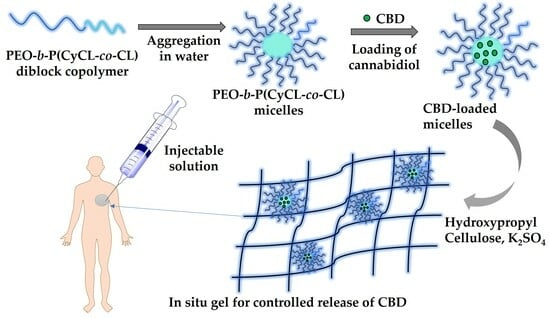
In Situ Gelling Hydroxypropyl Cellulose Formulation Comprising Cannabidiol-Loaded Block Copolymer Micelles for Sustained Drug Delivery
Abstract
:1. Introduction
2. Results and Discussion
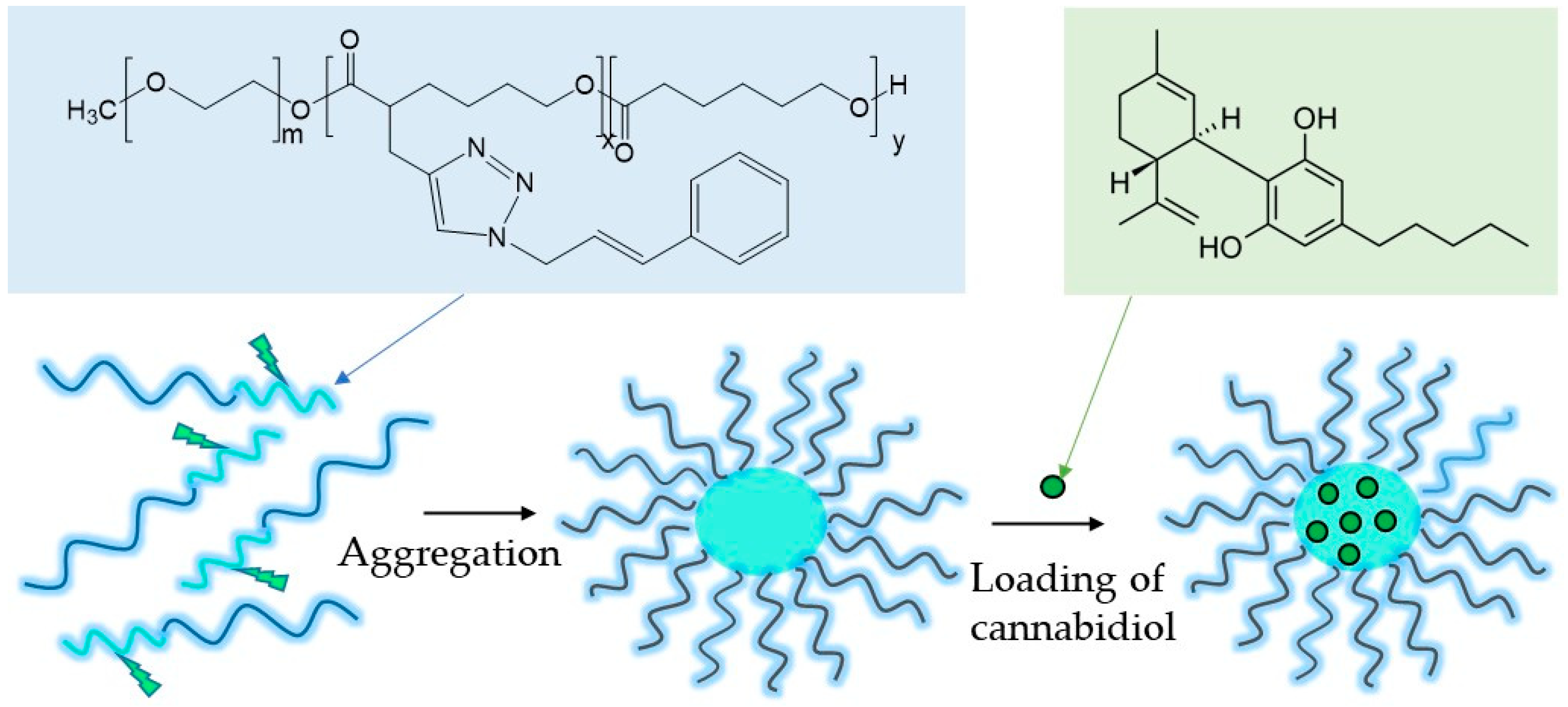
2.1. Preparation of Cannabidiol-Loaded Polymeric Micelles
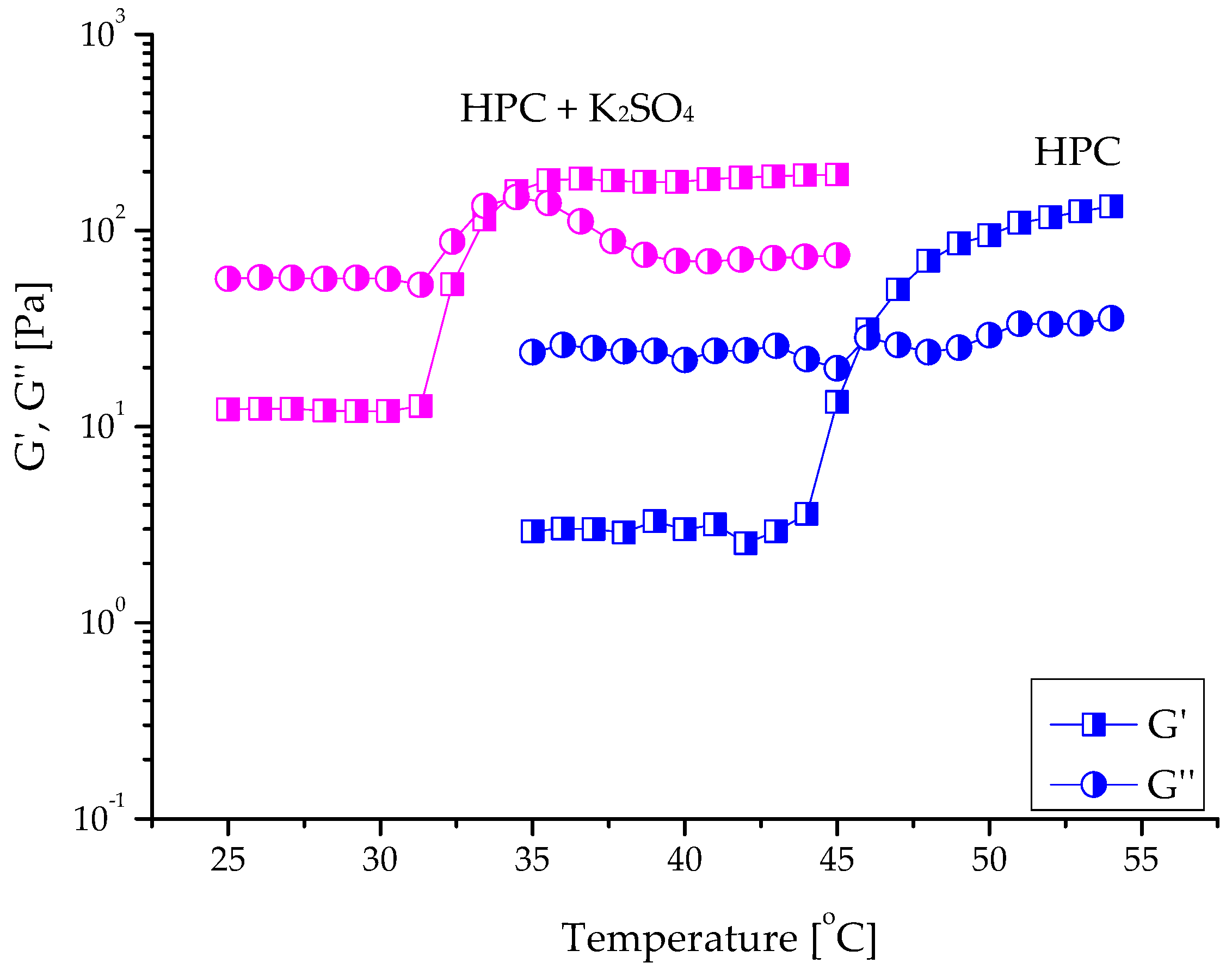
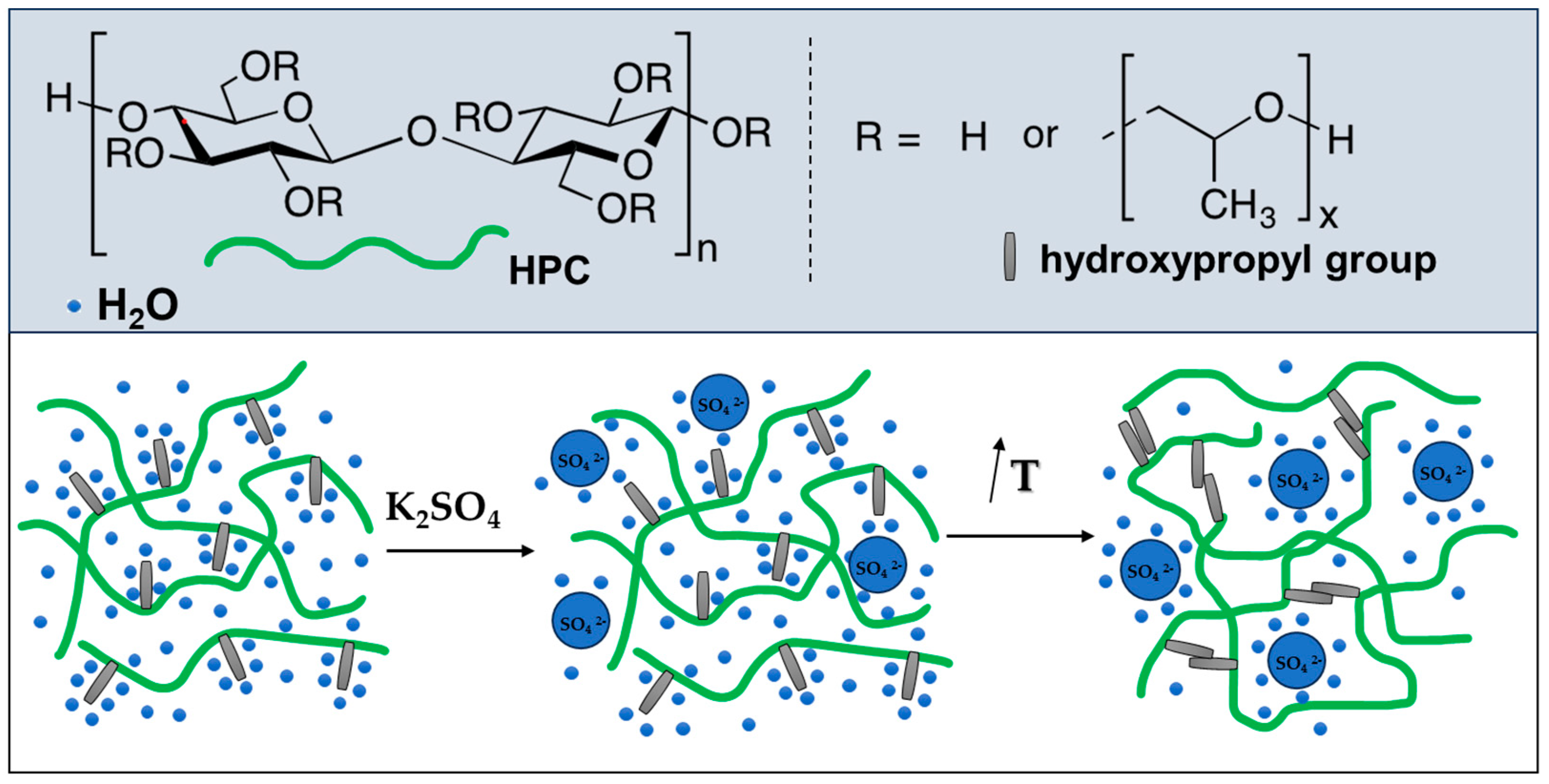
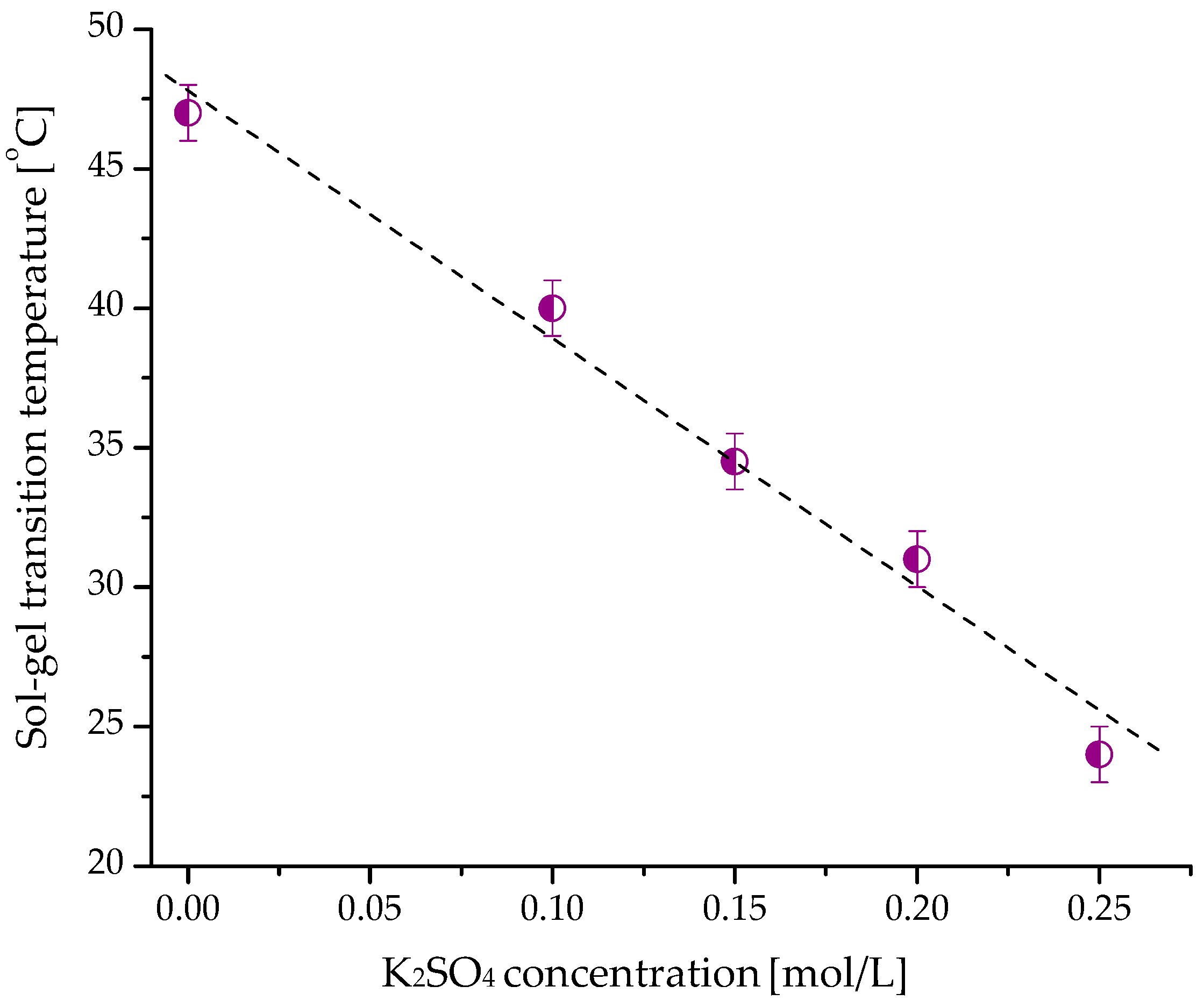
2.2. Gelation of Hydroxypropyl Cellulose in Water in the Presence of K2SO4
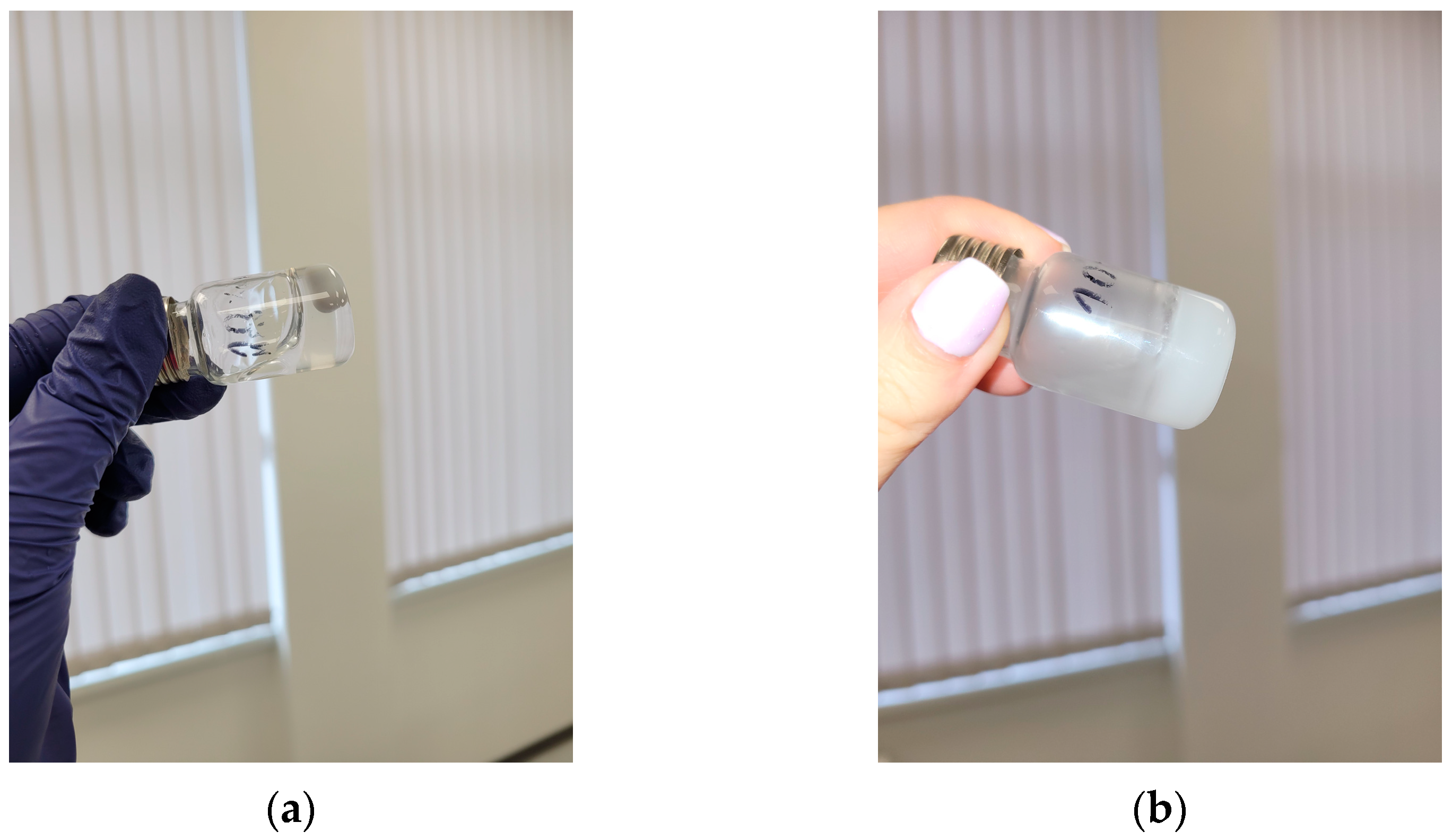
2.3. Fabrication of Injectable Nanocomposite In Situ Hydrogels Comprising Cannabidiol
2.4. Drug Release Study
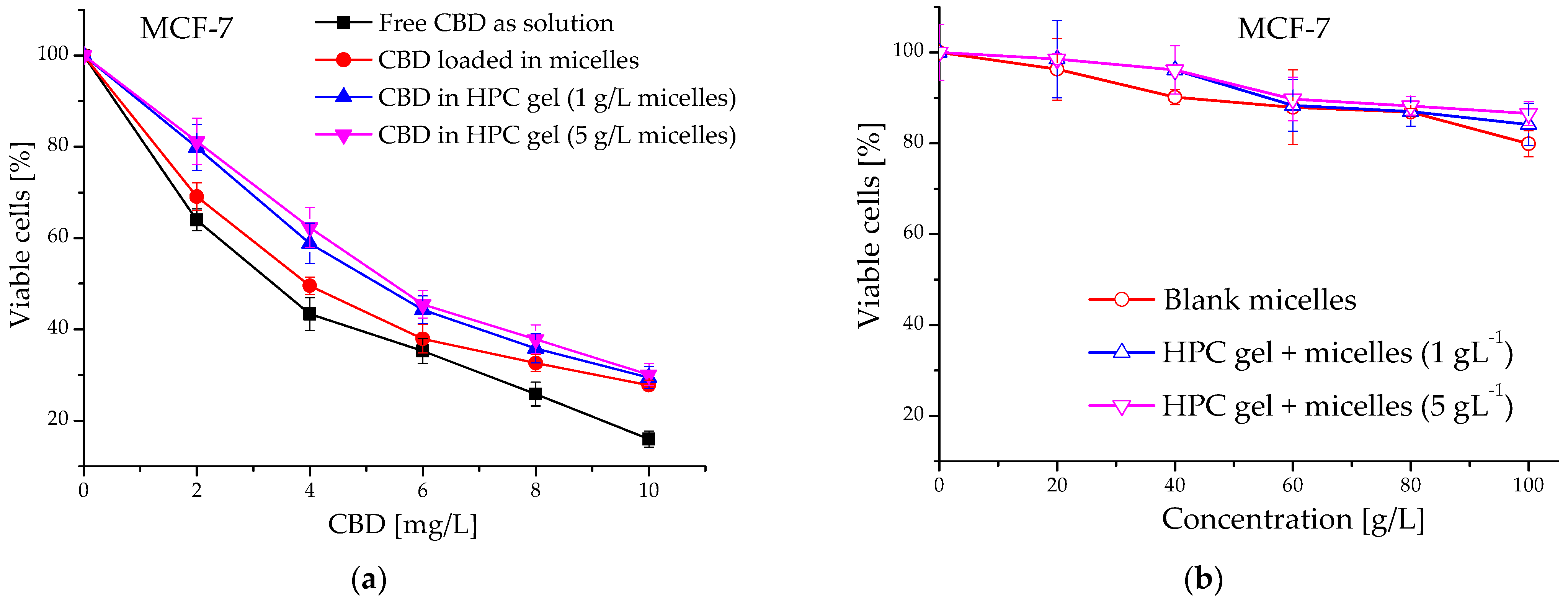
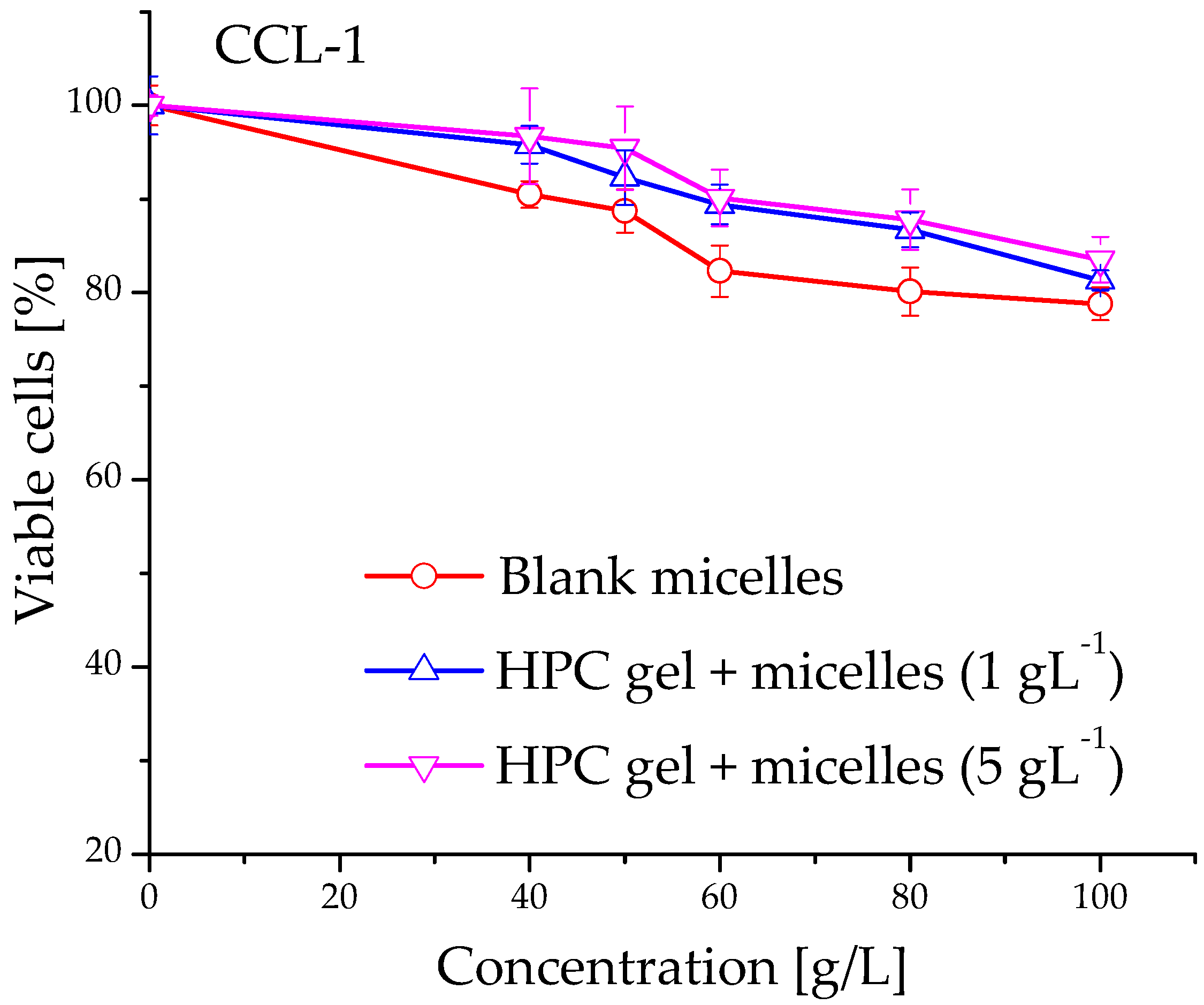
2.5. Evaluation of Cytotoxicity of Cannabidiol-Loaded In Situ Thermoresponsive Gels
3. Materials and Methods
3.1. Materials
3.2. Preparation of Block Copolymer Micelles
3.3. Loading of Cannabidiol into Micellar Carriers
3.4. Preparation of Blank HPC Hydrogels
3.5. Preparation of Nanocomposite HPC Hydrogels
3.6. Analysis
3.7. In Vitro Drug Release
3.8. Evaluation of Cytotoxicity
3.8.1. Cell Lines and Culture Conditions
3.8.2. MTT Colorimetric Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Jeong, B.; Kim, S.W.; Bae, Y.H. Thermosensitive sol-gel reversible hydrogels. Adv. Drug. Deliv. Rev. 2002, 54, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Van Tomme, S.R.; Storm, G.; Hennink, W.E. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int. J. Pharm. 2008, 355, 1–18. [Google Scholar] [CrossRef]
- Liow, S.S.; Dou, Q.; Kai, D.; Karim, A.A.; Zhang, K.; Xu, F.; Loh, X.J. Thermogels: In Situ Gelling Biomaterial. ACS Biomater. Sci. Eng. 2016, 2, 295–316. [Google Scholar] [CrossRef]
- Alonso, J.M.; Andrade del Olmo, J.; Perez Gonzalez, R.; Saez-Martinez, V. Injectable Hydrogels: From Laboratory to Industrialization. Polymers 2021, 13, 650. [Google Scholar] [CrossRef]
- Mathew, A.P.; Uthaman, S.; Cho, K.H.; Cho, C.S.; Park, I.K. Injectable hydrogels for delivering biotherapeutic molecules. Int. J. Biol. Macromol. 2018, 110, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Thambi, T.; Li, Y.; Lee, D.S. Injectable hydrogels for sustained release of therapeutic agents. J. Control. Release 2017, 267, 57–66. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, M.; Ma, Q.; Dia, X.; Wu, G. A bio-inspired fluorescent nano-injectable hydrogel as a synergistic drug delivery system. New J. Chem. 2021, 45, 3079–3087. [Google Scholar] [CrossRef]
- Patel, M.; Moon, H.J.; Jung, B.K.; Jeong, B. Microsphere-Incorporated Hybrid Thermogel for Neuronal Differentiation of Tonsil Derived Mesenchymal Stem Cells. Adv. Healthc. Mater. 2015, 4, 1565–1574. [Google Scholar] [CrossRef]
- Payyappilly, S.; Dhara, S.; Chattopadhyay, S. Thermoresponsive biodegradable PEG-PCL-PEG based injectable hydrogel for pulsatile insulin delivery. J. Biomed. Mater. Res. A 2014, 102, 1500–1509. [Google Scholar] [CrossRef]
- Zubik, K.; Singhsa, P.; Wang, Y.; Manuspiya, H.; Narain, R. Thermo-responsive poly(N-isopropylacrylamide)-cellulose nanocrystals hybrid hydrogels for wound dressing. Polymers 2017, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.D.; Ye, J.; Yang, Y.C.; Huang, Y.Y.; Xiao, M.T. Self-healing polysaccharide-based injectable hydrogels with antibacterial activity for wound healing. Carbohydr. Polym. 2022, 275, 118770. [Google Scholar] [CrossRef] [PubMed]
- Klouda, L. Thermoresponsive hydrogels in biomedical applications: A seven-year update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Csóka, G.; Gelencsér, A.; Makó, A.; Marton, S.; Zelkó, R.; Klebovich, I.; Antal, I. Potential application of Metolose in a thermoresponsive transdermal therapeutic system. Int. J. Pharm. 2007, 338, 15–20. [Google Scholar] [CrossRef]
- Miao, L.; Hu, J.; Lu, M.; Tu, Y.; Chen, X.; Li, Y.; Lin, S.; Li, F.; Hu, S. Alkynyl-functionalization of hydroxypropyl cellulose and thermoresponsive hydrogel thereof prepared with P(NIPAAm-co-HEMAPCL). Carbohydr. Polym. 2016, 137, 433–440. [Google Scholar] [CrossRef]
- Gosecki, M.; Setälä, H.; Virtanen, T.; Ryan, A.J. A facile method to control the phase behavior of hydroxypropyl cellulose. Carbohydr. Polym. 2021, 251, 117015. [Google Scholar] [CrossRef]
- Joshi, S.C. Sol-Gel Behavior of Hydroxypropyl Methylcellulose (HPMC) in Ionic Media Including Drug Release. Materials 2011, 4, 1861–1905. [Google Scholar] [CrossRef]
- Scuderi, C.; Filippis, D.D.; Iuvone, T.; Blasio, A.; Steardo, A.; Esposito, G. Cannabidiol in medicine: A review of its therapeutic potential in CNS disorders. Phytother. Res. 2009, 23, 597–602. [Google Scholar] [CrossRef]
- Seltzer, E.S.; Watters, A.K.; MacKenzie, D., Jr.; Granat, L.M.; Zhang, D. Cannabidiol (CBD) as a promising anti-cancer drug. Cancers 2020, 12, 3203. [Google Scholar] [CrossRef]
- Millar, S.A.; Maguire, R.F.; Yates, A.S.; O’Sullivan, S.E. Towards Better Delivery of Cannabidiol (CBD). Pharmaceuticals 2020, 13, 219. [Google Scholar] [CrossRef]
- Momekova, D.; Ivanov, E.; Konstantinov, S.; Ublekov, F.; Petrov, P.D. Nanocomposite cryogel carriers from 2-hydroxyethyl cellulose network and cannabidiol-loaded polymeric micelles for sustained topical delivery. Polymers 2020, 12, 1172. [Google Scholar] [CrossRef] [PubMed]
- Chountoulesi, M.; Selianitis, D.; Pispas, S.; Pippa, N. Recent Advances on PEO-PCL Block and Graft Copolymers as Nanocarriers for Drug Delivery Applications. Materials 2023, 16, 2298. [Google Scholar] [CrossRef]
- Grancharov, G.; Atanasova, M.D.; Aluani, D.; Yoncheva, K.; Tzankova, V.; Trusheva, B.; Forys, A.; Trzebicka, B.; Petrov, P.D. Functional block copolymers bearing pendant cinnamyl groups for enhanced solubilization of caffeic acid phenethyl ester. Polym. J. 2020, 52, 435–447. [Google Scholar] [CrossRef]
- Atanasova, M.-D.; Grancharov, G.; Petrov, P.D. Poly(ethylene oxide)-block-poly(α-cinnamyl-ε-caprolactone-co-ε-caprolactone) diblock copolymer nanocarriers for enhanced solubilization of caffeic acid phenethyl ester. J. Polym. Sci. 2021, 59, 251–260. [Google Scholar] [CrossRef]
- Gil, E.S.; Hudson, S.M. Stimuli-reponsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Pencheva, V.; Margaritova, E.; Borinarova, M.; Slavkova, M.; Momekova, D.; Petrov, P.D. A novel approach for fabricating nanocomposite materials by embedding stabilized core-shell micelles into polysaccharide cryogel matrix. Carbohydr. Polym. 2018, 183, 165–172. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Fernández-Carballido, A.; Simancas-Herbada, R.; Martin-Sabroso, C.; Torres-Suárez, A.I. CBD loaded microparticles as a potential formulation to improve paclitaxel and doxorubicin-based chemotherapy in breast cancer. Int. J. Pharm. 2020, 574, 118916. [Google Scholar] [CrossRef]
- D’Aloia, A.; Ceriani, M.; Tisi, R.; Stucchi, S.; Sacco, E.; Costa, B. Cannabidiol Antiproliferative Effect in Triple-Negative Breast Cancer MDA-MB-231 Cells Is Modulated by its Physical State and by IGF-1. Int. J. Mol. Sci. 2022, 23, 7145. [Google Scholar] [CrossRef]
- Nie, S.; Hsiao, W.L.; Pan, W.; Yang, Z. Thermoreversible Pluronic F127-based hydrogel containing liposomes for the controlled delivery of paclitaxel: In vitro drug release, cell cytotoxicity, and uptake studies. Int. J. Nanomed. 2011, 6, 151–166. [Google Scholar]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2017.
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
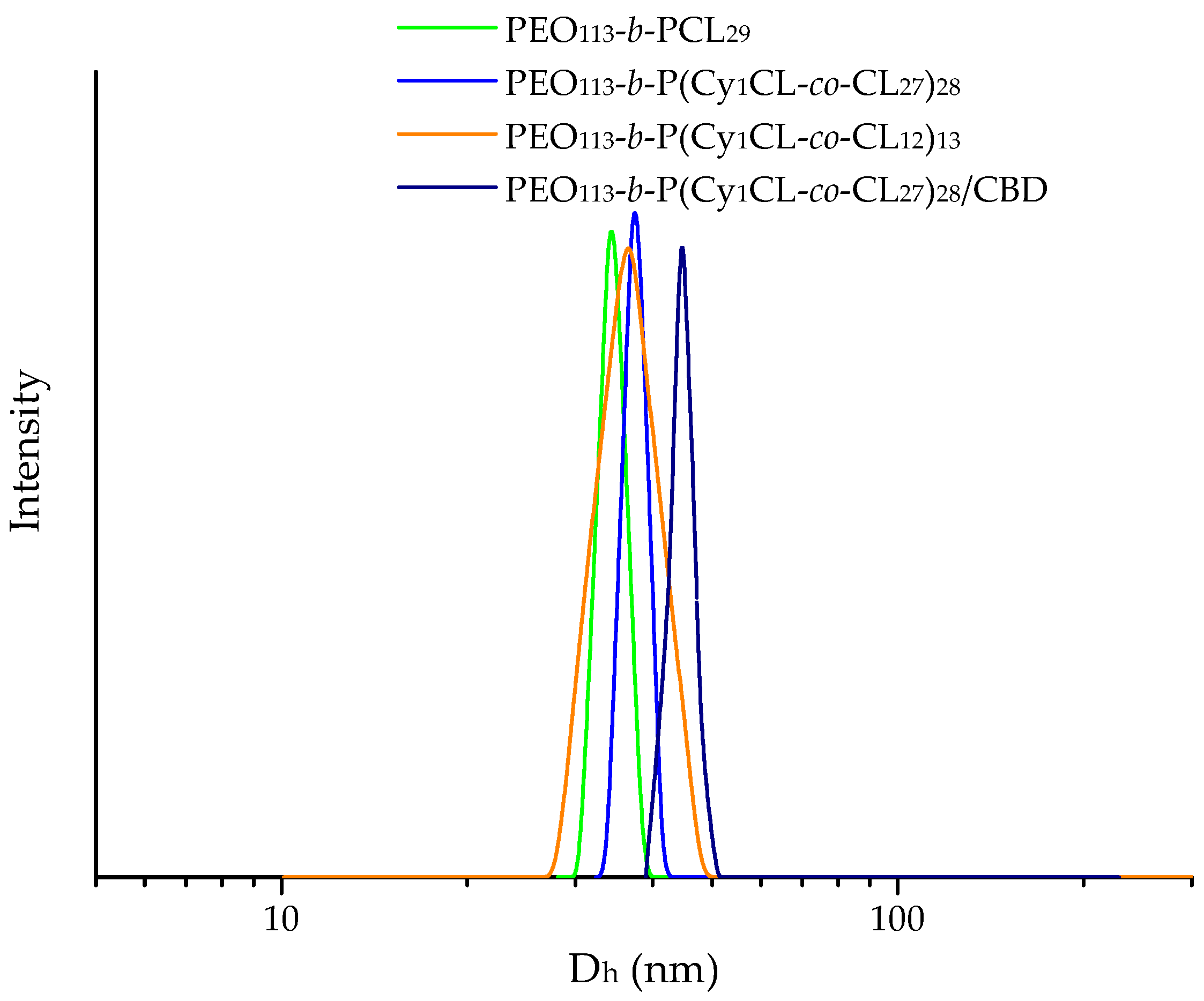


| Copolymer | Dh (nm) | ζ Potential (mV) | ||
|---|---|---|---|---|
| Blank | Loaded 1 | Blank | Loaded 1 | |
| PEO113-b-PCL29 | 34 ± 3 | 37 ± 3 | −4.7 ± 0.7 | −11.3 ± 0.7 |
| PEO113-b-P(CyCL1-co-CL27)28 | 37 ± 3 | 44 ± 4 | −7.9 ± 0.9 | −12.3 ± 0.6 |
| PEO113-b-P(CyCL1-co-CL12)13 | 36 ± 2 | 40 ± 4 | −3.9 ± 0.6 | −5.2 ± 0.4 |
| Formulation | Mass Ratio | DLC (%) | EE (%) |
|---|---|---|---|
| PEO113-b-PCL29/CBD | 10:1 | 8 ± 0.7 | 82 ± 3 |
| 7:1 | 9 ± 0.8 | 67 ± 3 | |
| 5:1 | 13 ± 0.7 | 66 ± 4 | |
| PEO113-b-P(CyCL1-co-CL27)28/CBD | 10:1 | 9 ± 0.7 | 89 ± 3 |
| 7:1 | 11 ± 0.7 | 83 ± 4 | |
| 5:1 | 16 ± 0.8 | 82 ± 3 | |
| PEO113-b-P(CyCL1-co-CL12)13/CBD | 10:1 | 7 ± 0.7 | 75 ± 3 |
| 7:1 | 10 ± 0.8 | 69 ± 3 | |
| 5:1 | 14 ± 0.8 | 68 ± 3 |
| Sample | G′sol 1 (Pa) | G″sol (Pa) | G′gel (Pa) | G″gel (Pa) | Tsol-gel (°C) |
|---|---|---|---|---|---|
| HPC 10% | 5 ± 1 | 26 ± 3 | 94 ± 4 | 29 ± 3 | 45.8 ± 0.3 |
| HPC 12% | 26 ± 2 | 76 ± 4 | 211 ± 14 | 88 ± 4 | 46.1 ± 0.4 |
| HPC 15% | 160 ± 14 | 327 ± 22 | 747 ± 32 | 270 ± 10 | 46.2 ± 0.4 |
| HPC 10% + K2SO4 | 12 ± 2 | 57 ± 3 | 180 ± 12 | 89 ± 8 | 34.2 ± 0.3 |
| HPC 12% + K2SO4 | 32 ± 2 | 81 ± 4 | 246 ± 13 | 117 ± 12 | 34.6 ± 0.3 |
| HPC 15% + K2SO4 | 167 ± 11 | 343 ± 22 | 940 ± 35 | 346 ± 15 | 33.4 ± 0.2 |
| Sample | IC50 (mg/L) | IC50 (µM) |
|---|---|---|
| CBD (as solution in ethanol) | 3.36 | 10.7 |
| PEO113-b-P(CyCL1-co-CL27)28/CBD-loaded micelles | 3.98 | 12.7 |
| HPC gels + 1 g/L PEO113-b-P(CyCL1-co-CL27)28/CBD-loaded micelles | 5.19 | 16.5 |
| HPC gels + 5 g/L PEO113-b-P(CyCL1-co-CL27)28/CBD-loaded micelles | 5.44 | 17.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamenova, K.; Momekova, D.; Grancharov, G.; Prancheva, A.; Toncheva-Moncheva, N.; Ivanov, E.; Konstantinov, S.; Petrov, P.D. In Situ Gelling Hydroxypropyl Cellulose Formulation Comprising Cannabidiol-Loaded Block Copolymer Micelles for Sustained Drug Delivery. Int. J. Mol. Sci. 2023, 24, 16534. https://doi.org/10.3390/ijms242216534
Kamenova K, Momekova D, Grancharov G, Prancheva A, Toncheva-Moncheva N, Ivanov E, Konstantinov S, Petrov PD. In Situ Gelling Hydroxypropyl Cellulose Formulation Comprising Cannabidiol-Loaded Block Copolymer Micelles for Sustained Drug Delivery. International Journal of Molecular Sciences. 2023; 24(22):16534. https://doi.org/10.3390/ijms242216534
Chicago/Turabian StyleKamenova, Katya, Denitsa Momekova, Georgy Grancharov, Anna Prancheva, Natalia Toncheva-Moncheva, Ervin Ivanov, Spiro Konstantinov, and Petar D. Petrov. 2023. "In Situ Gelling Hydroxypropyl Cellulose Formulation Comprising Cannabidiol-Loaded Block Copolymer Micelles for Sustained Drug Delivery" International Journal of Molecular Sciences 24, no. 22: 16534. https://doi.org/10.3390/ijms242216534
APA StyleKamenova, K., Momekova, D., Grancharov, G., Prancheva, A., Toncheva-Moncheva, N., Ivanov, E., Konstantinov, S., & Petrov, P. D. (2023). In Situ Gelling Hydroxypropyl Cellulose Formulation Comprising Cannabidiol-Loaded Block Copolymer Micelles for Sustained Drug Delivery. International Journal of Molecular Sciences, 24(22), 16534. https://doi.org/10.3390/ijms242216534