Genome-Wide Identification of Catalase Gene Family and the Function of SmCAT4 in Eggplant Response to Salt Stress
Abstract
:1. Introduction
2. Results
2.1. Identification and Physicochemical Property Analysis of Catalase Proteins in Eggplant
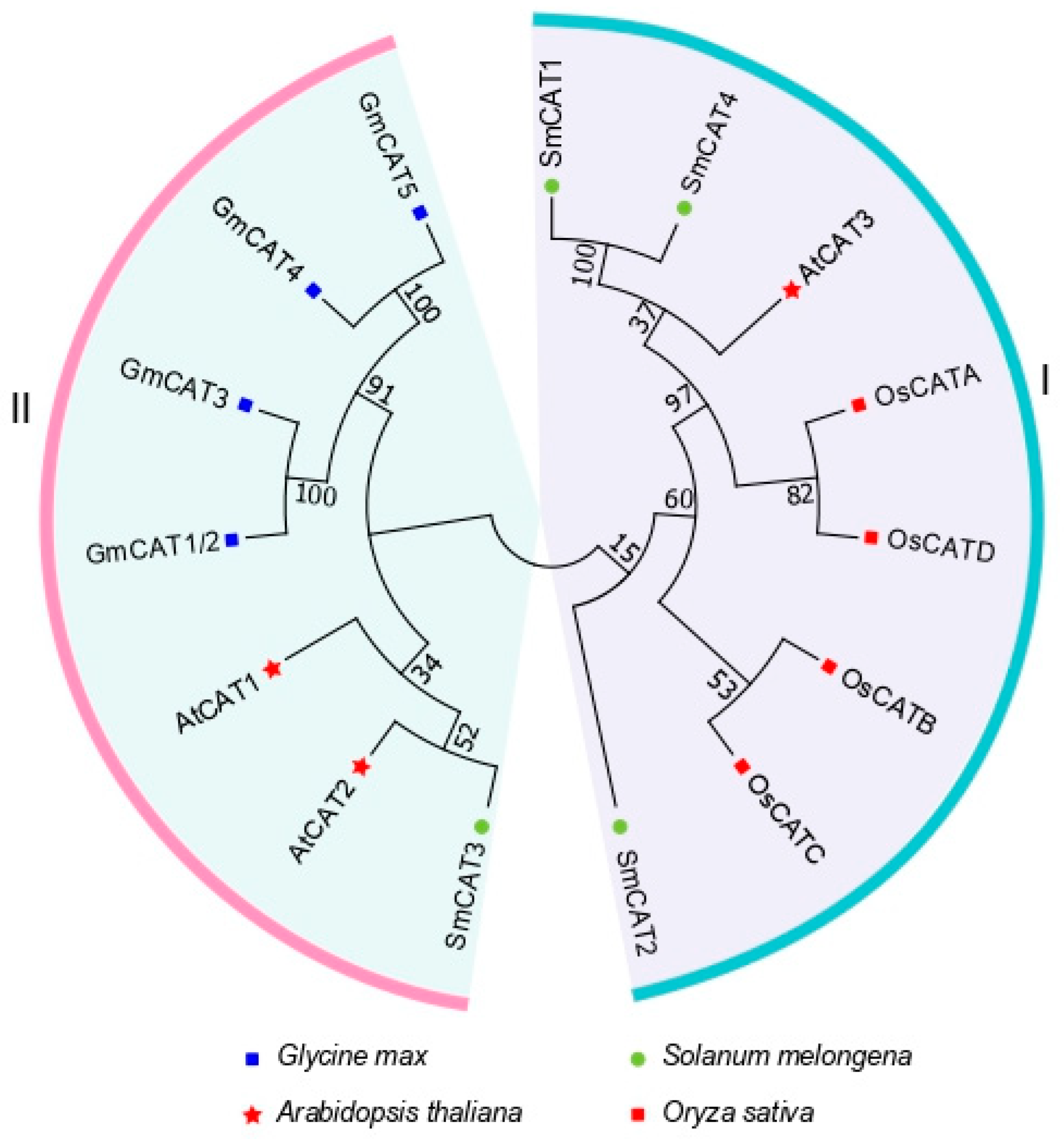
2.2. Phylogenic Analysis of Catalase Proteins
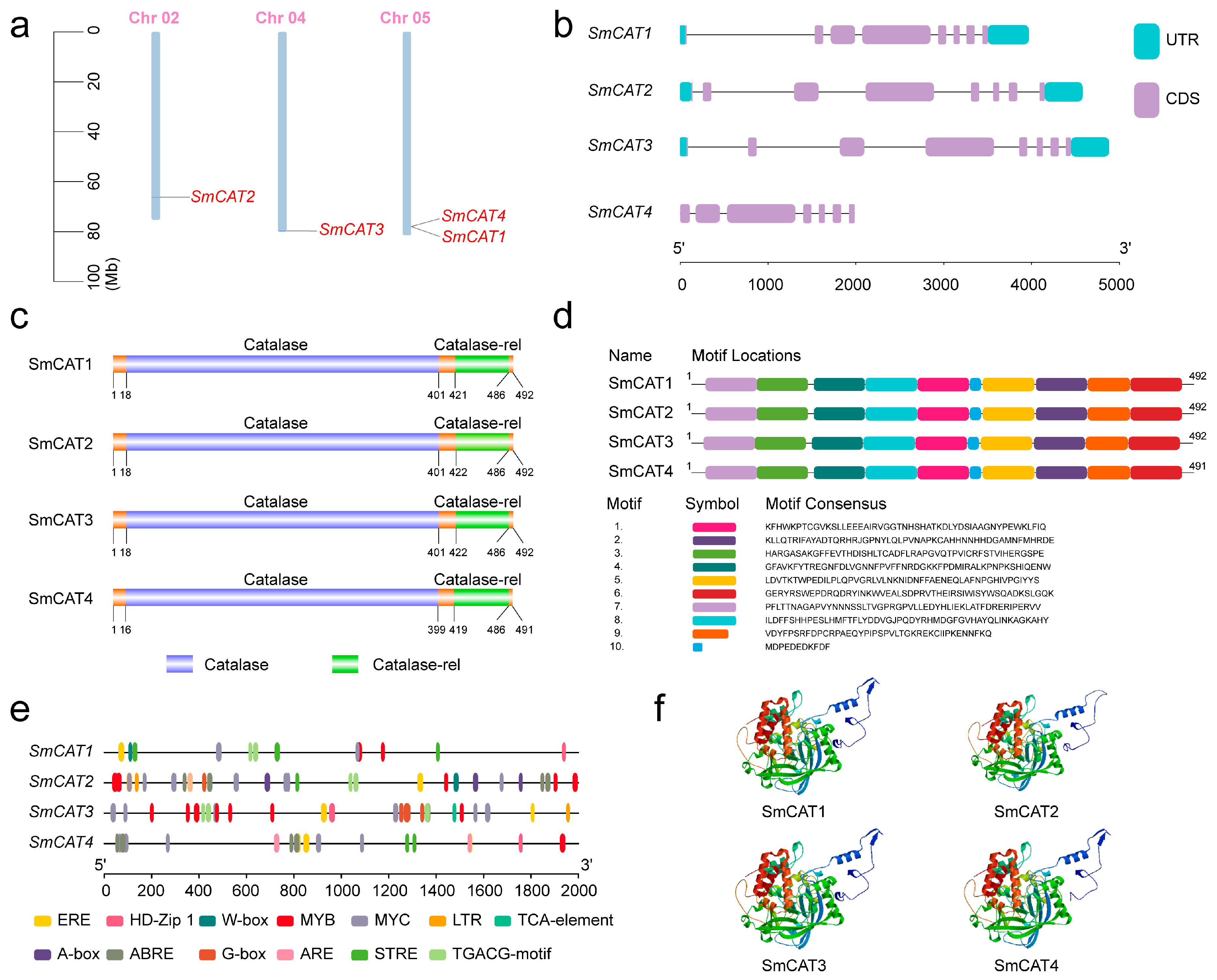
2.3. Sequence Analysis of CAT Genes in Eggplant
2.4. Collinearity Analysis of Eggplant CAT Genes and Their Homologs from Arabidopsis and Tomato
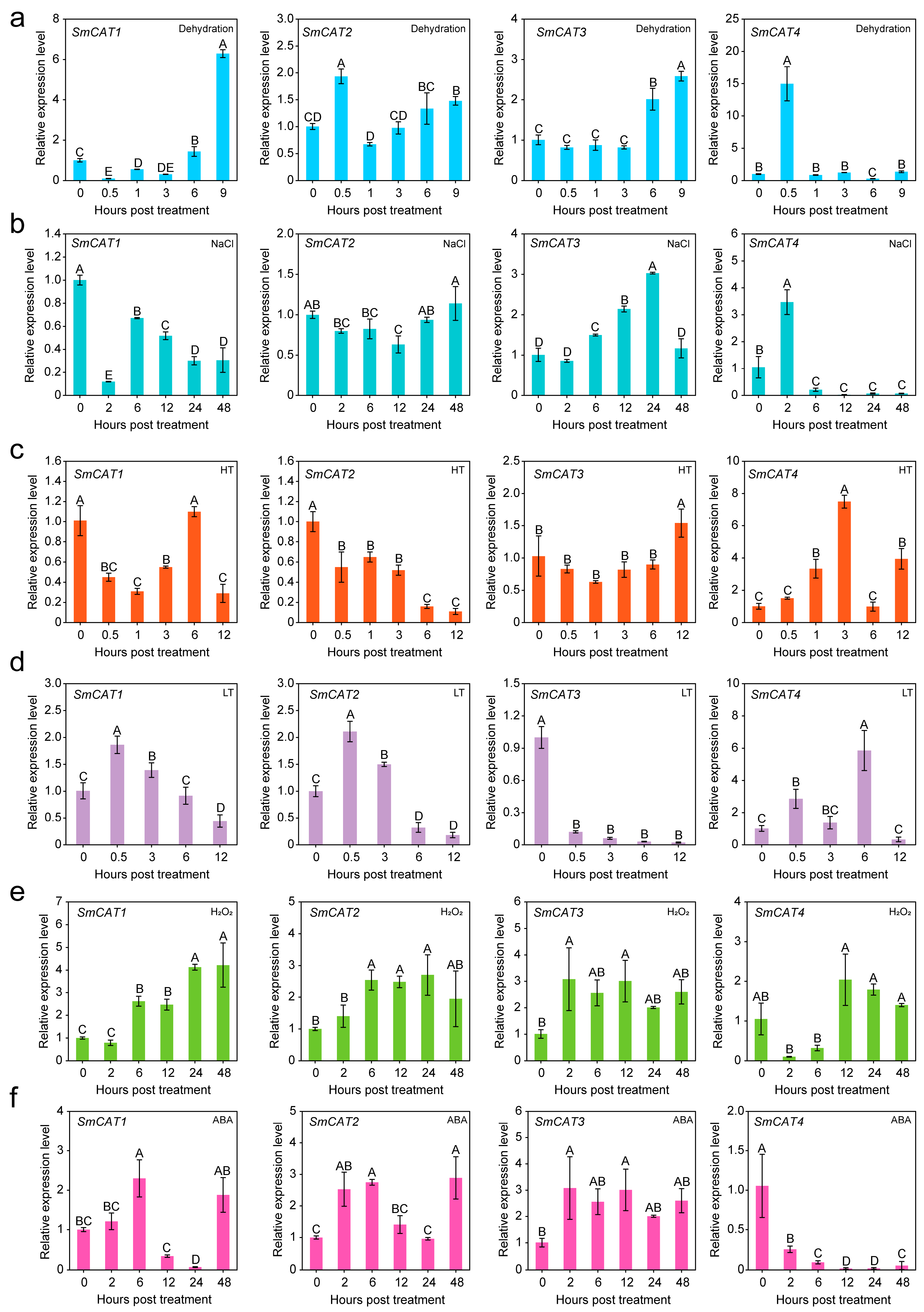
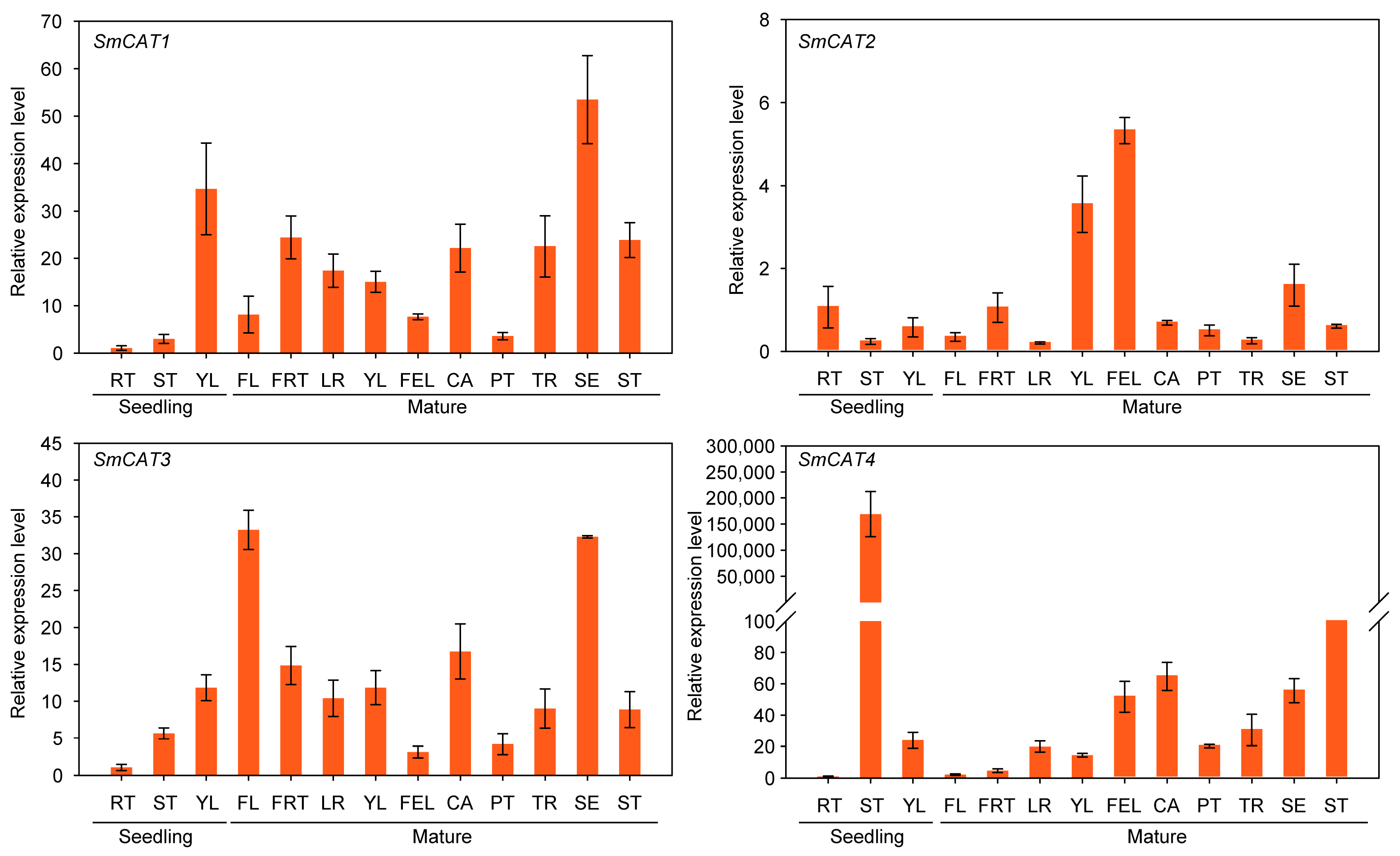
2.5. Expression Analysis of Eggplant CATs under Abiotic Stress Treatment and in Different Tissues
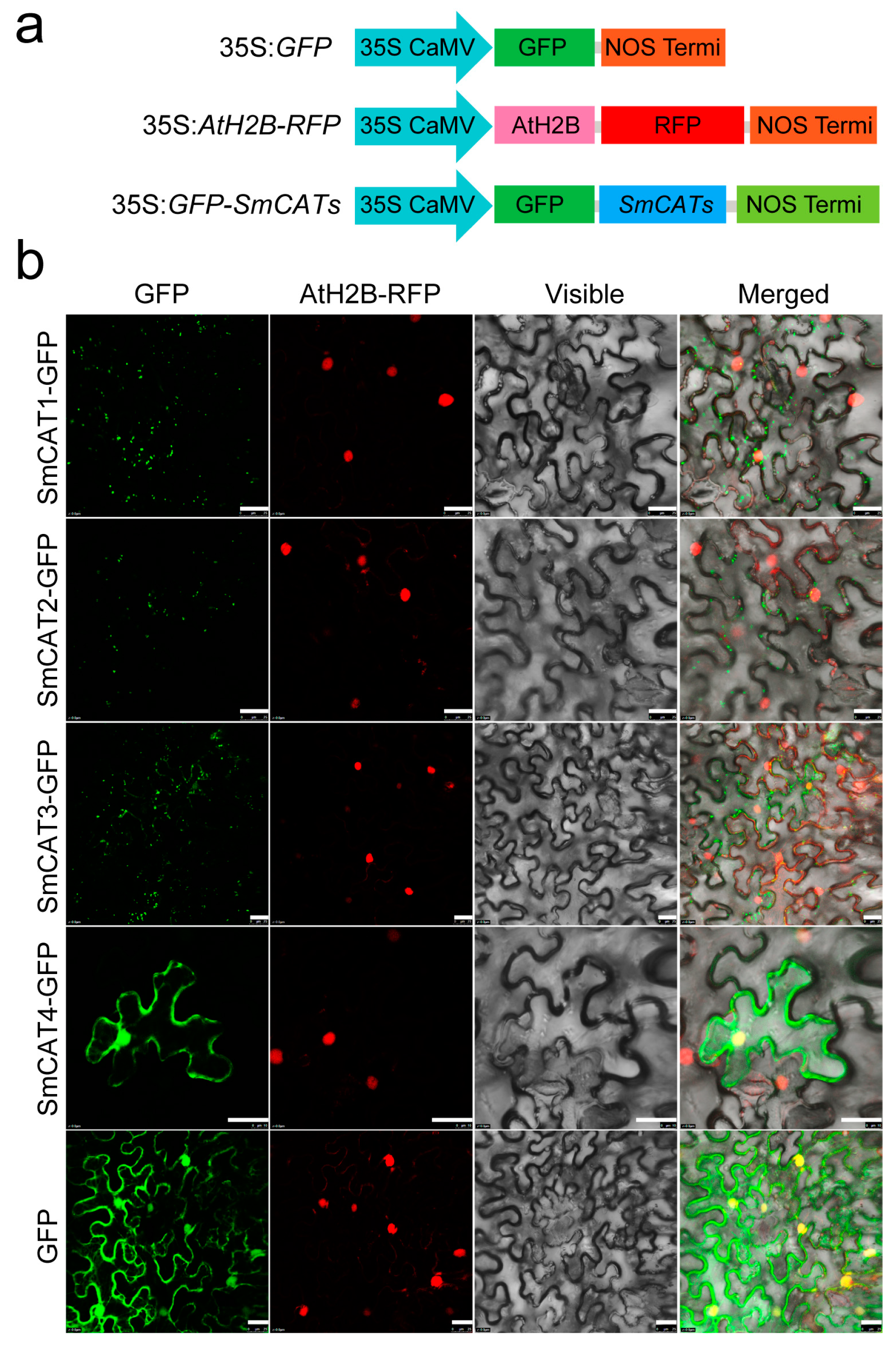
2.6. Subcellular Localization of Eggplant CAT Proteins
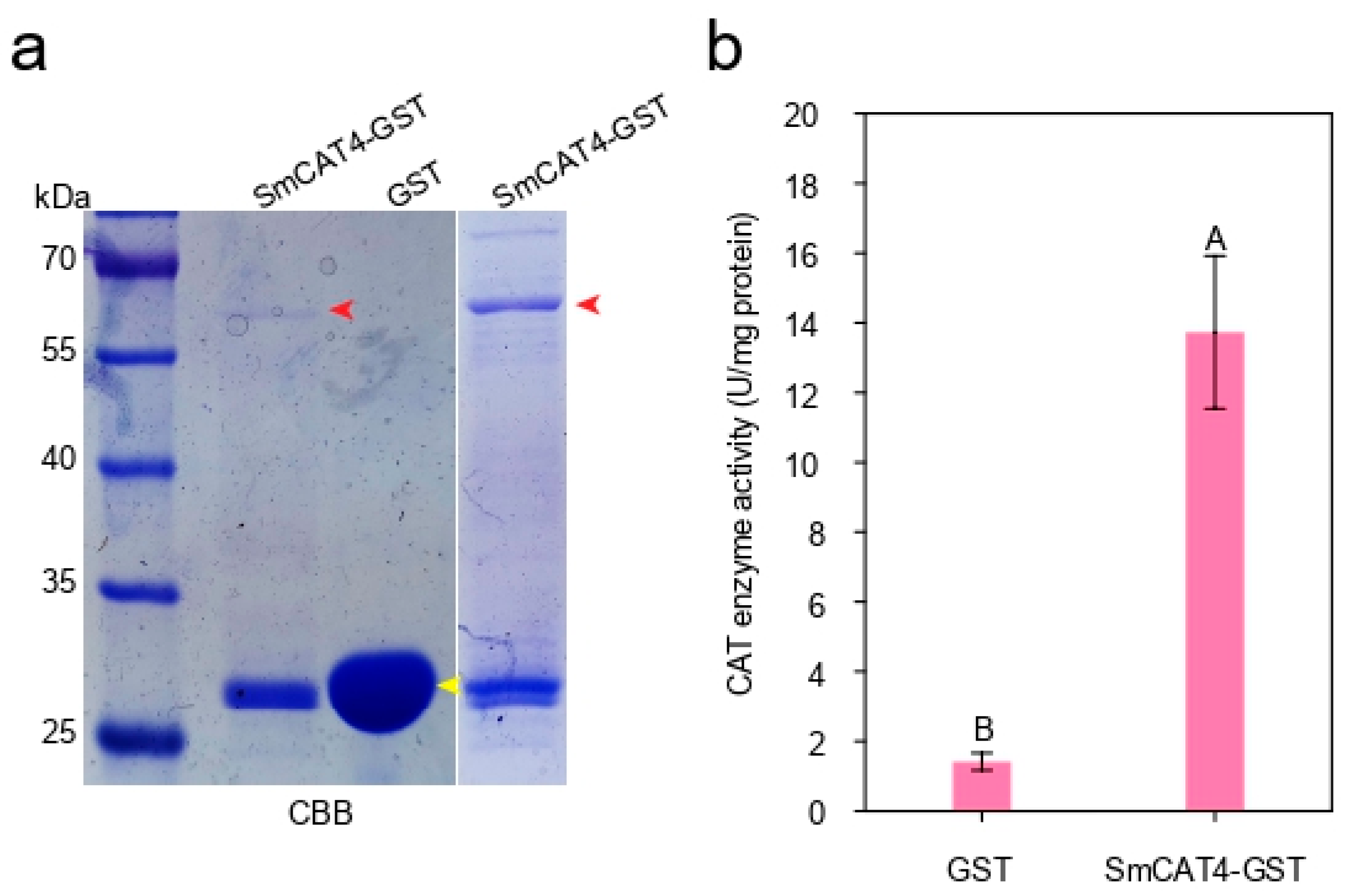
2.7. Recombinant SmCAT4 Enzyme Activity Analysis
2.8. Silencing of SmCAT4 Enhanced Susceptibility of Eggplant to Salt Stress
3. Discussion
4. Material and Methods
4.1. Identification of CAT Genes in Solanum melongena Genome
4.2. Physicochemical Properties Analysis of CAT Proteins in Eggplant
4.3. Multiple Sequence Alignment and Phylogenetic Analysis
4.4. Analysis of Chromosomal Location, Gene Structure, Conserved Domain and Motif, cis-Elements, and Tertiary Structure of Eggplant CATs
4.5. Collinearity Analysis
4.6. Plant Materials and Growth Conditions
4.7. Salt Stress, Dehydration, High Temperature, Low Temperature, ABA, and H2O2 Treatment
4.8. Plant Total RNA Extraction, cDNA Synthesis and RT-qPCR Analysis
4.9. Vector Construction
4.10. Agrobacterium tumefaciens Cultivation and Infiltration and Subcellular Localization Analysis
4.11. VIGS Assay
4.12. Prokaryotic Expression and Purification of Recombinant Protein
4.13. Recombinant CAT Enzyme Assay
4.14. Physiological Index Measurement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Chen, M.; Khan, A.H.; Ma, Y.; He, X.; Yang, J.; Zhang, R.; Ma, H.; Zuo, C.; Li, Y.; et al. Histone H3 lysine 27 trimethylation suppresses jasmonate biosynthesis and signaling to affect male fertility under high temperature in cotton. Plant Commun. 2023, 4, 100660. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Song, Z.; Xie, Y.; Cheng, H.; Yan, H.; Sun, F.; Liu, H.; Shen, J.; Li, L.; He, X.; et al. High temperature inhibits vascular development via the PIF4-miR166-HB15 module in Arabidopsis. Curr. Biol. 2023, 33, 3203–3214.e4. [Google Scholar] [CrossRef]
- Gu, S.; Zhang, Z.; Li, J.; Sun, J.; Cui, Z.; Li, F.; Zhuang, J.; Chen, W.; Su, C.; Wu, L.; et al. Natural variation in OsSEC13 HOMOLOG 1 modulates redox homeostasis to confer cold tolerance in rice. Plant Physiol. 2023, 193, 2180–2196. [Google Scholar] [CrossRef]
- An, J.P.; Liu, Z.Y.; Zhang, X.W.; Wang, D.R.; Zeng, F.; You, C.X.; Han, Y. Brassinosteroid signaling regulator BIM1 integrates brassinolide and jasmonic acid signaling during cold tolerance in apple. Plant Physiol. 2023, 193, 1652–1674. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.; Yang, J.; Mei, Q.; Jia, D.; Yan, P.; Feng, B.; Mamat, A.; Gong, X.; Guan, Q.; Mao, K.; et al. MdNAC104 positively regulates apple cold tolerance via CBF-dependent and CBF-independent pathways. Plant Biotechnol. J. 2023, 21, 2057–2073. [Google Scholar] [CrossRef]
- Hu, Y.; Zeng, L.; Lv, X.; Guo, J.; Li, X.; Zhang, X.; Wang, D.; Wang, J.; Bi, J.; Julkowska, M.M.; et al. NIGT1.4 maintains primary root elongation in response to salt stress through induction of ERF1 in Arabidopsis. Plant J. 2023, 116, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qiu, L.; Mei, Q.; Sun, Y.; Li, N.; Gong, X.; Ma, F.; Mao, K. MdHB7-like positively modulates apple salt tolerance by promoting autophagic activity and Na(+) efflux. Plant J. 2023, 116, 669–689. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, W.; Ding, C.; Hu, Z.; Fan, C.; Zhang, J.; Li, Z.; Diao, S.; Shen, L.; Zhang, B.; et al. A breeding strategy for improving drought and salt tolerance of poplar based on CRISPR/Cas9. Plant Biotechnol. J. 2023, 21, 2160–2162. [Google Scholar] [CrossRef]
- Gautam, N.; Tiwari, M.; Kidwai, M.; Dutta, P.; Chakrabarty, D. Functional characterization of rice metallothionein OsMT-I-Id: Insights into metal binding and heavy metal tolerance mechanisms. J. Hazard. Mater. 2023, 458, 131815. [Google Scholar] [CrossRef]
- Xia, J.; Guo, Z.; Yang, Z.; Han, H.; Wang, S.; Xu, H.; Yang, X.; Yang, F.; Wu, Q.; Xie, W.; et al. Whitefly hijacks a plant detoxification gene that neutralizes plant toxins. Cell 2021, 184, 1693–1705.e17. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Y.; Liu, X.; Francis, F.; Fan, J.; Liu, H.; Wang, Q.; Sun, Y.; Zhang, Y.; Chen, J. SmCSP4 from aphid saliva stimulates salicylic acid-mediated defence responses in wheat by interacting with transcription factor TaWKRY76. Plant Biotechnol. J. 2023, 21, 2389–2407. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, X.; Song, J.; Wang, H.; Ruan, C.; Zhang, W.; Guo, Z.; Li, W.; Guo, W. Cotton peroxisome-localized lysophospholipase counteracts the toxic effects of Verticillium dahliae NLP1 and confers wilt resistance. Plant J. 2023, 115, 452–469. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Cai, W.; Wu, R.; Huang, Y.; Lu, Q.; Hui, W.; Huang, X.; Zhang, Y.; Wu, Q.; Cheng, X.; et al. Differential CaKAN3-CaHSF8 associations underlie distinct immune and heat responses under high temperature and high humidity conditions. Nat. Commun. 2023, 14, 4477. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.B.; Ghosh, A. Comprehensive analysis and transcript profiling of Arabidopsis thaliana and Oryza sativa catalase gene family suggests their specific roles in development and stress responses. Plant Physiol. Biochem. 2018, 123, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Mhamdi, A.; Queval, G.; Chaouch, S.; Vanderauwera, S.; Van Breusegem, F.; Noctor, G. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J. Exp. Bot. 2010, 61, 4197–4220. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Wang, M.R.; Wang, Q.C. ROS-induced oxidative stress in plant cryopreservation: Occurrence and alleviation. Planta 2021, 254, 124. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Ahmed, W.; Reiter, R.J.; Niu, M.; Hameed, S. Melatonin: Current status and future perspectives in plant science. Front. Plant Sci. 2015, 6, 1230. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic acid and abiotic stress tolerance in crop plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, L.; Yun, L.; Ji, L.; Li, G.; Ji, M.; Shi, Y.; Zheng, X. Catalase (CAT) gene family in wheat (Triticum aestivum L.): Evolution, expression pattern and function analysis. Int. J. Mol. Sci. 2022, 23, 524. [Google Scholar] [CrossRef]
- Hu, L.; Yang, Y.; Jiang, L.; Liu, S. The catalase gene family in cucumber: Genome-wide identification and organization. Genet. Mol. Biol. 2016, 39, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.Y.; Wang, P.C.; Chen, J.; Song, C.P. Comprehensive functional analysis of the catalase gene family in Arabidopsis thaliana. J. Integr. Plant Biol. 2008, 50, 1318–1326. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Ye, Q.; Wu, Z.; Zhang, Q.; Wang, L.; Liu, J.; Hu, X.; Guo, D.; Wang, X.; Zhang, Z.; et al. Analysis of CAT gene family and functional identification of OsCAT3 in rice. Genes 2023, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cheng, Y.; Chen, D.; Liu, D.; Hu, M.; Dong, J.; Zhang, X.; Song, L.; Shen, F. The catalase gene family in cotton: Genome-wide characterization and bioinformatics analysis. Cells 2019, 8, 86. [Google Scholar] [CrossRef]
- Raza, A.; Su, W.; Gao, A.; Mehmood, S.S.; Hussain, M.A.; Nie, W.; Lv, Y.; Zou, X.; Zhang, X. Catalase (CAT) gene family in rapeseed (Brassica napus L.): Genome-wide analysis, identification, and expression pattern in response to multiple hormones and abiotic stress conditions. Int. J. Mol. Sci. 2021, 22, 4281. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef] [PubMed]
- Bi, C.; Ma, Y.; Wu, Z.; Yu, Y.T.; Liang, S.; Lu, K.; Wang, X.F. Arabidopsis ABI5 plays a role in regulating ROS homeostasis by activating CATALASE 1 transcription in seed germination. Plant Mol. Biol. 2017, 94, 197–213. [Google Scholar] [CrossRef]
- Lee, S.H.; An, C.S. Differential expression of three catalase genes in hot pepper (Capsicum annuum L.). Mol. Cells 2005, 20, 247–255. [Google Scholar]
- Zhu, X.; Guo, L.; Zhu, R.; Zhou, X.; Zhang, J.; Li, D.; He, S.; Qiao, Y. Phytophthora sojae effector PsAvh113 associates with the soybean transcription factor GmDPB to inhibit catalase-mediated immunity. Plant Biotechnol. J. 2023, 21, 1393–1407. [Google Scholar] [CrossRef]
- You, X.; Zhang, F.; Liu, Z.; Wang, M.; Xu, X.; He, F.; Wang, D.; Wang, R.; Wang, Y.; Wang, G.; et al. Rice catalase OsCATC is degraded by E3 ligase APIP6 to negatively regulate immunity. Plant Physiol. 2022, 190, 1095–1099. [Google Scholar] [CrossRef]
- Xu, J.; Duan, X.; Yang, J.; Beeching, J.R.; Zhang, P. Coupled expression of Cu/Zn-superoxide dismutase and catalase in cassava improves tolerance against cold and drought stresses. Plant Signal Behav. 2013, 8, e24525. [Google Scholar] [CrossRef] [PubMed]
- Tian, G.; Wang, S.; Wu, J.; Wang, Y.; Wang, X.; Liu, S.; Han, D.; Xia, G.; Wang, M. Allelic variation of TaWD40-4B.1 contributes to drought tolerance by modulating catalase activity in wheat. Nat. Commun. 2023, 14, 1200. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, J.; Wang, W.; Hu, T.; Hu, H.; Bao, C. A high-quality chromosome-level genome assembly reveals genetics for important traits in eggplant. Hortic. Res. 2020, 7, 153. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Han, Y.; Meng, D.; Li, D.; Jiao, C.; Jin, Q.; Lin, Y.; Cai, Y. B-BOX genes: Genome-wide identification, evolution and their contribution to pollen growth in pear (Pyrus bretschneideri Rehd.). BMC Plant Biol. 2017, 17, 156. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Kaushal, N.; Balhara, R.; Singh, K. Genome-wide analysis of Catalase gene family reveal insights into abiotic stress response mechanism in Brassica juncea and B. rapa. Plant Sci. 2023, 330, 111620. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.F.; Li, T.T.; Liu, W.C. Mutual promotion of LAP2 and CAT2 synergistically regulates plant salt and osmotic stress tolerance. Front. Plant Sci. 2021, 12, 672672. [Google Scholar] [CrossRef]
- Luo, X.; Wu, J.; Li, Y.; Nan, Z.; Guo, X.; Wang, Y.; Zhang, A.; Wang, Z.; Xia, G.; Tian, Y. Synergistic effects of GhSOD1 and GhCAT1 overexpression in cotton chloroplasts on enhancing tolerance to methyl viologen and salt stresses. PLoS ONE 2013, 8, e54002. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Y.; Ding, Y.; Chen, C.; Chen, X.; Su, N.; Zhang, X.; Pan, Y.; Li, J. Novel flavin-containing monooxygenase protein FMO1 interacts with CAT2 to negatively regulate drought tolerance through ROS homeostasis and ABA signaling pathway in tomato. Hortic. Res. 2023, 10, uhad037. [Google Scholar] [CrossRef]
- Zhao, Q.; Hu, R.S.; Liu, D.; Liu, X.; Wang, J.; Xiang, X.H.; Li, Y.Y. The AP2 transcription factor NtERF172 confers drought resistance by modifying NtCAT. Plant Biotechnol. J. 2020, 18, 2444–2455. [Google Scholar] [CrossRef]
- Zou, J.J.; Li, X.D.; Ratnasekera, D.; Wang, C.; Liu, W.X.; Song, L.F.; Zhang, W.Z.; Wu, W.H. Arabidopsis CALCIUM-DEPENDENT PROTEIN KINASE8 and CATALASE3 function in abscisic acid-mediated signaling and H2O2 homeostasis in stomatal guard cells under drought stress. Plant Cell 2015, 27, 1445–1460. [Google Scholar] [CrossRef]
- Ono, M.; Isono, K.; Sakata, Y.; Taji, T. CATALASE2 plays a crucial role in long-term heat tolerance of Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2021, 534, 747–751. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Wang, L.; Zhou, M.; Nian, J.; Chen, M.; Lu, X.; Liu, X.; Wang, Z.; Cen, J.; et al. SEMI-ROLLED LEAF 10 stabilizes catalase isozyme B to regulate leaf morphology and thermotolerance in rice (Oryza sativa L.). Plant Biotechnol. J. 2023, 21, 819–838. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, Y.; Zou, W.; Pan, Y.B.; Lin, P.; Xu, L.; Grisham, M.P.; Ding, Q.; Su, Y.; Que, Y. Genome-wide characterization of sugarcane catalase gene family identifies a ScCAT1 gene associated disease resistance. Int. J. Biol. Macromol. 2023, 232, 123398. [Google Scholar] [CrossRef]
- Liao, Y.; Ali, A.; Xue, Z.; Zhou, X.; Ye, W.; Guo, D.; Liao, Y.; Jiang, P.; Wu, T.; Zhang, H.; et al. Disruption of LLM9428/OsCATC represses starch metabolism and confers enhanced blast resistance in rice. Int. J. Mol. Sci. 2022, 23, 3827. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, R.F.; Yuan, H.M.; Li, T.T.; Wang, L.F.; Lu, K.K.; Guo, J.X.; Liu, W.C. Overexpressing the N-terminus of CATALASE2 enhances plant jasmonic acid biosynthesis and resistance to necrotrophic pathogen Botrytis cinerea B05.10. Mol. Plant Pathol. 2021, 22, 1226–1238. [Google Scholar] [CrossRef]
- Sun, T.; Liu, F.; Wang, W.; Wang, L.; Wang, Z.; Li, J.; Que, Y.; Xu, L.; Su, Y. The role of sugarcane catalase gene ScCAT2 in the defense response to pathogen challenge and adversity stress. Int. J. Mol. Sci. 2018, 19, 2686. [Google Scholar] [CrossRef] [PubMed]
- Giri, M.K.; Singh, N.; Banday, Z.Z.; Singh, V.; Ram, H.; Singh, D.; Chattopadhyay, S.; Nandi, A.K. GBF1 differentially regulates CAT2 and PAD4 transcription to promote pathogen defense in Arabidopsis thaliana. Plant J. 2017, 91, 802–815. [Google Scholar] [CrossRef]
- Yuan, H.; Jin, C.; Pei, H.; Zhao, L.; Li, X.; Li, J.; Huang, W.; Fan, R.; Liu, W.; Shen, Q.H. The powdery mildew effector CSEP0027 interacts with barley catalase to regulate host immunity. Front. Plant Sci. 2021, 12, 733237. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Tian, Y.; Cao, Y.; Wang, J.; Zhan, B.; Zhao, Z.; Sun, B.; Guo, C.; Ma, W.; Liao, Z.; et al. A novel pathogenicity determinant hijacks maize catalase 1 to enhance viral multiplication and infection. New Phytol. 2021, 230, 1126–1141. [Google Scholar] [CrossRef] [PubMed]
- Rasool, B.; Karpinska, B.; Pascual, J.; Kangasjarvi, S.; Foyer, C.H. Catalase, glutathione, and protein phosphatase 2A-dependent organellar redox signalling regulate aphid fecundity under moderate and high irradiance. Plant Cell Environ. 2020, 43, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Mhamdi, A.; Noctor, G. Analysis of catalase mutants underscores the essential role of CATALASE2 for plant growth and day length-dependent oxidative signalling. Plant Cell Environ. 2019, 42, 688–700. [Google Scholar] [CrossRef]
- Chen, H.J.; Wu, S.D.; Huang, G.J.; Shen, C.Y.; Afiyanti, M.; Li, W.J.; Lin, Y.H. Expression of a cloned sweet potato catalase SPCAT1 alleviates ethephon-mediated leaf senescence and H2O2 elevation. J. Plant Physiol. 2012, 169, 86–97. [Google Scholar] [CrossRef]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014, 65, 1241–1257. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant 2008, 133, 481–489. [Google Scholar] [CrossRef]
- FOYER, C.H.; NOCTOR, G. Oxidant and antioxidant signalling in plants: A re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 2005, 28, 1056–1071. [Google Scholar] [CrossRef]
- Hanada, K.; Zou, C.; Lehti-Shiu, M.D.; Shinozaki, K.; Shiu, S.H. Importance of lineage-specific expansion of plant tandem duplicates in the adaptive response to environmental stimuli. Plant Physiol. 2008, 148, 993–1003. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.; Qi, Y.; Zhao, Y.; Duan, C.; Wang, Y.; Meng, Z.; Zhang, Q. Genome-wide identification of PYL/PYR-PP2C (A)-SnRK2 genes in Eutrema and their co-expression analysis in response to ABA and abiotic stresses. Int. J. Biol. Macromol. 2023, 253, 126701. [Google Scholar] [CrossRef]
- Hackenberg, T.; Juul, T.; Auzina, A.; Gwizdz, S.; Malolepszy, A.; Van Der Kelen, K.; Dam, S.; Bressendorff, S.; Lorentzen, A.; Roepstorff, P.; et al. Catalase and NO CATALASE ACTIVITY1 promote autophagy-dependent cell death in Arabidopsis. Plant Cell 2013, 25, 4616–4626. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Du, N.; Dong, T.; Zhang, H.; Xue, T.; Zhao, F.; Zhao, F.; Duan, Y.; Xue, J. A novel Pinellia ternata catalase gene PtCAT2 regulates drought tolerance in Arabidopsis by modulating ROS balance. Front. Plant Sci. 2023, 14, 1206798. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wen, L.; Gao, X.; Jin, C.; Xue, Y.; Yao, X. DOG 1.0: Illustrator of protein domain structures. Cell Res. 2009, 19, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, Z.; Gu, Y.; Li, W.; Wang, W.; Yuan, X.; Zhang, Y.; Yuan, M.; Du, J.; Zhao, Q. Genome-wide identification of the soybean cytokinin oxidase/dehydrogenase gene family and its diverse roles in response to multiple abiotic stress. Front. Plant Sci. 2023, 14, 1163219. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yang, T.; Peng, Q.; Lin, H.; Xi, D. Alpha-momorcharin preserves catalase activity to inhibit viral infection by disrupting the 2b-CAT interaction in Solanum lycopersicum. Mol. Plant Pathol. 2023, 24, 107–122. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Z.; Wei, H.; Cheng, S.; Hao, P.; Yu, S.; Wang, H. Silencing of GhKEA4 and GhKEA12 revealed their potential functions under salt and potassium stresses in upland cotton. Front. Plant Sci. 2021, 12, 789775. [Google Scholar] [CrossRef]


| Gene Name | Base Number | Amino Acid Number | Molecular Weight | Instability Index | Aliphatic Index | Grand Average of Hydropathicity | Average of Hydropathicity | Theoretical pI | Subcellular Localization |
|---|---|---|---|---|---|---|---|---|---|
| SmCAT1 | 1479 | 492 | 56,594.07 | 39.78 | 71.91 | −0.515 | −0.515 | 6.86 | Peroxisome |
| SmCAT2 | 1479 | 492 | 56,996.23 | 40.25 | 68.94 | −0.582 | −0.582 | 6.88 | Peroxisome |
| SmCAT3 | 1479 | 492 | 56,934.43 | 38.18 | 70.35 | −0.552 | −0.552 | 6.80 | Peroxisome |
| SmCAT4 | 1476 | 491 | 56,545.00 | 38.37 | 71.87 | −0.575 | −0.575 | 7.31 | Peroxisome |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, L.; Xia, X.; Zhang, L.; Yang, S.; Yang, X. Genome-Wide Identification of Catalase Gene Family and the Function of SmCAT4 in Eggplant Response to Salt Stress. Int. J. Mol. Sci. 2023, 24, 16979. https://doi.org/10.3390/ijms242316979
Shen L, Xia X, Zhang L, Yang S, Yang X. Genome-Wide Identification of Catalase Gene Family and the Function of SmCAT4 in Eggplant Response to Salt Stress. International Journal of Molecular Sciences. 2023; 24(23):16979. https://doi.org/10.3390/ijms242316979
Chicago/Turabian StyleShen, Lei, Xin Xia, Longhao Zhang, Shixin Yang, and Xu Yang. 2023. "Genome-Wide Identification of Catalase Gene Family and the Function of SmCAT4 in Eggplant Response to Salt Stress" International Journal of Molecular Sciences 24, no. 23: 16979. https://doi.org/10.3390/ijms242316979
APA StyleShen, L., Xia, X., Zhang, L., Yang, S., & Yang, X. (2023). Genome-Wide Identification of Catalase Gene Family and the Function of SmCAT4 in Eggplant Response to Salt Stress. International Journal of Molecular Sciences, 24(23), 16979. https://doi.org/10.3390/ijms242316979






