Nanoplatforms for the Delivery of Nucleic Acids into Plant Cells
Abstract
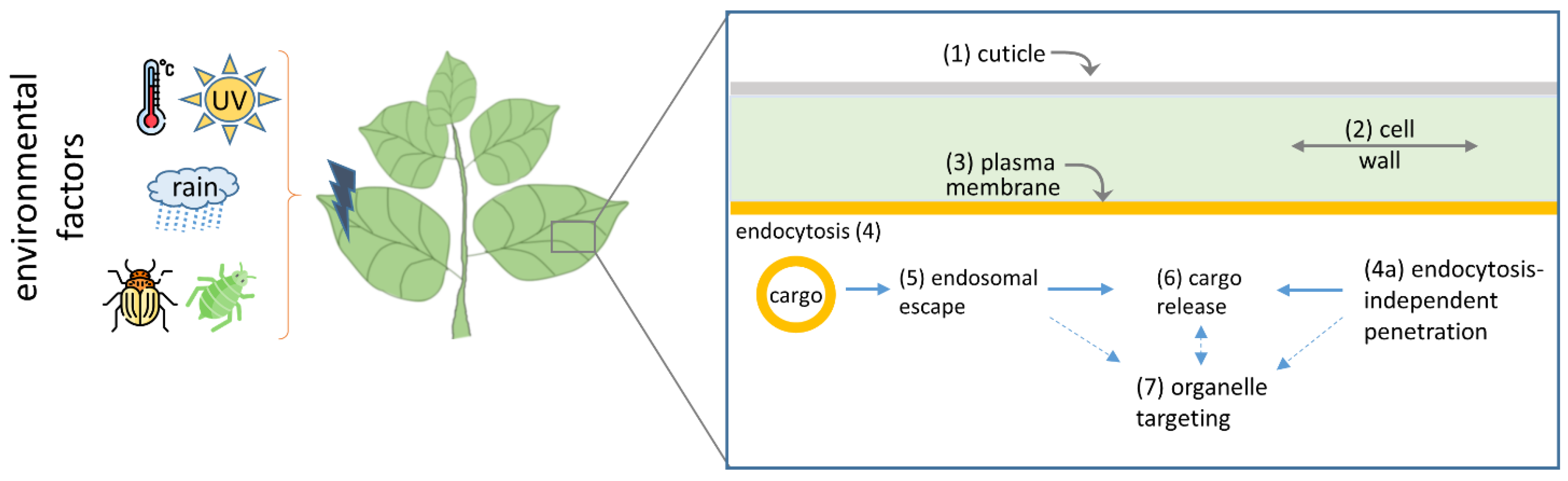
:1. Introduction
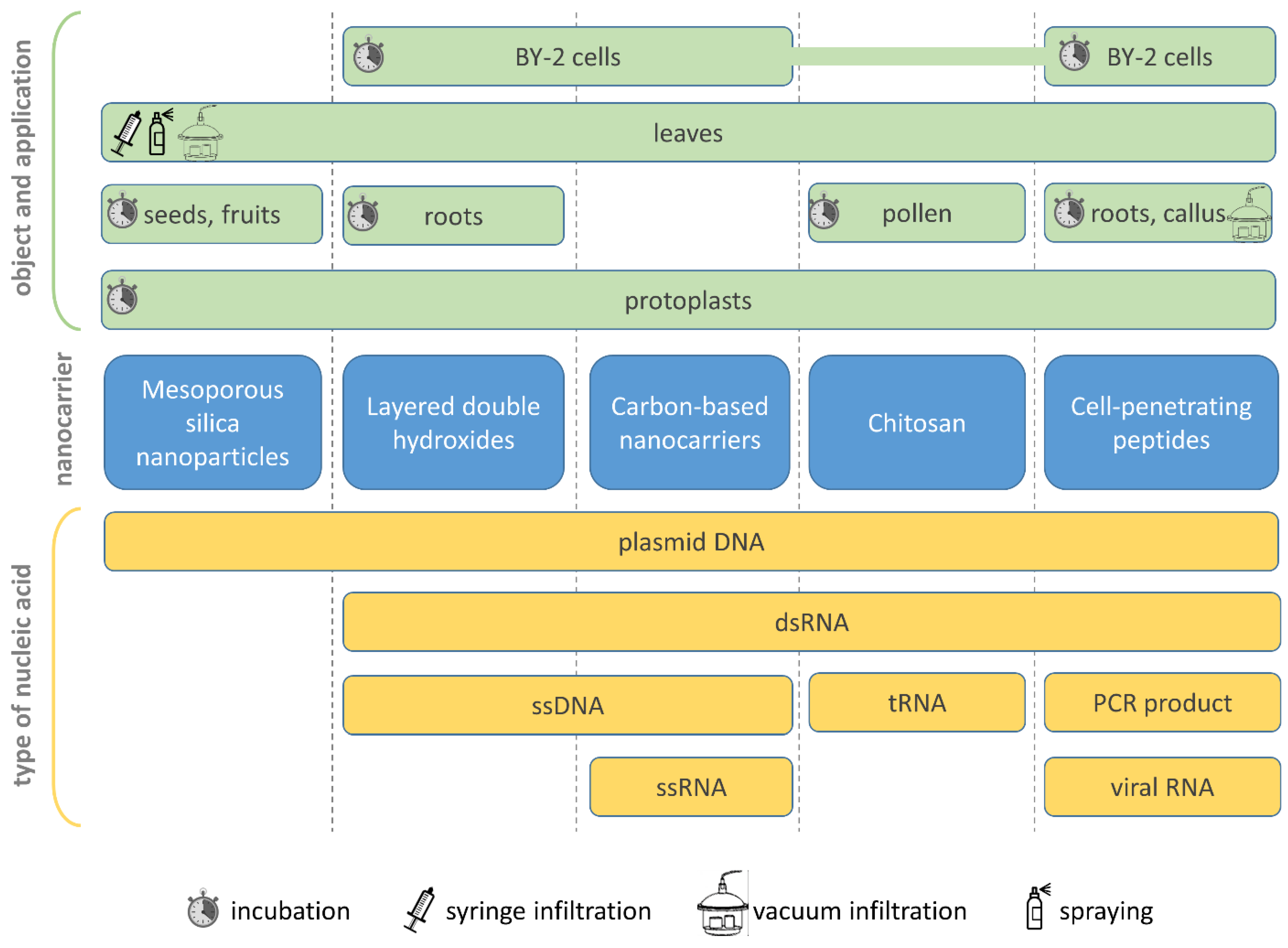
| Nanoplatform | Cargo or Label | Object (Cells, Plants, etc.) | Application Method | Analysis Technique, Functional Tests | Reference |
|---|---|---|---|---|---|
| Mesoporous silica nanoparticles (MSN) | |||||
| FITC- or RITC-doped MSNs functionalized with APTMS, TMAPS, THPMP | pDNA | N. tabacum protoplasts, A. thaliana roots | Root incubation in 1/2 MS media supplemented with MSN for 24 h or with MSN/DNA complex for 48 h | FITC- or RITC-labeled MSN intracellular uptake was confirmed using fluorescent microscopy; MSN/DNA complex internalization shown using confocal microscopy (fluorescence) and transmission electron micriscopy (TEM) (immunogold) of mCherry expressed from the delivered DNA | [39] |
| FITC- or β-oestradiol-doped MSNs, gold-capped MSNs | pDNA | N. tabacum protoplasts, Zea mays embryos | Protoplast incubation with MSNs; tissue bombardment | MSN/DNA complex penetration confirmed using fluorescent microscopy (FITC monitoring or GFP detection) or TEM (for gold-capped MSN) | [40] |
| ~40 nm APTES-functionalized MSNs | pDNA | S. lycopersicum leaves | Spraying the abaxial surface of leaves; injection into the shoot or leaves | Intracellular delivery of pDNA confirmed using RT-PCR and detection of β-glucuronidase (GUS) activity in leaves or protection against Tuta absoluta when cry1-encoding pDNA was delivered. Injection of the shoots is not effective. Leaf injection was demonstrated to be more efficient than spraying | [41] |
| FITC- or RITC-doped MSNs | No cargo | A. thaliana protoplasts, Z. mays, Triticum aestivum and Lupinus sp. roots | Root incubation in the MSN solution | Penetration of the FITC- or RITC-labeled MSNs into the cell wall and vasculature confirmed using TEM and fluorescent microscopy | [42] |
| MSN–APTES–FITC | No cargo | A. thaliana, T. aestivum seeds, Lupinus sp. | Vacuum infiltration of A. thaliana seedlings, lupin root incubation, wheat seed germination in growth medium supplemented with MSNs | Root uptake, presence in the intercellular space after vacuum infiltration and cellular uptake were confirmed using fluorescent microscopy | [43] |
| APTES-functionalized MSNs | pDNA | S. lycopersicum fruits at early ripening stage | Injection into fruits | The successful delivery of pDNA in a APTES-MSN/DNA complex into seeds of ripening fruits was confirmed by obtaining stably transformed plants after the germination of these seeds | [44] |
| Layered double hydroxides (LDHs) | |||||
| LDH-lactate nanosheets | FITC-, TRITC-conjugated LDH, ssDNA–FITC | BY-2 cells, A. thaliana seedling roots | Co-incubation | Microscopy Fluorescent dyes penetrated cells even in the presence of endocytosis inhibitors | [45] |
| 50 nm LDH nanoparticles | FITC-labeled LDH, dsRNA–Cy3, dsRNA | S. lycopersicum developing pollen | Co-incubation | Microscopy of FITC-labeled LDH, dsRNA–Cy3, functional testing, transgene (GUS) silencing | [46] |
| 40 nm LDH nanoparticles | siRNA, Cy5-labeled 21-bp DNA, siRNA | N. benthamiana, A. thaliana, T. aestivum leaves | Infiltration | Confirmed leaf cell penetration, apoplast and vasculature distribution, microscopy and functional tests, silencing of the transgene (16C line) | [47] |
| BioClay (LDH sheets) | dsRNA | N. tabacum, Vigna unguiculata leaves | Topical application, spraying | Prolonged effect: dsRNA detected on the leaf surface for up to 30 days; microscopy of Cy3-labeled dsRNA and functional tests confirmed antiviral (CMV, PMMoV) effect | [23] |
| Colloidal LDH nanosheets | dsRNA | S. lycopersicum leaves, roots, fruit; Fusarium oxysporum | Spraying on plant leaves, leaf petioles adsorption, dipping the plant roots; in vitro solution application on F. oxysporum micelium | In vitro antifungal activity of the LDH–dsRNA on mycelial growth and virulence; silencing of essential F. oxysporum genes using LDH–dsRNA; topical spraying provided protection from Fusarium crown and root rot for up to 60 days | [48] |
| LDH nanosheets | YOYO-labeled pDNA, pDNA encoding artificial microRNA | S. lycopersicum, N. benthamiana leaves, Allium cepa epidermis | Spraying plant leaves with an atomizer | Confirmed delivery of pDNA labeled with YOYO-1 dye into onion epidermis and N. benthamiana leaf cells using microscopy; systemic transport of pDNA-YOYO was observed up to 35 days after treatment in N. benthamiana and S. lycopersicum. Tomato yellow leaf curl virus (TYLCV) challenge was performed on plants pre-treated with pDNA–LDH: increased resistance of pre-treated plants was observed during 35 days | [31] |
| Carbon dots (CDs) and single-walled carbon nanotubes (SWNT) | |||||
| PEI-functionalized CDs | pDNA | O. sativa, T. aestivum, Phaseolus radiatus leaves and O. sativa roots | Wheat leaf topical application (twice a day), rice seedling root soaking, vacuum infiltration of rice calli | Expression of the pDNA-encoding genes (hygromycin resistance, GUS enzymatic assay, eGFP and mCherry fluorescence microscopy) | [49] |
| PEI-functionalized CDs | dsRNA, FITC-labeled dsRNA | Cucumis sativus seedlings | Spraying under pressure of 2.5 bar | Fluorescent microscopy of FITC-labeled dsRNA; qRT-PCR | [50] |
| PEI-functionalized CDs | siRNA (22-mer) | N. benthamiana 16C line leaves; wild-type N. benthamiana; transgenic GFP-expressing S. lycopersicum | Topical application via spraying in presence of 0.4% nonionic surfactant | Systemic silencing of (i) GFP in 16C N. benthamiana or transgenic S. lycopersicum plants and (ii) endogenous CHLH and CHLI genes encoding the H and I subunits of magnesium chelatase. Confirmed using visualization and qRT-PCR | [29] |
| PEI/PEG-functionalized CDs | dsRNA, FITC-labeled chitosan and Cy3-labeled dsRNA | N. benthamiana | Leaf infiltration and spraying, root soaking | Confocal microscopy of labeled dsRNA and nanoparticles; functional tests confirming antiviral effect against PVY (qRT-PCR and Western blotting); miRNA sequencing confirming RNA interference induction | [51] |
| SWNTs, SWNT/FITC | ssDNA, FITC-labeled DNA | N. tabacum BY-2 cells | Co-incubation | Fluorescent microscopy of SWNT/FITC and SWNT/DNA–FITC complexes | [52] |
| PEI-SWNTs | pDNA | O. sativa leaves and embryos | Infiltration | qRT-PCR analysis, GFP and YFP confocal imaging, GUS histochemical test, PDS knock-out phenotype observation | [28] |
| PEI-SWNTs | Cy3-tagged pDNA, pDNA | Wild-type and transgenic mGFP5 N. benthamiana, Eruca sativa, T. aestivum and Gossypium hirsutum leaves, E. sativa protoplasts | Leaf infiltration, protoplasts co-incubation | Transmission electron microscopy and direct near-infrared imaging, confocal microscopy, qRT-PCR analysis of pDNA expression, droplet digital PCR | [53,54] |
| SWNT-PM-CytKH9, SWNT-PM-KH9 | SWNT-PM conjugated with CytKH9 peptide labeled with DyLight488 fluorescent dye, pDNA | Seven-day-old A. thaliana seedlings, roots | Vacuum/pressure infiltration | Confocal laser scanning microscopy, confocal Raman microscopy, Western blotting, luciferase activity assay | [55] |
| Chitosan–SWNT | pDNA, Cy3-labeled DNA | E. sativa, Nasturtium officinale, N. tabacum, Spinacia oleracea plants and A. thaliana protoplasts | Co-incubation with protoplasts; whole plant leaf infiltration | Confocal microscopy, detection of near-infrared fluorescence of SWNT and YFP expressed from pDNA | [56] |
| Chitosan | |||||
| TPP crosslinked chitosan | FITC-labeled BSA and Cy3-labeled tRNA | N. benthamiana leaves | Syringe leaf infiltration | Confocal microscopy | [57] |
| TPP crosslinked chitosan | Cas9 endonuclease in complex with guide RNA | S. tuberosum apical meristem | Vacuum infiltration | Gene (coilin or phytoene desaturase) editing confirmed by sequencing and RT-PCR | [58,59,60] |
| N-2-hydroxypropyl trimethyl ammonium chloride chitosan (HACC) | pDNA, FITC-labeled HACC | N. benthamiana leaves | Syringe leaf infiltration | Confocal microscopy, functional tests on antiviral resistance | [61] |
| HACC | dsRNA, FITC-labeled chitosan and Cy3-labeled dsRNA | Laboratory experiments: A. thaliana protoplasts, N. benthamiana plants Field experiments: N. tabacum, S. lycopersicum, Capsicum annuum | Leaf infiltration and spraying, root soaking | Confocal microscopy of labeled dsRNA and nanoparticles; functional tests confirming antiviral effect against PVY (qRT-PCR and western-blot); miRNA sequencing | [51] |
| HACC | dsRNA, FITC- and Cy3-labeled dsRNA | A. thaliana protoplasts, N. tabacum plants and pollen | Leaf infiltration and spraying, pollen co-incubation, root soaking | Confocal microscopy of labeled dsRNA and nanoparticles; functional tests confirming antiviral effect against TMV (qRT-PCR and western-blot); siRNA sequencing confirming RNA interference induction | [62] |
| Cell-penetrating peptides (CPPs) | |||||
| CPP from capsid proteins of plant viruses: BMV, BYDV, TCSV, BeYDV | FlAsH dye, BMV RNA, dsRNA | A. thaliana protoplasts and seedlings; Hordeum vulgare protoplasts, roots and mesophyll | Protoplast incubation, seedling and root soaking | Protoplast and root uptake was confirmed by microscopy, Western- and Northern-blot, qRT-PCR | [63] |
| Tat and its doubled variant Tat2, transportan, pVEC | CF-labeled CPPs | Triticale mesophyll protoplasts, A. cepa epidermal cells, leaf bases and root tips of seven-day old triticale seedlings | Protoplast incubation, root soaking | Protoplast and root uptake was confirmed by microscopy and fluorimetric analysis | [64,65] |
| Tat and its doubled variant Tat2, transportan, pVEC | GUS, pDNA | T. aestivum immature embryos | Embryos were permeabilized with toluene ⁄ ethanol and incubated CPP or CPP/cargo complexes solution | Fluorescent microscopy, GUS histochemical tests | [66] |
| Arginine-rich peptides (R9, R12) | Cy3-labeled pDNA, R9-GFP fusion protein, FITC-labeled dsRNA (0.9 or 0.4 kb) | V. radiata and Glycine max roots; A. cepa and S. lycopersicum roots; N. tabacum suspension culture | Roots incubation in the solution of R9-GFP or R9/pDNA-Cy3 complex; suspension culture incubation with R12/dsRNA complexes | Fluorescent label internalization was confirmed by microscopy; R9/pDNA and R12/dsRNA complex formation confirmed by gel retardation assay; dsRNA internalization induced silencing of transgene in suspension culture | [67,68,69] |
| 55 CPP library | TAMRA-labeled CPP | BY-2 cells, leaves of N. benthamiana, A. thaliana, S. lycopersicum, poplar, and O. sativa callus | Incubation with BY-2 cells, leaves infiltration, rice callus was treated with CPP solution | TAMRA-CPP cellular uptake confirmed by confocal microscopy | [70] |
| Synthetic CPPs combining either amphipathic BP100 peptide or Tat peptide with polycationic peptide (Lys/Arg/His in different combinations) | pDNA, Cy3-labeled pDNA | N. benthamiana and A. thaliana leaves | Infiltration | Registration of protein products synthesized from plasmid pDNA–luciferase and GFP; microscopy of Cy3-labeled pDNA intracellular distribution | [27] |
| BP100CH7 (with -S-S- bonds) | pDNA, Cy3-labeled pDNA | A. thaliana leaves | Infiltration | Fluorescent microscopy, luciferase activity assay | [71] |
| BP100(KH)9, BP100CH7 (with -S-S- bonds) | Citrine yellow fluorescent protein | O. sativa callus | Vacuum infiltration | Fluorescent microscopy, western-blot | [72] |
| (KH)9-BP100 | Cy3-lableled dsRNA | A. thaliana leaves | Infiltration | Fluorescent microscopy, local silencing of YFP-encoding transgene | [26] |
| MAL-TEG-based micelles decorated with CPP (Tat, BP100 or KAibA peptide) and EDPs | pDNA, Cy3-labeled pDNA | A. thaliana seedlings | Vacuum infiltration | Fluorescent microscopy: Cy3-pDNA or GFP produced from pDNA; luciferase activity assay | [73,74] |
| BP100 conjugated with cationic peptides; CPPs with chloroplast-targeting signal | Cy3-labeled pDNA, dsRNA, siRNA | A. thaliana, G. max, S. lycopersicum, N. tabacum leaves | Topical application via spray | Fluorescent microscopy: Cy3-labeled DNA internalization, transgene (GFP, YFP) silencing by siRNA or dsRNA; GUS histochemical tests (expression from the plasmid); in chloroplasts-luciferase activity assay (expression from the plasmid), chloroplast transgene (GFP in transplastomic tobacco) silencing by siRNA | [75] |
| CPPs with chloroplast-targeting signal (AtOEP34)/mixture of CPP and CTP peptides | pDNA, siRNA, | N. tabacum, O. sativa, A. thaliana and N. benthamiana leaves, tomato fruit and roots; S. tuberosum tubers | Topical application via spray, leaf and tomato fruit infiltration, tomato roots and potato tubers vacuum infiltration | Fluorescent microscopy, luciferase assay, western-blot; chloroplast genome integration via homologous recombination confirmed by Southern-blot and reporter gene expression | [76,77,78,79] |
| CPPs with mitochondria-targeting signal | pDNA | A. thaliana leaves and seedlings; N. tabacum seedlings | Infiltration, vacuum infiltration | Fluorescent microscopy, detection of reporter genes expression: luciferase assay, western-blot; mitochondrial genome integration via homologous recombination confirmed by Southern-blot and reporter gene expression | [76,80] |
2. Mesoporous Silica Nanoparticles
3. Layered Double Hydroxides
4. Carbon-Based Nanoplatforms
4.1. Carbon Dots
4.2. Carbon Nanotubes
5. Chitosan-Based Nanocarriers
6. Cell-Penetrating Peptides
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APTES | Aminopropyl triethoxysilane |
| APTMS | 3-aminopropyltrimethoxysilane |
| BeYDV | Bean yellow dwarf virus |
| BMV | Brome mosaic virus |
| BYDV | Barley yellow dwarf virus |
| CD | Carbon dot |
| CF | Carboxyfluorescein |
| CMV | Cucumber mosaic virus |
| CPP | Cell-penetrating peptide |
| GUS | β-glucuronidase |
| EDP | Endosome-disrupting peptide |
| FITC | Fluorescein isothiocyanate |
| HACC | N-2-hydroxypropyl trimethyl ammonium chloride chitosan |
| KAibA | synthetic CPP with a lysine/α-aminoisobutyric acid/alanine repeat |
| LDH | Layered double hydroxide |
| MSN | Mesoporous silica nanoparticles |
| MTP | Mitochondria-targeting peptide |
| pDNA | Plasmid DNA |
| PEI | Polyethylenimine |
| PMMoV | Pepper mild mottle virus |
| pVEC | 18-amino-acid peptide derived from the murine vascular endothelial cadherin protein |
| PVY | Potato virus Y |
| RITC | Rhodamine B isothiocyanate |
| SWNT | Single-walled carbon nanotube |
| TAMRA | Tetramethylrhodamine |
| Tat | HIV-1 Tat basic domain peptide (RKKRRQRRR) |
| TCSV | Tobacco curly shoot virus |
| TEM | Transmission electron microscopy |
| THPMP | (3-trihydroxysilyl)propylmethylphosphonate |
| TMAPS | N-trimethoxysilylpropyl N,N,N-trimethylammonium chloride |
| TMV | Tobacco mosaic virus |
| TPP | Tripolyphosphate |
| TRITC | Tetramethylrhodamine isothiocyanate |
| TYLCV | Tomato yellow leaf curl virus |
References
- Zhao, J.; Feng, S.-S. Nanocarriers for Delivery of siRNA and Co-Delivery of siRNA and Other Therapeutic Agents. Nanomedicine 2015, 10, 2199–2228. [Google Scholar] [CrossRef] [PubMed]
- Edis, Z.; Wang, J.; Waqas, M.K.; Ijaz, M.; Ijaz, M. Nanocarriers-Mediated Drug Delivery Systems for Anticancer Agents: An Overview and Perspectives. Int. J. Nanomed. 2021, 16, 1313–1330. [Google Scholar] [CrossRef] [PubMed]
- Khizar, S.; Alrushaid, N.; Alam Khan, F.; Zine, N.; Jaffrezic-Renault, N.; Errachid, A.; Elaissari, A. Nanocarriers Based Novel and Effective Drug Delivery System. Int. J. Pharm. 2023, 632, 122570. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.; Deikman, J.; Hendrix, B.; Iandolino, A. Barriers to Efficient Foliar Uptake of dsRNA and Molecular Barriers to dsRNA Activity in Plant Cells. Front. Plant Sci. 2020, 11, 816. [Google Scholar] [CrossRef] [PubMed]
- Landry, M.P.; Mitter, N. How Nanocarriers Delivering Cargos in Plants Can Change the GMO Landscape. Nat. Nanotechnol. 2019, 14, 512–514. [Google Scholar] [CrossRef] [PubMed]
- Napoli, C.; Lemieux, C.; Jorgensen, R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in Trans. Plant Cell 1990, 2, 279–289. [Google Scholar] [CrossRef]
- Covey, S.N.; Al-Kaff, N.S.; Lángara, A.; Turner, D.S. Plants Combat Infection by Gene Silencing. Nature 1997, 385, 781–782. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and Specific Genetic Interference by Double-Stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 6669. [Google Scholar] [CrossRef]
- Dalakouras, A.; Wassenegger, M.; Dadami, E.; Ganopoulos, I.; Pappas, M.L.; Papadopoulou, K. Genetically Modified Organism-Free RNA Interference: Exogenous Application of RNA Molecules in Plants. Plant Physiol. 2020, 182, 38–50. [Google Scholar] [CrossRef]
- Hoang, B.T.L.; Fletcher, S.J.; Brosnan, C.A.; Ghodke, A.B.; Manzie, N.; Mitter, N. RNAi as a Foliar Spray: Efficiency and Challenges to Field Applications. Int. J. Mol. Sci. 2022, 23, 6639. [Google Scholar] [CrossRef]
- Koeppe, S.; Kawchuk, L.; Kalischuk, M. RNA Interference Past and Future Applications in Plants. Int. J. Mol. Sci. 2023, 24, 9755. [Google Scholar] [CrossRef] [PubMed]
- Dubrovina, A.S.; Kiselev, K.V. Exogenous RNAs for Gene Regulation and Plant Resistance. Int. J. Mol. Sci. 2019, 20, 2282. [Google Scholar] [CrossRef]
- Uslu, V.V.; Wassenegger, M. Critical View on RNA Silencing-Mediated Virus Resistance Using Exogenously Applied RNA. Curr. Opin. Virol. 2020, 42, 18–24. [Google Scholar] [CrossRef]
- Taliansky, M.; Samarskaya, V.; Zavriev, S.K.; Fesenko, I.; Kalinina, N.O.; Love, A.J. RNA-Based Technologies for Engineering Plant Virus Resistance. Plants 2021, 10, 82. [Google Scholar] [CrossRef]
- Rêgo-Machado, C.M.; Inoue-Nagata, A.K.; Nakasu, E.Y.T. Topical Application of dsRNA for Plant Virus Control: A Review. Trop. Plant Pathol. 2023, 48, 11–22. [Google Scholar] [CrossRef]
- Bachman, P.; Fischer, J.; Song, Z.; Urbanczyk-Wochniak, E.; Watson, G. Environmental Fate and Dissipation of Applied dsRNA in Soil, Aquatic Systems, and Plants. Front. Plant Sci. 2020, 11, 21. [Google Scholar] [CrossRef]
- SCHREIBER, L. Polar Paths of Diffusion across Plant Cuticles: New Evidence for an Old Hypothesis. Ann. Bot. 2005, 95, 1069–1073. [Google Scholar] [CrossRef]
- Yeats, T.H.; Rose, J.K.C. The Formation and Function of Plant Cuticles1. Plant Physiol. 2013, 163, 5–20. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, Y.; Zhang, L.; Zhou, Y. The Plant Cell Wall: Biosynthesis, Construction, and Functions. J. Integr. Plant Biol. 2021, 63, 251–272. [Google Scholar] [CrossRef]
- Flexas, J.; Clemente-Moreno, M.J.; Bota, J.; Brodribb, T.J.; Gago, J.; Mizokami, Y.; Nadal, M.; Perera-Castro, A.V.; Roig-Oliver, M.; Sugiura, D.; et al. Cell Wall Thickness and Composition Are Involved in Photosynthetic Limitation. J. Exp. Bot. 2021, 72, 3971–3986. [Google Scholar] [CrossRef]
- Carpita, N.; Sabularse, D.; Montezinos, D.; Delmer, D.P. Determination of the Pore Size of Cell Walls of Living Plant Cells. Science 1979, 205, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, T.R.; Lenka, S.K.; Arya, S.S.; Bansal, K.C. A Short History and Perspectives on Plant Genetic Transformation. Methods Mol. Biol. 2020, 2124, 39–68. [Google Scholar] [CrossRef] [PubMed]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G.; Taochy, C.; Fletcher, S.J.; Carroll, B.J.; Lu, G.Q.M.; Xu, Z.P. Clay Nanosheets for Topical Delivery of RNAi for Sustained Protection against Plant Viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.; Cerana, R. Recent Advances of Chitosan Applications in Plants. Polymers 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Fu, J.; Ma, J.; Wang, X.; Gao, C.; Zhuang, C.; Wan, J.; Jiang, L. Isolation, Culture, and Transient Transformation of Plant Protoplasts. Curr. Protoc. Cell Biol. 2014, 63, 2.8.1–2.8.17. [Google Scholar] [CrossRef]
- Numata, K.; Ohtani, M.; Yoshizumi, T.; Demura, T.; Kodama, Y. Local Gene Silencing in Plants via Synthetic dsRNA and Carrier Peptide. Plant Biotechnol. J. 2014, 12, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanan, M.; Kodama, Y.; Yoshizumi, T.; Sudesh, K.; Numata, K. Rapid and Efficient Gene Delivery into Plant Cells Using Designed Peptide Carriers. Biomacromolecules 2013, 14, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, T.; Tsakirpaloglou, N.; Septiningsih, E.M.; Thomson, M.J. Carbon Nanotube-Mediated Plasmid DNA Delivery in Rice Leaves and Seeds. Int. J. Mol. Sci. 2022, 23, 4081. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Hendrix, B.; Hoffer, P.; Sanders, R.A.; Zheng, W. Carbon Dots for Efficient Small Interfering RNA Delivery and Gene Silencing in Plants. Plant Physiol. 2020, 184, 647–657. [Google Scholar] [CrossRef]
- Song, L.; Wang, J.; Jia, H.; Kamran, A.; Qin, Y.; Liu, Y.; Hao, K.; Han, F.; Zhang, C.; Li, B.; et al. Identification and Functional Characterization of NbMLP28, a Novel MLP-like Protein 28 Enhancing Potato Virus Y Resistance in Nicotiana Benthamiana. BMC Microbiol. 2020, 20, 55. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Xu, K.; Li, D.; Hu, H.; Zhou, F.; Song, P.; Yu, Y.; Wei, Q.; Liu, Q.; et al. Clay Nanosheet-Mediated Delivery of Recombinant Plasmids Expressing Artificial miRNAs via Leaf Spray to Prevent Infection by Plant DNA Viruses. Hortic. Res. 2020, 7, 179. [Google Scholar] [CrossRef]
- Bhat, S.; Folimonova, S.Y.; Cole, A.B.; Ballard, K.D.; Lei, Z.; Watson, B.S.; Sumner, L.W.; Nelson, R.S. Influence of Host Chloroplast Proteins on Tobacco Mosaic Virus Accumulation and Intercellular Movement. Plant Physiol. 2013, 161, 134–147. [Google Scholar] [CrossRef]
- Hashimoto, M.; Neriya, Y.; Yamaji, Y.; Namba, S. Recessive Resistance to Plant Viruses: Potential Resistance Genes Beyond Translation Initiation Factors. Front. Microbiol. 2016, 7, 1695. [Google Scholar] [CrossRef]
- Ershova, N.; Sheshukova, E.; Kamarova, K.; Arifulin, E.; Tashlitsky, V.; Serebryakova, M.; Komarova, T. Nicotiana Benthamiana Kunitz Peptidase Inhibitor-like Protein Involved in Chloroplast-to-Nucleus Regulatory Pathway in Plant-Virus Interaction. Front. Plant Sci. 2022, 13, 1041867. [Google Scholar] [CrossRef]
- Kamarova, K.A.; Ershova, N.M.; Sheshukova, E.V.; Arifulin, E.A.; Ovsiannikova, N.L.; Antimonova, A.A.; Kudriashov, A.A.; Komarova, T.V. Nicotiana Benthamiana Class 1 Reversibly Glycosylated Polypeptides Suppress Tobacco Mosaic Virus Infection. Int. J. Mol. Sci. 2023, 24, 12843. [Google Scholar] [CrossRef]
- Shaw, J.; Love, A.J.; Makarova, S.S.; Kalinina, N.O.; Harrison, B.D.; Taliansky, M.E. Coilin, the Signature Protein of Cajal Bodies, Differentially Modulates the Interactions of Plants with Viruses in Widely Different Taxa. Nucleus 2014, 5, 85–94. [Google Scholar] [CrossRef]
- Garcia-Ruiz, H. Susceptibility Genes to Plant Viruses. Viruses 2018, 10, 484. [Google Scholar] [CrossRef]
- Ershova, N.; Kamarova, K.; Sheshukova, E.; Antimonova, A.; Komarova, T. A Novel Cellular Factor of Nicotiana Benthamiana Susceptibility to Tobamovirus Infection. Front. Plant Sci. 2023, 14, 1224958. [Google Scholar] [CrossRef]
- Chang, F.-P.; Kuang, L.-Y.; Huang, C.-A.; Jane, W.-N.; Hung, Y.; Hsing, Y.-I.C.; Mou, C.-Y. A Simple Plant Gene Delivery System Using Mesoporous Silica Nanoparticles as Carriers. J. Mater. Chem. B 2013, 1, 5279–5287. [Google Scholar] [CrossRef]
- Torney, F.; Trewyn, B.G.; Lin, V.S.-Y.; Wang, K. Mesoporous Silica Nanoparticles Deliver DNA and Chemicals into Plants. Nat. Nanotechnol. 2007, 2, 295–300. [Google Scholar] [CrossRef]
- Hajiahmadi, Z.; Shirzadian-Khorramabad, R.; Kazemzad, M.; Sohani, M.M. Enhancement of Tomato Resistance to Tuta Absoluta Using a New Efficient Mesoporous Silica Nanoparticle-Mediated Plant Transient Gene Expression Approach. Sci. Hortic. 2019, 243, 367–375. [Google Scholar] [CrossRef]
- Sun, D.; Hussain, H.I.; Yi, Z.; Siegele, R.; Cresswell, T.; Kong, L.; Cahill, D.M. Uptake and Cellular Distribution, in Four Plant Species, of Fluorescently Labeled Mesoporous Silica Nanoparticles. Plant Cell Rep. 2014, 33, 1389–1402. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.I.; Yi, Z.; Rookes, J.E.; Kong, L.X.; Cahill, D.M. Mesoporous Silica Nanoparticles as a Biomolecule Delivery Vehicle in Plants. J. Nanopart Res. 2013, 15, 1676. [Google Scholar] [CrossRef]
- Hajiahmadi, Z.; Shirzadian-Khorramabad, R.; Kazemzad, M.; Sohani, M.M.; Khajehali, J. A Novel, Simple, and Stable Mesoporous Silica Nanoparticle-Based Gene Transformation Approach in Solanum Lycopersicum. 3 Biotech. 2020, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Wang, J.; Wang, Q.; O’Hare, D.; Wan, Y. Layered Double Hydroxide Nanotransporter for Molecule Delivery to Intact Plant Cells. Sci. Rep. 2016, 6, 26738. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Zhang, R.; Bi, S.; Li, P.; Sun, L.; Mitter, N.; Carroll, B.J.; Xu, Z.P. Sheet-like Clay Nanoparticles Deliver RNA into Developing Pollen to Efficiently Silence a Target Gene. Plant Physiol. 2021, 187, 886–899. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Wu, M.; Zhang, R.; Bi, S.; Mann, C.W.G.; Mitter, N.; Carroll, B.J.; Xu, Z.P. Clay Nanoparticles Efficiently Deliver Small Interfering RNA to Intact Plant Leaf Cells. Plant Physiol. 2022, 190, 2187–2202. [Google Scholar] [CrossRef]
- Mosa, M.A.; Youssef, K. Topical Delivery of Host Induced RNAi Silencing by Layered Double Hydroxide Nanosheets: An Efficient Tool to Decipher Pathogenicity Gene Function of Fusarium Crown and Root Rot in Tomato. Physiol. Mol. Plant Pathol. 2021, 115, 101684. [Google Scholar] [CrossRef]
- Wang, B.; Huang, J.; Zhang, M.; Wang, Y.; Wang, H.; Ma, Y.; Zhao, X.; Wang, X.; Liu, C.; Huang, H.; et al. Carbon Dots Enable Efficient Delivery of Functional DNA in Plants. ACS Appl. Bio Mater. 2020, 3, 8857–8864. [Google Scholar] [CrossRef]
- Delgado-Martín, J.; Delgado-Olidén, A.; Velasco, L. Carbon Dots Boost dsRNA Delivery in Plants and Increase Local and Systemic siRNA Production. Int. J. Mol. Sci. 2022, 23, 5338. [Google Scholar] [CrossRef]
- Xu, X.; Yu, T.; Zhang, D.; Song, H.; Huang, K.; Wang, Y.; Shen, L.; Li, Y.; Wang, F.; Zhang, S.; et al. Evaluation of the Anti-Viral Efficacy of Three Different dsRNA Nanoparticles against Potato Virus Y Using Various Delivery Methods. Ecotoxicol. Environ. Saf. 2023, 255, 114775. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, B.; Wang, Q.; Shi, X.; Xiao, Z.; Lin, J.; Fang, X. Carbon Nanotubes as Molecular Transporters for Walled Plant Cells. Nano Lett. 2009, 9, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Demirer, G.S.; Zhang, H.; Goh, N.S.; González-Grandío, E.; Landry, M.P. Carbon Nanotube–Mediated DNA Delivery without Transgene Integration in Intact Plants. Nat. Protoc. 2019, 14, 2954–2971. [Google Scholar] [CrossRef] [PubMed]
- Demirer, G.S.; Zhang, H.; Matos, J.L.; Goh, N.S.; Cunningham, F.J.; Sung, Y.; Chang, R.; Aditham, A.J.; Chio, L.; Cho, M.-J.; et al. High Aspect Ratio Nanomaterials Enable Delivery of Functional Genetic Material without DNA Integration in Mature Plants. Nat. Nanotechnol. 2019, 14, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Law, S.S.Y.; Liou, G.; Nagai, Y.; Giménez-Dejoz, J.; Tateishi, A.; Tsuchiya, K.; Kodama, Y.; Fujigaya, T.; Numata, K. Polymer-Coated Carbon Nanotube Hybrids with Functional Peptides for Gene Delivery into Plant Mitochondria. Nat. Commun. 2022, 13, 2417. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.-Y.; Lew, T.T.S.; Sweeney, C.J.; Koman, V.B.; Wong, M.H.; Bohmert-Tatarev, K.; Snell, K.D.; Seo, J.S.; Chua, N.-H.; Strano, M.S. Chloroplast-Selective Gene Delivery and Expression in Planta Using Chitosan-Complexed Single-Walled Carbon Nanotube Carriers. Nat. Nanotechnol. 2019, 14, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Makhotenko, A.V.; Snigir, E.A.; Kalinina, N.O.; Makarov, V.V.; Taliansky, M.E. Data on a Delivery of Biomolecules into Nicothiana Benthamiana Leaves Using Different Nanoparticles. Data Brief. 2017, 16, 1034–1037. [Google Scholar] [CrossRef]
- Khromov, A.; Makhotenko, A.V.; Snigir, E.V.; Makarova, S.S.; Makarov, V.; Suprunova, T.; Miroshnichenko, D.; Kalinina, N.; Dolgov, S.; Tliansky, M.E. Delivery of CRISPR/Cas9 Ribonucleoprotein Complex to Apical Meristem Cells for DNA-Free Editing of Potato Solanum Tuberosum Genome. Biotekhnologiya 2018, 34, 51–58. [Google Scholar] [CrossRef]
- Khromov, A.V.; Makhotenko, A.V.; Makarova, S.S.; Suprunova, T.P.; Kalinina, N.O.; Taliansky, M.E. Delivery of CRISPR/Cas9 Ribonucleoprotein Complex into Plant Apical Meristem Cells Leads to Large Deletions in an Editing Gene. Russ. J. Bioorg. Chem. 2020, 46, 1242–1249. [Google Scholar] [CrossRef]
- Makhotenko, A.V.; Khromov, A.V.; Snigir, E.A.; Makarova, S.S.; Makarov, V.V.; Suprunova, T.P.; Kalinina, N.O.; Taliansky, M.E. Functional Analysis of Coilin in Virus Resistance and Stress Tolerance of Potato Solanum Tuberosum Using CRISPR-Cas9 Editing. Dokl. Biochem. Biophys. 2019, 484, 88–91. [Google Scholar] [CrossRef]
- Zhang, D.; Song, L.; Lin, Z.; Huang, K.; Liu, C.; Wang, Y.; Liu, D.; Zhang, S.; Yang, J. HACC-Based Nanoscale Delivery of the NbMLP28 Plasmid as a Crop Protection Strategy for Viral Diseases. ACS Omega 2021, 6, 33953–33960. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jiao, Y.; Shen, L.; Li, Y.; Mei, Y.; Yang, W.; Li, C.; Cao, Y.; Chen, F.; Li, B.; et al. Nanoparticle-dsRNA Treatment of Pollen and Root Systems of Diseased Plants Effectively Reduces the Rate of Tobacco Mosaic Virus in Contemporary Seeds. ACS Appl. Mater. Interfaces 2023, 15, 29052–29063. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Droste, T.; Kao, C.C. Cell-Penetrating Peptides Derived from Viral Capsid Proteins. Mol. Plant Microbe Interact. 2011, 24, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Chugh, A.; Eudes, F. Translocation and Nuclear Accumulation of Monomer and Dimer of HIV-1 Tat Basic Domain in Triticale Mesophyll Protoplasts. Biochim. Et Biophys. Acta (BBA) Biomembr. 2007, 1768, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Chugh, A.; Eudes, F. Cellular Uptake of Cell-Penetrating Peptides pVEC and Transportan in Plants. J. Pept. Sci. 2008, 14, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Chugh, A.; Eudes, F. Study of Uptake of Cell Penetrating Peptides and Their Cargoes in Permeabilized Wheat Immature Embryos. FEBS J. 2008, 275, 2403–2414. [Google Scholar] [CrossRef] [PubMed]
- Unnamalai, N.; Kang, B.G.; Lee, W.S. Cationic Oligopeptide-Mediated Delivery of dsRNA for Post-Transcriptional Gene Silencing in Plant Cells. FEBS Lett. 2004, 566, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Chou, J.-C.; Lee, H.-J. Cellular Internalization of Fluorescent Proteins via Arginine-Rich Intracellular Delivery Peptide in Plant Cells. Plant Cell Physiol. 2005, 46, 482–488. [Google Scholar] [CrossRef]
- Chen, C.-P.; Chou, J.-C.; Liu, B.R.; Chang, M.; Lee, H.-J. Transfection and Expression of Plasmid DNA in Plant Cells by an Arginine-Rich Intracellular Delivery Peptide without Protoplast Preparation. FEBS Lett. 2007, 581, 1891–1897. [Google Scholar] [CrossRef]
- Numata, K.; Horii, Y.; Oikawa, K.; Miyagi, Y.; Demura, T.; Ohtani, M. Library Screening of Cell-Penetrating Peptide for BY-2 Cells, Leaves of Arabidopsis, Tobacco, Tomato, Poplar, and Rice Callus. Sci. Rep. 2018, 8, 10966. [Google Scholar] [CrossRef]
- Chuah, J.-A.; Numata, K. Stimulus-Responsive Peptide for Effective Delivery and Release of DNA in Plants. Biomacromolecules 2018, 19, 1154–1163. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Itami, J.; Oikawa, K.; Motoda, Y.; Kigawa, T.; Numata, K. Native Protein Delivery into Rice Callus Using Ionic Complexes of Protein and Cell-Penetrating Peptides. PLoS ONE 2019, 14, e0214033. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Tsuchiya, K.; Numata, K. Block Copolymer/Plasmid DNA Micelles Postmodified with Functional Peptides via Thiol–Maleimide Conjugation for Efficient Gene Delivery into Plants. Biomacromolecules 2019, 20, 653–661. [Google Scholar] [CrossRef]
- Miyamoto, T.; Tsuchiya, K.; Numata, K. Endosome-Escaping Micelle Complexes Dually Equipped with Cell-Penetrating and Endosome-Disrupting Peptides for Efficient DNA Delivery into Intact Plants. Nanoscale 2021, 13, 5679–5692. [Google Scholar] [CrossRef]
- Thagun, C.; Horii, Y.; Mori, M.; Fujita, S.; Ohtani, M.; Tsuchiya, K.; Kodama, Y.; Odahara, M.; Numata, K. Non-Transgenic Gene Modulation via Spray Delivery of Nucleic Acid/Peptide Complexes into Plant Nuclei and Chloroplasts. ACS Nano 2022, 16, 3506–3521. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, T.; Oikawa, K.; Chuah, J.-A.; Kodama, Y.; Numata, K. Selective Gene Delivery for Integrating Exogenous DNA into Plastid and Mitochondrial Genomes Using Peptide–DNA Complexes. Biomacromolecules 2018, 19, 1582–1591. [Google Scholar] [CrossRef]
- Thagun, C.; Chuah, J.-A.; Numata, K. Targeted Gene Delivery into Various Plastids Mediated by Clustered Cell-Penetrating and Chloroplast-Targeting Peptides. Adv. Sci. 2019, 6, 1902064. [Google Scholar] [CrossRef]
- Oikawa, K.; Tateishi, A.; Odahara, M.; Kodama, Y.; Numata, K. Imaging of the Entry Pathway of a Cell-Penetrating Peptide–DNA Complex From the Extracellular Space to Chloroplast Nucleoids Across Multiple Membranes in Arabidopsis Leaves. Front. Plant Sci. 2021, 12, 759871. [Google Scholar] [CrossRef]
- Odahara, M.; Horii, Y.; Itami, J.; Watanabe, K.; Numata, K. Functional Peptide-Mediated Plastid Transformation in Tobacco, Rice, and Kenaf. Front. Plant Sci. 2022, 13, 989310. [Google Scholar] [CrossRef]
- Chuah, J.-A.; Yoshizumi, T.; Kodama, Y.; Numata, K. Gene Introduction into the Mitochondria of Arabidopsis Thaliana via Peptide-Based Carriers. Sci. Rep. 2015, 5, 7751. [Google Scholar] [CrossRef]
- Fang, I.-J.; Trewyn, B.G. Chapter Three—Application of Mesoporous Silica Nanoparticles in Intracellular Delivery of Molecules and Proteins. In Methods in Enzymology; Düzgüneş, N., Ed.; Nanomedicine; Academic Press: Cambridge, MA, USA, 2012; Volume 508, pp. 41–59. [Google Scholar]
- Niculescu, V.-C. Mesoporous Silica Nanoparticles for Bio-Applications. Front. Mater. 2020, 7, 36. [Google Scholar] [CrossRef]
- Slowing, I.I.; Vivero-Escoto, J.L.; Trewyn, B.G.; Lin, V.S.-Y. Mesoporous Silica Nanoparticles: Structural Design and Applications. J. Mater. Chem. 2010, 20, 7924–7937. [Google Scholar] [CrossRef]
- Cha, W.; Fan, R.; Miao, Y.; Zhou, Y.; Qin, C.; Shan, X.; Wan, X.; Li, J. Mesoporous Silica Nanoparticles as Carriers for Intracellular Delivery of Nucleic Acids and Subsequent Therapeutic Applications. Molecules 2017, 22, 782. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, Q.R.; Zhang, J.; Xia, W.; Gu, H. The Packaging of siRNA within the Mesoporous Structure of Silica Nanoparticles. Biomaterials 2011, 32, 9546–9556. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.R.; Baeza, A.; Vallet-Regí, M. Recent Applications of the Combination of Mesoporous Silica Nanoparticles with Nucleic Acids: Development of Bioresponsive Devices, Carriers and Sensors. Biomater. Sci. 2017, 5, 353–377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xiang, T.; Li, P.; Bao, L. Transgenic Plants of Petunia Hybrida Harboring the CYP2E1 Gene Efficiently Remove Benzene and Toluene Pollutants and Improve Resistance to Formaldehyde. Genet. Mol. Biol. 2011, 34, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Buchman, J.T.; Elmer, W.H.; Ma, C.; Landy, K.M.; White, J.C.; Haynes, C.L. Chitosan-Coated Mesoporous Silica Nanoparticle Treatment of Citrullus Lanatus (Watermelon): Enhanced Fungal Disease Suppression and Modulated Expression of Stress-Related Genes. ACS Sustain. Chem. Eng. 2019, 7, 19649–19659. [Google Scholar] [CrossRef]
- Nair, R.; Poulose, A.C.; Nagaoka, Y.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Uptake of FITC Labeled Silica Nanoparticles and Quantum Dots by Rice Seedlings: Effects on Seed Germination and Their Potential as Biolabels for Plants. J. Fluoresc. 2011, 21, 2057–2068. [Google Scholar] [CrossRef]
- Martin-Ortigosa, S.; Valenstein, J.S.; Lin, V.S.-Y.; Trewyn, B.G.; Wang, K. Gold Functionalized Mesoporous Silica Nanoparticle Mediated Protein and DNA Codelivery to Plant Cells Via the Biolistic Method. Adv. Funct. Mater. 2012, 22, 3576–3582. [Google Scholar] [CrossRef]
- Martin-Ortigosa, S.; Peterson, D.J.; Valenstein, J.S.; Lin, V.S.-Y.; Trewyn, B.G.; Lyznik, L.A.; Wang, K. Mesoporous Silica Nanoparticle-Mediated Intracellular Cre Protein Delivery for Maize Genome Editing via loxP Site Excision. Plant Physiol. 2014, 164, 537–547. [Google Scholar] [CrossRef]
- Fu, Y.; Li, L.; Wang, H.; Jiang, Y.; Liu, H.; Cui, X.; Wang, P.; Lü, C. Silica Nanoparticles-Mediated Stable Genetic Transformation in Nicotiana Tabacum. Chem. Res. Chin. Univ. 2015, 31, 976–981. [Google Scholar] [CrossRef]
- Mishra, G.; Dash, B.; Pandey, S. Layered Double Hydroxides: A Brief Review from Fundamentals to Application as Evolving Biomaterials. Appl. Clay Sci. 2018, 153, 172–186. [Google Scholar] [CrossRef]
- Verma, S.K.; Das, A.K.; Gantait, S.; Kumar, V.; Gurel, E. Applications of Carbon Nanomaterials in the Plant System: A Perspective View on the Pros and Cons. Sci. Total Environ. 2019, 667, 485–499. [Google Scholar] [CrossRef]
- Pierrat, P.; Wang, R.; Kereselidze, D.; Lux, M.; Didier, P.; Kichler, A.; Pons, F.; Lebeau, L. Efficient in Vitro and in Vivo Pulmonary Delivery of Nucleic Acid by Carbon Dot-Based Nanocarriers. Biomaterials 2015, 51, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Choi, Y.; Park, G.; Won, C.; Park, Y.-J.; Lee, Y.; Kim, B.-S.; Min, D.-H. Highly Efficient Gene Silencing and Bioimaging Based on Fluorescent Carbon Dots in Vitro and in Vivo. Nano Res. 2017, 10, 503–519. [Google Scholar] [CrossRef]
- Tripathi, S.; Sarkar, S. Influence of Water Soluble Carbon Dots on the Growth of Wheat Plant. Appl. Nanosci. 2015, 5, 609–616. [Google Scholar] [CrossRef]
- Chen, J.; Dou, R.; Yang, Z.; Wang, X.; Mao, C.; Gao, X.; Wang, L. The Effect and Fate of Water-Soluble Carbon Nanodots in Maize (Zea Mays L.). Nanotoxicology 2016, 10, 818–828. [Google Scholar] [CrossRef]
- Qian, K.; Guo, H.; Chen, G.; Ma, C.; Xing, B. Distribution of Different Surface Modified Carbon Dots in Pumpkin Seedlings. Sci. Rep. 2018, 8, 7991. [Google Scholar] [CrossRef]
- Santana, I.; Wu, H.; Hu, P.; Giraldo, J.P. Targeted Delivery of Nanomaterials with Chemical Cargoes in Plants Enabled by a Biorecognition Motif. Nat. Commun. 2020, 11, 2045. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon Dots: A New Type of Carbon-Based Nanomaterial with Wide Applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Li, J.; Gong, X. The Emerging Development of Multicolor Carbon Dots. Small 2022, 18, e2205099. [Google Scholar] [CrossRef] [PubMed]
- Giordano, M.G.; Seganti, G.; Bartoli, M.; Tagliaferro, A. An Overview on Carbon Quantum Dots Optical and Chemical Features. Molecules 2023, 28, 2772. [Google Scholar] [CrossRef] [PubMed]
- Sobhanan, J.; Rival, J.V.; Anas, A.; Sidharth Shibu, E.; Takano, Y.; Biju, V. Luminescent Quantum Dots: Synthesis, Optical Properties, Bioimaging and Toxicity. Adv. Drug Deliv. Rev. 2023, 197, 114830. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, C.; Shen, G.; Liu, H.; Fu, H.; Cui, D. Fluorescent Carbon Dots as an Efficient siRNA Nanocarrier for Its Interference Therapy in Gastric Cancer Cells. J. Nanobiotechnol. 2014, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zheng, Y.; Zhang, H.; Liu, Z.; Su, W.; Chen, S.; Liu, Y.; Zhuang, J.; Lei, B. Phytotoxicity, Uptake, and Translocation of Fluorescent Carbon Dots in Mung Bean Plants. ACS Appl. Mater. Interfaces 2016, 8, 19939–19945. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, M.; Song, Y.; Li, H.; Huang, H.; Shao, M.; Liu, Y.; Kang, Z. Carbon Dots Promote the Growth and Photosynthesis of Mung Bean Sprouts. Carbon 2018, 136, 94–102. [Google Scholar] [CrossRef]
- Zheng, Y.; Xie, G.; Zhang, X.; Chen, Z.; Cai, Y.; Yu, W.; Liu, H.; Shan, J.; Li, R.; Liu, Y.; et al. Bioimaging Application and Growth-Promoting Behavior of Carbon Dots from Pollen on Hydroponically Cultivated Rome Lettuce. ACS Omega 2017, 2, 3958–3965. [Google Scholar] [CrossRef]
- Sai, L.; Liu, S.; Qian, X.; Yu, Y.; Xu, X. Nontoxic Fluorescent Carbon Nanodot Serving as a Light Conversion Material in Plant for UV Light Utilization. Colloids Surf. B Biointerfaces 2018, 169, 422–428. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Dadashzadeh, A.; Moghassemi, S.; Ashrafizadeh, M.; Dehshahri, A.; Pardakhty, A.; Sassan, H.; Sohrevardi, S.-M.; Mandegary, A. Shedding Light on Gene Therapy: Carbon Dots for the Minimally Invasive Image-Guided Delivery of Plasmids and Noncoding RNAs—A Review. J. Adv. Res. 2019, 18, 81–93. [Google Scholar] [CrossRef]
- Kam, N.W.S.; Liu, Z.; Dai, H. Carbon Nanotubes as Intracellular Transporters for Proteins and DNA: An Investigation of the Uptake Mechanism and Pathway. Angew. Chem. Int. Ed. 2006, 45, 577–581. [Google Scholar] [CrossRef]
- Liu, Z.; Robinson, J.T.; Tabakman, S.M.; Yang, K.; Dai, H. Carbon Materials for Drug Delivery & Cancer Therapy. Mater. Today 2011, 14, 316–323. [Google Scholar] [CrossRef]
- Zaytseva, O.; Neumann, G. Carbon Nanomaterials: Production, Impact on Plant Development, Agricultural and Environmental Applications. Chem. Biol. Technol. Agric. 2016, 3, 17. [Google Scholar] [CrossRef]
- Huang, H.; Zou, M.; Xu, X.; Liu, F.; Li, N.; Wang, X. Near-Infrared Fluorescence Spectroscopy of Single-Walled Carbon Nanotubes and Its Applications. TrAC Trends Anal. Chem. 2011, 30, 1109–1119. [Google Scholar] [CrossRef]
- Ali-Boucetta, H.; Al-Jamal, K.T.; Kostarelos, K. Cytotoxic Assessment of Carbon Nanotube Interaction with Cell Cultures. Methods Mol. Biol. 2011, 726, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Charbgoo, F.; Behmanesh, M.; Nikkhah, M. Enhanced Reduction of Single-Wall Carbon Nanotube Cytotoxicity in Vitro: Applying a Novel Method of Arginine Functionalization. Biotechnol. Appl. Biochem. 2015, 62, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Wang, H.; Yan, L.; Wang, X.; Pei, R.; Yan, T.; Zhao, Y.; Guo, X. Cytotoxicity of Carbon Nanomaterials: Single-Wall Nanotube, Multi-Wall Nanotube, and Fullerene. Environ. Sci. Technol. 2005, 39, 1378–1383. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C. Cytotoxicity of Carbon Nanotubes, Graphene, Fullerenes, and Dots. Nanomaterials 2023, 13, 1458. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, V.; Sadanandan, B.; Venkataramanaiah Raghu, A. Single Walled Carbon Nanotubes in High Concentrations Is Cytotoxic to the Human Neuronal Cell LN18. Results Chem. 2022, 4, 100484. [Google Scholar] [CrossRef]
- González-Grandío, E.; Demirer, G.S.; Jackson, C.T.; Yang, D.; Ebert, S.; Molawi, K.; Keller, H.; Landry, M.P. Carbon Nanotube Biocompatibility in Plants Is Determined by Their Surface Chemistry. J. Nanobiotechnol. 2021, 19, 431. [Google Scholar] [CrossRef]
- Velikova, V.; Petrova, N.; Kovács, L.; Petrova, A.; Koleva, D.; Tsonev, T.; Taneva, S.; Petrov, P.; Krumova, S. Single-Walled Carbon Nanotubes Modify Leaf Micromorphology, Chloroplast Ultrastructure and Photosynthetic Activity of Pea Plants. Int. J. Mol. Sci. 2021, 22, 4878. [Google Scholar] [CrossRef]
- Jackson, C.T.; Wang, J.W.; González-Grandío, E.; Goh, N.S.; Mun, J.; Krishnan, S.; Geyer, F.L.; Keller, H.; Ebert, S.; Molawi, K.; et al. Polymer-Conjugated Carbon Nanotubes for Biomolecule Loading. ACS Nano 2022, 16, 1802–1812. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-X.; Zhang, Q.-F.; Li, J.; Bi, F.-C.; Yao, N. Induction of Programmed Cell Death in Arabidopsis and Rice by Single-Wall Carbon Nanotubes. Am. J. Bot. 2010, 97, 1602–1609. [Google Scholar] [CrossRef] [PubMed]
- Demirer, G.S.; Zhang, H.; Goh, N.S.; Pinals, R.L.; Chang, R.; Landry, M.P. Carbon Nanocarriers Deliver siRNA to Intact Plant Cells for Efficient Gene Knockdown. Sci. Adv. 2020, 6, eaaz0495. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Phillips, J.A.; Liu, H.; Yang, R.; Tan, W. Carbon Nanotubes Protect DNA Strands during Cellular Delivery. ACS Nano 2008, 2, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Golestanipour, A.; Nikkhah, M.; Aalami, A.; Hosseinkhani, S. Gene Delivery to Tobacco Root Cells with Single-Walled Carbon Nanotubes and Cell-Penetrating Fusogenic Peptides. Mol. Biotechnol. 2018, 60, 863–878. [Google Scholar] [CrossRef]
- Demirer, G.S.; Landry, M.P. Efficient Transient Gene Knock-down in Tobacco Plants Using Carbon Nanocarriers. Bio. Protoc. 2021, 11, e3897. [Google Scholar] [CrossRef] [PubMed]
- Thambiliyagodage, C.; Jayanetti, M.; Mendis, A.; Ekanayake, G.; Liyanaarachchi, H.; Vigneswaran, S. Recent Advances in Chitosan-Based Applications—A Review. Materials 2023, 16, 2073. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Román-Doval, R.; Torres-Arellanes, S.P.; Tenorio-Barajas, A.Y.; Gómez-Sánchez, A.; Valencia-Lazcano, A.A. Chitosan: Properties and Its Application in Agriculture in Context of Molecular Weight. Polymers 2023, 15, 2867. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical Applications of Chitosan. Adv. Colloid. Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Bandara, S.; Du, H.; Carson, L.; Bradford, D.; Kommalapati, R. Agricultural and Biomedical Applications of Chitosan-Based Nanomaterials. Nanomaterials 2020, 10, 1903. [Google Scholar] [CrossRef] [PubMed]
- Manigandan, V.; Karthik, R.; Ramachandran, S.; Rajagopal, S. Chapter 15—Chitosan Applications in Food Industry. In Biopolymers for Food Design; Grumezescu, A.M., Holban, A.M., Eds.; Handbook of Food Bioengineering; Academic Press: Cambridge, MA, USA, 2018; pp. 469–491. ISBN 978-0-12-811449-0. [Google Scholar]
- Katas, H.; Alpar, H.O. Development and Characterisation of Chitosan Nanoparticles for siRNA Delivery. J. Control Release 2006, 115, 216–225. [Google Scholar] [CrossRef]
- Mao, S.; Sun, W.; Kissel, T. Chitosan-Based Formulations for Delivery of DNA and siRNA. Adv. Drug Deliv. Rev. 2010, 62, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Rudzinski, W.E.; Aminabhavi, T.M. Chitosan as a Carrier for Targeted Delivery of Small Interfering RNA. Int. J. Pharm. 2010, 399, 19530–19535. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Debnath, N.; Cui, Y.; Unrine, J.; Palli, S.R. Chitosan, Carbon Quantum Dot, and Silica Nanoparticle Mediated dsRNA Delivery for Gene Silencing in Aedes Aegypti: A Comparative Analysis. ACS Appl. Mater. Interfaces 2015, 7, 19530–19535. [Google Scholar] [CrossRef]
- Ramesh Kumar, D.; Saravana Kumar, P.; Gandhi, M.R.; Al-Dhabi, N.A.; Paulraj, M.G.; Ignacimuthu, S. Delivery of Chitosan/dsRNA Nanoparticles for Silencing of Wing Development Vestigial (vg) Gene in Aedes Aegypti Mosquitoes. Int. J. Biol. Macromol. 2016, 86, 89–95. [Google Scholar] [CrossRef]
- Gurusamy, D.; Mogilicherla, K.; Palli, S.R. Chitosan Nanoparticles Help Double-Stranded RNA Escape from Endosomes and Improve RNA Interference in the Fall Armyworm, Spodoptera Frugiperda. Arch. Insect Biochem. Physiol. 2020, 104, e21677. [Google Scholar] [CrossRef]
- Qiao, H.; Zhao, J.; Wang, X.; Xiao, L.; Zhu-Salzman, K.; Lei, J.; Xu, D.; Xu, G.; Tan, Y.; Hao, D. An Oral dsRNA Delivery System Based on Chitosan Induces G Protein-Coupled Receptor Kinase 2 Gene Silencing for Apolygus Lucorum Control. Pestic. Biochem. Physiol. 2023, 194, 105481. [Google Scholar] [CrossRef]
- Yan, S.; Ren, B.-Y.; Shen, J. Nanoparticle-Mediated Double-Stranded RNA Delivery System: A Promising Approach for Sustainable Pest Management. Insect Sci. 2021, 28, 21–34. [Google Scholar] [CrossRef]
- An, C.; Sun, C.; Li, N.; Huang, B.; Jiang, J.; Shen, Y.; Wang, C.; Zhao, X.; Cui, B.; Wang, C.; et al. Nanomaterials and Nanotechnology for the Delivery of Agrochemicals: Strategies towards Sustainable Agriculture. J. Nanobiotechnol. 2022, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Zhang, H.; Bao, L.; Song, Z.; Liu, C.; Jiang, Z.; Zheng, Y. NCs-Delivered Pesticides: A Promising Candidate in Smart Agriculture. Int. J. Mol. Sci. 2021, 22, 13043. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, D.; Geetha, N.; Khawar, K.M.; Jogaiah, S.; Mujtaba, M. Current Trends and Challenges in the Synthesis and Applications of Chitosan-Based Nanocomposites for Plants: A Review. Carbohydr. Polym. 2021, 261, 117904. [Google Scholar] [CrossRef] [PubMed]
- Khairy, A.M.; Tohamy, M.R.A.; Zayed, M.A.; Mahmoud, S.F.; El-Tahan, A.M.; El-Saadony, M.T.; Mesiha, P.K. Eco-Friendly Application of Nano-Chitosan for Controlling Potato and Tomato Bacterial Wilt. Saudi J. Biol. Sci. 2022, 29, 2199–2209. [Google Scholar] [CrossRef] [PubMed]
- Elagamey, E.; Abdellatef, M.A.E.; Arafat, M.Y. Proteomic Insights of Chitosan Mediated Inhibition of Fusarium Oxysporum f. Sp. Cucumerinum. J. Proteom. 2022, 260, 104560. [Google Scholar] [CrossRef] [PubMed]
- El-Morsy, E.-S.M.; Elmalahy, Y.S.; Mousa, M.M.A. Biocontrol of Fusarium Equiseti Using Chitosan Nanoparticles Combined with Trichoderma Longibrachiatum and Penicillium Polonicum. Fungal Biol. Biotechnol. 2023, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Abdelkhalek, A.; Qari, S.H.; Abu-Saied, M.A.A.-R.; Khalil, A.M.; Younes, H.A.; Nehela, Y.; Behiry, S.I. Chitosan Nanoparticles Inactivate Alfalfa Mosaic Virus Replication and Boost Innate Immunity in Nicotiana Glutinosa Plants. Plants 2021, 10, 2701. [Google Scholar] [CrossRef]
- El-Ganainy, S.M.; Soliman, A.M.; Ismail, A.M.; Sattar, M.N.; Farroh, K.Y.; Shafie, R.M. Antiviral Activity of Chitosan Nanoparticles and Chitosan Silver Nanocomposites against Alfalfa Mosaic Virus. Polymers 2023, 15, 2961. [Google Scholar] [CrossRef]
- Frankel, A.D.; Pabo, C.O. Cellular Uptake of the Tat Protein from Human Immunodeficiency Virus. Cell 1988, 55, 1189–1193. [Google Scholar] [CrossRef]
- Green, M.; Loewenstein, P.M. Autonomous Functional Domains of Chemically Synthesized Human Immunodeficiency Virus Tat Trans-Activator Protein. Cell 1988, 55, 1179–1188. [Google Scholar] [CrossRef]
- Fawell, S.; Seery, J.; Daikh, Y.; Moore, C.; Chen, L.L.; Pepinsky, B.; Barsoum, J. Tat-Mediated Delivery of Heterologous Proteins into Cells. Proc. Natl. Acad. Sci. USA 1994, 91, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Vivès, E.; Brodin, P.; Lebleu, B. A Truncated HIV-1 Tat Protein Basic Domain Rapidly Translocates through the Plasma Membrane and Accumulates in the Cell Nucleus. J. Biol. Chem. 1997, 272, 16010–16017. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.; Bhalla, S.; Usmani, S.S.; Singh, S.; Chaudhary, K.; Raghava, G.P.S.; Gautam, A. CPPsite 2.0: A Repository of Experimentally Validated Cell-Penetrating Peptides. Nucleic Acids Res. 2016, 44, D1098–D1103. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-W.; Lee, H.-J.; Tolliver, L.M.; Aronstam, R.S. Delivery of Nucleic Acids and Nanomaterials by Cell-Penetrating Peptides: Opportunities and Challenges. Biomed. Res. Int. 2015, 2015, 834079. [Google Scholar] [CrossRef]
- Durzyńska, J.; Przysiecka, Ł.; Nawrot, R.; Barylski, J.; Nowicki, G.; Warowicka, A.; Musidlak, O.; Goździcka-Józefiak, A. Viral and Other Cell-Penetrating Peptides as Vectors of Therapeutic Agents in Medicine. J. Pharmacol. Exp. Ther. 2015, 354, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Bi, Y.; Zhang, H.; Dong, S.; Teng, L.; Lee, R.J.; Yang, Z. Cell-Penetrating Peptides in Diagnosis and Treatment of Human Diseases: From Preclinical Research to Clinical Application. Front. Pharmacol. 2020, 11, 697. [Google Scholar] [CrossRef]
- Elmquist, A.; Lindgren, M.; Bartfai, T.; Langel, U. null VE-Cadherin-Derived Cell-Penetrating Peptide, pVEC, with Carrier Functions. Exp. Cell Res. 2001, 269, 237–244. [Google Scholar] [CrossRef]
- Pooga, M.; Hällbrink, M.; Zorko, M.; Langel, U. Cell Penetration by Transportan. FASEB J. 1998, 12, 67–77. [Google Scholar] [CrossRef]
- Smith, A.E.; Helenius, A. How Viruses Enter Animal Cells. Science 2004, 304, 237–242. [Google Scholar] [CrossRef]
- Mercer, J.; Helenius, A. Vaccinia Virus Uses Macropinocytosis and Apoptotic Mimicry to Enter Host Cells. Science 2008, 320, 531–535. [Google Scholar] [CrossRef]
- Nakase, I.; Hirose, H.; Tanaka, G.; Tadokoro, A.; Kobayashi, S.; Takeuchi, T.; Futaki, S. Cell-Surface Accumulation of Flock House Virus-Derived Peptide Leads to Efficient Internalization via Macropinocytosis. Mol. Ther. 2009, 17, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.K.; Motoda, Y.; Watanabe, S.; Sofiman Othman, A.; Kigawa, T.; Kodama, Y.; Numata, K. Intracellular Delivery of Proteins via Fusion Peptides in Intact Plants. PLoS ONE 2016, 11, e0154081. [Google Scholar] [CrossRef] [PubMed]
- Eggenberger, K.; Mink, C.; Wadhwani, P.; Ulrich, A.S.; Nick, P. Using the Peptide Bp100 as a Cell-Penetrating Tool for the Chemical Engineering of Actin Filaments within Living Plant Cells. ChemBioChem 2011, 12, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.R.; Chen, C.-W.; Huang, Y.-W.; Lee, H.-J. Cell-Penetrating Peptides for Use in Development of Transgenic Plants. Molecules 2023, 28, 3367. [Google Scholar] [CrossRef]
- Terada, K.; Gimenez-Dejoz, J.; Kurita, T.; Oikawa, K.; Uji, H.; Tsuchiya, K.; Numata, K. Synthetic Mitochondria-Targeting Peptides Incorporating α-Aminoisobutyric Acid with a Stable Amphiphilic Helix Conformation in Plant Cells. ACS Biomater. Sci. Eng. 2021, 7, 1475–1484. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komarova, T.; Ilina, I.; Taliansky, M.; Ershova, N. Nanoplatforms for the Delivery of Nucleic Acids into Plant Cells. Int. J. Mol. Sci. 2023, 24, 16665. https://doi.org/10.3390/ijms242316665
Komarova T, Ilina I, Taliansky M, Ershova N. Nanoplatforms for the Delivery of Nucleic Acids into Plant Cells. International Journal of Molecular Sciences. 2023; 24(23):16665. https://doi.org/10.3390/ijms242316665
Chicago/Turabian StyleKomarova, Tatiana, Irina Ilina, Michael Taliansky, and Natalia Ershova. 2023. "Nanoplatforms for the Delivery of Nucleic Acids into Plant Cells" International Journal of Molecular Sciences 24, no. 23: 16665. https://doi.org/10.3390/ijms242316665
APA StyleKomarova, T., Ilina, I., Taliansky, M., & Ershova, N. (2023). Nanoplatforms for the Delivery of Nucleic Acids into Plant Cells. International Journal of Molecular Sciences, 24(23), 16665. https://doi.org/10.3390/ijms242316665






