Zn2+ Differentially Influences the Neutralisation of Heparins by HRG, Fibrinogen, and Fibronectin
Abstract
1. Introduction
2. Results and Discussion
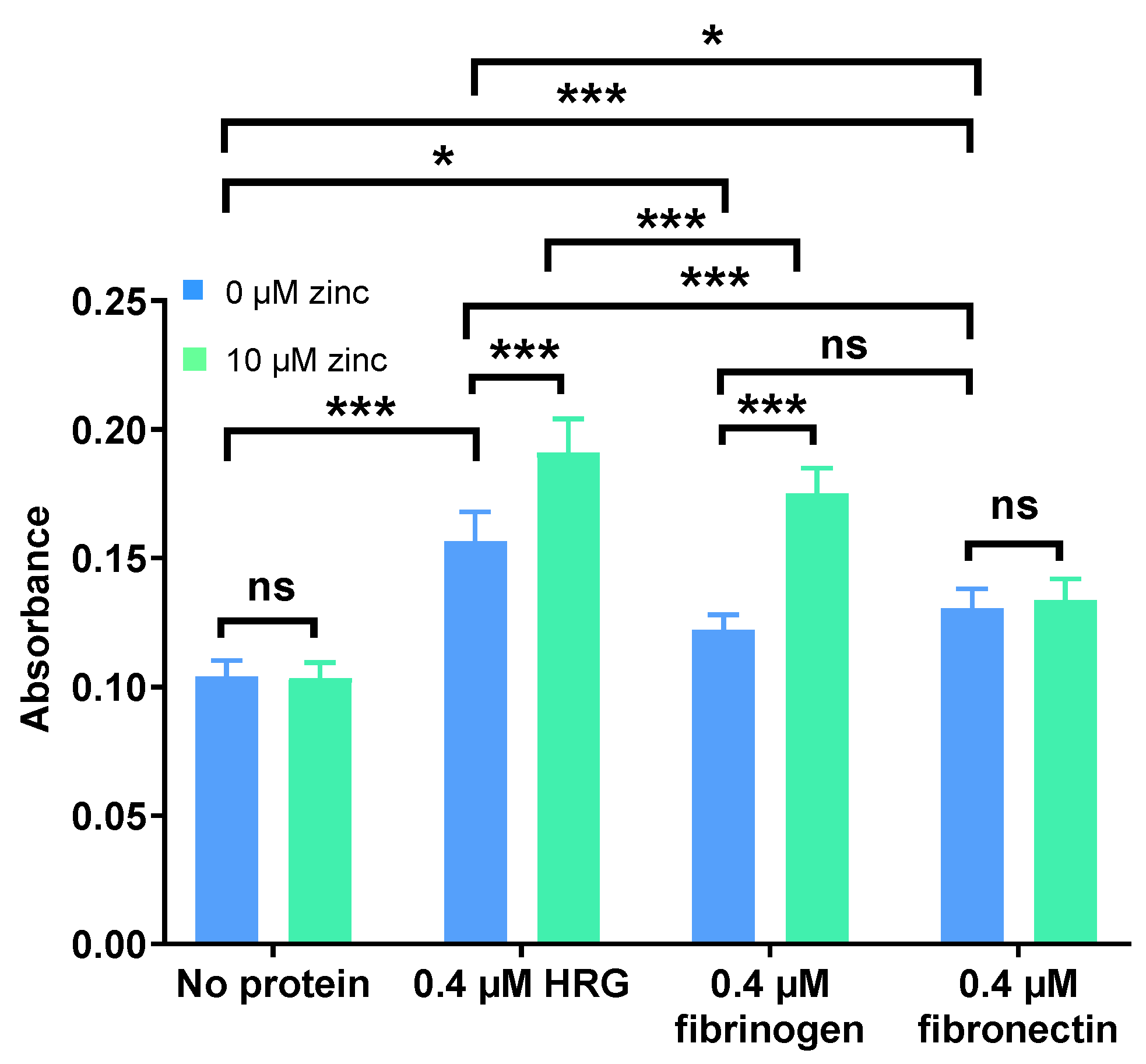
2.1. Zn2+ Modulates Neutralisation of Heparins by HRG and Fibrinogen but Not Fibronectin
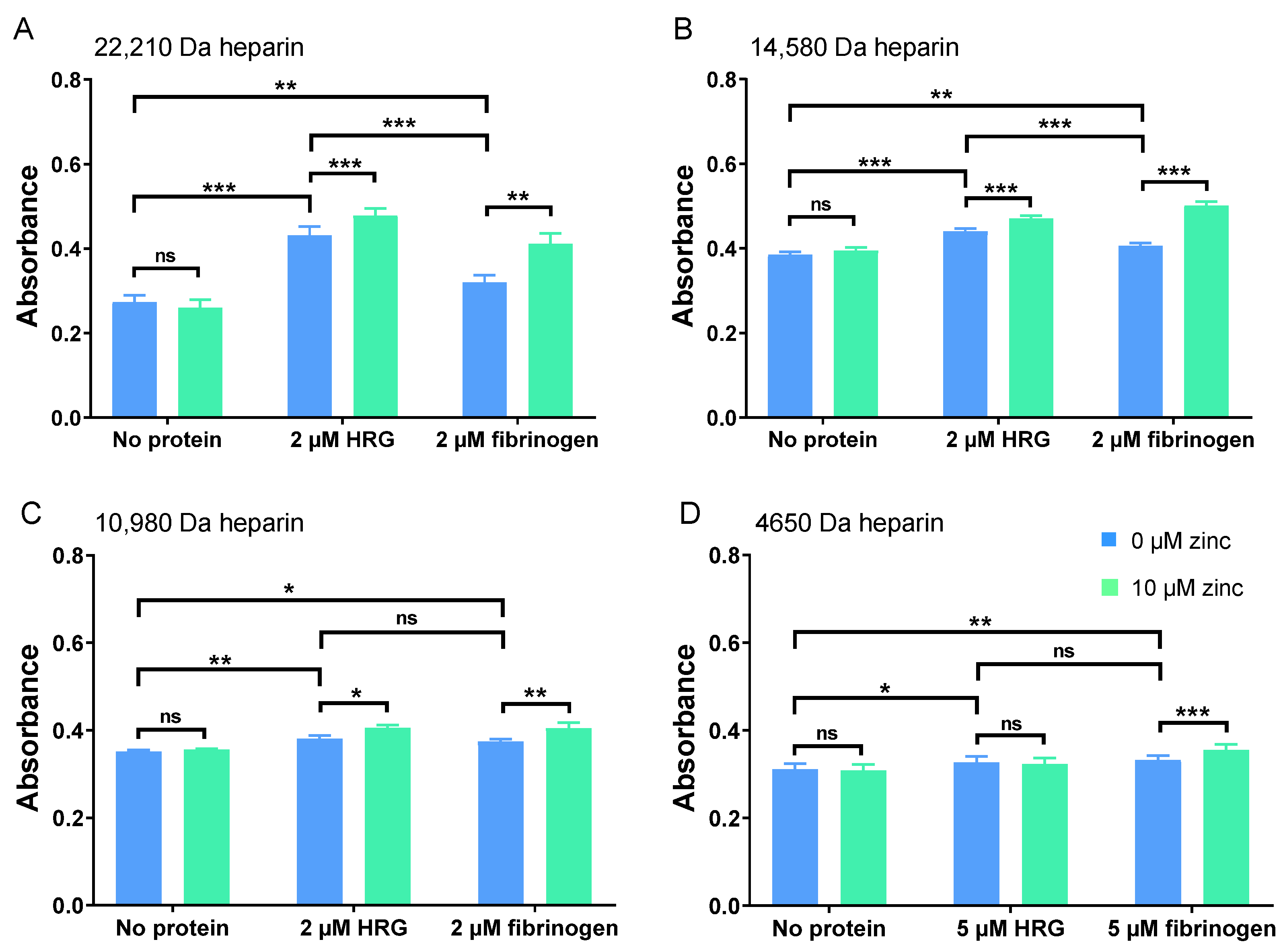
2.2. HRG and Fibrinogen Have Differential Effects on Neutralisation of Different Heparins
2.3. Zn2+ Impacts UFH Neutralisation by HRG and Fibrinogen, but Not Fibronectin, to Enhance Thrombin Activity
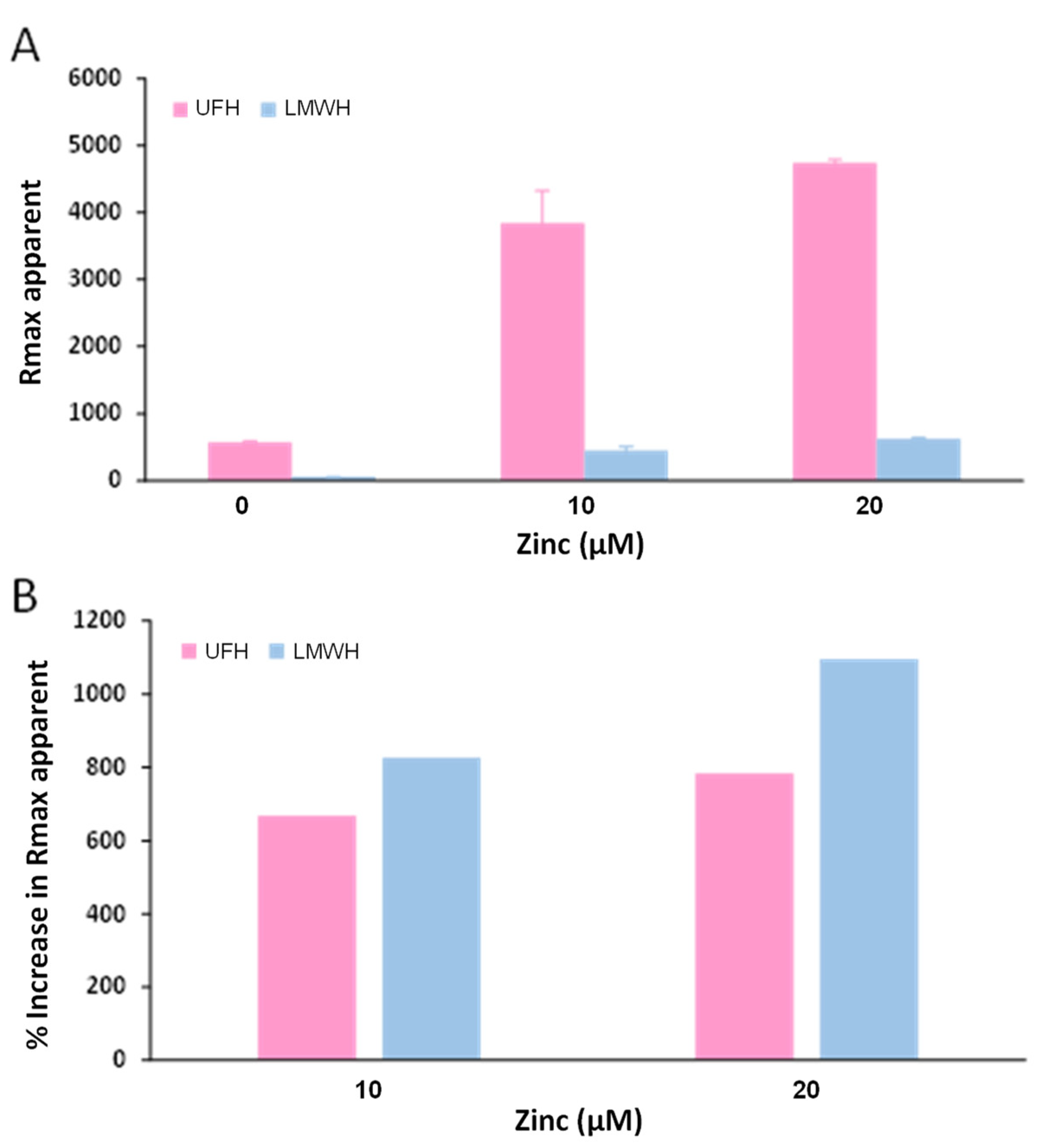
2.4. Zn2+ Enhances Fibrinogen Binding to Heparin
3. Materials and Methods
3.1. Materials
3.2. Purification of HRG from Human Plasma
3.3. Anti-FXa and Thrombin Assays
3.4. Surface Plasmon Resonance (SPR)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sobczak, A.I.S.; Pitt, S.J.; Stewart, A.J. Glycosaminoglycan neutralization in coagulation control. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Ourri, B.; Vial, L. Lost in (clinical) translation: Recent advances in heparin neutralization and monitoring. ACS Chem. Biol. 2019, 14, 2512–2526. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, A.I.S.; Pitt, S.J.; Stewart, A.J. Influence of zinc on glycosaminoglycan neutralisation during coagulation. Metallomics 2018, 10, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.T.; Fredenburgh, J.C.; Weitz, J.I. Zinc, an important cofactor in haemostasis and thrombosis. Thomb. Haemost. 2013, 109, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.A.; Pugh, N. The contribution of zinc to platelet behaviour during haemostasis and thrombosis. Metallomics 2016, 8, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, B. Metal-protein interactions in transport, accumulation, and excretion of metals. Biol. Trace Elem. Res. 1989, 21, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Giroux, E.L.; Henkin, R.I. Macromolecular ligands of exchangeable copper, zinc and cadmium in human serum. Bioinorg. Chem. 1973, 2, 125–133. [Google Scholar] [CrossRef]
- Kelly, E.; Mathew, J.; Kohler, J.E.; Blass, A.L.; Soybel, D.I. Redistribution of labile plasma zinc during mild surgical stress in the rat. Transl Res. 2011, 157, 139–149. [Google Scholar] [CrossRef][Green Version]
- Kassaar, O.; Schwarz-Linek, U.; Blindauer, C.A.; Stewart, A.J. Plasma free fatty acid levels influence Zn2+-dependent histidine-rich glycoprotein–heparin interactions via an allosteric switch on serum albumin. J. Thromb. Haemost. 2015, 13, 101–110. [Google Scholar] [CrossRef]
- Marx, G. Zinc binding to fibrinogen and fibrin. Arch. Biochem. Biophys. 1988, 266, 285–288. [Google Scholar] [CrossRef]
- Yakovlev, S.; Gorlatov, S.; Ingham, K.; Medved, L. Interaction of fibrin(ogen) with heparin: Further characterization and localization of the heparin-binding site. Biochemistry 2003, 42, 7709–7716. [Google Scholar] [CrossRef] [PubMed]
- Fredenburgh, J.C.; Leslie, B.A.; Stafford, A.R.; Lim, T.; Chan, H.H.; Weitz, J.I. Zn2+ mediates high affinity binding of heparin to the αC domain of fibrinogen. J. Biol. Chem. 2013, 288, 29394–29402. [Google Scholar] [CrossRef] [PubMed]
- Ingham, K.C.; Brew, S.A.; Atha, D.H. Interaction of heparin with fibronectin and isolated fibronectin domains. Biochem. J. 1990, 272, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Lovett, B.M.; Hill, K.E.; Randolph, E.M.; Wang, L.; Schwarzbauer, J.E. Nucleation of fibronectin fibril assembly requires binding between heparin and the 13th type III module of fibronectin. J. Biol. Chem. 2023, 299, 104622. [Google Scholar] [CrossRef] [PubMed]
- Heras-Parets, A.; Ginebra, M.P.; Manero, J.M.; Guillem-Marti, J. Guiding fibroblast activation using an RGD-mutated heparin binding II fragment of fibronectin for gingival titanium integration. Adv. Healthc. Mater. 2023, 12, e2203307. [Google Scholar] [CrossRef] [PubMed]
- Askari, J.A.; Thornton, D.J.; Humphries, J.D.; Buckley, P.A.; Humphries, M.J. The alternatively spliced type III connecting segment of fibronectin is a zinc-binding module. Matrix Biol. 2007, 26, 485–493. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gmeiner, B.; Leibl, H.; Zerlauth, G.; Seelos, C. Affinity binding of distinct functional fibronectin domains to immobilized metal chelates. Arch. Biochem. Biophys. 1995, 321, 40–42. [Google Scholar] [CrossRef]
- Lambert-Vidmar, S.; Lottspeich, F.; Emod, I.; Imhoff, J.M.; Keil-Dlouha, V. Collagen-binding domain of human plasma fibronectin contains a latent type-IV collagenase. Eur. J. Biochem. 1991, 201, 79–84. [Google Scholar] [CrossRef]
- Lane, D.; Pejler, G.; Flynn, A.; Thompson, E.; Lindahl, U. Neutralization of heparin-related saccharides by histidine-rich glycoprotein and platelet factor-4. J. Biol. Chem. 1986, 261, 3980–3986. [Google Scholar] [CrossRef]
- Hogg, P.J.; Jackson, C.M. Fibrin monomer protects thrombin from inactivation by heparin-antithrombin III: Implications for heparin efficacy. Proc. Natl. Acad. Sci. USA 1989, 86, 3619–3623. [Google Scholar] [CrossRef]
- Chan, H.H.; Leslie, B.A.; Stafford, A.R.; Roberts, R.S.; Al-Aswad, N.N.; Fredenburgh, J.C.; Weitz, J.I. By increasing the affinity of heparin for fibrin, Zn2+ promotes the formation of a ternary heparin-thrombin-fibrin complex that protects thrombin from inhibition by antithrombin. Biochemistry 2012, 51, 7964–7973. [Google Scholar] [CrossRef] [PubMed]
- Weisel, J.W.; Litvinov, R.I. Fibrin formation, structure and properties. Subcell. Biochem. 2017, 82, 405–456. [Google Scholar] [PubMed]
- Lord, M.S.; Cheng, B.; Farrugia, B.L.; McCarthy, S.; Whitelock, J.M. Platelet factor 4 binds to vascular proteoglycans and controls both growth factor activities and platelet activation. J. Biol. Chem. 2017, 292, 4054–4063. [Google Scholar] [CrossRef] [PubMed]
- Käser-Glanzmann, R.; Jakábová, M.; Lüscher, E.F. Heparin-neutralizing factor (platelet factor 4) from human blood platelets and its reactivity with fibrinogen and soluble fibrin-monomer complexes. Pathophysiol. Thromb. Haemost. 1972, 1, 136–147. [Google Scholar] [CrossRef]
- Sobczak, A.I.S.; Katundu, K.G.H.; Phoenix, F.A.; Khazaipoul, S.; Yu, R.; Lampiao, F.; Stefanowicz, F.; Blindauer, C.A.; Pitt, S.J.; Smith, T.K.; et al. Albumin-mediated alteration of plasma zinc speciation by fatty acids modulates blood clotting in type-2 diabetes. Chem Sci. 2021, 12, 4079–4093. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.L.; Hulett, M.D.; Parish, C.R. Histidine-rich glycoprotein: A novel adaptor protein in plasma that modulates the immune, vascular and coagulation systems. Immunol. Cell. Biol. 2005, 83, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Corrigan, J.J.; Jeter, M.A.; Bruck, D.; Feinberg, W.M. Histidine-rich glycoprotein levels in children: The effect of age. Thromb. Res. 1990, 59, 681–686. [Google Scholar] [CrossRef]
- Tennent, G.A.; Brennan, S.O.; Stangou, A.J.; O’Grady, J.; Hawkins, P.N.; Pepys, M.B. Human plasma fibrinogen is synthesized in the liver. Blood 2007, 109, 1971–1974. [Google Scholar] [CrossRef]
- Tseng, P.-Y.; Rele, S.M.; Sun, X.-L.; Chaikof, E.L. Fabrication and characterization of heparin functionalized membrane-mimetic assemblies. Biomaterials 2006, 27, 2627–2636. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobczak, A.I.S.; Ajjan, R.A.; Stewart, A.J. Zn2+ Differentially Influences the Neutralisation of Heparins by HRG, Fibrinogen, and Fibronectin. Int. J. Mol. Sci. 2023, 24, 16667. https://doi.org/10.3390/ijms242316667
Sobczak AIS, Ajjan RA, Stewart AJ. Zn2+ Differentially Influences the Neutralisation of Heparins by HRG, Fibrinogen, and Fibronectin. International Journal of Molecular Sciences. 2023; 24(23):16667. https://doi.org/10.3390/ijms242316667
Chicago/Turabian StyleSobczak, Amélie I. S., Ramzi A. Ajjan, and Alan J. Stewart. 2023. "Zn2+ Differentially Influences the Neutralisation of Heparins by HRG, Fibrinogen, and Fibronectin" International Journal of Molecular Sciences 24, no. 23: 16667. https://doi.org/10.3390/ijms242316667
APA StyleSobczak, A. I. S., Ajjan, R. A., & Stewart, A. J. (2023). Zn2+ Differentially Influences the Neutralisation of Heparins by HRG, Fibrinogen, and Fibronectin. International Journal of Molecular Sciences, 24(23), 16667. https://doi.org/10.3390/ijms242316667






