Macrocystis pyrifera Lipids Reduce Cytokine-Induced Pro-Inflammatory Signalling and Barrier Dysfunction in Human Keratinocyte Models
Abstract
:1. Introduction
2. Results
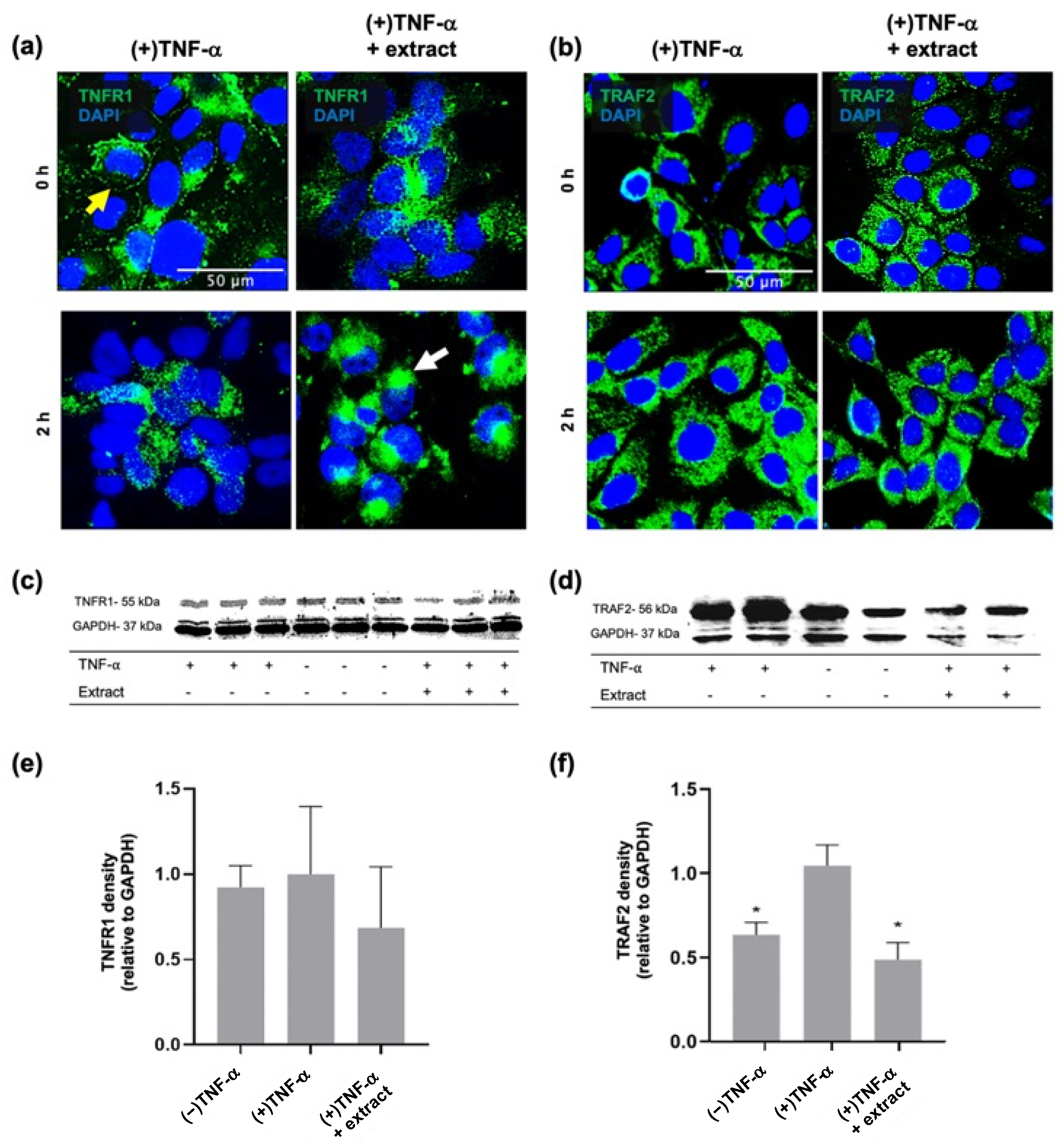
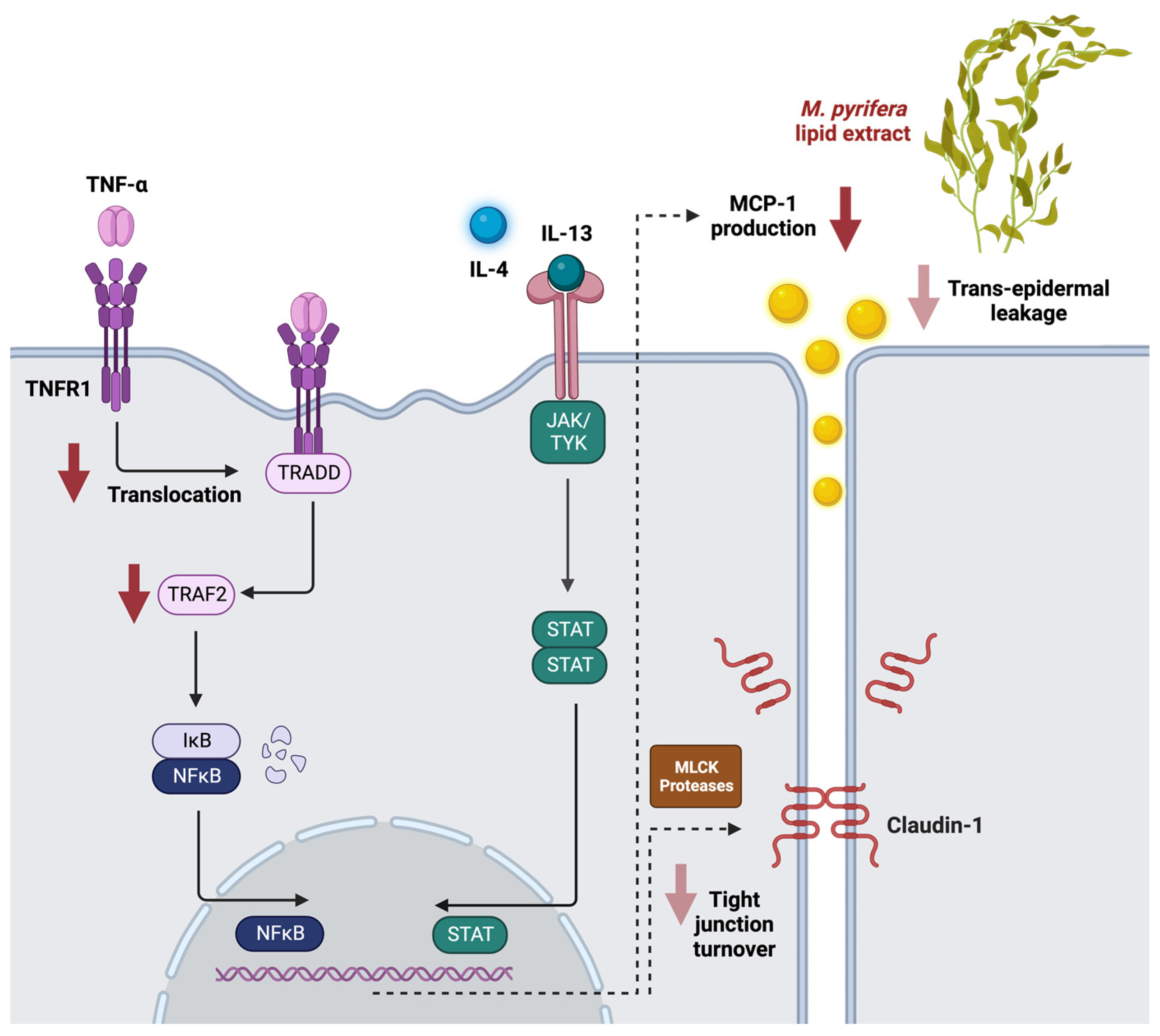
2.1. M. pyrifera Lipids Inhibit Pro-Inflammatory Signalling in Keratinocyte Monolayers Stimulated by the Th1 Cytokine TNF-α
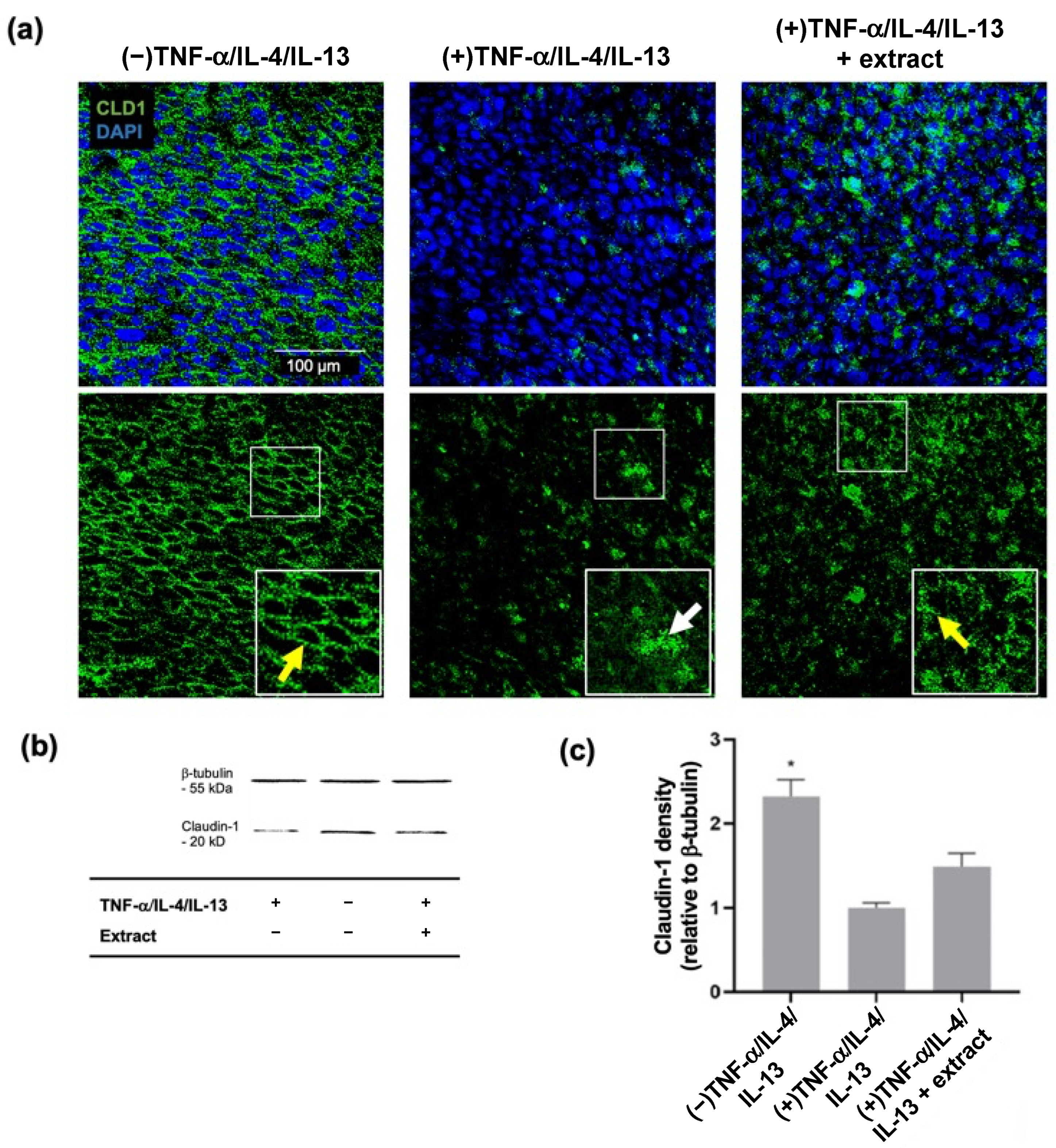
2.2. M. pyrifera Lipids Do Not Prevent Disruption of Claudin-1 Tight Junctions in Keratinocyte Monolayers Stimulated by Th1 and Th2 Cytokines
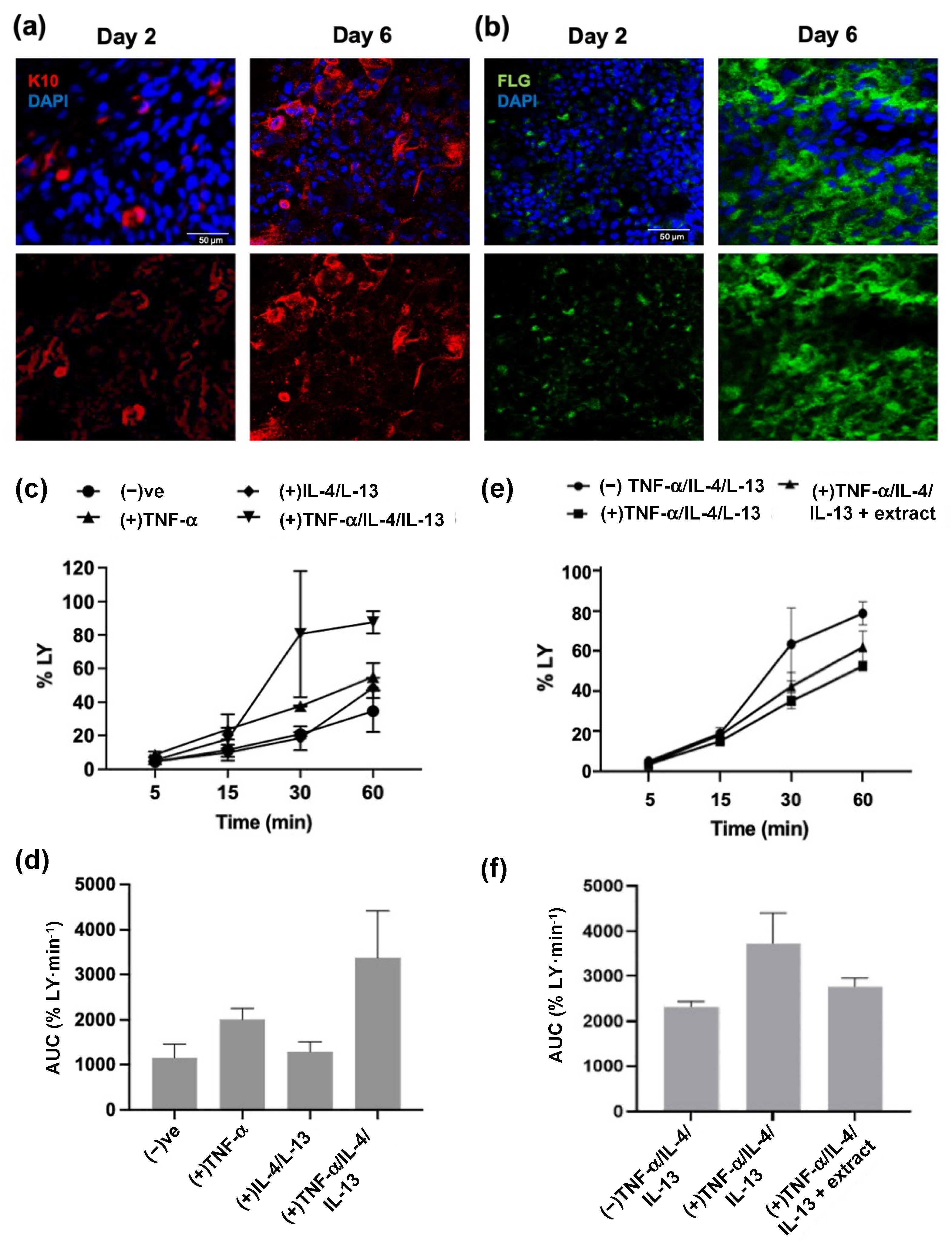
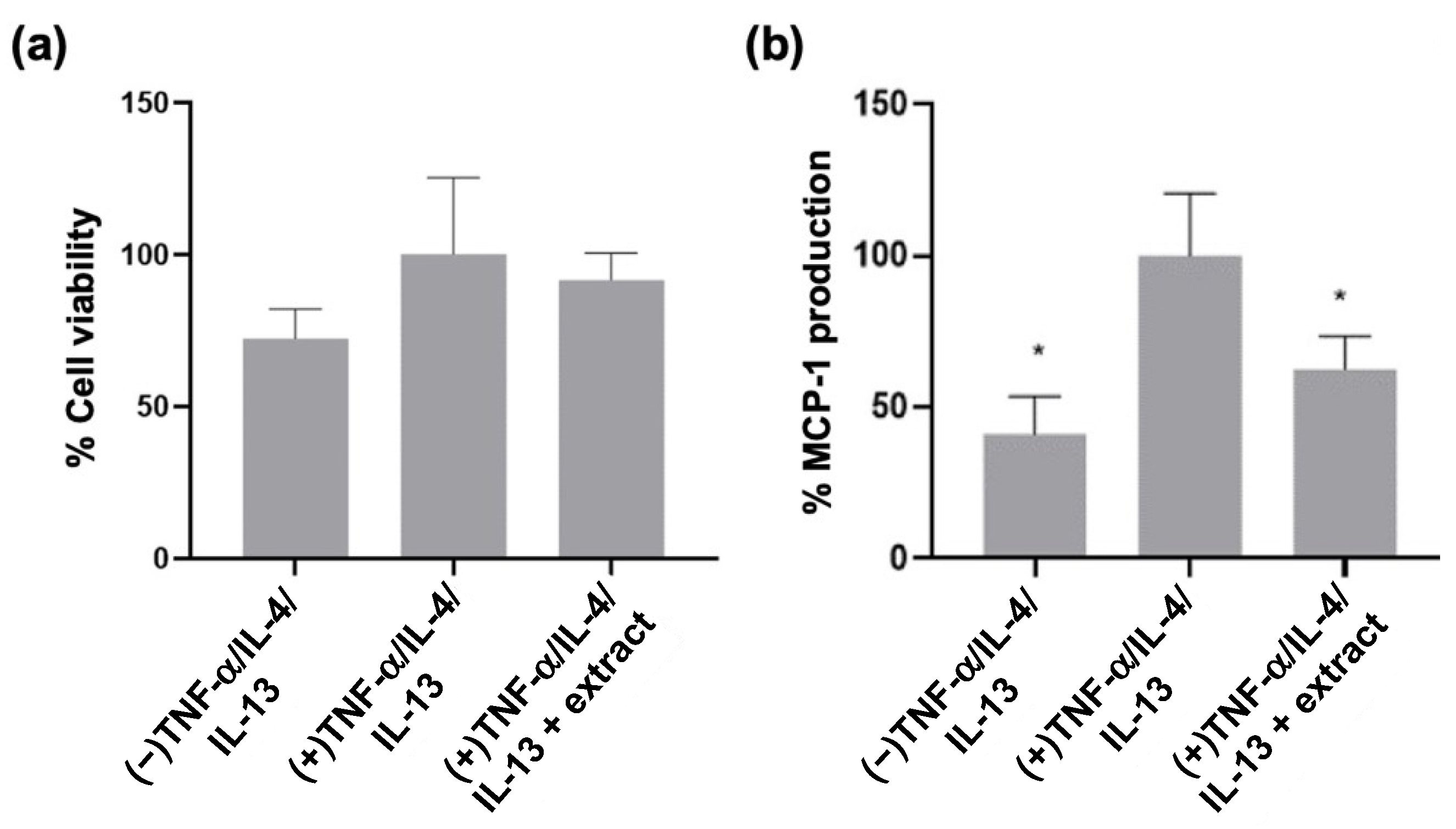
2.3. M. pyrifera Lipids, in Part, Reduce Trans-Epidermal Leakage, Chemokine Production, and Claudin-1 Tight Junction Disruption in 3D Epidermal Constructs Stimulated by Th1 and Th2 Cytokines
2.4. Fatty Acid Composition of M. pyrifera Lipid Extracts
3. Discussion
4. Materials and Methods
4.1. Total Lipid Extracts
4.2. HaCaT Cell Monolayer Culture, Treatment, and Processing
4.3. 3D Epidermal Construct Culture, Treatment, and Processing
4.4. Cytotoxicity Assays
4.5. ELISAs
4.6. Immunocytochemistry
4.7. Whole-Mount Immunocytochemistry and Confocal Microscopy
4.8. Western Blotting
4.9. Lucifer Yellow Dye Exclusion Assay
4.10. GC-MS
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Archer, C.B. Atopic dermatitis. Medicine 2021, 49, 370–373. [Google Scholar] [CrossRef]
- Peng, W.; Novak, N. Pathogenesis of atopic dermatitis. Clin. Exp. Allergy 2015, 45, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.X.; Landén, N.X. New insights into T cells and their signature cytokines in atopic dermatitis. IUBMB Life 2015, 67, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Koria, P.; Andreadis, S.T. Epidermal morphogenesis: The transcriptional program of human keratinocytes during stratification. J. Investig. Dermatol. 2006, 126, 1834–1841. [Google Scholar] [CrossRef]
- Lefèvre-Utile, A.; Braun, C.; Haftek, M.; Aubin, F. Five Functional Aspects of the Epidermal Barrier. Int. J. Mol. Sci. 2021, 22, 11676. [Google Scholar] [CrossRef]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef]
- Knox, S.; O’Boyle, N.M. Skin lipids in health and disease: A review. Chem. Phys. Lipids 2021, 236, 105055. [Google Scholar] [CrossRef]
- Brandner, J.; Zorn-Kruppa, M.; Yoshida, T.; Moll, I.; Beck, L.; De Benedetto, A. Epidermal tight junctions in health and disease. Tissue Barriers 2015, 3, e974451. [Google Scholar] [CrossRef]
- Kirschner, N.; Rosenthal, R.; Furuse, M.; Moll, I.; Fromm, M.; Brandner, J.M. Contribution of Tight Junction Proteins to Ion, Macromolecule, and Water Barrier in Keratinocytes. J. Investig. Dermatol. 2013, 133, 1161–1169. [Google Scholar] [CrossRef]
- Tokumasu, R.; Tamura, A.; Tsukita, S. Time- and dose-dependent claudin contribution to biological functions: Lessons from claudin-1 in skin. Tissue Barriers 2017, 5, e1336194. [Google Scholar] [CrossRef]
- Volksdorf, T.; Heilmann, J.; Eming, S.A.; Schawjinski, K.; Zorn-Kruppa, M.; Ueck, C.; Vidal-y-Sy, S.; Windhorst, S.; Jücker, M.; Moll, I. Tight junction proteins claudin-1 and occludin are important for cutaneous wound healing. Am. J. Path 2017, 187, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: A lesson from claudin-1-deficient mice. J. Cell Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef]
- Yokouchi, M.; Kubo, A.; Kawasaki, H.; Yoshida, K.; Ishii, K.; Furuse, M.; Amagai, M. Epidermal tight junction barrier function is altered by skin inflammation, but not by filaggrin-deficient stratum corneum. J. Dermatol. Sci. 2015, 77, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, S.; von Buenau, B.; Vidal-y-Sy, S.; Haftek, M.; Wladykowski, E.; Houdek, P.; Lezius, S.; Duplan, H.; Bäsler, K.; Dähnhardt-Pfeiffer, S. Claudin-1 decrease impacts epidermal barrier function in atopic dermatitis lesions dose-dependently. Sci. Rep. 2020, 10, 2024. [Google Scholar] [CrossRef]
- Proksch, E.; Brasch, J. Abnormal epidermal barrier in the pathogenesis of contact dermatitis. Clin. Dermatol. 2012, 30, 335–344. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.E.; Berdyshev, E.; Bronova, I.; Bin, L.; Bae, J.; Kim, S.; Kim, H.-Y.; Lee, U.H.; Kim, M.S.; et al. Staphylococcus aureus causes aberrant epidermal lipid composition and skin barrier dysfunction. Allergy 2023, 78, 1292–1306. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Yoon, J.-S.; Yoshihara, T.; Iwasaki, T.; Nishifuji, K. Increased transepidermal water loss and decreased ceramide content in lesional and non-lesional skin of dogs with atopic dermatitis. Vet. Dermatol. 2009, 20, 541–546. [Google Scholar] [CrossRef]
- Yen, C.-H.; Dai, Y.-S.; Yang, Y.-H.; Wang, L.-C.; Lee, J.-H.; Chiang, B.-L. Linoleic acid metabolite levels and transepidermal water loss in children with atopic dermatitis. Ann. Allergy Asthma Immunol. 2008, 100, 66–73. [Google Scholar] [CrossRef]
- Chen, L.; Martinez, O.; Overbergh, L.; Mathieu, C.; Prabhakar, B.S.; Chan, L.S. Early up-regulation of Th2 cytokines and late surge of Th1 cytokines in an atopic dermatitis model. Clin. Exp. Immunol. 2004, 138, 375–387. [Google Scholar] [CrossRef]
- Fania, L.; Moretta, G.; Antonelli, F.; Scala, E.; Abeni, D.; Albanesi, C.; Madonna, S. Multiple Roles for Cytokines in Atopic Dermatitis: From Pathogenic Mediators to Endotype-Specific Biomarkers to Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 2684. [Google Scholar] [CrossRef]
- Gruber, R.; Börnchen, C.; Rose, K.; Daubmann, A.; Volksdorf, T.; Wladykowski, E.; Vidal-y-Sy, S.; Peters, E.M.; Danso, M.; Bouwstra, J.A. Diverse regulation of claudin-1 and claudin-4 in atopic dermatitis. Am. J. Pathol. 2015, 185, 2777–2789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chinnappan, M.; Prestwood, C.A.; Edwards, M.; Artami, M.; Thompson, B.M.; Eckert, K.M.; Vale, G.; Zouboulis, C.C.; McDonald, J.G. Interleukins 4 and 13 drive lipid abnormalities in skin cells through regulation of sex steroid hormone synthesis. Proc. Nat. Acad. Sci. USA 2021, 118, e2100749118. [Google Scholar] [CrossRef] [PubMed]
- Banno TGazel, A.; Blumenberg, M.; Adachi, M.; Mukkamala, L. Effects of tumor necrosis factor-α (TNF-α) in epidermal keratinocytes revealed using global transcriptional profiling. J. Biol. Chem. 2004, 279, 32633–32642. [Google Scholar] [CrossRef]
- Giustizieri, M.L.; Mascia, F.; Frezzolini, A.; De Pità, O.; Chinni, L.M.; Giannetti, A.; Girolomoni, G.; Pastore, S. Keratinocytes from patients with atopic dermatitis and psoriasis show a distinct chemokine production profile in response to T cell–derived cytokines. J. Allergy Clin. Immunol. 2001, 107, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Muppidi, J.R.; Tschopp, J.; Siegel, R.M. Life And Death Decisions: Secondary Complexes and Lipid Rafts in TNF Receptor Family Signal Transduction. Immunity 2004, 21, 461–465. [Google Scholar] [CrossRef]
- Mayba, J.N.; Gooderham, M.J. Review of Atopic Dermatitis and Topical Therapies. J. Cutan. Med. Surg. 2017, 21, 227–236. [Google Scholar] [CrossRef]
- Hon, K.L.; Kung, J.S.C.; Ng, W.G.G.; Leung, T.F. Emollient treatment of atopic dermatitis: Latest evidence and clinical considerations. Drugs Context 2018, 7, 212530. [Google Scholar] [CrossRef]
- Anderson, J.; Maghfour, J.; Hamp, A.; Christensen, A.; Dellavalle, R.P. From the Cochrane Library: Emollients and Moisturizers for Eczema. Dermatology 2021, 238, 594–596. [Google Scholar] [CrossRef]
- Johnson, B.B.; Franco, A.I.; Beck, L.A.; Prezzano, J.C. Treatment-resistant atopic dermatitis: Challenges and solutions. Clin. Cosmet. Investig. Dermatol. 2019, 12, 181–192. [Google Scholar] [CrossRef]
- Netzlaff, F.; Lehr, C.-M.; Wertz, P.; Schaefer, U. The human epidermis models EpiSkin®, SkinEthic® and EpiDerm®: An evaluation of morphology and their suitability for testing phototoxicity, irritancy, corrosivity, and substance transport. Eur. J. Pharm. Biopharm. 2005, 60, 167–178. [Google Scholar] [CrossRef]
- Zhang, Z.; Michniak-Kohn, B.B. Tissue engineered human skin equivalents. Pharmaceutics 2012, 4, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Sutaria, N.; Roh, Y.S.; Bordeaux, Z.; Alphonse, M.P.; Kwatra, S.G.; Kwatra, M.M. Translational Relevance of Mouse Models of Atopic Dermatitis. J. Clin. Med. 2021, 10, 613. [Google Scholar] [CrossRef] [PubMed]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D. HaCaT cells as a reliable in vitro differentiation model to dissect the inflammatory/repair response of human keratinocytes. Mediators Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef] [PubMed]
- Strudwick, X.L.; Lang, D.L.; Smith, L.E.; Cowin, A.J. Combination of low calcium with Y-27632 rock inhibitor increases the proliferative capacity, expansion potential and lifespan of primary human keratinocytes while retaining their capacity to differentiate into stratified epidermis in a 3D skin model. PLoS ONE 2015, 10, e0123651. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Baek, J.; Lee, J.R.; Roh, J.Y.; Jung, Y. Optimization of cytokine milieu to reproduce atopic dermatitis-related gene expression in HaCaT keratinocyte cell line. Immune Netw. 2018, 18, e9. [Google Scholar] [CrossRef]
- Tebbe, B.; Mankertz, J.; Schwarz, C.; Amasheh, S.; Fromm, M.; Assaf, C.; Schultz-Ehrenburg, U.; Sanchez Ruderisch, H.; Schulzke, J.-D.; Orfanos, C. Tight junction proteins: A novel class of integral membrane proteins. Arch. Dermatol. Res. 2002, 294, 14–18. [Google Scholar] [CrossRef]
- Schoop, V.M.; Fusenig, N.E.; Mirancea, N. Epidermal organization and differentiation of HaCaT keratinocytes in organotypic coculture with human dermal fibroblasts. J. Investig. Dermatol. 1999, 112, 343–353. [Google Scholar] [CrossRef]
- Breitkreutz, D.; Schoop, V.M.; Mirancea, N.; Baur, M.; Stark, H.-J.; Fusenig, N.E. Epidermal differentiation and basement membrane formation by HaCaT cells in surface transplants. Eur. J. Cell Biol. 1998, 75, 273–286. [Google Scholar] [CrossRef]
- Gallegos-Alcalá, P.; Jiménez, M.; Cervantes-García, D.; Salinas, E. The Keratinocyte as a Crucial Cell in the Predisposition, Onset, Progression, Therapy and Study of the Atopic Dermatitis. Int. J. Mol. Sci. 2021, 22, 10661. [Google Scholar] [CrossRef] [PubMed]
- Klicks, J.; von Molitor, E.; Ertongur-Fauth, T.; Rudolf, R.; Hafner, M. In vitro skin three-dimensional models and their applications. J. Cell Biotechnol. 2017, 3, 21–39. [Google Scholar] [CrossRef]
- Wu, S.; Pang, Y.; He, Y.; Zhang, X.; Peng, L.; Guo, J.; Zeng, J. A comprehensive review of natural products against atopic dermatitis: Flavonoids, alkaloids, terpenes, glycosides and other compounds. Biomed. Pharmacother. 2021, 140, 111741. [Google Scholar] [CrossRef] [PubMed]
- Jesumani, V.; Du, H.; Aslam, M.; Pei, P.; Huang, N. Potential Use of Seaweed Bioactive Compounds in Skincare-A Review. Mar. Drugs 2019, 17, 688. [Google Scholar] [CrossRef]
- López-Hortas, L.; Flórez-Fernández, N.; Torres, M.D.; Ferreira-Anta, T.; Casas, M.P.; Balboa, E.M.; Falqué, E.; Domínguez, H. Applying Seaweed Compounds in Cosmetics, Cosmeceuticals and Nutricosmetics. Mar. Drugs 2021, 19, 552. [Google Scholar] [CrossRef] [PubMed]
- Kok, J.M.L.; Dowd, G.; Cabral, J.; Wise, L. Time-Dependent Anti-inflammatory Effects of a Lipid Extract from Macrocystis pyrifera on Toll-Like Receptor 2 Signaling in Human THP-1 Monocytes. Planta Med. Int. Open 2022, 9, e80–e89. [Google Scholar] [CrossRef]
- Khan, M.N.; Choi, J.S.; Lee, M.C.; Kim, E.; Nam, T.J.; Fujii, H.; Hong, Y.K. Anti-inflammatory activities of methanol extracts from various seaweed species. J. Environ. Biol. 2008, 29, 465–469. [Google Scholar]
- Le, Q.-T.; Li, Y.; Qian, Z.-J.; Kim, M.-M.; Kim, S.-K. Inhibitory effects of polyphenols isolated from marine alga Ecklonia cava on histamine release. Process Biochem. 2009, 44, 168–176. [Google Scholar] [CrossRef]
- Hwang, P.-A.; Chien, S.-Y.; Chan, Y.-L.; Lu, M.-K.; Wu, C.-H.; Kong, Z.-L.; Wu, C.-J. Inhibition of lipopolysaccharide (LPS)-induced inflammatory responses by Sargassum hemiphyllum sulfated polysaccharide extract in RAW 264.7 macrophage cells. J. Agric. Food Chem. 2011, 59, 2062–2068. [Google Scholar] [CrossRef]
- Sanjeewa, K.; Jayawardena, T.U.; Kim, H.-S.; Kim, S.-Y.; Ahn, G.; Kim, H.-J.; Fu, X.; Jee, Y.; Jeon, Y.-J. Ethanol extract separated from Sargassum horneri (Turner) abate LPS-induced inflammation in RAW 264.7 macrophages. Fish. Aquat. Sci. 2019, 22, 6. [Google Scholar] [CrossRef]
- Gil, T.-Y.; Kang, Y.-M.; Eom, Y.-J.; Hong, C.-H.; An, H.-J. Anti-atopic dermatitis effect of seaweed fulvescens extract via inhibiting the STAT1 pathway. Mediators Inflamm. 2019, 2019, 3760934. [Google Scholar] [CrossRef]
- Yang, H.-S.; Haj, F.G.; Lee, M.; Kang, I.; Zhang, G.; Lee, Y. Laminaria japonica extract enhances intestinal barrier function by altering inflammatory response and tight junction-related protein in lipopolysaccharide-stimulated Caco-2 cells. Nutrients 2019, 11, 1001. [Google Scholar] [CrossRef]
- Iraha, A.; Chinen, H.; Hokama, A.; Yonashiro, T.; Kinjo, T.; Kishimoto, K.; Nakamoto, M.; Hirata, T.; Kinjo, N.; Higa, F. Fucoidan enhances intestinal barrier function by upregulating the expression of claudin-1. World J. Gastroenterol. 2013, 19, 5500. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.-A.; Phan, N.N.; Lu, W.-J.; Hieu, B.T.N.; Lin, Y.-C. Low-molecular-weight fucoidan and high-stability fucoxanthin from brown seaweed exert prebiotics and anti-inflammatory activities in Caco-2 cells. Food Nutr. Res. 2016, 60, 32033. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-H.; Lim, Y.; Kim, E.J.; Ko, Y.W.; Hong, I.-S.; Kim, S.; Jung, Y. Antarctic Marine Algae Extracts as a Potential Natural Resource to Protect Epithelial Barrier Integrity. Marine Drugs 2022, 20, 562. [Google Scholar] [CrossRef]
- Albanesi, C.; Fairchild, H.R.; Madonna, S.; Scarponi, C.; De Pità, O.; Leung, D.Y.M.; Howell, M.D. IL-4 and IL-13 Negatively Regulate TNF-α- and IFN-γ-Induced β-Defensin Expression through STAT-6, Suppressor of Cytokine Signaling (SOCS)-1, and SOCS-31. J. Immunol. 2007, 179, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Morgner, B.; Tittelbach, J.; Wiegand, C. Induction of psoriasis- and atopic dermatitis-like phenotypes in 3D skin equivalents with a fibroblast-derived matrix. Sci. Rep. 2023, 13, 1807. [Google Scholar] [CrossRef]
- Sirover, M.A. The role of posttranslational modification in moonlighting glyceraldehyde-3-phosphate dehydrogenase structure and function. Amino Acids 2021, 53, 507–515. [Google Scholar] [CrossRef] [PubMed]
- White, M.R.; Garcin, E.D. D-Glyceraldehyde-3-Phosphate Dehydrogenase Structure and Function. In Macromolecular Protein Complexes: Structure and Function; Harris, J.R., Marles-Wright, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 413–453. [Google Scholar]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.P.; Craigie, J.S.; Hafting, J.T.; Critchley, A.T. Polar lipids from the marine macroalga Palmaria palmata inhibit lipopolysaccharide-induced nitric oxide production in RAW264.7 macrophage cells. Phytochemistry 2014, 101, 101–108. [Google Scholar] [CrossRef]
- Boelsma, E.; Verhoeven, M.C.; Ponec, M. Reconstruction of a human skin equivalent using a spontaneously transformed keratinocyte cell line (HaCaT). J. Investig. Dermatol. 1999, 112, 489–498. [Google Scholar] [CrossRef]
- Moran, M.C.; Pandya, R.P.; Leffler, K.A.; Yoshida, T.; Beck, L.A.; Brewer, M.G. Characterization of Human Keratinocyte Cell Lines for Barrier Studies. JID Innov. 2021, 1, 100018. [Google Scholar] [CrossRef]
- Marunaka, K.; Kobayashi, M.; Shu, S.; Matsunaga, T.; Ikari, A. Brazilian Green Propolis Rescues Oxidative Stress-Induced Mislocalization of Claudin-1 in Human Keratinocyte-Derived HaCaT Cells. Int. J. Mol. Sci. 2019, 20, 3869. [Google Scholar] [CrossRef]
- Youssefian, L.; Niaziorimi, F.; Saeidian, A.H.; South, A.P.; Khosravi-Bachehmir, F.; Khodavaisy, S.; Vahidnezhad, H.; Uitto, J. Knockdown of SDR9C7 Impairs Epidermal Barrier Function. J. Investig. Dermatol. 2021, 141, 1754–1764.e1. [Google Scholar] [CrossRef] [PubMed]
- Han, E.J.; Kim, H.-S.; Jung, K.; Sanjeewa, K.K.A.; Herath, K.H.I.N.M.; Lee, W.; Jee, Y.; Jeon, Y.-J.; Lee, J.; Kim, T. Sargassum horneri ethanol extract ameliorates TNF-α/IFN-γ-induced inflammation in human keratinocytes and TPA-induced ear edema in mice. Food Biosci. 2021, 39, 100831. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Brit. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.Y.; Barhoumi, R.; Fan, Y.-Y.; Rivera, G.M.; Hannoush, R.N.; McMurray, D.N.; Chapkin, R.S. n-3 polyunsaturated fatty acids suppress CD4+ T cell proliferation by altering phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P2] organization. Biochim. Biophys. Acta Biomembr. 2016, 1858, 85–96. [Google Scholar] [CrossRef]
- Siddiqui, R.A.; Harvey, K.A.; Zaloga, G.P.; Stillwell, W. Modulation of Lipid Rafts by Ω-3 Fatty Acids in Inflammation and Cancer: Implications for Use of Lipids During Nutrition Support. Nutr. Clin. Pract. 2007, 22, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, H.-S.; Jeon, Y.-J.; Jee, Y.; Kim, K.-N.; Lee, K.; Fernando, I.P.S.; Ahn, G. Sargassum horneri (Turner) C. Agardh ethanol extract attenuates fine dust-induced inflammatory responses and impaired skin barrier functions in HaCaT keratinocytes. J. Ethnopharmacol. 2021, 273, 114003. [Google Scholar] [CrossRef]
- Nieto, N.; Torres, M.I.; Ríos, A.; Gil, A. Dietary polyunsaturated fatty acids improve histological and biochemical alterations in rats with experimental ulcerative colitis. J. Nutr. 2002, 132, 11–19. [Google Scholar] [CrossRef]
- Xiao, K.; Xu, Q.; Liu, C.; He, P.; Qin, Q.; Zhu, H.; Zhang, J.; Gin, A.; Zhang, G.; Liu, Y. Docosahexaenoic acid alleviates cell injury and improves barrier function by suppressing necroptosis signalling in TNF-α-challenged porcine intestinal epithelial cells. Innate Immun. 2020, 26, 653–665. [Google Scholar] [CrossRef]
- Capaldo, C.T.; Nusrat, A. Cytokine regulation of tight junctions. Biochim. Biophys. Acta 2009, 1788, 864–871. [Google Scholar] [CrossRef]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef]
- Nava, P.; Kamekura, R.; Nusrat, A. Cleavage of transmembrane junction proteins and their role in regulating epithelial homeostasis. Tissue Barriers 2013, 1, e24783. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Braubach, P.; Schilpp, C.; Lochbaum, R.; Neuland, K.; Thompson, K.; Jonigk, D.; Frick, M.; Dietl, P.; Wittekindt, O.H. IL-13 Impairs Tight Junctions in Airway Epithelia. Int. J. Mol. Sci. 2019, 20, 3222. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, Q.; Zhang, M.; Wang, C.; Zhu, Z.; Li, N.; Li, J. Effect of n-3 polyunsaturated fatty acids on membrane microdomain localization of tight junction proteins in experimental colitis. FEBS J. 2008, 275, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.D.; Kang, T.J.; Lee, C.H.; Lee, A.Y.; Noh, M. HaCaT Keratinocytes and Primary Epidermal Keratinocytes Have Different Transcriptional Profiles of Cornified Envelope-Associated Genes to T Helper Cell Cytokines. Biomol. Ther. 2012, 20, 171–176. [Google Scholar] [CrossRef]
- Schürer, N.; Köhne, A.; Schliep, V.; Barlag, K.; Goerz, G. Lipid composition and synthesis of HaCaT cells, an immortalized human keratinocyte line, in comparison with normal human adult keratinocytes. Exp. Dermatol. 1993, 2, 179–185. [Google Scholar] [CrossRef]
- Lopes, D.; Rey, F.; Leal, M.C.; Lillebø, A.I.; Calado, R.; Domingues, M.R. Bioactivities of Lipid Extracts and Complex Lipids from Seaweeds: Current Knowledge and Future Prospects. Marine Drugs 2021, 19, 686. [Google Scholar] [CrossRef]
- Miyashita, K.; Mikami, N.; Hosokawa, M. Chemical and nutritional characteristics of brown seaweed lipids: A review. J. Funct. Foods 2013, 5, 1507–1517. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; Lall, S.; Craigie, J.S.; Hafting, J.T. Lipids isolated from the cultivated red alga Chondrus crispus inhibit nitric oxide production. J. App Phycol. 2014, 26, 1565–1571. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kok, J.M.L.; Dowd, G.C.; Cabral, J.D.; Wise, L.M. Macrocystis pyrifera Lipids Reduce Cytokine-Induced Pro-Inflammatory Signalling and Barrier Dysfunction in Human Keratinocyte Models. Int. J. Mol. Sci. 2023, 24, 16383. https://doi.org/10.3390/ijms242216383
Kok JML, Dowd GC, Cabral JD, Wise LM. Macrocystis pyrifera Lipids Reduce Cytokine-Induced Pro-Inflammatory Signalling and Barrier Dysfunction in Human Keratinocyte Models. International Journal of Molecular Sciences. 2023; 24(22):16383. https://doi.org/10.3390/ijms242216383
Chicago/Turabian StyleKok, Jamie M. L., Georgina C. Dowd, Jaydee D. Cabral, and Lyn M. Wise. 2023. "Macrocystis pyrifera Lipids Reduce Cytokine-Induced Pro-Inflammatory Signalling and Barrier Dysfunction in Human Keratinocyte Models" International Journal of Molecular Sciences 24, no. 22: 16383. https://doi.org/10.3390/ijms242216383
APA StyleKok, J. M. L., Dowd, G. C., Cabral, J. D., & Wise, L. M. (2023). Macrocystis pyrifera Lipids Reduce Cytokine-Induced Pro-Inflammatory Signalling and Barrier Dysfunction in Human Keratinocyte Models. International Journal of Molecular Sciences, 24(22), 16383. https://doi.org/10.3390/ijms242216383






