Genome-Wide Identification, Expression, and Molecular Characterization of the CONSTANS-like Gene Family in Seven Orchid Species
Abstract
:1. Introduction
2. Results
2.1. Basic Characterization of COL Genes in Orchidaceae
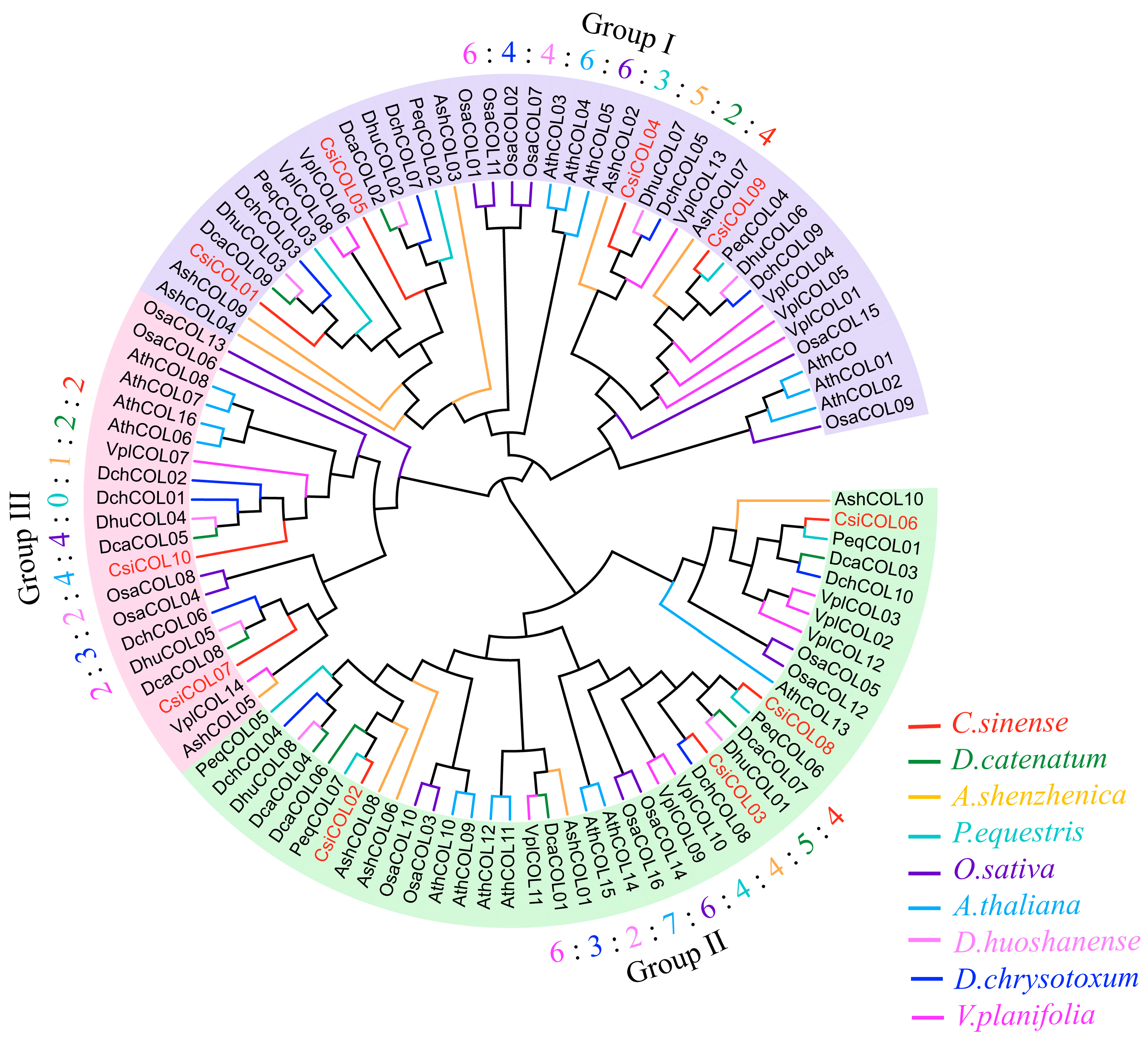
2.2. Phylogenetic Analysis of COL Genes
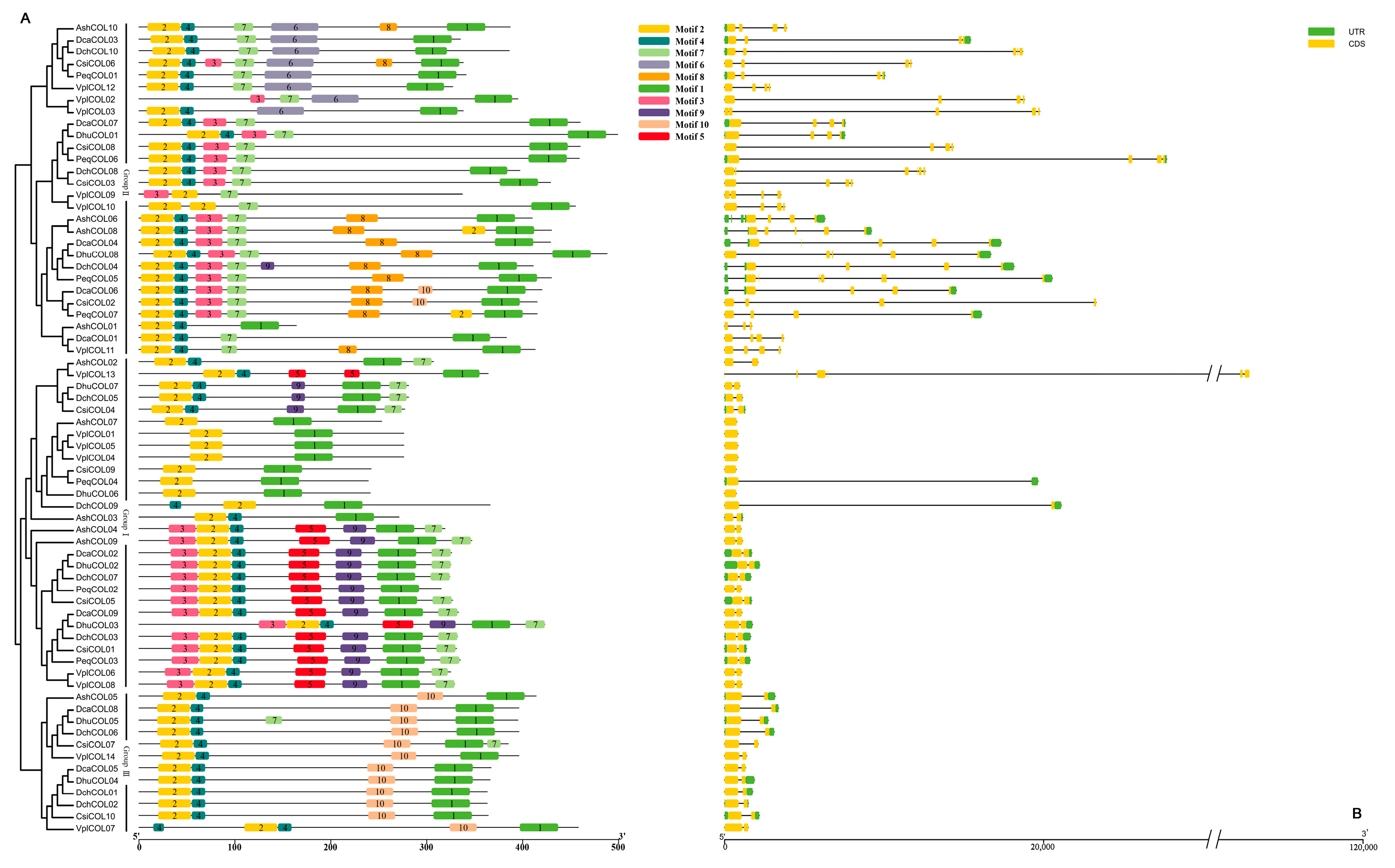
2.3. Sequence Structure Analysis of COL Members
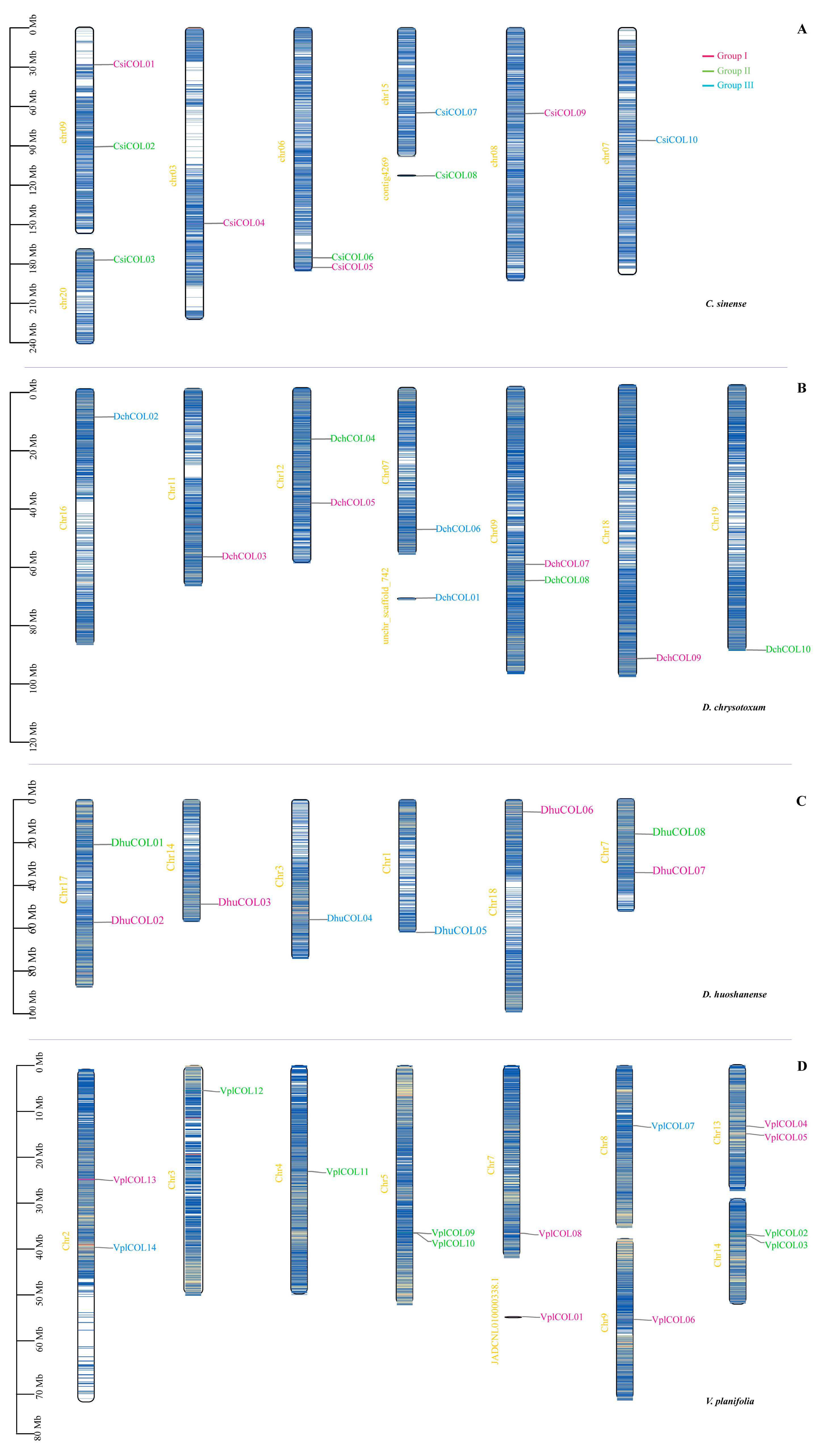
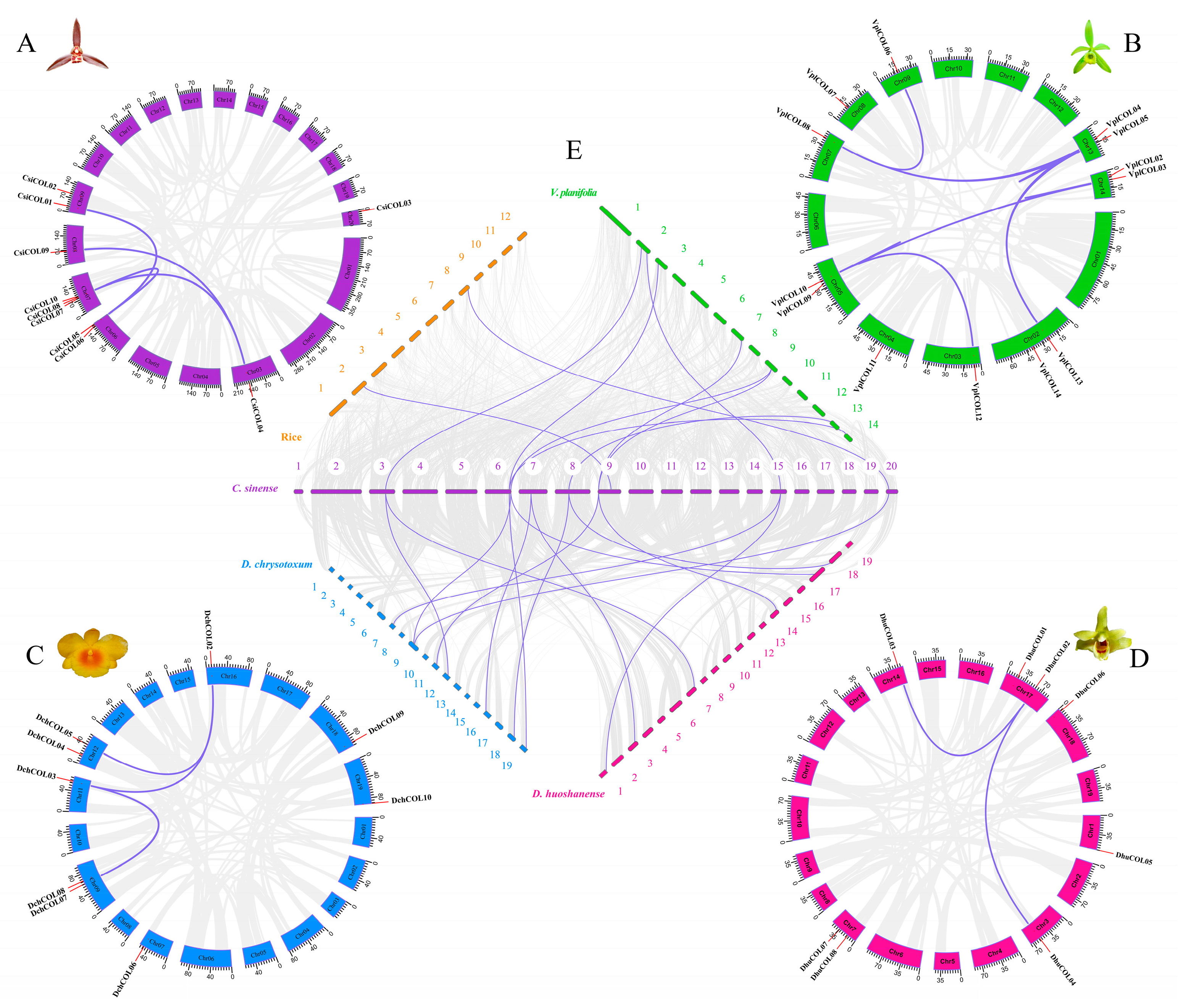
2.4. Chromosomal Location, Collinearity and Evolutionary Analysis of Orchid COL Genes
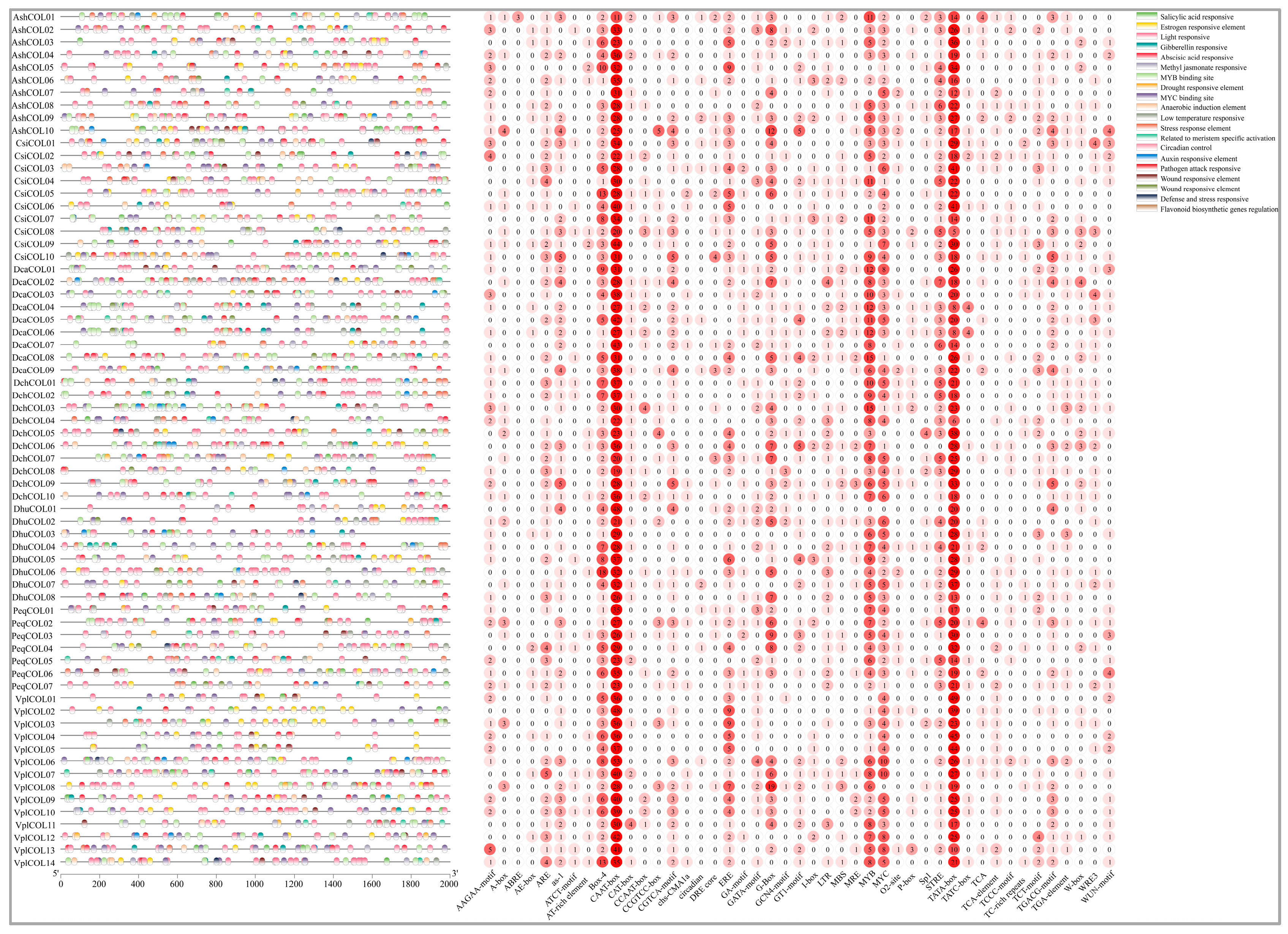
2.5. Cis-Acting Elements in the Promoters of Orchid COL Genes
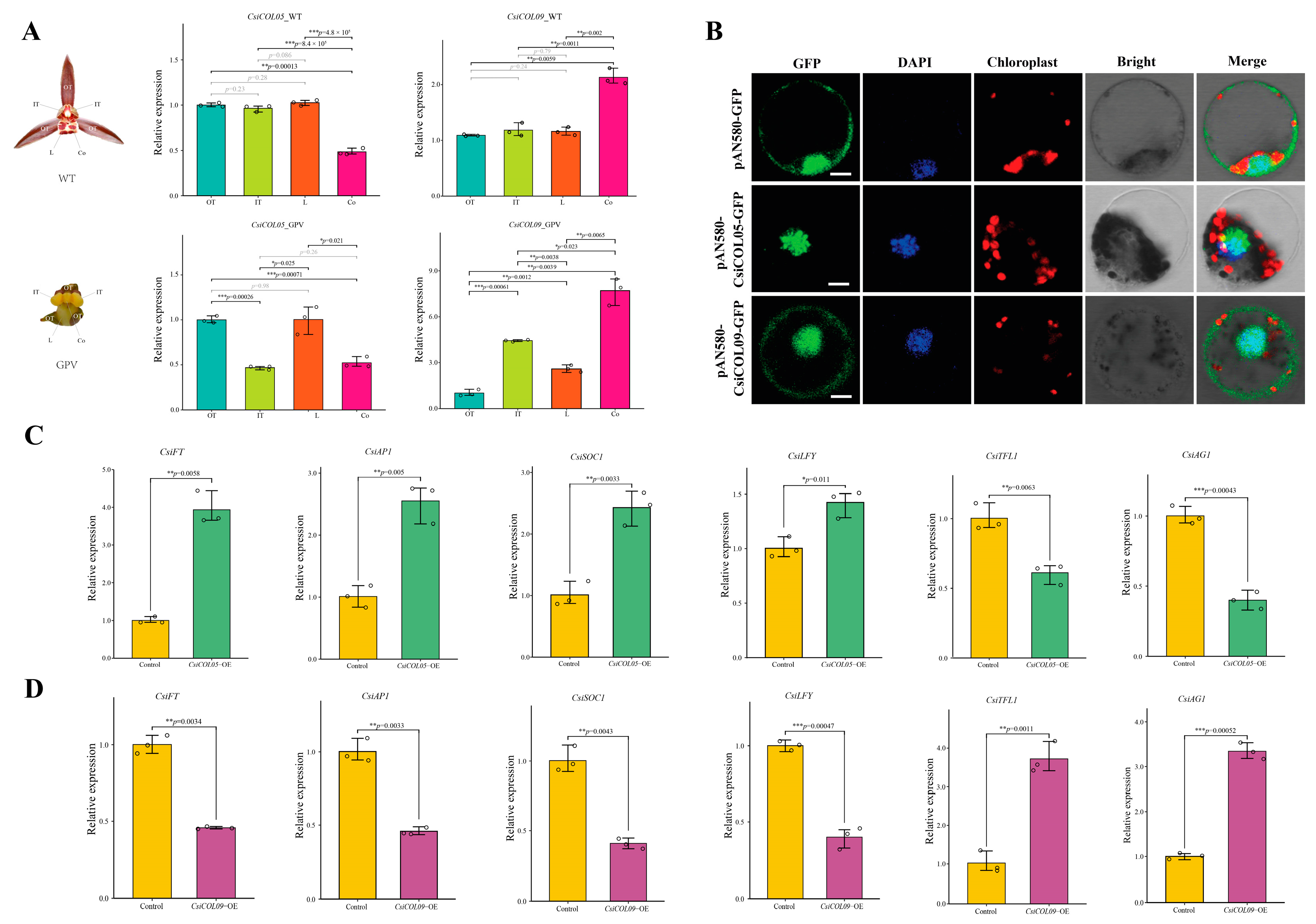
2.6. Expression Profiles of Orchid COL Genes in Different Tissues and Differentially Expressed CsiCOL Genes during ABA Treatment
2.7. Protein Structure Prediction
2.8. Correlation Analysis between CsiCOLs and Regulation of Co-Activity-Related Transcription Factors
2.9. Exploration of Subcellular Localization and Regulatory Analysis by Transient Overexpression of CsiCOLs in Cymbidium Protoplasts
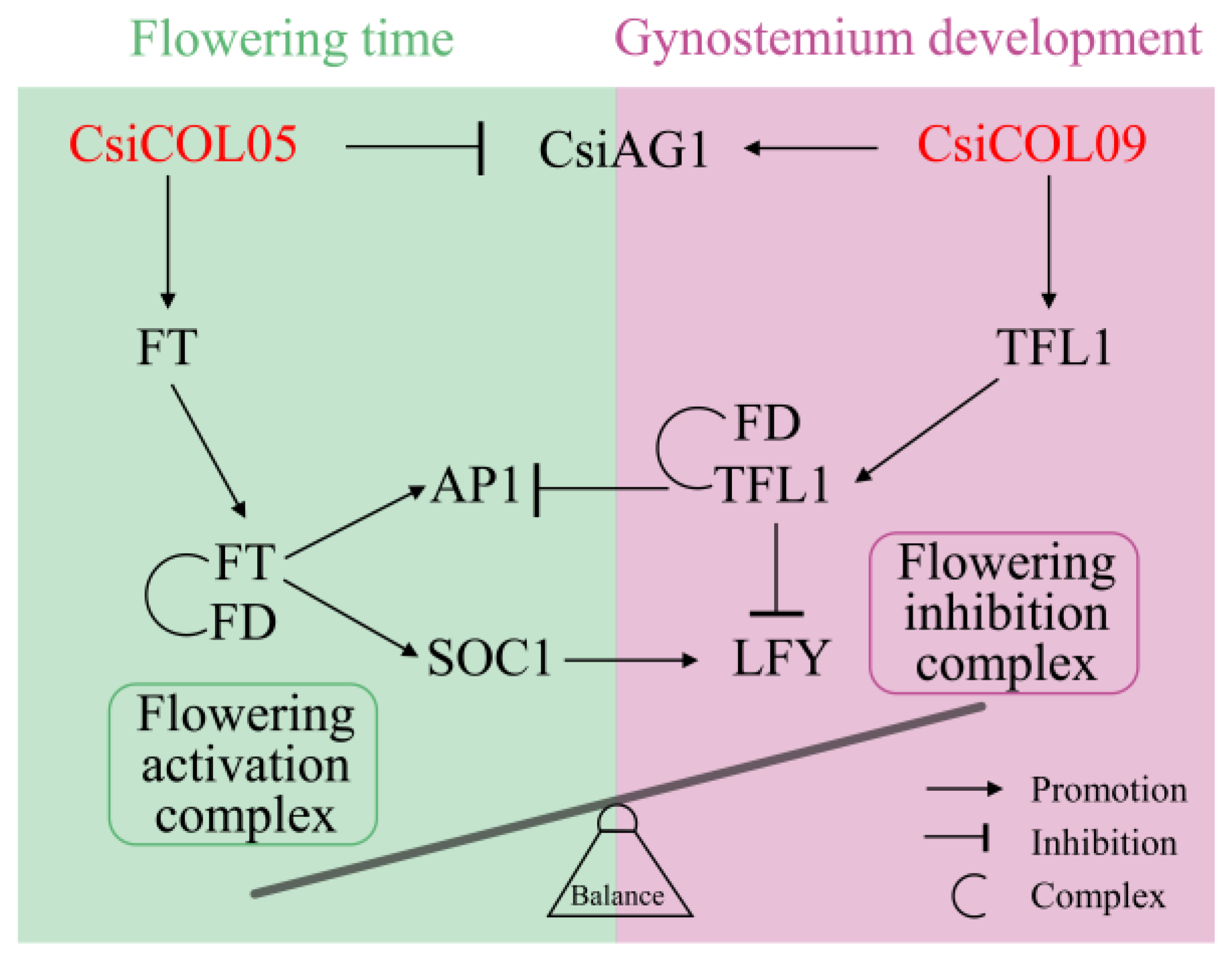
3. Discussion
4. Materials and Methods
4.1. Basic Characterization of COL Genes in Orchidaceae
4.2. Phylogenetic Tree Construction
4.3. Chromosomal Location, Comparative Genome Collinearity Analysis, and Selective Pressure
4.4. Conserved Motifs of Orchidaceae COL Genes
4.5. Analysis of the Cis-Acting Elements
4.6. Growth Conditions of C. sinense, Sample Collection, and Abscisic Acid (ABA) Treatments
4.7. Protein Tertiary Structure Prediction
4.8. Correlation Analysis between CsiCOLs and Transcription Factors
4.9. C. sinense Leaf Protoplast Transformation and Subcellular Localization
4.10. RNA Extraction and qRT-PCR Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fay, M.F.; Chase, M.W. Orchid biology: From Linnaeus via Darwin to the 21st century. Preface. Ann. Bot. 2009, 104, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Salazar, G.; van den Berg, C.; Schuiteman, A. An updated classification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174. [Google Scholar] [CrossRef]
- Kindlmann, P.; Kull, T.; McCormick, M. The Distribution and Diversity of Orchids. Diversity 2023, 15, 810. [Google Scholar] [CrossRef]
- Mérillon, J.-M.; Kodja, H. Orchids Phytochemistry, Biology and Horticulture; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, X.; Li, Y.; Ke, S.; Yin, W.; Lan, S.; Liu, Z. Advances and prospects of orchid research and industrialization. Hortic. Res. 2022, 9, uhac220. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, B.; Yu, H. Molecular genetic insights into orchid reproductive development. J. Exp. Bot. 2022, 73, 1841–1852. [Google Scholar] [CrossRef]
- Hsiao, Y.Y.; Fu, C.H.; Ho, S.Y.; Li, C.I.; Chen, Y.Y.; Wu, W.L.; Wang, J.S.; Zhang, D.Y.; Hu, W.Q.; Yu, X.; et al. OrchidBase 4.0: A database for orchid genomics and molecular biology. BMC Plant Biol. 2021, 21, 371. [Google Scholar] [CrossRef]
- Liaqat, F.; Xu, L.; Khazi, M.I.; Ali, S.; Rahman, M.U.; Zhu, D. Extraction, purification, and applications of vanillin: A review of recent advances and challenges. Ind. Crop. Prod. 2023, 204, 117372. [Google Scholar] [CrossRef]
- Das, P.; Chandra, T.; Negi, A.; Jaiswal, S.; Iquebal, M.A.; Rai, A.; Kumar, D. A comprehensive review on genomic resources in medicinally and industrially important major spices for future breeding programs: Status, utility and challenges. Curr. Res. Food Sci. 2023, 7, 100579. [Google Scholar] [CrossRef]
- Hammer, K.; Khoshbakht, K. A domestication assessment of the big five plant families. Genet. Resour. Crop Evol. 2015, 62, 665–689. [Google Scholar] [CrossRef]
- Freudenstein, J.V.; Chase, M.W. Phylogenetic relationships in Epidendroideae (Orchidaceae), one of the great flowering plant radiations: Progressive specialization and diversification. Ann. Bot. 2015, 115, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Lin, Y.; Farag, M.A.; Li, Z.; Shao, P. Dendrobium as a new natural source of bioactive for the prevention and treatment of digestive tract diseases: A comprehensive review with future perspectives. Phytomedicine 2023, 114, 154784. [Google Scholar] [CrossRef] [PubMed]
- Hew, C.S.; Wong, Y.S. Chinese Cymbidium Orchid: History of Chinese Cymbidium; World Scientific: Singapore, 2023. [Google Scholar] [CrossRef]
- Boss, P.K.; Bastow, R.M.; Mylne, J.S.; Dean, C. Multiple pathways in the decision to flower: Enabling, promoting, and resetting. Plant Cell 2004, 16 (Suppl. S1), S18–S31. [Google Scholar] [CrossRef] [PubMed]
- Baurle, I.; Dean, C. The timing of developmental transitions in plants. Cell 2006, 125, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Wickland, D.P.; Hanzawa, Y. The FLOWERING LOCUS T/TERMINAL FLOWER 1 Gene Family: Functional Evolution and Molecular Mechanisms. Mol. Plant 2015, 8, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Chong, K. Remembering winter through vernalisation. Nat. Plants 2018, 4, 997–1009. [Google Scholar] [CrossRef]
- Taylor, C.M.; Kamphuis, L.G.; Zhang, W.; Garg, G.; Berger, J.D.; Mousavi-Derazmahalleh, M.; Bayer, P.E.; Edwards, D.; Singh, K.B.; Cowling, W.A.; et al. INDEL variation in the regulatory region of the major flowering time gene LanFTc1 is associated with vernalization response and flowering time in narrow-leafed lupin (Lupinus angustifolius L.). Plant Cell Environ. 2019, 42, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yuan, J.; Cheng, T.; Tang, M.J.; Sun, K.; Song, S.L.; Xu, F.J.; Dai, C.C. Flowering-mediated root-fungus symbiosis loss is related to jasmonate-dependent root soluble sugar deprivation. Plant Cell Environ. 2019, 42, 3208–3226. [Google Scholar] [CrossRef] [PubMed]
- Robson, F.; Costa, M.M.; Hepworth, S.R.; Vizir, I.; Pineiro, M.; Reeves, P.H.; Putterill, J.; Coupland, G. Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J. 2001, 28, 619–631. [Google Scholar] [CrossRef]
- Jin, S.; Nasim, Z.; Susila, H.; Ahn, J.H. Evolution and functional diversification of FLOWERING LOCUS T/TERMINAL FLOWER 1 family genes in plants. Semin. Cell Dev. Biol. 2021, 109, 20–30. [Google Scholar] [CrossRef]
- Nam, J.; dePamphilis, C.W.; Ma, H.; Nei, M. Antiquity and evolution of the MADS-box gene family controlling flower development in plants. Mol. Biol. Evol. 2003, 20, 1435–1447. [Google Scholar] [CrossRef]
- Huang, J.; BolañosVillegas, P.; Chen, F. Regulation of Flowering in Orchids. In The Orchid Genome; Chen, F., Chin, S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 73–94. [Google Scholar] [CrossRef]
- Imaizumi, T.; Schultz, T.F.; Harmon, F.G.; Ho, L.A.; Kay, S.A. FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science 2005, 309, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Kronmiller, B.; Maszle, D.R.; Coupland, G.; Holm, M.; Mizuno, T.; Wu, S.H. The Arabidopsis B-box zinc finger family. Plant Cell 2009, 21, 3416–3420. [Google Scholar] [CrossRef] [PubMed]
- Gangappa, S.N.; Botto, J.F. The BBX family of plant transcription factors. Trends Plant Sci. 2014, 19, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Putterill, J.; Robson, F.; Lee, K.; Simon, R.; Coupland, G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 1995, 80, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xue, J.; Dai, W.; Tang, Y.; Gong, P.; Wang, Y.; Zhang, C. Genome-wide Identification, Phylogenetic Analysis, and Expression Profiling of CONSTANS-like (COL) Genes in Vitis vinifera. J. Plant Growth Regul. 2019, 38, 631–643. [Google Scholar] [CrossRef]
- Cao, D.; Lin, Z.; Huang, L.; Damaris, R.N.; Li, M.; Yang, P. A CONSTANS-LIKE gene of Nelumbo nucifera could promote potato tuberization. Planta 2021, 253, 65. [Google Scholar] [CrossRef]
- Li, J.; Gao, K.; Yang, X.; Khan, W.U.; Guo, B.; Guo, T.; An, X. Identification and characterization of the CONSTANS-like gene family and its expression profiling under light treatment in Populus. Int. J. Biol. Macromol. 2020, 161, 999–1010. [Google Scholar] [CrossRef] [PubMed]
- Khatun, K.; Debnath, S.; Robin, A.H.K.; Wai, A.H.; Nath, U.K.; Lee, D.J.; Kim, C.K.; Chung, M.Y. Genome-wide identification, genomic organization, and expression profiling of the CONSTANS-like (COL) gene family in petunia under multiple stresses. BMC Genom. 2021, 22, 727. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, S.; Dunford, R.P.; Coupland, G.; Laurie, D.A. The evolution of CONSTANS-like gene families in barley, rice, and Arabidopsis. Plant Physiol. 2003, 131, 1855–1867. [Google Scholar] [CrossRef]
- Song, N.; Xu, Z.; Wang, J.; Qin, Q.; Jiang, H.; Si, W.; Li, X. Genome-wide analysis of maize CONSTANS-LIKE gene family and expression profiling under light/dark and abscisic acid treatment. Gene 2018, 673, 1–11. [Google Scholar] [CrossRef]
- Niu, T.; Wang, X.; Abbas, M.; Shen, J.; Liu, R.; Wang, Z.; Liu, A. Expansion of CONSTANS-like genes in sunflower confers putative neofunctionalization in the adaptation to abiotic stresses. Ind. Crops Prod. 2022, 176, 114400. [Google Scholar] [CrossRef]
- Chaurasia, A.K.; Patil, H.B.; Azeez, A.; Subramaniam, V.R.; Krishna, B.; Sane, A.P.; Sane, P.V. Molecular characterization of CONSTANS-Like (COL) genes in banana (Musa acuminata L. AAA Group, cv. Grand Nain). Physiol. Mol. Biol. Plants 2016, 22, 1–15. [Google Scholar] [CrossRef]
- Song, X.; Duan, W.; Huang, Z.; Liu, G.; Wu, P.; Liu, T.; Li, Y.; Hou, X. Comprehensive analysis of the flowering genes in Chinese cabbage and examination of evolutionary pattern of CO-like genes in plant kingdom. Sci. Rep. 2015, 5, 14631. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Liu, H.; Sang, N.; Huang, X. Identification and characterization of CONSTANS-like (COL) gene family in upland cotton (Gossypium hirsutum L.). PLoS ONE 2017, 12, e0179038. [Google Scholar] [CrossRef] [PubMed]
- Perez-Escobar, O.A.; Bogarín, D.; Przelomska, N.A.; Ackerman, J.D.; Balbuena, J.A.; Bellot, S.; Bühlmann, R.P.; Cabrera, B.; Cano, J.A.; Charitonidou, M. The Origin and Speciation of Orchids. bioRxiv 2023. bioRxiv:2023.09.10.556973. [Google Scholar] [CrossRef]
- Gamage, D.G.; Gunaratne, A.; Periyannan, G.R.; Russell, T.G. Applicability of Instability Index for In vitro Protein Stability Prediction. Protein Pept. Lett. 2019, 26, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.; Podolec, R.; Chappuis, R.; Ulm, R.; Hothorn, M. Plant photoreceptors and their signaling components compete for COP1 binding via VP peptide motifs. EMBO J. 2019, 38, e102140. [Google Scholar] [CrossRef]
- Tian, Z.; Qin, X.; Wang, H.; Li, J.; Chen, J. Genome-Wide Identification and Expression Analyses of CONSTANS-Like Family Genes in Cucumber (Cucumis sativus L.). J. Plant Growth Regul. 2022, 41, 1627–1641. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, J.; Zhao, X.; Wang, J.; Wong, G.K.; Yu, J. KaKs_Calculator: Calculating Ka and Ks Through Model Selection and Model Averaging. Genom. Proteom. Bioinform. 2006, 4, 259–263. [Google Scholar] [CrossRef]
- Simon, S.; Ruhl, M.; de Montaigu, A.; Wotzel, S.; Coupland, G. Evolution of CONSTANS Regulation and Function after Gene Duplication Produced a Photoperiodic Flowering Switch in the Brassicaceae. Mol. Biol. Evol. 2015, 32, 2284–2301. [Google Scholar] [CrossRef] [PubMed]
- Campoli, C.; Drosse, B.; Searle, I.; Coupland, G.; von Korff, M. Functional characterisation of HvCO1, the barley (Hordeum vulgare) flowering time ortholog of CONSTANS. Plant J. 2012, 69, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, R.; Kawahigashi, H.; Oshima, M.; Ando, T.; Handa, H. The differential expression of HvCO9, a member of the CONSTANS-like gene family, contributes to the control of flowering under short-day conditions in barley. J. Exp. Bot. 2012, 63, 773–784. [Google Scholar] [CrossRef]
- Cheng, X.F.; Wang, Z.Y. Overexpression of COL9, a CONSTANS-LIKE gene, delays flowering by reducing expression of CO and FT in Arabidopsis thaliana. Plant J. 2005, 43, 758–768. [Google Scholar] [CrossRef]
- Zeng, X.; Lv, X.; Liu, R.; He, H.; Liang, S.; Chen, L.; Zhang, F.; Chen, L.; He, Y.; Du, J. Molecular basis of CONSTANS oligomerization in FLOWERING LOCUS T activation. J. Integr. Plant Biol. 2022, 64, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, B.; Yu, H. GSK3s: Nodes of multilayer regulation of plant development and stress responses. Trends Plant Sci. 2021, 26, 1286–1300. [Google Scholar] [CrossRef] [PubMed]
- Hayama, R.; Sarid-Krebs, L.; Richter, R.; Fernandez, V.; Jang, S.; Coupland, G. PSEUDO RESPONSE REGULATORs stabilize CONSTANS protein to promote flowering in response to day length. EMBO J. 2017, 36, 904–918. [Google Scholar] [CrossRef]
- Xu, Y.; Li, R.; Luo, H.; Wang, Z.; Li, M.W.; Lam, H.M.; Huang, C. Protoplasts: Small cells with big roles in plant biology. Trends Plant Sci. 2022, 27, 828–829. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Debernardi, J.M.; Dubcovsky, J.; Gallavotti, A. Recent advances in crop transformation technologies. Nat. Plants 2022, 8, 1343–1351. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Wang, Y.; Yu, Y.; He, Y.; Wang, L. Coordinative regulation of plants growth and development by light and circadian clock. aBIOTECH 2021, 2, 176–189. [Google Scholar] [CrossRef]
- Wang, S.-L.; An, H.R.; Tong, C.-G.; Jang, S. Flowering and flowering genes: From model plants to orchids. Hortic. Environ. Biotechnol. 2021, 62, 135–148. [Google Scholar] [CrossRef]
- Teo, Z.W.N.; Zhou, W.; Shen, L. Dissecting the Function of MADS-Box Transcription Factors in Orchid Reproductive Development. Front. Plant Sci. 2019, 10, 1474. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.X.; Gao, J.; Wei, Y.L.; Ren, R.; Zhang, G.Q.; Lu, C.Q.; Jin, J.P.; Ai, Y.; Wang, Y.Q.; Chen, L.J.; et al. The genome of Cymbidium sinense revealed the evolution of orchid traits. Plant Biotechnol. J. 2021, 19, 2501–2516. [Google Scholar] [CrossRef]
- Su, S.; Shao, X.; Zhu, C.; Xu, J.; Tang, Y.; Luo, D.; Huang, X. An AGAMOUS-like factor is associated with the origin of two domesticated varieties in Cymbidium sinense (Orchidaceae). Hortic. Res.-Engl. 2018, 5, 48. [Google Scholar] [CrossRef]
- Chugh, S.; Guha, S.; Rao, I.U. Micropropagation of orchids: A review on the potential of different explants. Sci. Hortic. 2009, 122, 507–520. [Google Scholar] [CrossRef]
- Chao, Y.T.; Yen, S.H.; Yeh, J.H.; Chen, W.C.; Shih, M.C. Orchidstra 2.0-A Transcriptomics Resource for the Orchid Family. Plant Cell Physiol. 2017, 58, e9. [Google Scholar] [CrossRef] [PubMed]
- Libault, M.; Wan, J.; Czechowski, T.; Udvardi, M.; Stacey, G. Identification of 118 Arabidopsis transcription factor and 30 ubiquitin-ligase genes responding to chitin, a plant-defense elicitor. Mol. Plant-Microbe Interact. 2007, 20, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Shalmani, A.; Jing, X.Q.; Shi, Y.; Muhammad, I.; Zhou, M.R.; Wei, X.Y.; Chen, Q.Q.; Li, W.Q.; Liu, W.T.; Chen, K.M. Characterization of B-BOX gene family and their expression profiles under hormonal, abiotic and metal stresses in Poaceae plants. BMC Genom. 2019, 20, 27. [Google Scholar] [CrossRef]
- Gnesutta, N.; Kumimoto, R.W.; Swain, S.; Chiara, M.; Siriwardana, C.; Horner, D.S.; Holt, B.F., 3rd; Mantovani, R. CONSTANS Imparts DNA Sequence Specificity to the Histone Fold NF-YB/NF-YC Dimer. Plant Cell 2017, 29, 1516–1532. [Google Scholar] [CrossRef]
- Ordoñez-Herrera, N.; Trimborn, L.; Menje, M.; Henschel, M.; Robers, L.; Kaufholdt, D.; Hansch, R.; Adrian, J.; Ponnu, J.; Hoecker, U. The Transcription Factor COL12 Is a Substrate of the COP1/SPA E3 Ligase and Regulates Flowering Time and Plant Architecture. Plant Physiol. 2018, 176, 1327–1340. [Google Scholar] [CrossRef]
- Plant, A.R.; Larrieu, A.; Causier, B. Repressor for hire! The vital roles of TOPLESS-mediated transcriptional repression in plants. New Phytol. 2021, 231, 963–973. [Google Scholar] [CrossRef] [PubMed]
- Fjellheim, S.; Young, D.A.; Paliocha, M.; Johnsen, S.S.; Schubert, M.; Preston, J.C. Major niche transitions in Pooideae correlate with variation in photoperiodic flowering and evolution of CCT domain genes. J. Exp. Bot. 2022, 73, 4079–4093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Hu, Y.; Huang, M.Z.; Huang, W.C.; Liu, D.K.; Zhang, D.; Hu, H.; Downing, J.L.; Liu, Z.J.; Ma, H. Comprehensive phylogenetic analyses of Orchidaceae using nuclear genes and evolutionary insights into epiphytism. J. Integr. Plant Biol. 2023, 65, 1204–1225. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.M.; Hofmann, H.A. Seeing is believing: Dynamic evolution of gene families. Proc. Natl. Acad. Sci. USA 2015, 112, 1252–1253. [Google Scholar] [CrossRef]
- Kinoshita, A.; Richter, R. Genetic and molecular basis of floral induction in Arabidopsis thaliana. J. Exp. Bot. 2020, 71, 2490–2504. [Google Scholar] [CrossRef] [PubMed]
- Osnato, M.; Cota, I.; Nebhnani, P.; Cereijo, U.; Pelaz, S. Photoperiod Control of Plant Growth: Flowering Time Genes Beyond Flowering. Front. Plant Sci. 2021, 12, 805635. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yuan, J.; Zhang, Y.; Chen, C.; Zhang, B.; Shi, X.; Niu, R.; Lin, F. Multi-layered roles of BBX proteins in plant growth and development. Stress Biol. 2023, 3, 1. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Shen, Y.; Chang, H.; Hou, Y.; Harris, A.; Ma, S.F.; McPartland, M.; Hymus, G.J.; Adam, L.; Marion, C.; et al. The flowering time regulator CONSTANS is recruited to the FLOWERING LOCUS T promoter via a unique cis-element. New Phytol. 2010, 187, 57–66. [Google Scholar] [CrossRef]
- Zhu, Y.; Klasfeld, S.; Wagner, D. Molecular regulation of plant developmental transitions and plant architecture via PEPB family proteins: An update on mechanism of action. J. Exp. Bot. 2021, 72, 2301–2311. [Google Scholar] [CrossRef]
- Ke, Y. The Study of Phalaenopsis CONSTANS-like Gene Regulatory Networks; National Central University: Taoyuan City, Taiwan, 2020. [Google Scholar]
- Zhang, J.; Zhao, X.; Tian, R.; Zeng, S.; Wu, K.; Teixeira da Silva, J.A.; Duan, J. Molecular Cloning and Functional Analysis of Three CONSTANS-Like Genes from Chinese Cymbidium. J. Plant Growth Regul. 2020, 39, 1061–1074. [Google Scholar] [CrossRef]
- Hou, C.; Yang, C. Functional Analysis of FT and TFL1 Orthologs from Orchid (Oncidium Gower Ramsey) that Regulate the Vegetative to Reproductive Transition. Plant Cell Physiol. 2009, 50, 1544–1557. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, B.; Wang, Y.; Gong, X.; Yu, H. DOTFL1 affects the floral transition in orchid Dendrobium Chao Praya Smile. Plant Physiol. 2021, 186, 2021–2036. [Google Scholar] [CrossRef]
- Zhu, Y.; Klasfeld, S.; Jeong, C.W.; Jin, R.; Goto, K.; Yamaguchi, N.; Wagner, D. TERMINAL FLOWER 1-FD complex target genes and competition with FLOWERING LOCUS T. Nat. Commun. 2020, 11, 5118. [Google Scholar] [CrossRef]
- Bratzel, F.; Turck, F. Molecular memories in the regulation of seasonal flowering: From competence to cessation. Genome Biol. 2015, 16, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, L.; Chen, X.; Zeng, L.; Su, Y.; Liu, Z. Characterization of Two AGAMOUS-like Genes and Their Promoters from the Cymbidium faberi (Orchidaceae). Plants 2023, 12, 2740. [Google Scholar] [CrossRef] [PubMed]
- Almada, R.; Cabrera, N.; Casaretto, J.A.; Ruiz-Lara, S.; Gonzalez Villanueva, E. VvCO and VvCOL1, two CONSTANS homologous genes, are regulated during flower induction and dormancy in grapevine buds. Plant Cell Rep. 2009, 28, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, J.Y.; Wang, J.N.; Kuang, J.F.; Shan, W.; Lu, W.J. Molecular characterization and expression profiles of MaCOL1, a CONSTANS-like gene in banana fruit. Gene 2012, 496, 110–117. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, H.; Li, T.; Wu, J.; Nagarajan, R.; Lei, L.; Powers, C.; Kan, C.C.; Hua, W.; Liu, Z.; et al. TaCol-B5 modifies spike architecture and enhances grain yield in wheat. Science 2022, 376, 180–183. [Google Scholar] [CrossRef]
- Kishor, P.B.K.; Tiozon, R.N.; Fernie, A.R.; Sreenivasulu, N. Abscisic acid and its role in the modulation of plant growth, development, and yield stability. Trends Plant Sci. 2022, 27, 1283–1295. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Q.; Liu, W.; Wu, X.; Li, Z.; Xu, Y.; Li, Y.; Imaizumi, T.; Hou, X.; Liu, T. BrABF3 promotes flowering through the direct activation of CONSTANS transcription in pak choi. Plant J. 2022, 111, 134–148. [Google Scholar] [CrossRef]
- Sevilleno, S.; Cabahug-Braza, R.; An, H.; Lim, K.; Hwang, Y. The role of cytogenetic tools in orchid breeding. Korean J. Agric. Sci. 2023, 50, 193–206. [Google Scholar] [CrossRef]
- Balilashaki, K.; Dehghanian, Z.; Gougerdchi, V.; Kavusi, E.; Feizi, F.; Tang, X.; Vahedi, M.; Hossain, M.M. Progress and Prospect of Orchid Breeding: An Overview. In Advances in Orchid Biology, Biotechnology and Omics; Springer: Berlin/Heidelberg, Germany, 2023; pp. 261–283. [Google Scholar] [CrossRef]
- Ramya, M.; Lee, S.Y.; An, H.R.; Park, P.M.; Kim, N.S.; Park, P.H. MYB1 transcription factor regulation through floral scent in Cymbidium cultivar ‘Sael Bit’. Phytochem. Lett. 2019, 32, 181–187. [Google Scholar] [CrossRef]
- Yam, T.W.; Nair, H.; Hew, C.S.; Arditti, J. History-Seeds: Orchid Seeds and their Germination: An Historical Account. In Orchid Biology: Reviews and Perspectives, VIII; Springer: Berlin/Heidelberg, Germany, 2002; pp. 387–504. [Google Scholar] [CrossRef]
- Lee, Y.-I.; Yeung, E.C. The orchid seed coat: A developmental and functional perspective. Bot. Stud. 2023, 64, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; He, X.; Tang, Y.; Chen, Z.; Zhou, L.; Li, X.; Zhang, C.; Huang, X.; Yang, Y.; Zhang, W. Photoperiod controls plant seed size in a CONSTANS-dependent manner. Nat. Plants 2023, 9, 343–354. [Google Scholar] [CrossRef]
- Achary, R.K.; Majee, M. CONSTANS, a key-player connecting day length to seed size. Trends Plant Sci. 2023, 28, 975–977. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.H.; Jin, H.; Marler, B.; Guo, H.; et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An information aesthetic for comparative genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- Zhang, Z. KaKs_calculator 3.0: Calculating selective pressure on coding and non-coding sequences. Genom. Proteom. Bioinform. 2022, 20, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Schwede, T.; Kopp, J.; Guex, N.; Peitsch, M.C. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef] [PubMed]
- Geourjon, C.; Deléage, G. SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput. Appl. Biosci. 1995, 11, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Hempton, A.K.; Imaizumi, T. Photoperiodic flowering in Arabidopsis: Multilayered regulatory mechanisms of CONSTANS and the florigen FLOWERING LOCUS T. Plant Commun. 2023, 4, 100552. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Gao, J.; Lu, C.; Wei, Y.; Jin, J.; Wong, S.M.; Zhu, G.; Yang, F. Highly Efficient Protoplast Isolation and Transient Expression System for Functional Characterization of Flowering Related Genes in Cymbidium Orchids. Int. J. Mol. Sci. 2020, 21, 2264. [Google Scholar] [CrossRef]
- Ren, R.; Gao, J.; Yin, D.; Li, K.; Lu, C.; Ahmad, S.; Wei, Y.; Jin, J.; Zhu, G.; Yang, F. Highly Efficient Leaf Base Protoplast Isolation and Transient Expression Systems for Orchids and Other Important Monocot Crops. Front. Plant Sci. 2021, 12, 626015. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Y.; Jin, J.; Lin, Z.; Lu, C.; Gao, J.; Li, J.; Xie, Q.; Zhu, W.; Zhu, G.; Yang, F. Genome-Wide Identification, Expression, and Molecular Characterization of the CONSTANS-like Gene Family in Seven Orchid Species. Int. J. Mol. Sci. 2023, 24, 16825. https://doi.org/10.3390/ijms242316825
Wei Y, Jin J, Lin Z, Lu C, Gao J, Li J, Xie Q, Zhu W, Zhu G, Yang F. Genome-Wide Identification, Expression, and Molecular Characterization of the CONSTANS-like Gene Family in Seven Orchid Species. International Journal of Molecular Sciences. 2023; 24(23):16825. https://doi.org/10.3390/ijms242316825
Chicago/Turabian StyleWei, Yonglu, Jianpeng Jin, Zengyu Lin, Chuqiao Lu, Jie Gao, Jie Li, Qi Xie, Wei Zhu, Genfa Zhu, and Fengxi Yang. 2023. "Genome-Wide Identification, Expression, and Molecular Characterization of the CONSTANS-like Gene Family in Seven Orchid Species" International Journal of Molecular Sciences 24, no. 23: 16825. https://doi.org/10.3390/ijms242316825
APA StyleWei, Y., Jin, J., Lin, Z., Lu, C., Gao, J., Li, J., Xie, Q., Zhu, W., Zhu, G., & Yang, F. (2023). Genome-Wide Identification, Expression, and Molecular Characterization of the CONSTANS-like Gene Family in Seven Orchid Species. International Journal of Molecular Sciences, 24(23), 16825. https://doi.org/10.3390/ijms242316825







