The Potential of Endophytes in Improving Salt–Alkali Tolerance and Salinity Resistance in Plants
Abstract
:1. Introduction
2. Plant-Associated Endophytes
3. Plant Salt Stress and Mechanism Underlying Salt Tolerance in Plants
4. Endophytes and Management of Salt Stress
4.1. Probiotic Effects of Endophyte
4.2. Endophytes for Saline-Land Management
5. Mechanisms Whereby Plant Endophytes Mitigate Salt Stress
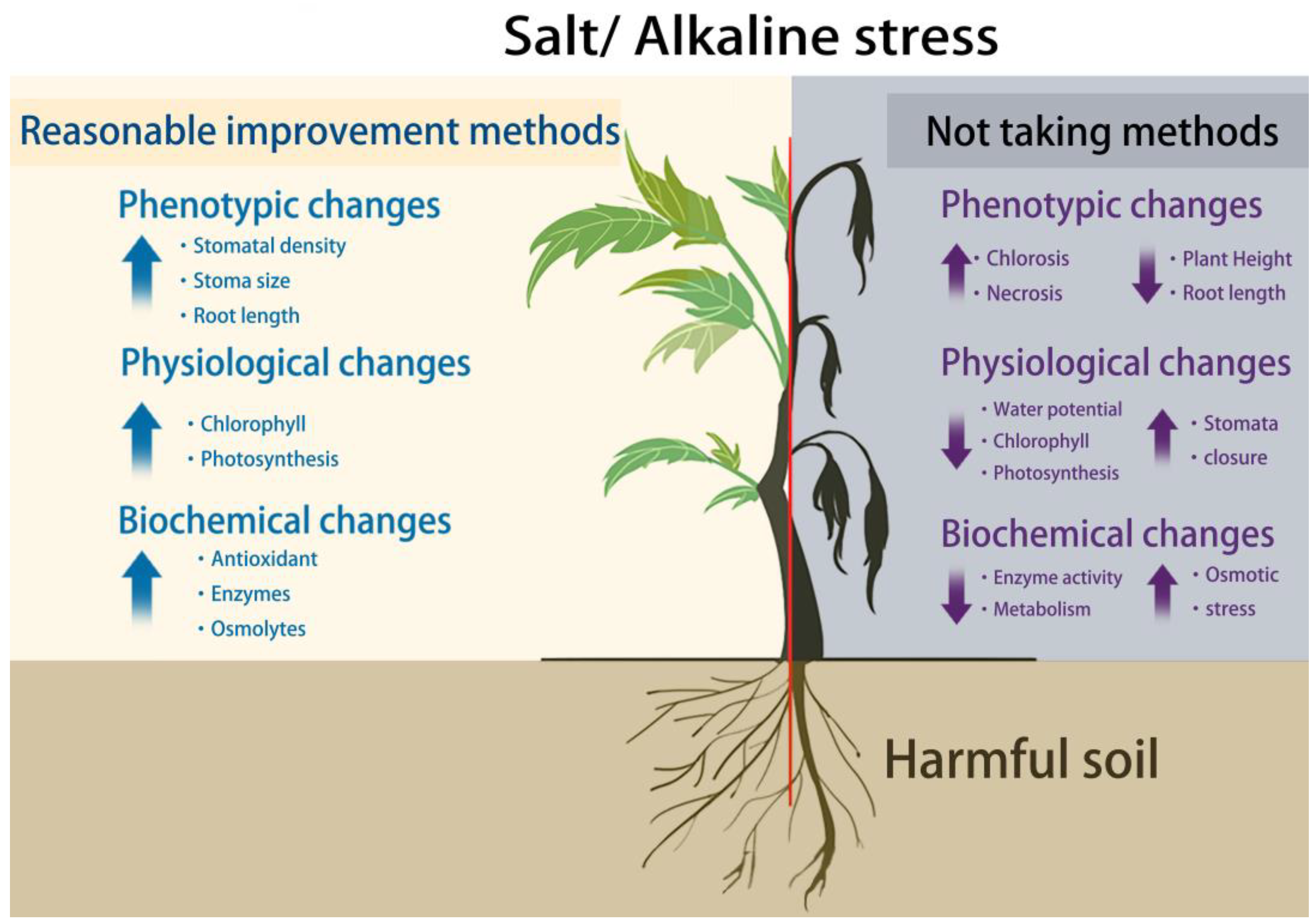
5.1. Hazards of High Salt Penetration to Plants
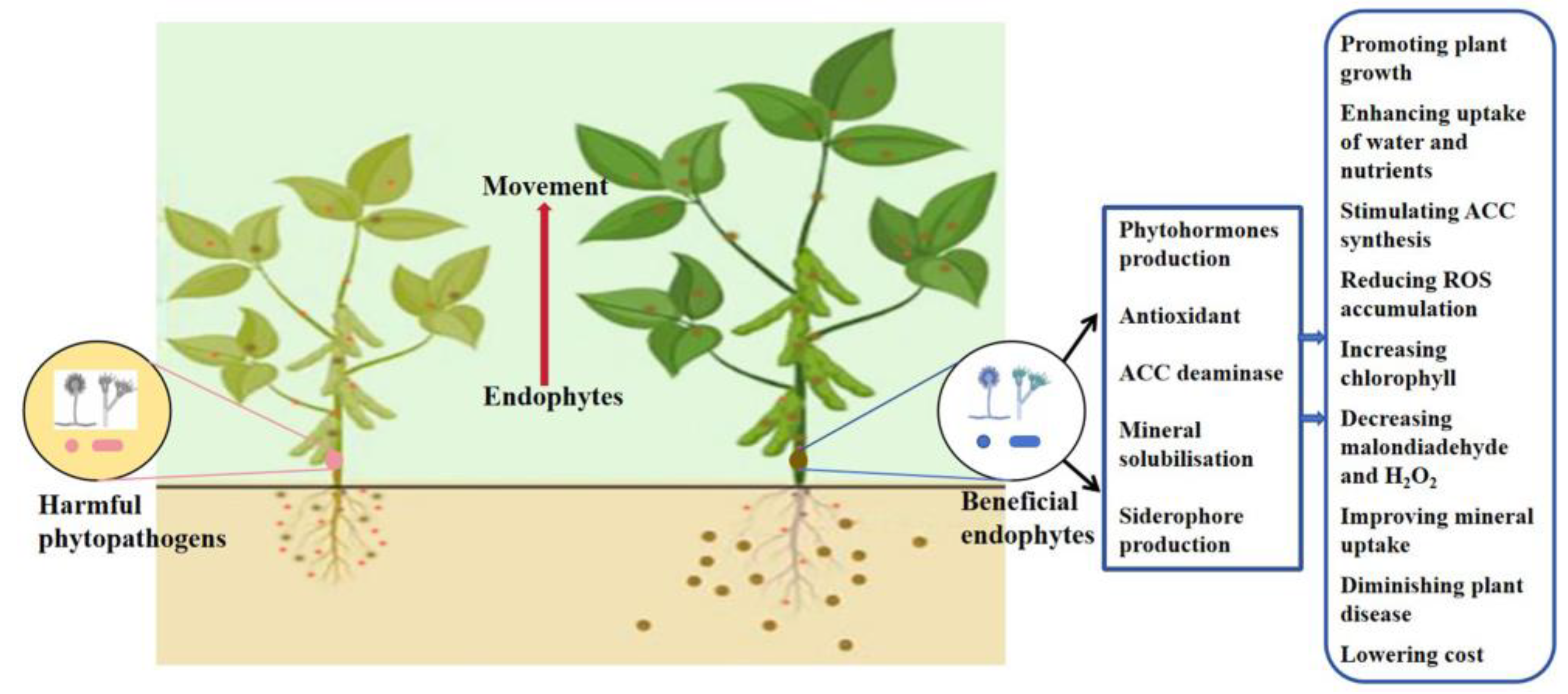
5.2. Direct Action of Endophytes
5.3. Indirect Action of Endophytes
5.3.1. Accumulation of Plant Hormones
5.3.2. Accumulation of Osmoprotectants
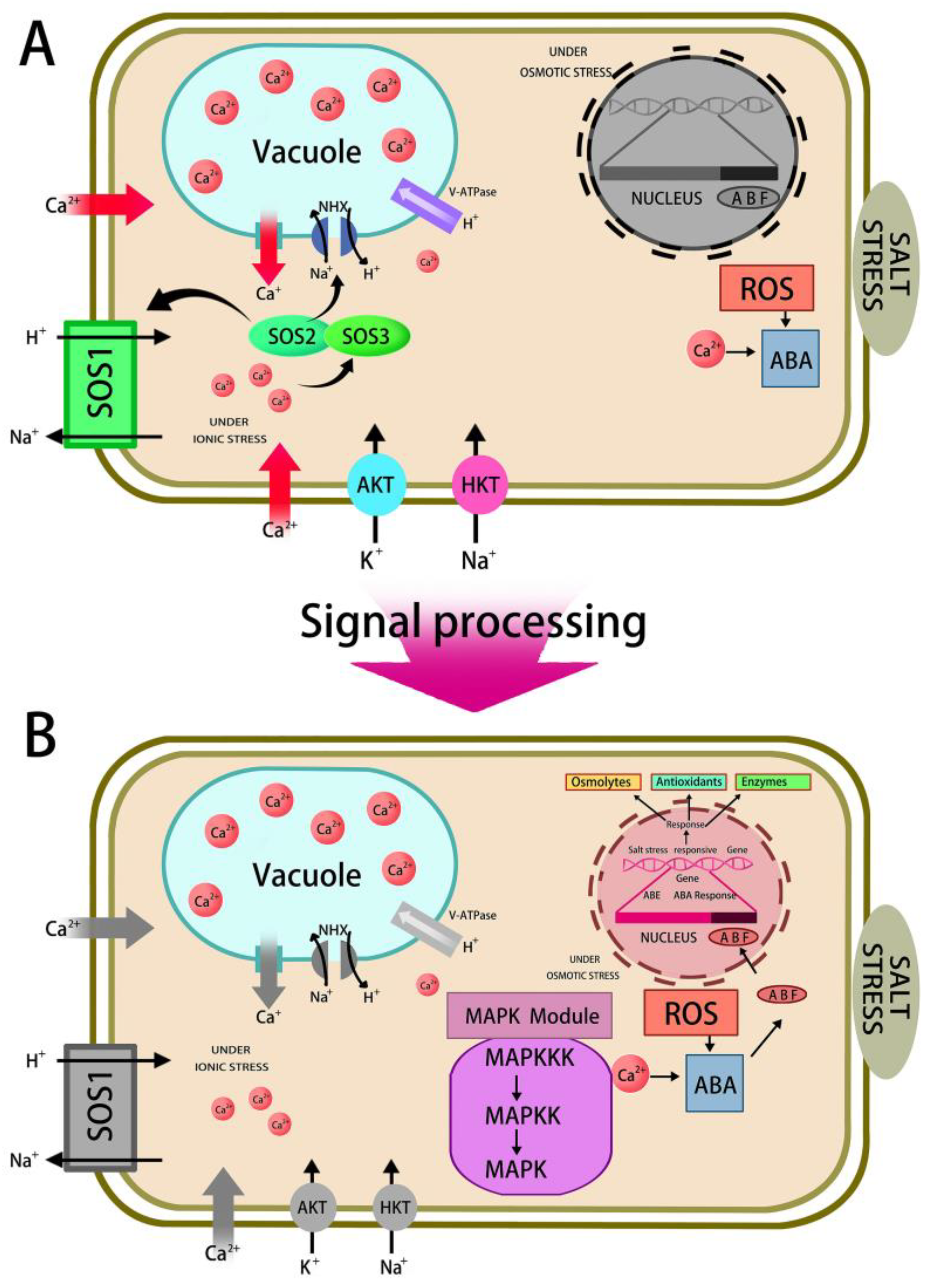
5.3.3. Regulation of Ion Transportation
5.3.4. Salt-Responsive Gene Expression
6. Application of Endophyte in Mitigating Salt Stress
6.1. Application of Single Endophyte
6.2. Combined Application of Multiple Endophytes
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fang, S.; Hou, X.; Liang, X. Response Mechanisms of Plants Under Saline-Alkali Stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Peng, X.; Hua, X.; Sun, S.; Wang, Y.; Yan, X. Effects of arbuscular mycorrhizal fungi on Leymus chinensis seedlings under salt–alkali stress and nitrogen deposition conditions: From osmotic adjustment and ion balance. RSC Adv. 2018, 8, 14500–14509. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Peng, T.; Xue, S. Mechanisms of plant saline-alkaline tolerance. J. Plant Physiol. 2023, 281, 153916. [Google Scholar] [CrossRef]
- Mann, A.; Lata, C.; Kumar, N.; Kumar, A.; Kumar, A.; Sheoran, P. Halophytes as new model plant species for salt tolerance strategies. Front. Plant Sci. 2023, 14, 1137211. [Google Scholar] [CrossRef]
- Liu, X.; Guo, K.; Feng, X.; Sun, H. Exploring the efficient use of saline resources in agriculture. Chin. J. Eco-Agric. 2023, 31, 345–353. [Google Scholar]
- Sagar, A.; Rai, S.; Ilyas, N.; Sayyed, R.Z.; Al-Turki, A.I.; El Enshasy, H.A.; Simarmata, T. Halotolerant Rhizobacteria for Salinity-Stress Mitigation: Diversity, Mechanisms and Molecular Approaches. Sustainability 2022, 14, 490. [Google Scholar] [CrossRef]
- Mijti, M. An analysis of saline land management techniques. Xinjiang Agric. Sci. Technol. 2021, 23–25. [Google Scholar]
- Meshram, V.; Elazar, M.; Maymon, M.; Sharma, G.; Shawahna, R.; Belausov, E.; Charuvi, D.; Freeman, S. Endophytic Fusarium clavum confers growth and salt tolerance in Cucumis melo. Environ. Exp. Bot. 2023, 206, 105153. [Google Scholar] [CrossRef]
- Moghaddam, M.S.H.; Safaie, N.; Soltani, J.; Hagh-Doust, N. Desert-adapted fungal endophytes induce salinity and drought stress resistance in model crops. Plant Physiol. Biochem. 2021, 160, 225–238. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Opportunity and challenges of phenotyping plant salt tolerance. Trends Plant Sci. 2023, 28, 552–566. [Google Scholar] [CrossRef]
- Giannelli, G.; Potestio, S.; Visioli, G. The Contribution of PGPR in Salt Stress Tolerance in Crops: Unravelling the Molecular Mechanisms of Cross-Talk between Plant and Bacteria. Plants 2023, 12, 2197. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.; Palombo, E.A.; Castillo, A.J.; Zaferanloo, B. Endophytes in Agriculture: Potential to Improve Yields and Tolerances of Agricultural Crops. Microorganisms 2023, 11, 1276. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yao, D.; Li, B.; Li, M.; Zhan, F.; Zu, Y.; Li, Y. The role of microbial communities in the remediation of heavy metal contaminated soils. Jiangsu J. Agric. Sci. 2020, 36, 1322–1331. [Google Scholar]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621, Correction in 2021, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Solanki, M.K.; Solanki, A.C.; Singh, A.; Kashyap, B.K.; Rai, S.; Malviya, M.K. Chapter 1—Microbial endophytes’ association and application in plant health: An overview. In Microbial Endophytes and Plant Growth; Solanki, M.K., Yadav, M.K., Singh, B.P., Gupta, V.K., Eds.; Academic Press: Salt Lake City, UT, USA, 2023; pp. 1–18. [Google Scholar]
- Hou, Y.; Luo, J.; Li, X.; Cui, X.; Jia, B.; Diao, F.; Wang, L.; Guo, W. Effect of AM fungi on the growth of small-fruited white spurge under salt and heavy metal stresses. J. South China Agric. Univ. 2022, 43, 68–78. [Google Scholar]
- Kandasamy, G.D.; Kathirvel, P. Insights into bacterial endophytic diversity and isolation with a focus on their potential applications—A review. Microbiol. Res. 2023, 266, 127256. [Google Scholar] [CrossRef]
- Pandey, S.S.; Jain, R.; Bhardwaj, P.; Thakur, A.; Kumari, M.; Bhushan, S.; Kumar, S. Plant probiotics—Endophytes pivotal to plant health. Microbiol. Res. 2022, 263, 127148. [Google Scholar] [CrossRef]
- Fan, D.; Subramanian, S.; Smith, D.L. Plant endophytes promote growth and alleviate salt stress in Arabidopsis thaliana. Sci. Rep. 2020, 10, 12740. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Liu, Q.; Yang, H.; Wang, Z.; Zhang, W.; Liu, T. Progress of research on combined plant-endophyte treatment of environmental pollutants. Chin. J. Appl. Environ. Biol. 2021, 27, 1706–1715. [Google Scholar]
- Yan, L.; Zhu, J.; Zhao, X.; Shi, J.; Jiang, C.; Shao, D. Beneficial effects of endophytic fungi colonization on plants. Appl. Microbiol. Biotechnol. 2019, 103, 3327–3340. [Google Scholar] [CrossRef]
- Taheri, E.; Tarighi, S.; Taheri, P. An endophytic bacterium with biocontrol activity against important wheat pathogens. Biol. Control. 2023, 183. [Google Scholar] [CrossRef]
- Zhang, F.; Feng, N.; Bin, H.; Li, Y.; Zhao, H.; Xiang, Q.; Cai, Q.; Mo, X. Mechanisms of colonization, promotion and reduction of organic pollution in crops by endophytic bacteria (I): Colonization and promotion. J. Agro-Environ. Sci. 2022, 41, 1619–1628. [Google Scholar]
- Singh, S.; Kumar, V.; Dhanjal, D.S.; Sidhu, G.K.; Datta, S.; Kumar, S.; Singh, J. Endophytic microbes in abiotic stress management. In Microbial Endophytes; Kumar, A., Singh, V.K., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 91–123. [Google Scholar]
- Anand, U.; Pal, T.; Yadav, N.; Singh, V.K.; Tripathi, V.; Choudhary, K.K.; Shukla, A.K.; Sunita, K.; Kumar, A.; Bontempi, E.; et al. Current Scenario and Future Prospects of Endophytic Microbes: Promising Candidates for Abiotic and Biotic Stress Management for Agricultural and Environmental Sustainability. Microb. Ecol. 2023, 86, 1455–1486. [Google Scholar] [CrossRef] [PubMed]
- Durand, A.; Leglize, P.; Benizri, E. Are endophytes essential partners for plants and what are the prospects for metal phytoremediation? Plant Soil 2021, 460, 1–30. [Google Scholar] [CrossRef]
- Omomowo, I.O.; Amao, J.A.; Abubakar, A.; Ogundola, A.F.; Ezediuno, L.O.; Bamigboye, C.O. A review on the trends of endophytic fungi bioactivities. Sci. Afr. 2023, 20, e01594. [Google Scholar] [CrossRef]
- Adhikari, P.; Joshi, K.; Pandey, A. Taxus associated fungal endophytes: Anticancerous to other biological activities. Fungal Biol. Rev. 2023, 45, 100308. [Google Scholar] [CrossRef]
- Martinez-Klimova, E.; Rodríguez-Peña, K.; Sánchez, S. Endophytes as sources of antibiotics. Biochem. Pharmacol. 2017, 134, 1–17. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef]
- Roy, S.; Chakraborty, A.P.; Chakraborty, R. Understanding the potential of root microbiome influencing salt-tolerance in plants and mechanisms involved at the transcriptional and translational level. Physiol. Plant. 2021, 173, 1657–1681. [Google Scholar] [CrossRef]
- Fu, H.; Yang, Y. How plants tolerate salt stress. Curr. Issues Mol. Biol. 2023, 45, 5914–5934. [Google Scholar] [CrossRef]
- Ali, A.; Petrov, V.; Yun, D.-J.; Gechev, T. Revisiting plant salt tolerance: Novel components of the SOS pathway. Trends Plant Sci. 2023, 28, 1060–1069. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.-u.-D.; Tanveer, M.; Shaukat, R.; Ali, M.; Pirdad, F. An overview of salinity tolerance mechanism in plants. In Salt and Drought Stress Tolerance in Plants: Signaling Networks and Adaptive Mechanisms; Hasanuzzaman, M., Tanveer, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–16. [Google Scholar]
- Cheng, P.; Gao, J.; Feng, Y.; Zhang, Z.; Liu, Y.; Fang, W.; Chen, S.; Chen, F.; Jiang, J. The chrysanthemum leaf and root transcript profiling in response to salinity stress. Gene 2018, 674, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, J.; Zhang, Y.; Fan, F.; Li, W.; Wang, F.; Zhong, W.; Wang, C.; Yang, J. Comparative transcriptome analysis reveals molecular response to salinity stress of salt-tolerant and sensitive genotypes of indica rice at seedling stage. Sci. Rep. 2018, 8, 2085. [Google Scholar] [CrossRef] [PubMed]
- Bouzroud, S.; Henkrar, F.; Fahr, M.; Smouni, A. Salt stress responses and alleviation strategies in legumes: A review of the current knowledge. 3 Biotech 2023, 13, 287. [Google Scholar] [CrossRef]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef]
- Amir, R.; Munir, F.; Kubra, G.; Nauman, I.; Noor, N. Role of Signaling Pathways in Improving Salt Stress in Plants. In Salt Stress, Microbes, and Plant Interactions: Mechanisms and Molecular Approaches: Volume 2; Akhtar, M.S., Ed.; Springer: Singapore, 2019; pp. 183–211. [Google Scholar]
- Anwar, Z.; Ijaz, A.; Ditta, A.; Wang, B.; Liu, F.; Khan, S.M.-U.; Haidar, S.; Hassan, H.M.; Khan, M.K.R. Genomic Dynamics and Functional Insights under Salt Stress in Gossypium hirsutum L. Genes 2023, 14, 1103. [Google Scholar] [CrossRef] [PubMed]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; van Themaat, E.V.L.; Schulze-Lefert, P. Structure and Functions of the Bacterial Microbiota of Plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Cordovez, V.; Dini-Andreote, F.; Carrión, V.J.; Raaijmakers, J.M. Ecology and Evolution of Plant Microbiomes. Annu. Rev. Microbiol. 2019, 73, 69–88. [Google Scholar] [CrossRef]
- Frank, A.C.; Guzmán, J.P.S.; Shay, J.E. Transmission of Bacterial Endophytes. Microorganisms 2017, 5, 70. [Google Scholar] [CrossRef]
- Lòpez-Fernàndez, S.; Mazzoni, V.; Pedrazzoli, F.; Pertot, I.; Campisano, A. A Phloem-Feeding Insect Transfers Bacterial Endophytic Communities between Grapevine Plants. Front. Microbiol. 2017, 8, 834. [Google Scholar] [CrossRef]
- Kaga, H.; Mano, H.; Tanaka, F.; Watanabe, A.; Kaneko, S.; Morisaki, H. Rice Seeds as Sources of Endophytic Bacteria. Microbes Environ. 2009, 24, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Luo, Y.; Teng, Y.; Li, X. Progress of phytoremediation of heavy metal contaminated soil enhanced by endophytic bacteria. Acta Pedol. Sin. 2013, 50, 195–202. [Google Scholar]
- Galeano, R.M.S.; Silva, S.M.; Yonekawa, M.K.A.; Guimarães, N.C.d.A.; Giannesi, G.C.; Masui, D.C.; Corrêa, B.O.; Brasil, M.d.S.; Zanoelo, F.F. Penicillium chrysogenum strain 34-P promotes plant growth and improves initial development of maize under saline conditions. Rhizosphere 2023, 26, 100710. [Google Scholar] [CrossRef]
- El-Sawah, A.M.; Abdel-Fattah, G.G.; Holford, P.; Korany, S.M.; Alsherif, E.A.; AbdElgawad, H.; Ulhassan, Z.; Jośko, I.; Ali, B.; Sheteiwy, M.S. Funneliformis constrictum modulates polyamine metabolism to enhance tolerance of Zea mays L. to salinity. Microbiol. Res. 2023, 266, 127254. [Google Scholar] [CrossRef]
- Chen, L.; Fang, R.; Wu, J.; Zhang, L. Advances in methods for the determination of endophytic bacteria in plants. Microbiol. China 2022, 49, 1105–1119. [Google Scholar]
- Deng, Z.; Cao, L. Fungal endophytes and their interactions with plants in phytoremediation: A review. Chemosphere 2017, 168, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Dini-Andreote, F. Endophytes: The second layer of plant defense. Trends Plant Sci. 2020, 25, 4. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.K.; Makampara, R.A.; Kalaria, R.K.; Joshi, M.P. Endophytes: A novel tool for sustainable agriculture. In Endophytic Association: What, Why and How; Shah, M., Deka, D., Eds.; Academic Press: Salt Lake City, UT, USA, 2023; pp. 37–55. [Google Scholar]
- Rajkumar, M.; Ae, N.; Freitas, H. Endophytic bacteria and their potential to enhance heavy metal phytoextraction. Chemosphere 2009, 77, 153–160. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Ma, M.; Gao, M.; Chai, W.; Feng, Y. Effect of mixed saline and alkaline stresses on seed germination of saline alkali ponts. Microbiol. China 2020, 47, 47–53. [Google Scholar]
- Liu, J.; Zhang, H.; Zou, R.; Yang, X.; Zhu, J.; Zhang, H. Advances in physiological growth mechanisms and Na+ reverse transport in different types of saline plants adapted to salt stress. Biotechnol. Bull. 2023, 39, 59–71. [Google Scholar]
- Zhou, X.; Yin, Y.; Wang, G.; Amombo, E.; Li, X.; Xue, Y.; Fu, J. Mitigation of salt stress on low temperature in bermudagrass: Resistance and forage quality. Front. Plant Sci. 2022, 13, 1042855. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Zeyad, M.T.; Syed, A.; Singh, U.B.; Mohamed, A.; Bahkali, A.H.; Elgorban, A.M.; Pichtel, J. Stress-tolerant endophytic isolate Priestia aryabhattai BPR-9 modulates physio-biochemical mechanisms in wheat (Triticum aestivum L.) for enhanced salt tolerance. Int. J. Environ. Res. Public Health 2022, 19, 10883. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, H.; Ahmad, F. Soil-Plant and Microbial Interaction in Improving Salt Stress. In Salt Stress, Microbes, and Plant Interactions: Causes and Solution: Volume 1; Akhtar, M.S., Ed.; Springer: Singapore, 2019; pp. 217–235. [Google Scholar]
- Zhang, L.; Hou, Y.; Wang, L. Effects of saline and alkaline stress on plants and methods to improve their saline and alkaline tolerance. J. Northeast Agric. Sci. 2018, 43, 11–16. [Google Scholar]
- Hu, L.; Hu, J.; Wang, L.; Zhang, X.; Chen, Z. Mechanisms of plant response, regulation and adaptation to salt stress. Hubei Agric. Sci. 2015, 54, 1–7. [Google Scholar]
- Lanza, M.; Haro, R.; Conchillo, L.B.; Benito, B. The endophyte Serendipita indica reduces the sodium content of Arabidopsis plants exposed to salt stress: Fungal ENA ATPases are expressed and regulated at high pH and during plant co-cultivation in salinity. Environ. Microbiol. 2019, 21, 3364–3378. [Google Scholar] [CrossRef] [PubMed]
- Azzeme, A.M.; Abdullah, S.N.A. Adaptive Mechanisms of Plants Against Salt Stress and Salt Shock. In Salt Stress, Microbes, and Plant Interactions: Mechanisms and Molecular Approaches: Volume 2; Akhtar, M.S., Ed.; Springer: Singapore, 2019; pp. 27–47. [Google Scholar]
- Phurailatpam, L.; Mishra, S. Role of Plant Endophytes in Conferring Abiotic Stress Tolerance. In Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives II: Mechanisms of Adaptation and Stress Amelioration; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; pp. 603–628. [Google Scholar]
- Lu, Y.; Fricke, W. Salt Stress—Regulation of Root Water Uptake in a Whole-Plant and Diurnal Context. Int. J. Mol. Sci. 2023, 24, 8070. [Google Scholar] [CrossRef]
- Guo, R.; Li, F.; Zhou, J.; Li, H.; Xia, X.; Liu, Q. Physiological characteristics of flax in response to salt and alkali stresses. Chin. J. Plant Ecol. 2016, 40, 69–79. [Google Scholar]
- Siddiqui, Z.S.; Wei, X.; Umar, M.; Abideen, Z.; Zulfiqar, F.; Chen, J.; Hanif, A.; Dawar, S.; Dias, D.A.; Yasmeen, R. Scrutinizing the Application of Saline Endophyte to Enhance Salt Tolerance in Rice and Maize Plants. Front. Plant Sci. 2022, 12, 770084. [Google Scholar] [CrossRef]
- Choudhury, A.R.; Trivedi, P.; Madhaiyan, M.; Choi, J.; Choi, W.; Park, J.-H.; Walitang, D.I.; Sa, T. ACC deaminase producing endophytic bacteria enhances cell viability of rice (Oryza sativa L.) under salt stress by regulating ethylene emission pathway. Environ. Exp. Bot. 2023, 213, 105411. [Google Scholar] [CrossRef]
- Della Mónica, I.F.; Vignale, M.V.; Scervino, J.M.; Iannone, L.J.; Novas, M.V. Role of fungal endophytes on mycorrhizal-plant association and its impact on plant fitness. In Microbial Endophytes and Plant Growth; Solanki, M.K., Yadav, M.K., Singh, B.P., Gupta, V.K., Eds.; Academic Press: Salt Lake City, UT, USA, 2023; pp. 117–136. [Google Scholar]
- Feng, Z.; Chen, S.; Wang, W.; Yang, H.; Deng, Z.; Li, Z.; Wang, S.; Xu, Y. Screening and identification of several strains of phosphorus solubilising bacteria and their phosphorus solubilising effect. J. Nanjing Agric. Univ. 2017, 40, 842–849. [Google Scholar]
- Shu, Z.; Han, R.; Wang, Z.; Xing, J.; Wang, R.; Zhu, D. Advances in plant growth promotion and metabolic regulation by halophilic microorganisms in saline soils. Jiangsu Agric. Sci. 2022, 50, 27–36. [Google Scholar]
- Li, C.; Ge, B.; Senhao, J.; Tang, B. Research progress on bioremediation of saline and contaminated soils by alkali puffs. Chin. J. Soil Sci. 2014, 45, 1014–1019. [Google Scholar]
- Cui, C.; MeixueDai; Xia, Z. Isolation and phylogenetic diversity analysis of endophytic moderately halophilic bacteria from saline alkali ponts. Microbiol. China 2010, 37, 204–210. [Google Scholar]
- Li, Y.; Zhuo, W.; Wang, N. Progress in the research and application of salt-tolerant bacteria in saline alkali flora. Food Res. Dev. 2015, 36, 188–190. [Google Scholar]
- Xi, J.; Qian, K.; Shan, L.; Huang, J.; Yan, Y. The potential of mineral weathering of halophilic-endophytic bacteria isolated from Suaeda salsa and Spartina anglica. Arch. Microbiol. 2022, 204, 561. [Google Scholar] [CrossRef]
- Singh, A.V.; Khan, A.; Joshi, M. Plant–microbe interaction: A sustainable strategy to elevate salinity tolerance in plants. In Microbes and Signaling Biomolecules Against Plant Stress: Strategies of Plant- Microbe Relationships for Better Survival; Sharma, A., Ed.; Springer: Singapore, 2021; pp. 37–54. [Google Scholar]
- Lu, H.; Wu, T.; Yao, Z.; Xie, W.; Liu, J.; DaixiangDuan; Zhang, X. Isolation and characterisation of endophytic hydrocarbon solubilising bacteria from saline plants. Anhui Agric. Sci. Bull. 2017, 23, 18–20. [Google Scholar]
- Kukla, M.; Płociniczak, T.; Piotrowska-Seget, Z. Diversity of endophytic bacteria in Lolium perenne and their potential to degrade petroleum hydrocarbons and promote plant growth. Chemosphere 2014, 117, 40–46. [Google Scholar] [CrossRef]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How Plant Hormones Mediate Salt Stress Responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef]
- Kushwaha, P.; Kashyap, P.L.; Bhardwaj, A.K.; Kuppusamy, P.; Srivastava, A.K.; Tiwari, R.K. Bacterial endophyte mediated plant tolerance to salinity: Growth responses and mechanisms of action. World J. Microbiol. Biotechnol. 2020, 36, 26. [Google Scholar] [CrossRef]
- Jalil, S.U.; Ansari, M.I. Role of Phytohormones in Recuperating Salt Stress. In Salt Stress, Microbes, and Plant Interactions: Mechanisms and Molecular Approaches: Volume 2; Akhtar, M.S., Ed.; Springer: Singapore, 2019; pp. 91–104. [Google Scholar]
- Wen, D.; Gao, C.; Zhang, Q.; Lu, Z.; Jin, D.; Li, J.; Lu, J. Progress of research on potassium-solubilising bacteria and their application in agricultural production. J. Shanxi Agric. Sci. 2014, 42, 921–924. [Google Scholar]
- Niu, S.; Gao, Y.; Zi, H.; Liu, Y.; Liu, X.; Xiong, X.; Yao, Q.; Qin, Z.; Chen, N.; Guo, L.; et al. The osmolyte-producing endophyte Streptomyces albidoflavus OsiLf-2 induces drought and salt tolerance in rice via a multi-level mechanism. Crop. J. 2022, 10, 375–386. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting Rhizobacteria for Enhancing Crop Productivity of Saline Soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Iqbal, A.; Ahmed, F.; Ahmad, M. Phytobeneficial and salt stress mitigating efficacy of IAA producing salt tolerant strains in Gossypium hirsutum. Saudi J. Biol. Sci. 2021, 28, 5317–5324. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; He, W.; Li, Z.; Ge, L.; Zhang, J.; Liu, T. Salt-tolerant endophytic bacterium Enterobacter ludwigii B30 enhance bermudagrass growth under salt stress by modulating plant physiology and changing rhizosphere and root bacterial community. Front. Plant Sci. 2022, 13, 959427. [Google Scholar] [CrossRef] [PubMed]
- Bouzouina, M.; Kouadria, R.; Lotmani, B. Fungal endophytes alleviate salt stress in wheat in terms of growth, ion homeostasis and osmoregulation. J. Appl. Microbiol. 2020, 130, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.K.; Dey, R.; Sherathia, D.N.; Devidayal; Mangalassery, S.; Kumar, A.; Rupapara, R.B.; Mandaliya, M.; Rawal, P.; Bhadania, R.A.; et al. Alleviation of Salinity Stress in Peanut by Application of Endophytic Bacteria. Front. Microbiol. 2021, 12, 650771. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, Y.; Lu, P.; Liu, M.; Sun, P.; Zhang, Z. Roles of endophytic bacteria in Suaeda salsa grown in coastal wetlands: Plant growth characteristics and salt tolerance mechanisms. Environ. Pollut. 2021, 287, 117641. [Google Scholar] [CrossRef]
- Chen, T.; White, J.F.; Li, C. Fungal endophyte Epichloë bromicola infection regulates anatomical changes to account for salt stress tolerance in wild barley (Hordeum brevisubulatum). Plant Soil 2021, 461, 533–546. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef]
- Liu, H.; Tang, H.; Ni, X.; Zhang, Y.; Wang, Y. Interactive Effects of Epichloë Endophytes and Arbuscular Mycorrhizal Fungi on Saline-Alkali Stress Tolerance in Tall Fescue. Front. Microbiol. 2022, 13, 855890. [Google Scholar] [CrossRef]
- Bharti, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016, 6, 34768. [Google Scholar] [CrossRef]
- Pinedo, I.; Ledger, T.; Greve, M.; Poupin, M.J. Burkholderia phytofirmans PsJN induces long-term metabolic and transcriptional changes involved in Arabidopsis thaliana salt tolerance. Front. Plant Sci. 2015, 6, 466. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Paul, S.C.; Parveen, S.; Alam, S.; Rahman, N.; Jannat, B.; Hoque, S.; Rahman, M.T.; Karim, M.M. Isolation and identification of salt-tolerant plant-growth-promoting rhizobacteria and their application for rice cultivation under salt stress. Can. J. Microbiol. 2020, 66, 144–160. [Google Scholar] [CrossRef]
- Kumar, V.; Raghuvanshi, N.; Pandey, A.K.; Kumar, A.; Thoday-Kennedy, E.; Kant, S. Role of Halotolerant Plant Growth-Promoting Rhizobacteria in Mitigating Salinity Stress: Recent Advances and Possibilities. Agriculture 2023, 13, 168. [Google Scholar] [CrossRef]
- Gupta, A.; Shaw, B.P. Augmenting salt tolerance in rice by regulating uptake and tissue specific accumulation of Na+ through Ca2+ induced alteration of biochemical events. Plant Biol. 2021, 23, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, M.E.; Kim, D.; Ali, S.; Fedoroff, N.V.; Al-Babili, S. The endophytic fungus Piriformospora indica enhances Arabidopsis thaliana growth and modulates Na+/K+ homeostasis under salt stress conditions. Plant Sci. 2017, 263, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; He, R.; Chai, Q.; Li, C.; Nan, Z. Transcriptomic analyses giving insights into molecular regulation mechanisms involved in cold tolerance by Epichloë endophyte in seed germination of Achnatherum inebrians. Plant Growth Regul. 2016, 80, 367–375. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, G.; Lü, X.; Zhou, D.; Han, X. Salt tolerance during seed germination and early seedling stages of 12 halophytes. Plant Soil 2014, 388, 229–241. [Google Scholar] [CrossRef]
- Mushtaq, A.; Khan, Z.; Khan, S.; Rizwan, S.; Jabeen, U.; Bashir, F.; Ismail, T.; Anjum, S.; Masood, A. Effect of silicon on antioxidant enzymes of wheat (Triticum aestivum L.) grown under salt stress. Silicon 2020, 12, 2783–2788. [Google Scholar] [CrossRef]
- Dong, Z.-Y.; Rao, M.P.N.; Wang, H.-F.; Fang, B.-Z.; Liu, Y.-H.; Li, L.; Xiao, M.; Li, W.-J. Transcriptomic analysis of two endophytes involved in enhancing salt stress ability of Arabidopsis thaliana. Sci. Total. Environ. 2019, 686, 107–117. [Google Scholar] [CrossRef]
- Yoo, S.-J.; Weon, H.-Y.; Song, J.; Sang, M.K. Induced Tolerance to Salinity Stress by Halotolerant Bacteria Bacillus aryabhattai H19-1 and B. mesonae H20-5 in Tomato Plants. J. Microbiol. Biotechnol. 2019, 29, 1124–1136. [Google Scholar] [CrossRef]
- Sampangi-Ramaiah, M.H.; Jagadheesh; Dey, P.; Jambagi, S.; Kumari, M.M.V.; Oelmüller, R.; Nataraja, K.N.; Ravishankar, K.V.; Ravikanth, G.; Shaanker, R.U. An endophyte from salt-adapted Pokkali rice confers salt-tolerance to a salt-sensitive rice variety and targets a unique pattern of genes in its new host. Sci. Rep. 2020, 10, 3237. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, H.; Zhao, D.; Shi, Y.; Liu, A. Isolation and identification of endophytic salt-tolerant strain YN1 from wheat and its biological properties. Shandong Agric. Sci. 2019, 51, 63–68. [Google Scholar]
- Manjunatha, N.; Manjunatha, N.; Li, H.; Sivasithamparam, K.; Jones, M.G.; Edwards, I.; Wylie, S.J.; Agarrwal, R. Fungal endophytes from salt-adapted plants confer salt tolerance and promote growth in wheat (Triticum aestivum L.) at early seedling stage. Microbiology 2022, 168, 001225. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, L.; Xing, H.; Luo, Y.; Wei, Z. Effect of endophytic bacteria on proline and malondialdehyde in wheat seedlings under salt stress. Acta Ecol. Sin. 2020, 40, 3726–3737. [Google Scholar]
- Du, X.; Yang, L.; Jiang, M.; Zeng, Z.; Li, M.; Li, Z.; Li, S. Mitigation effect of endophytic bacteria on wheat seedlings under NaCl stress. Anhui Agric. Sci. Bull. 2022, 28, 15–18. [Google Scholar]
- Fu, L.; Li, X.; Gao, L.; Jiang, J.; Sun, J. Effect of endophytic bacteria of the genus Pantoea on germination and physiology of hybrid wolfsbane under salt stress. Pratacultural Sci. 2017, 34, 2099–2108. [Google Scholar]
- Miao, Y.; Zhang, Y.; Song, B.; Liu, X.; Zhang, A.; Lv, J.; Zhang, H.; Zhang, X.; Ouyang, J.; Li, W.; et al. Effect of inter-root and endophytic bacterial strains of alkali ponts on the growth of alfalfa under saline and alkaline stresses. Acta Prataculturae Sin. 2022, 31, 107–117. [Google Scholar]
- Wang, Q.; Feng, L.; Li, Y.; Chu, G.; Sun, Y. Screening and characterisation of inter-root salt-tolerant bacteria of Suaeda dendroides in Xinjiang. Microbiol. China 2019, 46, 2569–2578. [Google Scholar]
- Jha, Y.; Subramanian, R.B.; Patel, S. Combination of endophytic and rhizospheric plant growth promoting rhizobacteria in Oryza sativa shows higher accumulation of osmoprotectant against saline stress. Acta Physiol. Plant. 2010, 33, 797–802. [Google Scholar] [CrossRef]
- Elgharably, A.; Nafady, N.A. Inoculation with Arbuscular mycorrhizae, Penicillium funiculosum and Fusarium oxysporum enhanced wheat growth and nutrient uptake in the saline soil. Rhizosphere 2021, 18, 100345. [Google Scholar] [CrossRef]

| Edophyte | Host | Mechanism | References |
|---|---|---|---|
| Funneliformis mosseae | Nitraria sibirica | It alleviates salt and heavy-metal stress by promoting nutrient absorption, regulating ion balance, and affecting Na+ and Cd absorption in plants. | [16] |
| Bacillus japanicum | Soybean, wheat | Reduce ethylene levels to achieve resistance to salt stress. | [83] |
| B. cereus, B. subtilis, B. paramycoides, | Cotton | Synthesize indole, indole-3-butylamide, benzyl malonic acid and 4-methyl-2-pyrrolidone. | [84] |
| Enterobacter ludwigii | Cynodon dactylon | The content of indoleacetic acid was increased, and the content of abscisic acid was decreased under salt stress to the host. | [85] |
| Streptomyces albidoflavus | Rice | Help rice produce rich osmotic-pressure substances, including proline, polysaccharide, and exotin, to increase the osmoregulation ability of rice. | [82] |
| Bacillus firmus; B. tequilensis | Peanut | Enhance accumulation of proline, reduced level of phenol and H2O2, and enhanced uptake of potassium. | [87] |
| Sphingomonas prati, S. zeicaulis | Suaeda salsa | Improved intracellular osmotic metabolism and promoted the production of CAT in the antioxidant enzyme system and retained permeability. | [88] |
| Bacillus mobilis, Rhizobium jaguaris | Arabidopsis thaliana | Regulate osmolytes and antioxidant enzymes. | [19] |
| Claroideoglomus etunicatum | Lolium arundinaceum | Increase shoot and root biomass and nutrient uptake (organic carbon, total nitrogen, and total phosphorus concentration), and accumulate K+, while decreasing Na+ concentration. | [91] |
| Serendipita indica | Arabidopsis | Produce a reduction in Na+ content in the plant roots and upregulation of chlorophyll a reductase. | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Peng, W.; Xu, X.; Xie, K.; Yang, X. The Potential of Endophytes in Improving Salt–Alkali Tolerance and Salinity Resistance in Plants. Int. J. Mol. Sci. 2023, 24, 16917. https://doi.org/10.3390/ijms242316917
Guo X, Peng W, Xu X, Xie K, Yang X. The Potential of Endophytes in Improving Salt–Alkali Tolerance and Salinity Resistance in Plants. International Journal of Molecular Sciences. 2023; 24(23):16917. https://doi.org/10.3390/ijms242316917
Chicago/Turabian StyleGuo, Xueying, Wanrong Peng, Xinyi Xu, Kangwei Xie, and Xingyong Yang. 2023. "The Potential of Endophytes in Improving Salt–Alkali Tolerance and Salinity Resistance in Plants" International Journal of Molecular Sciences 24, no. 23: 16917. https://doi.org/10.3390/ijms242316917
APA StyleGuo, X., Peng, W., Xu, X., Xie, K., & Yang, X. (2023). The Potential of Endophytes in Improving Salt–Alkali Tolerance and Salinity Resistance in Plants. International Journal of Molecular Sciences, 24(23), 16917. https://doi.org/10.3390/ijms242316917







