Gas Chromatography–Mass Spectrometry Detection of Thymoquinone in Oil and Serum for Clinical Pharmacokinetic Studies
Abstract
:1. Introduction
2. Results
2.1. Method Development and Validation Results
2.2. Quantification of TQ in Different NS Oil Products
2.3. Quantification of TQ in Serum Samples and Detection of TQ Metabolites in Urine: Preliminary Results
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Chemicals and Reagents
4.3. Human Serum Samples
4.4. Calibration Standards and Quality Control Samples
4.4.1. Method Validation
4.4.2. Clinical Application
4.5. Sample Preparation
4.5.1. Method Validation
4.5.2. Clinical Application
4.6. GC-MS Analysis
4.6.1. Method Validation
4.6.2. Linearity
4.6.3. Precision
4.6.4. LLOQ
4.6.5. Stability
4.6.6. Matrix Effect
4.6.7. Carry-Over
4.6.8. Dilution Integrity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bent, S. Herbal medicine in the United States: Review of efficacy, safety, and regulation: Grand rounds at University of California, San Francisco Medical Center. J. Gen. Intern. Med. 2008, 23, 854–859. [Google Scholar] [PubMed]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Khare, C. Encyclopedia of Indian Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Tekbas, A.; Huebner, J.; Settmacher, U.; Dahmen, U. Plants and Surgery: The Protective Effects of Thymoquinone on Hepatic Injury-A Systematic Review of In Vivo Studies. Int. J. Mol. Sci. 2018, 19, 1085. [Google Scholar] [CrossRef] [PubMed]
- Mollazadeh, H.; Hosseinzadeh, H. The protective effect of Nigella sativa against liver injury: A review. Iran. J. Basic Med. Sci. 2014, 17, 958–966. [Google Scholar] [PubMed]
- El-Najjar, N.; Ketola, R.A.; Nissila, T.; Mauriala, T.; Antopolsky, M.; Janis, J.; Gali-Muhtasib, H.; Urtti, A.; Vuorela, H. Impact of protein binding on the analytical detectability and anticancer activity of thymoquinone. J. Chem. Biol. 2011, 4, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Maurer, H.H.; Pfleger, K.; Weber, A.A. Mass Spectral and GC Data of Drugs, Poisons, Pesticides, Pollutants, and Their Metabolites, 5th ed.; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Maurer, H.H.; Wissenbach, D.K.; Weber, A.A. Maurer/Wissenbach/Weber LC-MSn Library of Drugs, Poisons, and Their Metabolites, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2019. [Google Scholar]
- Johnson-Ajinwo, O.R.; Li, W.W. Stable isotope dilution gas chromatography-mass spectrometry for quantification of thymoquinone in black cumin seed oil. J. Agric. Food Chem. 2014, 62, 5466–5471. [Google Scholar] [CrossRef] [PubMed]
- Botnick, I.; Xue, W.; Bar, E.; Ibdah, M.; Schwartz, A.; Joel, D.M.; Lev, E.; Fait, A.; Lewinsohn, E. Distribution of primary and specialized metabolites in Nigella sativa seeds, a spice with vast traditional and historical uses. Molecules 2012, 17, 10159–10177. [Google Scholar] [CrossRef] [PubMed]
- Ghosheh, O.A.; Houdi, A.A.; Crooks, P.A. High performance liquid chromatographic analysis of the pharmacologically active quinones and related compounds in the oil of the black seed (Nigella sativa L.). J. Pharm. Biomed. Anal. 1999, 19, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Michelitsch, A.; Rittmannsberger, A. A simple differential pulse polarographic method for the determination of thymoquinone in black seed oil. Phytochem. Anal. 2003, 14, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Velho-Pereira, R.M.; Barhate, C.R.; Kulkarni, S.R.; Jagtap, A.G. Validated high-performance thin-layer chromatographic method for the quantification of thymoquinone in Nigella sativa extracts and formulations. Phytochem. Anal. 2011, 22, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Land, E.J.; Ramsden, C.A.; Riley, P.A. Quinone chemistry and melanogenesis. Methods Enzymol. 2004, 378, 88–109. [Google Scholar] [PubMed]
- Li, W.W.; Heinze, J.; Haehnel, W. Site-specific binding of quinones to proteins through thiol addition and addition-elimination reactions. J. Am. Chem. Soc. 2005, 127, 6140–6141. [Google Scholar] [CrossRef] [PubMed]
- Alkharfy, K.M.; Ahmad, A.; Khan, R.M.A.; Al-Asmari, M. High-performance liquid chromatography of thymoquinone in rabbit plasma and its application to pharmacokinetics. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 2242–2250. [Google Scholar] [CrossRef]
- Alkharfy, K.M.; Ahmad, A.; Khan, R.M.; Al-Shagha, W.M. Pharmacokinetic plasma behaviors of intravenous and oral bioavailability of thymoquinone in a rabbit model. Eur. J. Drug Metab. Pharmacokinet. 2015, 40, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Javeed, A.; Sattar, A.; Tanvir, R. Pharmacokinetics of thymoquinone in layer chickens following oral and intravenous administration. J. Vet. Pharmacol. Ther. 2019, 42, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Zakarial Ansar, F.H.; Latifah, S.Y.; Wan Kamal, W.H.B.; Khong, K.C.; Ng, Y.; Foong, J.N.; Gopalsamy, B.; Ng, W.K.; How, C.W.; Ong, Y.S.; et al. Pharmacokinetics and Biodistribution of Thymoquinone-loaded Nanostructured Lipid Carrier After Oral and Intravenous Administration into Rats. Int. J. Nanomed. 2020, 15, 7703–7717. [Google Scholar] [CrossRef] [PubMed]
- Khalife, K.H.; Lupidi, G. Nonenzymatic reduction of thymoquinone in physiological conditions. Free Radic. Res. 2007, 41, 153–161. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Guideline on Bioanalytical Method Validation. 2012. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 16 November 2020).
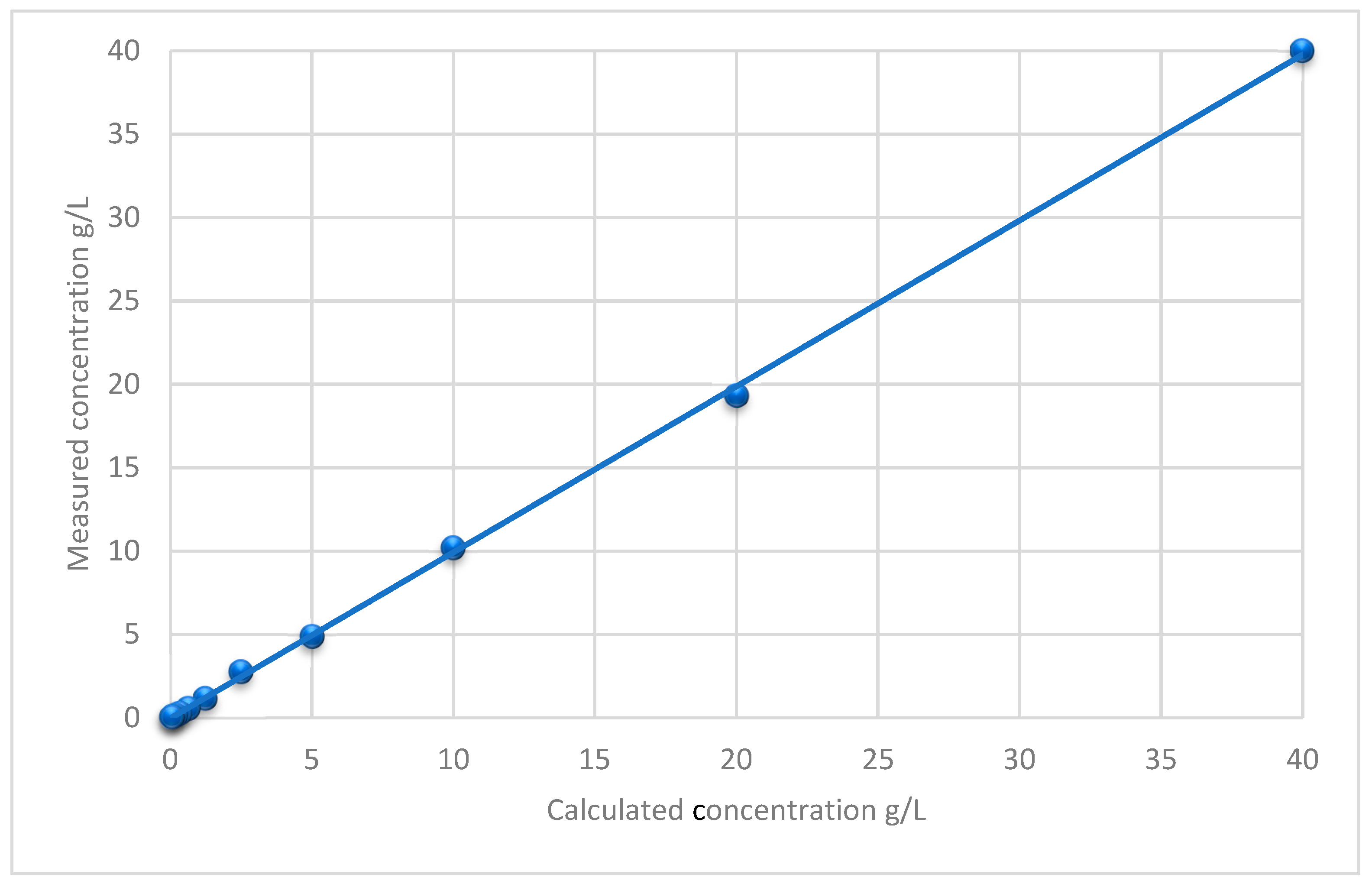


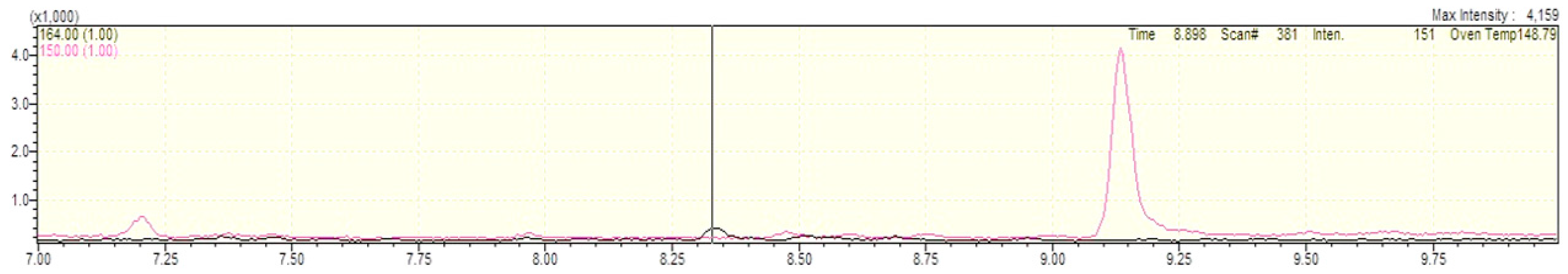
| Storage Duration | Storage Condition | Deviation from RT in% |
|---|---|---|
| 15 days | 4–8 °C | 1.3 |
| −20 °C | 3 | |
| 30 days | 4–8 °C | 0.4 |
| −20 °C | 1.6 |
| NS Oil Product | Mean TQ in g/L | RSD in% |
|---|---|---|
| No. 1 | 1.0 | 23.9 |
| No. 2 | 4.8 | 0.4 |
| No. 3 | 24.4 | 8.9 |
| No. 4 | 1.15 | 3.4 |
| No. 5 | 21.1 | 4.7 |
| No. 6 | 0.9 | 5.6 |
| No. 7 | 4.8 | 5.1 |
| No. 8 | 1.3 | 1.9 |
| No. 9 | 1.0 | 2.8 |
| No. 10 | 2.5 | 2.1 |
| No. 11 | 2.7 | 14.6 |
| No. 12 | 0.6 | 13.3 |
| No. 13 | 0.6 | 3.1 |
| No. 14 | 1.3 | 9.1 |
| No. 15 | 6.7 | 15.4 |
| No. 16 | 1.0 | 2.2 |
| No. 17 | 36.6 | 9.3 |
| No. 18 | 3.5 | 5.6 |
| No. 19 | 27.9 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tekbaş, A.; Bremer-Streck, S.; Wissenbach, D.K.; Peters, F.T.; von Lilienfeld-Toal, M.; Soonawalla, Z.; Rauchfuß, F.; Settmacher, U.; Dahmen, U. Gas Chromatography–Mass Spectrometry Detection of Thymoquinone in Oil and Serum for Clinical Pharmacokinetic Studies. Int. J. Mol. Sci. 2023, 24, 16431. https://doi.org/10.3390/ijms242216431
Tekbaş A, Bremer-Streck S, Wissenbach DK, Peters FT, von Lilienfeld-Toal M, Soonawalla Z, Rauchfuß F, Settmacher U, Dahmen U. Gas Chromatography–Mass Spectrometry Detection of Thymoquinone in Oil and Serum for Clinical Pharmacokinetic Studies. International Journal of Molecular Sciences. 2023; 24(22):16431. https://doi.org/10.3390/ijms242216431
Chicago/Turabian StyleTekbaş, A., S. Bremer-Streck, D. K. Wissenbach, F. T. Peters, M. von Lilienfeld-Toal, Z. Soonawalla, F. Rauchfuß, U. Settmacher, and U. Dahmen. 2023. "Gas Chromatography–Mass Spectrometry Detection of Thymoquinone in Oil and Serum for Clinical Pharmacokinetic Studies" International Journal of Molecular Sciences 24, no. 22: 16431. https://doi.org/10.3390/ijms242216431
APA StyleTekbaş, A., Bremer-Streck, S., Wissenbach, D. K., Peters, F. T., von Lilienfeld-Toal, M., Soonawalla, Z., Rauchfuß, F., Settmacher, U., & Dahmen, U. (2023). Gas Chromatography–Mass Spectrometry Detection of Thymoquinone in Oil and Serum for Clinical Pharmacokinetic Studies. International Journal of Molecular Sciences, 24(22), 16431. https://doi.org/10.3390/ijms242216431


_Kim.png)




