Heparan Sulfate and Sialic Acid in Viral Attachment: Two Sides of the Same Coin?
Abstract
:1. Introduction
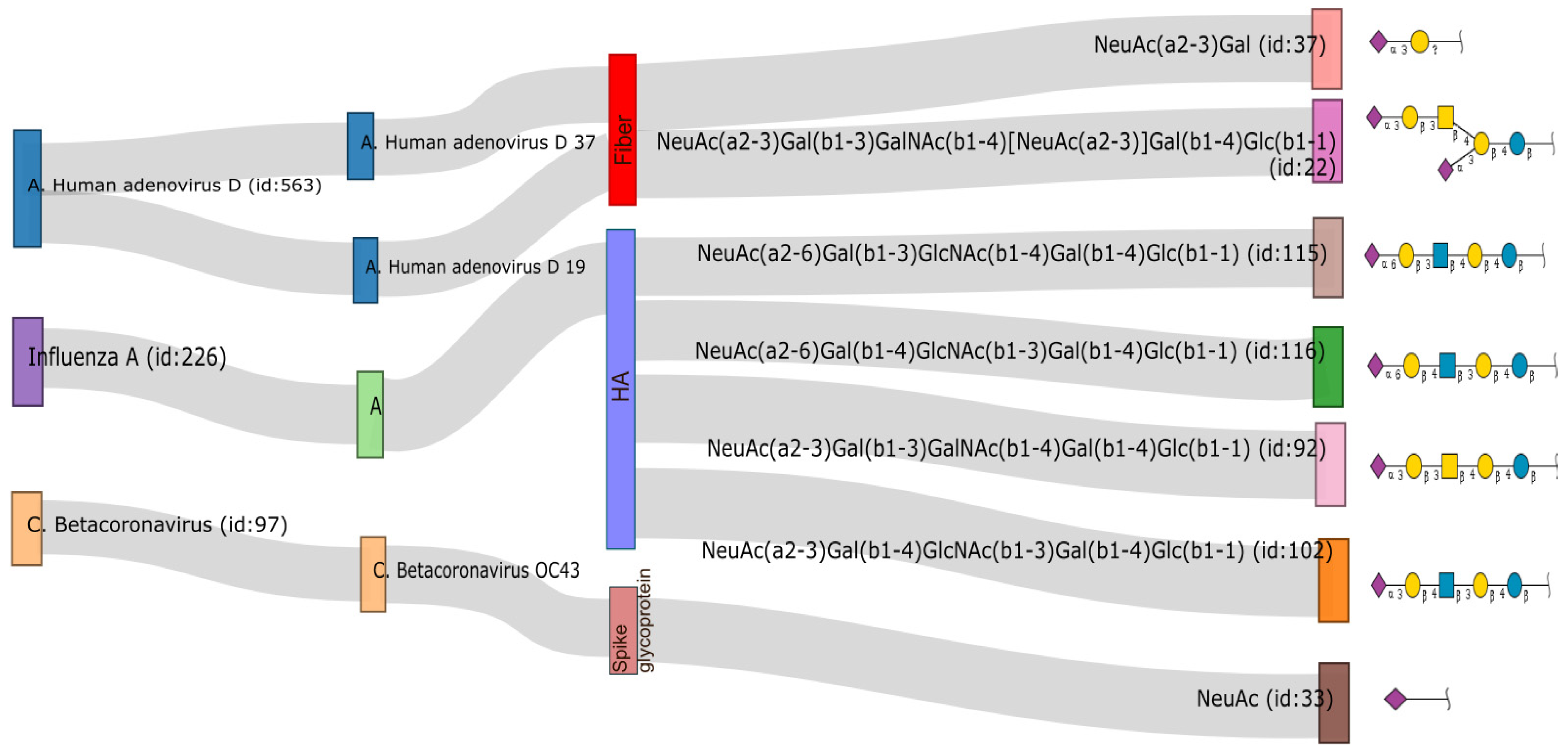
| Heparan Sulfate |
|---|
| Herpes Simplex Virus type 1 [10] |
| Dengue virus [11] |
| Zika virus [12] |
| Hepatitis C virus [13] |
| Rabies virus [14] |
| Human papillomavirus [15] |
| SARS-CoV-2 [16] |
| Cytomegalovirus [17] |
| Sialic Acid |
| Enterovirus D68 [18] |
| Influenza A virus [19] |
| Adenovirus [20] |
| Human polyomavirus [21] |
| Reovirus [22] |
| Porcine rubulavirus [23] |
| Parainfluenza virus type 3 [24] |
| Porcine reproductive and respiratory syndrome virus (PRRSV) [25] |
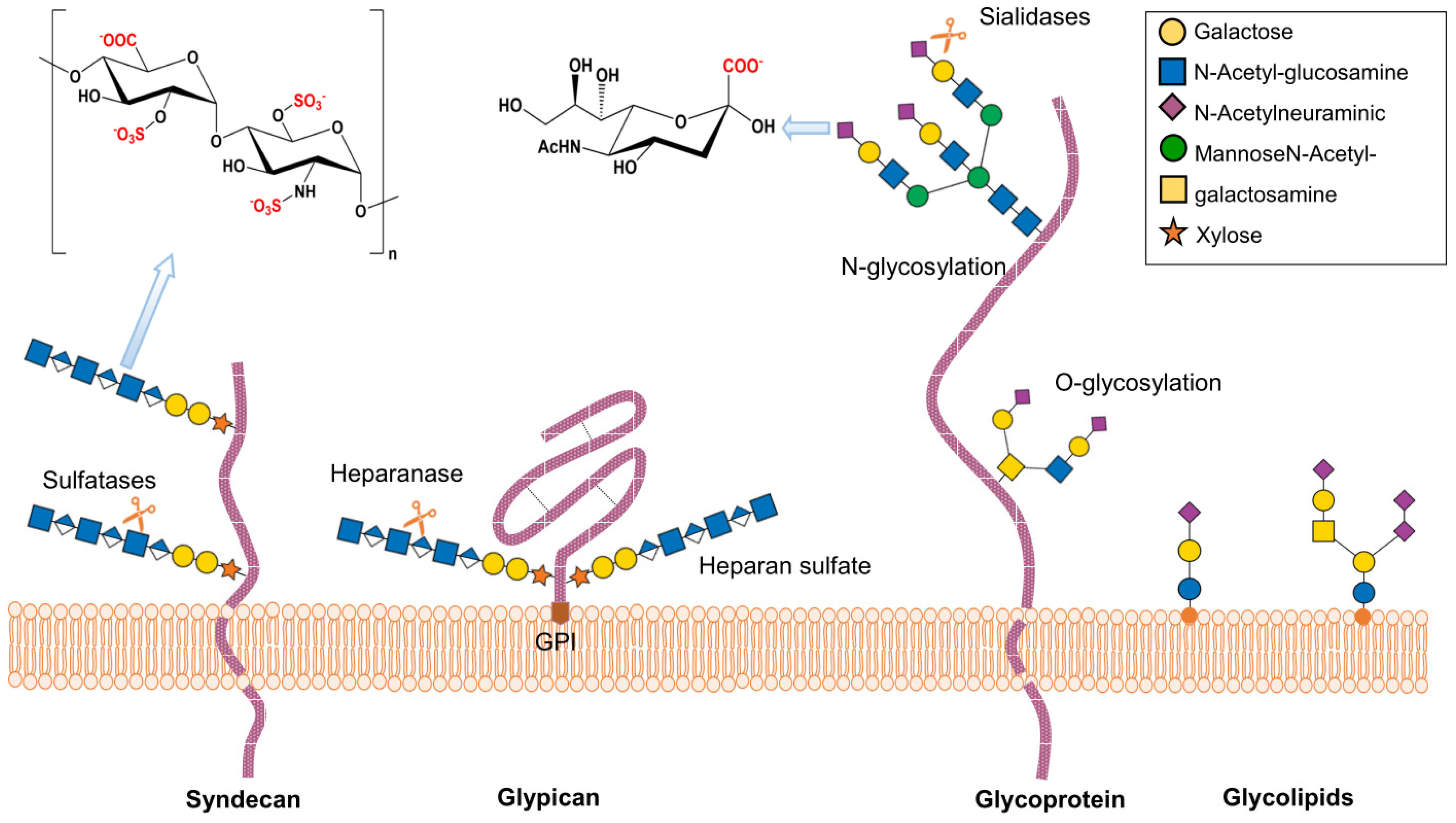
2. Structure and Function of Heparan Sulfate Proteoglycans
3. Structure and Function of Sialic Acid
4. Herpes Simplex Virus
4.1. HSV Receptors
4.2. HSV and Sialic Acids
5. Influenza A Virus
5.1. IAV Receptors
5.2. IAV and Heparan Sulfates
6. SARS-CoV-2
6.1. SARS-CoV-2 Receptors
6.2. SARS-CoV-2 and Sialic Acids
7. Human Papillomavirus
7.1. HPV Receptors
7.2. HPV and Sialic Acids
8. Adenovirus
8.1. Adenovirus Receptors
8.2. Adenovirus and Heparan Sulfates
9. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apweiler, R.; Hermjakob, H.; Sharon, N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim. Biophys. Acta 1999, 1473, 4–8. [Google Scholar] [CrossRef]
- Langford-Smith, A.; Day, A.J.; Bishop, P.N.; Clark, S.J. Complementing the Sugar Code: Role of GAGs and Sialic Acid in Complement Regulation. Front. Immunol. 2015, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Schnaar, R.; Schauer, R. Sialic Acids and Other Nonulosonic Acids. In Essentials of Glycobiology, 3rd ed.; Varki, A., Cummings, R., Esko, J., Eds.; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2017. [Google Scholar]
- Cagno, V.; Tseligka, E.D.; Jones, S.T.; Tapparel, C. Heparan sulfate proteoglycans and viral attachment: True receptors or adaptation bias? Viruses 2019, 11, 596. [Google Scholar] [CrossRef] [PubMed]
- Matrosovich, M.; Herrler, G.; Klenk, H.D. Sialic Acid Receptors of Viruses. Top. Curr. Chem. 2015, 367, 1–28. [Google Scholar]
- Suenaga, T.; Satoh, T.; Somboonthum, P.; Kawaguchi, Y.; Mori, Y.; Arase, H. Myelin-associated glycoprotein mediates membrane fusion and entry of neurotropic herpesviruses. Proc. Natl. Acad. Sci. USA 2010, 107, 866–871. [Google Scholar] [CrossRef] [PubMed]
- WuDunn, D.; Spear, P.G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 1989, 63, 52–58. [Google Scholar] [CrossRef]
- Brauer, R.; Ge, L.; Schlesinger, S.Y.; Birkland, T.P.; Huang, Y.; Parimon, T.; Lee, V.; McKinney, B.L.; McGuire, J.K.; Parks, W.C.; et al. Syndecan-1 Attenuates Lung Injury during Influenza Infection by Potentiating c-Met Signaling to Suppress Epithelial Apoptosis. Am. J. Respir. Crit. Care Med. 2016, 194, 333–344. [Google Scholar] [CrossRef]
- Wang, S.; Luo, Y.; Huang, L.; Wang, S.; Hao, C.; Sun, L.; Zhang, Y.; Wang, W.; Li, C. The inhibition effects and mechanisms of sulfated chitooligosaccharides on influenza A virus in vitro and in vivo. Carbohydr. Polym. 2022, 286, 119316. [Google Scholar] [CrossRef]
- Shukla, D.; Liu, J.; Blaiklock, P.; Shworak, N.W.; Bai, X.; Esko, J.D.; Cohen, G.H.; Eisenberg, R.J.; Rosenberg, R.D.; Spear, P.G. A Novel Role for 3-O-Sulfated Heparan Sulfate in Herpes Simplex Virus 1 Entry. Cell 1999, 99, 13–22. [Google Scholar] [CrossRef]
- Dalrymple, N.; Mackow, E.R. Productive dengue virus infection of human endothelial cells is directed by heparan sulfate-containing proteoglycan receptors. J. Virol. 2011, 85, 9478–9485. [Google Scholar] [CrossRef]
- Gao, H.; Lin, Y.; He, J.; Zhou, S.; Liang, M.; Huang, C.; Li, X.; Liu, C.; Zhang, P. Role of heparan sulfate in the Zika virus entry, replication, and cell death. Virology 2019, 529, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Heo, T.H. A potential role of the heparan sulfate in the hepatitis C virus attachment. Acta Virol. 2008, 52, 7–15. [Google Scholar] [PubMed]
- Sasaki, M.; Anindita, P.D.; Ito, N.; Sugiyama, M.; Carr, M.; Fukuhara, H.; Ose, T.; Maenaka, K.; Takada, A.; Hall, W.W.; et al. The Role of Heparan Sulfate Proteoglycans as an Attachment Factor for Rabies Virus Entry and Infection. J. Infect. Dis. 2018, 217, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Surviladze, Z.; Sterkand, R.T.; Ozbun, M.A. Interaction of human papillomavirus type 16 particles with heparan sulfate and syndecan-1 molecules in the keratinocyte extracellular matrix plays an active role in infection. J. Gen. Virol. 2015, 96, 2232–2241. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.M.; Sandoval, D.R.; Spliid, C.B.; Pihl, J.; Perrett, H.R.; Painter, C.D.; Narayanan, A.; Majowicz, S.A.; Kwong, E.M.; McVicar, R.N.; et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 2020, 183, 1043–1057.e15. [Google Scholar] [CrossRef] [PubMed]
- Mitra, D.; Hasan, M.H.; Bates, J.T.; Bierdeman, M.A.; Ederer, D.R.; Parmar, R.C.; Fassero, L.A.; Liang, Q.; Qiu, H.; Tiwari, V.; et al. The degree of polymerization and sulfation patterns in heparan sulfate are critical determinants of cytomegalovirus entry into host cells. PLoS Pathog. 2021, 17, e1009803. [Google Scholar] [CrossRef]
- Liu, Y.; Sheng, J.; Baggen, J.; Meng, G.; Xiao, C.; Thibaut, H.J.; van Kuppeveld, F.J.M.; Rossmann, M.G. Sialic acid-dependent cell entry of human enterovirus D68. Nat. Commun. 2015, 6, 8865. [Google Scholar] [CrossRef]
- Matrosovich, M.N.; Matrosovich, T.Y.; Gray, T.; Roberts, N.A.; Klenk, H.-D. Human and avian influenza viruses target different cell types in cultures of human airway epithelium. Proc. Natl. Acad. Sci. USA 2004, 101, 4620–4624. [Google Scholar] [CrossRef]
- Lenman, A.; Liaci, A.M.; Liu, Y.; Årdahl, C.; Rajan, A.; Nilsson, E.; Bradford, W.; Kaeshammer, L.; Jones, M.S.; Frängsmyr, L.; et al. Human adenovirus 52 uses sialic acid-containing glycoproteins and the coxsackie and adenovirus receptor for binding to target cells. PLoS Pathog. 2015, 11, e1004657. [Google Scholar] [CrossRef]
- Dugan, A.S.; Eash, S.; Atwood, W.J. An N-linked glycoprotein with alpha(2,3)-linked sialic acid is a receptor for BK virus. J. Virol. 2005, 79, 14442–14445. [Google Scholar] [CrossRef]
- Barton, E.S.; Youree, B.E.; Ebert, D.H.; Forrest, J.C.; Connolly, J.L.; Valyi-Nagy, T.; Washington, K.; Wetzel, J.D.; Dermody, T.S. Utilization of sialic acid as a coreceptor is required for reovirus-induced biliary disease. J. Clin. Investig. 2003, 111, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Magaña, M.L.; Godoy-Martinez, D.V.; Guerrero-Cazares, H.; Rodriguez-Peredo, A.; Dueñas-Jimenez, J.M.; Dueñas-Jiménez, S.H.; Ramírez-Herrera, M.A. Blue eye disease porcine rubulavirus (PoRv) infects pig neurons and glial cells using sialo-glycoprotein as receptor. Vet. J. 2007, 173, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Fu, Y.; Meng, F.; Krüger, N.; Valentin-Weigand, P.; Herrler, G. The Sialic Acid Binding Activity of Human Parainfluenza Virus 3 and Mumps Virus Glycoproteins Enhances the Adherence of Group B Streptococci to HEp-2 Cells. Front. Cell Infect. Microbiol. 2018, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Van Breedam, W.; Van Gorp, H.; Zhang, J.Q.; Crocker, P.R.; Delputte, P.L.; Nauwynck, H.J. The M/GP(5) glycoprotein complex of porcine reproductive and respiratory syndrome virus binds the sialoadhesin receptor in a sialic acid-dependent manner. PLoS Pathog. 2010, 6, e1000730. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.E.; Troeberg, L. Heparan sulfate as a regulator of inflammation and immunity. J. Leukoc. Biol. 2019, 105, 81–92. [Google Scholar] [CrossRef]
- Maïza, A.; Chantepie, S.; Vera, C.; Fifre, A.; Huynh, M.B.; Stettler, O.; Ouidja, M.O.; Papy-Garcia, D. The role of heparan sulfates in protein aggregation and their potential impact on neurodegeneration. FEBS Lett. 2018, 592, 3806–3818. [Google Scholar] [CrossRef]
- Marques, C.; Reis, C.A.; Vivès, R.R.; Magalhães, A. Heparan Sulfate Biosynthesis and Sulfation Profiles as Modulators of Cancer Signalling and Progression. Front. Oncol. 2021, 11, 778752. [Google Scholar] [CrossRef]
- Li, J.-P.; Kusche-Gullberg, M. Heparan Sulfate: Biosynthesis, Structure, and Function. Int. Rev. Cell Mol. Biol. 2016, 325, 215–273. [Google Scholar]
- Thacker, B.E.; Xu, D.; Lawrence, R.; Esko, J.D. Heparan sulfate 3-O-sulfation: A rare modification in search of a function. Matrix Biol. 2014, 35, 60–72. [Google Scholar] [CrossRef]
- Lamanna, W.C.; Frese, M.-A.; Balleininger, M.; Dierks, T. Sulf loss influences N-, 2-O-, and 6-O-sulfation of multiple heparan sulfate proteoglycans and modulates fibroblast growth factor signaling. J. Biol. Chem. 2008, 283, 27724–27735. [Google Scholar] [CrossRef]
- Beurskens, D.M.H.; Huckriede, J.P.; Schrijver, R.; Hemker, H.C.; Reutelingsperger, C.P.; Nicolaes, G.A.F. The Anticoagulant and Nonanticoagulant Properties of Heparin. Thromb. Haemost. 2020, 120, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R.; Kamerling, J.P. Exploration of the Sialic Acid World. Adv. Carbohydr. Chem. Biochem. 2018, 75, 1–213. [Google Scholar]
- Rustmeier, N.H.; Strebl, M.; Stehle, T. The Symmetry of Viral Sialic Acid Binding Sites-Implications for Antiviral Strategies. Viruses 2019, 11, 947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, M.; Hart, G.; Kinoshita, T. Glycosylphosphatidylinositol Anchors. In Essentials of Glycobiology, 3rd ed.; Varki, A., Cummings, R., Esko, J., Eds.; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2017. [Google Scholar]
- Mikolajczyk, K.; Kaczmarek, R.; Czerwinski, M. How glycosylation affects glycosylation: The role of N-glycans in glycosyltransferase activity. Glycobiology 2020, 30, 941–969. [Google Scholar] [CrossRef] [PubMed]
- Burzyńska, P.; Sobala, Ł.F.; Mikołajczyk, K.; Jodłowska, M.; Jaśkiewicz, E. Sialic Acids as Receptors for Pathogens. Biomolecules 2021, 11, 831. [Google Scholar] [CrossRef]
- Lee, S.W.H.; Gottlieb, S.L.; Chaiyakunapruk, N. Healthcare resource utilisation pattern and costs associated with herpes simplex virus diagnosis and management: A systematic review. BMJ Open 2022, 12, e049618. [Google Scholar] [CrossRef]
- Krummenacher, C.; Baribaud, F.; Ponce de Leon, M.; Baribaud, I.; Whitbeck, J.C.; Xu, R.; Cohen, G.H.; Eisenberg, R.J. Comparative usage of herpesvirus entry mediator A and nectin-1 by laboratory strains and clinical isolates of herpes simplex virus. Virology 2004, 322, 286–299. [Google Scholar] [CrossRef]
- Atanasiu, D.; Saw, W.T.; Cohen, G.H.; Eisenberg, R.J. Cascade of events governing cell-cell fusion induced by herpes simplex virus glycoproteins gD, gH/gL, and gB. J. Virol. 2010, 84, 12292–12299. [Google Scholar] [CrossRef]
- Yoon, M.; Zago, A.; Shukla, D.; Spear, P.G. Mutations in the N termini of herpes simplex virus type 1 and 2 gDs alter functional interactions with the entry/fusion receptors HVEM, nectin-2, and 3-O-sulfated heparan sulfate but not with nectin-1. J. Virol. 2003, 77, 9221–9231. [Google Scholar] [CrossRef]
- Denys, A.; Allain, F. The Emerging Roles of Heparan Sulfate 3-O-Sulfotransferases in Cancer. Front. Oncol. 2019, 9, 507. [Google Scholar] [CrossRef]
- Huynh, M.B.; Ouidja, M.O.; Chantepie, S.; Carpentier, G.; Maïza, A.; Zhang, G.; Vilares, J.; Raisman-Vozari, R.; Papy-Garcia, D. Glycosaminoglycans from Alzheimer’s disease hippocampus have altered capacities to bind and regulate growth factors activities and to bind tau. PLoS ONE 2019, 14, e0209573. [Google Scholar]
- Tiwari, V.; O’Donnell, C.D.; Oh, M.-J.; Valyi-Nagy, T.; Shukla, D. A role for 3-O-sulfotransferase isoform-4 in assisting HSV-1 entry and spread. Biochem. Biophys. Res. Commun. 2005, 338, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Chen, J.; Tiwari, V.; Ju, W.; Li, J.-P.; Malmström, A.; Shukla, D.; Liu, J. Heparan Sulfate 3-O-Sulfotransferase Isoform 5 Generates Both an Antithrombin-binding Site and an Entry Receptor for Herpes Simplex Virus, Type 1. J. Biol. Chem. 2002, 277, 37912–37919. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Tiwari, V.; Xia, G.; Clement, C.; Shukla, D.; Liu, J. Characterization of heparan sulphate 3-O-sulphotransferase isoform 6 and its role in assisting the entry of herpes simplex virus type 1. Biochem. J. 2005, 385 Pt 2, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Yakoub, A.M.; Rawal, N.; Maus, E.; Baldwin, J.; Shukla, D.; Tiwari, V. Comprehensive analysis of herpes simplex virus 1 (HSV-1) entry mediated by zebrafish 3-O-Sulfotransferase isoforms: Implications for the development of a zebrafish model of HSV-1 infection. J. Virol. 2014, 88, 12915–12922. [Google Scholar] [CrossRef]
- Bacsa, S.; Karasneh, G.; Dosa, S.; Liu, J.; Valyi-Nagy, T.; Shukla, D. Syndecan-1 and syndecan-2 play key roles in herpes simplex virus type-1 infection. J. Gen. Virol. 2011, 92 Pt 4, 733–743. [Google Scholar] [CrossRef]
- Hadigal, S.R.; Agelidis, A.M.; Karasneh, G.A.; Antoine, T.E.; Yakoub, A.M.; Ramani, V.C.; Djalilian, A.R.; Sanderson, R.D.; Shukla, D. Heparanase is a host enzyme required for herpes simplex virus-1 release from cells. Nat. Commun. 2015, 6, 6985. [Google Scholar] [CrossRef]
- Karasneh, G.A.; Kapoor, D.; Bellamkonda, N.; Patil, C.D.; Shukla, D. Protease, Growth Factor, and Heparanase-Mediated Syndecan-1 Shedding Leads to Enhanced HSV-1 Egress. Viruses 2021, 13, 1748. [Google Scholar] [CrossRef]
- Nahmias, A.J.; Kibrick, S. Inhibitory effect of heparin on herpes simplex virus. J. Bacteriol. 1964, 87, 1060–1066. [Google Scholar] [CrossRef]
- Ali, M.M.; Karasneh, G.A.; Jarding, M.J.; Tiwari, V.; Shukla, D. A 3-O-sulfated heparan sulfate binding peptide preferentially targets herpes simplex virus 2-infected cells. J. Virol. 2012, 86, 6434–6443. [Google Scholar] [CrossRef]
- Tiwari, V.; Clement, C.; Xu, D.; Valyi-Nagy, T.; Yue, B.Y.J.T.; Liu, J.; Shukla, D. Role for 3-O-sulfated heparan sulfate as the receptor for herpes simplex virus type 1 entry into primary human corneal fibroblasts. J. Virol. 2006, 80, 8970–8980. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-P.; Lin, S.-Y.; Huang, C.-Y.; Zulueta, M.M.L.; Liu, J.-Y.; Chang, W.; Hung, S.-C. Synthesis of 3-O-sulfonated heparan sulfate octasaccharides that inhibit the herpes simplex virus type 1 host-cell interaction. Nat. Chem. 2011, 3, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Gangji, R.N.; Sankaranarayanan, N.V.; Elste, J.; Al-Horani, R.A.; Afosah, D.K.; Joshi, R.; Tiwari, V.; Desai, U.R. Inhibition of Herpes Simplex Virus-1 Entry into Human Cells by Nonsaccharide Glycosaminoglycan Mimetics. ACS Med. Chem. Lett. 2018, 9, 797–802. [Google Scholar] [CrossRef]
- Deback, C.; Rousseau, A.; Breckler, M.; Molet, L.; Boutolleau, D.; Burrel, S.; Roque-Afonso, A.-M.; Labetoulle, M. Antiviral effects of Cacicol(®), a heparan sulfate biomimetic for corneal regeneration therapy, for herpes simplex virus type-1 and varicella zoster virus infection. Antivir. Ther. 2018, 23, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Teuton, J.R.; Brandt, C.R. Sialic acid on herpes simplex virus type 1 envelope glycoproteins is required for efficient infection of cells. J. Virol. 2007, 81, 3731–3739. [Google Scholar] [CrossRef]
- Hasegawa, K.; Motsuchi, W.; Tanaka, S.; Dosako, S. Inhibition with lactoferrin of in vitro infection with human herpes virus. Jpn. J. Med. Sci. Biol. 1994, 47, 73–85. [Google Scholar] [CrossRef]
- Maya-Badillo, B.A.; Ojeda-Flores, R.; Chaves, A.; Reveles-Félix, S.; Orta-Pineda, G.; Martínez-Mercado, M.J.; Saavedra-Montañez, M.; Segura-Velázquez, R.; Sanvicente, M.; Sánchez-Betancourt, J.I. Eco-Epidemiological Evidence of the Transmission of Avian and Human Influenza A Viruses in Wild Pigs in Campeche, Mexico. Viruses 2020, 12, 528. [Google Scholar] [CrossRef]
- Nuwarda, R.F.; Alharbi, A.A.; Kayser, V. An Overview of Influenza Viruses and Vaccines. Vaccines 2021, 9, 1032. [Google Scholar] [CrossRef]
- Bui, C.H.T.; Kuok, D.I.T.; Yeung, H.W.; Ng, K.-C.; Chu, D.K.W.; Webby, R.J.; Nicholls, J.M.; Peiris, J.S.M.; Hui, K.P.Y.; Chan, M.C.W. Risk Assessment for Highly Pathogenic Avian Influenza A(H5N6/H5N8) Clade 2.3.4.4 Viruses. Emerg. Infect. Dis. 2021, 27, 2619–2627. [Google Scholar] [CrossRef]
- Saavedra-Montañez, M.; Vaca, L.; Ramírez-Mendoza, H.; Gaitán-Peredo, C.; Bautista-Martínez, R.; Segura-Velázquez, R.; Cervantes-Torres, J.; Sánchez-Betancourt, J.I. Identification and genomic characterization of influenza viruses with different origin in Mexican pigs. Transbound. Emerg. Dis. 2019, 66, 186–194. [Google Scholar] [CrossRef]
- Yang, W.; Schountz, T.; Ma, W. Bat Influenza Viruses: Current Status and Perspective. Viruses 2021, 13, 547. [Google Scholar] [CrossRef] [PubMed]
- van Riel, D.; Munster, V.J.; de Wit, E.; Rimmelzwaan, G.F.; Fouchier, R.A.M.; Osterhaus, A.D.M.E.; Kuiken, T. Human and avian influenza viruses target different cells in the lower respiratory tract of humans and other mammals. Am. J. Pathol. 2007, 171, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.Y.; Gan, S.K.-E. Peering into Avian Influenza A(H5N8) for a Framework towards Pandemic Preparedness. Viruses 2021, 13, 2276. [Google Scholar] [CrossRef] [PubMed]
- Byrd-Leotis, L.; Jia, N.; Dutta, S.; Trost, J.F.; Gao, C.; Cummings, S.F.; Braulke, T.; Müller-Loennies, S.; Heimburg-Molinaro, J.; Steinhauer, D.A.; et al. Influenza binds phosphorylated glycans from human lung. Sci. Adv. 2019, 5, eaav2554. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Shi, Y.; Lu, X.; He, J.; Gao, F.; Yan, J.; Qi, J.; Gao, G.F. Bat-derived influenza hemagglutinin H17 does not bind canonical avian or human receptors and most likely uses a unique entry mechanism. Cell Rep. 2013, 3, 769–778. [Google Scholar] [CrossRef]
- Guo, C.T.; Wong, C.H.; Kajimoto, T.; Miura, T.; Ida, Y.; Juneja, L.R.; Kim, M.-J.; Masuda, H.; Suzuki, T.; Suzuki, Y. Synthetic sialylphosphatidylethanolamine derivatives bind to human influenza A viruses and inhibit viral infection. Glycoconj. J. 1998, 15, 1099–1108. [Google Scholar] [CrossRef]
- Papp, I.; Sieben, C.; Ludwig, K.; Roskamp, M.; Böttcher, C.; Schlecht, S.; Herrmann, A.; Haag, R. Inhibition of influenza virus infection by multivalent sialic-acid-functionalized gold nanoparticles. Small 2010, 6, 2900–2906. [Google Scholar] [CrossRef]
- Han, X.; Si, L.-L.; Shi, Y.-Y.; Fan, Z.-B.; Wang, S.-X.; Tian, Z.-Y.; Li, M.; Sun, J.-Q.; Jiao, P.-X.; Ran, F.-X.; et al. Synthesis and In Vitro Anti-Influenza Virus Evaluation of Novel Sialic Acid (C-5 and C-9)-Pentacyclic Triterpene Derivatives. Molecules 2017, 22, 1018. [Google Scholar] [CrossRef]
- Scala, M.C.; Sala, M.; Pietrantoni, A.; Spensiero, A.; Di Micco, S.; Agamennone, M.; Bertamino, A.; Novellino, E.; Bifulco, G.; Gomez-Monterrey, I.M.; et al. Lactoferrin-derived Peptides Active towards Influenza: Identification of Three Potent Tetrapeptide Inhibitors. Sci. Rep. 2017, 7, 10593. [Google Scholar] [CrossRef]
- Matsubara, T.; Onishi, A.; Saito, T.; Shimada, A.; Inoue, H.; Taki, T.; Nagata, K.; Okahata, Y.; Sato, T. Sialic acid-mimic peptides as hemagglutinin inhibitors for anti-influenza therapy. J. Med. Chem. 2010, 53, 4441–4449. [Google Scholar] [CrossRef]
- Jeyaram, R.A.; Radha, C.A.; Gromiha, M.M.; Veluraja, K. Design of fluorinated sialic acid analog inhibitor to H5 hemagglutinin of H5N1 influenza virus through molecular dynamics simulation study. J. Biomol. Struct. Dyn. 2020, 38, 3504–3513. [Google Scholar] [CrossRef] [PubMed]
- de Castro, S.; Ginex, T.; Vanderlinden, E.; Laporte, M.; Stevaert, A.; Cumella, J.; Gago, F.; Camarasa, M.J.; Luque, F.J.; Naesens, L.; et al. N-benzyl 4,4-disubstituted piperidines as a potent class of influenza H1N1 virus inhibitors showing a novel mechanism of hemagglutinin fusion peptide interaction. Eur. J. Med. Chem. 2020, 194, 112223. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-J.; Yeh, C.-Y.; Cheng, J.-C.; Huang, Y.-Q.; Hsu, K.-C.; Lin, Y.-F.; Lu, C.-H. Potent sialic acid inhibitors that target influenza A virus hemagglutinin. Sci. Rep. 2021, 11, 8637. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lu, F.; Tian, H.; Hu, H.; Ning, F.; Shang, Q.; Hao, D.; Zhu, W.; Kong, G.; Ma, X.; et al. Association between plasma glycocalyx component levels and poor prognosis in severe influenza type A (H1N1). Sci. Rep. 2022, 12, 163. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Gupta, P.; Johns, S.C.; Zuniga, E.I.; Teijaro, J.R.; Fuster, M.M. Genetic alteration of heparan sulfate in CD11c + immune cells inhibits inflammation and facilitates pathogen clearance during influenza A virus infection. Sci. Rep. 2022, 12, 5382. [Google Scholar] [CrossRef]
- Skidmore, M.A.; Kajaste-Rudnitski, A.; Wells, N.M.; Guimond, S.E.; Rudd, T.R.; Yates, E.A.; Vicenzi, E. Inhibition of influenza H5N1 invasion by modified heparin derivatives. Med. Chem. Commun. 2015, 6, 640–646. [Google Scholar] [CrossRef]
- Kosono, S.; Kasai, A.; Komaba, S.; Matsubara, T.; Sato, T.; Takahashi, D.; Toshima, K. Novel hemagglutinin-binding sulfated oligofucosides and their effect on influenza virus infection. Chem. Commun. 2018, 54, 7467–7470. [Google Scholar] [CrossRef]
- Singh, D.; Yi, S.V. On the origin and evolution of SARS-CoV-2. Exp. Mol. Med. 2021, 53, 537–547. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Suryadevara, N.; Shrihari, S.; Gilchuk, P.; VanBlargan, L.A.; Binshtein, E.; Zost, S.J.; Nargi, R.S.; Sutton, R.E.; Winkler, E.S.; Chen, E.C.; et al. Neutralizing and protective human monoclonal antibodies recognizing the N-terminal domain of the SARS-CoV-2 spike protein. Cell 2021, 184, 2316–2331.e15. [Google Scholar] [CrossRef]
- Koch, J.; Uckeley, Z.M.; Doldan, P.; Stanifer, M.; Boulant, S.; Lozach, P.-Y. TMPRSS2 expression dictates the entry route used by SARS-CoV-2 to infect host cells. EMBO J. 2021, 40, e107821. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Tandon, R.; Sankaranarayanan, N.V.; Beer, J.C.; Kohlmeir, E.K.; Swanson-Mungerson, M.; Desai, U.R. Preferential recognition and antagonism of SARS-CoV-2 spike glycoprotein binding to 3- O -sulfated heparan sulfate. bioRxiv 2020. [Google Scholar] [CrossRef]
- Bermejo-Jambrina, M.; Eder, J.; Kaptein, T.M.; van Hamme, J.L.; Helgers, L.C.; Vlaming, K.E.; Brouwer, P.J.; van Nuenen, A.C.; Spaargaren, M.; de Bree, G.J.; et al. Infection and transmission of SARS-CoV-2 depend on heparan sulfate proteoglycans. EMBO J. 2021, 40, e106765. [Google Scholar] [CrossRef] [PubMed]
- Hudák, A.; Veres, G.; Letoha, A.; Szilák, L.; Letoha, T. Syndecan-4 Is a Key Facilitator of the SARS-CoV-2 Delta Variant’s Superior Transmission. Int. J. Mol. Sci. 2022, 23, 796. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Zhang, W.; Mitra, D.; McCandless, M.G.; Sharma, P.; Tandon, R.; Zhang, F.; Linhardt, R.J. The structure-activity relationship of the interactions of SARS-CoV-2 spike glycoproteins with glucuronomannan and sulfated galactofucan from Saccharina japonica. Int. J. Biol. Macromol. 2020, 163, 1649–1658. [Google Scholar] [CrossRef]
- Ennemoser, M.; Rieger, J.; Muttenthaler, E.; Gerlza, T.; Zatloukal, K.; Kungl, A.J. Enoxaparin and Pentosan Polysulfate Bind to the SARS-CoV-2 Spike Protein and Human ACE2 Receptor, Inhibiting Vero Cell Infection. Biomedicines 2021, 10, 49. [Google Scholar] [CrossRef]
- Zhang, F.; He, P.; Rodrigues, A.L.; Jeske, W.; Tandon, R.; Bates, J.T.; Bierdeman, M.A.; Fareed, J.; Dordick, J.; Linhardt, R.J. Potential Anti-SARS-CoV-2 Activity of Pentosan Polysulfate and Mucopolysaccharide Polysulfate. Pharmaceuticals 2022, 15, 258. [Google Scholar] [CrossRef]
- Tu, B.; Wang, H.; An, X.; Qu, J.; Li, Q.; Gao, Y.; Shi, M.; Qiu, H.; Huang, Y. Inhaled heparin polysaccharide nanodecoy against SARS-CoV-2 and variants. Acta Pharm. Sin. B 2022, 12, 3187–3194. [Google Scholar] [CrossRef]
- Suryawanshi, R.K.; Patil, C.D.; Koganti, R.; Singh, S.K.; Ames, J.M.; Shukla, D. Heparan Sulfate Binding Cationic Peptides Restrict SARS-CoV-2 Entry. Pathogens 2021, 10, 803. [Google Scholar] [CrossRef]
- Unione, L.; Moure, M.J.; Lenza, M.P.; Oyenarte, I.; Ereño-Orbea, J.; Ardá, A.; Jiménez-Barbero, J. The SARS-CoV-2 Spike Glycoprotein Directly Binds Exogeneous Sialic Acids: A NMR View. Angew. Chem. Int. Ed. Engl. 2022, 61, e202201432. [Google Scholar] [CrossRef]
- Nguyen, L.; McCord, K.A.; Bui, D.T.; Bouwman, K.M.; Kitova, E.N.; Elaish, M.; Kumawat, D.; Daskhan, G.C.; Tomris, I.; Han, L.; et al. Sialic acid-containing glycolipids mediate binding and viral entry of SARS-CoV-2. Nat. Chem. Biol. 2022, 18, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Hu, B.; Huang, X.; Chai, Y.; Zhou, D.; Wang, Y.; Shuai, H.; Yang, D.; Hou, Y.; Zhang, X.; et al. Host and viral determinants for efficient SARS-CoV-2 infection of the human lung. Nat. Commun. 2021, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Watanabe, Y.; Chawla, H.; Newby, M.L.; Crispin, M. Subtle Influence of ACE2 Glycan Processing on SARS-CoV-2 Recognition. J. Mol. Biol. 2021, 433, 166762. [Google Scholar] [CrossRef]
- Sanda, M.; Morrison, L.; Goldman, R. N- and O-Glycosylation of the SARS-CoV-2 Spike Protein. Anal. Chem. 2021, 93, 2003–2009. [Google Scholar] [CrossRef]
- Perez-Zsolt, D.; Muñoz-Basagoiti, J.; Rodon, J.; Elosua-Bayes, M.; Raïch-Regué, D.; Risco, C.; Sachse, M.; Pino, M.; Gumber, S.; Paiardini, M.; et al. SARS-CoV-2 interaction with Siglec-1 mediates trans-infection by dendritic cells. Cell. Mol. Immunol. 2021, 18, 2676–2678. [Google Scholar] [CrossRef] [PubMed]
- Lempp, F.A.; Soriaga, L.B.; Montiel-Ruiz, M.; Benigni, F.; Noack, J.; Park, Y.-J.; Bianchi, S.; Walls, A.C.; Bowen, J.E.; Zhou, J.; et al. Lectins enhance SARS-CoV-2 infection and influence neutralizing antibodies. Nature 2021, 598, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Ma, B.; Li, Z.; Wang, X.; Gao, X.; Li, Y.; Qin, B.; Shang, S.; Cui, S.; Tan, Z. Binding of the SARS-CoV-2 spike protein to glycans. Sci. Bull. 2021, 66, 1205–1214. [Google Scholar] [CrossRef]
- Yousefi, Z.; Aria, H.; Ghaedrahmati, F.; Bakhtiari, T.; Azizi, M.; Bastan, R.; Hosseini, R.; Eskandari, N. An Update on Human Papilloma Virus Vaccines: History, Types, Protection, and Efficacy. Front. Immunol. 2021, 12, 805695. [Google Scholar] [CrossRef]
- Mattox, A.K.; Roelands, J.; Saal, T.M.; Cheng, Y.; Rinchai, D.; Hendrickx, W.; Young, G.D.; Diefenbach, T.J.; Berger, A.E.; Westra, W.H.; et al. Myeloid Cells Are Enriched in Tonsillar Crypts, Providing Insight into the Viral Tropism of Human Papillomavirus. Am. J. Pathol. 2021, 191, 1774–1786. [Google Scholar] [CrossRef]
- Wu, D.-D.; Long, F.-Q.; Gao, J.; Zhong, L.; Sun, C. HPV6 and HPV11 Genome Methylation in Condyloma Accuminatum Measured by Bisulfite Sequencing. Am. J. Dermatopathol. 2019, 41, 534–535. [Google Scholar] [CrossRef]
- Mane, A.; Patil, L.; Limaye, S.; Nirmalkar, A.; Kulkarni-Kale, U. Characterization of major capsid protein (L1) variants of Human papillomavirus type 16 by cervical neoplastic status in Indian women: Phylogenetic and functional analysis. J. Med. Virol. 2020, 92, 1303–1308. [Google Scholar] [CrossRef] [PubMed]
- Culp, T.D.; Budgeon, L.R.; Marinkovich, M.P.; Meneguzzi, G.; Christensen, N.D. Keratinocyte-secreted laminin 5 can function as a transient receptor for human papillomaviruses by binding virions and transferring them to adjacent cells. J. Virol. 2006, 80, 8940–8950. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Greune, L.; Schmidt, M.A.; Schelhaas, M. Extracellular Conformational Changes in the Capsid of Human Papillomaviruses Contribute to Asynchronous Uptake into Host Cells. J. Virol. 2018, 92, e02106-17. [Google Scholar] [CrossRef] [PubMed]
- Buck, C.B.; Thompson, C.D.; Roberts, J.N.; Müller, M.; Lowy, D.R.; Schiller, J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006, 2, e69. [Google Scholar] [CrossRef] [Green Version]
- Lembo, D.; Donalisio, M.; Rusnati, M.; Bugatti, A.; Cornaglia, M.; Cappello, P.; Giovarelli, M.; Oreste, P.; Landolfo, S. Sulfated K5 Escherichia coli polysaccharide derivatives as wide-range inhibitors of genital types of human papillomavirus. Antimicrob. Agents Chemother. 2008, 52, 1374–1381. [Google Scholar] [CrossRef]
- Drobni, P.; Näslund, J.; Evander, M. Lactoferrin inhibits human papillomavirus binding and uptake in vitro. Antivir. Res. 2004, 64, 63–68. [Google Scholar] [CrossRef]
- Mistry, N.; Drobni, P.; Näslund, J.; Sunkari, V.G.; Jenssen, H.; Evander, M. The anti-papillomavirus activity of human and bovine lactoferricin. Antivir. Res. 2007, 75, 258–265. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, Q.; Chen, Y.; Dong, B.; Xue, H.; Lei, H.; Lu, Y.; Wei, X.; Sun, P. Changes of the vaginal microbiota in HPV infection and cervical intraepithelial neoplasia: A cross-sectional analysis. Sci. Rep. 2022, 12, 2812. [Google Scholar] [CrossRef]
- Greber, U.F.; Flatt, J.W. Adenovirus Entry: From Infection to Immunity. Annu. Rev. Virol. 2019, 6, 177–197. [Google Scholar] [CrossRef]
- King, C.R.; Zhang, A.; Mymryk, J.S. The Persistent Mystery of Adenovirus Persistence. Trends Microbiol. 2016, 24, 323–324. [Google Scholar] [CrossRef]
- Stasiak, A.C.; Stehle, T. Human adenovirus binding to host cell receptors: A structural view. Med. Microbiol. Immunol. 2020, 209, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Wickham, T.J.; Mathias, P.; Cheresh, D.A.; Nemerow, G.R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell 1993, 73, 309–319. [Google Scholar] [CrossRef]
- Lenman, A.; Liaci, A.M.; Liu, Y.; Frängsmyr, L.; Frank, M.; Blaum, B.S.; Chai, W.; Podgorski, I.I.; Harrach, B.; Benkő, M.; et al. Polysialic acid is a cellular receptor for human adenovirus 52. Proc. Natl. Acad. Sci. USA 2018, 115, E4264–E4273. [Google Scholar] [CrossRef]
- Burmeister, W.P.; Guilligay, D.; Cusack, S.; Wadell, G.; Arnberg, N. Crystal structure of species D adenovirus fiber knobs and their sialic acid binding sites. J. Virol. 2004, 78, 7727–7736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, E.C.; Storm, R.J.; Bauer, J.; Johansson, S.M.C.; Lookene, A.; Ångström, J.; Hedenström, M.; Eriksson, T.L.; Frängsmyr, L.; Rinaldi, S.; et al. The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat. Med. 2011, 17, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Mahsoub, H.M.; Yuan, L.; Pierson, F.W. Turkey adenovirus 3, a siadenovirus, uses sialic acid on N-linked glycoproteins as a cellular receptor. J. Gen. Virol. 2020, 101, 760–771. [Google Scholar] [CrossRef]
- Rademacher, C.; Bru, T.; McBride, R.; Robison, E.; Nycholat, C.M.; Kremer, E.J.; Paulson, J.C. A Siglec-like sialic-acid-binding motif revealed in an adenovirus capsid protein. Glycobiology 2012, 22, 1086–1091. [Google Scholar] [CrossRef]
- Baker, A.T.; Mundy, R.M.; Davies, J.A.; Rizkallah, P.J.; Parker, A.L. Human adenovirus type 26 uses sialic acid-bearing glycans as a primary cell entry receptor. Sci. Adv. 2019, 5, eaax3567. [Google Scholar] [CrossRef]
- Chandra, N.; Frängsmyr, L.; Imhof, S.; Caraballo, R.; Elofsson, M.; Arnberg, N. Sialic Acid-Containing Glycans as Cellular Receptors for Ocular Human Adenoviruses: Implications for Tropism and Treatment. Viruses 2019, 11, 395. [Google Scholar] [CrossRef]
- Johansson, S.M.C.; Arnberg, N.; Elofsson, M.; Wadell, G.; Kihlberg, J. Multivalent HSA conjugates of 3′-sialyllactose are potent inhibitors of adenoviral cell attachment and infection. ChemBioChem 2005, 6, 358–364. [Google Scholar] [CrossRef]
- Caraballo, R.; Saleeb, M.; Bauer, J.; Liaci, A.M.; Chandra, N.; Storm, R.J.; Frängsmyr, L.; Qian, W.; Stehle, T.; Arnberg, N.; et al. Triazole linker-based trivalent sialic acid inhibitors of adenovirus type 37 infection of human corneal epithelial cells. Org. Biomol. Chem. 2015, 13, 9194–9205. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Caraballo, R.; Mistry, N.; Zocher, G.; Qian, W.; Andersson, C.D.; Hurdiss, D.L.; Chandra, N.; Thompson, R.; Frängsmyr, L.; et al. Pentavalent Sialic Acid Conjugates Block Coxsackievirus A24 Variant and Human Adenovirus Type 37-Viruses That Cause Highly Contagious Eye Infections. ACS Chem. Biol. 2020, 15, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Heida, R.; Bhide, Y.C.; Gasbarri, M.; Kocabiyik, Ö.; Stellacci, F.; Huckriede, A.L.W.; Hinrichs, W.L.; Frijlink, H.W. Advances in the development of entry inhibitors for sialic-acid-targeting viruses. Drug Discov. Today 2021, 26, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Dechecchi, M.C.; Melotti, P.; Bonizzato, A.; Santacatterina, M.; Chilosi, M.; Cabrini, G. Heparan sulfate glycosaminoglycans are receptors sufficient to mediate the initial binding of adenovirus types 2 and 5. J. Virol. 2001, 75, 8772–8780. [Google Scholar] [CrossRef] [Green Version]
- Bayo-Puxan, N.; Cascallo, M.; Gros, A.; Huch, M.; Fillat, C.; Alemany, R. Role of the putative heparan sulfate glycosaminoglycan-binding site of the adenovirus type 5 fiber shaft on liver detargeting and knob-mediated retargeting. J. Gen. Virol. 2006, 87, 2487–2495. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, A.K.; Foley, E.M.; Lawrence, R.; Schneider, L.S.; Hoveida, H.; Secrest, P.; Catapang, A.B.; Yamaguchi, Y.; Alemany, R.; Shayakhmetov, D.M.; et al. Hepatocyte Heparan Sulfate Is Required for Adeno-Associated Virus 2 but Dispensable for Adenovirus 5 Liver Transduction In Vivo. J. Virol. 2016, 90, 412–420. [Google Scholar] [CrossRef]
- Chandra, N.; Liu, Y.; Liu, J.-X.; Frängsmyr, L.; Wu, N.; Silva, L.M.; Lindström, M.; Chai, W.; Domellöf, F.P.; Feizi, T.; et al. Sulfated Glycosaminoglycans as Viral Decoy Receptors for Human Adenovirus Type 37. Viruses 2019, 11, 247. [Google Scholar] [CrossRef]
- Tuve, S.; Wang, H.; Jacobs, J.D.; Yumul, R.C.; Smith, D.F.; Lieber, A. Role of Cellular Heparan Sulfate Proteoglycans in Infection of Human Adenovirus Serotype 3 and 35. PLoS Pathog. 2008, 4, e1000189. [Google Scholar] [CrossRef]
- Dechecchi, M.C.; Tamanini, A.; Bonizzato, A.; Cabrini, G. Heparan Sulfate Glycosaminoglycans Are Involved in Adenovirus Type 5 and 2-Host Cell Interactions. Virology 2000, 268, 382–390. [Google Scholar] [CrossRef]
- Lenaerts, L.; van Dam, W.; Persoons, L.; Naesens, L. Interaction between mouse adenovirus type 1 and cell surface heparan sulfate proteoglycans. PLoS ONE 2012, 7, e31454. [Google Scholar]
- Raman, S.; Hsu, T.-H.; Ashley, S.L.; Spindler, K.R. Usage of integrin and heparan sulfate as receptors for mouse adenovirus type 1. J. Virol. 2009, 83, 2831–2838. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, M.I.; Lenman, A.E.; Frängsmyr, L.; Nyberg, C.; Abdullahi, M.; Arnberg, N. Coagulation factors IX and X enhance binding and infection of adenovirus types 5 and 31 in human epithelial cells. J. Virol. 2009, 83, 3816–3825. [Google Scholar] [CrossRef]
- He, W.-T.; Hou, X.; Zhao, J.; Sun, J.; He, H.; Si, W.; Wang, J.; Jiang, Z.; Yan, Z.; Xing, G.; et al. Virome characterization of game animals in China reveals a spectrum of emerging pathogens. Cell 2022, 185, 1117–1129.e8. [Google Scholar] [CrossRef] [PubMed]
- Mariethoz, J.; Khatib, K.; Alocci, D.; Campbell, M.P.; Karlsson, N.G.; Packer, N.H.; Mullen, E.H.; Lisacek, F. SugarBindDB, a resource of glycan-mediated host-pathogen interactions. Nucleic Acids Res. 2016, 44, D1243–D1250. [Google Scholar] [CrossRef] [PubMed]
- Van Breedam, W.; Pöhlmann, S.; Favoreel, H.W.; de Groot, R.J.; Nauwynck, H.J. Bitter-sweet symphony: Glycan-lectin interactions in virus biology. FEMS Microbiol. Rev. 2014, 38, 598–632. [Google Scholar] [CrossRef] [Green Version]
- Colpitts, C.C.; Schang, L.M. A small molecule inhibits virion attachment to heparan sulfate- or sialic acid-containing glycans. J. Virol. 2014, 88, 7806–7817. [Google Scholar] [CrossRef]
- Zafar, H.; Atif, M.; Atia-Tul-Wahab; Choudhary, M.I. Fucosyltransferase 2 inhibitors: Identification via docking and STD-NMR studies. PLoS ONE 2021, 16, e0257623. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Martínez, I.E.; Ramos-Martínez, E.; Segura-Velázquez, R.Á.; Saavedra-Montañez, M.; Cervantes-Torres, J.B.; Cerbón, M.; Papy-Garcia, D.; Zenteno, E.; Sánchez-Betancourt, J.I. Heparan Sulfate and Sialic Acid in Viral Attachment: Two Sides of the Same Coin? Int. J. Mol. Sci. 2022, 23, 9842. https://doi.org/10.3390/ijms23179842
Ramos-Martínez IE, Ramos-Martínez E, Segura-Velázquez RÁ, Saavedra-Montañez M, Cervantes-Torres JB, Cerbón M, Papy-Garcia D, Zenteno E, Sánchez-Betancourt JI. Heparan Sulfate and Sialic Acid in Viral Attachment: Two Sides of the Same Coin? International Journal of Molecular Sciences. 2022; 23(17):9842. https://doi.org/10.3390/ijms23179842
Chicago/Turabian StyleRamos-Martínez, Ivan Emmanuel, Edgar Ramos-Martínez, René Álvaro Segura-Velázquez, Manuel Saavedra-Montañez, Jacquelynne Brenda Cervantes-Torres, Marco Cerbón, Dulce Papy-Garcia, Edgar Zenteno, and José Ivan Sánchez-Betancourt. 2022. "Heparan Sulfate and Sialic Acid in Viral Attachment: Two Sides of the Same Coin?" International Journal of Molecular Sciences 23, no. 17: 9842. https://doi.org/10.3390/ijms23179842
APA StyleRamos-Martínez, I. E., Ramos-Martínez, E., Segura-Velázquez, R. Á., Saavedra-Montañez, M., Cervantes-Torres, J. B., Cerbón, M., Papy-Garcia, D., Zenteno, E., & Sánchez-Betancourt, J. I. (2022). Heparan Sulfate and Sialic Acid in Viral Attachment: Two Sides of the Same Coin? International Journal of Molecular Sciences, 23(17), 9842. https://doi.org/10.3390/ijms23179842





