The Role of the Innate Immune Response in Oral Mucositis Pathogenesis
Abstract
1. Introduction
2. Current Management of OM
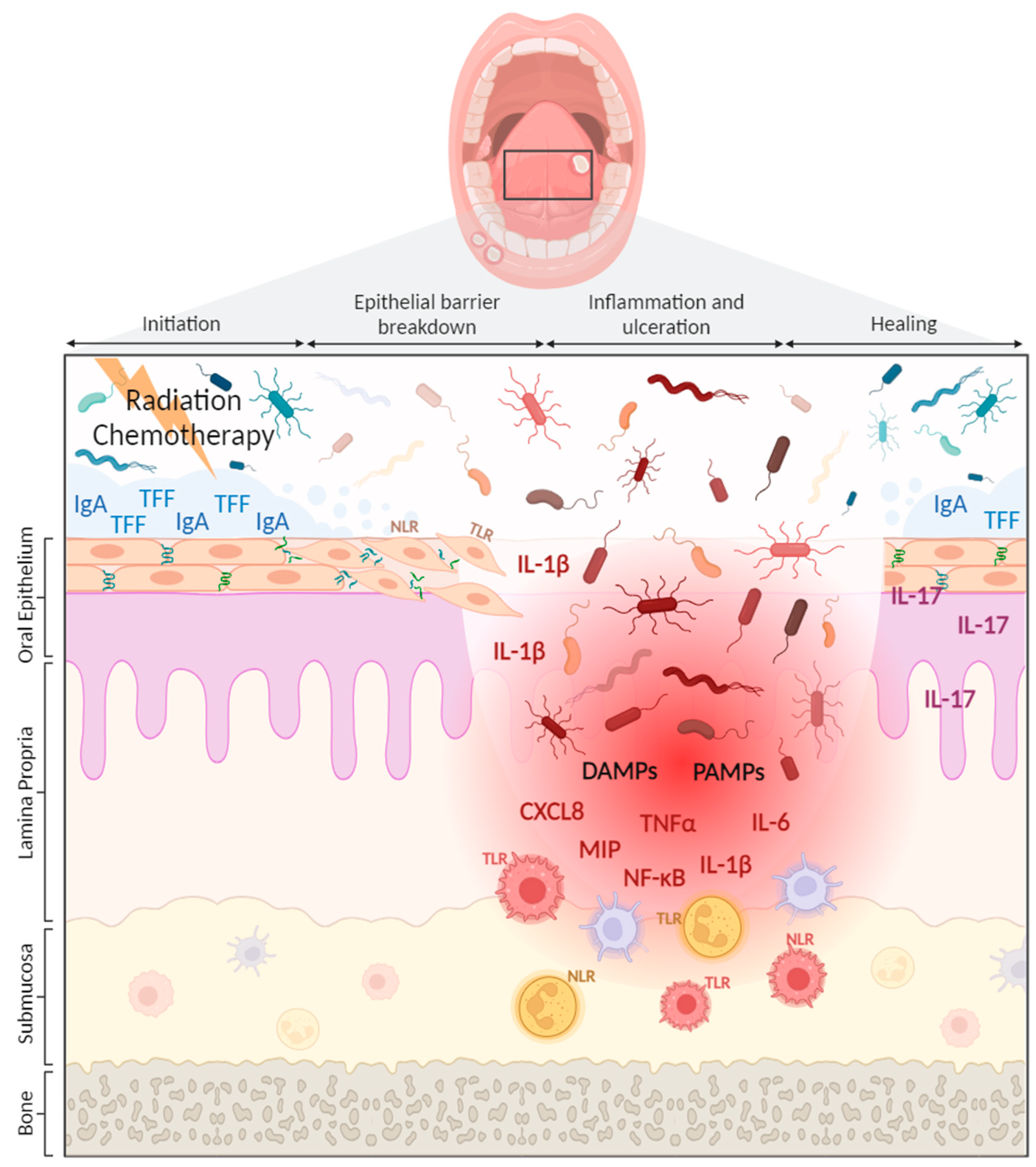
3. The Innate Immune System in OM Development
3.1. Pattern Recognition Receptors and OM Development
3.2. Oral Microbiome—Innate Immune Interactions and OM Development
4. Innate Immune Response Targeted Interventions for OM
5. Future Directions
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Logan, R.M.; Gibson, R.J.; Bowen, J.M.; Stringer, A.M.; Sonis, S.T.; Keefe, D.M.K. Characterisation of mucosal changes in the alimentary tract following administration of irinotecan: Implications for the pathobiology of mucositis. Cancer Chemother. Pharmacol. 2008, 62, 33–41. [Google Scholar] [CrossRef]
- Jiang, R.; Liu, Y.; Zhang, H.; Chen, Y.; Liu, T.; Zeng, J.; Nie, E.; Chen, S.; Tan, J. Distinctive microbiota of delayed healing of oral mucositis after radiotherapy of nasopharyngeal carcinoma. Front. Cell. Infect. Microbiol. 2022, 12, 1070322. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Vesty, A.; Gear, K.; Biswas, K.; Mackenzie, B.W.; Taylor, M.W.; Douglas, R.G. Oral microbial influences on oral mucositis during radiotherapy treatment of head and neck cancer. Support. Care Cancer 2020, 28, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.; Srivastava, R.; Lalla, R. Oral mucosal injury in oncology patients: Perspectives on maturation of a field. Oral Dis. 2015, 21, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B. Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004, 100, 1995–2025. [Google Scholar] [CrossRef]
- Peterson, D.E.; Keefe, D.M.; Sonis, S.T. New frontiers in mucositis. In American Society of Clinical Oncology Educational Book; American Society of Clinical Oncology: Alexandria, VA, USA, 2012; pp. 545–551. [Google Scholar]
- Sonis, S.T. Precision medicine for risk prediction of oral complications of cancer therapy-The example of oral mucositis in patients receiving radiation therapy for cancers of the head and neck. Front. Oral Health 2022, 3, 917860. [Google Scholar] [CrossRef]
- Sonis, S.T. A hypothesis for the pathogenesis of radiation-induced oral mucositis: When biological challenges exceed physiologic protective mechanisms. Implications for pharmacological prevention and treatment. Support. Care Cancer 2021, 29, 4939–4947. [Google Scholar] [CrossRef]
- Pulito, C.; Cristaudo, A.; La Porta, C.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral mucositis: The hidden side of cancer therapy. J. Exp. Clin. Cancer Res. 2020, 39, 210. [Google Scholar] [CrossRef]
- Bowen, J.M.; Wardill, H.R. Advances in the understanding and management of mucositis during stem cell transplantation. Curr. Opin. Support. Palliat. Care 2017, 11, 341–346. [Google Scholar] [CrossRef]
- McCullough, R.W. US oncology-wide incidence, duration, costs and deaths from chemoradiation mucositis and antimucositis therapy benefits. Future Oncol. 2017, 13, 2823–2852. [Google Scholar] [CrossRef] [PubMed]
- Elting, L.S.; Chang, Y.C. Costs of Oral Complications of Cancer Therapies: Estimates and a Blueprint for Future Study. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz010. [Google Scholar] [CrossRef]
- Villa, A.; Sonis, S.T. Mucositis: Pathobiology and management. Curr. Opin. Oncol. 2015, 27, 159–164. [Google Scholar] [CrossRef]
- Lacouture, M.; Sibaud, V. Toxic Side Effects of Targeted Therapies and Immunotherapies Affecting the Skin, Oral Mucosa, Hair, and Nails. Am. J. Clin. Dermatol. 2018, 19 (Suppl. S1), 31–39. [Google Scholar] [CrossRef] [PubMed]
- Dougan, M. Understanding and Overcoming the Inflammatory Toxicities of Immunotherapy. Cancer Immunol. Res. 2020, 8, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Cheng, K.K.F.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- Raymond, B.M.; Agyeman-Yeboah, J. Nurses’ Knowledge on Assessment and Management of Cancer Therapy-Associated Oral Mucositis. Nurs. Open 2023, 10, 7292–7300. [Google Scholar] [CrossRef]
- Mirabile, A.; Airoldi, M.; Ripamonti, C.; Bolner, A.; Murphy, B.; Russi, E.; Numico, G.; Licitra, L.; Bossi, P. Pain management in head and neck cancer patients undergoing chemo-radiotherapy: Clinical practical recommendations. Crit. Rev. Oncol. Hematol. 2016, 99, 100–106. [Google Scholar] [CrossRef]
- Hong, C.H.L.; On behalf of the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society for Oral Oncology (MASCC/ISOO); Gueiros, L.A.; Fulton, J.S.; Cheng, K.K.F.; Kandwal, A.; Galiti, D.; Fall-Dickson, J.M.; Johansen, J.; Ameringer, S.; et al. Systematic review of basic oral care for the management of oral mucositis in cancer patients and clinical practice guidelines. Support. Care Cancer 2019, 27, 3949–3967. [Google Scholar]
- Sonis, S.T. Superoxide Dismutase as an Intervention for Radiation Therapy-Associated Toxicities: Review and Profile of Avasopasem Manganese as a Treatment Option for Radiation-Induced Mucositis. Drug Des. Dev. Ther. 2021, 15, 1021–1029. [Google Scholar] [CrossRef]
- Ariyawardana, A.; On behalf of the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society for Oral Oncology (MASCC/ISOO); Cheng, K.K.F.; Kandwal, A.; Tilly, V.; Al-Azri, A.R.; Galiti, D.; Chiang, K.; Vaddi, A.; Ranna, V.; et al. Systematic review of anti-inflammatory agents for the management of oral mucositis in cancer patients and clinical practice guidelines. Support. Care Cancer 2019, 27, 3985–3995. [Google Scholar]
- Senel, S. An Overview of Physical, Microbiological and Immune Barriers of Oral Mucosa. Int. J. Mol. Sci. 2021, 22, 7821. [Google Scholar] [CrossRef] [PubMed]
- Gunther, J.; Seyfert, H.M. The first line of defence: Insights into mechanisms and relevance of phagocytosis in epithelial cells. Semin. Immunopathol. 2018, 40, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Levy, M.; Suez, J.; Elinav, E. The interplay between the innate immune system and the microbiota. Curr. Opin. Immunol. 2014, 26, 41–48. [Google Scholar] [CrossRef]
- Samiei, M.; Ahmadian, E.; Eftekhari, A.; Eghbal, M.A.; Rezaie, F.; Vinken, M. Cell junctions and oral health. EXCLI J. 2019, 18, 317–330. [Google Scholar]
- Wardill, H.R.; Logan, R.M.; Bowen, J.M.; Van Sebille, Y.Z.A.; Gibson, R.J. Tight junction defects are seen in the buccal mucosa of patients receiving standard dose chemotherapy for cancer. Support. Care Cancer 2016, 24, 1779–1788. [Google Scholar] [CrossRef]
- Sonis, S.T. Pathobiology of oral mucositis: Novel insights and opportunities. J. Support Oncol. 2007, 5 (Suppl. S4), 3–11. [Google Scholar]
- Müller, V.J.; Belibasakis, G.N.; Bosshard, P.P.; Wiedemeier, D.B.; Bichsel, D.; Rücker, M.; Stadlinger, B. Change of saliva composition with radiotherapy. Arch. Oral Biol. 2019, 106, 104480. [Google Scholar] [CrossRef]
- Groeger, S.; Meyle, J. Oral Mucosal Epithelial Cells. Front. Immunol. 2019, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Stolte, K.N.; Pelz, C.; Yapto, C.V.; Raguse, J.D.; Dommisch, H.; Danker, K. IL-1beta strengthens the physical barrier in gingival epithelial cells. Tissue Barriers 2020, 8, 1804249. [Google Scholar] [CrossRef] [PubMed]
- Bossi, P.; Bergamini, C.; Miceli, R.; Cova, A.; Orlandi, E.; Resteghini, C.; Locati, L.; Alfieri, S.; Imbimbo, M.; Granata, R.; et al. Salivary Cytokine Levels and Oral Mucositis in Head and Neck Cancer Patients Treated With Chemotherapy and Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 959–966. [Google Scholar] [CrossRef]
- Logan, R.M.; Stringer, A.M.; Bowen, J.M.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M. Is the pathobiology of chemotherapy-induced alimentary tract mucositis influenced by the type of mucotoxic drug administered? Cancer Chemother. Pharmacol. 2009, 63, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.; Khodadadi, H.; Baban, B. Innate immunity and oral microbiome: A personalized, predictive, and preventive approach to the management of oral diseases. EPMA J. 2019, 10, 43–50. [Google Scholar] [CrossRef]
- Bonan, P.; Kaminagakura, E.; Pires, F.; Vargas, P.; de Almeida, O. Histomorphometry and immunohistochemical features of grade I (WHO) oral radiomucositis. Oral Dis. 2007, 13, 170–176. [Google Scholar] [CrossRef]
- Homa-Mlak, I.; Brzozowska, A.; Mlak, R.; Szudy-Szczyrek, A.; Małecka-Massalska, T. Neutrophil-to-Lymphocyte Ratio as a Factor Predicting Radiotherapy Induced Oral Mucositis in Head Neck Cancer Patients Treated with Radiotherapy. J. Clin. Med. 2021, 10, 4444. [Google Scholar] [CrossRef]
- Cua, D.J.; Tato, C.M. Innate IL-17-producing cells: The sentinels of the immune system. Nat. Rev. Immunol. 2010, 10, 479–489. [Google Scholar] [CrossRef]
- Saul-McBeth, J.; Dillon, J.; Lee, A.; Launder, D.; Kratch, J.M.; Abutaha, E.; Williamson, A.A.; Schroering, A.G.; Michalski, G.; Biswas, P.; et al. Tissue Damage in Radiation-Induced Oral Mucositis Is Mitigated by IL-17 Receptor Signaling. Front. Immunol. 2021, 12, 687627. [Google Scholar] [CrossRef]
- Conti, H.R.; Bruno, V.M.; Childs, E.E.; Daugherty, S.; Hunter, J.P.; Mengesha, B.G.; Saevig, D.L.; Hendricks, M.R.; Coleman, B.M.; Brane, L.; et al. IL-17 Receptor Signaling in Oral Epithelial Cells Is Critical for Protection against Oropharyngeal Candidiasis. Cell Host Microbe 2016, 20, 606–617. [Google Scholar] [CrossRef]
- Blijlevens, N.M.; Logan, R.M.; Netea, M.G. The changing face of febrile neutropenia-from monotherapy to moulds to mucositis. Mucositis: From febrile neutropenia to febrile mucositis. J. Antimicrob. Chemother. 2009, 63 (Suppl. S1), i36–i40. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Janeway, C.A., Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989, 54 Pt 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Levy, M.; Itav, S.; Elinav, E. Integration of Innate Immune Signaling. Trends Immunol. 2016, 37, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Kaczmarek, A.; Brinkman, B.M.; Heyndrickx, L.; Vandenabeele, P.; Krysko, D.V. Severity of doxorubicin-induced small intestinal mucositis is regulated by the TLR-2 and TLR-9 pathways. J. Pathol. 2012, 226, 598–608. [Google Scholar] [CrossRef]
- Bruning, E.E.; Coller, J.K.; Wardill, H.R.; Bowen, J.M. Site-specific contribution of Toll-like receptor 4 to intestinal homeostasis and inflammatory disease. J. Cell. Physiol. 2021, 236, 877–888. [Google Scholar] [CrossRef]
- Wardill, H.R.; Gibson, R.J.; Van Sebille, Y.Z.; Secombe, K.R.; Coller, J.K.; White, I.A.; Manavis, J.; Hutchinson, M.R.; Staikopoulos, V.; Logan, R.M.; et al. Irinotecan-Induced Gastrointestinal Dysfunction and Pain Are Mediated by Common TLR4-Dependent Mechanisms. Mol. Cancer Ther. 2016, 15, 1376–1386. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Piccinini, A.M.; Midwood, K.S. DAMPening inflammation by modulating TLR signalling. Mediat. Inflamm. 2010, 2010, 672395. [Google Scholar] [CrossRef]
- Qi, W.; Yang, X.; Ye, N.; Li, S.; Han, Q.; Huang, J.; Wu, B. TLR4 gene in the regulation of periodontitis and its molecular mechanism. Exp. Ther. Med. 2019, 18, 1961–1966. [Google Scholar] [CrossRef]
- Lukova, O.A.; Zaslavskaya, M.I.; Makhrova, T.V.; Kropotov, V.S.; Kitaeva, E.V. Expression of toll-like and adhesive receptors on epithelial cells of the oral mucosa in periodontitis. Klin Lab Diagn 2020, 65, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Hao, S.; Wang, J.; Zou, J.; Wang, Y. Roles of Toll-Like Receptors in Radiotherapy- and Chemotherapy-Induced Oral Mucositis: A Concise Review. Front. Cell. Infect. Microbiol. 2022, 12, 831387. [Google Scholar] [CrossRef] [PubMed]
- Karasneh, J.; Bani-Hani, M.; Alkhateeb, A.; Hassan, A.; Alzoubi, F.; Thornhill, M. TLR2, TLR4 and CD86 gene polymorphisms in recurrent aphthous stomatitis. J. Oral Pathol. Med. 2015, 44, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, J.; Du, X.; Hu, L.; Chen, L. The expression of hBDs in the gingival tissue and keratinocytes from healthy subjects and periodontitis patients. Arch. Oral Biol. 2014, 59, 193–198. [Google Scholar] [CrossRef]
- Shang, L.; Deng, D.; Buskermolen, J.K.; Roffel, S.; Janus, M.M.; Krom, B.P.; Crielaard, W.; Gibbs, S. Commensal and Pathogenic Biofilms Alter Toll-Like Receptor Signaling in Reconstructed Human Gingiva. Front. Cell. Infect. Microbiol. 2019, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Hug, H.; Mohajeri, M.H.; La Fata, G. Toll-Like Receptors: Regulators of the Immune Response in the Human Gut. Nutrients 2018, 10, 203. [Google Scholar] [CrossRef]
- Stringer, A.M.; Logan, R.M. The role of oral flora in the development of chemotherapy-induced oral mucositis. J. Oral Pathol. Med. 2015, 44, 81–87. [Google Scholar] [CrossRef]
- Vasconcelos, R.; Sanfilippo, N.; Paster, B.; Kerr, A.; Li, Y.; Ramalho, L.; Queiroz, E.; Smith, B.; Sonis, S.; Corby, P. Host-Microbiome Cross-talk in Oral Mucositis. J. Dent. Res. 2016, 95, 725–733. [Google Scholar] [CrossRef]
- Yamanobe, H.; Yamamoto, K.; Kishimoto, S.; Nakai, K.; Oseko, F.; Yamamoto, T.; Mazda, O.; Kanamura, N. Anti-Inflammatory Effects of beta-Cryptoxanthin on 5-Fluorouracil-Induced Cytokine Expression in Human Oral Mucosal Keratinocytes. Molecules 2023, 28, 2935. [Google Scholar] [CrossRef]
- Im, K.-I.; Nam, Y.-S.; Kim, N.; Song, Y.; Lee, E.-S.; Lim, J.-Y.; Jeon, Y.-W.; Cho, S.-G. Regulation of HMGB1 release protects chemoradiotherapy-associated mucositis. Mucosal Immunol. 2019, 12, 1070–1081. [Google Scholar] [CrossRef]
- He, H.; Hao, Y.; Fan, Y.; Li, B.; Cheng, L. The interaction between innate immunity and oral microbiota in oral diseases. Expert Rev. Clin. Immunol. 2023, 19, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Martinon, F.; Tschopp, J. NLRs join TLRs as innate sensors of pathogens. Trends Immunol. 2005, 26, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, H.; Wang, H.; Wang, B.; Meng, L.; Xin, Y.; Jiang, X. The role of NLRP3 inflammasome activation in radiation damage. Biomed. Pharmacother. 2019, 118, 109217. [Google Scholar] [CrossRef] [PubMed]
- Bedoui, S.; Herold, M.J.; Strasser, A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat. Rev. Mol. Cell Biol. 2020, 21, 678–695. [Google Scholar] [CrossRef] [PubMed]
- Al-Qadami, G.; Bowen, J.; Van Sebille, Y.; Secombe, K.; Dorraki, M.; Verjans, J.; Wardill, H.; Le, H. Baseline gut microbiota composition is associated with oral mucositis and tumour recurrence in patients with head and neck cancer: A pilot study. Support. Care Cancer 2023, 31, 98. [Google Scholar] [CrossRef]
- Cario, E. Toll-like receptors in the pathogenesis of chemotherapy-induced gastrointestinal toxicity. Curr. Opin. Support. Palliat. Care 2016, 10, 157–164. [Google Scholar] [CrossRef]
- Vanhoecke, B.; De Ryck, T.; Stringer, A.; Van de Wiele, T.; Keefe, D. Microbiota and their role in the pathogenesis of oral mucositis. Oral Dis. 2015, 21, 17–30. [Google Scholar] [CrossRef]
- Nassar, M.; Tabib, Y.; Capucha, T.; Mizraji, G.; Nir, T.; Pevsner-Fischer, M.; Zilberman-Schapira, G.; Heyman, O.; Nussbaum, G.; Bercovier, H.; et al. GAS6 is a key homeostatic immunological regulator of host-commensal interactions in the oral mucosa. Proc. Natl. Acad. Sci. USA 2017, 114, E337–E346. [Google Scholar] [CrossRef]
- Zenobia, C.; Luo, X.L.; Hashim, A.; Abe, T.; Jin, L.; Chang, Y.; Jin, Z.C.; Sun, J.X.; Hajishengallis, G.; Curtis, M.A.; et al. Commensal bacteria-dependent select expression of CXCL2 contributes to periodontal tissue homeostasis. Cell. Microbiol. 2013, 15, 1419–1426. [Google Scholar] [CrossRef]
- Takahashi, M.; Toyosaki, M.; Matsui, K.; Machida, S.; Kikkawa, E.; Ota, Y.; Kaneko, A.; Ogawa, Y.; Ando, K.; Onizuka, M. An analysis of oral microbial flora by T-RFLP in patients undergoing hematopoietic stem cell transplantation. Int. J. Hematol. 2020, 112, 690–696. [Google Scholar] [CrossRef]
- Mojdami, Z.D.; Barbour, A.; Oveisi, M.; Sun, C.; Fine, N.; Saha, S.; Marks, C.; Elebyary, O.; Watson, E.; Tenenbaum, H.; et al. The Effect of Intensity-Modulated Radiotherapy to the Head and Neck Region on the Oral Innate Immune Response and Oral Microbiome: A Prospective Cohort Study of Head and Neck Tumour Patients. Int. J. Mol. Sci. 2022, 23, 9594. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.S.; Al-Qadami, G.H.; Laheij, A.M.G.A.; Bossi, P.; Fregnani, E.R.; Wardill, H.R. From Pathogenesis to Intervention: The Importance of the Microbiome in Oral Mucositis. Int. J. Mol. Sci. 2023, 24, 8274. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.A.; King, K.Y.; Baldridge, M.T. Mouse Microbiota Models: Comparing Germ-Free Mice and Antibiotics Treatment as Tools for Modifying Gut Bacteria. Front. Physiol. 2018, 9, 1534. [Google Scholar] [CrossRef] [PubMed]
- Al-Qadami, G.; Van Sebille, Y.; Bowen, J.; Wardill, H. Oral-Gut Microbiome Axis in the Pathogenesis of Cancer Treatment-Induced Oral Mucositis. Front. Oral Health 2022, 3, 881949. [Google Scholar] [CrossRef]
- Khosravi, A.; Yáñez, A.; Price, J.G.; Chow, A.; Merad, M.; Goodridge, H.S.; Mazmanian, S.K. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe 2014, 15, 374–381. [Google Scholar] [CrossRef]
- Mortha, A.; Chudnovskiy, A.; Hashimoto, D.; Bogunovic, M.; Spencer, S.P.; Belkaid, Y.; Merad, M. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science 2014, 343, 1249288. [Google Scholar] [CrossRef]
- Constantinides, M.G. Interactions between the microbiota and innate and innate-like lymphocytes. J. Leukoc. Biol. 2018, 103, 409–419. [Google Scholar] [CrossRef]
- Namuangchan, Y.; Chailertwanich, O.; Susinsamphan, S.; Supakalin, N.; Supaadirek, C.; Krusun, S.; Pesee, M.; Thamronganantasakul, K. Prophylaxis of Oral Mucositis with Iodine Solution during Concurrent Chemoradiation of Head and Neck Cancer: Preliminary Results of a Double-Blind, Randomized Controlled Trial. Asian Pac. J. Cancer Prev. 2023, 24, 2445–2454. [Google Scholar] [CrossRef]
- Pitten, F.-A.; Kiefer, T.; Buth, C.; Doelken, G.; Kramer, A. Do cancer patients with chemotherapy-induced leukopenia benefit from an antiseptic chlorhexidine-based oral rinse? A double-blind, block-randomized, controlled study. J. Hosp. Infect. 2003, 53, 283–291. [Google Scholar] [CrossRef]
- Spijkervet, F.; van Saene, H.; Panders, A.; Vermey, A.; van Saene, J.; Mehta, D.; Fidler, V. Effect of chlorhexidine rinsing on the oropharyngeal ecology in patients with head and neck cancer who have irradiation mucositis. Oral Surg. Oral Med. Oral Pathol. 1989, 67, 154–161. [Google Scholar] [CrossRef]
- Stokman, M.A.; Spijkervet, F.K.L.; Burlage, F.R.; Dijkstra, P.U.; Manson, W.L.; de Vries, E.G.E.; Roodenburg, J.L.N. Oral mucositis and selective elimination of oral flora in head and neck cancer patients receiving radiotherapy: A double-blind randomised clinical trial. Br. J. Cancer 2003, 88, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Wijers, O.B.; Levendag, P.C.; Harms, E.R.; Gan-Teng, A.; Schmitz, P.I.; Hendriks, W.; Wilms, E.B.; van der Est, H.; Visch, L.L. Mucositis reduction by selective elimination of oral flora in irradiated cancers of the head and neck: A placebo-controlled double-blind randomized study. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Li, P.; Yu, B.; Huang, S.; Chen, Y. The effectiveness of probiotics in prevention and treatment of cancer therapy-induced oral mucositis: A systematic review and meta-analysis. Oral Oncol. 2020, 102, 104559. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, V.; Belgioia, L.; Cante, D.; La Porta, M.R.; Caspiani, O.; Guarnaccia, R.; Argenone, A.; Muto, P.; Musio, D.; De Felice, F.; et al. Lactobacillus brevis CD2 for Prevention of Oral Mucositis in Patients With Head and Neck Tumors: A Multicentric Randomized Study. Anticancer Res. 2019, 39, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics Mechanism of Action on Immune Cells and Beneficial Effects on Human Health. Cells 2023, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Fan, Y.; Li, Y.; Dong, J.; Zhang, S.; Wang, B.; Liu, J.; Liu, X.; Fan, S.; Guan, J.; et al. Oral microbiota transplantation fights against head and neck radiotherapy-induced oral mucositis in mice. Comput. Struct. Biotechnol. J. 2021, 19, 5898–5910. [Google Scholar] [CrossRef]
- Zhu, X.-X.; Yang, X.-J.; Chao, Y.-L.; Zheng, H.-M.; Sheng, H.-F.; Liu, H.-Y.; He, Y.; Zhou, H.-W. The Potential Effect of Oral Microbiota in the Prediction of Mucositis During Radiotherapy for Nasopharyngeal Carcinoma. EBioMedicine 2017, 18, 23–31. [Google Scholar] [CrossRef]
- Burdelya, L.G.; Gleiberman, A.S.; Toshkov, I.; Aygun-Sunar, S.; Bapardekar, M.; Manderscheid-Kern, P.; Bellnier, D.; Krivokrysenko, V.I.; Feinstein, E.; Gudkov, A.V. Toll-like receptor 5 agonist protects mice from dermatitis and oral mucositis caused by local radiation: Implications for head-and-neck cancer radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, 228–234. [Google Scholar] [CrossRef]
- Ko, J.; Kim, J.; Choi, Y.-K.; Nahm, S.-S.; Kim, J.; Seo, S.-M.; Seo, J.-S.; Lee, W.; Chung, W.K.; Eom, K. Clinical evaluation of toll-like receptor-5 agonist for radiation-induced oral mucositis in beagle dogs. Front. Vet. Sci. 2022, 9, 839467. [Google Scholar] [CrossRef]
- Lee, H.-S.; Cho, D.-W.; Han, J.-S.; Han, S.-C.; Woo, S.K.; Jun, S.-Y.; Lee, W.-J.; Yoon, S.; Pak, S.-I.; Lee, S.-J.; et al. KMRC011, an agonist of toll-like receptor 5, mitigates irradiation-induced tissue damage and mortality in cynomolgus monkeys. J. Immunotoxicol. 2020, 17, 31–42. [Google Scholar] [CrossRef]
- Ortiz, F.; Acuña-Castroviejo, D.; Doerrier, C.; Dayoub, J.C.; Lopez, L.C.; Venegas, C.; García, J.A.; López, A.; Volt, H.; Sánchez, M.L.; et al. Melatonin blunts the mitochondrial/NLRP3 connection and protects against radiation-induced oral mucositis. J. Pineal Res. 2015, 58, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Pulsipher, A.; Savage, J.R.; Kennedy, T.P.; Gupta, K.; Cuiffo, B.G.; Sonis, S.T.; Lee, W.Y. GM-1111 reduces radiation-induced oral mucositis in mice by targeting pattern recognition receptor-mediated inflammatory signaling. PLoS ONE 2021, 16, e0249343. [Google Scholar] [CrossRef] [PubMed]
- Kudrimoti, M.; Curtis, A.; Azawi, S.; Worden, F.; Katz, S.; Adkins, D.; Bonomi, M.; Scott, Z.; Elder, J.; Sonis, S.T.; et al. Dusquetide: Reduction in oral mucositis associated with enduring ancillary benefits in tumor resolution and decreased mortality in head and neck cancer patients. Biotechnol. Rep. 2017, 15, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Watkins, B.; Pouliot, K.; Fey, E.; Tuthill, C.; Sonis, S. Attenuation of radiation- and chemoradiation-induced mucositis using gamma-D-glutamyl-L-tryptophan (SCV-07). Oral Dis. 2010, 16, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Ozcicek, F.; Kara, A.V.; Akbas, E.M.; Kurt, N.; Yazici, G.N.; Cankaya, M.; Mammadov, R.; Ozcicek, A.; Suleyman, H. Effects of anakinra on the small intestine mucositis induced by methotrexate in rats. Exp. Anim. 2020, 69, 144–152. [Google Scholar] [CrossRef]
- Kullenberg, F.; Peters, K.; Sjöblom, M.; Heindryckx, F.; Dahlgren, D.; Lennernäs, H. Anakinra and dexamethasone treatment of idarubicin-induced mucositis and diarrhoea in rats. Basic Clin. Pharmacol. Toxicol. 2023, 132, 511–520. [Google Scholar] [CrossRef]
- Wardill, H.R.; de Mooij, C.E.M.; Ferreira, A.R.D.S.; Havinga, H.; Harmsen, H.J.M.; van der Velden, W.J.F.M.; van Groningen, L.F.J.; Tissing, W.J.E.; Blijlevens, N.M.A. Supporting the gastrointestinal microenvironment during high-dose chemotherapy and stem cell transplantation by inhibiting IL-1 signaling with anakinra. Sci. Rep. 2022, 12, 6803. [Google Scholar] [CrossRef]
- de Mooij, C.E.; van Groningen, L.F.; de Haan, A.F.; Biemond, B.J.; Bakker, M.; van der Velden, W.J.; Blijlevens, N.M. Anakinra: Efficacy in the management of fever during neutropenia and mucositis in autologous stem cell transplantation (AFFECT-2)-study protocol for a multicenter randomized double-blind placebo-controlled trial. Trials 2020, 21, 948. [Google Scholar] [CrossRef]
- Chen, K.-J.; Chen, Y.-L.; Ueng, S.-H.; Hwang, T.-L.; Kuo, L.-M.; Hsieh, P.-W. Neutrophil elastase inhibitor (MPH-966) improves intestinal mucosal damage and gut microbiota in a mouse model of 5-fluorouracil-induced intestinal mucositis. Biomed. Pharmacother. 2021, 134, 111152. [Google Scholar] [CrossRef]
- Ahmed, A.; Tait, S.W.G. Targeting immunogenic cell death in cancer. Mol. Oncol. 2020, 14, 2994–3006. [Google Scholar] [CrossRef]
- Arbour, K.C.; Mezquita, L.; Long, N.; Rizvi, H.; Auclin, E.; Ni, A.; Martínez-Bernal, G.; Ferrara, R.; Lai, W.V.; Hendriks, L.E.L.; et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2872–2878. [Google Scholar] [CrossRef]
- von Itzstein, M.S.; Gonugunta, A.S.; Sheffield, T.; Homsi, J.; Dowell, J.E.; Koh, A.Y.; Raj, P.; Fattah, F.; Wang, Y.; Basava, V.S.; et al. Association between Antibiotic Exposure and Systemic Immune Parameters in Cancer Patients Receiving Checkpoint Inhibitor Therapy. Cancers 2022, 14, 1327. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Iaculli, A.; Signorelli, D.; Ghidini, A.; Dottorini, L.; Perego, G.; Ghidini, M.; Zaniboni, A.; Gori, S.; Inno, A. Survival of Patients Treated with Antibiotics and Immunotherapy for Cancer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 1458. [Google Scholar] [CrossRef] [PubMed]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 2013, 342, 967–970. [Google Scholar] [CrossRef]
| Intervention Type | Research Methodology | Effective? | Reference | ||||
|---|---|---|---|---|---|---|---|
| Therapeutic Compound | Study Design | Study Subjects | Yes | No | |||
| Clinical | Antiseptic/antibiotic approaches | Iodine-based mouthwash | Double-blind, randomized controlled trial (n = 20) | Patients receiving chemoradiation for head and neck cancer | X | [79] | |
| 0.3% chlorhexidine-based mouthwash | Double-blind, randomized controlled trial (n = 47) | Patients receiving chemotherapy | X | [80] | |||
| 0.1% chlorhexidine-based mouthwash | Double-blind, randomized controlled trial (n = 30) | Patients receiving radiotherapy for head and neck cancer | X | [81] | |||
| Lozenges containing polymyxin E, tobramycin, and amphotericin B | Double-blind, randomized controlled trial (n = 65) | Patients receiving radiotherapy for head and neck cancer | X | [82] | |||
| Oral paste containing polymyxin E, tobramycin, and amphotericin B | Double-blind, randomized controlled trial (n = 77) | Patients receiving radiotherapy for head and neck cancer | X | [83] | |||
| Microbiota-targeted therapeutics | Lactobacillus brevis CD2 lozenges | Double-blind, randomized controlled trial (n = 31) | Patients receiving chemoradiation for head and neck cancer | X | [85] | ||
| Capsule containing Bifidobacterium longum, Lactobacillus lactis, and Enterococcus faecium | Double-blind, randomized controlled trial (n = 99) | Patients receiving chemoradiation for head and neck cancer | ✓ | [84] | |||
| Preclinical | Oral microbiota transplantation (OMT) | Mice received OMT after treatment | Mice receiving head and neck irradiation | ✓ | [87] | ||
| Novel innate immune-targeted therapeutics | TLR5 agonist CBLB502 | Mice were injected subcutaneously with CBLB502 after treatment | Mouse model of head and neck cancer treated with radiotherapy | ✓ | [89] | ||
| TLR5 agonist KMRC011 | Beagle dogs were administered with KMRC011 up to 48 h after treatment | Beagle dogs receiving head and neck irradiation | ✓ | [90] | |||
| Melatonin | Rats were administered with melatonin gel up to 21 days after treatment | Rats receiving tongue irradiation | ✓ | [92] | |||
| Synthetic glycosaminoglycan GM-1111 | Mice were administered with GM-1111 subcutaneously daily | Mice receiving head and neck irradiation | ✓ | [93] | |||
| Immunomodulator peptide SCV-07 | Golden Syrian hamsters were administered SCV-07 | Hamsters receiving irradiation of buccal mucosa +/− chemotherapy | ✓ | [95] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bowen, J.; Cross, C. The Role of the Innate Immune Response in Oral Mucositis Pathogenesis. Int. J. Mol. Sci. 2023, 24, 16314. https://doi.org/10.3390/ijms242216314
Bowen J, Cross C. The Role of the Innate Immune Response in Oral Mucositis Pathogenesis. International Journal of Molecular Sciences. 2023; 24(22):16314. https://doi.org/10.3390/ijms242216314
Chicago/Turabian StyleBowen, Joanne, and Courtney Cross. 2023. "The Role of the Innate Immune Response in Oral Mucositis Pathogenesis" International Journal of Molecular Sciences 24, no. 22: 16314. https://doi.org/10.3390/ijms242216314
APA StyleBowen, J., & Cross, C. (2023). The Role of the Innate Immune Response in Oral Mucositis Pathogenesis. International Journal of Molecular Sciences, 24(22), 16314. https://doi.org/10.3390/ijms242216314






