The Future of HER2-Targeted Treatment for Osteosarcoma: Lessons from the Negative Trastuzumab Deruxtecan Results
Abstract
:1. Development of HER2-Targeted Therapy in Oncology
1.1. HER2 Biology
1.2. Development of HER2-Targeted Drugs
2. HER2 in Osteosarcoma
2.1. HER2 Amplification or Overexpression in Osteosarcoma
2.2. HER2-Targeted Therapies for Osteosarcoma
3. Trastuzumab Deruxtecan (T-DXd) for the Treatment of Osteosarcoma
3.1. The Mechanism of Action and Clinical Efficacy of T-DXd
3.2. T-DXd Treatment for Osteosarcoma—An Unexpected Result
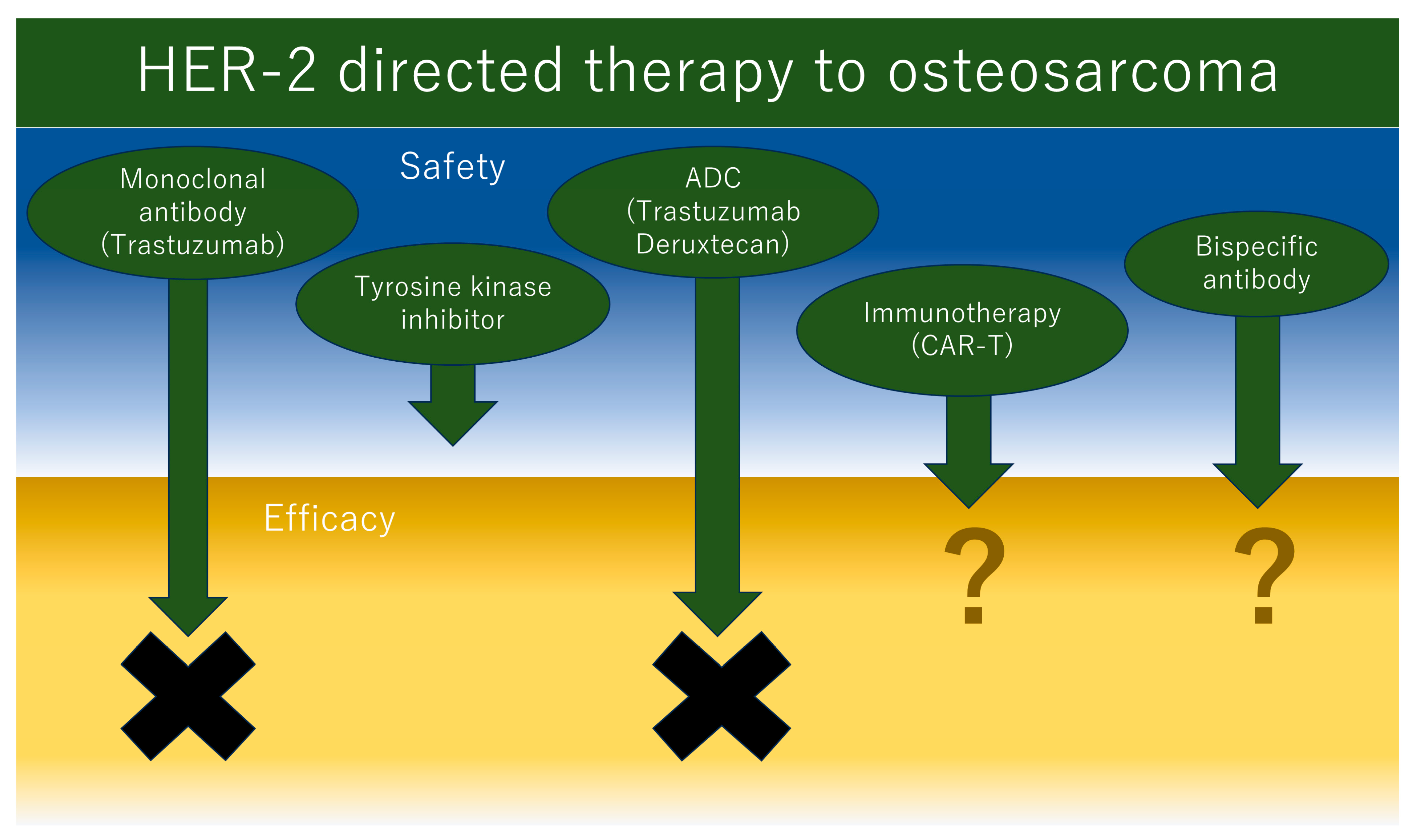
4. Future Direction of HER2-Targeted Therapy Development to Osteosarcoma
4.1. Bispecific Antibody Including HER2
4.2. Immunotherapy Targeted to HER2 in Osteosarcoma
Funding
Conflicts of Interest
Abbreviations
References
- Raghav, K.P.S.; Moasser, M.M. Molecular pathways and mechanisms of HER2 in cancer therapy. Clin. Cancer Res. 2023, 29, 2351–2361. [Google Scholar] [CrossRef]
- Guy, C.T.; Webster, M.A.; Schaller, M.; Parsons, T.J.; Cardiff, R.D.; Muller, W.J. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc. Natl. Acad. Sci. USA 1992, 89, 10578–10582. [Google Scholar] [CrossRef] [PubMed]
- Giordano, S.H.; Franzoi, M.A.B.; Temin, S.; Anders, C.K.; Chandarlapaty, S.; Crews, J.R.; Kirshner, J.J.; Krop, I.E.; Lin, N.U.; Morikawa, A.; et al. Systemic therapy for advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO guideline update. J. Clin. Oncol. 2022, 40, 2612–2635. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Somerfield, M.R.; Dowsett, M.; Hammond, M.E.H.; Hayes, D.F.; McShane, L.M.; Saphner, T.J.; Spears, P.A.; Allison, K.H. Human epidermal growth factor receptor 2 testing in breast cancer: ASCO-College of American Pathologists Guideline Update. J. Clin. Oncol. 2023, 41, 3867–3872. [Google Scholar] [CrossRef] [PubMed]
- Bartley, A.N.; Washington, M.K.; Colasacco, C.; Ventura, C.B.; Ismaila, N.; Benson, A.B., 3rd; Carrato, A.; Gulley, M.L.; Jain, D.; Kakar, S.; et al. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: Guideline from the College of American Pathologists, American Society for Clinical Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol. 2017, 35, 446–464. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Bang, Y.J. HER2-targeted therapies—A role beyond breast cancer. Nat. Rev. Clin. Oncol. 2020, 17, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, S.; Feng, W.; Luo, Y.; Lai, H.; Li, Q.; Xiu, B.; Li, Y.; Li, Y.; Huang, S.; et al. A pan-cancer analysis of HER2 index revealed transcriptional pattern for precise selection of HER2-targeted therapy. EBioMedicine 2020, 62, 103074. [Google Scholar] [CrossRef]
- Schubert, L.; Elliott, A.; Le, A.T.; Estrada-Bernal, A.; Doebele, R.C.; Lou, E.; Borghaei, H.; Demeure, M.J.; Kurzrock, R.; Reuss, J.E.; et al. ERBB family fusions are recurrent and actionable oncogenic targets across cancer types. Front. Oncol. 2023, 13, 1115405. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Takahashi, H.; Tada, Y.; Saotome, T.; Akazawa, K.; Ojiri, H.; Fushimi, C.; Masubuchi, T.; Matsuki, T.; Tani, K.; Osamura, R.Y.; et al. Phase II trial of trastuzumab and docetaxel in patients with human epidermal growth factor receptor 2-positive salivary duct carcinoma. J. Clin. Oncol. 2019, 37, 125–134. [Google Scholar] [CrossRef]
- Bennett, C.L.; Schoen, M.W.; Hoque, S.; Witherspoon, B.J.; Aboulafia, D.M.; Hwang, C.S.; Ray, P.; Yarnold, P.R.; Chen, B.K.; Schooley, B.; et al. Improving oncology biosimilar launches in the EU, the USA, and Japan: An updated Policy Review from the Southern Network on Adverse Reactions. Lancet Oncol. 2020, 21, e575–e588, Erratum in Lancet Oncol. 2021, 22, e42. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllidi, E.; Triantafillidis, J.K. Systematic review on the use of biosimilars of trastuzumab in HER2+ breast cancer. Biomedicines 2022, 10, 2045. [Google Scholar] [CrossRef] [PubMed]
- Ismael, G.; Hegg, R.; Muehlbauer, S.; Heinzmann, D.; Lum, B.; Kim, S.-B.; Pienkowski, T.; Lichinitser, M.; Semiglazov, V.; Melichar, B.; et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): A phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012, 13, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Ataseven, B.; Azim, H.; Al-Sakaff, N.; Lauer, S.; Shing, M.; Koroveshi, D.; Bouzid, K.; Casalnuovo, M.; Cascallar, D.; Korbenfeld, E.P.; et al. Safety and tolerability of subcutaneous trastuzumab for the adjuvant treatment of human epidermal growth factor receptor 2-positive early breast cancer: SafeHer phase III study’s primary analysis of 2573 patients. Eur. J. Cancer 2017, 82, 237–246. [Google Scholar] [CrossRef]
- Baselga, J.; Cortés, J.; Kim, S.-B.; Im, S.-A.; Hegg, R.; Im, Y.-H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A.; et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N. Engl. J. Med. 2017, 377, 122–131, Erratum in N. Engl. J. Med. 2017, 377, 702; Erratum in N. Engl. J. Med. 2018, 379, 1585. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.J.; Hainsworth, J.D.; Bose, R.; Burris, H.A.; Kurzrock, R.; Swanton, C.; Friedman, C.F.; Spigel, D.R.; Szado, T.; Schulze, K.; et al. MyPathway Human Epidermal Growth Factor Receptor 2 Basket Study: Pertuzumab + trastuzumab treatment of a tissue-agnostic cohort of patients with human epidermal growth factor receptor 2-altered advanced solid tumors. J. Clin. Oncol. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.R.; Im, S.-A.; Mattar, A.; Colomer, R.; Stroyakovskii, D.; Nowecki, Z.; De Laurentiis, M.; Pierga, J.-Y.; Jung, K.H.; Schem, C.; et al. Fixed-dose combination of pertuzumab and trastuzumab for subcutaneous injection plus chemotherapy in HER2-positive early breast cancer (FeDeriCa): A randomised, open-label, multicentre, non-inferiority, phase 3 study. Lancet Oncol. 2021, 22, 85–97, Erratum in Lancet Oncol. 2021, 22, e42. [Google Scholar] [CrossRef]
- Rugo, H.S.; Im, S.-A.; Cardoso, F.; Cortés, J.; Curigliano, G.; Musolino, A.; Pegram, M.D.; Wright, G.S.; Saura, C.; Escrivá-De-Romaní, S.; et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: A phase 3 randomized clinical trial. JAMA Oncol. 2021, 7, 573–584. [Google Scholar] [CrossRef]
- Royce, M.; Osgood, C.L.; Amatya, A.K.; Fiero, M.H.; Chang, C.G.; Ricks, T.K.; Shetty, K.A.; Kraft, J.; Qiu, J.; Song, P.; et al. FDA Approval Summary: Margetuximab plus chemotherapy for advanced or metastatic HER2-positive breast cancer. Clin. Cancer Res. 2022, 28, 1487–1492. [Google Scholar] [CrossRef]
- Ryan, Q.; Ibrahim, A.; Cohen, M.H.; Johnson, J.; Ko, C.W.; Sridhara, R.; Justice, R.; Pazdur, R. FDA drug approval summary: Lapatinib in combination with capecitabine for previously treated metastatic breast cancer that overexpresses HER-2. Oncologist 2008, 13, 1114–1119. [Google Scholar] [CrossRef] [PubMed]
- Saura, C.; Oliveira, M.; Feng, Y.-H.; Dai, M.-S.; Chen, S.-W.; Hurvitz, S.A.; Kim, S.-B.; Moy, B.; Delaloge, S.; Gradishar, W.; et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: Phase III NALA Trial. J. Clin. Oncol. 2020, 38, 3138–3149. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Delaloge, S.; Holmes, F.A.; Moy, B.; Iwata, H.; Harvey, V.J.; Robert, N.J.; Silovski, T.; Gokmen, E.; von Minckwitz, G.; et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): A multicentre, randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016, 17, 367–377. [Google Scholar] [CrossRef]
- Park, J.W.; Liu, M.C.; Yee, D.; Yau, C.; Veer, L.J.v.; Symmans, W.F.; Paoloni, M.; Perlmutter, J.; Hylton, N.M.; Hogarth, M.; et al. Adaptive randomization of neratinib in early breast cancer. N. Engl. J. Med. 2016, 375, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N. Engl. J. Med. 2020, 382, 597–609, Erratum in N. Engl. J. Med. 2020, 382, 586. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Borges, V.; Anders, C.; Murthy, R.K.; Paplomata, E.; Hamilton, E.; Hurvitz, S.; Loi, S.; Okines, A.; Abramson, V.; et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB Trial. J. Clin. Oncol. 2020, 38, 2610–2619. [Google Scholar] [CrossRef] [PubMed]
- Casak, S.J.; Horiba, M.N.; Yuan, M.; Cheng, J.; Lemery, S.J.; Shen, Y.L.; Fu, W.; Moore, J.N.; Li, Y.; Bi, Y.; et al. FDA Approval Summary: Tucatinib with Trastuzumab for Advanced Unresectable or Metastatic, Chemotherapy Refractory, HER2-Positive RAS Wild-Type Colorectal Cancer. Clin. Cancer Res. 2023, 29, 4326–4330. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791, Erratum in N. Engl. J. Med. 2013, 368, 2442. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Thuss-Patience, P.C.; Shah, M.A.; Ohtsu, A.; Van Cutsem, E.; Ajani, J.A.; Castro, H.; Mansoor, W.; Chung, H.C.; Bodoky, G.; Shitara, K.; et al. Trastuzumab emtansine versus taxane use for previously treated HER2-positive locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma (GATSBY): An international randomised, open-label, adaptive, phase 2/3 study. Lancet Oncol. 2017, 18, 640–653. [Google Scholar] [CrossRef]
- Meltzer, P.S.; Helman, L.J. New horizons in the treatment of osteosarcoma. N. Engl. J. Med. 2021, 385, 2066–2076. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Zhu, B.; Koster, R.; Karlins, E.; Dean, M.; Yeager, M.; Gianferante, M.; Spector, L.G.; Morton, L.M.; Karyadi, D.; et al. Frequency of pathogenic germline variants in cancer-susceptibility genes in patients with osteosarcoma. JAMA Oncol. 2020, 6, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.; Hingorani, P.; Roth, M.; Gorlick, R. HER2-targeted therapy in osteosarcoma. Adv. Exp. Med. Biol. 2020, 1257, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Hughes, D.P.; Thomas, D.G.; Giordano, T.J.; Baker, L.H.; McDonagh, K.T. Cell surface expression of epidermal growth factor receptor and Her-2 with nuclear expression of Her-4 in primary osteosarcoma. Cancer Res. 2004, 64, 2047–2053, Erratum in Cancer Res. 2013, 73, 4169. [Google Scholar] [CrossRef] [PubMed]
- Ebb, D.; Meyers, P.; Grier, H.; Bernstein, M.; Gorlick, R.; Lipshultz, S.E.; Krailo, M.; Devidas, M.; Barkauskas, D.A.; Siegal, G.P.; et al. Phase II trial of trastuzumab in combination with cytotoxic chemotherapy for treatment of metastatic osteosarcoma with human epidermal growth factor receptor 2 overexpression: A report from the children’s oncology group. J. Clin. Oncol. 2012, 30, 2545–2551. [Google Scholar] [CrossRef] [PubMed]
- Long, X.-H.; Zhang, G.-M.; Peng, A.-F.; Luo, Q.-F.; Zhang, L.; Wen, H.-C.; Zhou, R.-P.; Gao, S.; Zhou, Y.; Liu, Z.-L. Lapatinib alters the malignant phenotype of osteosarcoma cells via downregulation of the activity of the HER2-PI3K/AKT-FASN axis in vitro. Oncol. Rep. 2014, 31, 328–334. [Google Scholar] [CrossRef]
- Ogitani, Y.; Aida, T.; Hagihara, K.; Yamaguchi, J.; Ishii, C.; Harada, N.; Soma, M.; Okamoto, H.; Oitate, M.; Arakawa, S.; et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. 2016, 22, 5097–5108. [Google Scholar] [CrossRef]
- Staudacher, A.H.; Brown, M.P. Antibody drug conjugates and bystander killing: Is antigen-dependent internalisation required? Br. J. Cancer 2017, 117, 1736–1742. [Google Scholar] [CrossRef]
- Ogitani, Y.; Hagihara, K.; Oitate, M.; Naito, H.; Agatsuma, T. Bystander killing effect of DS-8201a, a novel anti-human epidermal growth factor receptor 2 antibody-drug conjugate, in tumors with human epidermal growth factor receptor 2 heterogeneity. Cancer Sci. 2016, 107, 1039–1046. [Google Scholar] [CrossRef]
- Tsurutani, J.; Iwata, H.; Krop, I.; Jänne, P.A.; Doi, T.; Takahashi, S.; Park, H.; Redfern, C.; Tamura, K.; Wise-Draper, T.M.; et al. Targeting HER2 with trastuzumab deruxtecan: A dose-expansion, phase I study in multiple advanced solid tumors. Cancer Discov. 2020, 10, 688–701, Erratum in Cancer Discov. 2020, 10, 1078. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.-B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab deruxtecan versus trastuzumab emtansine for breast cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Bang, Y.-J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.-C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef]
- Li, B.T.; Smit, E.F.; Goto, Y.; Nakagawa, K.; Udagawa, H.; Mazières, J.; Nagasaka, M.; Bazhenova, L.; Saltos, A.N.; Felip, E.; et al. Trastuzumab deruxtecan in HER2-mutant non-small-cell lung cancer. N. Engl. J. Med. 2022, 386, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Siena, S.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Fakih, M.; Elez, E.; et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2021, 22, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Hasegawa, K.; Matsumoto, K.; Mori, M.; Hirashima, Y.; Takehara, K.; Ariyoshi, K.; Kato, T.; Yagishita, S.; Hamada, A.; et al. Trastuzumab deruxtecan for human epidermal growth factor receptor 2-expressing advanced or recurrent uterine carcinosarcoma (NCCH1615): The STATICE Trial. J. Clin. Oncol. 2023, 41, 2789–2799. [Google Scholar] [CrossRef]
- Ohba, A.; Morizane, C.; Ueno, M.; Kobayashi, S.; Kawamoto, Y.; Komatsu, Y.; Ikeda, M.; Sasaki, M.; Okano, N.; Furuse, J.; et al. Multicenter phase II trial of trastuzumab deruxtecan for HER2-positive unresectable or recurrent biliary tract cancer: HERB trial. Future Oncol. 2022, 18, 2351–2360. [Google Scholar] [CrossRef] [PubMed]
- Kou, L.; Chen, X.; Xie, X.; Wen, Q.; Li, J.; Li, Y. The efficacy and safety of trastuzumab deruxtecan (T-DXd) in HER2-expressing solid tumours: A single-arm meta-analysis. Jpn. J. Clin. Oncol. 2023, 53, 722–729. [Google Scholar] [CrossRef]
- Jørgensen, J.T. The potential of trastuzumab deruxtecan as a tissue agnostic drug. Oncology 2023, in press. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.-Y.; Banerjee, S.N.; Martin, A.G.; Jung, K.H.; Lugowska, I.A.; Manso, L.; Manzano, A.; et al. Efficacy and safety of trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-expressing solid tumors: DESTINY-PanTumor02 (DP-02) interim results. J. Clin. Oncol. 2023, 41, LBA3000. [Google Scholar] [CrossRef]
- Narayan, P.; Dilawari, A.; Osgood, C.; Feng, Z.; Bloomquist, E.; Pierce, W.F.; Jafri, S.; Kalavar, S.; Kondratovich, M.; Jha, P.; et al. US Food and Drug Administration Approval Summary: Fam-Trastuzumab Deruxtecan-nxki for Human Epidermal Growth Factor Receptor 2-Low Unresectable or Metastatic Breast Cancer. J. Clin. Oncol. 2023, 41, 2108–2116. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Bang, Y.-J.; Iwasa, S.; Sugimoto, N.; Ryu, M.-H.; Sakai, D.; Chung, H.C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab deruxtecan in anti-human epidermal growth factor receptor 2 treatment-naive patients with human epidermal growth factor receptor 2-low gastric or gastroesophageal junction adenocarcinoma: Exploratory cohort results in a phase II trial. J. Clin. Oncol. 2023, 41, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, P.; Modi, S.; Tolaney, S.M.; Cortés, J.; Hamilton, E.P.; Kim, S.-B.; Toi, M.; Andrè, F.; Curigliano, G. Interstitial lung disease induced by anti-ERBB2 antibody-drug conjugates: A review. JAMA Oncol. 2021, 7, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Abuhelwa, Z.; Alloghbi, A.; Alqahtani, A.; Nagasaka, M. Trastuzumab deruxtecan-induced interstitial lung disease/pneumonitis in ERBB2-positive advanced solid malignancies: A systematic review. Drugs 2022, 82, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, P.; Zhang, W.; Zhang, Z.; Xu, Z.; Wang, W.-L.; Roth, M.E.; Wang, Y.; Gill, J.B.; Harrison, D.J.; Teicher, B.A.; et al. Trastuzumab deruxtecan, antibody-drug conjugate targeting HER2, is effective in pediatric malignancies: A report by the Pediatric Preclinical Testing Consortium. Mol. Cancer Ther. 2022, 21, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Reed, D.R.; Janeway, K.A.; Minard, C.G.; Hall, D.; Crompton, B.D.; Lazar, A.J.; Wang, W.-L.; Voss, S.D.; Militano, O.; Gorlick, R.G.; et al. PEPN1924, a phase 2 study of trastuzumab deruxtecan (DS-8201a, T-DXd) in adolescents and young adults with recurrent HER2+ osteosarcoma: A Children’s Oncology Group pediatric early-phase clinical trial network study. J. Clin. Oncol. 2023, 41, 11527. [Google Scholar] [CrossRef]
- Cosetti, M.; Wexler, L.H.; Calleja, E.; Trippett, T.; LaQuaglia, M.; Huvos, A.G.; Gerald, W.; Healey, J.H.; Meyers, P.A.; Gorlick, R. Irinotecan for pediatric solid tumors: The Memorial Sloan-Kettering experience. J. Pediatr. Hematol. Oncol. 2002, 24, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- Hyman, D.M.; Puzanov, I.; Subbiah, V.; Faris, J.E.; Chau, I.; Blay, J.-Y.; Wolf, J.; Raje, N.S.; Diamond, E.L.; Hollebecque, A.; et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N. Engl. J. Med. 2015, 373, 726–736, Erratum in N. Engl. J. Med. 2018, 379, 1585. [Google Scholar] [CrossRef]
- Subbiah, V.; Kreitman, R.J.; Wainberg, Z.A.; Gazzah, A.; Lassen, U.; Stein, A.; Wen, P.Y.; Dietrich, S.; de Jonge, M.J.A.; Blay, J.-Y.; et al. Dabrafenib plus trametinib in BRAFV600E-mutated rare cancers: The phase 2 ROAR trial. Nat. Med. 2023, 29, 1103–1112. [Google Scholar] [CrossRef]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Grothey, A.; Van Cutsem, E.; Yaeger, R.; Wasan, H.; Yoshino, T.; Desai, J.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600E-mutant metastatic colorectal cancer: Updated survival results and subgroup analyses from the BEACON Study. J. Clin. Oncol. 2021, 39, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Bolejack, V.; Ryan, C.W.; Ganjoo, K.N.; Loggers, E.T.; Chawla, S.; Agulnik, M.; Livingston, M.B.; Reed, D.; Keedy, V.; et al. Randomized double-blind phase ii study of regorafenib in patients with metastatic osteosarcoma. J. Clin. Oncol. 2019, 37, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Duffaud, F.; Mir, O.; Boudou-Rouquette, P.; Piperno-Neumann, S.; Penel, N.; Bompas, E.; Delcambre, C.; Kalbacher, E.; Italiano, A.; Collard, O.; et al. Efficacy and safety of regorafenib in adult patients with metastatic osteosarcoma: A non-comparative, randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2019, 20, 120–133. [Google Scholar] [CrossRef]
- Kantarjian, H.; Stein, A.; Gökbuget, N.; Fielding, A.K.; Schuh, A.C.; Ribera, J.-M.; Wei, A.; Dombret, H.; Foà, R.; Bassan, R.; et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N. Engl. J. Med. 2017, 376, 836–847. [Google Scholar] [CrossRef] [PubMed]
- Thieblemont, C.; Phillips, T.; Ghesquieres, H.; Cheah, C.Y.; Clausen, M.R.; Cunningham, D.; Do, Y.R.; Feldman, T.; Gasiorowski, R.; Jurczak, W.; et al. Epcoritamab, a novel, subcutaneous CD3xCD20 bispecific T-cell-engaging antibody, in relapsed or refractory large B-cell lymphoma: Dose expansion in a phase I/II trial. J. Clin. Oncol. 2023, 41, 2238–2247. [Google Scholar] [CrossRef] [PubMed]
- Holstein, S.A.; Grant, S.J.; Wildes, T.M. Chimeric antigen receptor T-cell and bispecific antibody therapy in multiple myeloma: Moving into the future. J. Clin. Oncol. 2023, 41, 4416–4429. [Google Scholar] [CrossRef]
- Misorin, A.K.; Chernyshova, D.O.; Karbyshev, M.S. State-of-the-art approaches to heterologous expression of bispecific antibodies targeting solid tumors. Biochemistry 2023, 88, 1215–1231. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, X.; Wang, X.; Yuan, K.; Wang, G.; Hu, L.; Zhang, G.; Pei, W.; Wang, L.; Sun, C.; et al. Bispecific antibodies in cancer therapy: Target selection and regulatory requirements. Acta Pharm. Sin. B 2023, 13, 3583–3597. [Google Scholar] [CrossRef]
- Roth, M.; Linkowski, M.; Tarim, J.; Piperdi, S.; Sowers, R.; Geller, D.; Gill, J.; Gorlick, R. Ganglioside GD2 as a therapeutic target for antibody-mediated therapy in patients with osteosarcoma. Cancer 2014, 120, 5548–5554. [Google Scholar] [CrossRef]
- Ladenstein, R.; Pötschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Yaniv, I.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, P.; Krailo, M.; Buxton, A.; Hutson, P.; Sondel, P.M.; Diccianni, M.; Yu, A.; Morris, C.D.; Womer, R.B.; Crompton, B.; et al. Phase 2 study of anti-disialoganglioside antibody, dinutuximab, in combination with GM-CSF in patients with recurrent osteosarcoma: A report from the Children’s Oncology Group. Eur. J. Cancer 2022, 172, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Park, J.A.; Cheung, N.V. GD2 or HER2 targeting T cell engaging bispecific antibodies to treat osteosarcoma. J. Hematol. Oncol. 2020, 13, 172. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, N.F.; Reischl, G.; Seitz, C.; Dittmann, H.; Seith, F.; Scheuermann, S.; Feuchtinger, T.; Dombrowski, F.; Handgretinger, R.; Fuchs, J.; et al. First-in-humans PET/MRI of in vivo GD2 expression in osteosarcoma. J. Nucl. Med. 2023, 64, 337–338. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Le Cesne, A.; Marec-Berard, P.; Blay, J.-Y.; Gaspar, N.; Bertucci, F.; Penel, N.; Bompas, E.; Cousin, S.; Toulmonde, M.; Bessede, A.; et al. Programmed cell death 1 (PD-1) targeting in patients with advanced osteosarcomas: Results from the PEMBROSARC study. Eur. J. Cancer 2019, 119, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501, Erratum in Lancet Oncol. 2017, 18, e711; Erratum in Lancet Oncol. 2018, 19, e8. [Google Scholar] [CrossRef]
- Labanieh, L.; Mackall, C.L. CAR immune cells: Design principles, resistance and the next generation. Nature 2023, 614, 635–648, Erratum in Nature 2023, 619, E26. [Google Scholar] [CrossRef]
- Ahmed, N.; Brawley, V.S.; Hegde, M.; Robertson, C.; Ghazi, A.; Gerken, C.; Liu, E.; Dakhova, O.; Ashoori, A.; Corder, A.; et al. Human epidermal growth factor receptor 2 (HER2)-specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J. Clin. Oncol. 2015, 33, 1688–1696. [Google Scholar] [CrossRef]
- Disis, M.L.; Dang, Y.; Coveler, A.L.; Marzbani, E.; Kou, Z.C.; Childs, J.S.; Fintak, P.; Higgins, D.M.; Reichow, J.; Waisman, J.; et al. HER-2/neu vaccine-primed autologous T-cell infusions for the treatment of advanced stage HER-2/neu expressing cancers. Cancer Immunol. Immunother. 2014, 63, 101–109. [Google Scholar] [CrossRef]
- Disis, M.L.; Dang, Y.; Coveler, A.L.; Childs, J.S.; Higgins, D.M.; Liu, Y.; Zhou, J.; Mackay, S.; Salazar, L.G. A phase I/II trial of HER2 vaccine-primed autologous T-Cell infusions in patients with treatment refractory HER2-overexpressing breast cancer. Clin. Cancer Res. 2023, 29, 3362–3371. [Google Scholar] [CrossRef] [PubMed]
| Tumor Origin | HER2 Amplification (%) | HER2 Overexpression (%) | HER2 Mutation (%) |
|---|---|---|---|
| Salivary gland | 12–52 | 17–44 | 1 |
| Lung | 2–3 | 2.5 | 1–3 |
| Breast | 20 | 15–20 | 2 |
| Stomach | 11–16 | 20 | 3 |
| Biliary tract | 5–15 | 20 | 2 |
| Pancreas | 2 | 26 | <1 |
| Colorectum | 5.8 | 5 | 2 |
| Bladder | 8.6 | 12.4 | 9 |
| Prostate | 5.8–6 | 10 | <1 |
| Ovary | 7 | 27 | 1 |
| Uterus | 4–69 | 18–80 | 2 |
| Cervix | 0.5–14 | 21 | 3 |
| Drug | Route(s) | Indications | Year of First FDA Approval |
|---|---|---|---|
| Trastuzumab | Intravenous, subcutaneous | Breast cancer (early, advanced) Gastroesophageal cancer | 1998 |
| Lapatinib | p.o. | Breast cancer (advanced) | 2007 |
| Pertuzumab | Intravenous, subcutaneous | Breast cancer (early, advanced) | 2012 |
| Trastuzumab emtansine (T-DM1) | Intravenous | Breast cancer (early, advanced) | 2013 |
| Neratinib | p.o. | Breast cancer (early, advanced) | 2017 |
| Trastuzumab deruxtecan (T-DXd) | Intravenous | Breast cancer (advanced) NSCLC | 2019 |
| Tucatinib | p.o. | Breast cancer (advanced) Colorectal cancer | 2020 |
| Margetuximab | Intravenous | Breast cancer (advanced) | 2020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakano, K. The Future of HER2-Targeted Treatment for Osteosarcoma: Lessons from the Negative Trastuzumab Deruxtecan Results. Int. J. Mol. Sci. 2023, 24, 16823. https://doi.org/10.3390/ijms242316823
Nakano K. The Future of HER2-Targeted Treatment for Osteosarcoma: Lessons from the Negative Trastuzumab Deruxtecan Results. International Journal of Molecular Sciences. 2023; 24(23):16823. https://doi.org/10.3390/ijms242316823
Chicago/Turabian StyleNakano, Kenji. 2023. "The Future of HER2-Targeted Treatment for Osteosarcoma: Lessons from the Negative Trastuzumab Deruxtecan Results" International Journal of Molecular Sciences 24, no. 23: 16823. https://doi.org/10.3390/ijms242316823
APA StyleNakano, K. (2023). The Future of HER2-Targeted Treatment for Osteosarcoma: Lessons from the Negative Trastuzumab Deruxtecan Results. International Journal of Molecular Sciences, 24(23), 16823. https://doi.org/10.3390/ijms242316823





