Integration of Metabolomics and Transcriptomics to Explore Dynamic Alterations in Fruit Color and Quality in ‘Comte de Paris’ Pineapples during Ripening Processes
Abstract
:1. Introduction
2. Results
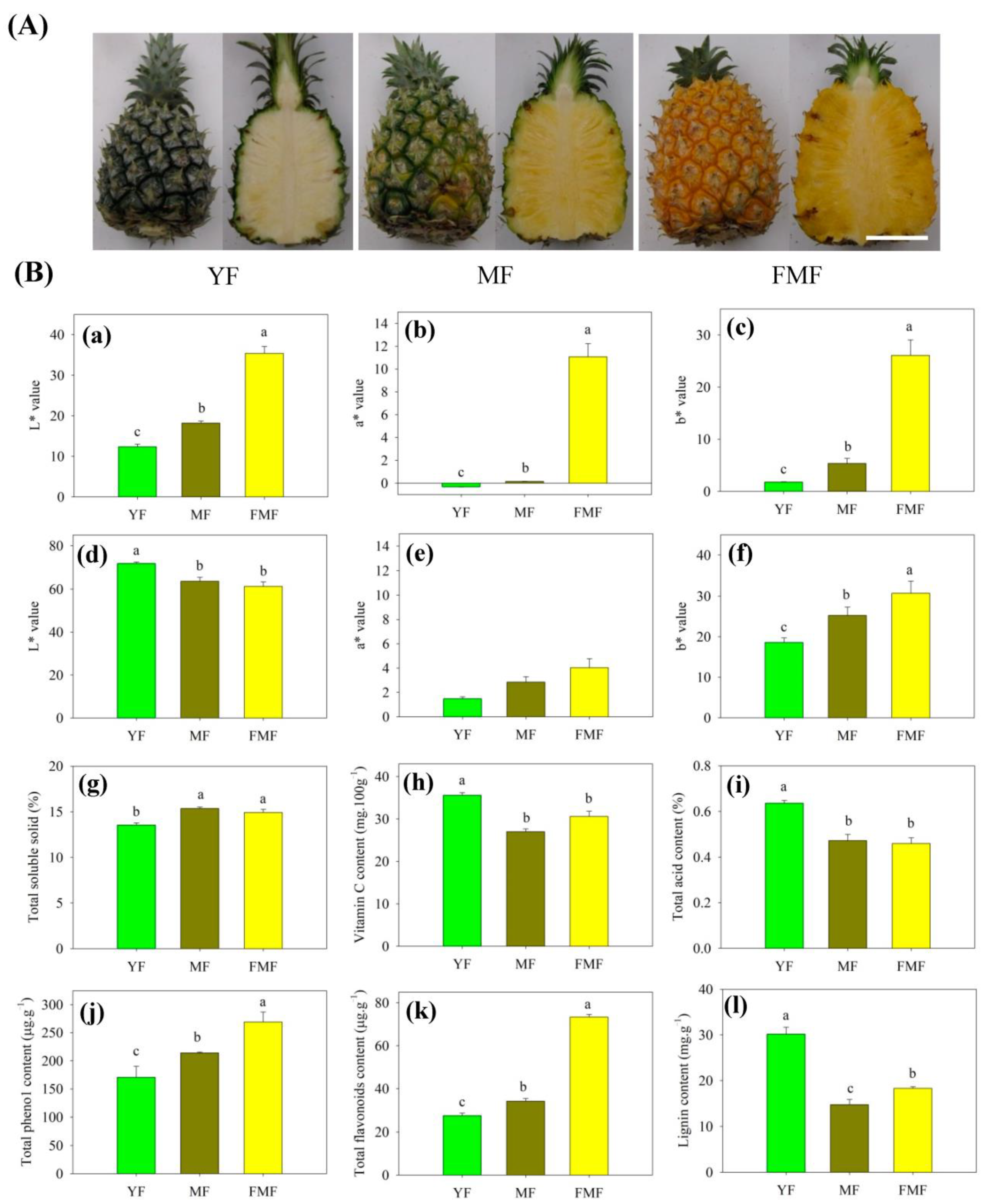
2.1. L*, a*, and b* Values of Peel and Pulp
2.2. Contents of SSC, TA, and Vc in Pulp
2.3. Contents of Total Phenolic, Total Flavonoids, and Lignin in Pulp
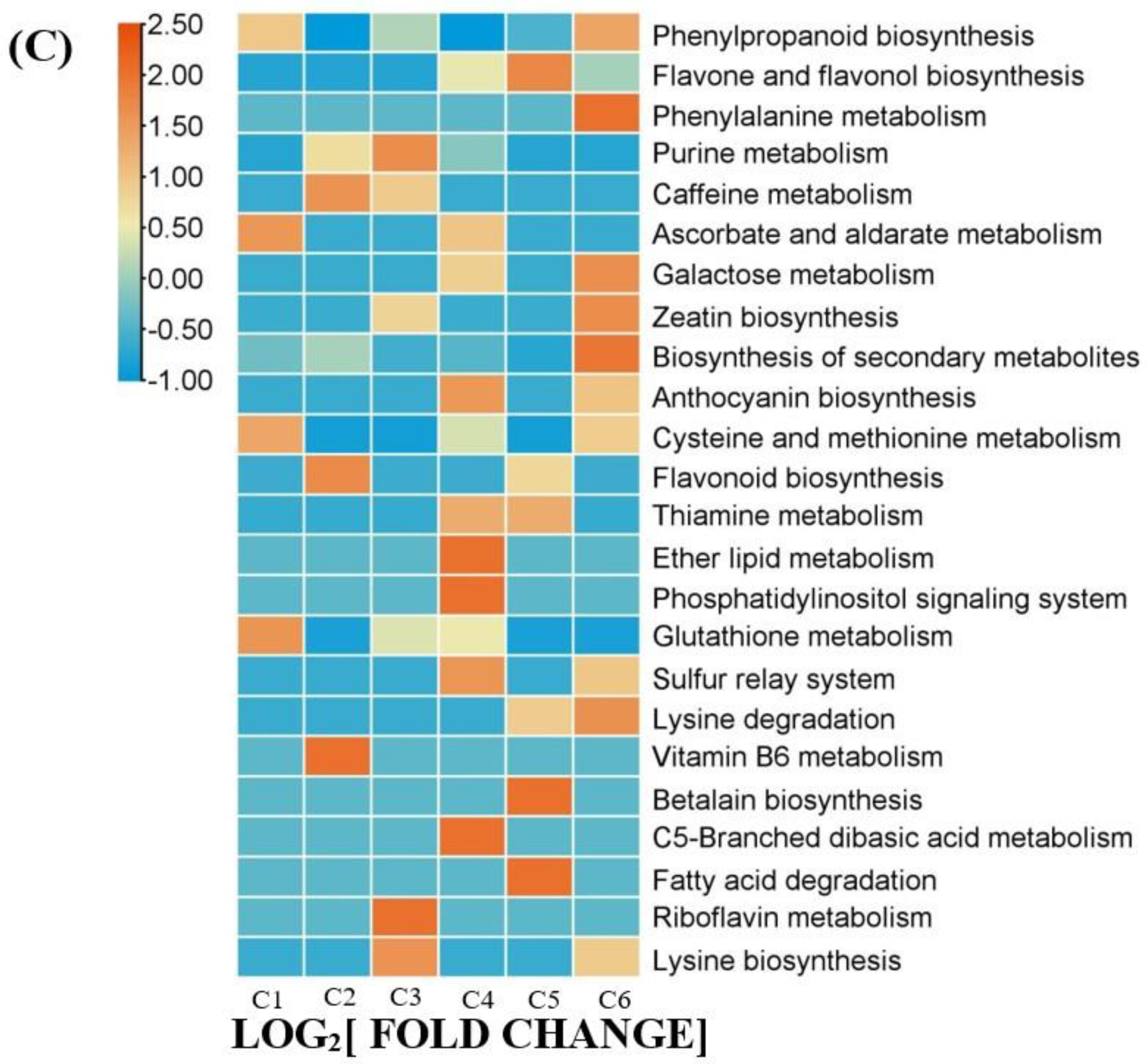
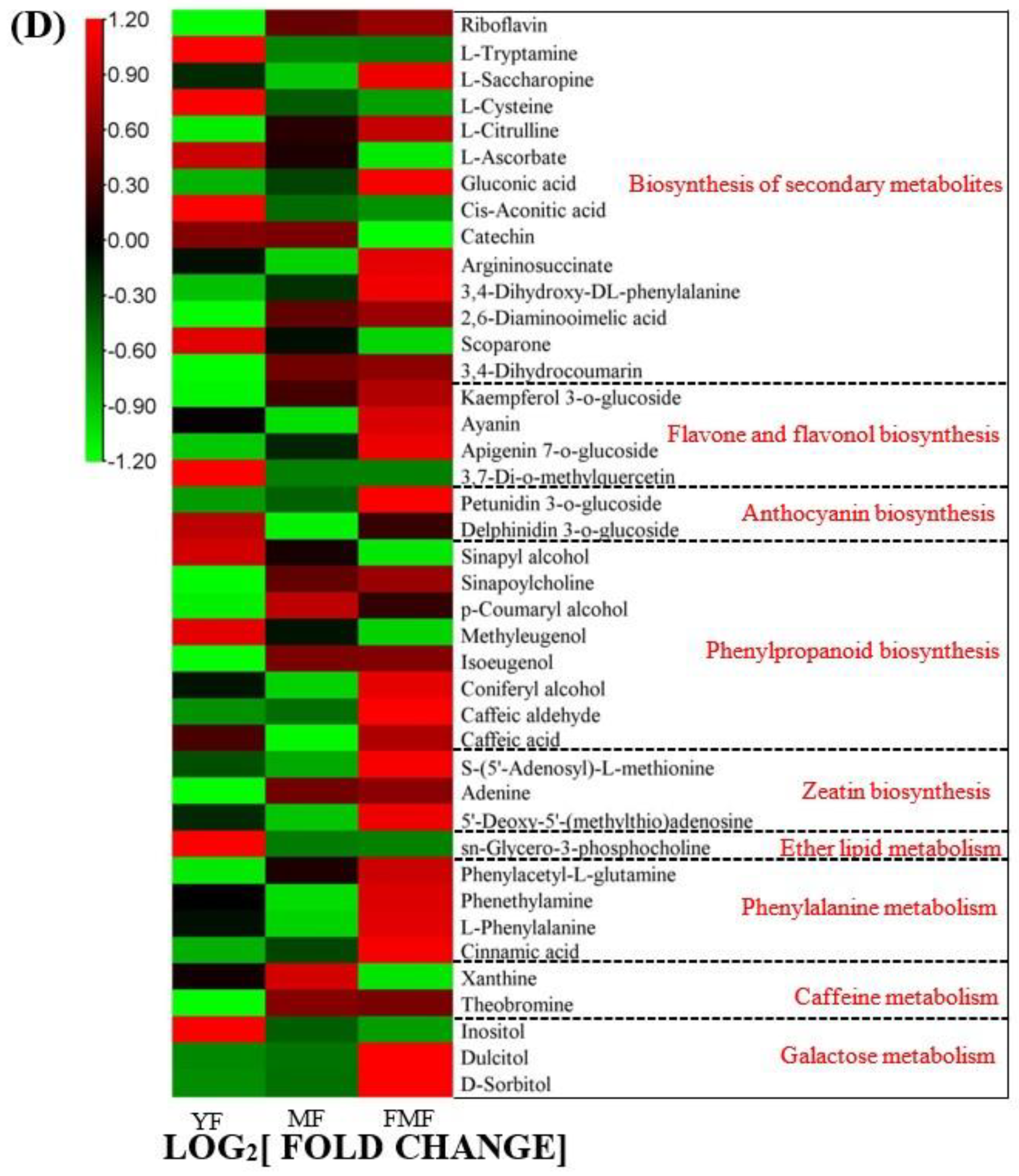
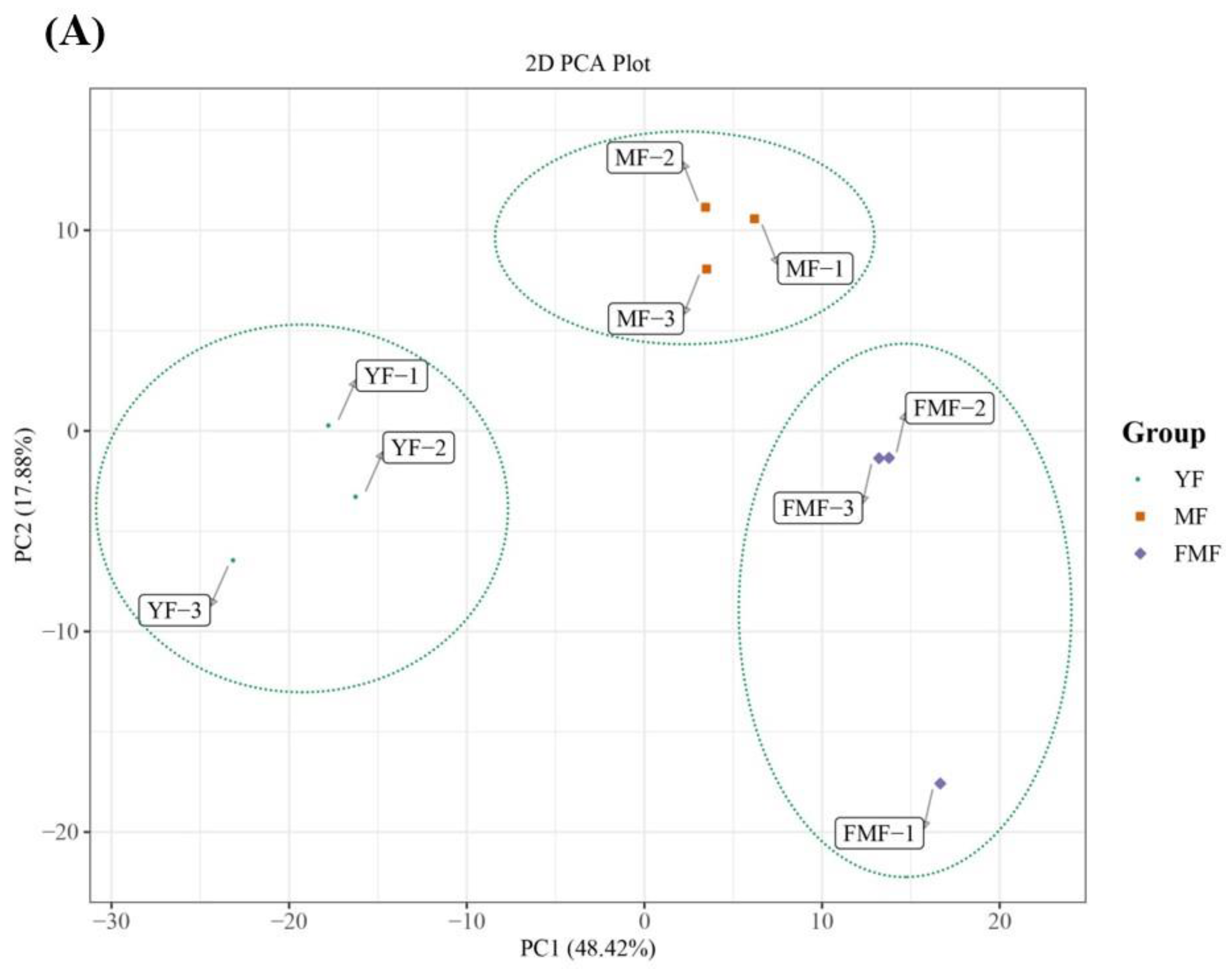
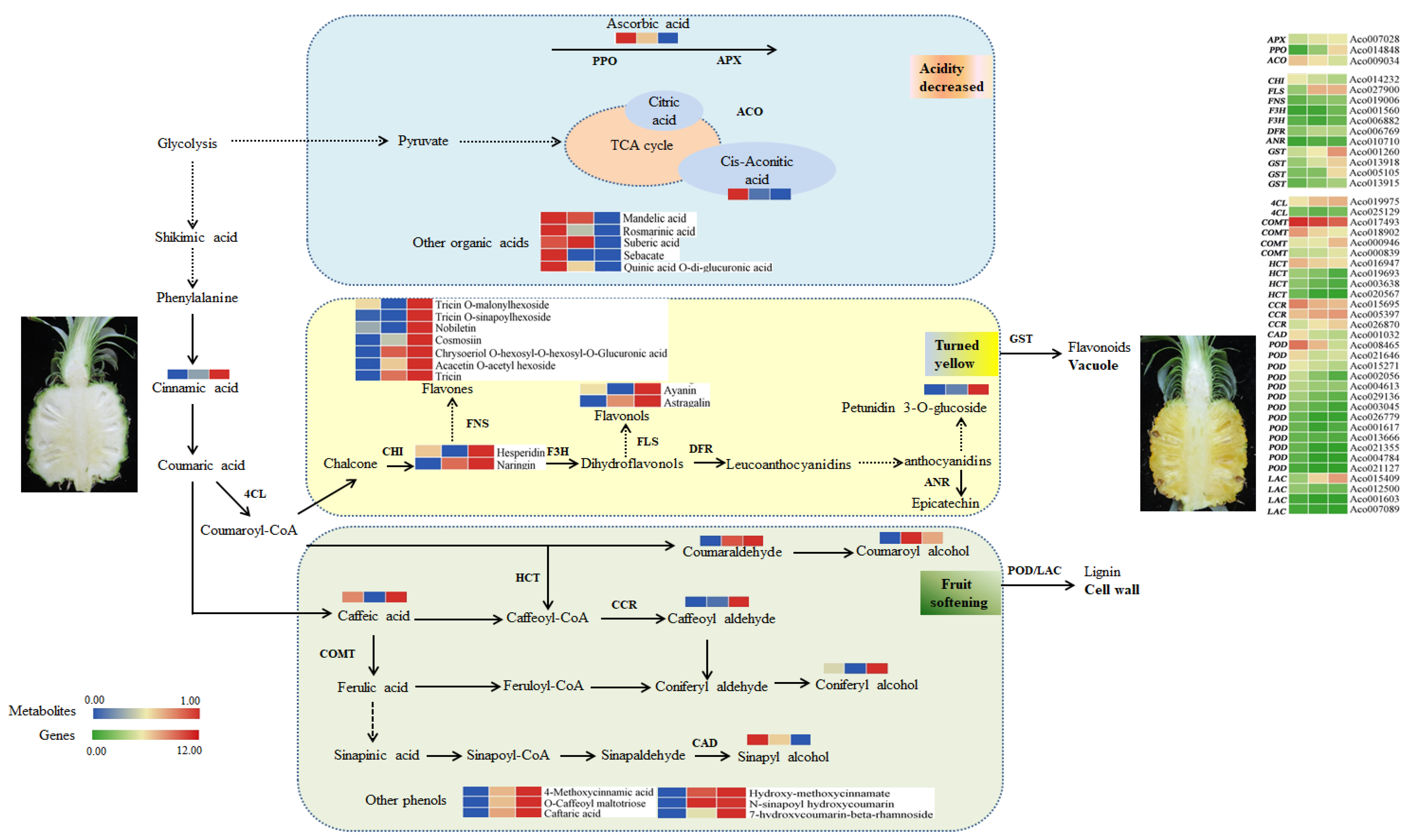
2.4. Differentially Accumulated Metabolites (DAMs) Annotated
2.5. DAMs Analysis
2.6. Transcriptome Profiling
2.6.1. Transcriptomics Analysis
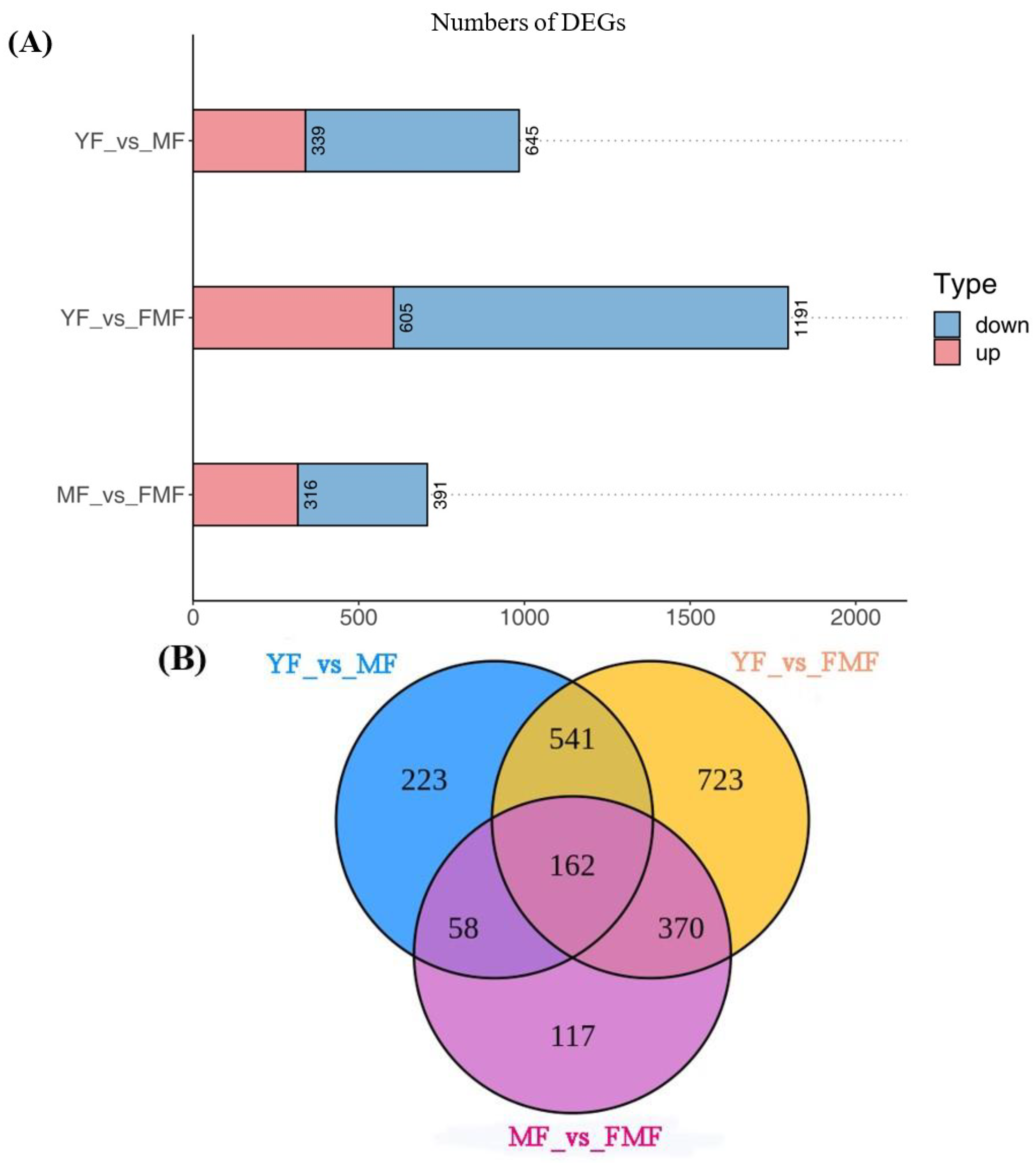
2.6.2. DEGs Analysis
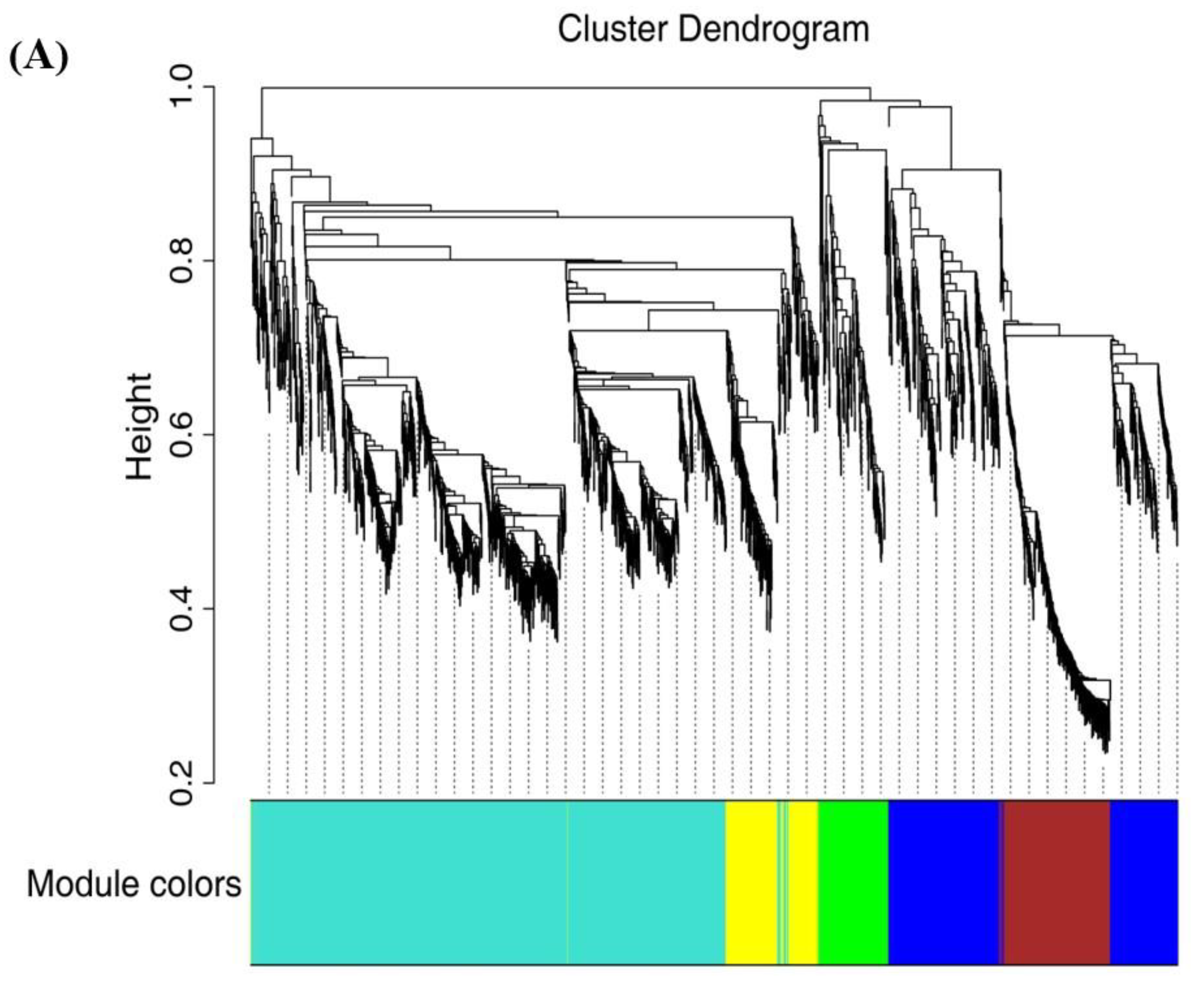
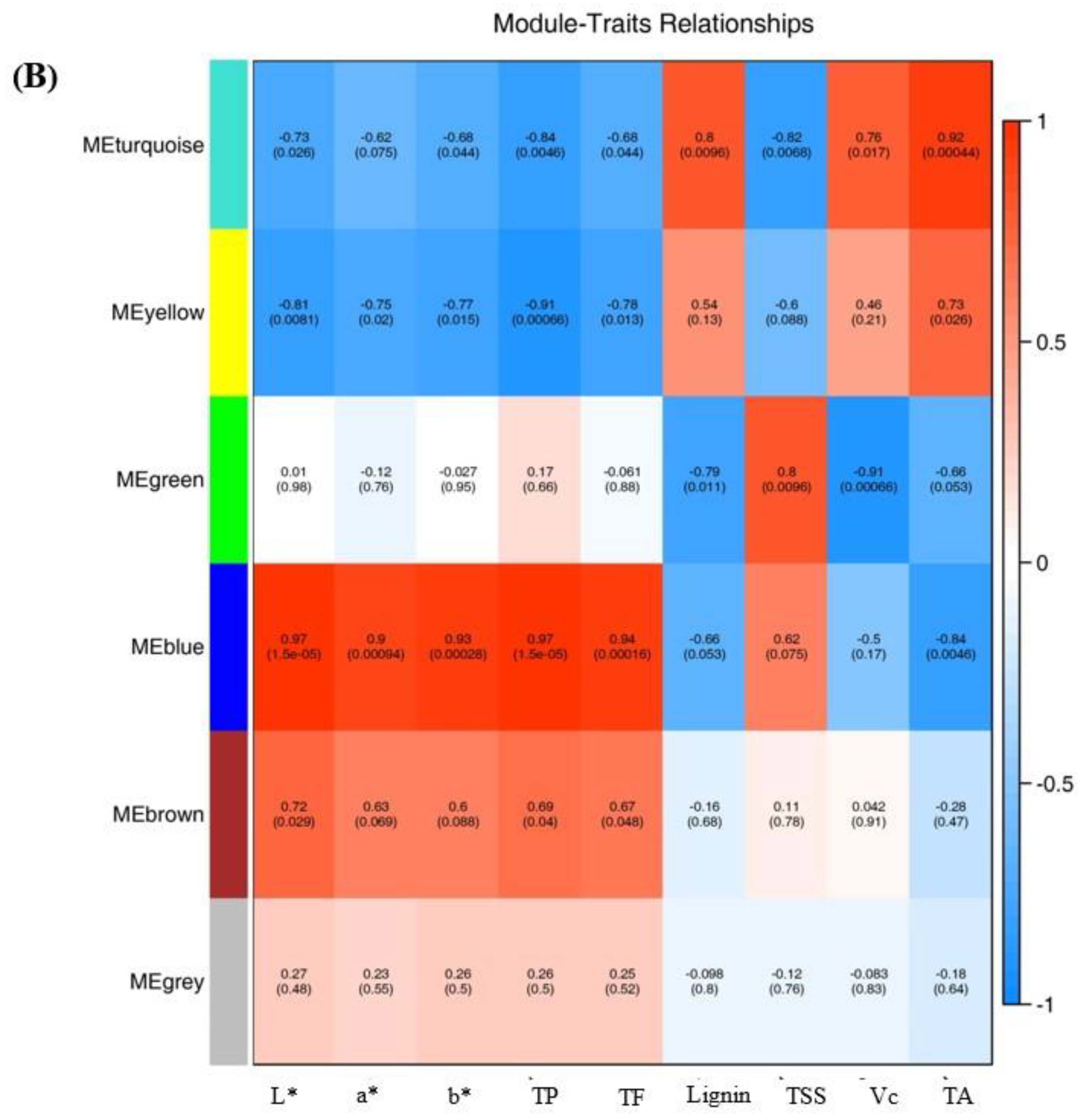
2.7. WGCNA Analysis and Gene Network Visualization
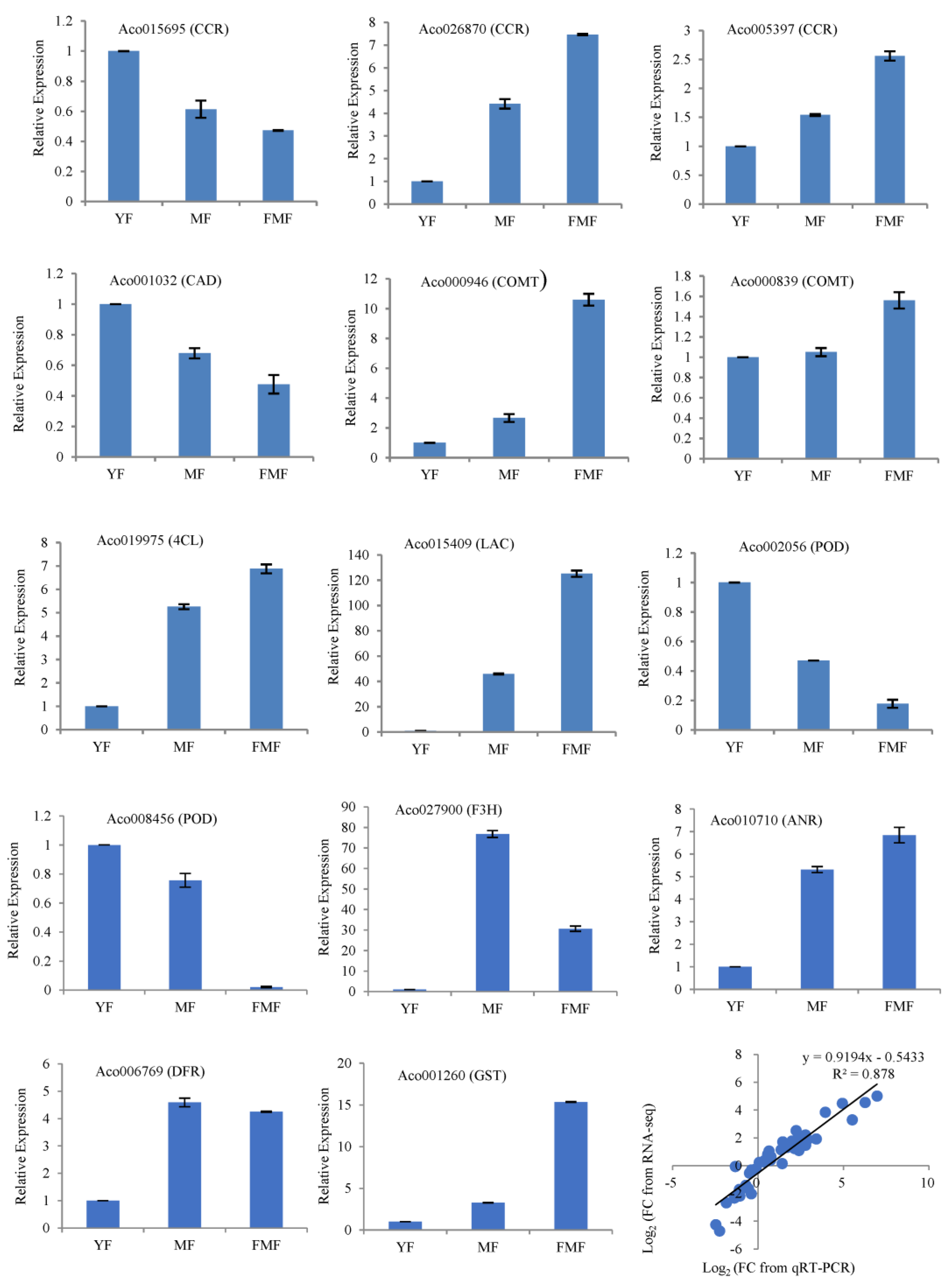
2.8. RT-qPCR Validation of DEGs
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Sampling
4.2. Evaluation of the Color Changes in Fruit
4.3. Measurements of Contents of Soluble Solids, Titratable Acid, and Vitamin C in Pulp
4.4. Extractions and Assays of Contents of Total Phenolic, Total Flavonoids, and Lignin in Pulp
4.5. RNA Extraction, Illumine Sequencing, and Transcriptomics Data Analysis
4.6. Weighted Gene Co-Expression Network Analysis and Gene Network Visualization
4.7. Quantitative Real-Time PCR (RT-qPCR) Verification
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, G.M.; Zhang, X.M.; Soler, A.; Marie-alphonsine, P.A. Nutritional composition of pineapple (Ananas comosus (L.) Merr.). In Nutritional Composition of Fruit Cultivars; Academic Press: Cambridge, MA, USA, 2016; pp. 609–637. [Google Scholar] [CrossRef]
- Ali, M.M.; Hashim, N.; Aziz, S.A.; Lasekan, O. Pineapple (Ananas comosus): A comprehensive review of nutritional values, volatile compounds, health benefits, and potential food products. Food Res. Int. 2020, 137, 109675. [Google Scholar] [CrossRef]
- Shuvo, M.S.H.; Rahman, S.B.; Mortuza, M.G.; Rahman, M.A. Changes in physicochemical properties of pineapple at different ripening stages during storage. J. Agrofor. Environ. 2019, 13, 1–2. [Google Scholar]
- Li, L.; Yi, P.; Sun, J.; Tang, J.; Liu, G.M.; Bi, J.F.; Teng, J.W.; Hu, M.J.; Yuan, F.; He, X.M.; et al. Genome-wide transcriptome analysis uncovers gene networks regulating fruit quality and volatile compounds in mango cultivar ‘Tainong’ during postharvest. Food Res. Int. 2023, 165, 112531. [Google Scholar] [CrossRef]
- Hassan, A.; Othman, Z.; Siriphanich, J. Pineapple (Ananas comosus L. Merr.). Postharvest Biol. Technol. Trop. Subtrop. Fruits. 2011, 10, 194–217. [Google Scholar] [CrossRef]
- Hu, Y.J.; Cheng, H.; Zhang, Y.; Zhang, J.; Niu, S.Q.; Wang, X.S.; Li, W.J.; Zhang, J.; Yao, Y.C. The MdMYB16/MdMYB1-miR7125-MdCCR module regulates the homeostasis between anthocyanin and lignin biosynthesis during light induction in apple. New Phytol. 2021, 231, 1105–1122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Wei, X.X.; Ali, M.M.; Rizwan, H.M.; Li, B.Q.; Li, H.; Jia, K.J.; Yang, X.L.; Ma, S.F.; Li, S.J.; et al. Changes in the content of organic acids and expression analysis of citric acid accumulation-related genes during fruit development of yellow (Passiflora edulis f. flavicarpa) and purple (Passiflora edulis f. edulis) passion fruits. Int. J. Mol. Sci. 2021, 22, 5765. [Google Scholar] [CrossRef] [PubMed]
- Palma, J.M.; Corpas, F.J.; Freschi, L.; Valpuesta, V. Editorial: Fruit ripening: From present knowledge to future development. Front. Plant Sci. 2019, 10, 545. [Google Scholar] [CrossRef]
- Song, K.H.; Gu, H.; John, B.G.; Penta, P.; Hou, X.W.; Zhang, L.B.; Hong, K.Q.; Yao, Q.S.; Zhang, X.M. Insight into the physiological and molecular mechanisms of hot air treatment which reduce internal browning in winter-harvested pineapples. Postharvest Biol. Technol. 2022, 194, 112066. [Google Scholar] [CrossRef]
- Koia, J.H.; Moyle, R.L.; Botella, J.R. Microarray analysis of gene expression profiles in ripening pineapple fruit. BMC Plant Biol. 2012, 12, 240. [Google Scholar] [CrossRef]
- Bartolomé, A.P.; Rupérez, P.; Fúster, C. Pineapple fruit: Morphological characteristics, chemical composition and sensory analysis of Red Spanish and Smooth Cayenne cultivars. Food Chem. 1995, 53, 75–79. [Google Scholar] [CrossRef]
- Ding, P.; Syazwani, S. Physicochemical quality, antioxidant compounds and activity of MD-2 pineapple fruit at five ripening stages. Int. Food Res. J. 2016, 23, 549–555. Available online: http://www.ifrj.upm.edu.my (accessed on 4 August 2015).
- Ikram, M.M.M.; Ridwani, S.; Putri, S.P.; Fukusaki, E. GC-MS based metabolite profiling to monitor ripening-specific metabolites in pineapple (Ananas comosus). Metabolites 2020, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.M.M.; Mizuno, R.; Putri, S.P.; Fukusaki, E. Comparative metabolomics and sensory evaluation of pineapple (Ananas comosus) reveal the importance of ripening stage compared to cultivar. J. Biosci. Bioeng. 2021, 132, 592–598. [Google Scholar] [CrossRef]
- Hong, K.Q.; Chen, L.; Gu, H.; Zhang, X.M.; Chen, J.; Shivraj, N.; Hu, M.J.; Gong, D.Q.; Song, K.H.; Hou, X.W.; et al. Novel insight into the relationship between metabolic profile and fatty acid accumulation altering cellular lipid content in pineapple fruits at different stages of maturity. J. Agric. Food Chem. 2021, 69, 8578–8589. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Zhu, X.; Hou, Y.Y.; Pan, Y.F.; Shi, L.; Li, X.H. Effects of harvest maturity stage on postharvest quality of winter jujube (Zizyphus jujuba Mill. Cv. Dongzao) fruit during cold storage. Sci. Hortic. 2021, 277, 109778. [Google Scholar] [CrossRef]
- Pillet, J.; Yu, H.W.; Chambers, A.H.; Whitaker, V.M.; Folta, K.M. Identification of candidate flavonoid pathway genes using transcriptome correlation network analysis in ripe strawberry (Fragaria × nannassa) fruit. J. Exp. Bot. 2015, 66, 4455–4467. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Ye, L.L.; Ding, X.C.; Gao, Q.Y.; Xiao, S.L.; Tan, Q.Q.; Huang, J.L.; Chen, W.X.; Li, X.P. Transcriptomic analysis reveals key factors in fruit ripening and rubbery texture caused by 1-MCP in papaya. BMC Plant Biol. 2019, 19, 309. [Google Scholar] [CrossRef]
- Zheng, B.B.; Zhao, L.; Jiang, X.H.; Cherono, S.; Liu, J.J.; Ogutu, C.; Ntini, C.; Zhang, X.J.; Han, Y.P. Assessment of organic acid accumulation and its related genes in peach. Food Chem. 2021, 334, 127567. [Google Scholar] [CrossRef]
- Cai, Y.B.; Yang, X.Y.; Sun, G.M.; Zhang, Z.L.; Huang, Q.; Liu, Y.Q.; Tang, Y.Y.; Wang, Y.R.; Li, M. Relationship among colors, pigments, and antioxidant activities of pineapple leaves. Plant Sci. J. 2017, 35, 283–290. [Google Scholar] [CrossRef]
- Liu, W.X.; Feng, Y.; Yu, S.H.; Fan, Z.Q.; Li, X.L.; Li, J.Y.; Yin, H.F. The flavonoid biosynthesis network in plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Qiao, F.; Zhang, K.M.; Zhou, L.Y.; Qiu, Q.S.; Chen, Z.N.; Lu, Y.H.; Wang, L.H.; Geng, G.G.; Xie, H.C. Analysis of flavonoid metabolism during fruit development of Lycium chinense. J. Plant Physiol. 2022, 279, 153856. [Google Scholar] [CrossRef]
- Fu, H.B.; Qiao, Y.J.; Wang, P.F.; Mu, X.P.; Zhang, J.C.; Fu, B.C.; Du, J.J. Changes of bioactive components and antioxidant potential during fruit development of Prunus humilis Bunge. PLoS ONE 2021, 16, e0251300. [Google Scholar] [CrossRef] [PubMed]
- Tohge, T.; Alseekh, S.; Fernie, A.R. On the regulation and function of secondary metabolism during fruit development and ripening. J. Exp. Bot. 2014, 65, 4599–4611. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.Q.; Lin, H.X. Contribution of phenylpropanoid metabolism to plant development and plant-environment interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef] [PubMed]
- Saradhuldhat, P.; Paull, R.E. Pineapple organic acid metabolism and accumulation during pineapple fruit development. Sci. Hortic. 2007, 112, 297–303. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Yao, Y.L.; Chen, X.; Wu, J.Y.; Wu, Q.S.; Liu, S.H.; Guo, A.P.; Zhang, X.M. Metabolomic and transcriptomic analyses reveal the mechanism of sweet-acidic taste formation during pineapple fruit development. Front. Plant Sci. 2022, 13, 971506. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Jin, Z.G.; Gu, M.; Li, J.B.; Tang, S.; Guo, Q.; Zhang, Y.P.; Zhang, W.; Li, Y.Z.; Huang, X.N.; et al. Microbial production of trans-aconitic acid. Metab. Eng. 2023, 78, 183–191. [Google Scholar] [CrossRef]
- Syazwani, S.; Nurliya, I.; Ding, P. Storage quality of ‘MD-2′ pineapple (Ananas comosus L.) fruits. Acta Hortic. 2012, 1012, 897–901. [Google Scholar] [CrossRef]
- Winkel-shirley, B. Flavonoid biosynthesis. A colorful model for genetics biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Albert, N.W.; Lorizzo, M.; Mengist, M.F.; Montanari, S.; Zalapa, J.; Maule, A.; Edger, P.P.; Yocca, A.E.; Platts, A.E.; Pucker, B.; et al. Vaccinium as a comparative system for understanding of complex flavonoid accumulation profiles and regulation in fruit. Plant Physiol. 2023, 192, 1696–1710. [Google Scholar] [CrossRef]
- Wang, L.X.; Chen, M.X.; Lam, P.Y.; Dini-Andreote, F.; Dai, W.; Wei, Z. Multifaceted roles of flavonoids mediating plant-microbe interactions. Microbiome 2022, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Niu, H.H.; Yun, Y.R.; Tian, J.; Lao, F.; Liao, X.J.; Gao, Z.Q.; Ren, D.B.; Zhou, L.Y. Analysis of coloration characteristics of Tunisian soft-seed pomegranate arils based on transcriptome and metabolome. Food Chem. 2022, 370, 131270. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.J.; Wu, H.X.; Yang, C.K.; Zhu, W.C.; Shi, B.; Zheng, B.; Wang, S.B.; Zhou, K.B.; Gao, A.P. RNA-seq reveals the key pathways and genes involved in the light-regulated flavonoids biosynthesis in mango (Mangifera indica L.) peel. Front. Plant Sci. 2023, 13, 1119384. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.N.; Sun, D.Y.; Wang, S.; Luo, S.; Fu, Y.Q.; Niu, L.X.; Shi, Q.Q.; Zhang, Y.L. Integrating full-length transcriptomics and metabolomics reveals the regulatory mechanisms underlying yellow pigmentation in tree peony (Paeonia suffruticosa Andr.) flowers. Hortic. Res. 2021, 8, 235. [Google Scholar] [CrossRef]
- Xu, B.; Escamilla-Treviño, L.L.; Sathitsuksanoh, N.; Shen, Z.X.; Shen, H.; Zhang, Y.P.; Dixon, R.A.; Zhao, B.Y. Silencing of 4-coumarate:coenzyme A ligase in switchgrass leads to reduced lignin content and improved fermentable sugar yields for biofuel production. New Phytol. 2011, 192, 611–625. [Google Scholar] [CrossRef]
- Eudes, A.; Pereira, J.H.; Yogiswara, S.; Wang, G.; Benites, V.T.; Baidoo, E.E.K.; Lee, T.S.; Adams, P.D.; Keasling, J.D.; Loqué, D. Exploiting the substrate promiscuity of hydroxycinnamoyl-CoA: Shikimate hydroxycinnamoyl transferase to reduce lignin. Plant Cell Physiol. 2016, 57, 568–579. [Google Scholar] [CrossRef]
- Yang, T.T.; Zhang, P.Y.; Pan, J.H.; Amanullah, S.; Luan, F.S.; Han, W.H.; Liu, H.Y.; Wang, X.Z. Genome-wide analysis of the peroxidase gene family and verification of lignin synthesis-related genes in watermelon. Int. J. Mol. Sci. 2022, 23, 642. [Google Scholar] [CrossRef]
- Kumari, A.; Dogra, V.; Joshi, R.; Kumar, S. Stress-responsive cis-regulatory elements underline podophyllotoxin biosynthesis and better performance of Sinopodophyllum hexandrum under water deficit conditions. Front. Plant Sci. 2022, 12, 2021. [Google Scholar] [CrossRef]
- Tanaka, N.; Murao, S. Difference between various copper-containing enzymes (Polyporus Laccase, Mushroom Tyrosinase and Cucumber Ascorbate Oxidase) and Bilirubin Oxidase. Agric. Biol. Chem. 1983, 47, 1627–1628. [Google Scholar] [CrossRef]
- Sadka, A.; Dahan, E.; Cohen, E.L.; March, K. Aconitase activity and expression during the development of lemon fruit. Physiol. Plant 2000, 108, 255–262. [Google Scholar] [CrossRef]
- Hong, K.Q.; Yao, Q.S.; Golding, J.B.; Pristijono, P.; Zhang, X.M.; Hou, X.W.; Yuan, D.B.; Li, Y.X.; Chen, L.; Song, K.H.; et al. Low temperature storage alleviates internal browning of ‘Comte de Paris’ winter pineapple fruit by reducing phospholipid degradation, phosphatidic acid accumulation and membrane lipid peroxidation processes. Food Chem. 2023, 404, 134656. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Y.; Li, H.; Xu, Z.H.; Guo, X.N.; Zhou, T.; Shi, J.L. Transcriptome profiling and identification of the candidate genes involved in early ripening in Ziziphus jujuba. Front. Genet. 2022, 13, 863746. [Google Scholar] [CrossRef]
- Hou, X.W.; Lu, Z.W.; Hong, K.Q.; Song, K.H.; Gu, H.; Hu, W.; Yao, Q.S. The class III peroxidase gene family is involved in ascorbic acid induced delay of internal browning in pineapple. Front. Plant Sci. 2022, 13, 953623. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.H.; Xu, D.Y.; Liu, C.H.; Chen, C.; Tian, M.X.; Jiang, A.L. Ascorbic acid treatment inhibits wound healing of fresh-cut potato strips by controlling phenylpropanoid metabolism. Postharvest Biol. Technol. 2021, 181, 111644. [Google Scholar] [CrossRef]
- Morrison, I.M. A semi-micro method for the determination of lignin and its use in predicting the digestibility of forage Crops. J. Sci. Food Agric. 1972, 23, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, K.; Zhang, X.; Liu, J.; Yao, Q.; Li, Y.; Hou, X.; Liu, S.; Qiu, X.; Yang, Y.; Chen, L.; et al. Integration of Metabolomics and Transcriptomics to Explore Dynamic Alterations in Fruit Color and Quality in ‘Comte de Paris’ Pineapples during Ripening Processes. Int. J. Mol. Sci. 2023, 24, 16384. https://doi.org/10.3390/ijms242216384
Song K, Zhang X, Liu J, Yao Q, Li Y, Hou X, Liu S, Qiu X, Yang Y, Chen L, et al. Integration of Metabolomics and Transcriptomics to Explore Dynamic Alterations in Fruit Color and Quality in ‘Comte de Paris’ Pineapples during Ripening Processes. International Journal of Molecular Sciences. 2023; 24(22):16384. https://doi.org/10.3390/ijms242216384
Chicago/Turabian StyleSong, Kanghua, Xiumei Zhang, Jiameng Liu, Quansheng Yao, Yixing Li, Xiaowan Hou, Shenghui Liu, Xunxia Qiu, Yue Yang, Li Chen, and et al. 2023. "Integration of Metabolomics and Transcriptomics to Explore Dynamic Alterations in Fruit Color and Quality in ‘Comte de Paris’ Pineapples during Ripening Processes" International Journal of Molecular Sciences 24, no. 22: 16384. https://doi.org/10.3390/ijms242216384
APA StyleSong, K., Zhang, X., Liu, J., Yao, Q., Li, Y., Hou, X., Liu, S., Qiu, X., Yang, Y., Chen, L., Hong, K., & Lin, L. (2023). Integration of Metabolomics and Transcriptomics to Explore Dynamic Alterations in Fruit Color and Quality in ‘Comte de Paris’ Pineapples during Ripening Processes. International Journal of Molecular Sciences, 24(22), 16384. https://doi.org/10.3390/ijms242216384




