverages: A Review. J. Culin. Sci. Technol. 2022, 1, 1–36. [Google Scholar] [CrossRef]
- Hegde, S.V.; Adhikari, P.; Nandini, M.; D’Souza, V. Effect of Daily Supplementation of Fruits on Oxidative Stress Indices and Glycaemic Status in Type 2 Diabetes Mellitus. Complement. Ther. Clin. Pract. 2013, 19, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Shidfar, F.; Froghifar, N.; Vafa, M.; Rajab, A.; Hosseini, S.; Shidfar, S.; Gohari, M. The Effects of Tomato Consumption on Serum Glucose, Apolipoprotein B, Apolipoprotein A-I, Homocysteine and Blood Pressure in Type 2 Diabetic Patients. Int. J. Food Sci. Nutr. 2011, 62, 289–294. [Google Scholar] [CrossRef]
- Potter, A.S.; Foroudi, S.; Stamatikos, A.; Patil, B.S.; Deyhim, F. Drinking Carrot Juice Increases Total Antioxidant Status and Decreases Lipid Peroxidation in Adults. Nutr. J. 2011, 10, 96. [Google Scholar] [CrossRef]
- Kim, M.; Hwang, S.; Park, E.; Bae, J. Strict Vegetarian Diet Improves the Risk Factors Associated with Metabolic Diseases by Modulating Gut Microbiota and Reducing Intestinal Inflammation. Environ. Microbiol. Rep. 2013, 5, 765–775. [Google Scholar] [CrossRef]
- Buscemi, S.; Rosafio, G.; Arcoleo, G.; Mattina, A.; Canino, B.; Montana, M.; Verga, S.; Rini, G. Effects of Red Orange Juice Intake on Endothelial Function and Inflammatory Markers in Adult Subjects with Increased Cardiovascular Risk. Am. J. Clin. Nutr. 2012, 95, 1089–1095. [Google Scholar] [CrossRef]
- Mirmiran, P. Functional Foods-Based Diet as a Novel Dietary Approach for Management of Type 2 Diabetes and Its Complications: A Review. World J. Diabetes 2014, 5, 267. [Google Scholar] [CrossRef]
- Alkhatib, A.; Tsang, C.; Tiss, A.; Bahorun, T.; Arefanian, H.; Barake, R.; Khadir, A.; Tuomilehto, J. Functional Foods and Lifestyle Approaches for Diabetes Prevention and Management. Nutrients 2017, 9, 1310. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.; López Sánchez, G.F.; Veronese, N.; Soysal, P.; Oh, H.; Barnett, Y.; Keyes, H.; Butler, L.; Allen, P.; Kostev, K.; et al. Fruit and Vegetable Intake and Non-Communicable Diseases among Adults Aged ≥50 Years in Low- and Middle-Income Countries. J. Nutr. Health Aging 2022, 26, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, Y.; Li, S.; Zhang, P.; Zhou, T.; Xu, D.-P.; Li, H.-B. Effects and Mechanisms of Fruit and Vegetable Juices on Cardiovascular Diseases. Int. J. Mol. Sci. 2017, 18, 555. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health Effects of Dietary Risks in 195 Countries, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- FAO. WHO Fruit and Vegetables for Health. Rep. a Jt. FAO WHO Work. 2004, 10, 1–46. [Google Scholar]
- Li, M.; Fan, Y.; Zhang, X.; Hou, W.; Tang, Z. Fruit and Vegetable Intake and Risk of Type 2 Diabetes Mellitus: Meta-Analysis of Prospective Cohort Studies. BMJ Open 2014, 4, e005497. [Google Scholar] [CrossRef]
- Anderson, R.A.; Broadhurst, C.L.; Polansky, M.M.; Schmidt, W.F.; Khan, A.; Flanagan, V.P.; Schoene, N.W.; Graves, D.J. Isolation and Characterization of Polyphenol Type-A Polymers from Cinnamon with Insulin-like Biological Activity. J. Agric. Food Chem. 2004, 52, 65–70. [Google Scholar] [CrossRef]
- Babbar, N.; Oberoi, H.S.; Sandhu, S.K.; Bhargav, V.K. Influence of Different Solvents in Extraction of Phenolic Compounds from Vegetable Residues and Their Evaluation as Natural Sources of Antioxidants. J. Food Sci. Technol. 2014, 51, 2568–2575. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Survay, N.S.; Ko, E.; Upadhyay, C.P.; Mi, J.; Park, S.W.; Lee, D.; Jung, Y.-S.; Yoon, D.-Y.; Hong, S. Hypoglycemic Effects of Fruits and Vegetables in Hyperglycemic Rats for Prevention of Type-2 Diabetes. Korean J. Hortic. Sci. Technol. 2010, 28, 850–856. [Google Scholar]
- Jayaprakasam, B.; Vareed, S.K.; Olson, L.K.; Nair, M.G. Insulin Secretion by Bioactive Anthocyanins and Anthocyanidins Present in Fruits. J. Agric. Food Chem. 2005, 53, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Wedick, N.M.; Pan, A.; Cassidy, A.; Rimm, E.B.; Sampson, L.; Rosner, B.; Willett, W.; Hu, F.B.; Sun, Q.; Van Dam, R.M. Dietary Flavonoid Intakes and Risk of Type 2 Diabetes in US Men and Women. Am. J. Clin. Nutr. 2012, 95, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Mazlan, F.A.; Suffian, M.; Sharifuddin, Y. Biotransformation of Momordica Charantia Fresh Juice by Lactobacillus Plantarum BET003 and Its Putative Anti-Diabetic Potential. PeerJ 2015, 2015, e1376. [Google Scholar] [CrossRef]
- Badejo, A.A.; Falarunu, A.J.; Duyilemi, T.I.; Fasuhanmi, O.S. Antioxidative and Anti-Diabetic Potentials of Tigernut (Cyperus esculentus) Sedge Beverages Fortified with Vernonia Amygdalina and Momordica Charantia. J. Food Meas. Charact. 2020, 14, 2790–2799. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Samoticha, J. Evaluation of Phytochemicals, Antioxidant Capacity, and Antidiabetic Activity of Novel Smoothies from Selected Prunus Fruits. J. Funct. Foods 2016, 25, 397–407. [Google Scholar] [CrossRef]
- Ujiroghene, O.J.; Liu, L.; Zhang, S.; Lu, J.; Zhang, C.; Pang, X.; Lv, J. Potent α-Amylase Inhibitory Activity of Sprouted Quinoa-Based Yoghurt Beverages Fermented with Selected Anti-Diabetic Strains of Lactic Acid Bacteria. RSC Adv. 2019, 9, 9486–9493. [Google Scholar] [CrossRef]
- Zhong, H.; Abdullah; Zhao, M.; Tang, J.; Deng, L.; Feng, F. Probiotics-Fermented Blueberry Juices as Potential Antidiabetic Product: Antioxidant, Antimicrobial and Antidiabetic Potentials. J. Sci. Food Agric. 2021, 101, 4420–4427. [Google Scholar] [CrossRef]
- Etxeberria, U.; De La Garza, A.L.; Campin, J.; Martnez, J.A.; Milagro, F.I. Antidiabetic Effects of Natural Plant Extracts via Inhibition of Carbohydrate Hydrolysis Enzymes with Emphasis on Pancreatic Alpha Amylase. Expert Opin. Ther. Targets 2012, 16, 269–297. [Google Scholar] [CrossRef]
- Rubilar, M.; Jara, C.; Poo, Y.; Acevedo, F.; Gutierrez, C.; Sineiro, J.; Shene, C. Extracts of Maqui (Aristotelia chilensis) and Murta (Ugni molinae Turcz.): Sources of Antioxidant Compounds and α-Glucosidase/α-Amylase Inhibitors. J. Agric. Food Chem. 2011, 59, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, Y.; Huang, D.; Chen, S.; Xia, Y.; Zhu, S. The Inhibitory Mechanism of Chlorogenic Acid and Its Acylated Derivatives on α-Amylase and α-Glucosidase. Food Chem. 2022, 372, 131334. [Google Scholar] [CrossRef] [PubMed]
- Nazir, N.; Zahoor, M.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.E. Curative Effect of Catechin Isolated from Elaeagnus umbellata Thunb. Berries for Diabetes and Related Complications in Streptozotocin-Induced Diabetic Rats Model. Molecules 2020, 26, 137. [Google Scholar] [CrossRef] [PubMed]
- Costamagna, M.S.; Zampini, I.C.; Alberto, M.R.; Cuello, S.; Torres, S.; Pérez, J.; Quispe, C.; Schmeda-Hirschmann, G.; Isla, M.I. Polyphenols Rich Fraction from Geoffroea decorticans Fruits Flour Affects Key Enzymes Involved in Metabolic Syndrome, Oxidative Stress and Inflammatory Process. Food Chem. 2016, 190, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Batubara, I.; Kuspradini, H.; Muddathir, A.M.; Mitsunaga, T. Intsia palembanica Wood Extracts and Its Isolated Compounds as Propionibacterium Acnes Lipase Inhibitor. J. Wood Sci. 2014, 60, 169–174. [Google Scholar] [CrossRef]
- Dalar, A.; Türker, M.; Zabaras, D.; Konczak, I. Phenolic Composition, Antioxidant and Enzyme Inhibitory Activities of Eryngium bornmuelleri Leaf. Plant Foods Hum. Nutr. 2014, 69, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.F.; El Ashry, F.E.Z.Z.; El Maraghy, N.N.; Fahmy, A. Studies on the Antidiabetic Activities of Momordica charantia Fruit Juice in Streptozotocin-Induced Diabetic Rats. Pharm. Biol. 2017, 55, 758–765. [Google Scholar] [CrossRef]
- Vhora, N.; Naskar, U.; Hiray, A.; Kate, A.S.; Jain, A. Recent Advances In In-Vitro Assays for Type 2 Diabetes Mellitus: An Overview. Rev. Diabet. Stud. 2020, 16, 13–23. [Google Scholar] [CrossRef]
- Zhu, C.; Lü, H.; Du, L.; Li, J.; Chen, H.; Zhao, H.; Wu, W.; Chen, J.; Li, W. Five Blueberry Anthocyanins and Their Antioxidant, Hypoglycemic, and Hypolipidemic Effects In Vitro. Front. Nutr. 2023, 10, 1172982. [Google Scholar] [CrossRef]
- Castro-Acosta, M.L.; Stone, S.G.; Mok, J.E.; Mhajan, R.K.; Fu, C.I.; Lenihan-Geels, G.N.; Corpe, C.P.; Hall, W.L. Apple and Blackcurrant Polyphenol-Rich Drinks Decrease Postprandial Glucose, Insulin and Incretin Response to a High-Carbohydrate Meal in Healthy Men and Women. J. Nutr. Biochem. 2017, 49, 53–62. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Kan, W.-C.; Cheng, T.-J.; Yu, S.-H.; Chang, L.-H.; Chuu, J.-J. Differential Anti-Diabetic Effects and Mechanism of Action of Charantin-Rich Extract of Taiwanese Momordica charantia between Type 1 and Type 2 Diabetic Mice. Food Chem. Toxicol. 2014, 69, 347–356. [Google Scholar] [CrossRef]
- Tian, M.; Zeng, X.-Q.; Song, H.-L.; Hu, S.-X.; Wang, F.-J.; Zhao, J.; Hu, Z.-B. Molecular Diversity and Hypoglycemic Polypeptide-P Content of Momordica charantia in Different Accessions and Different Seasons. J. Sci. Food Agric. 2015, 95, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Mahwish; Saeed, F.; Sultan, M.T.; Riaz, A.; Ahmed, S.; Bigiu, N.; Amarowicz, R.; Manea, R. Bitter Melon (Momordica charantia L.) Fruit Bioactives Charantin and Vicine Potential for Diabetes Prophylaxis and Treatment. Plants 2021, 10, 730. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Xiao, X. Antidiabetic Effect of Hydro-Methanol Extract of Prunus cerasus L Fruits and Identification of Its Bioactive Compounds. Trop. J. Pharm. Res. 2021, 18, 597–602. [Google Scholar] [CrossRef]
- Koprivica, M.R.; Trifković, J.Đ.; Dramićanin, A.M.; Gašić, U.M.; Akšić, M.M.F.; Milojković-Opsenica, D.M. Determination of the Phenolic Profile of Peach (Prunus persica L.) Kernels Using UHPLC–LTQ OrbiTrap MS/MS Technique. Eur. Food Res. Technol. 2018, 244, 2051–2064. [Google Scholar] [CrossRef]
- Iglesias-Carres, L.; Mas-Capdevila, A.; Bravo, F.I.; Bladé, C.; Arola-Arnal, A.; Muguerza, B. Optimization of Extraction Methods for Characterization of Phenolic Compounds in Apricot Fruit (Prunus armeniaca). Food Funct. 2019, 10, 6492–6502. [Google Scholar] [CrossRef] [PubMed]
- Mihailović, N.R.; Mihailović, V.B.; Kreft, S.; Ćirić, A.R.; Joksović, L.G.; Đurđević, P.T. Analysis of Phenolics in the Peel and Pulp of Wild Apples (Malus sylvestris (L.) Mill.). J. Food Compos. Anal. 2018, 67, 1–9. [Google Scholar] [CrossRef]
- Vagiri, M.; Ekholm, A.; Andersson, S.C.; Johansson, E.; Rumpunen, K. An Optimized Method for Analysis of Phenolic Compounds in Buds, Leaves, and Fruits of Black Currant (Ribes nigrum L.). J. Agric. Food Chem. 2012, 60, 10501–10510. [Google Scholar] [CrossRef]
- Pandya, A.; Thiele, B.; Köppchen, S.; Zurita-Silva, A.; Usadel, B.; Fiorani, F. Characterization of Bioactive Phenolic Compounds in Seeds of Chilean Quinoa (Chenopodium quinoa Willd.) Germplasm. Agronomy 2023, 13, 2170. [Google Scholar] [CrossRef]
- Mohamed, M.; Soliman, A.; Abbas, M.; Abd el-lateaf, H.; Ismael, A. Comparative Study for Physico-Chemical Characteristics of Crude Moringa Peregrina, Terminalia bellerica and Tiger Nut Oils. Egypt. J. Chem. 2021, 64, 1–2. [Google Scholar] [CrossRef]
- Varo, M.Á.; Martín-Gómez, J.; Mérida, J.; Serratosa, M.P. Bioactive Compounds and Antioxidant Activity of Highbush Blueberry (Vaccinium corymbosum) Grown in Southern Spain. Eur. Food Res. Technol. 2021, 247, 1199–1208. [Google Scholar] [CrossRef]
- Pop, R.M.; Socaciu, C.; Pintea, A.; Buzoianu, A.D.; Sanders, M.G.; Gruppen, H.; Vincken, J. UHPLC/PDA–ESI/MS Analysis of the Main Berry and Leaf Flavonol Glycosides from Different Carpathian Hippophaë rhamnoides L. Varieties. Phytochem. Anal. 2013, 24, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Guo, X.; Li, T.; Fu, X.; Liu, R.H. Comparative Assessment of Phytochemical Profiles, Antioxidant and Antiproliferative Activities of Sea Buckthorn (Hippophaë rhamnoides L.) Berries. Food Chem. 2017, 221, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Teleszko, M.; Wojdyło, A.; Rudzińska, M.; Oszmiański, J.; Golis, T. Analysis of Lipophilic and Hydrophilic Bioactive Compounds Content in Sea Buckthorn (Hippophaë rhamnoides L.) Berries. J. Agric. Food Chem. 2015, 63, 4120–4129. [Google Scholar] [CrossRef]
- Nambiar, S.S.; Paramesha, M.; Shetty, N.P. Comparative Analysis of Phytochemical Profile, Antioxidant Activities and Foam Prevention Abilities of Whole Fruit, Pulp and Seeds of Emblica officinalis. J. Food Sci. Technol. 2015, 52, 7254–7262. [Google Scholar] [CrossRef]
- Almeida, É.S.; de Oliveira, D.; Hotza, D. Properties and Applications of Morinda citrifolia (Noni): A Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 883–909. [Google Scholar] [CrossRef]
- Ali, M.Y.; Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional Composition and Bioactive Compounds in Tomatoes and Their Impact on Human Health and Disease: A Review. Foods 2021, 10, 45. [Google Scholar] [CrossRef]
- Banihani, S.; Swedan, S.; Alguraan, Z. Pomegranate and Type 2 Diabetes. Nutr. Res. 2013, 33, 341–348. [Google Scholar] [CrossRef]
- Ballistreri, G.; Fabroni, S.; Romeo, F.V.; Timpanaro, N.; Amenta, M.; Rapisarda, P. Anthocyanins and Other Polyphenols in Citrus Genus: Biosynthesis, Chemical Profile, and Biological Activity. In Polyphenols in Plants; Elsevier: Amsterdam, The Netherlands, 2019; pp. 191–215. ISBN 9780128137680. [Google Scholar] [CrossRef]
- Cai, R.; Hettiarachchy, N.S.; Jalaluddin, M. High-Performance Liquid Chromatography Determination of Phenolic Constituents in 17 Varieties of Cowpeas. J. Agric. Food Chem. 2003, 51, 1623–1627. [Google Scholar] [CrossRef]
- Salas-Arias, K.; Irías-Mata, A.; Sánchez-Kopper, A.; Hernández-Moncada, R.; Salas-Morgan, B.; Villalta-Romero, F.; Calvo-Castro, L.A. Strawberry Fragaria x ananassa Cv. Festival: A Polyphenol-Based Phytochemical Characterization in Fruit and Leaf Extracts. Molecules 2023, 28, 1865. [Google Scholar] [CrossRef]
- Šedbarė, R.; Siliņa, D.; Janulis, V. Evaluation of the Phytochemical Composition of Phenolic and Triterpene Compounds in Fruit of Large Cranberries (Vaccinium macrocarpon Aiton) Grown in Latvia. Plants 2022, 11, 2725. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.; Talcott, S.T. Chemical Composition, Antioxidant Properties, and Thermal Stability of a Phytochemical Enriched Oil from Açai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef]
- Cebadera, L.; Dias, M.I.; Barros, L.; Fernández-Ruiz, V.; Cámara, R.M.; Del Pino, Á.; Santos-Buelga, C.; Ferreira, I.C.F.R.; Morales, P.; Cámara, M. Characterization of Extra Early Spanish Clementine Varieties (Citrus clementina Hort Ex Tan) as a Relevant Source of Bioactive Compounds with Antioxidant Activity. Foods 2020, 9, 642. [Google Scholar] [CrossRef]
- Gargi, B.; Semwal, P.; Jameel Pasha, S.B.; Singh, P.; Painuli, S.; Thapliyal, A.; Cruz-Martins, N. Revisiting the Nutritional, Chemical and Biological Potential of Cajanus cajan (L.) Millsp. Molecules 2022, 27, 6877. [Google Scholar] [CrossRef]
- Kottaisamy, C.P.D.; Raj, D.S.; Prasanth Kumar, V.; Sankaran, U. Experimental Animal Models for Diabetes and Its Related Complications—A Review. Lab. Anim. Res. 2021, 37, 23. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, G.; Su, X.; Yang, H.; Zhang, J. Antiobesity Action of a Daidzein Derivative on Male Obese Mice Induced by a High-Fat Diet. Nutr. Res. 2009, 29, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Nerurkar, P.V.; Nishioka, A.; Eck, P.O.; Johns, L.M.; Volper, E.; Nerurkar, V.R. Regulation of Glucose Metabolism via Hepatic forkhead Transcription Factor 1 (FoxO1) by Morinda citrifolia (noni) in High-Fat Diet-Induced Obese Mice. Br. J. Nutr. 2012, 108, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Lee, J.; Choi, R.; Lee, H.-I.; Lee, J.-H.; Jeong, Y.; Kim, M.; Lee, M. Anti-Obesity and Anti-Insulin Resistance Effects of Tomato Vinegar Beverage in Diet-Induced Obese Mice. Food Funct. 2014, 5, 1579. [Google Scholar] [CrossRef] [PubMed]
- Chudnovskiy, R.; Thompson, A.; Tharp, K.; Hellerstein, M.; Napoli, J.L.; Stah, A. Consumption of Clarified Grapefruit Juice Ameliorates High-Fat Diet Induced Insulin Resistance and Weight Gain in Mice. PLoS ONE 2014, 9, e108408. [Google Scholar] [CrossRef] [PubMed]
- Variya, B.C.; Bakrania, A.K.; Patel, S.S. Antidiabetic Potential of Gallic Acid from Emblica officinalis: Improved Glucose Transporters and Insulin Sensitivity through PPAR-γ and Akt Signaling. Phytomedicine 2020, 73, 152906. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, M.; Sato, Y.; Kondo, S.; Adachi, S.I.; Yoshizawa, F.; Sato, Y. Quercetin enhances fatty acid β-oxidation by inducing lipophagy in AML12 hepatocytes. Heliyon 2021, 7, e07324. [Google Scholar] [CrossRef]
- van Raalte, D.H.; Li, M.; Pritchard, P.H.; Wasan, K.M. Peroxisome Proliferator-Activated Receptor (PPAR)-: A Pharmacological Target with a Promising Future. Pharm. Res. 2004, 21, 1531–1538. [Google Scholar] [CrossRef] [PubMed]
- Ariviani, S.; Affandi, D.R.; Listyaningsih, E.; Handajani, S. The Potential of Pigeon Pea (Cajanus cajan) Beverage as an Anti-diabetic Functional Drink. IOP Conf. Ser. Earth Environ. Sci. 2018, 102, 012054. [Google Scholar] [CrossRef]
- Bolsinger, J.; Pronczuk, A.; Sambanthamurthi, R.; Hayes, K.C. Anti-Diabetic Effects of Palm Fruit Juice in the Nile Rat (Arvicanthis niloticus). J. Nutr. Sci. 2014, 3, e5. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wen, J.-J.; Hu, J.-L.; Nie, Q.-X.; Chen, H.H.; Xiong, T.; Nie, S.-P.; Xie, M.-Y. Fermented Momordica charantia L. Juice Modulates Hyperglycemia, Lipid Profile, and Gut Microbiota in Type 2 Diabetic Rats. Food Res. Int. 2019, 121, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Iwansyah, A.C.; Luthfiyanti, R.; Ardiansyah, R.C.E.; Rahman, N.; Andriana, Y.; Hamid, H.A. Antidiabetic Activity of Physalis angulata L. Fruit juIce on Streptozotocin-Induced Diabetic Rats. S. Afr. J. Bot. 2022, 145, 313–319. [Google Scholar] [CrossRef]
- Hu, J.; Nie, S.; Xie, M. Antidiabetic Mechanism of Dietary Polysaccharides Based on Their Gastrointestinal Functions. J. Agric. Food Chem. 2018, 66, 4781–4786. [Google Scholar] [CrossRef] [PubMed]
- Association, A.D. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2009, 32, S62–S67. [Google Scholar] [CrossRef]
- Shi, G.-J.; Li, Y.; Cao, Q.-H.; Wu, H.-X.; Tang, X.-Y.; Gao, X.-H.; Yu, J.-Q.; Chen, Z.; Yang, Y. In Vitro and In Vivo Evidence That Quercetin Protects against Diabetes and Its Complications: A Systematic Review of the Literature. Biomed. Pharmacother. 2019, 109, 1085–1099. [Google Scholar] [CrossRef]
- Youl, E.; Bardy, G.; Magous, R.; Cros, G.; Sejalon, F.; Virsolvy, A.; Richard, S.; Quignard, J.F.; Gross, R.; Petit, P.; et al. Quercetin Potentiates Insulin Secretion and Protects INS-1 Pancreatic-Cells against Oxidative Damage via the ERK1/2 Pathway. Br. J. Pharmacol. 2010, 161, 799–814. [Google Scholar] [CrossRef]
- Adewole, S.; Caxton-Martins, E.; Ojewole, J. Protective Effect of Quercetin on the Morphology of Pancreatic β-Cells of Streptozotocin-Treated Diabetic Rats. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.K.; Gao, J.; Zhu, D.N. Kaempferol and Quercetin Isolated from Euonymus alatus Improve Glucose Uptake of 3T3-L1 Cells without Adipogenesis Activity. Life Sci. 2008, 82, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, D.; Gao, X.; Parry, J.; Liu, K.; Liu, B.; Wang, M. Quercetin and Quercetin-3-O-Glucuronide Are Equally Effective in Ameliorating Endothelial Insulin Resistance through Inhibition of Reactive Oxygen Species-Associated Inflammation. Mol. Nutr. Food Res. 2013, 57, 1037–1045. [Google Scholar] [CrossRef]
- Soltesova-Prnova, M.; Milackova, I.; Stefek, M. 3′-O-(3-Chloropivaloyl)Quercetin, α-Glucosidase Inhibitor with Multi-Targeted Therapeutic Potential in Relation to Diabetic Complications. Chem. Pap. 2016, 70, 1439–1444. [Google Scholar] [CrossRef]
- Kamiya, K.; Hamabe, W.; Harada, S.; Murakami, R.; Tokuyama, S.; Satake, T. Chemical Constituents of Morinda citrifolia Roots Exhibit Hypoglycemic Effects in Streptozotocin-Induced Diabetic Mice. Biol. Pharm. Bull. 2008, 31, 935–938. [Google Scholar] [CrossRef]
- O’Neill, H.M. AMPK and Exercise: Glucose Uptake and Insulin Sensitivity. Diabetes Metab 2013, 37, 155. [Google Scholar] [CrossRef]
- Prabhakar, P.K.; Doble, M. Mechanism of Action of Natural Products Used in the Treatment of Diabetes Mellitus. Chin. J. Integr. Med. 2011, 17, 563–574. [Google Scholar] [CrossRef]
- Pitipanapong, J.; Chitprasert, S.; Goto, M.; Jiratchariyakul, W.; Sasaki, M.; Shotipruk, A. New Approach for Extraction of Charantin from Momordica charantia with Pressurized Liquid Extraction. Sep. Purif. Technol. 2007, 52, 416–422. [Google Scholar] [CrossRef]
- Eze, E.D.; Tanko, Y.; Tende, J.A.; Ehinomhen, U.A. Effects of Lycopene on Liver Markers and Glucokinase Activity in Experimentally-induced Diabetes Mellitus Rat Model. J. Basic Appl. Res 2016, 2, 353–362. [Google Scholar]
- Huang, S.; Czech, M.P. The GLUT4 Glucose Transporter. Cell Metab. 2007, 5, 237–252. [Google Scholar] [CrossRef]
- Kouznetsova, V.L.; Hauptschein, M.; Tsigelny, I.F. Glucose and Lipid Transporters Roles in Type 2 Diabetes. Integr. Obes. Diabetes 2017, 3, 1–6. [Google Scholar] [CrossRef]
- Wang, D.; Yan, J.; Chen, J.; Wu, W.; Zhu, X.; Wang, Y. Naringin Improves Neuronal Insulin Signaling, Brain Mitochondrial Function, and Cognitive Function in High-Fat Diet-Induced Obese Mice. Cell. Mol. Neurobiol. 2015, 35, 1061–1071. [Google Scholar] [CrossRef]
- Leow, S.; Bolsinger, J.; Pronczuk, A.; Hayes, K.C.; Sambanthamurthi, R. Hepatic Transcriptome Implications for Palm Fruit Juice Deterrence of Type 2 Diabetes Mellitus in Young Male Nile Rats. Genes Nutr. 2016, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayo, C.; Salomon, C.; Zuñiga, F.A. Association between Insulin Resistance and the Development of Cardiovascular Disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.J. Insulin Resistance as the Core Defect in Type 2 Diabetes Mellitus. Am. J. Cardiol. 2002, 90, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Vergès, B. Pathophysiology of Diabetic Dyslipidaemia: Where Are We? Diabetologia 2015, 58, 886–899. [Google Scholar] [CrossRef] [PubMed]
- Dikshit, P.; Shukla, K.; Tyagi, M.K.; Garg, P.; Gambhir, J.K.; Shukla, R. Antidiabetic and Antihyperlipidemic Effects of the Stem of Musa sapientum Linn. in Streptozotocin-Induced Diabetic Rats. J. Diabetes 2012, 4, 378–385. [Google Scholar] [CrossRef]
- Koebnick, C.; Garcia, A.L.; Dagnelie, P.C.; Strassner, C.; Lindemans, J.; Katz, N.; Leitzmann, C.; Hoffmann, I. Long-Term Consumption of a Raw Food Diet is Associated with Favorable Serum LDL Cholesterol and Triglycerides But Also with Elevated Plasma Homocysteine and Low Serum HDL Cholesterol in Humans. J. Nutr. 2005, 135, 2372–2378. [Google Scholar] [CrossRef]
- Ullah, A.; Khan, A.; Khan, I. Diabetes Mellitus and Oxidative Stress—A Concise Review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef]
- Banihani, S.A.; Makahleh, S.M.; El-Akawi, Z.; Al-Fashtaki, R.A.; Khabour, O.F.; Gharibeh, M.Y.; Saadah, N.A.; Al-Hashimi, F.H.; Al-Khasieb, N.J. Fresh Pomegranate Juice Ameliorates Insulin Resistance, Enhances β-Cell Function, and Decreases Fasting Serum Glucose in Type 2 Diabetic Patients. Nutr. Res. 2014, 34, 862–867. [Google Scholar] [CrossRef]
- Hontecillas, R.; O’Shea, M.; Einerhand, A.; Diguardo, M.; Bassaganya-Riera, J. Activation of PPAR γ and α by Punicic Acid Ameliorates Glucose Tolerance and Suppresses Obesity-Related Inflammation. J. Am. Coll. Nutr. 2009, 28, 184–195. [Google Scholar] [CrossRef]
- Koren-Gluzer, M.; Aviram, M.; Meilin, E.; Hayek, T. The Antioxidant HDL-Associated Paraoxonase-1 (PON1) Attenuates Diabetes Development and Stimulates β-Cell Insulin Release. Atherosclerosis 2011, 219, 510–518. [Google Scholar] [CrossRef]
- Devaki, C.S.; Premavalli, K.S. Evaluation of Supplementation of Bittergourd Fermented Beverage to Diabetic Subjects. J. Pharm. Nutr. Sci. 2014, 4, 27–36. [Google Scholar] [CrossRef]
- Paquette, M.; Medina Larqué, A.S.; Weisnagel, S.J.; Desjardins, Y.; Marois, J.; Pilon, G.; Dudonné, S.; Marette, A.; Jacques, H. Strawberry and Cranberry Polyphenols Improve Insulin Sensitivity in Insulin-Resistant, Non-Diabetic Adults: A Parallel, Double-Blind, Controlled and Randomised Clinical Trial. Br. J. Nutr. 2017, 117, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Simbo, S.Y.; Fang, C.; McAlister, L.; Roque, A.; Banerjee, N.; Talcott, S.T.; Zhao, H.; Kreider, R.B.; Mertens-Talcott, S.U. Açaí (Euterpe oleracea Mart.) Beverage Consumption Improves Biomarkers for Inflammation but Not Glucose- or Lipid-Metabolism in Individuals with Metabolic Syndrome in a Randomized, Double-Blinded, Placebo-Controlled Clinical Trial. Food Funct. 2018, 9, 3097–3103. [Google Scholar] [CrossRef] [PubMed]
- Aktan, A.; Ozcelik, A.; Cure, E.; Cure, M.; Yuce, S. Profound Hypoglycemia-Induced by Vaccinium corymbosum Juice and Laurocerasus Fruit. Indian J. Pharmacol. 2014, 46, 446–447. [Google Scholar] [CrossRef] [PubMed]
- Hasniyati, R.; Yuniritha, E.; Fadri, R.A. The Efficacy of Therapeutic-Diabetes Mellitus Functional Drink on Blood Glucose and Plasma Malondialdehyde (MDA) Levels of Type 2 Diabetes Mellitus Patients. In Proceedings of the 1st Lekantara Annual Conference on Natural Science and Environment (LeNS), Malang, Indonesia, 30 September 2021; Volume 1097, p. 012021. [Google Scholar] [CrossRef]
- Sequeira, I.; Poppitt, S. Unfolding Novel Mechanisms of Polyphenol Flavonoids for Better Glycaemic Control: Targeting Pancreatic Islet Amyloid Polypeptide (IAPP). Nutrients 2017, 9, 788. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified Anthocyanin Supplementation Reduces Dyslipidemia, Enhances Antioxidant Capacity, and Prevents Insulin Resistance in Diabetic Patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef]
- Cladis, D.P.; Li, S.; Reddivari, L.; Cox, A.; Ferruzzi, M.G.; Weaver, C.M. A 90 Day Oral Toxicity Study of Blueberry Polyphenols in Ovariectomized Sprague-Dawley Rats. Food Chem. Toxicol. 2020, 139, 111254. [Google Scholar] [CrossRef]
- Cladis, D.P.; Weaver, C.M.; Ferruzzi, M.G. (Poly)Phenol Toxicity In Vivo Following Oral Administration: A Targeted Narrative Review of (Poly)Phenols from Green Tea, Grape, and Anthocyanin-Rich Extracts. Phyther. Res. 2022, 36, 323–335. [Google Scholar] [CrossRef]
- Patel, S.S.; Goyal, R.K. Prevention of Diabetes-Induced Myocardial Dysfunction in Rats Using the Juice of the Emblica officinalis Fruit. Exp. Clin. Cardiol. 2011, 16, 87–91. [Google Scholar] [PubMed]
- Dhuique-mayer, C.; Gence, L.; Portet, K.; Tousch, D.; Poucheret, P. Preventive Action of Retinoids in Metabolic Syndrome/Type 2 Diabetic Rats Fed with Citrus Functional Food Enriched in β-Cryptoxanthin Claudie. Food Funct. 2020, 11, 9263–9271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, S.; Zhou, T.; Zhang, P.; Li, H. Bin Alcoholic Beverage Consumption and Chronic Diseases. Int. J. Environ. Res. Public Health 2016, 13, 522. [Google Scholar] [CrossRef] [PubMed]
- Conigrave, K.M.; Rimm, E.B. Alcohol for the Prevention of Type 2 Diabetes Mellitus? Treat. Endocrinol. 2003, 2, 145–152. [Google Scholar] [CrossRef]
- Sanz, M. Inositols and Carbohydrates in Different Fresh Fruit Juices. Food Chem. 2004, 87, 325–328. [Google Scholar] [CrossRef]
- Lifschitz, C.H. Carbohydrate Absorption From Fruit Juices in Infants. Pediatrics 2000, 105, e4. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Li, T.Y.; Joshipura, K.J.; Hu, F.B. Intake of Fruit, Vegetables, and Fruit Juices and Risk of Diabetes in Women. Diabetes Care 2008, 31, 1311–1317. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, F.; Lahlou, R.A.; Pires, P.; Salgado, M.; Silva, L.R. Natural Functional Beverages as an Approach to Manage Diabetes. Int. J. Mol. Sci. 2023, 24, 16977. https://doi.org/10.3390/ijms242316977
Carvalho F, Lahlou RA, Pires P, Salgado M, Silva LR. Natural Functional Beverages as an Approach to Manage Diabetes. International Journal of Molecular Sciences. 2023; 24(23):16977. https://doi.org/10.3390/ijms242316977
Chicago/Turabian StyleCarvalho, Filomena, Radhia Aitfella Lahlou, Paula Pires, Manuel Salgado, and Luís R. Silva. 2023. "Natural Functional Beverages as an Approach to Manage Diabetes" International Journal of Molecular Sciences 24, no. 23: 16977. https://doi.org/10.3390/ijms242316977
APA StyleCarvalho, F., Lahlou, R. A., Pires, P., Salgado, M., & Silva, L. R. (2023). Natural Functional Beverages as an Approach to Manage Diabetes. International Journal of Molecular Sciences, 24(23), 16977. https://doi.org/10.3390/ijms242316977






