Assessment of Neurotoxic Effects of Oxycodone and Naloxone in SH-SY5Y Cell Line
Abstract
1. Introduction
2. Results
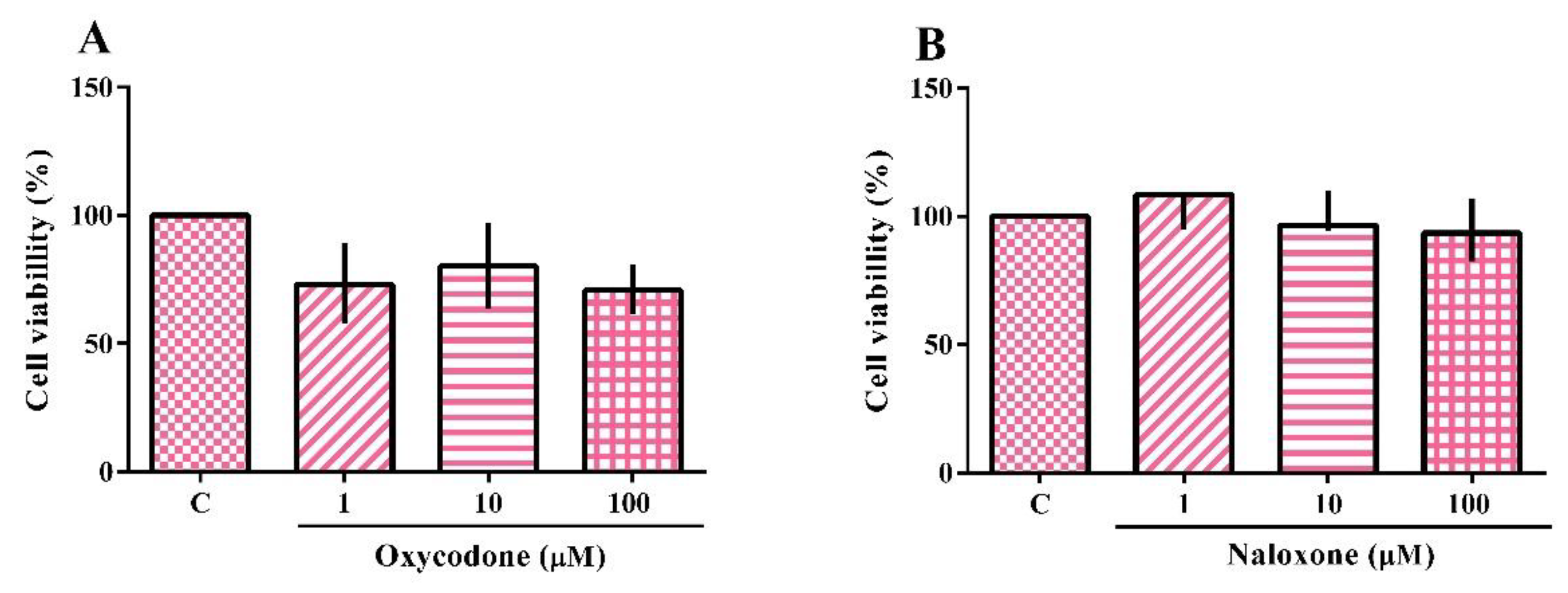
2.1. Cytotoxicity
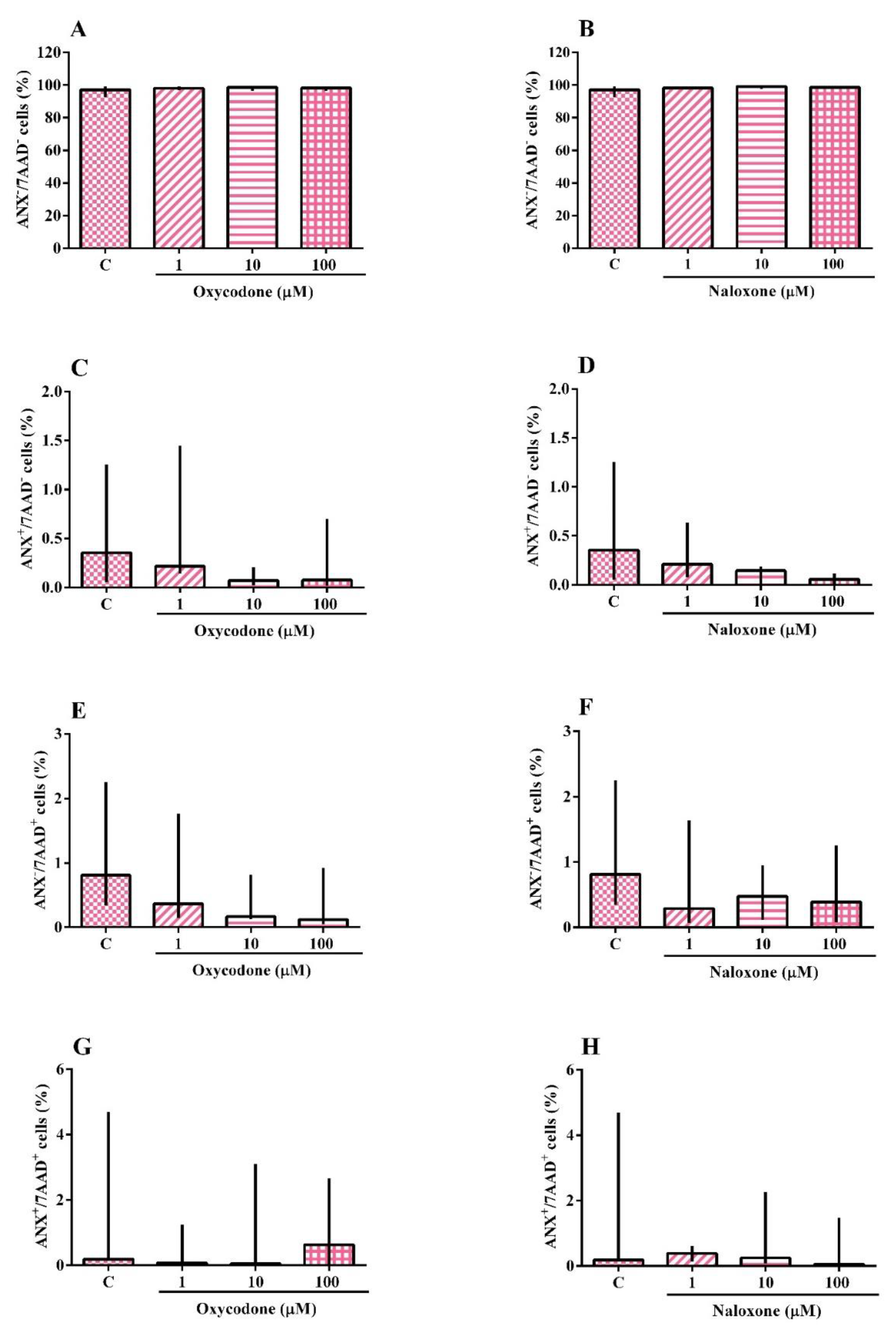
2.2. Identification of Apoptotic and Necrotic Cells
2.3. DNA Damage
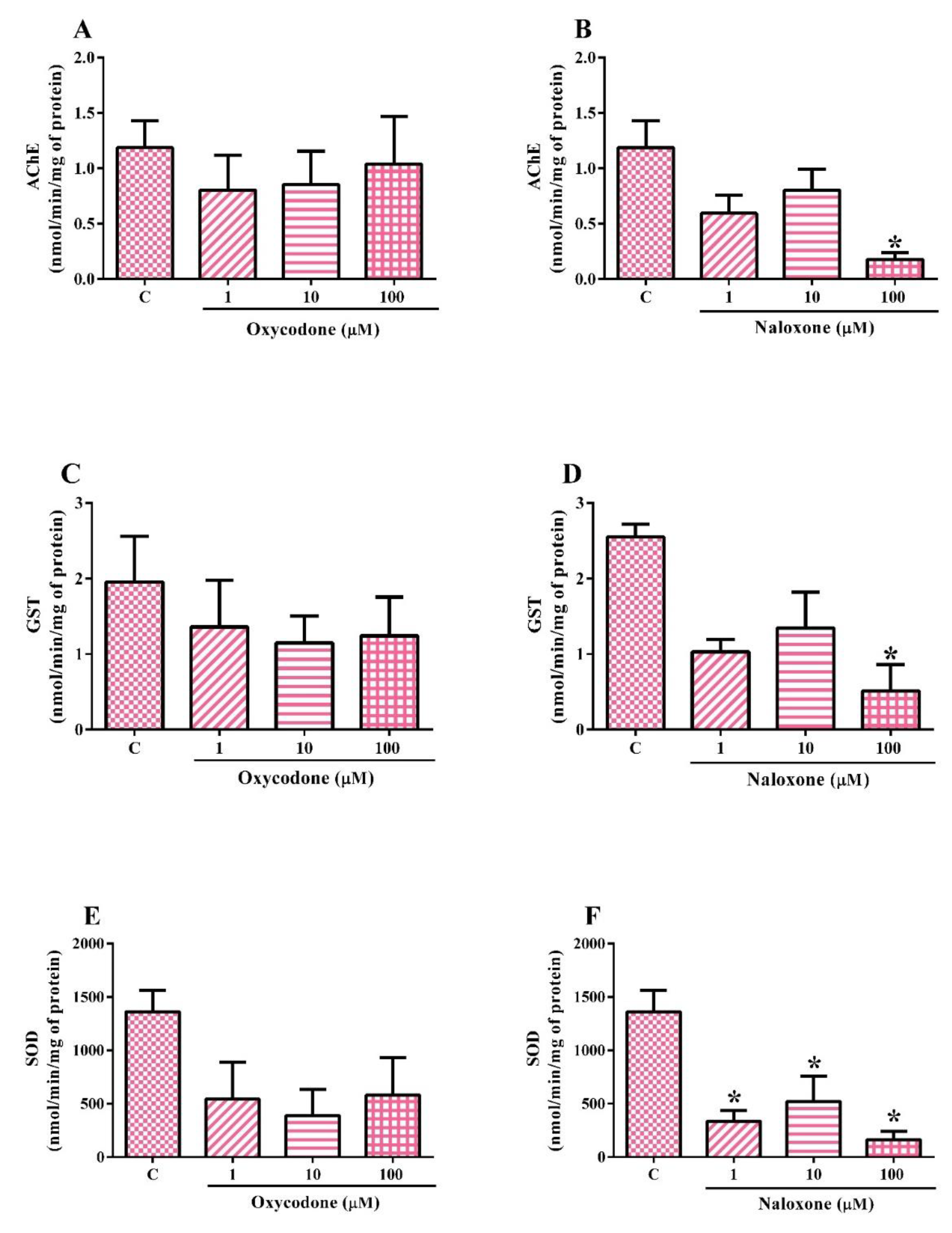
2.4. Biochemical Analysis
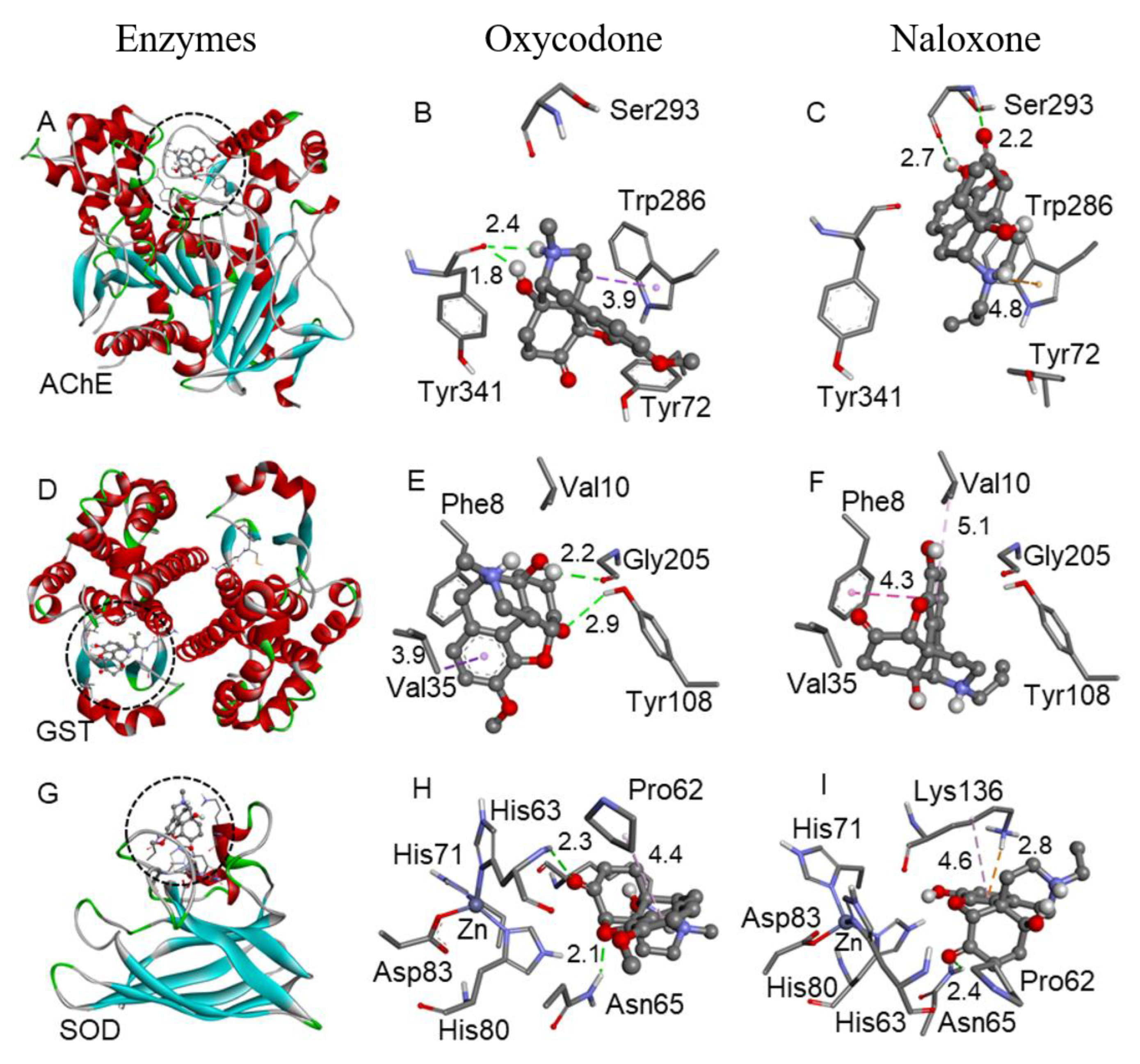
2.5. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Neuroblastoma Cell Line
4.2. Cell Cultivation
4.3. Oxycodone and Naloxone Exposure
4.4. Cytotoxicity Assay
4.5. Identification of Apoptotic and Necrotic Cells
4.6. DNA Damage
4.7. Biochemical Analyses
4.7.1. Sample Preparation
4.7.2. Acetylcholinesterase
4.7.3. Glutathione S-Transferase
4.7.4. Superoxide Dismutase
4.7.5. Protein Quantification
4.8. Molecular Docking
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trescot, A.M.; Datta, S.; Lee, M.; Hansen, H. Opioid pharmacology. Pain Physician 2008, 11, 133–153. [Google Scholar] [CrossRef]
- Chan, P.C.W. China’s Approaches to International Law since the Opium War. Leiden J. Int. Law 2014, 27, 859–892. [Google Scholar] [CrossRef]
- Pathan, H.; Williams, J. Basic opioid pharmacology: An update. Br. J. Pain 2012, 6, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Huddart, R.; Clarke, M.; Altman, R.B.; Klein, T.E. PharmGKB summary: Oxycodone pathway, pharmacokinetics. Pharmacogenet. Genom. 2018, 28, 230. [Google Scholar] [CrossRef]
- Smith, H.S. Opioid Metabolism. Mayo Clin Proc. 2009, 84, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.H.; Camargo, M.M.A. Opiáceos e Opioides. In Fundamentos da Toxicologia, 4th ed.; OGA, S., Camargo, M.M.A., Batistuzzo, J.A.O., Eds.; Atheneu: São Paulo, Brazil, 2014. [Google Scholar]
- National Institute on Drug Abuse—NIDA. Naloxone Drug Facts. January 2022. Available online: https://nida.nih.gov/publications/drugfacts/naloxone. (accessed on 13 June 2022).
- Dowell, D.; Haegerich, T.M.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. Recomm. Rep. 2016, 65, 1–49. [Google Scholar]
- Tyan, P.; Carey, E.T. Physiological Response to Opioids. Clin. Obstet. Gynecol. 2019, 62, 11–21. [Google Scholar] [CrossRef]
- Chahl, L.A. Opioids—Mechanisms of action. Exp. Clin. Pharmacol. 1996, 19, 63–65. [Google Scholar]
- Parkin, S.; Neale, J.; Brown, C.; Campbell, A.N.C.; Castillo, F.; Jones, J.D.; Strang, J.; Comer, S.D. Opioid overdose reversals using naloxone in New York City by people who use opioids: Implications for public health and overdose harm reduction approaches from a qualitative study. Int. J. Drug Policy 2020, 79, 102751. [Google Scholar] [CrossRef]
- Motaghinejad, M.S.M.; Motaghinejad, O.; Shabab, B.; Asadighaleni, M.; Fatima, S. The effect of various morphine weaning regimens on the sequelae of opioid tolerance involving physical dependency, anxiety and hippocampus cell neurodegeneration in rats. Fundam. Clin. Pharmacol. 2015, 29, 299–309. [Google Scholar] [CrossRef]
- Bates, D.D.; Gallagher, K.; Yu, H.; Uyeda, J.; Murakami, A.M.; Setty, B.N.; Anderson, S.W.; Clement, M.O. Acute radiologic manifestations of America’s opioid epidemic. RadioGraphics 2018, 3, 109–123. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Death Rate Maps & Graphs, Drug Overdose Deaths Remain High. 2 June 2022. Available online: https://www.cdc.gov/drugoverdose/deaths/index.html (accessed on 25 June 2022).
- Anekar, A.A.; Cascella, M. WHO Analgesic Ladder. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Moon, J.M.; Chun, B.J. Fentanyl Intoxication Caused by Abuse of Transdermal Fentanyl. J. Emer. Med. 2011, 40, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Pujol, C.N.; Paasche, C.; Laprevote, V.; Trojak, B.; Vidailhet, P.; Bacon, E.; Lalanne, L. Cognitive effects of labeled addictolytic medications. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 81, 306–332. [Google Scholar] [CrossRef] [PubMed]
- Marshansky, S.; Mayer, P.; Rizzo, D.; Baltzan, M.; Denis, R.; Lavigne, G.J. Sleep, chronic pain, and opioid risk for apnea. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 87, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, V.; García-Campos, M.; Sáez-González, E.; Delpozo, P.; Garrigues, V. A concise review of opioid-induced esophageal dysfunction: Is this a new clinical entity? Dis. Esophagus 2018, 31, 1–6. [Google Scholar] [CrossRef]
- Whitman, Z.; O’neil, D.H.R. Gastric disorders: Modifications of gastric content, antacids and drugs influencing gastric secretions and motility. Anaesth. Intensive Care Med. 2018, 19, 25–29. [Google Scholar] [CrossRef]
- Rai, Y.; Pathak, R.; Kumari, N.; Sah, D.K.; Pandey, S.; Kalra, N.; Soni, R.; Dwarakanath, B.S.; Bhatt, A.N. Mitochondrial biogenesis and metabolic hyperactivation limits the application of MTT assay in the estimation of radiation induced growth inhibition. Sci. Rep. 2018, 8, 1531. [Google Scholar] [CrossRef]
- Kokki, M.; Pesonen, M.; Vehviläinen, P.; Litmala, O.; Pasanen, M.; Kokki, H. Cytotoxicity of oxycodone and morphine in human neuroblastoma and mouse motoneuronal cells: A comparative approach. Drugs R&D 2016, 16, 155–163. [Google Scholar]
- Faria, J.; Barbosa, J.; Queirós, O.; Moreira, R.; Carvalho, F.; Dinis-Oliveira, R.J. Comparative study of the neurotoxicological effects of tramadol and tapentadol in SH-SY5Y cells. Toxicology 2016, 359, 1–10. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Y.J.; Li, Q.; Hou, Y.Y.; Hong, M.H.; Cao, Y.L.; Chi, Z.Q.; Liu, J.G. Chronic high-dose morphine treatment promotes SH-SY5Y cell apoptosis via c-Jun N-terminal kinase-mediated activation of mitochondria-dependent pathway. FEBS J. 2009, 276, 2022–2036. [Google Scholar] [CrossRef]
- Aramjoo, H.; Riahi-Zanjani, B.; Farkhondeh, T.; Forouzanfar, F.; Sadeghi, M. Modulatory effect of opioid administration on the activity of cholinesterase enzyme: A systematic review of mice/rat models. Environ. Sci. Pollut. Res. 2021, 28, 52675–52688. [Google Scholar] [CrossRef] [PubMed]
- Motel, W.C.; Coop, A.; Cunningham, C.W. Cholinergic modulation by opioid receptor ligands: Potential application to Alzheimer’s disease. Mini-Rev. Med. Chem. 2013, 13, 456–466. [Google Scholar]
- Lannutti, F.; Marrone, A.; Re, N. Binding of GSH conjugates to π-GST: A cross-docking approach. J. Mol. Graph. Model. 2012, 32, 9–18. [Google Scholar] [CrossRef]
- Payabvash, S.; Beheshtian, A.; Salmasi, A.H.; Kiumehr, S.; Ghahremani, M.H.; Tavangar, S.M.; Sabzevari, O.; Dehpour, A.R. Chronic morphine treatment induces oxidant and apoptotic damage in the mice liver. Life Sci. 2006, 79, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Afshari, R.; Farkhondeh, T. Effect of long-term treatment of morphine on enzymes, oxidative stress indices and antioxidant status in male rat liver. Int. J. Clin. Exp. Med. 2014, 7, 1449. [Google Scholar] [PubMed]
- Hashemi, S.A.; Karami, M.; Bathaie, S.Z. Saffron carotenoids change the superoxide dismutase activity in breast cancer: In vitro, in vivo and in silico studies. Int. J. Biol. Macromol. 2020, 158, 845–853. [Google Scholar] [CrossRef]
- Sadat-Shirazi, M.S.; Zarrindast, M.R.; Ashabi, G. Oxidative stress enzymes are changed in opioid abusers and multidrug abusers. J. Clin. Neurosci. 2020, 72, 365–369. [Google Scholar] [CrossRef]
- Ma, J.; Yuan, X.; Qu, H.; Zhang, J.; Wang, D.; Sun, X.; Zheng, Q. The role of reactive oxygen species in morphine addiction of SH-SY5Y cells. Life Sci. 2015, 124, 128–135. [Google Scholar] [CrossRef]
- Salarian, A.; Kadkhodaee, M.; Zahmatkesh, M.; Seifi, B.; Bakhshi, E.; Akhondzadeh, S.; Adeli, S.; Askari, H.; Arbabi, M. Opioid use disorder induces oxidative stress and inflammation: The attenuating effect of methadone maintenance treatment. Iran. J. Psychiatry 2018, 13, 46. [Google Scholar]
- Khomula, E.V.; Araldi, D.; Bonet, I.J.; Levine, J.D. Opioid-induced hyperalgesic priming in single nociceptors. J. Neurosci. 2021, 41, 31–46. [Google Scholar] [CrossRef]
- Tobore, T.O. Towards a Comprehensive Theory of Non-Cancer Acute and Chronic Pain Management: The Critical Role of Reactive Oxygen and Nitrogen Species in Pain, and Opioid Dependence, Addiction, Hyperalgesia, and Tolerance. Adv. Redox Res. 2021, 2, 100003. [Google Scholar] [CrossRef]
- Hearing, M. Prefrontal-accumbens opioid plasticity: Implications for relapse and dependence. Pharmacol. Res. 2019, 139, 158–165. [Google Scholar] [CrossRef]
- Eckroat, T.J.; Manross, D.L.; Cowan, S.C. Merged tacrine-based, multitarget-directed acetylcholinesterase inhibitors 2015–present: Synthesis and biological activity. Int. J. Mol. Sci. 2020, 21, 5965. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Sierra, J.V.; García, F.I.S.; Brooks, J.H.; Derefinko, K.J.; Mozhui, K. Effect of short-term prescription opioids on DNA methylation of the OPRM1 promoter. Clin. Epigenetics 2020, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, H.; Shoda, T.; Mizota, T.; Fukuda, K. Morphine induces DNA damage and P53 activation in CD3+ T cells. Biochim. Biophys. Acta—Gen. Subj. 2009, 1790, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Tylleskar, I.; Skarra, S.; Skulberg, A.K.; Dale, O. The pharmacokinetic interaction between nasally administered naloxone and the opioid remifentanil in human volunteers. Eur. J. Clin. Pharmacol. 2021, 77, 1901–1908. [Google Scholar] [CrossRef]
- Campos, C.S.; Gradowski, F.C.N.T.; Almeida, B.P.F.; França, J.N.; Santos, R.B.; Cavalli, L.R.; Elifio-Esposito, S. An overview of neuroblastoma cell lineage phenotypes and in vitro models. Exp. Biol. Med. 2020, 245, 1637–1647. [Google Scholar] [CrossRef]
- Sumantran, V.N. Cellular chemosensitivity assays: An overview. In Cancer Cell Culture; Springer: Berlin/Heidelberg, Germany, 2011; pp. 219–236. [Google Scholar]
- Vermes, I.; Haanen, C.; Reutelingsperger, C. Flow cytometry of apoptotic cell death. J. Immunol. Methods 2000, 243, 167–190. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Ferraro, M.V.M.; Fenocchio, A.S.; Mantovani, M.S.; Ribeiro, C.O.; Cestari, M.M. Mutagenic effects of lead (Pb II) on the fish H. malabaricus as evaluated using the comet assay, piscine micronucleus and chromosome aberrations tests. Genet. Mol. Biol. 2004, 27, 103–107. [Google Scholar] [CrossRef]
- Collins, A.R.; Ai-Guo, M.; Duthie, S.J. The kinetics of repair of oxidative DNA damage (strand breaks and oxidised pyrimidines) in human cells. Mutat. Res. /DNA Repair. 1995, 336, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Keen, J.H.; Habig, W.H.; Jakoby, W.B. Mechanism for the several activities of the glutathione S-transferases. J. Biol. Chem. 1976, 251, 6183–6188. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Yuan, Z.; Zhao, Z.; Gao, X. Mechanism of pyrogallol autoxidation and determination of superoxide dismutase enzyme activity. Bioelect. Bioenerg. 1998, 45, 41–45. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Arya, A.; Azarmehr, N.; Mansourian, M.; Doustimotlagh, A.H. Inactivation of the superoxide dismutase by malondialdehyde in the nonalcoholic fatty liver disease: A combined molecular docking approach to clinical studies. Arch. Physiol. Biochem. 2021, 127, 557–564. [Google Scholar] [CrossRef]
- Kobzar, O.L.; Shulha, Y.V.; Buldenko, V.M.; Mrug, G.P.; Kolotylo, M.V.; Stanko, O.V.; Onysko, P.P.; Vovk, I. Alkyl and aryl α-ketophosphonate derivatives as photoactive compounds targeting glutathione S -transferases. Phosphorus Sulfur Silicon Relat. Ele. 2021, 96, 672–678. [Google Scholar] [CrossRef]
- Silva, F.D.; Nogara, P.A.; Ochoa-Rodríguez, E.; Nunez-Figueredo, Y.; Wong-Guerra, M.; Rosemberg, D.B.; Rocha, J.B.T. Molecular docking and in vitro evaluation of a new hybrid molecule (JM-20) on cholinesterase activity from different sources. Biochimie 2020, 168, 297–306. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Stewart Computational Chemistry—MOPAC Home Page. 2016. Available online: http://openmopac.net/home.html (accessed on 13 June 2022).
- Møllendal, H.; Balcells, D.; Eisenstein, O.; Syversen, L.; Suissa, M.R. Conformational complexity of morphine and morphinum in the gas phase and in water. A DFT and MP2 study. RSC Adv. 2014, 4, 24729–24735. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated Docking Using a Lamarckian Genetic Algorithm and an Empirical Binding Free Energy Function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- BIOVIA Discovery Studio. Accelrys Discovery Studio [WWW Document]. 2017. Available online: https://discover.3ds.com (accessed on 13 June 2022).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, L.S.; da Costa, N.d.S.; Galiciolli, M.E.A.; Pereira, M.E.; Almeida, W.; Margarete Cestari, M.; Nogara, P.A.; Irioda, A.C.; Oliveira, C.S. Assessment of Neurotoxic Effects of Oxycodone and Naloxone in SH-SY5Y Cell Line. Int. J. Mol. Sci. 2023, 24, 1424. https://doi.org/10.3390/ijms24021424
Lima LS, da Costa NdS, Galiciolli MEA, Pereira ME, Almeida W, Margarete Cestari M, Nogara PA, Irioda AC, Oliveira CS. Assessment of Neurotoxic Effects of Oxycodone and Naloxone in SH-SY5Y Cell Line. International Journal of Molecular Sciences. 2023; 24(2):1424. https://doi.org/10.3390/ijms24021424
Chicago/Turabian StyleLima, Luíza Siqueira, Nayara de Souza da Costa, Maria Eduarda Andrade Galiciolli, Meire Ellen Pereira, William Almeida, Marta Margarete Cestari, Pablo Andrei Nogara, Ana Carolina Irioda, and Cláudia Sirlene Oliveira. 2023. "Assessment of Neurotoxic Effects of Oxycodone and Naloxone in SH-SY5Y Cell Line" International Journal of Molecular Sciences 24, no. 2: 1424. https://doi.org/10.3390/ijms24021424
APA StyleLima, L. S., da Costa, N. d. S., Galiciolli, M. E. A., Pereira, M. E., Almeida, W., Margarete Cestari, M., Nogara, P. A., Irioda, A. C., & Oliveira, C. S. (2023). Assessment of Neurotoxic Effects of Oxycodone and Naloxone in SH-SY5Y Cell Line. International Journal of Molecular Sciences, 24(2), 1424. https://doi.org/10.3390/ijms24021424









