Polyphenolic Compounds Nanostructurated with Gold Nanoparticles Enhance Wound Repair
Abstract
:1. Introduction
2. Results
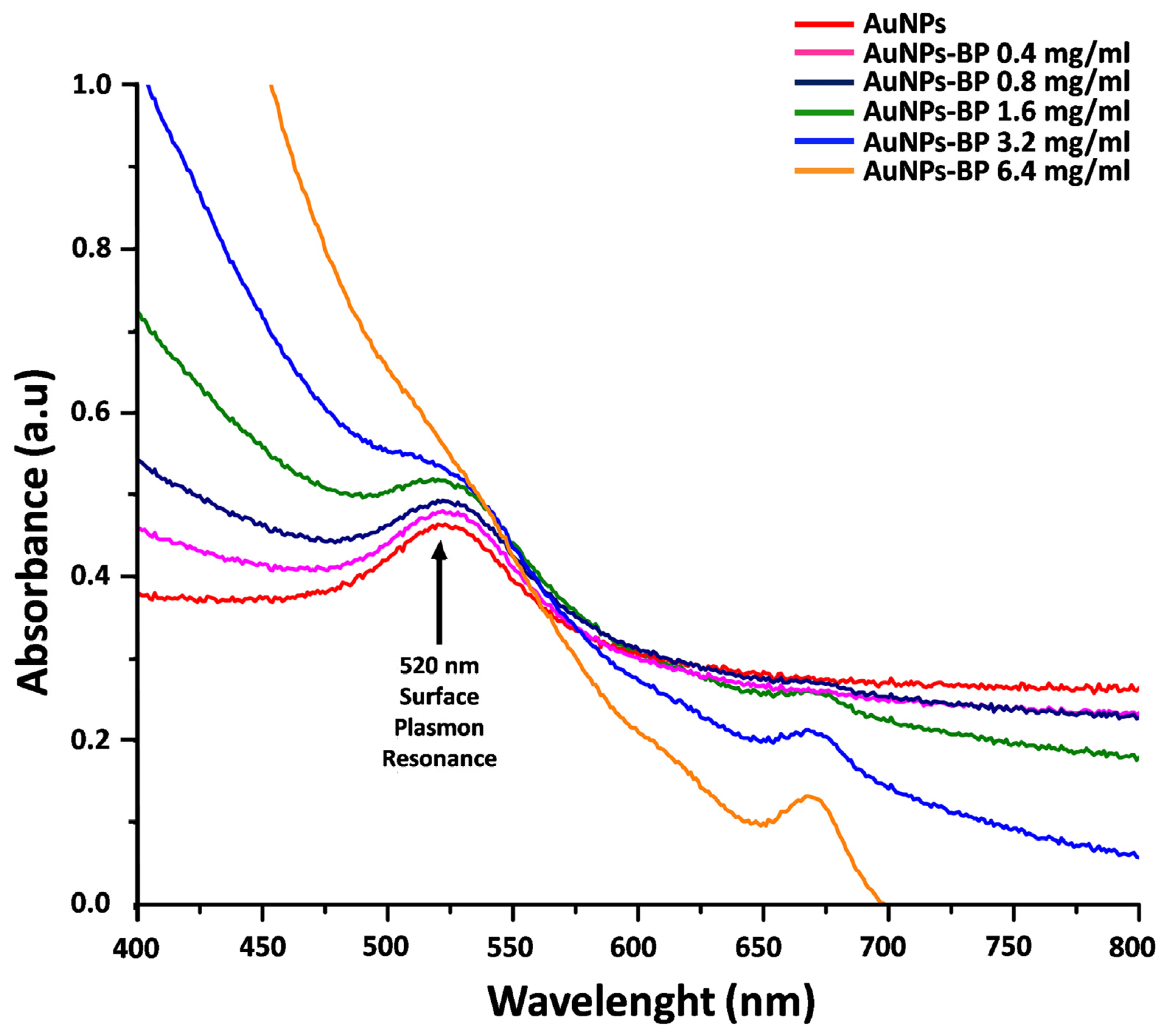
2.1. UV-Visible Spectrophotometry Analyses of Nanofunctionalized Gold Nanoparticles with Polyphenolic Compounds of B. procumbens (AuNP-BP)
2.1.1. FTIR Spectroscopy of the AuNP-BP
| Band | AuNPs | BP | AuNP-BP 1.6 mg/mL | Functional Group | References |
|---|---|---|---|---|---|
| 1 | 1742 | - | - | O-H on AuNPs | [16] |
| 2 | 1712 | - | - | N-H on AuNPs | [16] |
| 3 | - | 1703 | 1703 | C=O | [18] |
| 4 | 1637 | - | - | N-H on AuNPs | [17] |
| 5 | 1612 | 1608 | 1608 | aromatic double bond | [18] |
| 6 | 1589 | 1589 | 1589 | CH2-O on AuNPs | [17] |
| 7 | 1512 | 1512 | 1510 | C-C flavonoids and aromatic rings | [19] |
| 8 | 1470 | - | - | C-C on AuNPs | [16] |
| 9 | 1402 | - | - | C=O on AuNPs | [17] |
| 10 | - | 1258 | 1259 | C-O on polyols | [19] |
| 11 | - | 1230 | 1229 | C-O on polyols | [19] |
| 12 | 1171 | 1165 | C-O and -OH of primary alcohols | [19] | |
| 13 | 1076 | 1076 | 1074 | C-O alcohols, phenols, carboxylic anions | [16,19] |
| 14 | - | 1045 | 1055 | C-O and -OH of tertiary alcohols | [19] |
2.1.2. Dynamic Light Scattering Measurements (DLS) and Electrophoretic Light Scattering (ELS)
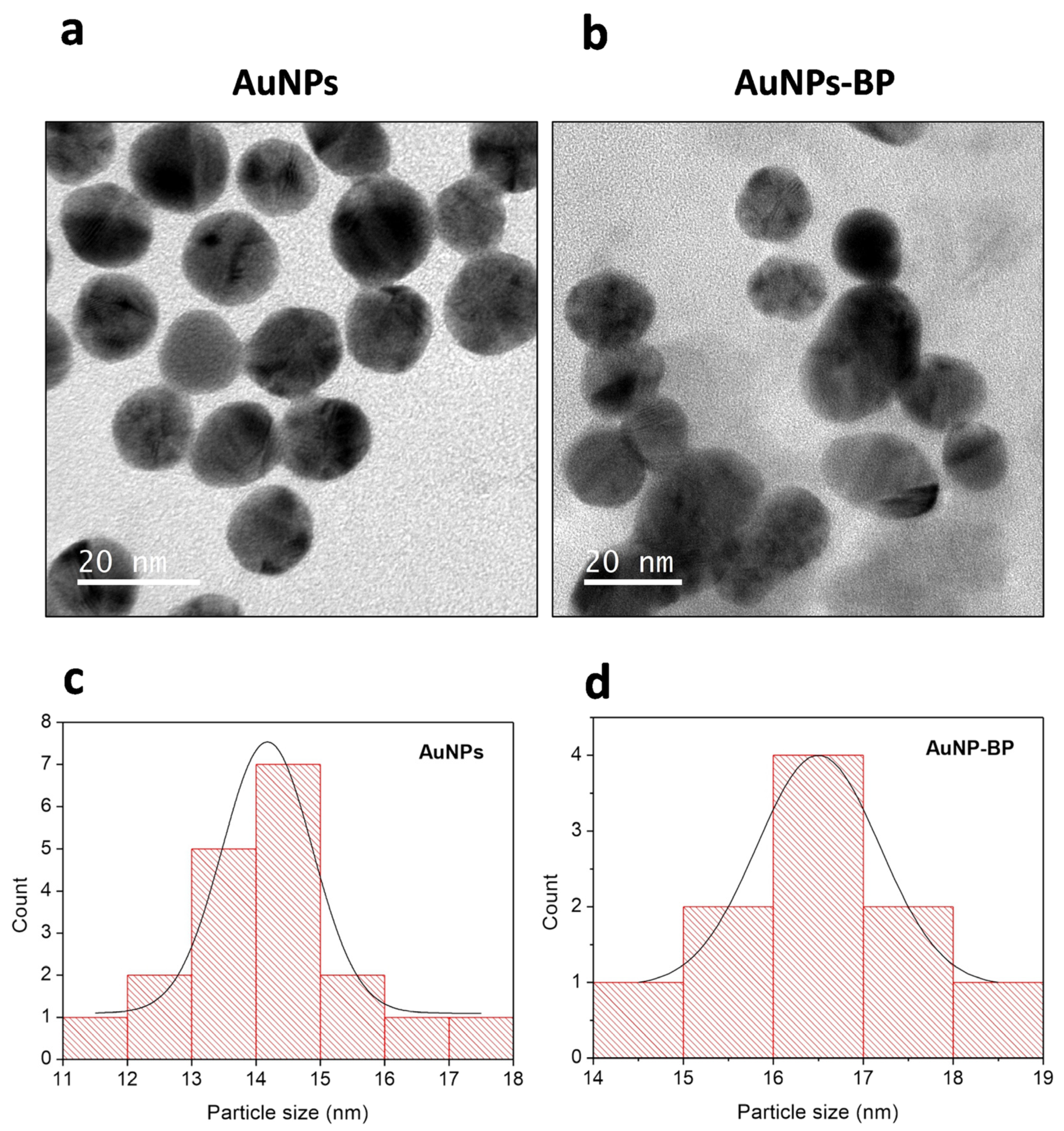
2.1.3. Transmission Electron Microscopy (TEM) of AuNP and AuNP-BP
2.2. Effects of Topical Application of AuNP-BP Hydrogel in a Rat Wound Healing Model
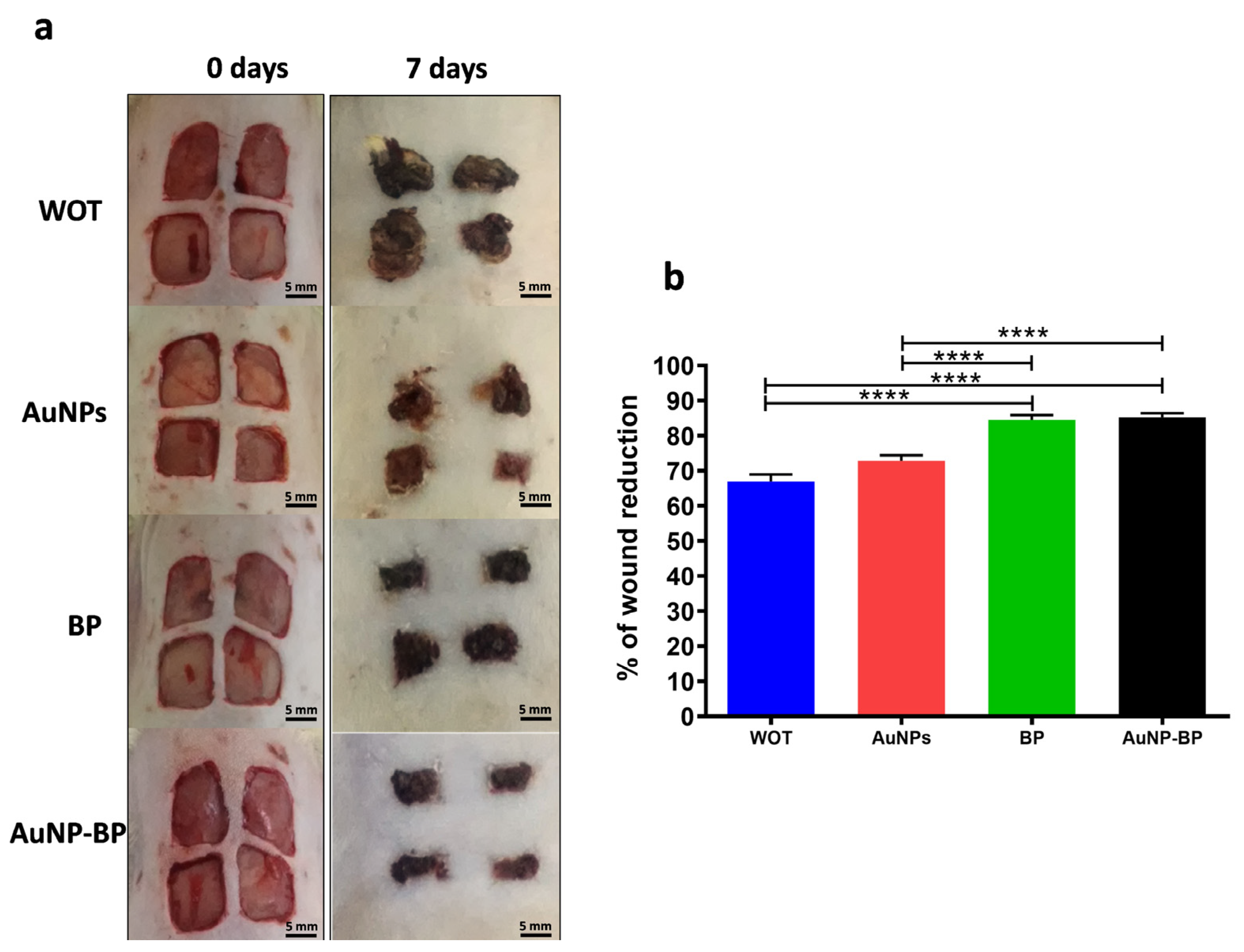
2.2.1. Macroscopic Changes Induced by AuNP-BP Hydrogel
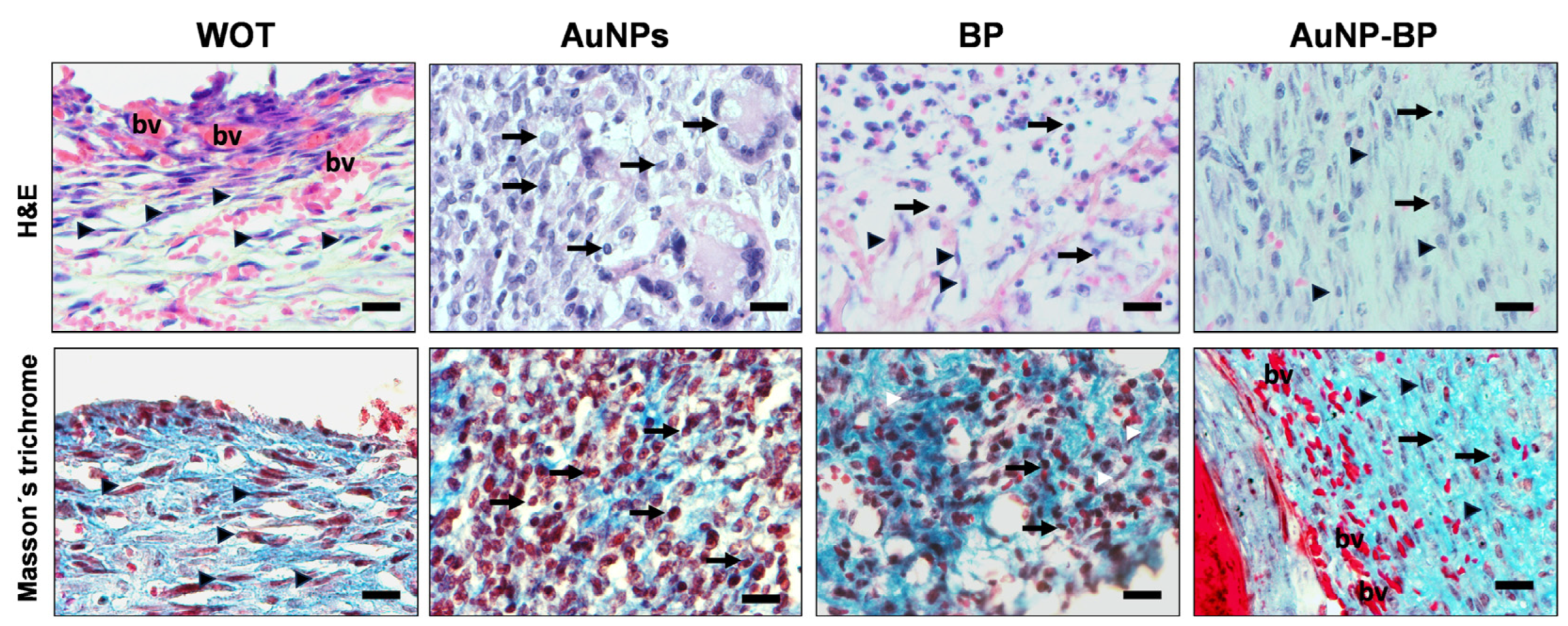
2.2.2. AuNP-BP Hydrogel Enhances Histological Wound Healing
2.2.3. ATR-FTIR Spectra Changes on Skin Wound Healing Induced by AuNP-BP
3. Discussion
4. Materials and Methods
4.1. Extraction of Polyphenolic Compounds from Bacopa Procumbens
4.2. Gold Nanoparticles Synthesis
4.3. Gold Nanoparticles Conjugation with Polyphenolic Compounds of Bacopa Procumbens
4.4. Instrumentation
4.5. In Vivo Skin Wound Model
4.5.1. Animals
4.5.2. Morphometric Analysis
4.5.3. Histopathological Analysis
4.5.4. ATR-FTIR Spectra Analysis
4.6. Statistical Analysis
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwan, K.H.; Liu, X.; To, M.K.; Yeung, K.W.; Ho, C.M.; Wong, K.K. Modulation of collagen alignment by silver nanoparticles results in better mechanical properties in wound healing. Nanomedicine 2011, 7, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Spampinato, S.F.; Caruso, G.I.; De Pasquale, R.; Sortino, M.A.; Merlo, S. The Treatment of Impaired Wound Healing in Diabetes: Looking among Old Drugs. Pharmaceuticals 2020, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Naskar, A.; Kim, K.S. Recent Advances in Nanomaterial-Based Wound-Healing Therapeutics. Pharmaceutics 2020, 12, 499. [Google Scholar] [CrossRef] [PubMed]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid. Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef] [PubMed]
- Hajialyani, M.; Tewari, D.; Sobarzo-Sánchez, E.; Nabavi, S.M.; Farzaei, M.H.; Abdollahi, M. Natural product-based nanomedicines for wound healing purposes: Therapeutic targets and drug delivery systems. Int. J. Nanomed. 2018, 13, 5023–5043. [Google Scholar] [CrossRef] [PubMed]
- Melguizo-Rodríguez, L.; de Luna-Bertos, E.; Ramos-Torrecillas, J.; Illescas-Montesa, R.; Costela-Ruiz, V.J.; García-Martínez, O. Potential Effects of Phenolic Compounds That Can Be Found in Olive Oil on Wound Healing. Foods 2021, 10, 1642. [Google Scholar] [CrossRef]
- Martínez-Cuazitl, A.; Gómez-García, M.D.C.; Hidalgo-Alegria, O.; Flores, O.M.; Núñez-Gastélum, J.A.; Martínez, E.S.M.; Ríos-Cortés, A.M.; Garcia-Solis, M.; Pérez-Ishiwara, D.G. Characterization of Polyphenolic Compounds from Bacopa procumbens and Their Effects on Wound-Healing Process. Molecules 2022, 27, 6521. [Google Scholar] [CrossRef]
- Cucci, L.M.; Trapani, G.; Hansson, Ö.; La Mendola, D.; Satriano, C. Gold Nanoparticles Functionalized with Angiogenin for Wound Care Application. Nanomaterials 2021, 11, 201. [Google Scholar] [CrossRef]
- Grzelczak, M.; Pérez-Juste, J.; Mulvaney, P.; Liz-Marzán, L.M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 2008, 37, 1783–1791. [Google Scholar] [CrossRef]
- Kus-Liśkiewicz, M.; Fickers, P.; Ben Tahar, I. Biocompatibility and Cytotoxicity of Gold Nanoparticles: Recent Advances in Methodologies and Regulations. Int. J. Mol. Sci. 2021, 22, 10952. [Google Scholar] [CrossRef] [PubMed]
- Kadhim, R.J.; Karsh, E.H.; Taqi, Z.J.; Jabir, M.S. Biocompatibility of gold nanoparticles: In-vitro and In-vivo study. Mater. Today Proc. 2021, 42, 3041–3045. [Google Scholar] [CrossRef]
- Wang, A.; Ng, H.P.; Xu, Y.; Li, Y.; Zheng, Y.; Yu, J.; Han, F.; Peng, F.; Fu, L. Gold nanoparticles: Synthesis, stability test, and application for the rice growth. J. Nanomater. 2014, 2014, 451232. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization techniques for nanoparticles: Comparison and complementarity upon studying nanoparticle properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Sandiningtyas, R.D.; Suendo, V. Isolation of chlorophyll from spinach and its modification using Fe2+ in photostability study. In Proceedings of the Third International Conference on Mathematics and Natural Science, Bandung, Indonesia, 23’25 November 2010; Faculty of Mathematics and Natural Sciences, School of Life Sciences and Technology, and School of Pharmacy at Institut Teknologi Bandung: Bandung, Indonesia, 2010; pp. 859–873. [Google Scholar]
- Aravinthan, A.; Kamala-Kannan, S.; Govarthanan, M.; Kim, J.H. Accumulation of biosynthesized gold nanoparticles and its impact on various organs of Sprague Dawley rats: A systematic study. Toxicol. Res. 2016, 5, 1530–1538. [Google Scholar] [CrossRef]
- Nghiem, T.H.L.; La, T.H.; Vu, X.H.; Chu, V.H.; Nguyen, T.H.; Le, Q.H.; Fort, E.; Do, Q.H.; Tran, H.N. Synthesis, capping and binding of colloidal Gold Nanoparticles to Proteins. Adv. Nat. Sci. Nanosci. Nanotechnol. 2010, 1, 025009. [Google Scholar] [CrossRef]
- Sanna, V.; Pala, N.; Dessì, G.; Manconi, P.; Mariani, A.; Dedola, S.; Rassu, M.; Crosio, C.; Iaccarino, C.; Sechi, M. Single-step green synthesis and characterization of gold-conjugated polyphenol nanoparticles with antioxidant and biological activities. Int. J. Nanomed. 2014, 9, 4935–4951. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Mancini, M.C.; De Oliveira, F.C.S.; Passos, T.M.; Quilty, B.; Da Silva Moreira Thiré, R.M.; McGuinness, G.B. FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Matéria 2016, 21, 767–779. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Eklouh-Molinier, C.; Sebiskveradze, D.; Feru, J.; Terryn, C.; Manfait, M.; Brassart-Pasco, S.; Piot, O. Changes of skin collagen orientation associated with chronological aging as probed by polarized-FTIR micro-imaging. Analyst 2014, 139, 2482–2488. [Google Scholar] [CrossRef]
- Sanden, K.W.; Kohler, A.; Afseth, N.K.; Böcker, U.; Rønning, S.B.; Liland, K.H.; Pedersen, M.E. The use of Fourier-transform infrared spectroscopy to characterize connective tissue components in skeletal muscle of Atlantic cod (Gadus morhua L.). J. Biophotonics 2019, 12, e201800436. [Google Scholar] [CrossRef]
- Vazquez-Zapien, G.J.; Martinez-Cuazitl, A.; Granados-Jimenez, A.; Sanchez-Brito, M.; Guerrero-Ruiz, M.; Camacho-Ibarra, A.; Miranda-Ruiz, M.A.; Dox-Aguillón, I.S.; Ramirez-Torres, J.A.; Mata-Miranda, M.M. Skin wound healing improvement in diabetic mice through FTIR microspectroscopy after implanting pluripotent stem cells. APL Bioeng. 2023, 7, 016109. [Google Scholar] [CrossRef]
- Aji, A.; Oktafiani, D.; Yuniarto, A.; Kurnia, A.A. Biosynthesis of gold nanoparticles using Kapok (Ceiba pentandra) leaf aqueous extract and investigating their antioxidant activity. J. Mol. Struct. 2022, 1270, 133906. [Google Scholar] [CrossRef]
- González, V.; Kharisov, B.; Gómez, I. Preparation, optical characterization and stability of gold nanoparticles by facile methods. Rev. Mex. Fis. 2019, 65, 690–698. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV-vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Elbagory, A.M.; Hussein, A.A.; Meyer, M. The In Vitro Immunomodulatory Effects of Gold Nanoparticles Synthesized From Hypoxis hemerocallidea Aqueous Extract and Hypoxoside on Macrophage and Natural Killer Cells. Int. J. Nanomed. 2019, 14, 9007–9018. [Google Scholar] [CrossRef] [PubMed]
- Korani, S.; Rashidi, K.; Hamelian, M.; Jalalvand, A.R.; Tajehmiri, A.; Korani, M.; Sathyapalan, T.; Sahebkar, A. Evaluation of Antimicrobial and Wound Healing Effects of Gold Nanoparticles Containing Abelmoschus esculentus (L.) Aqueous Extract. Bioinorg. Chem. Appl. 2021, 2021, 7019130. [Google Scholar] [CrossRef] [PubMed]
- Leu, J.G.; Chen, S.A.; Chen, H.M.; Wu, W.M.; Hung, C.F.; Yao, Y.D.; Tu, C.S.; Liang, Y.J. The effects of gold nanoparticles in wound healing with antioxidant epigallocatechin gallate and α-lipoic acid. Nanomedicine 2012, 8, 767–775. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef]
- Castro, P.A.A.; Lima, C.A.; Morais, M.R.P.T.; Zorn, T.M.T.; Zezell, D.M. Monitoring the Progress and Healing Status of Burn Wounds Using Infrared Spectroscopy. Appl. Spectrosc. 2020, 74, 758–766. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Thirupathi, A.; Guzzatti, M.F.M.; Corrêa, M.E.A.B.; Venturini, L.M.; Casagrande, L.R.; Lima, I.R.; Da Costa, C.; De Pieri, E.; Tietbohl, L.T.W.; Feuser, P.E.; et al. Green Synthesis of Gold Nanoparticles with Curcumin or Açai in the Tissue Repair of Palatal Wounds. Antioxidants 2023, 12, 1574. [Google Scholar] [CrossRef] [PubMed]
- De Campos Vidal, B.; Mello, M.L.S. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; He, X.; Sun, Z.; Huo, X.; Hou, Y.; Xu, X.; Wu, H.; Shi, L.; Ma, G. Natural carrier-free self-assembled diterpene nanoparticles with its efficient anti-inflammation through the inhibition of NF-κB pathway for accelerated wound healing. Biomed. Pharmacother. 2023, 165, 115041. [Google Scholar] [CrossRef] [PubMed]
- Zulkefli, N.; Che Zahari, C.N.M.; Sayuti, N.H.; Kamarudin, A.A.; Saad, N.; Hamezah, H.S.; Bunawan, H.; Baharum, S.N.; Mediani, A.; Ahmed, Q.U.; et al. Flavonoids as Potential Wound-Healing Molecules: Emphasis on Pathways Perspective. Int. J. Mol. Sci. 2023, 24, 4607. [Google Scholar] [CrossRef]





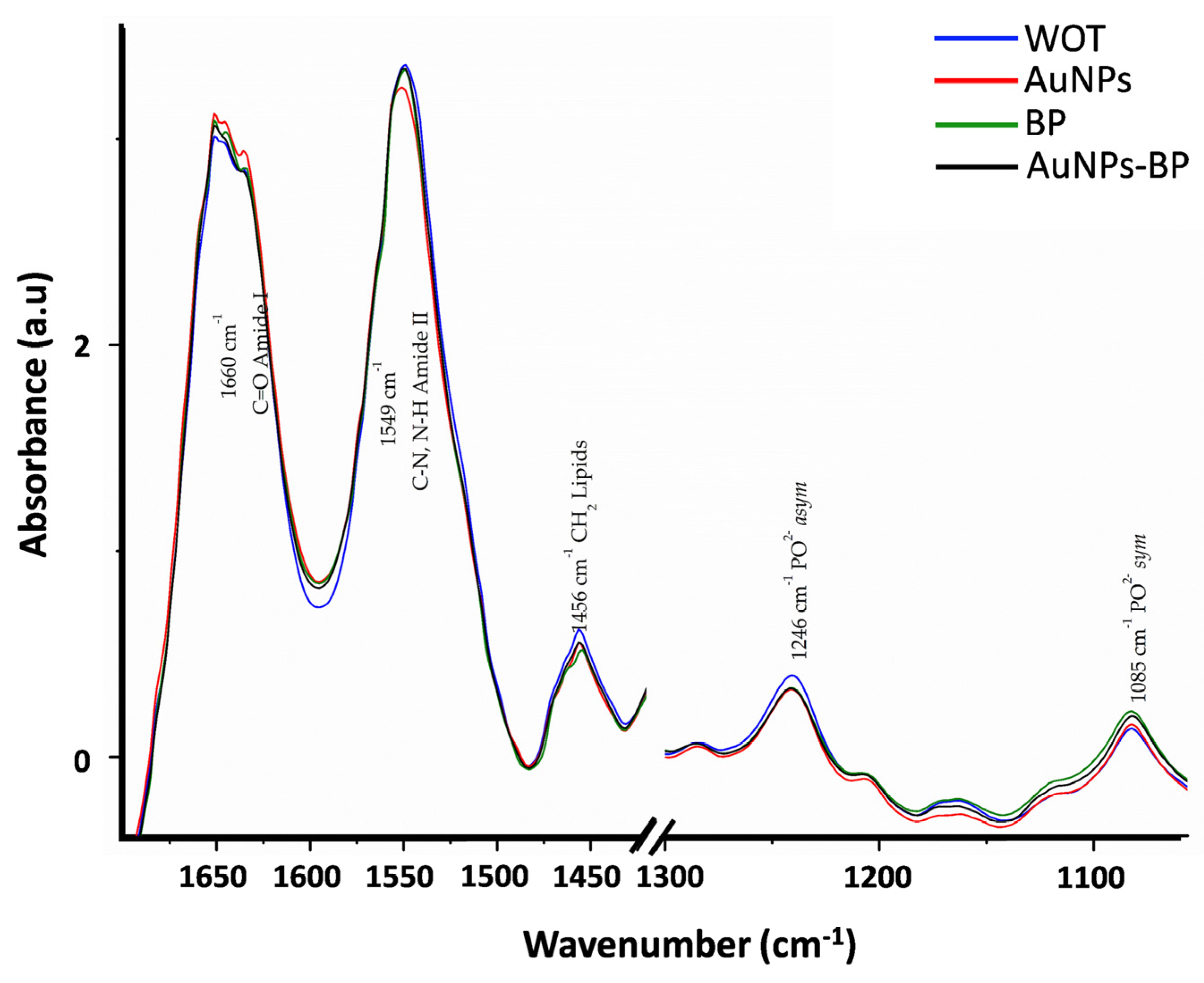
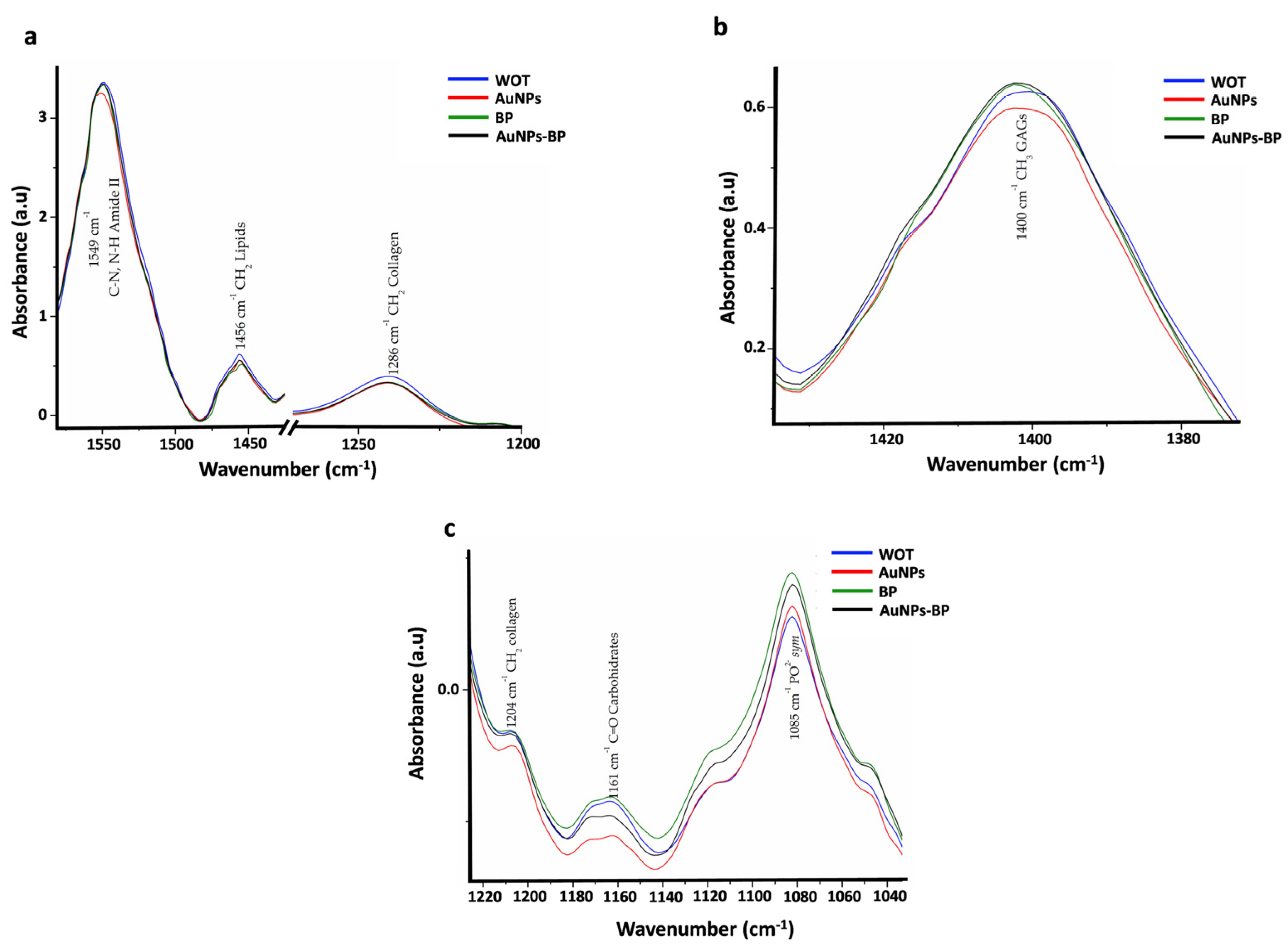
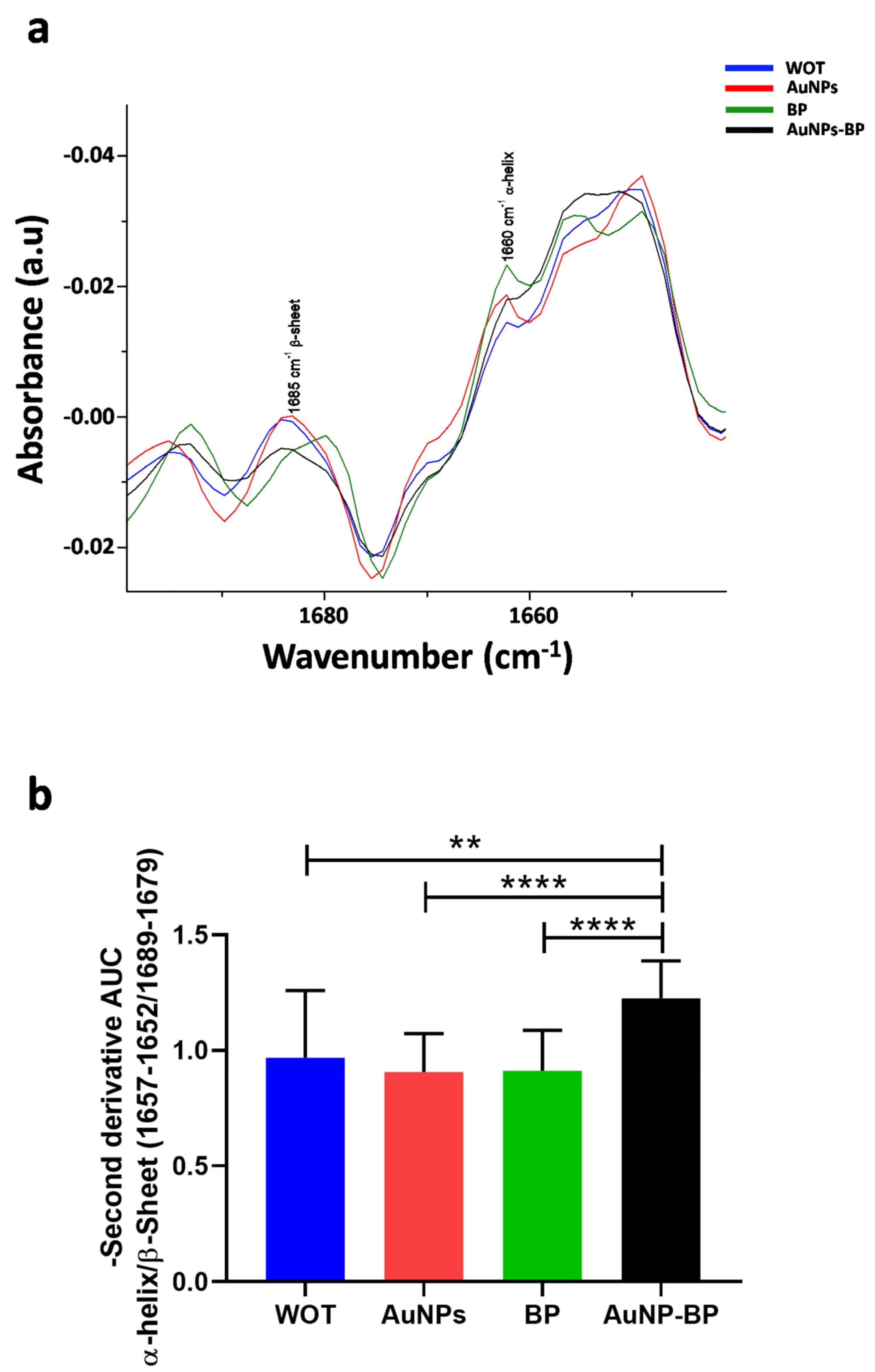
| Band | cm−1 | Assignment | References |
|---|---|---|---|
| 1 | 1737 | C=O lipids | [22] |
| 2 | 1660 | C=O Amide I | [21,22] |
| 3 | 1549 | C-N, N-H Amide II | [21,22] |
| 4 | 1456 | CH2 lipids | [21,22] |
| 5 | 1400 | CH3 GAGs | [21] |
| 6 | 1338 | CH2 Collagen type I | [21] |
| 7 | 1286 | CH2 collagen Amide III, glycine and proline | [21] |
| 8 | 1246 | PO2− asym Phospholipds | [22] |
| 9 | 1204 | CH2 Collagen Amide III | [21] |
| 10 | 1161 | C-O carbohydrate residues | [21] |
| 11 | 1085 | PO2− sym Phospholipds, C-O of carbohydrate on Collagen and PG | [21,22] |
| Band | WOT cm−1 Media (IQR) | AuNPs cm−1 Media (IQR) | BP cm−1 Media (IQR) | AuNP-BP cm−1 Media (IQR) | p |
|---|---|---|---|---|---|
| 1 | 1736 (1736, 1736) | 1736 (1736, 1736) | 1736 (1736, 1736) | 1736 (1736, 1736) | 0.069 |
| 2 | 1651 (1645, 1651) | 1651 (1651, 1651) a | 1651 (1651, 1651) a | 1651 (1651, 1651) a | 0.016 |
| 3 | 1549 (1549, 1549) | 1551 (1551, 1552) a | 1551 (1549, 1551) a | 1551 (1549, 1551) ab | 0.000 |
| 4 | 1456 (1454, 1456) | 1456 (1456, 1456) | 1456 (1454, 1656) b | 1456 (1456, 1456.) | 0.001 |
| 5 | 1402 (1400, 1402) | 1402 (1402, 1402) | 1402 (1402, 1402) | 1402 (1402, 1402) | 0.317 |
| 6 | 1339 (1339, 1340) | 1339 (1339, 1340) | 1339 (1337, 1340) | 1340.46 (1339, 1340) | 0.713 |
| 7 | 1285 (1283, 1286) | 1285 (1285, 1286) | 1286 (1285, 1288) | 1285 (1285, 1286) | 0.081 |
| 8 | 1240 (1240, 1240) | 1240 (1240, 1242) | 1240 (1240, 1242) | 1242 (1240, 1242) ab | 0.028 |
| 9 | 1207 (1207, 1211) | 1207 (1207, 1209) | 1209 (1207, 1211) | 1209 (1207, 1211) | 0.088 |
| 10 | 1163 (1161, 1171) | 1164 (1162, 1168) | 1163 (1163, 1171) | 1167 (1163, 1171) | 0.497 |
| 11 | 1082 (1082, 1082) | 1082 (1082, 1082) | 1082 (1082, 1084) ab | 1082 (1082, 1082) | 0.000 |
| Band | WOT Absorbance Media (IQR) | AuNPs Absorbance Media (IQR) | BP Absorbance Media (IQR) | AuNP-BP Absorbance Media (IQR) | p |
|---|---|---|---|---|---|
| 1 | 0.4 (0.16, 0.64) | 0.19(0.12, 0.29) | 0.28 (0.23, 0.62) | 0.28 (0.23, 0.34) | 0.08 |
| 2 | 4.03 (3.88, 4.17) | 4.08 (4.96, 4.13) | 4.09 (4.06, 4.2) | 4.07 (4.03, 4.1) | 0.128 |
| 3 | 4.36 (4.33, 4.45) | 4.21 (4.19, 4.24) a | 4.35 (4.24, 4.45) b | 4.28 (4.24, 4.33) ab | 0.0001 |
| 4 | 1.58 (1.52, 1.74) | 1.51 (1.47, 1.54) | 1.55 (1.51, 1.61) b | 1.53 (1.49, 1.55) a | 0.012 |
| 5 | 1.64 (1.55, 1.66) | 1.56 (1.53, 1.58) a | 1.63 (1.58, 1.74) b | 1.63 (1.56, 1.67) b | 0.001 |
| 6 | 1.06 (1.01, 1.13) | 1.06 (1.03, 1.08) | 1.09 (1.04, 1.15) | 1.06 (1.05, 1.08) | 0.305 |
| 7 | 1.06 (1, 1.12) | 1.01 (0.98, 1.04) a | 1.07 (0.99, 1.17) b | 1.02 (1.02, 1.04) | 0.04 |
| 8 | 1.36 (1.28, 1.44) | 1.3 (1.23, 1.34) | 1.36 (1.25, 1.48) | 1.28 (1.26, 1.33) | 0.091 |
| 9 | 0.89 (0.84, 0.99) | 0.85 (0.81, 0.89) a | 0.93 (0.84, 1.02) b | 0.89 (0.86, 0.93) b | 0.015 |
| 10 | 0.76 (0.72, 0.87) | 0.66 (0.63, 0.72) a | 0.79 (0.69, 0.92) b | 0.75 (0.69, 0.81) b | 0.003 |
| 11 | 1.14 (0.99, 1.22) | 1.11 (1.07, 1.17) | 1.19 (1.13, 1.35) ab | 1.2 (1.16, 1.23) b | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Cuazitl, A.; Gómez-García, M.d.C.; Pérez-Mora, S.; Rojas-López, M.; Delgado-Macuil, R.J.; Ocampo-López, J.; Vázquez-Zapién, G.J.; Mata-Miranda, M.M.; Pérez-Ishiwara, D.G. Polyphenolic Compounds Nanostructurated with Gold Nanoparticles Enhance Wound Repair. Int. J. Mol. Sci. 2023, 24, 17138. https://doi.org/10.3390/ijms242417138
Martínez-Cuazitl A, Gómez-García MdC, Pérez-Mora S, Rojas-López M, Delgado-Macuil RJ, Ocampo-López J, Vázquez-Zapién GJ, Mata-Miranda MM, Pérez-Ishiwara DG. Polyphenolic Compounds Nanostructurated with Gold Nanoparticles Enhance Wound Repair. International Journal of Molecular Sciences. 2023; 24(24):17138. https://doi.org/10.3390/ijms242417138
Chicago/Turabian StyleMartínez-Cuazitl, Adriana, María del Consuelo Gómez-García, Salvador Pérez-Mora, Marlon Rojas-López, Raúl Jacobo Delgado-Macuil, Juan Ocampo-López, Gustavo Jesús Vázquez-Zapién, Mónica Maribel Mata-Miranda, and David Guillermo Pérez-Ishiwara. 2023. "Polyphenolic Compounds Nanostructurated with Gold Nanoparticles Enhance Wound Repair" International Journal of Molecular Sciences 24, no. 24: 17138. https://doi.org/10.3390/ijms242417138
APA StyleMartínez-Cuazitl, A., Gómez-García, M. d. C., Pérez-Mora, S., Rojas-López, M., Delgado-Macuil, R. J., Ocampo-López, J., Vázquez-Zapién, G. J., Mata-Miranda, M. M., & Pérez-Ishiwara, D. G. (2023). Polyphenolic Compounds Nanostructurated with Gold Nanoparticles Enhance Wound Repair. International Journal of Molecular Sciences, 24(24), 17138. https://doi.org/10.3390/ijms242417138








