Mass Spectrometry Analysis of Shark Skin Proteins
Abstract
:1. Introduction
2. Results and Discussion
2.1. Protein Categorization
| Accession Number (UniProt) | Protein Name | Organism a | Function |
|---|---|---|---|
| A0A401P1E2 | Mucin-5B | CCS | Highly glycosylated and gel-forming macromolecular components of mucus secretions. [30]. Also named vWFD domain-containing protein, exhibiting an evolutionarily- conserved von Willebrand factor type D domain (vWD), found in mucins [31]. |
| A0A401QGB0 | FTP domain-containing protein (fragment) | CCS | Fucolectin tachylectin-4 pentraxin-1 domain-containing protein. Acts as a defensive agent and recognizes blood group fucosylated oligosaccharides including A, B, H, and Lewis B-type antigens (Uniprot) [87]. |
| A0A401PKI6 | GDP-mannose 4,6- dehydratase | CCS | See A0A4W3GT93 |
| A0A401PH36 | vWFD domain- containing protein | CCS | See A0A401PH36 |
| A0A401NTT0 | N(4)-(Beta-N-acetylglucosaminyl)-L- asparaginase | CCS | Has a role the catabolism of N-linked oligosaccharides of glycoproteins. It cleaves asparagine from N-acetylglucosamines as one of the final steps in the lysosomal breakdown of glycoproteins [88]. |
| A0A401PMW9 | N-acetylglucosamine-6-sulfatase | CCS | Degrades glycosaminoglycans such as heparin, heparan sulfate, and keratan sulfate [89]. |
| A0A401NXV8 | Intelectin | CCS | A Lectin that recognizes microbial carbohydrate chains in a Ca+2-dependent manner [90,91]. Binds to glycans from Gram-positive and Gram-negative bacteria, including K. pneumoniae, S. pneumoniae, Y. pestis, P. mirabilis, and P. vulgaris [91]. |
| A0A401NHG1 | Serotransferrin | CCS | See A0A401RZK0 |
| A0A401QHD1 | Annexin (fragment) | CCS | See A0A401SL10 |
| A0A401PZD5 | Annexin | CCS | See A0A401SL10 |
| A0A401PS39 | Annexin | CCS | See A0A401SL10 |
| A0A401P412 | Annexin (fragment) | CCS | See A0A401SL10 |
| A0A401NHG1 | Serotransferrin | CCS | See A0A401RZK0 |
| H9LEQ0 | Haptoglobin | NS | Acts as an antioxidant, has antibacterial activity, and plays a role in modulating the acute phase response (UniProt, [92]). |
| A0A401NHT1 | Sushi domain-containing protein | CCS | See A0A401S7A0 |
| A0A401NPB6 | Cystatin kininogen-type domain-containing protein | CCS | Glycoproteins related to cystatins. This protein participates in blood coagulation, inflammatory response, and vasodilation [93]. |
| A0A401PTT0 | Cathepsin L (fragment) | CCS | Active enzyme in the extracellular space of antigen presenting cells (APCs) during inflammation [94]. |
| A0A401P304 | Argininosuccinate synthase | CCS | This enzyme channels extracellular L-arginine to nitric oxide synthesis pathway during inflammation [52]. |
| Q6EE48 | Cathepsin B (fragment) | SSCS | See A0A401PTT0 |
| A0A401PKI6 | GDP-mannose 4,6- dehydratase | CCS | See A0A4W3GT93 |
| A0A401NTU8 | Syndecan binding protein | CCS | A glycoprotein involved in the immune system activation [37,38]. |
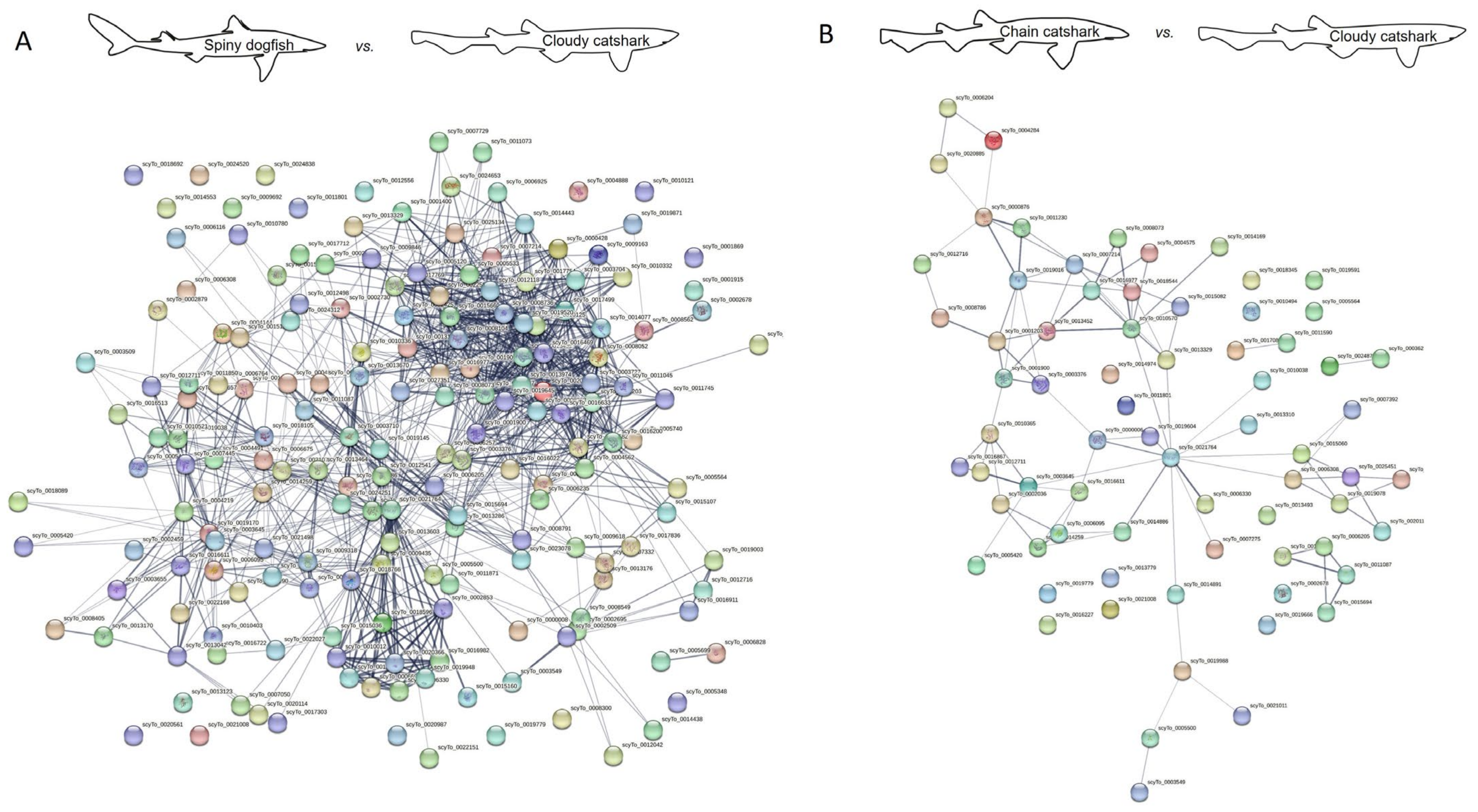
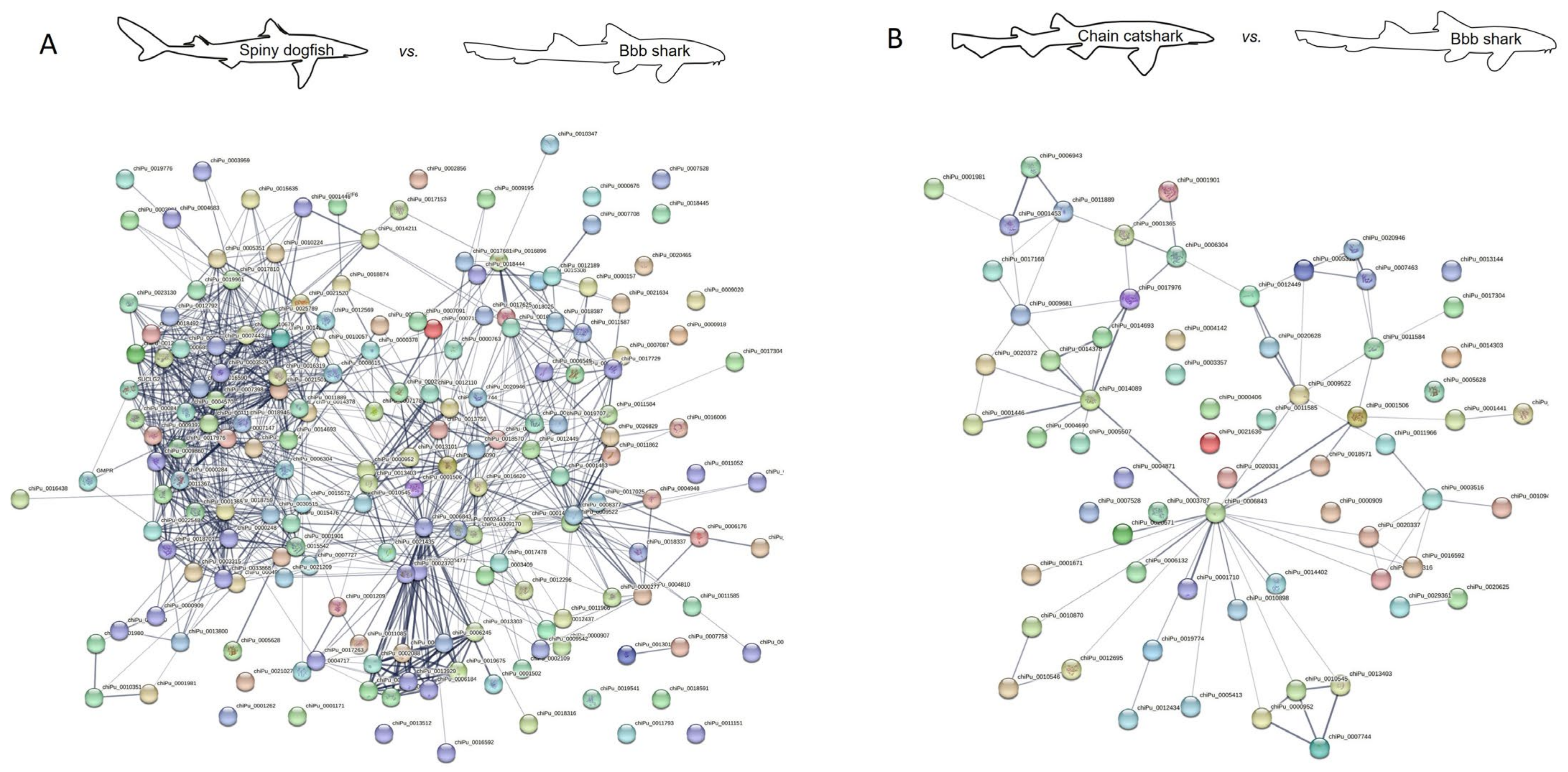
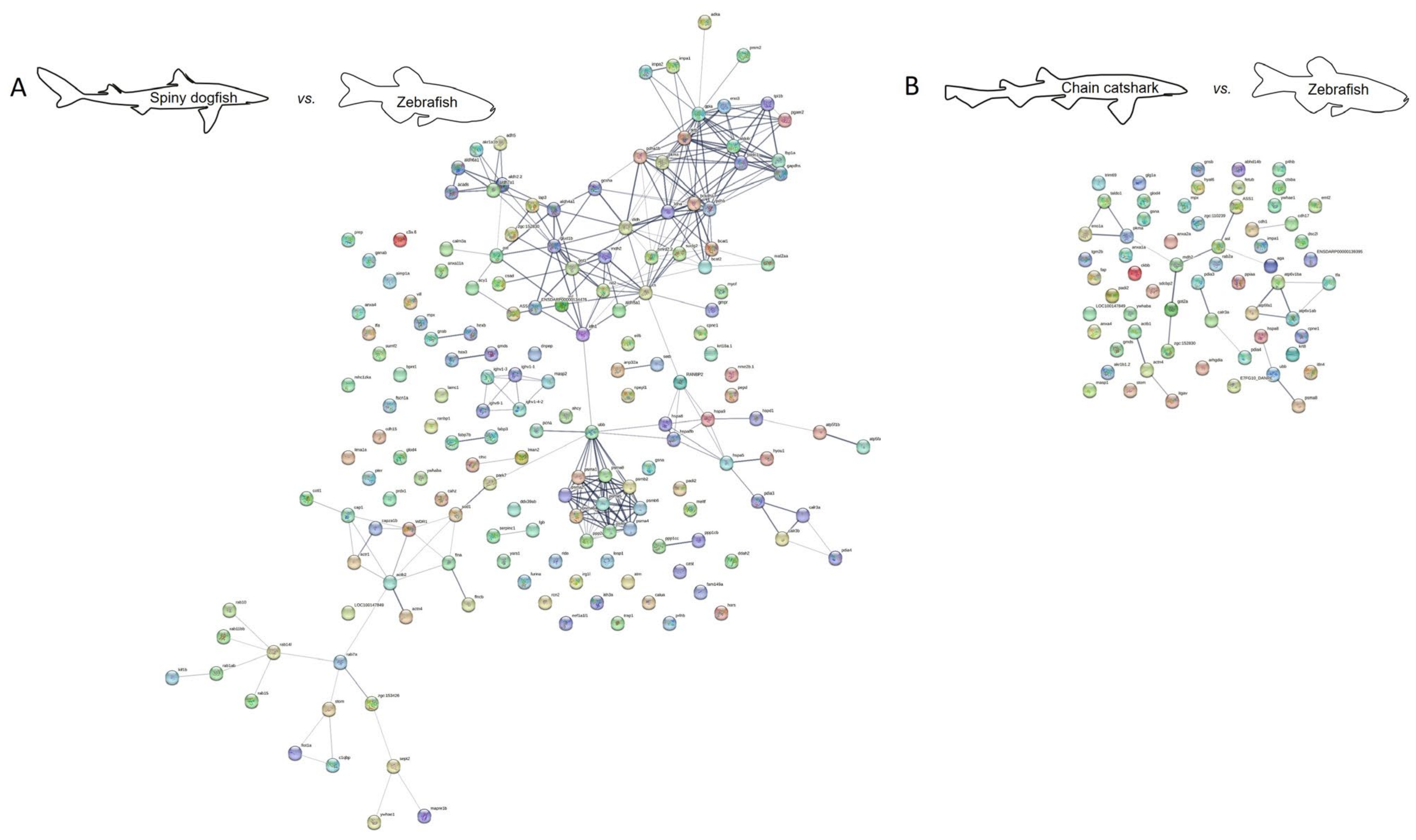
2.2. Protein Interaction
2.3. Therapeutic Implications and Human Relevance
2.4. Study Limitations
3. Methods
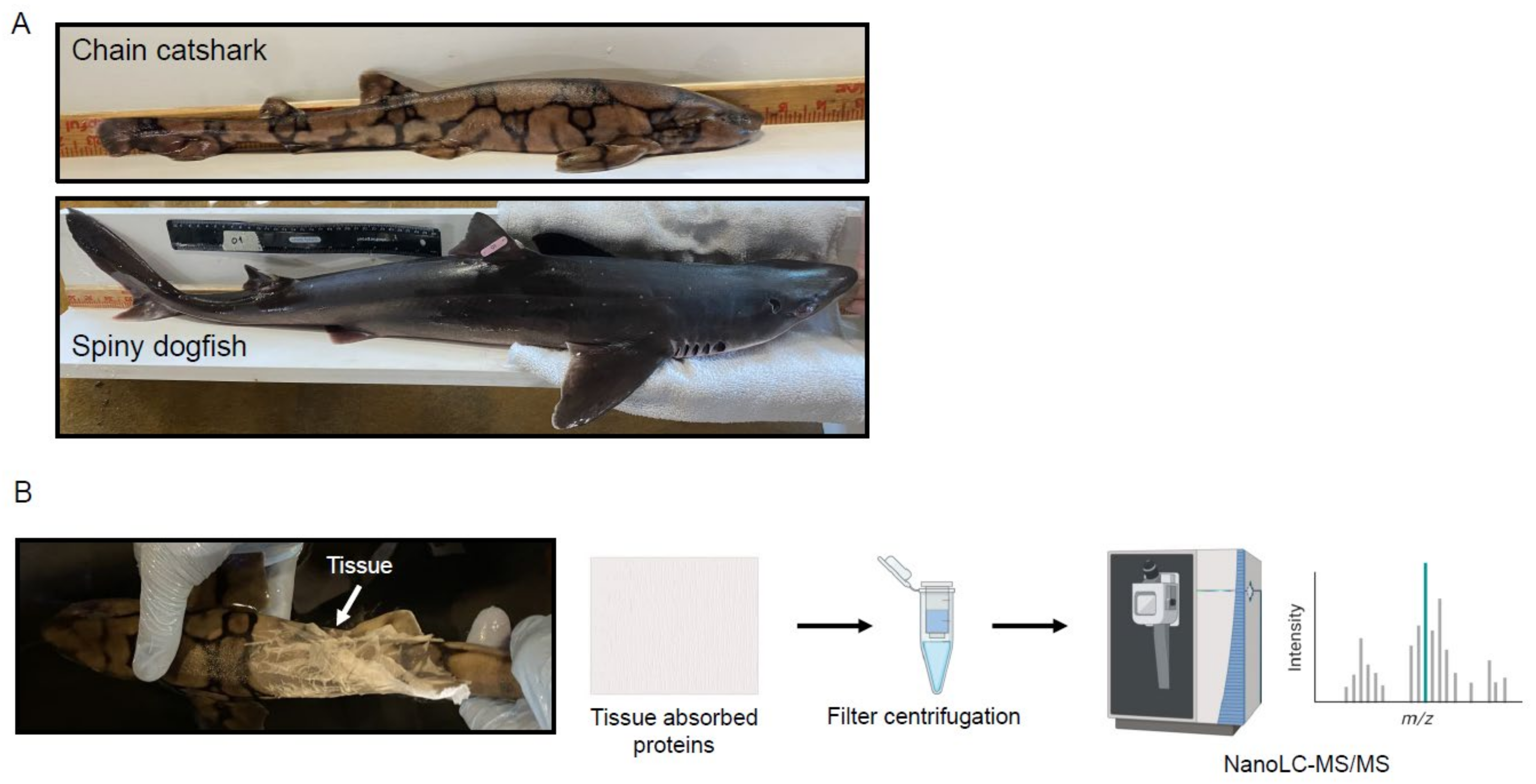
3.1. Animals
3.2. Skin Mucus Sampling
3.3. Proteomics
3.3.1. Sample Preparation
3.3.2. NanoLC/MS
3.3.3. Proteomic Data Analysis
3.4. Protein–Protein Interaction Network Analysis
3.5. Chemicals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Moore, K.S.; Wehrli, S.; Roder, H.; Rogers, M.; Forrest, J.N., Jr.; McCrimmon, D.; Zasloff, M. Squalamine: An aminosterol antibiotic from the shark. Proc. Natl. Acad. Sci. USA 1993, 90, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.N. The shark rectal gland model: A champion of receptor mediated chloride secretion through cftr. Trans. Am. Clin. Climatol. Assoc. 2016, 127, 162–175. [Google Scholar]
- Domel, A.G.; Saadat, M.; Weaver, J.C.; Haj-Hariri, H.; Bertoldi, K.; Lauder, G.V. Shark skin-inspired designs that improve aerodynamic performance. J. R. Soc. Interface 2018, 15, 20170828. [Google Scholar] [CrossRef]
- Meyer, W.; Seegers, U.; Stelzer, R. Sulphur, thiols, and disulphides in the fish epidermis, with remarks on keratinization. J. Fish Biol. 2007, 71, 1135–1144. [Google Scholar] [CrossRef]
- Webb, A.E.; Kimelman, D. Analysis of early epidermal development in zebrafish. Methods Mol. Biol. 2005, 289, 137–146. [Google Scholar] [PubMed]
- Noga, E.J. Review Article: Skin Ulcers in Fish: Pfiesteria and Other Etiologies. Toxicol. Pathol. 2000, 28, 807–823. [Google Scholar] [CrossRef]
- Quintana-Hayashi, M.P.; Padra, M.; Padra, J.T.; Benktander, J.; Lindén, S.K. Mucus-Pathogen Interactions in the Gastrointestinal Tract of Farmed Animals. Microorganisms 2018, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.G.; Livraghi-Butrico, A.; Fletcher, A.A.; McElwee, M.M.; Evans, S.E.; Boerner, R.M.; Alexander, S.N.; Bellinghausen, L.K.; Song, A.S.; Petrova, Y.M.; et al. Muc5b is required for airway defence. Nature 2013, 505, 412–416. [Google Scholar] [CrossRef]
- Hasnain, S.Z.; Evans, C.M.; Roy, M.; Gallagher, A.L.; Kindrachuk, K.N.; Barron, L.; Dickey, B.F.; Wilson, M.S.; Wynn, T.A.; Grencis, R.K.; et al. Muc5ac: A critical component mediating the rejection of enteric nematodes. J. Exp. Med. 2011, 208, 893–900. [Google Scholar] [CrossRef]
- McAuley, J.L.; Linden, S.K.; Png, C.W.; King, R.M.; Pennington, H.L.; Gendler, S.J.; Florin, T.H.; Hill, G.R.; Korolik, V.; McGuckin, M.A. MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J. Clin. Investig. 2007, 117, 2313–2324. [Google Scholar] [CrossRef]
- Bergstrom, K.S.B.; Kissoon-Singh, V.; Gibson, D.L.; Ma, C.; Montero, M.; Sham, H.P.; Ryz, N.; Huang, T.; Velcich, A.; Finlay, B.B.; et al. Muc2 Protects against Lethal Infectious Colitis by Disassociating Pathogenic and Commensal Bacteria from the Colonic Mucosa. PLoS Pathog. 2010, 6, e1000902. [Google Scholar] [CrossRef]
- Reverter, M.; Tapissier-Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Biological and Ecological Roles of External Fish Mucus: A Review. Fishes 2018, 3, 41. [Google Scholar] [CrossRef]
- Hara, Y.; Yamaguchi, K.; Onimaru, K.; Kadota, M.; Koyanagi, M.; Keeley, S.D.; Tatsumi, K.; Tanaka, K.; Motone, F.; Kageyama, Y.; et al. Shark genomes provide insights into elasmobranch evolution and the origin of vertebrates. Nat. Ecol. Evol. 2018, 2, 1761–1771. [Google Scholar] [CrossRef]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2022, 50, D20–D26. [Google Scholar] [CrossRef]
- Read, T.D.; Petit, R.A., 3rd; Joseph, S.J.; Alam, M.T.; Weil, M.R.; Ahmad, M.; Bhimani, R.; Vuong, J.S.; Haase, C.P.; Webb, D.H.; et al. Draft sequencing and assembly of the genome of the world’s largest fish, the whale shark: Rhincodon typus Smith 1828. BMC Genom. 2017, 18, 532. [Google Scholar] [CrossRef]
- Gomez, D.; Sunyer, J.O.; Salinas, I. The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish Shellfish. Immunol. 2013, 35, 1729–1739. [Google Scholar] [CrossRef]
- Jurado, J.; Fuentes-Almagro, C.A.; Guardiola, F.A.; Cuesta, A.; Esteban, M.Á.; Prieto-Álamo, M.-J. Proteomic profile of the skin mucus of farmed gilthead seabream (Sparus aurata). J. Proteom. 2015, 120, 21–34. [Google Scholar] [CrossRef]
- Sanahuja, I.; Ibarz, A. Skin mucus proteome of gilthead sea bream: A non-invasive method to screen for welfare indicators. Fish Shellfish Immunol. 2015, 46, 426–435. [Google Scholar] [CrossRef]
- Cordero, H.; Brinchmann, M.F.; Cuesta, A.; Meseguer, J.; Esteban, M.A. Skin mucus proteome map of European sea bass (Dicentrarchus labrax). Proteomics 2015, 15, 4007–4020. [Google Scholar] [CrossRef]
- Bechert, D.W.; Bartenwerfer, M. The viscous flow on surfaces with longitudinal ribs. J. Fluid Mech. 1989, 206, 105–129. [Google Scholar] [CrossRef]
- Bakke, F.K.; Gundappa, M.K.; Matz, H.; Stead, D.A.; Macqueen, D.J.; Dooley, H. Exploration of the Nurse Shark (Ginglymostoma cirratum) Plasma Immunoproteome Using High-Resolution LC-MS/MS. Front. Immunol. 2022, 13, 873390. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Valkova, N.; White, M.P.; Kültz, D. Proteomic identification of processes and pathways characteristic of osmoregulatory tissues in spiny dogfish shark (Squalus acanthias). Comp. Biochem. Physiol. Part D Genom. Proteom. 2006, 1, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Dowd, W.W.; Wood, C.M.; Kajimura, M.; Walsh, P.J.; Kültz, D. Natural feeding influences protein expression in the dogfish shark rectal gland: A proteomic analysis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2008, 3, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Dowd, W.W.; Harris, B.N.; Cech, J.J.; Kültz, D. Proteomic and physiological responses of leopard sharks (Triakis semifasciata) to salinity change. J. Exp. Biol. 2010, 213, 210–224. [Google Scholar] [CrossRef] [PubMed]
- Pearce, J.F.M.; Sequeira, A.M.M.; Kaur, P. State of Shark and Ray Genomics in an Era of Extinction. Front. Mar. Sci. 2021, 8, 744986. [Google Scholar] [CrossRef]
- Bachar-Wikstrom, E.; Thomsson, K.A.; Sihlbom, C.; Abbo, L.; Tartor, H.; Lindén, S.K.; Wikstrom, J.D. Identification of Novel Glycans in the Mucus Layer of Shark and Skate Skin. Int. J. Mol. Sci. 2023, 24, 14331. [Google Scholar] [CrossRef]
- Ivanova, L.; Rangel-Huerta, O.D.; Tartor, H.; Gjessing, M.C.; Dahle, M.K.; Uhlig, S. Fish Skin and Gill Mucus: A Source of Metabolites for Non-Invasive Health Monitoring and Research. Metabolites 2021, 12, 28. [Google Scholar] [CrossRef]
- Rajan, B.; Fernandes, J.M.; Caipang, C.M.; Kiron, V.; Rombout, J.H.; Brinchmann, M.F. Proteome reference map of the skin mucus of Atlantic cod (Gadus morhua) revealing immune competent molecules. Fish Shellfish Immunol. 2011, 31, 224–231. [Google Scholar] [CrossRef]
- Bonin-Debs, A.L.; Boche, I.; Gille, H.; Brinkmann, U. Development of secreted proteins as biotherapeutic agents. Expert Opin. Biol. Ther. 2004, 4, 551–558. [Google Scholar] [CrossRef]
- Ramachandran, P.; Boontheung, P.; Xie, Y.; Sondej, M.; Wong, D.T.; Loo, J.A. Identification of N-Linked Glycoproteins in Human Saliva by Glycoprotein Capture and Mass Spectrometry. J. Proteome Res. 2006, 5, 1493–1503. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Eng, E.T.; Zhu, J.; Lu, C.; Walz, T.; Springer, T.A. Sequence and structure relationships within von Willebrand factor. Blood 2012, 120, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Javitt, G.; Khmelnitsky, L.; Albert, L.; Bigman, L.S.; Elad, N.; Morgenstern, D.; Ilani, T.; Levy, Y.; Diskin, R.; Fass, D. Assembly Mechanism of Mucin and von Willebrand Factor Polymers. Cell 2020, 183, 717–729.e16. [Google Scholar] [CrossRef]
- Edelman, G.M. CAMs and Igs: Cell Adhesion and the Evolutionary Origins of Immunity. Immunol. Rev. 1987, 100, 11–45. [Google Scholar] [CrossRef] [PubMed]
- Lang, T.; Hansson, G.C.; Samuelsson, T. Gel-forming mucins appeared early in metazoan evolution. Proc. Natl. Acad. Sci. USA 2007, 104, 16209–16214. [Google Scholar] [CrossRef] [PubMed]
- Szabo, R.; Netzel-Arnett, S.; Hobson, J.P.; Antalis, T.M.; Bugge, T.H. Matriptase-3 is a novel phylogenetically preserved membrane-anchored serine protease with broad serpin reactivity. Biochem. J. 2005, 390, 231–242. [Google Scholar] [CrossRef]
- Carroll, M. Immunology: Exposure of an executioner. Nature 2006, 444, 159–160. [Google Scholar] [CrossRef]
- Stafford, J.L.; Belosevic, M. Transferrin and the innate immune response of fish: Identification of a novel mechanism of macrophage activation. Dev. Comp. Immunol. 2003, 27, 539–554. [Google Scholar] [CrossRef]
- Esmon, C.T. Interactions between the innate immune and blood coagulation systems. Trends Immunol. 2004, 25, 536–542. [Google Scholar] [CrossRef]
- Bezkorovainy, A. Antimicrobial properties of iron-binding proteins. Adv. Exp. Med. Biol. 1981, 135, 139–154. [Google Scholar]
- Tonetti, M.; Sturla, L.; Bisso, A.; Benatti, U.; De Flora, A. Synthesis of GDP-L-fucose by the Human FX Protein. J. Biol. Chem. 1996, 271, 27274–27279. [Google Scholar] [CrossRef]
- Berstein, R.M.; Schluter, S.F.; Shen, S.; Marchalonis, J.J. A new high molecular weight immunoglobulin class from the carcharhine shark: Implications for the properties of the primordial immunoglobulin. Proc. Natl. Acad. Sci. USA 1996, 93, 3289–3293. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, R.F. Fibrinogen and fibrin. Annu. Rev. Biochem. 1984, 53, 195–229. [Google Scholar] [CrossRef]
- Sim, R.B. The human complement system serine proteases C1r and C1s and their proenzymes. Methods Enzymol. 1981, 80 Pt C, 26–42. [Google Scholar] [PubMed]
- Lefranc, M.P. Immunoglobulin and T Cell Receptor Genes: IMGT((R)) and the Birth and Rise of Immunoinformatics. Front. Immunol. 2014, 5, 22. [Google Scholar] [CrossRef]
- Elvington, M.; Liszewski, M.K.; Atkinson, J.P. Evolution of the complement system: From defense of the single cell to guardian of the intravascular space. Immunol. Rev. 2016, 274, 9–15. [Google Scholar] [CrossRef]
- Crichton, R.R.; Charloteaux-Wauters, M. Iron transport and storage. JBIC J. Biol. Inorg. Chem. 1987, 164, 485–506. [Google Scholar] [CrossRef] [PubMed]
- Arcone, R.; Arpaia, G.; Ruoppolo, M.; Malorni, A.; Pucci, P.; Marino, G.; Ialenti, A.; DI Rosa, M.; Ciliberto, G. Structural characterization of a biologically active human lipocortin 1 expressed in Escherichia coli. JBIC J. Biol. Inorg. Chem. 1993, 211, 347–355. [Google Scholar] [CrossRef]
- Shalak, V.; Kaminska, M.; Mitnacht-Kraus, R.; Vandenabeele, P.; Clauss, M.; Mirande, M. The EMAPII cytokine is released from the mammalian multisynthetase complex after cleavage of its p43/proEMAPII component. J. Biol. Chem. 2001, 276, 23769–23776. [Google Scholar] [CrossRef]
- Chen-Goodspeed, M.; Sogorb, M.A.; Wu, F.; Hong, S.-B.; Raushel, F.M. Structural Determinants of the Substrate and Stereochemical Specificity of Phosphotriesterase. Biochemistry 2001, 40, 1325–1331. [Google Scholar] [CrossRef]
- Turk, D.; Janjic, V.; Stern, I.; Podobnik, M.; Lamba, D.; Dahl, S.W.; Lauritzen, C.; Pedersen, J.; Turk, V.; Turk, B. Structure of human dipeptidyl peptidase I (cathepsin C): Exclusion domain added to an endopeptidase framework creates the machine for activation of granular serine proteases. EMBO J. 2001, 20, 6570–6582. [Google Scholar] [CrossRef]
- Hulin, J.-A.; Gubareva, E.A.; Jarzebska, N.; Rodionov, R.N.; Mangoni, A.A.; Tommasi, S. Inhibition of Dimethylarginine Dimethylaminohydrolase (DDAH) Enzymes as an Emerging Therapeutic Strategy to Target Angiogenesis and Vasculogenic Mimicry in Cancer. Front. Oncol. 2020, 9, 1455. [Google Scholar] [CrossRef]
- Erez, A.; Nagamani, S.C.S.A.; Shchelochkov, O.; Premkumar, M.H.; Campeau, P.M.; Chen, Y.; Garg, H.K.; Li, L.; Mian, A.; Bertin, T.K.; et al. Requirement of argininosuccinate lyase for systemic nitric oxide production. Nat. Med. 2011, 17, 1619–1626. [Google Scholar] [CrossRef]
- Reid, K.B.; Day, A.J. Structure-function relationships of the complement components. Immunol. Today 1989, 10, 177–180. [Google Scholar] [CrossRef]
- Chaput, M.; Claes, V.; Portetelle, D.; Cludts, I.; Cravador, A.; Burny, A.; Gras, H.; Tartar, A. The neurotrophic factor neuroleukin is 90% homologous with phosphohexose isomerase. Nature 1988, 332, 454–455. [Google Scholar] [CrossRef]
- Saleh, M.; Abdel-Baki, A.-A.S.; Dkhil, M.A.; El-Matbouli, M.; Al-Quraishy, S. Proteins of the Ciliated Protozoan Parasite Ichthyophthirius multifiliis Identified in Common Carp Skin Mucus. Pathogens 2021, 10, 790. [Google Scholar] [CrossRef]
- Fernandes, J.M.; Molle, G.; Kemp, G.D.; Smith, V.J. Isolation and characterisation of oncorhyncin II, a histone H1-derived antimicrobial peptide from skin secretions of rainbow trout, Oncorhynchus mykiss. Dev. Comp. Immunol. 2003, 28, 127–138. [Google Scholar] [CrossRef]
- Sugahara, T.; Nakajima, H.; Shirahata, S.; Murakami, H. Purification and characterization of immunoglobulin production stimulating factor-II beta derived from Namalwa cells. Cytotechnology 1992, 10, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Aumailley, M. The laminin family. Cell Adhes. Migr. 2013, 7, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Giordano, D.; Paredi, G.; Marchesani, F.; Milazzo, L.; Altomonte, G.; Del Canale, P.; Abbruzzetti, S.; Ascenzi, P.; di Prisco, G.; et al. The Greenland shark Somniosus microcephalus—Hemoglobins and ligand-binding properties. PLoS ONE 2017, 12, e0186181. [Google Scholar] [CrossRef] [PubMed]
- Gouyer, V.; Dubuquoy, L.; Robbe-Masselot, C.; Neut, C.; Singer, E.; Plet, S.; Geboes, K.; Desreumaux, P.; Gottrand, F.; Desseyn, J.-L. Delivery of a mucin domain enriched in cysteine residues strengthens the intestinal mucous barrier. Sci. Rep. 2015, 5, srep09577. [Google Scholar] [CrossRef] [PubMed]
- Marel, M.; Adamek, M.; Gonzalez, S.F.; Frost, P.; Rombout, J.H.; Wiegertjes, G.F.; Savelkoul, H.F.; Steinhagen, D. Molecular cloning and expression of two beta-defensin and two mucin genes in common carp (Cyprinus carpio L.) and their up-regulation after beta-glucan feeding. Fish Shellfish Immunol. 2012, 32, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, R.; Su, B.; Luo, Y.; Terhune, J.; Beck, B.; Peatman, E. Evasion of mucosal defenses during Aeromonas hydrophila infection of channel catfish (Ictalurus punctatus) skin. Dev. Comp. Immunol. 2013, 39, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Keep, J.C.; Piehl, M.; Miller, A.; Oyasu, R. Invasive Carcinomas of the Urinary Bladder: Evaluation of Tunica Muscularis Mucosae Involvement. Am. J. Clin. Pathol. 1989, 91, 575–579. [Google Scholar] [CrossRef]
- Mashoof, S.; Criscitiello, M.F. Fish Immunoglobulins. Biology 2016, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Khalid, Z.; Chen, Y.; Yu, D.; Abbas, M.; Huan, M.; Naz, Z.; Mengist, H.M.; Cao, M.-J.; Jin, T. IgNAR antibody: Structural features, diversity and applications. Fish Shellfish Immunol. 2022, 121, 467–477. [Google Scholar] [CrossRef]
- Liu, J.L.; Anderson, G.P.; Delehanty, J.B.; Baumann, R.; Hayhurst, A.; Goldman, E.R. Selection of cholera toxin specific IgNAR single-domain antibodies from a naïve shark library. Mol. Immunol. 2007, 44, 1775–1783. [Google Scholar] [CrossRef]
- Greenberg, A.S.; Hughes, A.L.; Guo, J.; Avila, D.; McKinney, E.C.; Flajnik, M.F. A novel “chimeric” antibody class in cartilaginous fish: IgM may not be the primordial immunoglobulin. Eur. J. Immunol. 1996, 26, 1123–1129. [Google Scholar] [CrossRef]
- Rumfelt, L.L.; Lohr, R.L.; Dooley, H.; Flajnik, M.F. Diversity and repertoire of IgW and IgM VH families in the newborn nurse shark. BMC Immunol. 2004, 5, 8. [Google Scholar] [CrossRef]
- Honda, Y.; Kondo, H.; Caipang, C.M.A.; Hirono, I.; Aoki, T. cDNA cloning of the immunoglobulin heavy chain genes in banded houndshark Triakis scyllium. Fish Shellfish Immunol. 2010, 29, 854–861. [Google Scholar] [CrossRef]
- Thomès, L.; Bojar, D. The Role of Fucose-Containing Glycan Motifs Across Taxonomic Kingdoms. Front. Mol. Biosci. 2021, 8. [Google Scholar] [CrossRef]
- Ræder, I.L.U.; Paulsen, S.M.; Smalås, A.O.; Willassen, N.P. Effect of fish skin mucus on the soluble proteome of Vibrio salmonicida analysed by 2-D gel electrophoresis and tandem mass spectrometry. Microb. Pathog. 2007, 42, 36–45. [Google Scholar] [CrossRef]
- van Campenhout, A.; van Campenhout, C.M.; Lagrou, A.R.; Manuel-y-Keenoy, B. Transferrin modifications and lipid peroxidation: Implications in diabetes mellitus. Free Radic. Res. 2003, 37, 1069–1077. [Google Scholar] [CrossRef]
- Tagliabue, A.; Bowie, A.R.; Boyd, P.W.; Buck, K.N.; Johnson, K.S.; Saito, M.A. The integral role of iron in ocean biogeochemistry. Nature 2017, 543, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Walport, M.J. Complement. First of two parts. N. Engl. J. Med. 2001, 344, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Abu-Humaidan, A.H.; Elvén, M.; Sonesson, A.; Garred, P.; Sørensen, O.E. Persistent Intracellular Staphylococcus aureus in Keratinocytes Lead to Activation of the Complement System with Subsequent Reduction in the Intracellular Bacterial Load. Front. Immunol. 2018, 9, 396. [Google Scholar] [CrossRef] [PubMed]
- Panelius, J.; Meri, S. Complement system in dermatological diseases-fire under the skin. Front. Med. 2015, 2, 3–10. [Google Scholar]
- Ruediger, G.F.; Davis, D.J. Phagocytosis and opsonins in the lower animals. J. Infect. Dis. 1907, 4, 333–336. [Google Scholar] [CrossRef]
- Legler, D.W.; Evans, E.E. Comparative Immunology: Hemolytic Complement in Elasmobranchs. Sage J. 1967, 124, 30–34. [Google Scholar] [CrossRef]
- Fonseca, V.J.A.; Braga, A.L.; Filho, J.R.; Teixeira, C.S.; da Hora, G.C.; Morais-Braga, M.F.B. A review on the antimicrobial properties of lectins. Int. J. Biol. Macromol. 2021, 195, 163–178. [Google Scholar] [CrossRef]
- Tully, J.G. Interaction of Spiroplasmas with Plant, Arthropod, and Animal Hosts. Clin. Infect. Dis. 1982, 4, S193–S199. [Google Scholar] [CrossRef]
- Gao, B.; Adhikari, R.; Howarth, M.; Nakamura, K.; Gold, M.C.; Hill, A.B.; Knee, R.; Michalak, M.; Elliott, T. Assembly and Antigen-Presenting Function of MHC Class I Molecules in Cells Lacking the ER Chaperone Calreticulin. Immunity 2002, 16, 99–109. [Google Scholar] [CrossRef]
- Salerno, G.; Parisi, M.; Parrinello, D.; Benenati, G.; Vizzini, A.; Vazzana, M.; Vasta, G.; Cammarata, M. F-type lectin from the sea bass (Dicentrarchus labrax): Purification, cDNA cloning, tissue expression and localization, and opsonic activity. Fish Shellfish Immunol. 2009, 27, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-Y.; Kwon, J.; Vaidya, B.; Kim, J.-O.; Lee, S.; Jeong, E.-H.; Baik, K.S.; Choi, J.-S.; Bae, H.-J.; Oh, M.-J.; et al. Modulation of proteome expression by F-type lectin during viral hemorrhagic septicemia virus infection in fathead minnow cells. Fish Shellfish Immunol. 2014, 39, 464–474. [Google Scholar] [CrossRef] [PubMed]
- Tateno, H.; Saneyoshi, A.; Ogawa, T.; Muramoto, K.; Kamiya, H.; Saneyoshi, M. Isolation and Characterization of Rhamnose-binding Lectins from Eggs of Steelhead Trout (Oncorhynchus mykiss) Homologous to Low Density Lipoprotein Receptor Superfamily. J. Biol. Chem. 1998, 273, 19190–19197. [Google Scholar] [CrossRef]
- Tsutsui, S.; Dotsuta, Y.; Ono, A.; Suzuki, M.; Tateno, H.; Hirabayashi, J.; Nakamura, O. A C-type lectin isolated from the skin of Japanese bullhead shark (Heterodontus japonicus) binds a remarkably broad range of sugars and induces blood coagulation. J. Biochem. 2014, 157, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Racioppi, L.; Means, A.R. Calcium/calmodulin-dependent kinase IV in immune and inflammatory responses: Novel routes for an ancient traveller. Trends Immunol. 2008, 29, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Vasta, G.R.; Amzel, L.M.; Bianchet, M.A.; Cammarata, M.; Feng, C.; Saito, K. F-Type Lectins: A Highly Diversified Family of Fucose-Binding Proteins with a Unique Sequence Motif and Structural Fold, Involved in Self/Non-Self-Recognition. Front. Immunol. 2017, 8, 1648. [Google Scholar] [CrossRef]
- Saarela, J.; Laine, M.; Oinonen, C.; Jalanko, A.; Rouvinen, J.; Peltonen, L.; Tikkanen, R. Activation and Oligomerization of Aspartylglucosaminidase. J. Biol. Chem. 1998, 273, 25320–25328. [Google Scholar] [CrossRef]
- Robertson, D.A.; Freeman, C.; Nelson, P.V.; Morris, C.P.; Hopwood, J.J. Human glucosamine-6-sulfatase cDNA reveals homology with steroid sulfatase. Biochem. Biophys. Res. Commun. 1988, 157, 218–224. [Google Scholar] [CrossRef]
- Tsuji, S.; Uehori, J.; Matsumoto, M.; Suzuki, Y.; Matsuhisa, A.; Toyoshima, K.; Seya, T. Human Intelectin Is a Novel Soluble Lectin That Recognizes Galactofuranose in Carbohydrate Chains of Bacterial Cell Wall. J. Biol. Chem. 2001, 276, 23456–23463. [Google Scholar] [CrossRef]
- Wesener, D.; Wangkanont, K.; McBride, R.; Song, X.; Kraft, M.B.; Hodges, H.L.; Zarling, L.C.A.; Splain, R.; Smith, D.F.; Cummings, R.D.; et al. Recognition of microbial glycans by human intelectin-1. Nat. Struct. Mol. Biol. 2015, 22, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A. Zonulin and Its Regulation of Intestinal Barrier Function: The Biological Door to Inflammation, Autoimmunity, and Cancer. Physiol. Rev. 2011, 91, 151–175. [Google Scholar] [CrossRef] [PubMed]
- Kordiš, D.; Turk, V. Phylogenomic analysis of the cystatin superfamily in eukaryotes and prokaryotes. BMC Evol. Biol. 2009, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Felbor, U.; Dreier, L.; Bryant, R.A.; Ploegh, H.L.; Olsen, B.R.; Mothes, W. Secreted cathepsin L generates endostatin from collagen XVIII. EMBO J. 2000, 19, 1187–1194. [Google Scholar] [CrossRef]
- Zavašnik-Bergant, T.; Turk, B. Cysteine cathepsins in the immune response. Tissue Antigens 2006, 67, 349–355. [Google Scholar] [CrossRef]
- Subramanian, S.; MacKinnon, S.L.; Ross, N.W. A comparative study on innate immune parameters in the epidermal mucus of various fish species. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2007, 148, 256–263. [Google Scholar] [CrossRef]
- Fernández-Montero, Á.; Torrecillas, S.; Montero, D.; Acosta, F.; Prieto-Álamo, M.-J.; Abril, N.; Jurado, J. Proteomic profile and protease activity in the skin mucus of greater amberjack (Seriola dumerili) infected with the ectoparasite Neobenedenia girellae—An immunological approach. Fish Shellfish Immunol. 2021, 110, 100–115. [Google Scholar] [CrossRef]
- Jhaveri, P.; Papastamatiou, Y.P.; German, D.P. Digestive enzyme activities in the guts of bonnethead sharks (Sphyrna tiburo) provide insight into their digestive strategy and evidence for microbial digestion in their hindguts. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 189, 76–83. [Google Scholar] [CrossRef]
- Young, N.; Cooper, G.; Nowak, B.; Koop, B. Morrison Coordinated down-regulation of the antigen processing machinery in the gills of amoebic gill disease-affected Atlantic salmon (Salmo salar L.). Mol. Immunol. 2008, 45, 2581–2597. [Google Scholar] [CrossRef]
- Walsh, C.J.; Luer, C.A.; Yordy, J.E.; Cantu, T.; Miedema, J.; Leggett, S.R.; Leigh, B.; Adams, P.; Ciesla, M.; Bennett, C.; et al. Epigonal Conditioned Media from Bonnethead Shark, Sphyrna tiburo, Induces Apoptosis in a T-Cell Leukemia Cell Line, Jurkat E6-1. Mar. Drugs 2013, 11, 3224–3257. [Google Scholar] [CrossRef]
- Weeds, A. Actin-binding proteins—Regulators of cell architecture and motility. Nature 1982, 296, 811–816. [Google Scholar] [CrossRef]
- Mostowy, S.; Shenoy, A.R. The cytoskeleton in cell-autonomous immunity: Structural determinants of host defence. Nat. Rev. Immunol. 2015, 15, 559–573. [Google Scholar] [CrossRef]
- Easy, R.H.; Ross, N.W. Changes in Atlantic salmon (Salmo salar) epidermal mucus protein composition profiles following infection with sea lice (Lepeophtheirus salmonis). Comp. Biochem. Physiol. Part D Genom. Proteom. 2009, 4, 159–167. [Google Scholar] [CrossRef]
- Sandiford, S.L.; Dong, Y.; Pike, A.; Blumberg, B.J.; Bahia, A.C.; Dimopoulos, G. Cytoplasmic Actin Is an Extracellular Insect Immune Factor which Is Secreted upon Immune Challenge and Mediates Phagocytosis and Direct Killing of Bacteria, and Is a Plasmodium Antagonist. PLoS Pathog. 2015, 11, e1004631. [Google Scholar] [CrossRef]
- Molle, V.; Campagna, S.; Bessin, Y.; Ebran, N.; Saint, N.; Molle, G. First evidence of the pore-forming properties of a keratin from skin mucus of rainbow trout (Oncorhynchus mykiss, formerly Salmo gairdneri). Biochem. J. 2008, 411, 33–40. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; Khalifa, A.S.; Abdel-Wahab, M.F. Genetic intrathyroidal hormone defects. J. Egypt. Med. Assoc. 1970, 53, 1–12. [Google Scholar]
- Tzivion, G.; Avruch, J. 14-3-3 Proteins: Active Cofactors in Cellular Regulation by Serine/Threonine Phosphorylation. J. Biol. Chem. 2002, 277, 3061–3064. [Google Scholar] [CrossRef]
- Sadeghi Shaker, M.; Rokni, M.; Mahmoudi, M.; Farhadi, E. Ras family signaling pathway in immunopathogenesis of inflammatory rheumatic diseases. Front. Immunol. 2023, 14, 1151246. [Google Scholar] [CrossRef]
- Cordero, H.; Morcillo, P.; Cuesta, A.; Brinchmann, M.F.; Esteban, M.A. Differential proteome profile of skin mucus of gilthead seabream (Sparus aurata) after probiotic intake and/or overcrowding stress. J. Proteom. 2016, 132, 41–50. [Google Scholar] [CrossRef]
- Bergsson, G.; Agerberth, B.; Jörnvall, H.; Gudmundsson, G.H. Isolation and identification of antimicrobial components from the epidermal mucus of Atlantic cod (Gadus morhua). FEBS J. 2005, 272, 4960–4969. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Kuhn, M.; Simonovic, M.; Roth, A.; Minguez, P.; Doerks, T.; Stark, M.; Muller, J.; Bork, P.; et al. The STRING database in 2011: Functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res. 2010, 39, D561–D568. [Google Scholar] [CrossRef] [PubMed]
- Stein, R.W.; Mull, C.G.; Kuhn, T.S.; Aschliman, N.C.; Davidson, L.N.K.; Joy, J.B.; Smith, G.J.; Dulvy, N.K.; Mooers, A.O. Global priorities for conserving the evolutionary history of sharks, rays and chimaeras. Nat. Ecol. Evol. 2018, 2, 288–298. [Google Scholar] [CrossRef]
- Richards, V.P.; Suzuki, H.; Stanhope, M.J.; Shivji, M.S. Characterization of the heart transcriptome of the white shark (Carcharodon carcharias). BMC Genom. 2013, 14, 697. [Google Scholar] [CrossRef]
- Bhargava, P.; Marshall, J.L.; Dahut, W.; Rizvi, N.; Trocky, N.I.; Williams, J.; Hait, H.; Song, S.; Holroyd, K.J.; Hawkins, M.J. A phase I and pharmacokinetic study of squalamine, a novel antiangiogenic agent, in patients with advanced cancers. Clin. Cancer Res. 2001, 7, 3912–3919. [Google Scholar]
- Herbst, R.S.A.; Hammond, L.; Carbone, D.P.; Tran, H.T.; Holroyd, K.J.; Desai, A.I.; Williams, J.; Bekele, B.N.; Hait, H.; Allgood, V.; et al. A phase I/IIA trial of continuous five-day infusion of squalamine lactate (MSI-1256F) plus carboplatin and paclitaxel in patients with advanced non-small cell lung cancer. Clin. Cancer Res. 2003, 9, 4108–4115. [Google Scholar]
- Ajeeshkumar, K.K.; Vishnu, K.V.; Navaneethan, R.; Raj, K.; Remyakumari, K.R.; Swaminathan, T.R.; Suseela, M.; Asha, K.K.; Sreekanth, G.P. Proteoglycans isolated from the bramble shark cartilage show potential anti-osteoarthritic properties. Inflammopharmacology 2019, 27, 175–187. [Google Scholar] [CrossRef]
- Luer, C.A.; Walsh, C.J. Potential Human Health Applications from Marine Biomedical Research with Elasmobranch Fishes. Fishes 2018, 3, 47. [Google Scholar] [CrossRef]
- Haugen, J.B.; Curtis, T.H.; Fernandes, P.G.; Sosebee, K.A.; Rago, P.J. Sexual segregation of spiny dogfish (Squalus acanthias) off the northeastern United States: Implications for a male-directed fishery. Fish. Res. 2017, 193, 121–128. [Google Scholar] [CrossRef]
- Fæste, C.; Tartor, H.; Moen, A.; Kristoffersen, A.; Dhanasiri, A.; Anonsen, J.; Furmanek, T.; Grove, S. Proteomic profiling of salmon skin mucus for the comparison of sampling methods. J. Chromatogr. B 2019, 1138, 121965. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]

| Accession Number | Immune-Related |
|---|---|
| A0A401T5Y9 | Mucin-5B-like |
| A0A401RME8 | Mucin-2-like |
| A0A401SE28 | Ig-like domain-containing protein |
| A0A401PH36 | vWFD domain-containing protein |
| P23085 | Ig heavy chain C region (fragment) |
| A0A401NGS8 | vWFA domain-containing protein |
| A0A0H4IU03 | Antithrombin |
| A0A401RZK0 | Serotransferrin |
| A0A401Q3Q5 | GDP-L-fucose synthase (fragment) |
| A0A401RXA4 | Prothymosin alpha |
| U5NJK8 | Secreted IgW heavy chain |
| A0A401RMX1 | Fibrinogen beta chain |
| A0A401RJ78 | Complement component 1 Q subcomponent-binding protein, mitochondrial (fragment) |
| H9LDW9 | Complement protein 1S |
| Q8HWH7 | MHC class I antigen |
| P03983 | Ig heavy chain V region |
| A0A401SX93 | Prothymosin alpha-like |
| A0A088MN23 | C3 complement component |
| A0A401P9G0 | IRG1 decarboxylase (fragment) |
| A0A401Q3Q5 | GDP-L-fucose synthase (fragment) |
| A0A401SNF0 | Transferrin-like domain-containing protein |
| A0A401NHG1 | Serotransferrin |
| A0A4W3I3U6 | Serotransferrin |
| A0A401RZK4 | Serotransferrin |
| Accession Number | Genetic information processing |
| A0A401SY06 | Heat shock protein 70 (fragment) |
| A0A401S035 | Proteasome subunit alpha type |
| K4G7F1 | Proteasome subunit alpha type |
| A0A4W3H615 | Proteasome subunit beta |
| A0A4W3I1Z3 | Protein disulfide isomerase |
| A0A401NMQ4 | Heat shock cognate 71 kDa protein |
| A0A401SNV8 | Protein disulfide isomerase |
| A0A401RSV7 | Proteasome subunit beta (fragment) |
| A0A401SEG3 | 60 kDa heat shock protein, mitochondrial |
| A0A401SGF6 | Proteasome subunit alpha type |
| A0A401SL10 | Annexin |
| A0A401RYU8 | Proteasome subunit alpha type |
| V9KKG3 | Annexin (fragment) |
| A0A4W3K8Z3 | Protein disulfide isomerase |
| K4FY62 | Proteasome subunit alpha type |
| A0A401PZD5 | Annexin |
| A0A401SX58 | Protein disulfide isomerase (fragment) |
| A0A401PL09 | Proteasome subunit alpha type |
| A0A4W3GHI3 | Proteasome 20S subunit alpha 2 |
| Q9DEZ5 | Ubiquitin (fragment) |
| A0A4W3IZD3 | Tyrosine—tRNA ligase |
| A0A4W3IS83 | GMP reductase |
| A0A4W3KHY8 | RNA helicase |
| V9KVF9 | Protein SET-like protein (fragment) |
| A0A401SFW5 | Thioredoxin domain-containing protein |
| A0A4W3JL89 | Calreticulin |
| A0A401STB5 | Calreticulin |
| A0A4W3IN11 | Thioredoxin disulfide reductase |
| A0A4W3HJ00 | TNF receptor associated protein 1 |
| A0A401NGY9 | Calreticulin |
| V9LF43 | Aminoacyl tRNA synthase complex-interacting multifunctional protein 1 (fragment) |
| A0A401SW93 | Hypoxia upregulated 1 |
| A0A401SF18 | CN hydrolase domain-containing protein |
| A0A401PA35 | 78 kDa glucose-regulated protein |
| P27950 | Nucleoside diphosphate kinase (fragment) |
| A0A401RY79 | RING-type E3 ubiquitin transferase |
| A0A4W3JJD7 | RAN binding protein 2 |
| A0A4W3K2G6 | Eukaryotic translation initiation factor 6 |
| V9KJH1 | Septin-2 |
| A0A401QMS9 | Quinolinate phosphoribosyltransferase (decarboxylating) |
| K4G9R8 | Elongation factor 1-alpha |
| A0A401P0F5 | Delta-1-pyrroline-5-carboxylate dehydrogenase, mitochondrial |
| A0A4W3JW47 | Stress-70 protein, mitochondrial |
| A0A401PGN2 | Myoferlin |
| A0A401NIS3 | 2-iminobutanoate/2-iminopropanoate deaminase |
| A0A4W3HQT2 | Acyl-CoA dehydrogenase short chain |
| A0A401RYC2 | Proliferating cell nuclear antigen |
| A0A4W3HJ00 | TNF receptor associated protein 1 |
| A0A401SVW0 | Protein SET |
| A0A401NQG3 | BPNT1 nucleotidase (fragment) |
| A0A401SK27 | Calumenin |
| A0A411HEE0 | Carbonic anhydrase |
| A0A401RS85 | Phosphotriesterase-related protein |
| Accession Number | Protein metabolism |
| A0A401P304 | Argininosuccinate synthase |
| A0A401NVA0 | Protein arginine deiminase |
| K4G395 | Serine/threonine protein phosphatase |
| A0A401RW52 | Dipeptidyl peptidase 1 |
| A0A401P906 | Dipeptidyl peptidase 1 |
| A0A401PKX8 | Cytosol aminopeptidase |
| V9KUJ8 | S-adenosylmethionine synthase |
| A0A4W3JRE6 | Adenosylhomocysteinase |
| V9KVD7 | Dimethylargininase (fragment) |
| A0A401S0W3 | Serine/threonine protein phosphatase |
| A0A401SFC8 | Protein arginine deiminase |
| A0A401QF75 | Peptide-methionine (S)-S-oxide reductase (fragment) |
| A0A401Q2J1 | Argininosuccinate lyase |
| A0A401SZD6 | Aspartate aminotransferase |
| A0A401TGT7 | alanine transaminase |
| A0A401SPB6 | Glutamate dehydrogenase (NAD(P)(+)) |
| A0A401S7A0 | Sushi domain-containing protein |
| A0A401T2T9 | Protein deglycase |
| A0A401NMQ6 | Branched-chain amino acid aminotransferase |
| A0A401SIE1 | Ornithine aminotransferase |
| A0A401Q121 | Aspartyl aminopeptidase |
| A0A401SJG6 | Calpastatin (fragment) |
| A0A401PT33 | AMP_N domain-containing protein (fragment) |
| A0A4W3K041 | Serine/threonine protein phosphatase |
| A0A401RNN1 | Branched-chain amino acid aminotransferase |
| A0A401SFZ0 | LRRcap domain-containing protein |
| A0A401SLZ2 | 2-oxoisovalerate dehydrogenase subunit alpha (fragment) |
| V9KNP8 | N-acyl-aliphatic-L-amino acid amidohydrolase |
| A0A401NQW3 | P/Homo B domain-containing protein |
| A0A401T7U8 | FGE-sulfatase domain-containing protein (fragment) |
| A0A401S584 | Inter-alpha-trypsin inhibitor heavy chain H3 |
| A0A401RY12 | CYTOSOL_AP domain-containing protein |
| A0A401T3E2 | CYTOSOL_AP domain-containing protein |
| A0A401NQA1 | GADL1 decarboxylase (fragment) |
| A0A401RXJ2 | Prolyl endopeptidase |
| Accession Number | Carbohydrate metabolism |
| A0A401PAL6 | Transaldolase |
| V9LII8 | Inositol monophosphatase 2-like protein (fragment) |
| V9L3J0 | Inositol-1-monophosphatase |
| A0A401QAH0 | Neutral alpha-glucosidase AB (fragment) |
| A0A401PMW9 | N-acetylglucosamine-6-sulfatase |
| A0A401NYQ0 | 6-phosphogluconate dehydrogenase, decarboxylating |
| A0A4W3JXK3 | Glucose-6-phosphate isomerase |
| P00341 | L-lactate dehydrogenase A chain |
| A0A4W3GT93 | GDP-mannose 4,6-dehydratase |
| A0A401P2T7 | Pyruvate dehydrogenase E1 component subunit beta |
| A0A4W3JPP5 | Malate dehydrogenase, mitochondrial |
| A0A401P8K0 | Pyr_redox_dim domain-containing protein |
| A0A401P258 | S-(hydroxymethyl)glutathione dehydrogenase |
| A0A401NTL9 | TRANSKETOLASE_1 domain-containing protein |
| A0A401Q0I1 | Pyr_redox_2 domain-containing protein (fragment) |
| A0A401SRC5 | Pyruvate dehydrogenase E1 component subunit alpha |
| A0A401PZL6 | Malate dehydrogenase (fragment) |
| Q76BC4 | Fructose-bisphosphate aldolase (fragment) |
| V9KVQ5 | phosphopyruvate hydratase |
| A0A401SFI1 | Succinate-CoA ligase (GDP-forming) subunit beta, mitochondrial |
| A0A401P9Y7 | Inositol-1-monophosphatase |
| A0A401S408 | Aldehyde dehydrogenase (NAD(+)) |
| A0A401SF33 | Succinate-semialdehyde dehydrogenase |
| A0A401PGK1 | Pyruvate kinase |
| A0A401P3M5 | Isocitrate dehydrogenase (NADP) |
| Q7ZZL2 | Glyceraldehyde-3-phosphate dehydrogenase (fragment) |
| A0A401Q3Q5 | GDP-L-fucose synthase (fragment) |
| A0A4W3GRJ7 | Isocitrate dehydrogenase (NADP) |
| A0A401RXT3 | Transaldolase |
| Q9DDG8 | Phosphopyruvate hydratase (fragment) |
| A0A401PKI1 | Beta-hexosaminidase |
| A0A401T325 | Phosphomannomutase |
| A0A401NUA1 | Fructose bisphosphatase |
| A0A4W3IQ20 | Alcohol dehydrogenase (NADP(+)) |
| A0A401PRM1 | Aldedh domain-containing protein (fragment) |
| K4FRZ7 | Aldehyde dehydrogenase (NAD(+)) |
| A0A4W3JEX4 | Aldehyde dehydrogenase 6 family member A1 |
| A0A401SDE7 | Glycine cleavage system H protein |
| A0A401RGC3 | Citrate synthase |
| A0A401RJ17 | Cis-aconitate decarboxylase |
| A0A401P0M9 | L-type lectin-like domain-containing protein |
| V9KBN2 | Transketolase |
| Q801K6 | Triosephosphate isomerase (Fragment) |
| Accession Number | Cell communication |
| A0A4W3IRK1 | 14-3-3 protein zeta |
| K4FRV3 | Ras-related protein ORAB-1 |
| V9KM01 | 14-3-3 protein epsilon |
| A0A401RKJ5 | Ras-related protein Rab-10 |
| A0A401P7K5 | Ras-related protein Rab-14 |
| A0A4W3KI96 | RAB7A, member RAS oncogene family |
| A0A4W3I0U6 | RAB11B, member RAS oncogene family |
| A0A401RKJ5 | Ras-related protein Rab-10 |
| A0A401P7K5 | Ras-related protein Rab-14 |
| A0A4W3IRK1 | 14-3-3 protein zeta |
| V9KM01 | 14-3-3 protein epsilon |
| A0A4W3I6S4 | RAB15, member RAS oncogene family |
| A0A4W3KI96 | RAB7A, member RAS oncogene family |
| A0A4W3I0U6 | RAB11B, member RAS oncogene family |
| A0A401T632 | Calmodulin |
| K4FRV3 | Ras-related protein ORAB-1 |
| A0A4W3IZ94 | Family with sequence similarity 149 member A |
| A0A401NXB1 | Adenosine kinase |
| A0A401RV12 | ATP synthase subunit beta |
| A0A401SNF0 | Transferrin-like domain-containing protein |
| A0A401SSE0 | Reticulocalbin-2 |
| Q000H3 | Superoxide dismutase (Cu-Zn) |
| A0A401RP32 | LAMC1 protein (fragment) |
| A0A401NHG1 | Serotransferrin |
| A0A401SSQ1 | ATP synthase subunit alpha |
| A0A4W3I3U6 | Serotransferrin |
| C0HJZ2 | Hemoglobin subunit alpha (fragment) |
| A0A4W3JCS1 | Histidine—tRNA ligase |
| A0A401T5K2 | Histidine—tRNA ligase |
| A0A401RZK4 | Serotransferrin |
| V9L0X4 | RAN-binding protein 1 |
| Accession Number | Cytoskeleton-related |
| A0A401PU26 | Actin |
| A0A401SSQ7 | Tropomyosin 1 |
| A0A401NHE5 | Tropomyosin 1 |
| A0A401P520 | Actinin alpha 4 |
| A0A401S817 | F-actin-capping protein subunit alpha |
| A0A401TJ26 | Tropomyosin (fragment) O |
| K4G4H2 | Tubulin beta chain |
| A0A401RP09 | Cadherin-1 |
| A0A401SRU2 | PHB domain-containing protein |
| I0J0X5 | Beta actin (fragment) |
| A0A401PTK4 | MICOS complex subunit (fragment) |
| A0A401NQQ8 | Adenylyl cyclase-associated protein |
| A0A401NRV5 | Fascin |
| A0A401Q7Q0 | Filamin-A (fragment) |
| A0A4W3J6W4 | Filamin B |
| V9KC15 | Fascin |
| A0A401PKY4 | LIM and SH3 domain protein 1 |
| A0A401SZY2 | ADF-H domain-containing protein |
| A0A4W3H117 | Attractin |
| A0A4W3IN76 | Glyoxalase domain-containing 4 |
| A0A401Q4Q2 | IF rod domain-containing protein |
| A0A401P7L8 | Gelsolin |
| A0A401PMT2 | LIM zinc-binding domain-containing protein |
| A0A401Q9B7 | IF rod domain-containing protein (fragment) |
| A0A401SCI8 | EB1 C-terminal domain-containing protein |
| A0A401SX48 | HP domain-containing protein |
| A0A401RYA2 | Beta-centractin |
| Accession Number | Lipid metabolism |
| A0A401P0G6 | FABP domain-containing protein |
| A0A401NMH8 | FABP domain-containing protein |
| A0A4W3GDQ7 | Copine-3-like |
| A0A401PJH8 | Flotillin |
| Accession Number | Others |
| A0A401SQA2 | Structural protein |
| A0A401RDW7 | Uncharacterized protein |
| A0A401QEW8 | Uncharacterized protein (fragment) |
| Accession Number | Immune-Related |
|---|---|
| A0A401P1E2 | Mucin-5B |
| A0A401QGB0 | FTP domain-containing protein (fragment) |
| A0A401PKI6 | GDP-mannose 4,6-dehydratase |
| A0A401PH36 | vWFD domain-containing protein |
| A0A401NTT0 | N(4)-(Beta-N-acetylglucosaminyl)-L-asparaginase |
| A0A401PMW9 | N-acetylglucosamine-6-sulfatase |
| A0A401NXV8 | Intelectin |
| A0A401NHG1 | Serotransferrin |
| A0A401NTT0 | N(4)-(Beta-N-acetylglucosaminyl)-L-asparaginase |
| Accession Number | Genetic information processing |
| A0A401NGY9 | Calreticulin |
| A0A401QHD1 | Annexin (fragment) |
| A0A401PZD5 | Annexin |
| A0A401PS39 | Annexin |
| A0A401P412 | Annexin (fragment) |
| A0A401SX58 | Protein disulfide isomerase (fragment) |
| A0A401PWZ3 | Protein disulfide isomerase |
| A0A401NT99 | Protein disulfide isomerase (fragment) |
| A0A401PWD0 | Peptidyl-prolyl cis-trans isomerase (fragment) |
| A0A401PGJ3 | Protein disulfide isomerase |
| Q9DEZ5 | Ubiquitin (fragment) |
| A0A401NX33 | Zinc-binding protein A33-like |
| A0A401NHG1 | Serotransferrin |
| A0A4W3HGD1 | Heat shock cognate 71 kDa protein |
| A0A401RYU8 | Proteasome subunit alpha type |
| Accession Number | Protein metabolism |
| H9LEQ0 | Haptoglobin |
| A0A401S4Q4 | Creatine kinase |
| K4GLE3 | Dipeptidase B-like protein |
| A0A401NHT1 | Sushi domain-containing protein |
| A0A401NPB6 | Cystatin kininogen-type domain-containing protein |
| A0A401Q2J1 | Argininosuccinate lyase |
| A0A401PTT0 | Cathepsin L (fragment) |
| A0A401SZ08 | Aspartate aminotransferase (fragment) |
| A0A401P304 | Argininosuccinate synthase |
| Q6EE48 | Cathepsin B (fragment) |
| A0A401NVA0 | Protein arginine deiminase |
| A0A4W3J5I2 | H(+)-transporting two-sector ATPase |
| A0A401SSQ1 | ATP synthase subunit alpha |
| A0A401NZH2 | Dipeptidyl peptidase IV membrane form (Fragment) |
| A0A401PMW9 | N-acetylglucosamine-6-sulfatase |
| A0A401P4Q5 | TGc domain-containing protein |
| A0A401RY28 | Vacuolar proton pump subunit B |
| Accession Number | Carbohydrate metabolism |
| A0A401PMD4 | Phosphopyruvate hydratase |
| A0A401NWX3 | Malate dehydrogenase |
| A0A401PKI6 | GDP-mannose 4,6-dehydratase |
| A0A401PGK1 | Pyruvate kinase |
| A0A401P9Y7 | Inositol-1-monophosphatase |
| A0A401NTT0 | N(4)-(Beta-N-acetylglucosaminyl)-L-asparaginase |
| A0A401P1U4 | Aldo_ket_red domain-containing protein |
| A0A401PPR5 | Hyaluronidase (fragment) |
| A0A401PY00 | AB hydrolase-1 domain-containing protein |
| A0A401PAL6 | Transaldolase |
| Accession Number | Cell communication |
| V9KM01 | 14-3-3 protein epsilon |
| A0A401NTU8 | Syndecan binding protein |
| A0A401SV03 | Ras-related protein Rab-2A |
| A0A401PFE8 | 14_3_3 domain-containing protein |
| A0A401PHA8 | Integrin_alpha2 domain-containing protein |
| A0A401PR00 | C2 domain-containing protein (fragment) |
| A0A401PGG8 | Rho GDP-dissociation inhibitor 1 |
| Accession Number | Cytoskeleton-related |
| A0A401PN55 | Golgi apparatus protein 1 |
| A0A401NQV3 | Cadherin-17 |
| A0A401Q409 | Cadherin-1 |
| A0A401P520 | Actinin alpha 4 |
| A0A4W3HRB3 | Keratin, type II cytoskeletal 8-like |
| A0A401Q538 | Cadherin-1 |
| A0A401NX75 | HELP domain-containing protein |
| A0A401RHP4 | Tropomyosin alpha-4 chain (fragment) |
| A0A401NHE5 | Tropomyosin 1 |
| A0A401P7L8 | Gelsolin |
| A0A401NRJ6 | Protein tyrosine phosphatase |
| A0A4W3IN76 | Glyoxalase domain containing 4 |
| A0A4W3JRG2 | ACTB protein |
| A0A401PTK4 | MICOS complex subunit (fragment) |
| A0A401SRU2 | PHB domain-containing protein |
| A0A4W3JRG2 | ACTB protein |
| Accession Number | Lipid metabolism |
| A0A401PDN1 | Vitellogenin domain-containing protein |
| Accession Number | Others |
| A0A401S9B9 | Breast carcinoma amplified sequence 1 |
| A0A401Q2V9 | UPAR/Ly6 domain-containing protein (fragment) |
| A0A401PTQ1 | DUF3298 domain-containing protein (fragment) |
| Spiny Dogfish | Classification | Number of Proteins | % of Total (206) |
|---|---|---|---|
| Immune-related | 24 | 11.6 | |
| Genetic information processing | 53 | 26 | |
| Protein metabolism | 35 | 17 | |
| Carbohydrate metabolism | 43 | 21 | |
| Cell communication | 31 | 15 | |
| Cytoskeletal | 27 | 13 | |
| Lipid metabolism | 4 | 2 | |
| others | 3 | 2 | |
| Cellular location | Number of proteins | % of total (206) | |
| Secreted | 39 | 18.9 | |
| Cytoplasm (including organelles and nucleus) | 173 | 84 | |
| Membrane | 25 | 12.1 | |
| Chain catshark | Classification | Number of proteins | % of total (72) |
| Immune-related | 9 | 13 | |
| Genetic infomation processing | 15 | 21 | |
| Protein metabolism | 17 | 21 | |
| Carbohydrate metabolism | 10 | 14 | |
| Cell communication | 7 | 10 | |
| Cytoskeletal | 16 | 22 | |
| Lipid metabolism | 1 | 1.4 | |
| others | 4 | 6 | |
| Cellular location | Number of proteins | % of total (72) | |
| Secreted | 22 | 30.6 | |
| Cytoplasm (including organelles and nucleus) | 52 | 72 | |
| Membrane | 17 | 23.6 |
| Accession Number (UniProt) | Protein Name | Organism a | Function |
|---|---|---|---|
| A0A401T5Y9 | Mucin-5B-like | BBBS | Highly glycosylated and gel-forming macromolecular components of mucus secretions [30]. Also named vWFD domain-containing protein, exhibiting an evolutionarily-conserved von Willebrand factor type D domain (vWD), found in mucins [31]. |
| A0A401RME8 | Mucin-2-like | BBBS | Antimicrobial mucin gel that participates in innate immunity [32]. Also named vWFD domain-containing protein (see above). |
| A0A401SE28 | Ig-like domain containing protein | BBBS | Immunoglobulin [33]. |
| A0A401PH36 | vWFD domain-containing protein | CCS | See A0A401T5Y9 |
| P23085 | Ig heavy chain C region (Fragment) | HS | Immunoglobulin (UniProt, [34]). |
| A0A401NGS8 | vWFA domain-containing protein | CCS | Von Willebrand factor type A domain See A0A401T5Y9 and [34]. |
| A0A0H4IU03 | Antithrombin | BBBS | Regulates blood coagulation [35,36] and is involved in activation of the immune system [37,38] |
| A0A401RZK0 | Serotransferrin | BBBS | Delivers iron to cells via a receptor-mediated endocytic process as well to remove toxic free iron from the blood and to provide an antibacterial, low-iron environment [39]. |
| A0A401Q3Q5 | GDP-L-fucose synthase (Fragment) | CCS | Involved in fucosylation [40]. |
| U5NJK8 | Secreted IgW heavy chain | NS | Immunoglobulin found in spiny dogfish serum [41]. |
| A0A401RMX1 | Fibrinogen beta chain | BBBS | β-component of fibrinogen, which serves key roles in hemostasis and antimicrobial host defense [42] |
| H9LDW9 | Complement protein 1S | SDF | A component of the classical pathway of the complement system (UniProt, [43]). |
| P03983 | Ig heavy chain V region | HS | V region of the variable domain of immunoglobulin heavy chains participates in the antigen recognition [44]. |
| A0A088MN23 | C3 complement component | NS | C3 plays a central role in the activation of the complement system [45] |
| A0A401Q3Q5 | GDP-L-fucose synthase (Fragment) | CCS | See A0A401Q3Q5 |
| A0A401SNF0 | Transferrin-like domain-containing protein | BBBS | The transferrin-like domain contains conserved cysteine residues involved in disulfide bond formation [46]. |
| A0A401NHG1 | Serotransferrin | CCS | See A0A401RZK0 |
| A0A4W3I3U6 | Serotransferrin | GS | See A0A401RZK0 |
| A0A401RZK4 | Serotransferrin | BBBS | See A0A401RZK0 |
| A0A401SL10 | Annexin | BBBS | Plays important roles in the innate immune response as effector of glucocorticoid-mediated responses and regulator of the inflammatory process [47]. |
| V9KKG3 | Annexin (fragment) | GS | See A0A401SL10 |
| A0A401PZD5 | Annexin | CCS | See A0A401SL10 |
| V9LF43 | Aminoacyl tRNA synthase complex- interacting multifunctional protein 1 (fragment) | GS | A cytokine that is specifically induced by apoptosis, and it is involved in the control of angiogenesis, inflammation, and wound healing [48]. |
| A0A401RS85 | Phosphotriesterase- related protein | BBBS | Predicted to enable hydrolase activity, acting on ester bonds and zinc ion binding activity [49] |
| A0A401P906 | Dipeptidyl peptidase 1 | CCS | Lysosomal cysteine proteinase that activates serine proteinases in cells of the immune system [50]. |
| V9KVD7 | dimethylargininase (Fragment) | GS | Positive regulation of angiogenesis and vascular permeability (UniProt, [51]. |
| A0A401Q2J1 | Argininosuccinate lyase | CCS | Channels extracellular L-arginine to nitric oxide synthesis pathway during inflammation [52]. |
| A0A401S7A0 | Sushi domain-containing protein | BBBS | Sushi domains are known to be involved in many recognition processes, including the binding of several complement factors to fragments C3b and C4b [53] |
| A0A401S584 | Inter-alpha-trypsin inhibitor heavy chain H3 | BBBS | Heavy chain subunit of the pre-alpha-trypsin inhibitor complex. This complex stabilizes the extracellular matrix through its ability to bind hyaluronic acid, found in mucins (UniProt, [31]). |
| A0A4W3JXK3 | Glucose-6-phosphate isomerase | GS | Induces immunoglobulin secretion [54] |
| A0A4W3GT93 | GDP-mannose 4,6- dehydratase | GS | This enzyme converts GDP-mannose to GDP-4-dehydro-6-deoxy-D-mannose, the first of three steps for the conversion of GDP-mannose to GDP-fucose in animals, plants, and bacteria [55,56]. |
| A0A401Q3Q5 | GDP-L-fucose synthase (fragment) | CCS | See A0A401Q3Q5 |
| Q9DDG8 | Phosphopyruvate hydratase (fragment) | BBBS | Stimulates immunoglobulin production [57]. |
| A0A401SNF0 | Transferrin-like domain-containing protein | BBBS | See A0A401SNF0 |
| A0A401RP32 | LAMC1 protein (fragment) | BBSS | Role in cell adhesion, differentiation, migration, and signaling [58] |
| A0A401NHG1 | Serotransferrin | CCS | See A0A401RZK0 |
| A0A4W3I3U6 | Serotransferrin | GS | See A0A401RZK0 |
| C0HJZ2 | Hemoglobin subunit alpha (fragment) | GLSS | Involved in oxygen transport from gills to the various peripheral tissues [59] |
| A0A401RZK4 | Serotransferrin | BBBS | See A0A401RZK0 |
| No. of Nodes | No. of Edges | Interaction Source | |
|---|---|---|---|
| Spiny dogfish vs. zebrafish | 170 | 831 | All |
| 170 | 293 | Experiments and databases | |
| Spiny dogfish vs. cloudy catshark | 183 | 1597 | All |
| 183 | 1098 | Experiments and databases | |
| Spiny dogfish vs. brownbanded bamboo shark | 182 | 1412 | All |
| 182 | 959 | Experiments and databases | |
| Spiny dogfish vs. elephant shark | 167 | 665 | All |
| 167 | 271 | Experiments and databases |
| No. of Nodes | No. of Edges | Interaction Source | |
|---|---|---|---|
| Chain catshark vs. zebrafish | 62 | 62 | All |
| 62 | 21 | Experiments and databases | |
| Chain catshark vs. cloudy catsharks | 71 | 146 | All |
| 71 | 94 | Experiments and databases | |
| Chain catshark vs. brownbanded bamboo shark | 67 | 119 | All |
| 67 | 82 | Experiments and databases | |
| Chain catshark vs. elephant shark | 64 | 46 | All |
| 64 | 20 | Experiments and databases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bachar-Wikstrom, E.; Dhillon, B.; Gill Dhillon, N.; Abbo, L.; Lindén, S.K.; Wikstrom, J.D. Mass Spectrometry Analysis of Shark Skin Proteins. Int. J. Mol. Sci. 2023, 24, 16954. https://doi.org/10.3390/ijms242316954
Bachar-Wikstrom E, Dhillon B, Gill Dhillon N, Abbo L, Lindén SK, Wikstrom JD. Mass Spectrometry Analysis of Shark Skin Proteins. International Journal of Molecular Sciences. 2023; 24(23):16954. https://doi.org/10.3390/ijms242316954
Chicago/Turabian StyleBachar-Wikstrom, Etty, Braham Dhillon, Navi Gill Dhillon, Lisa Abbo, Sara K. Lindén, and Jakob D. Wikstrom. 2023. "Mass Spectrometry Analysis of Shark Skin Proteins" International Journal of Molecular Sciences 24, no. 23: 16954. https://doi.org/10.3390/ijms242316954
APA StyleBachar-Wikstrom, E., Dhillon, B., Gill Dhillon, N., Abbo, L., Lindén, S. K., & Wikstrom, J. D. (2023). Mass Spectrometry Analysis of Shark Skin Proteins. International Journal of Molecular Sciences, 24(23), 16954. https://doi.org/10.3390/ijms242316954





