NopC/T/L Signal Crosstalk Gene GmPHT1-4
Abstract
:1. Introduction
2. Results
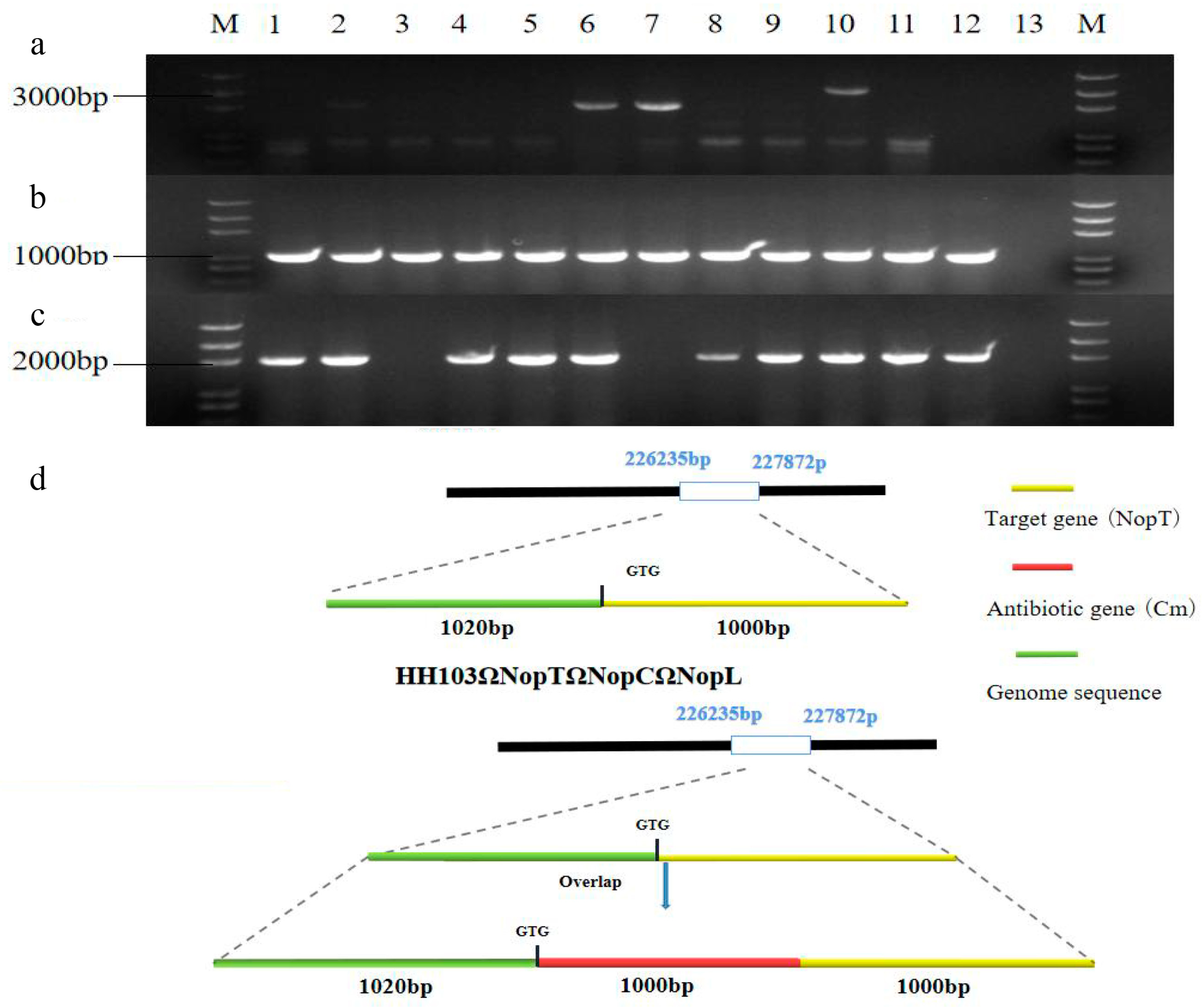
2.1. HH103ΩNopT&NopC&NopL Construction of Mutant
2.2. TCL Positively Regulates Nodules
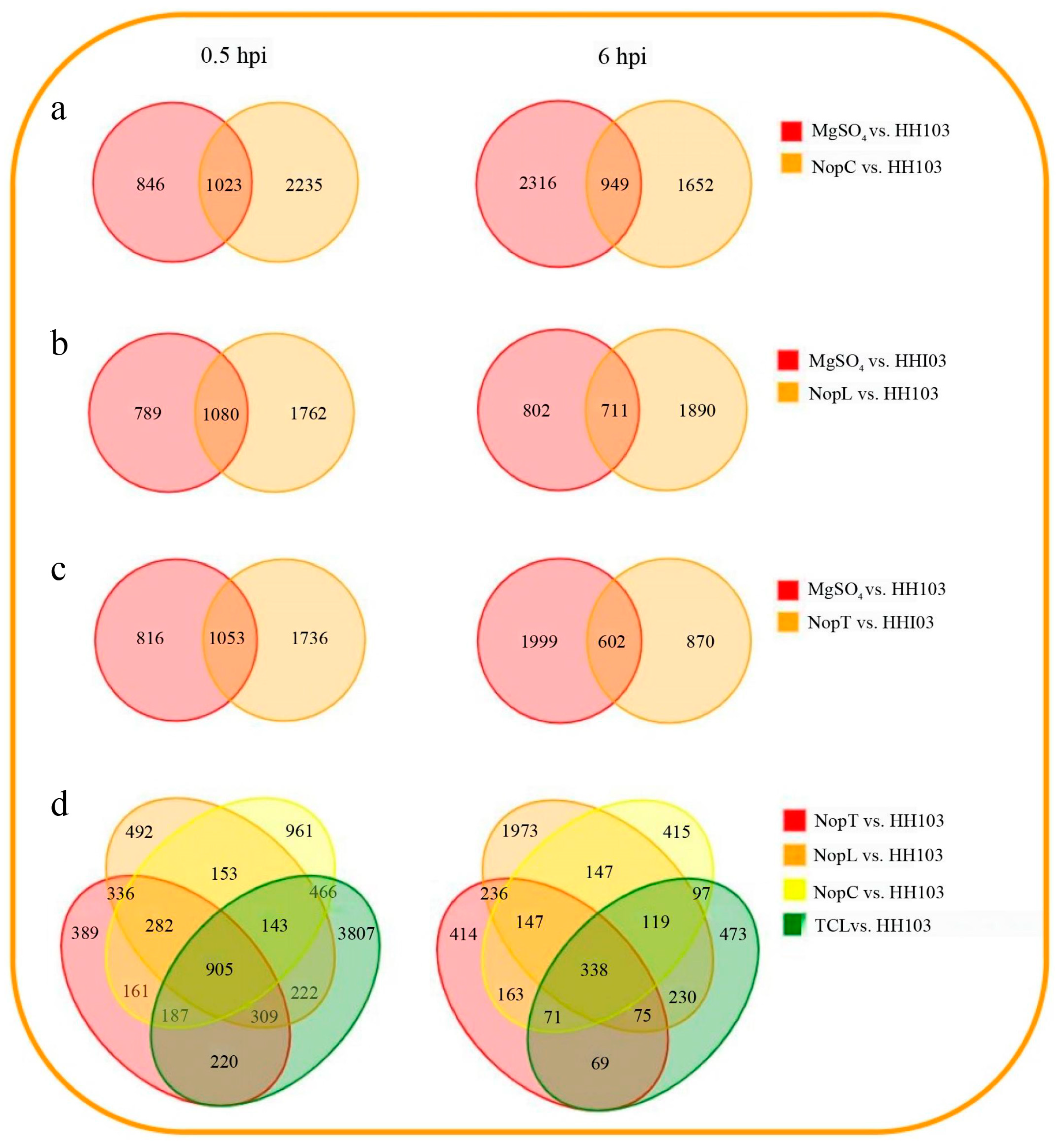
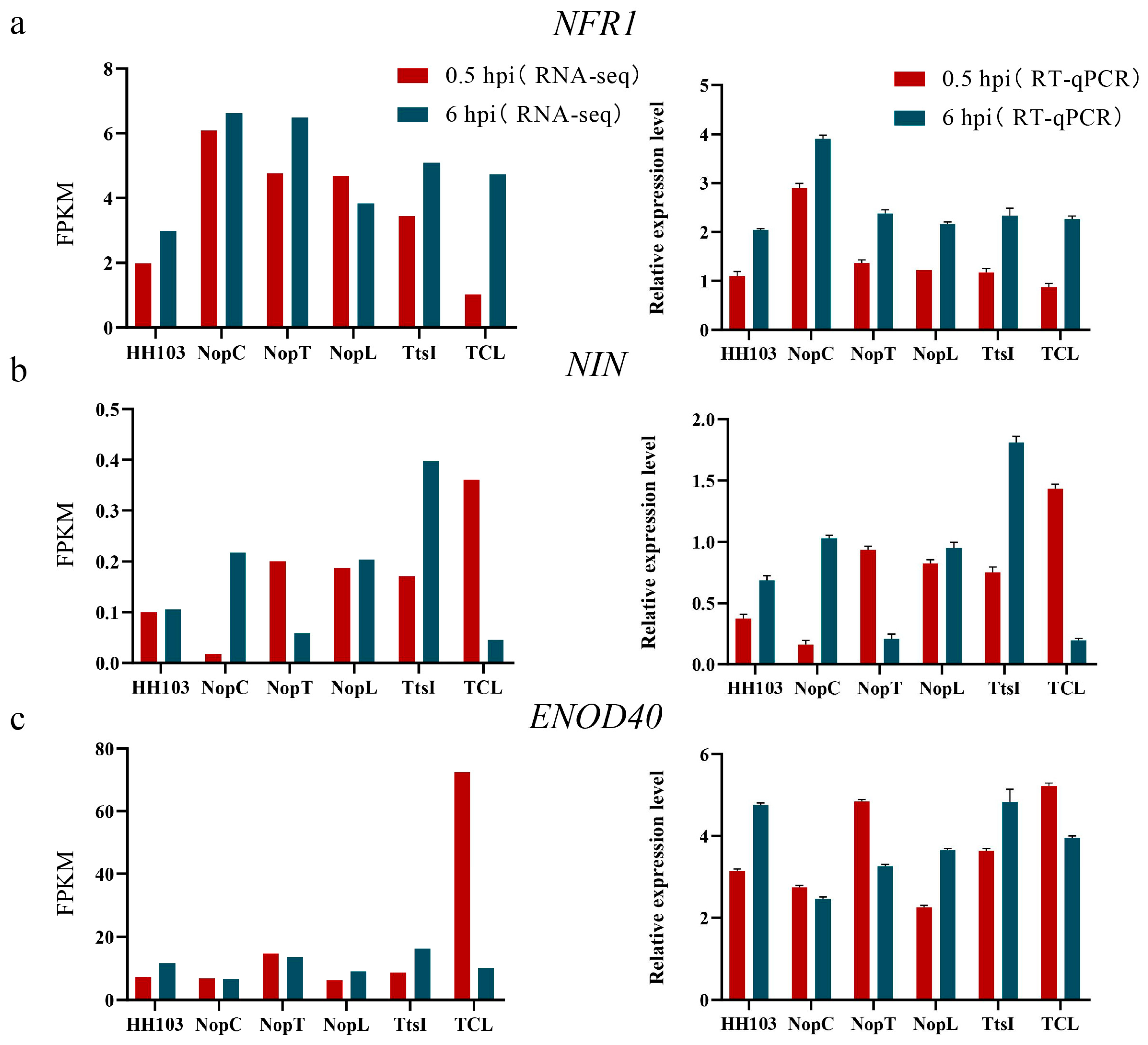
2.3. Identification of DEGs Induced by NopC, NopT, NopL, and TCL
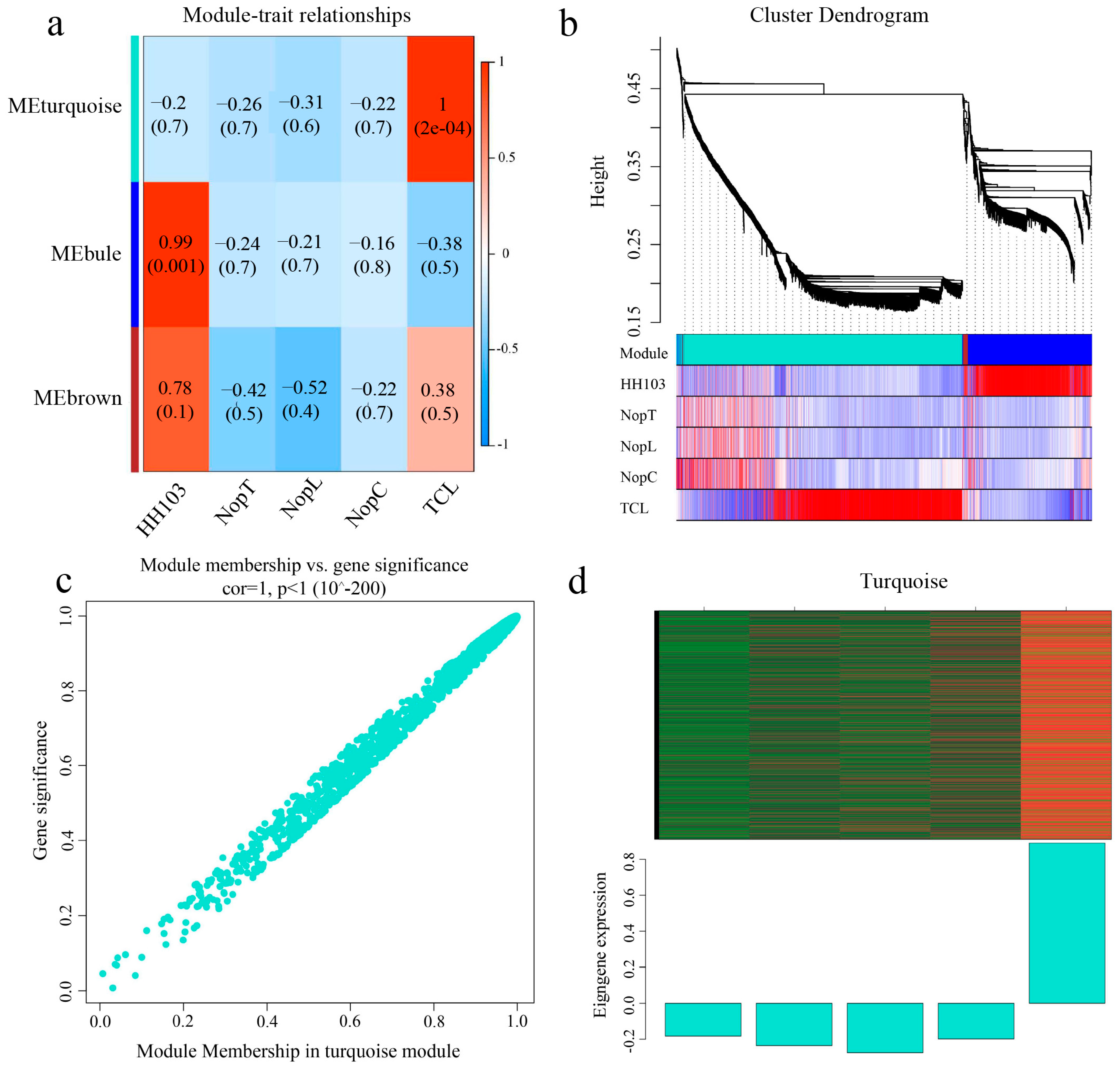
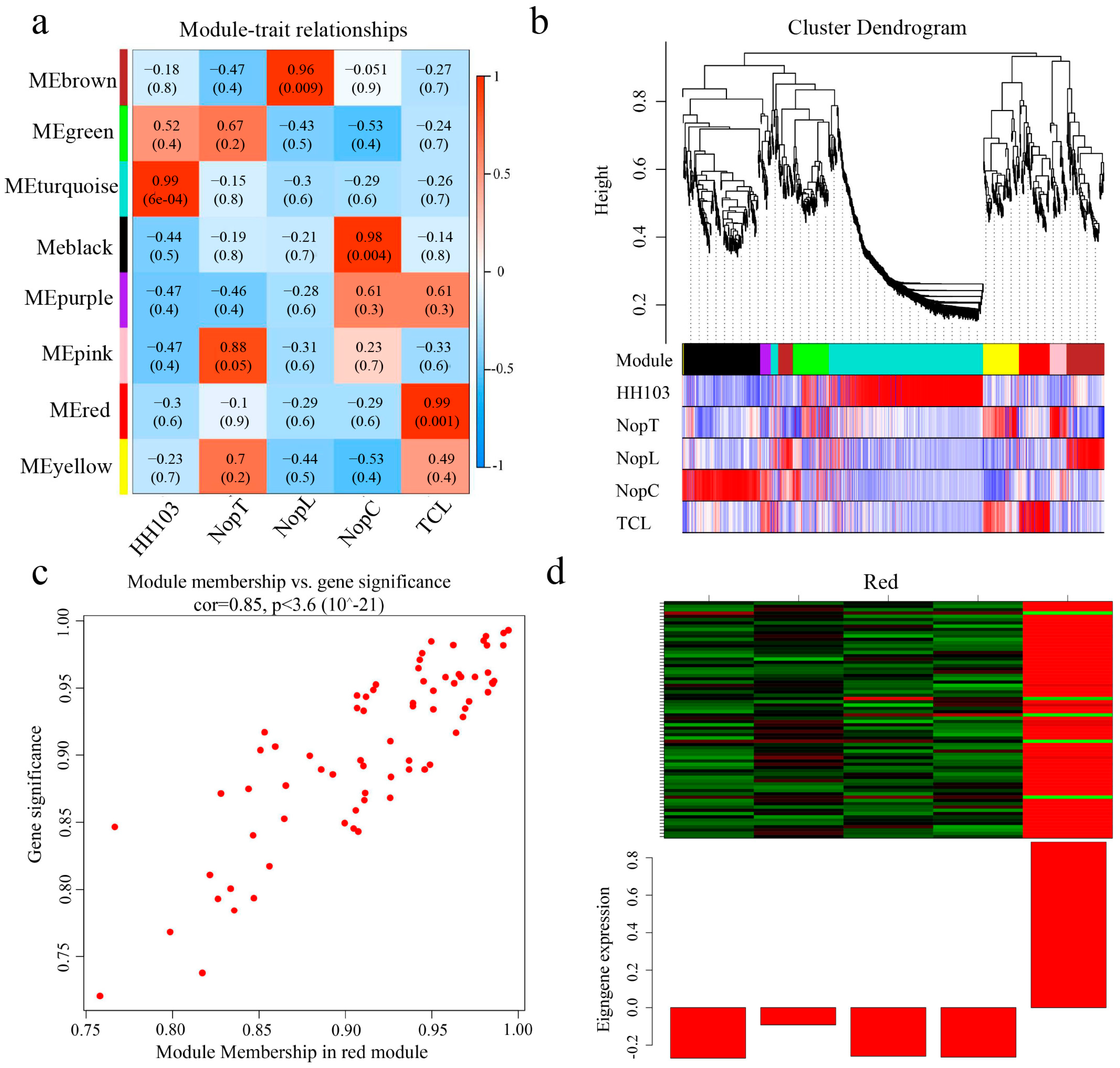
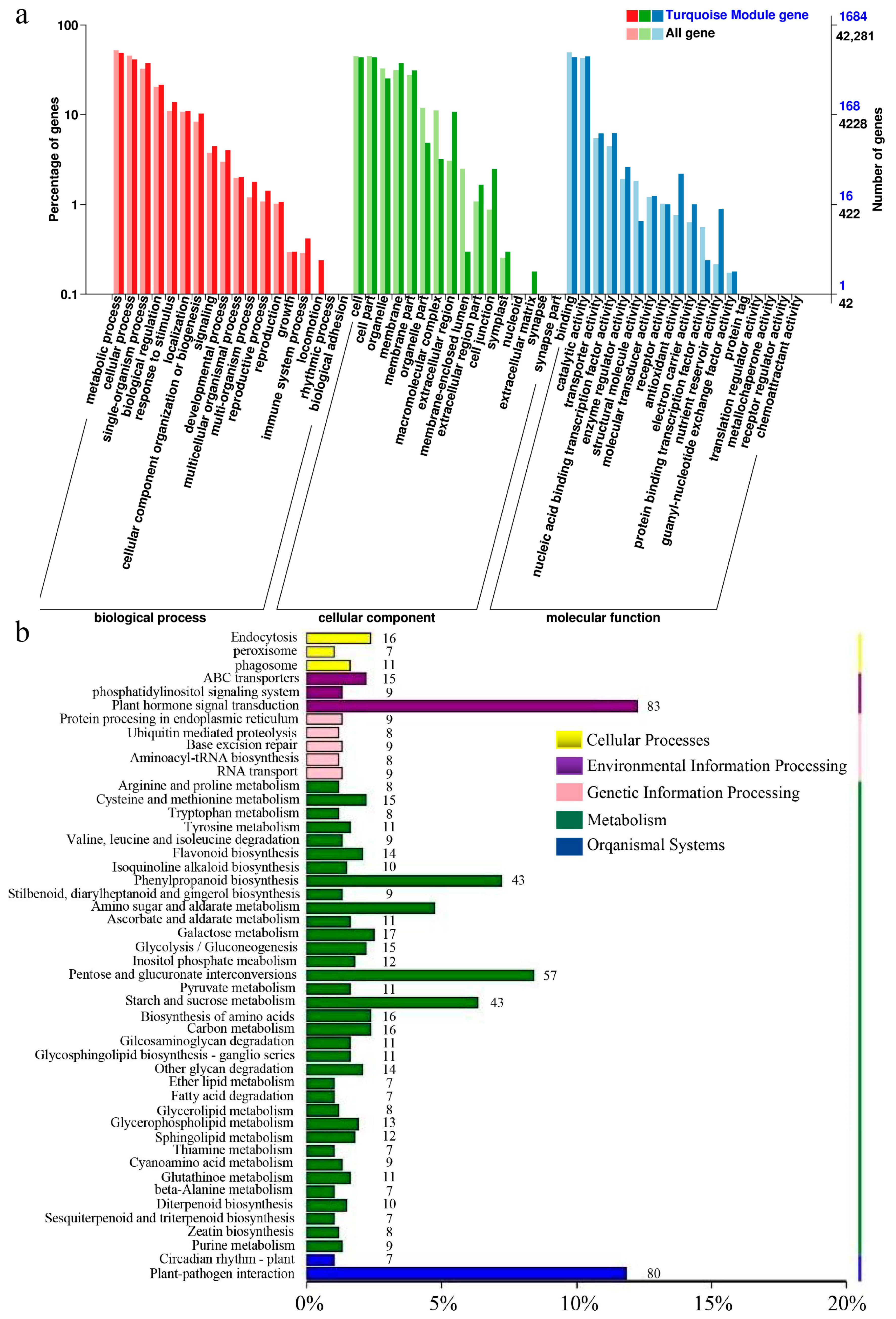
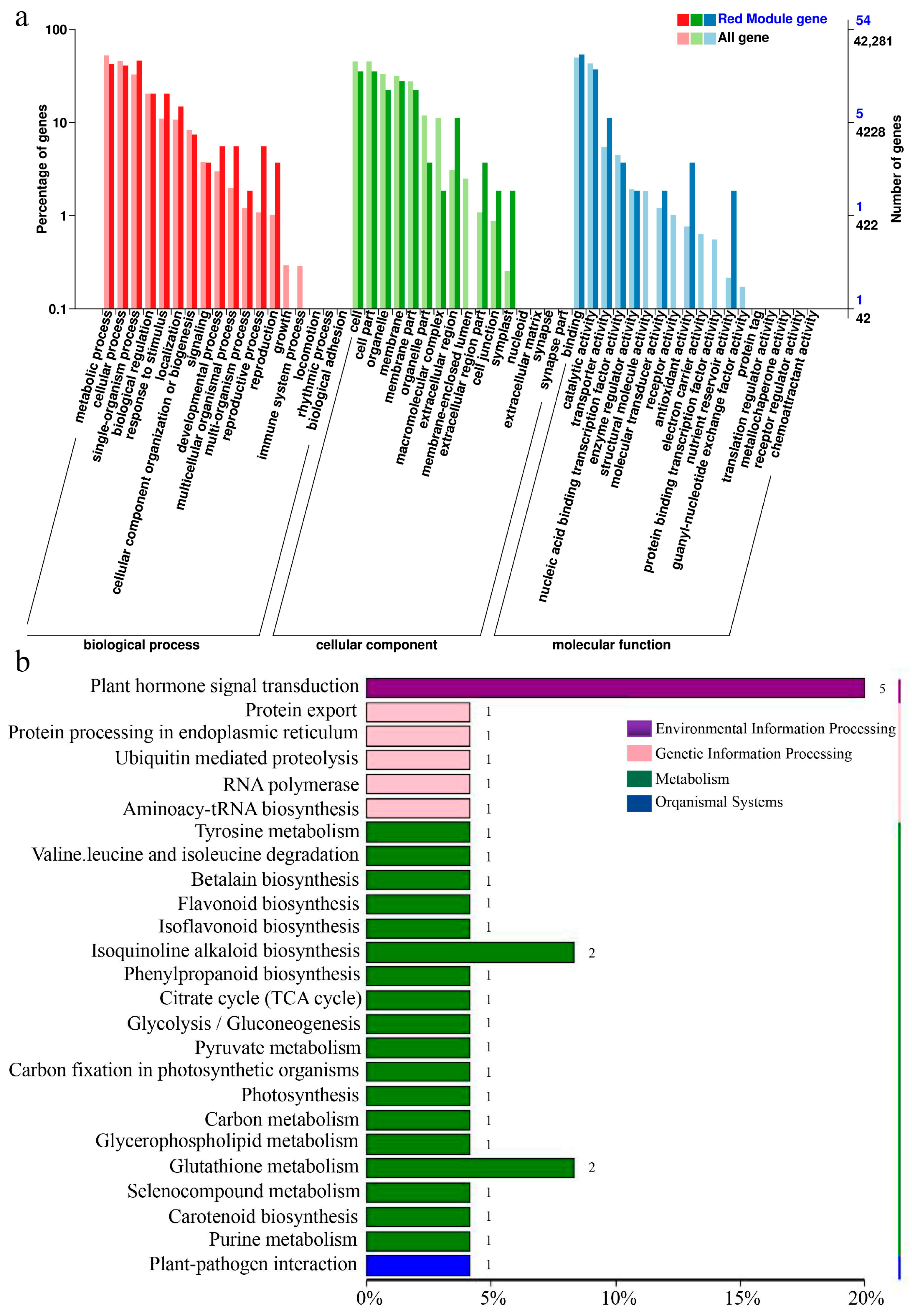
2.4. Weighted Gene Correlation Network Analysis (WGCNA)
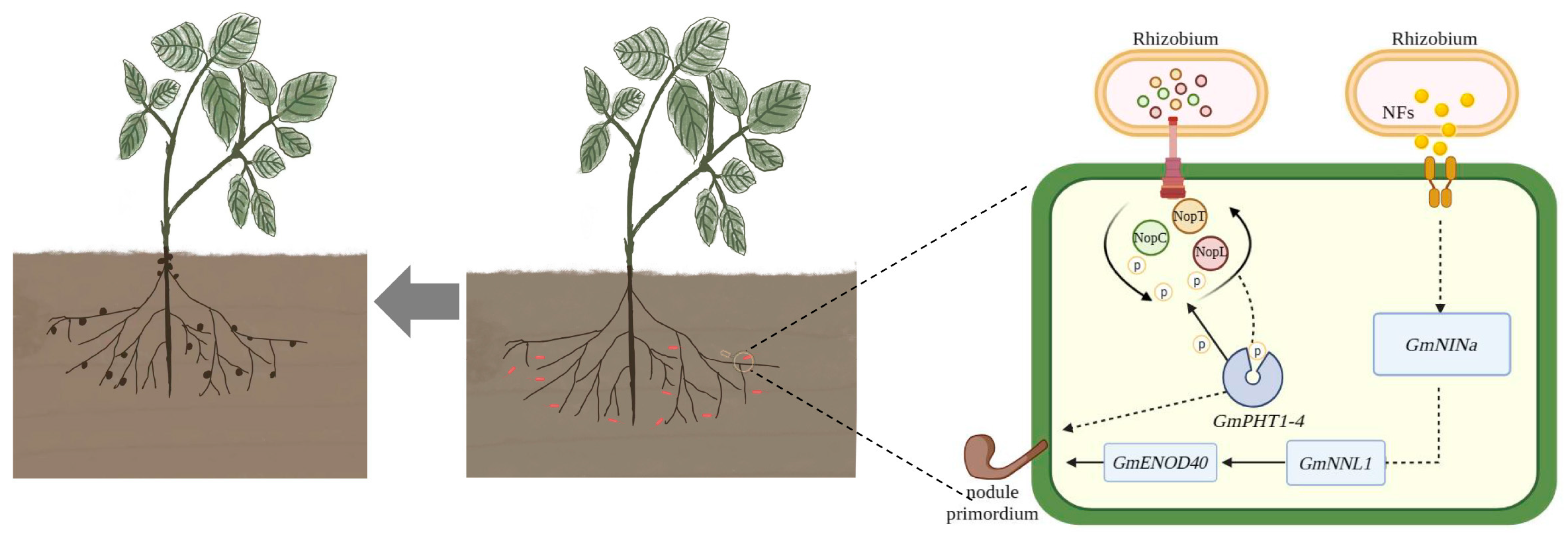
2.5. Screening for Signal Crosstalk Genes
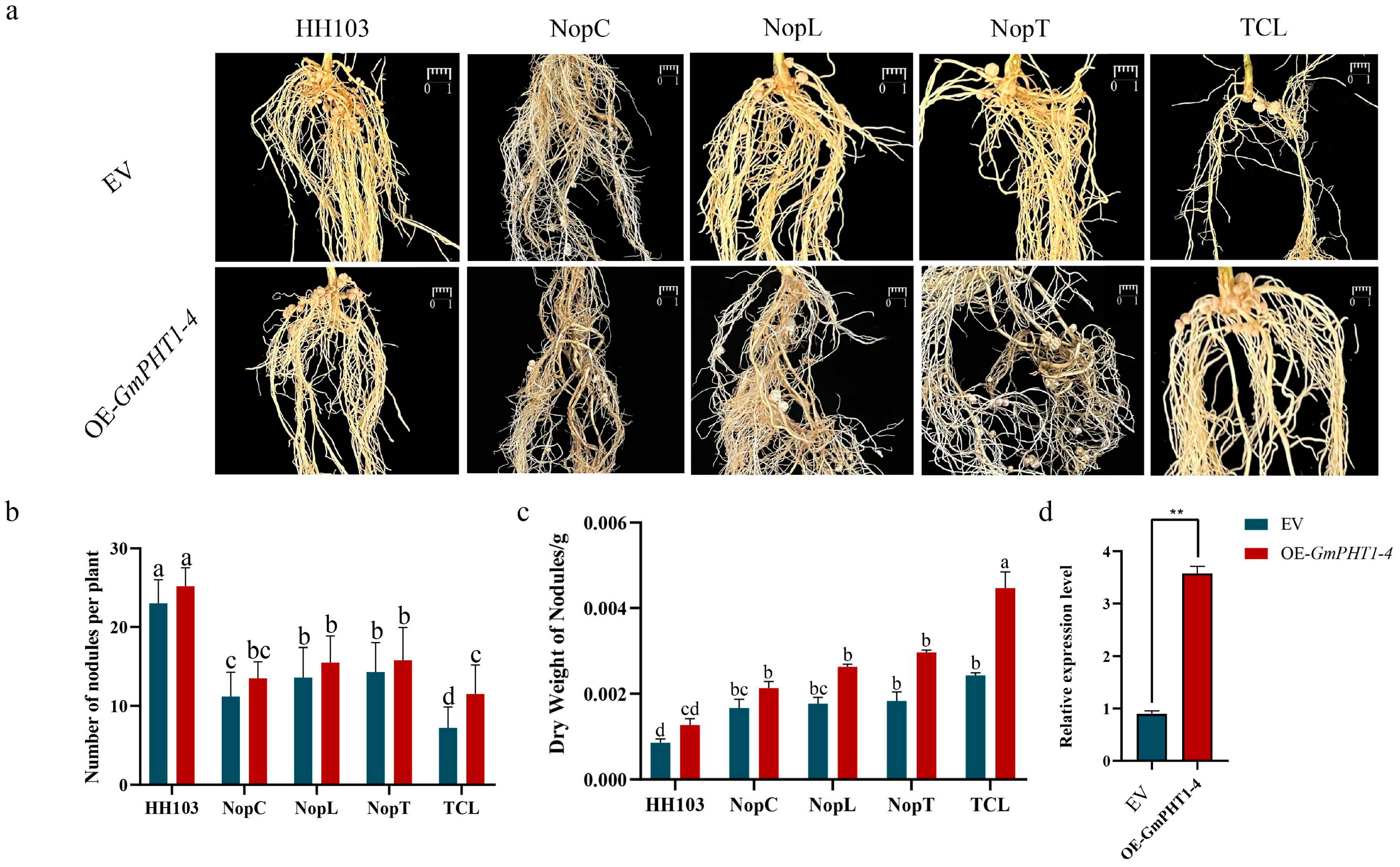
2.6. Effect of OE-GmPHT1-4 on the Nodulation
2.7. Analysis of Difference in GmPHT1-4 Expression after Inoculation with Various Rhizobia
3. Discussion
4. Materials and Methods
4.1. Acquisition of Triple Mutant HH103ΩNopT&NopC&NopL
4.2. PCR Verification of Bacterial Solution
4.3. Southern Blot
4.4. Soybean Nodulation Experiment
4.5. Phenotypic Statistics and Data Analysis
4.6. mRNA-Seq (mRNA Sequencing) Analysis
4.7. RNA Extraction and RT-qPCR Analysis
4.8. Hairy Root Transformation and Positive Soybean Root Detection
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Tian, Z.X. Genomics progress will facilitate molecular breeding in soybean. Sci. China Life Sci. 2015, 58, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhao, Q.; He, R.; Zhang, W.; Zhang, H.; Wang, H.; Ao, X.; Yao, X.; Xie, F. Rapid Effect of Enriched Nitrogen on Soybean Nitrogen Uptake, Distribution, and Assimilation During Early Flowering Stage. J. Soil Sci. Plant Nutr. 2022, 22, 3798–3810. [Google Scholar] [CrossRef]
- Qaswar, M.; Huang, J.; Ahmed, W.; Liu, S.; Li, D.; Zhang, L.; Liu, L.; Xu, Y.; Han, T.; Du, J.; et al. Substitution of inorganic nitrogen fertilizer with green manure (GM) increased yield stability by improving C input and nitrogen recovery efficiency in rice based cropping system. Agronomy 2019, 9, 609. [Google Scholar] [CrossRef]
- Jabborova, D.; Kannepalli, A.; Davranov, K.; Narimanov, A.; Enakiev, Y.; Syed, A.; Elgorban, A.M.; Bahkali, A.H.; Wirth, S.; Sayyed, R.Z.; et al. Co-inoculation of rhizobacteria promotes growth, yield, and nutrient contents in soybean and improves soil enzymes and nutrients under drought conditions. Sci. Rep. 2021, 11, 22081. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.; Ratu, S.T.N.; Yasuda, M.; Teaumroong, N.; Okazaki, S. Identification of Bradyrhizobium elkanii USDA61 type III effectors determining symbiosis with Vigna mungo. Genes 2020, 11, 474. [Google Scholar] [CrossRef]
- Siqueira, A.F.; Ormeño-Orrillo, E.; Souza, R.C.; Rodrigues, E.P.; Almeida, L.G.P.; Barcellos, F.G.; Batista, J.S.S.; Nakatani, A.S.; Martínez-Romero, E.; Vasconcelos, A.T.R.; et al. Comparative genomics of Bradyrhizobium japonicum CPAC 15 and Bradyrhizobium diazoefficiens CPAC 7: Elite model strains for understanding symbiotic performance with soybean. BMC Genom. 2014, 15, 420. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.; Zhu, H. Genetic and molecular mechanisms underlying symbiotic specificity in legume-rhizobium interactions. Front Plant Sci. 2018, 9, 313. [Google Scholar] [CrossRef]
- Dorival, J.; Philys, S.; Giuntini, E.; Brailly, R.; de Ruyck, J.; Czjzek, M.; Biondi, E.; Bompard, C. Structural and enzymatic characterisation of the Type III effector NopAA (=GunA) from Sinorhizobium fredii USDA257 reveals a Xyloglucan hydrolase activity. Sci. Rep. 2020, 10, 9932. [Google Scholar] [CrossRef]
- Coburn, B.; Sekirov, I.; Finlay, B.B. Type III secretion systems and disease. Clin. Microbiol. Rev. 2007, 20, 535–549. [Google Scholar] [CrossRef]
- Okazaki, S.; Okabe, S.; Higashi, M.; Shimoda, Y.; Sato, S.; Tabata, S.; Hashiguchi, M.; Akashi, R.; Göttfert, M.; Saeki, K. Identification and functional analysis of type III effector proteins in Mesorhizobium loti. Mol. Plant Microbe. Interact. 2010, 23, 223–234. [Google Scholar] [CrossRef]
- Sanchez-Garrido, J.; Ruano-Gallego, D.; Choudhary, J.S.; Frankel, G. The type III secretion system effector network hypothesis. Trends Microbiol. 2022, 30, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Huang, Q.; An, X.; Yang, Y.; Guan, W.; Zhao, T. Type III secretion system genes hrcJ and hrpE affect virulence, hypersensitive response and biofilm formation of group II strains of Acidovorax citrulli. Front. Sustain. Food Syst. 2022, 6, 995894. [Google Scholar] [CrossRef]
- Rodrigues, J.A.; Lopez-Baena, F.J.; Ollero, F.J.; Vinardell, J.M.; Espuny, M.d.R.; Bellogín, R.A.; Ruiz-Sainz, J.E.; Thomas, J.R.; Sumpton, D.; Ault, J.; et al. NopM and NopD are rhizobial nodulation outer proteins: Identification using LC-MALDI and LC-ESI with a monolithic capillary column. J. Phys. Chem. Lett. 2007, 6, 1029–1037. [Google Scholar] [CrossRef]
- Pérez-Montaño, F.; Jiménez-Guerrero, I.; Acosta-Jurado, S.; Navarro-Gómez, P.; Ollero, F.J.; Ruiz-Sainz, J.E.; López-Baena, F.J.; Vinardell, J.M. A transcriptomic analysis of the effect of genistein on Sinorhizobium fredii HH103 reveals novel rhizobial genes putatively involved in symbiosis. Sci. Rep. 2016, 6, 31592. [Google Scholar] [CrossRef] [PubMed]
- López-Baena, F.J.; Ruiz-Sainz, J.E.; Rodríguez-Carvajal, M.A.; Vinardell, J.M. Bacterial molecular signals in the Sinorhizobium fredii-soybean symbiosis. Int. J. Mol. Sci. 2016, 17, 755. [Google Scholar] [CrossRef]
- Arrighi, J.-F.; Barre, A.; Ben Amor, B.; Bersoult, A.; Soriano, L.C.; Mirabella, R.; de Carvalho-Niebel, F.; Journet, E.-P.; Ghérardi, M.; Huguet, T.; et al. The Medicago truncatula lysine motif-receptor-like kinase gene family includes NFP and new nodule-expressed genes. Plant Physiol. 2006, 142, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Lindner, F.; Milne-Davies, B.; Langenfeld, K.; Stiewe, T.; Diepold, A. LITESEC-T3SS-Light-controlled protein delivery into eukaryotic cells with high spatial and temporal resolution. Nat. Commun. 2020, 11, 2381. [Google Scholar] [CrossRef]
- LeBlanc, M.A.; Fink, M.R.; Perkins, T.T.; Sousa, M.C. Type III secretion system effector proteins are mechanically labile. Proc. Natl. Acad. Sci. USA 2021, 118, e2019566118. [Google Scholar] [CrossRef]
- Ratu, S.T.N.; Teulet, A.; Miwa, H.; Masuda, S.; Nguyen, H.P.; Yasuda, M.; Sato, S.; Kaneko, T.; Hayashi, M.; Giraud, E.; et al. Rhizobia use a pathogenic-like effector to hijack leguminous nodulation signalling. Sci. Rep. 2021, 11, 2034. [Google Scholar] [CrossRef]
- Jiménez-Guerrero, I.; Acosta-Jurado, S.; Medina, C.; Ollero, F.J.; Alias-Villegas, C.; Vinardell, J.M.; Pérez-Montaño, F.; López-Baena, F.J. The Sinorhizobium fredii HH103 type III secretion system effector NopC blocks nodulation with Lotus japonicus Gifu. J. Exp. Bot. 2020, 71, 6043–6056. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.J.; Lu, H.B.; Xie, Z.-P.; Staehelin, C. Functional analysis of the type 3 effector nodulation outer protein L (NopL) from Rhizobium sp. NGR234: Symbiotic effects, phosphorylation, and interference with mitogen-activated protein kinase signaling. J. Biol. Chem. 2011, 286, 32178–32187. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.Y.; Xiang, Q.W.; Wagner, C.; Zhang, D.; Xie, Z.-P.; Staehelin, C. The type 3 effector NopL of Sinorhizobium sp. strain NGR234 is a mitogen-activated protein kinase substrate. J. Exp. Bot. 2016, 67, 2483–2494. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Millar, A.J.; Davis, A.M.; Davis, S.J. Time for coffee encodes a nuclear regulator in the Arabidopsis thaliana circadian clock. Plant Cell 2007, 19, 1522–1536. [Google Scholar] [CrossRef]
- Bartsev, A.V.; Deakin, W.J.; Boukli, N.M.; McAlvin, C.B.; Stacey, G.; Malnoë, P.; Broughton, W.J.; Staehelin, C. NopL, an effector protein of Rhizobium sp. NGR234, thwarts activation of plant defense reactions. Plant Physiol. 2004, 134, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Marie, C.; Deakin, W.J.; Viprey, V.; Kopciñska, J.; Golinowski, W.; Krishnan, H.B.; Perret, X.; Broughton, W.J.; Liu, D.; Luo, Y.; et al. Characterization of Nops, nodulation outer proteins, secreted via the type III secretion system of NGR234. Mol. Plant Microbe Interact. 2003, 16, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.J.; Zeng, Y.; Xie, Z.P.; Staehelin, C. Symbiosis-promoting and deleterious effects of NopT, a novel type 3 effector of Rhizobium sp. strain NGR234. J. Bacteriol. 2008, 190, 5101–5110. [Google Scholar] [CrossRef] [PubMed]
- Fotiadis, C.T.; Dimou, M.; Georgakopoulos, D.G.; Katinakis, P.; Tampakaki, A.P. Functional characterization of NopT1 and NopT2, two type III effectors of Bradyrhizobium japonicum. Fems Microbiol. Lett. 2012, 327, 66–77. [Google Scholar] [CrossRef]
- Luo, Y.T.; Liu, D.Y.; Jiao, S.; Liu, S.; Wang, X.; Shen, X.; Wei, G. Identification of Robinia pseudoacacia target proteins responsive to Mesorhizobium amphore CCNWGS0123 effector protein NopT. J. Exp. Bot. 2020, 71, 7347–7363. [Google Scholar] [CrossRef]
- Khan, A.; Wadood, S.F.; Chen, M.; Wang, Y.; Xie, Z.-P.; Staehelin, C. Effector-triggered inhibition of nodulation: A rhizobial effector protease targets soybean kinase GmPBS1-1. Plant Physiol. 2022, 189, 2382–2395. [Google Scholar] [CrossRef]
- Ma, C.; Liu, C.; Yu, Y.; Ma, S.; Pan, S.; Feng, H.; Chen, Q.; Xin, D.; Wu, X.; Wang, J. GmTNRP1, associated with rhizobial type-III effector NopT, regulates nitrogenase activity in the nodules of soybean (Glycine max). Food Energy Secur. 2023, 12, e466. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, M.; Zhang, C.; Qu, X.; Liu, Y.; Yang, J.; Yuan, J. Isolation and functional characterisation of the PHT1 gene encoding a high-affinity phosphate transporter in Camellia oleifera. J. Hortic. Sci. Biotech. 2020, 95, 553–564. [Google Scholar] [CrossRef]
- Shin, H.; Shin, H.S.; Dewbre, G.R.; Harrison, M.J. Phosphate transport in Arabidopsis: Pht1; 1 and Pht1; 4 play a major role in phosphate acquisition from both low-and high-phosphate environments. Plant J. 2004, 39, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Ren, H.; Gu, M.; Zhao, J.; Sun, S.; Zhang, X.; Chen, J.; Wu, P.; Xu, G. The phosphate transporter gene OsPht1; 8 is involved in phosphate homeostasis in rice. Plant Physiol. 2011, 156, 1164–1175. [Google Scholar] [CrossRef]
- Bulgarelli, R.G.; De Oliveira, V.H.; Andrade, S.A.L. Arbuscular mycorrhizal symbiosis alters the expression of PHT1 phosphate transporters in roots and nodules of P-starved soybean plants. Theor. Exp. Plant Phys. 2020, 32, 243–253. [Google Scholar] [CrossRef]
- Lu, M.; Cheng, Z.; Zhang, X.M.; Huang, P.; Fan, C.; Yu, G.; Chen, F.; Xu, K.; Chen, Q.; Miao, Y.; et al. Spatial divergence of PHR-PHT1 modules maintains phosphorus homeostasis in soybean nodules. Plant Physiol. 2020, 184, 236–250. [Google Scholar] [CrossRef]
- Wei, X.; Fu, Y.; Yu, R.; Wu, L.; Wu, Z.; Tian, P.; Li, S.; Yang, X.; Yang, M. Comprehensive sequence and expression profile analysis of the phosphate transporter gene family in soybean. Sci. Rep. 2022, 12, 20883. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhu, Z.; Deng, X.; Zou, J.; Ma, C.; Li, C.; Yin, T.; Liu, C.; Wang, J.; Chen, Q.; et al. GmPBS1, a Hub Gene Interacting with Rhizobial Type-III Effectors NopT and NopP, Regulates Soybean Nodulation. Agronomy 2023, 13, 1242. [Google Scholar] [CrossRef]
- Dai, C.; Dai, X.; Qu, H.; Men, Q.; Liu, J.; Yu, L.; Gu, M.; Xu, G. The rice phosphate transporter OsPHT1; 7 plays a dual role in phosphorus redistribution and anther development. Plant Physiol. 2022, 188, 2272–2288. [Google Scholar] [CrossRef]
- Roy, S.; Liu, W.; Nandety, R.S.; Crook, A.; Mysore, K.S.; Pislariu, C.I.; Frugoli, J.; Dickstein, R.; Udvardi, M.K. Celebrating 20 years of genetic discoveries in legume nodulation and symbiotic nitrogen fixation. Plant Cell 2020, 32, 15–41. [Google Scholar] [CrossRef]
- Gao, J.P.; Xu, P.; Wang, M.; Zhang, X.; Yang, J.; Zhou, Y.; Murray, J.D.; Song, C.-P.; Wang, E. Nod factor receptor complex phosphorylates GmGEF2 to stimulate ROP signaling during nodulation. Curr. Biol. 2021, 31, 3538–3550.e5. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Q.; Liao, C.; Li, L.; Gou, C.; Chen, Z.; Wang, Y.; Liu, B.; Kong, F.; Chen, L. GmTOC1b inhibits nodulation by repressing GmNIN2a and GmENOD40-1 in soybean. Front. Plant Sci. 2022, 13, 1052017. [Google Scholar] [CrossRef] [PubMed]
- Kulich, I.; Vogler, F.; Bleckmann, A.; Cyprys, P.; Lindemeier, M.; Fuchs, I.; Krassini, L.; Schubert, T.; Steinbrenner, J.; Beynon, J.; et al. Armadillo repeat only proteins confine Rho GTPase signalling to polar growth sites. Nat. Plants 2020, 6, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Arai, K.; Aoi, Y.; Tanaka, Y.; Hira, H.; Guo, R.; Hu, Y.; Ge, C.; Zhao, Y.; Kasahara, H.; et al. The main oxidative inactivation pathway of the plant hormone auxin. Nat. Commun. 2021, 12, 6752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, S. Mitogen-activated protein kinase cascades in plant signaling. J. Integr. Plant Biol. 2022, 64, 301–341. [Google Scholar] [CrossRef] [PubMed]
- Kereszt, A.; Li, D.; Indrasumunar, A.; Nguyen, C.D.; Nontachaiyapoom, S.; Kinkema, M.; Gresshoff, P.M. Agrobacterium rhizogenes-mediated transformation of soybean to study root biology. Nat. Protoc. 2007, 2, 948–952. [Google Scholar] [CrossRef]


| GeneID | kME | Functional Annotation | Expression Position |
|---|---|---|---|
| Glyma.02G259300 | 0.989 | PEROXIDASE 52 | Root |
| Glyma.03G185400 | 0.982 | PECTATE LYASE 12-RELATED | Shoot |
| Glyma.06G174700 | 0.977 | probable pectate lyase 5 | Shoot |
| Glyma.07G203100 | 0.973 | rac-like GTP-binding protein RAC13 | Stem |
| Glyma.04G011900 | 0.956 | glucose-1-phosphate adenylyltransferase large subunit 1 | Nodules, Leaf, Stem, Shoot, Seed |
| Glyma.09G283900 | 0.949 | protein of unknown function | Seed |
| Glyma.12G035700 | 0.921 | ARG7 auxin-responsive family protein | Leaf |
| Glyma.10G247200 | 0.912 | protein of unknown function (DUF1677) (DUF1677) | Nodules |
| Glyma.15G100400 | 0.908 | calcofluor white hypersensitive protein precursor | Nodules |
| Glyma.08G038400 | 0.888 | transcription repressor OFP8-like | Shoot |
| Glyma.07G217900 | 0.882 | auxin efflux carrier component 3a | Leaf |
| Glyma.08G321400 | 0.882 | aspartyl protease family protein At5g10770 | Leaf |
| Glyma.12G116200 | 0.863 | leucine-rich repeat extensin-like protein 4 | Root |
| Glyma.08G042600 | 0.839 | plasmodesmata-located protein 6 | Shoot |
| Glyma.06G145300 | 0.836 | peroxidase 52-like | Root, Nodules |
| Glyma.10G036800 | 0.836 | inorganic phosphate transporter 1–4 | Nodules |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Yu, T.; Li, F.; Zhang, Y.; Liu, C.; Chen, Q.; Xin, D. NopC/T/L Signal Crosstalk Gene GmPHT1-4. Int. J. Mol. Sci. 2023, 24, 16521. https://doi.org/10.3390/ijms242216521
Zhu Z, Yu T, Li F, Zhang Y, Liu C, Chen Q, Xin D. NopC/T/L Signal Crosstalk Gene GmPHT1-4. International Journal of Molecular Sciences. 2023; 24(22):16521. https://doi.org/10.3390/ijms242216521
Chicago/Turabian StyleZhu, Zikun, Tong Yu, Fuxin Li, Yu Zhang, Chunyan Liu, Qingshan Chen, and Dawei Xin. 2023. "NopC/T/L Signal Crosstalk Gene GmPHT1-4" International Journal of Molecular Sciences 24, no. 22: 16521. https://doi.org/10.3390/ijms242216521
APA StyleZhu, Z., Yu, T., Li, F., Zhang, Y., Liu, C., Chen, Q., & Xin, D. (2023). NopC/T/L Signal Crosstalk Gene GmPHT1-4. International Journal of Molecular Sciences, 24(22), 16521. https://doi.org/10.3390/ijms242216521







