Mucositis and Infection in Hematology Patients
Abstract
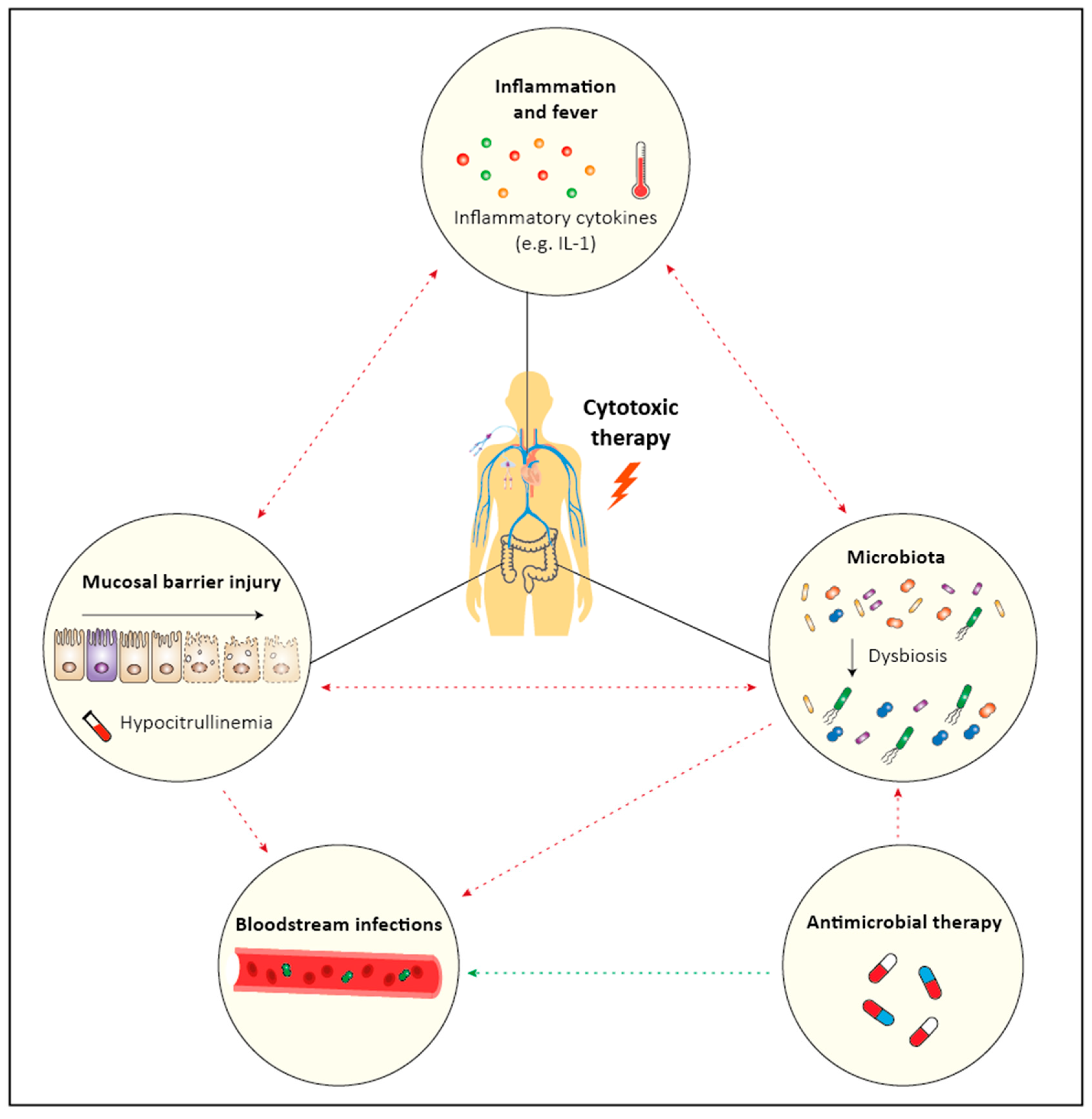
1. Introduction
2. Is Mucositis an Infectious Disease?
3. Does Antimicrobial Therapy Ameliorate Mucositis?
4. Might Insights of Microbiome Research Provide More Clinically Relevant Answers with Respect to Mucositis?
4.1. Oral Dysbiosis
4.2. Gut Dysbiosis
4.3. Bidirectional Relationship between Dysbiosis and Mucositis
5. Are Infections and Fever during Neutropenia Caused by Mucositis?
6. Treatment of Mucositis-Related Fever
7. Future Directions
8. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cordonnier, C.; Ljungman, P.; Cesaro, S.; Hirsch, H.H.; Maschmeyer, G.; von Lilienfeld-Toal, M.; Vehreschild, M.; Mikulska, M.; Emonts, M.; Gennery, A.R.; et al. The EHA Research Roadmap: Infections in Hematology. Hemasphere 2021, 5, e662. [Google Scholar] [CrossRef] [PubMed]
- Taplitz, R.A.; Kennedy, E.B.; Bow, E.J.; Crews, J.; Gleason, C.; Hawley, D.K.; Langston, A.A.; Nastoupil, L.J.; Rajotte, M.; Rolston, K.V.; et al. Antimicrobial Prophylaxis for Adult Patients with Cancer-Related Immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J. Clin. Oncol. 2018, 36, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- de la Court, J.R.; Bruns, A.H.W.; Roukens, A.H.E.; Baas, I.O.; van Steeg, K.; Toren-Wielema, M.L.; Tersmette, M.; Blijlevens, N.M.A.; Veld, R.A.G.H.I.; Wolfs, T.F.W.; et al. The Dutch Working Party on Antibiotic Policy (SWAB) Recommendations for the Diagnosis and Management of Febrile Neutropenia in Patients with Cancer. Infect. Dis. Ther. 2022, 11, 2063–2098. [Google Scholar] [CrossRef] [PubMed]
- Lehrnbecher, T.; Robinson, P.D.; Ammann, R.A.; Fisher, B.; Patel, P.; Phillips, R.; Beauchemin, M.P.; Carlesse, F.; Castagnola, E.; Davis, B.L.; et al. Guideline for the Management of Fever and Neutropenia in Pediatric Patients with Cancer and Hematopoietic Cell Transplantation Recipients: 2023 Update. J. Clin. Oncol. 2023, 41, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Rn, K.K.F.C.; Lalla, R.V.; Yarom, N.; Hong, C.; Logan, R.M.; Bowen, J.; Gibson, R.; Saunders, D.P.; Zadik, Y.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2020, 126, 4423–4431. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Silva, W.; On behalf of the Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society for Oral Oncology (MASCC/ISOO); Gomes-Silva, W.; Zadik, Y.; Yarom, N.; Al-Azri, A.R.; Hong, C.H.L.; Ariyawardana, A.; Saunders, D.P.; Correa, M.E.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis: Sub-analysis of current interventions for the management of oral mucositis in pediatric cancer patients. Support. Care Cancer 2021, 29, 3539–3562. [Google Scholar] [CrossRef] [PubMed]
- Elad, S.; Yarom, N.; Zadik, Y.; Kuten-Shorrer, M.; Sonis, S.T. The broadening scope of oral mucositis and oral ulcerative mucosal toxicities of anticancer therapies. CA Cancer J. Clin. 2022, 72, 57–77. [Google Scholar] [CrossRef]
- Blijlevens, N.; Schwenkglenks, M.; Bacon, P.; D’Addio, A.; Einsele, H.; Maertens, J.; Niederwieser, D.; Rabitsch, W.; Roosaar, A.; Ruutu, T.; et al. Prospective Oral Mucositis Audit: Oral Mucositis in Patients Receiving High-Dose Melphalan or BEAM Conditioning Chemotherapy—European Blood and Marrow Transplantation Mucositis Advisory Group. J. Clin. Oncol. 2008, 26, 1519–1525. [Google Scholar] [CrossRef]
- Dahlgren, D.; Lennernäs, H. Review on the effect of chemotherapy on the intestinal barrier: Epithelial permeability, mucus and bacterial translocation. Biomed. Pharmacother. 2023, 162, 114644. [Google Scholar] [CrossRef]
- Sonis, S.T. New thoughts on the initiation of mucositis. Oral Dis. 2010, 16, 597–600. [Google Scholar] [CrossRef]
- Lalla, R.V.; Brennan, M.T.; Gordon, S.M.; Sonis, S.T.; Rosenthal, D.I.; Keefe, D.M. Oral Mucositis Due to High-Dose Chemotherapy and/or Head and Neck Radiation Therapy. JNCI Monogr. 2019, 2019, lgz011. [Google Scholar] [CrossRef]
- Levy, O.; Teixeira-Pinto, A.; White, M.L.; Carroll, S.F.; Lehmann, L.; Wypij, D.; Guinan, E. Endotoxemia and elevation of lipopolysaccharide-binding protein after hematopoietic stem cell transplantation. Pediatr. Infect. Dis. J. 2003, 22, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, E.; Hiki, N.; Nomura, S.; Fukushima, R.; Kojima, J.-I.; Ogawa, T.; Mafune, K.-I.; Mimura, Y.; Kaminishi, M. Simultaneous onset of acute inflammatory response, sepsis-like symptoms and intestinal mucosal injury after cancer chemotherapy. Int. J. Cancer 2003, 107, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Clevers, H. Faculty Opinions recommendation of Reparative inflammation takes charge of tissue regeneration. Nature 2016, 529, 307–315. [Google Scholar] [CrossRef][Green Version]
- Mougeot, J.-L.C.; Stevens, C.B.; Morton, D.S.; Brennan, M.T.; Mougeot, F.B. Oral Microbiome and Cancer Therapy-Induced Oral Mucositis. JNCI Monogr. 2019, 2019, lgz002. [Google Scholar] [CrossRef]
- Laheij, A.M.; de Soet, J.J. Can the oral microflora affect oral ulcerative mucositis? Curr. Opin. Support. Palliat. Care 2014, 8, 180–187. [Google Scholar] [CrossRef]
- Rock, K.L.; Latz, E.; Ontiveros, F.; Kono, H. The Sterile Inflammatory Response. Annu. Rev. Immunol. 2010, 28, 321–342. [Google Scholar] [CrossRef][Green Version]
- Colella, G.; Boschetti, C.E.; Vitagliano, R.; Colella, C.; Jiao, L.; King-Smith, N.; Li, C.; Lau, Y.N.; Lai, Z.; Mohammed, A.I.; et al. Interventions for the Prevention of Oral Mucositis in Patients Receiving Cancer Treatment: Evidence from Randomised Controlled Trials. Curr. Oncol. 2023, 30, 967–980. [Google Scholar] [CrossRef]
- Diaz-Sanchez, R.M.; Pachon-Ibanez, J.; Marin-Conde, F.; Rodriguez-Caballero, A.; Pérez, J.L.G.; Torres-Lagares, D. Double-blind, randomized pilot study of bioadhesive chlorhexidine gel in the prevention and treatment of mucositis induced by chemoradiotherapy of head and neck cancer. Med. Oral Patol. Oral Cir. Bucal. 2015, 20, e378–e385. [Google Scholar] [CrossRef]
- Epstein, J.B.; Vickars, L.; Spinelli, J.; Reece, D. Efficacy of chlorhexidine and nystatin rinses in prevention of oral complications in leukemia and bone marrow transplantation. Oral Surg. Oral Med. Oral Pathol. 1992, 73, 682–689. [Google Scholar] [CrossRef]
- Dodd, M.J.; Larson, P.J.; Dibble, S.L.; Miaskowski, C.; Greenspan, D.; MacPhail, L.; Hauck, W.W.; Paul, S.M.; Ignoffo, R.; Shiba, G. Randomized clinical trial of chlorhexidine versus placebo for prevention of oral mucositis in patients receiving chemotherapy. Oncol. Nurs. Forum 1996, 23, 921–927. [Google Scholar] [PubMed]
- Sorensen, J.B.; Skovsgaard, T.; Bork, E.; Damstrup, L.; Ingeberg, S. Double-blind, placebo-controlled, randomized study of chlorhexidine prophylaxis for 5-fluorouracil-based chemotherapy-induced oral mucositis with nonblinded randomized comparison to oral cooling (cryotherapy) in gastrointestinal malignancies. Cancer 2008, 112, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Molassiotis, A.; Chang, A.; Wai, W.; Cheung, S. Evaluation of an oral care protocol intervention in the prevention of chemotherapy-induced oral mucositis in paediatric cancer patients. Eur. J. Cancer 2001, 37, 2056–2063. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Molassiotis, A.; Chang, A. An oral care protocol intervention to prevent chemotherapy-induced oral mucositis in paediatric cancer patients: A pilot study. Eur. J. Oncol. Nurs. 2002, 6, 66–73. [Google Scholar] [CrossRef]
- Giles, F.J.; Miller, C.B.; Hurd, D.D.; Wingard, J.R.; Fleming, T.R.; Sonis, S.T.; Bradford, W.Z.; Pulliam, J.G.; Anaissie, E.J.; Beveridge, R.A.; et al. A Phase III, Randomized, Double-blind, Placebo-controlled, Multinational Trial of Iseganan for the Prevention of Oral Mucositis in Patients Receiving Stomatotoxic Chemotherapy (PROMPT-CT Trial). Leuk. Lymphoma 2003, 44, 1165–1172. [Google Scholar] [CrossRef]
- Parkhideh, S.; Zeraatkar, M.; Moradi, O.; Hajifathali, A.; Mehdizadeh, M.; Tavakoli-Ardakani, M. Azithromycin oral suspension in prevention and management of oral mucositis in patients undergoing hematopoietic stem cell transplantation: A randomized controlled trial. Support. Care Cancer 2022, 30, 251–257. [Google Scholar] [CrossRef]
- Vokurka, S.; Bystricka, E.; Koza, V.; Scudlová, J.; Pavlicová, V.; Valentová, D.; Bockova, J.; Misaniová, L. The comparative effects of povidone-iodine and normal saline mouthwashes on oral mucositis in patients after high-dose chemotherapy and APBSCT—Results of a randomized multicentre study. Support. Care Cancer 2005, 13, 554–558. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Bellm, L.; Epstein, J.B.; Sonis, S.T.; Symonds, R.P. Antimicrobial therapy to prevent or treat oral mucositis. Lancet Infect. Dis. 2003, 3, 405–412. [Google Scholar] [CrossRef]
- Martino, C.; Dilmore, A.H.; Burcham, Z.M.; Metcalf, J.L.; Jeste, D.; Knight, R. Microbiota succession throughout life from the cradle to the grave. Nat. Rev. Genet. 2022, 20, 707–720. [Google Scholar] [CrossRef]
- Zaura, E.; Keijser, B.J.F.; Huse, S.M.; Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef][Green Version]
- Laheij, A.M.G.A.; Raber-Durlacher, J.E.; Koppelmans, R.G.A.; Huysmans, M.-C.D.N.J.M.; Potting, C.; van Leeuwen, S.J.M.; Hazenberg, M.D.; Brennan, M.T.; von Bültzingslöwen, I.; Johansson, J.-E.; et al. Microbial changes in relation to oral mucositis in autologous hematopoietic stem cell transplantation recipients. Sci. Rep. 2019, 9, 16929. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bruno, J.S.; Heidrich, V.; Knebel, F.H.; de Molla, V.C.; Parahyba, C.J.; Miranda-Silva, W.; Asprino, P.F.; Tucunduva, L.; Rocha, V.; Novis, Y.; et al. Commensal oral microbiota impacts ulcerative oral mucositis clinical course in allogeneic stem cell transplant recipients. Sci. Rep. 2022, 12, 17527. [Google Scholar] [CrossRef]
- Laheij, A.M.G.A.; de Soet, J.J.; Veerman, E.C.I.; Bolscher, J.G.M.; van Loveren, C. The Influence of Oral Bacteria on Epithelial Cell Migration In Vitro. Mediat. Inflamm. 2013, 2013, 154532. [Google Scholar] [CrossRef][Green Version]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Faculty Opinions recommendation of Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sommer, F.; Bäckhed, F. The gut microbiota—Masters of host development and physiology. Nat. Rev. Microbiol. 2013, 11, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.V.; Littman, D.R.; MacPherson, A.J. Interactions Between the Microbiota and the Immune System. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rashidi, A.; Kaiser, T.; Graiziger, C.; Holtan, S.G.; Rehman, T.U.; Weisdorf, D.J.; Dunny, G.M.; Khoruts, A.; Staley, C. Gut dysbiosis during antileukemia chemotherapy versus allogeneic hematopoietic cell transplantation. Cancer 2020, 126, 1434–1447. [Google Scholar] [CrossRef]
- Galloway-Peña, J.R.; Shi, Y.; Peterson, C.B.; Sahasrabhojane, P.; Gopalakrishnan, V.; Brumlow, C.E.; Daver, N.G.; Alfayez, M.; Boddu, P.C.; Khan, M.A.W.; et al. Gut Microbiome Signatures Are Predictive of Infectious Risk Following Induction Therapy for Acute Myeloid Leukemia. Clin. Infect. Dis. 2020, 71, 63–71. [Google Scholar] [CrossRef]
- Hakim, H.; Dallas, R.; Wolf, J.; Tang, L.; Schultz-Cherry, S.; Darling, V.; Johnson, C.; Karlsson, E.A.; Chang, T.-C.; Jeha, S.; et al. Gut Microbiome Composition Predicts Infection Risk During Chemotherapy in Children with Acute Lymphoblastic Leukemia. Clin. Infect. Dis. 2018, 67, 541–548. [Google Scholar] [CrossRef][Green Version]
- Peled, J.U.; Gomes, A.L.; Devlin, S.M.; Littmann, E.R.; Taur, Y.; Sung, A.D.; Weber, D.; Hashimoto, D.; Slingerland, A.E.; Slingerland, J.B.; et al. Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2020, 382, 822–834. [Google Scholar] [CrossRef]
- Khuat, L.T.; Dave, M.; Murphy, W.J. The emerging roles of the gut microbiome in allogeneic hematopoietic stem cell transplantation. Gut Microbes 2021, 13, 1966262. [Google Scholar] [CrossRef]
- McMahon, S.; Sahasrabhojane, P.; Kim, J.; Franklin, S.; Chang, C.C.; Jenq, R.R.; Hillhouse, A.E.; Shelburne, S.A.; Galloway-Peña, J. Contribution of the Oral and Gastrointestinal Microbiomes to Bloodstream Infections in Leukemia Patients. Microbiol. Spectr. 2023, e0041523. [Google Scholar] [CrossRef] [PubMed]
- Haak, B.W.; Littmann, E.R.; Chaubard, J.-L.; Pickard, A.; Fontana, E.; Adhi, F.; Gyaltshen, Y.; Ling, L.; Morjaria, S.M.; Peled, J.; et al. Impact of gut colonization with butyrate producing microbiota on respiratory viral infection following allo-HCT. Blood 2018, 131, 2978–2986. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jenq, R.R.; Ubeda, C.; Taur, Y.; Menezes, C.C.; Khanin, R.; Dudakov, J.A.; Liu, C.; West, M.L.; Singer, N.V.; Equinda, M.J.; et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J. Exp. Med. 2012, 209, 903–911. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Staffas, A.; Da Silva, M.B.; van den Brink, M.R. The intestinal microbiota in allogeneic hematopoietic cell transplant and graft-versus-host disease. Blood 2017, 129, 927–933. [Google Scholar] [CrossRef]
- Shono, Y.; Brink, M.R.M.V.D. Gut microbiota injury in allogeneic haematopoietic stem cell transplantation. Nat. Rev. Cancer 2018, 18, 283–295. [Google Scholar] [CrossRef]
- Peled, J.U.; Devlin, S.M.; Staffas, A.; Lumish, M.; Khanin, R.; Littmann, E.R.; Ling, L.; Kosuri, S.; Maloy, M.; Slingerland, J.B.; et al. Intestinal Microbiota and Relapse After Hematopoietic-Cell Transplantation. J. Clin. Oncol. 2017, 35, 1650–1659. [Google Scholar] [CrossRef][Green Version]
- Nishi, K.; Kanda, J.; Hishizawa, M.; Kitano, T.; Kondo, T.; Yamashita, K.; Takaori-Kondo, A. Impact of the Use and Type of Antibiotics on Acute Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2018, 24, 2178–2183. [Google Scholar] [CrossRef][Green Version]
- Lee, S.E.; Lim, J.Y.; Ryu, D.B.; Kim, T.W.; Park, S.S.; Jeon, Y.W.; Yoon, J.H.; Cho, B.S.; Eom, K.S.; Kim, Y.J.; et al. Alteration of the Intestinal Microbiota by Broad-Spectrum Antibiotic Use Correlates with the Occurrence of Intestinal Graft-versus-Host Disease. Biol. Blood Marrow Transplant. 2019, 25, 1933–1943. [Google Scholar] [CrossRef]
- Taur, Y.; Coyte, K.; Schluter, J.; Robilotti, E.; Figueroa, C.; Gjonbalaj, M.; Littmann, E.R.; Ling, L.; Miller, L.; Gyaltshen, Y.; et al. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci. Transl. Med. 2018, 10, eaap9489. [Google Scholar] [CrossRef][Green Version]
- Weber, D.; Jenq, R.R.; Peled, J.U.; Taur, Y.; Hiergeist, A.; Koestler, J.; Dettmer, K.; Weber, M.; Wolff, D.; Hahn, J.; et al. Microbiota Disruption Induced by Early Use of Broad-Spectrum Antibiotics Is an Independent Risk Factor of Outcome after Allogeneic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2017, 23, 845–852. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weber, D.; Hiergeist, A.; Weber, M.; Dettmer, K.; Wolff, D.; Hahn, J.; Herr, W.; Gessner, A.; Holler, E. Detrimental Effect of Broad-spectrum Antibiotics on Intestinal Microbiome Diversity in Patients After Allogeneic Stem Cell Transplantation: Lack of Commensal Sparing Antibiotics. Clin. Infect. Dis. 2019, 68, 1303–1310. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shono, Y.; Docampo, M.D.; Peled, J.U.; Perobelli, S.M.; Velardi, E.; Tsai, J.J.; Slingerland, A.E.; Smith, O.M.; Young, L.F.; Gupta, J.; et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci. Transl. Med. 2016, 8, 339ra71. [Google Scholar] [CrossRef][Green Version]
- Stein-Thoeringer, C.K.; Nichols, K.B.; Lazrak, A.; Docampo, M.D.; Slingerland, A.E.; Slingerland, J.B.; Clurman, A.G.; Armijo, G.; Gomes, A.L.C.; Shono, Y.; et al. Lactose drives Enterococcus expansion to promote graft-versus-host disease. Science 2019, 366, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Chou, W.C.; Lai, Y.; Liang, K.; Tam, J.W.; Brickey, W.J.; Chen, L.; Montgomery, N.D.; Li, X.; Bohannon, L.M.; et al. Multi-omics analyses of radiation survivors identify radioprotective microbes and metabolites. Science 2020, 370, eaay9097. [Google Scholar] [CrossRef]
- Montassier, E.; Gastinne, T.; Vangay, P.; Al-Ghalith, G.A.; Bruley des Varannes, S.; Massart, S.; Moreau, P.; Potel, G.; De La Cochetière, M.F.; Batard, E.; et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment. Pharmacol. Ther. 2015, 42, 515–528. [Google Scholar] [CrossRef][Green Version]
- Lakhdari, O.; Tap, J.; Béguet-Crespel, F.; Le Roux, K.; De Wouters, T.; Cultrone, A.; Nepelska, M.; Lefevre, F.; Doré, J.; Blottiere, H.M. Identification of NF-kappaB modulation capabilities within human intestinal commensal bacteria. J. Biomed. Biotechnol. 2011, 2011, 282356. [Google Scholar] [CrossRef][Green Version]
- Khokhlova, E.V.; Smeianov, V.V.; Efimov, B.A.; Kafarskaia, L.I.; Pavlova, S.I.; Shkoporov, A.N. Anti-inflammatory properties of intestinal Bifidobacterium strains isolated from healthy infants. Microbiol. Immunol. 2012, 56, 27–39. [Google Scholar] [CrossRef]
- Meedt, E.; Hiergeist, A.; Gessner, A.; Dettmer, K.; Liebisch, G.; Ghimire, S.; Poeck, H.; Edinger, M.; Wolff, D.; Herr, W.; et al. Prolonged Suppression of Butyrate-Producing Bacteria Is Associated with Acute Gastrointestinal Graft-vs-Host Disease and Transplantation-Related Mortality After Allogeneic Stem Cell Transplantation. Clin. Infect. Dis. 2022, 74, 614–621. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Hueso, T.; Ekpe, K.; Mayeur, C.; Gatse, A.; Curt, M.J.-C.; Gricourt, G.; Rodriguez, C.; Burdet, C.; Ulmann, G.; Neut, C.; et al. Impact and consequences of intensive chemotherapy on intestinal barrier and microbiota in acute myeloid leukemia: The role of mucosal strengthening. Gut Microbes 2020, 12, 1800897. [Google Scholar] [CrossRef] [PubMed]
- De Pietri, S.; Ingham, A.C.; Frandsen, T.L.; Rathe, M.; Krych, L.; Castro-Mejía, J.L.; Nielsen, D.S.; Nersting, J.; Wehner, P.S.; Schmiegelow, K.; et al. Gastrointestinal toxicity during induction treatment for childhood acute lymphoblastic leukemia: The impact of the gut microbiota. Int. J. Cancer 2020, 147, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Caminero, A.; Guzman, M.; Libertucci, J.; Lomax, A.E. The emerging roles of bacterial proteases in intestinal diseases. Gut Microbes 2023, 15, 2181922. [Google Scholar] [CrossRef]
- Shen, Z.-H.; Zhu, C.-X.; Quan, Y.-S.; Yang, Z.-Y.; Wu, S.; Luo, W.-W.; Tan, B.; Wang, X.-Y. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018, 24, 5–14. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet–microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef]
- Wardill, H.R.; de Mooij, C.E.M.; Ferreira, A.R.D.S.; van de Peppel, I.P.; Havinga, R.; Harmsen, H.J.M.; Tissing, W.J.E.; Blijlevens, N.M.A. Translational model of melphalan-induced gut toxicity reveals drug-host-microbe interactions that drive tissue injury and fever. Cancer Chemother. Pharmacol. 2021, 88, 173–188. [Google Scholar] [CrossRef]
- Herbers, A.H.; Feuth, T.; Donnelly, J.P.; Blijlevens, N.M. Citrulline-based assessment score: First choice for measuring and monitoring intestinal failure after high-dose chemotherapy. Ann. Oncol. 2010, 21, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, A.; Peled, J.U.; Ebadi, M.; Rehman, T.U.; Elhusseini, H.; Marcello, L.T.; Halaweish, H.; Kaiser, T.; Holtan, S.G.; Khoruts, A.; et al. Protective Effect of Intestinal Blautia Against Neutropenic Fever in Allogeneic Transplant Recipients. Clin. Infect. Dis. 2022, 75, 1912–1920. [Google Scholar] [CrossRef]
- van der Velden, W.J.; Herbers, A.H.; Netea, M.G.; Blijlevens, N.M. Mucosal barrier injury, fever and infection in neutropenic patients with cancer: Introducing the paradigm febrile mucositis. Br. J. Haematol. 2014, 167, 441–452. [Google Scholar] [CrossRef]
- de Mooij, C.E.M.; van Groningen, L.F.J.; Molendijk, E.B.D.; Wardill, H.R.; van der Velden, W.J.F.M.; Blijlevens, N.M.A. Blautia Abundance and Mucosal Barrier Injury: A Complex Play of Cause and Effect. Clin. Infect. Dis. 2022, 76, 1152–1153. [Google Scholar] [CrossRef]
- Shouval, R.; Waters, N.R.; Gomes, A.L.C.; Brambilla, C.Z.; Fei, T.; Devlin, S.M.; Nguyen, C.L.; Markey, K.A.; Dai, A.; Slingerland, J.B.; et al. Conditioning Regimens are Associated with Distinct Patterns of Microbiota Injury in Allogeneic Hematopoietic Cell Transplantation. Clin. Cancer Res. 2022, 29, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Wardill, H.v.G.L.; Molendijk, E.; Ferreira Da Silva, A.R.; Kurilshikov, A.; Dorraki, M.; Ryan, F.; Harmsen, H.; Tissing, W.; van der Velden, W.; Blijlevens, N. Unravelling the factors that shape gut dysbiosis in AHCT recipients. 2023; Submitted manuscript. [Google Scholar]
- Fattizzo, B.; Cavallaro, F.; Folino, F.; Barcellini, W. Recent insights into the role of the microbiome in malignant and benign hematologic diseases. Crit. Rev. Oncol. 2021, 160, 103289. [Google Scholar] [CrossRef]
- Ustun, C.; Young, J.-A.; Papanicolaou, G.A.; Kim, S.; Ahn, K.W.; Chen, M.; Abdel-Azim, H.; Aljurf, M.; Beitinjaneh, A.; Brown, V.; et al. Bacterial blood stream infections (BSIs), particularly post-engraftment BSIs, are associated with increased mortality after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2018, 54, 1254–1265. [Google Scholar] [CrossRef]
- Lindsay, J.; Kerridge, I.; Wilcox, L.; Tran, S.; O’Brien, T.A.; Greenwood, M.; Chen, S.C.-A.; Kong, D.C.; Pergam, S.A.; Liu, C.; et al. Infection-Related Mortality in Adults and Children Undergoing Allogeneic Hematopoietic Cell Transplantation: An Australian Registry Report. Transplant. Cell. Ther. 2021, 27, 798.e1–798.e10. [Google Scholar] [CrossRef] [PubMed]
- Bow, E.J. Management of the febrile neutropenic cancer patient: Lessons from 40 years of study. Clin. Microbiol. Infect. 2005, 11 (Suppl. S5), 24–29. [Google Scholar] [CrossRef][Green Version]
- Averbuch, D.; Orasch, C.; Cordonnier, C.; Livermore, D.M.; Mikulska, M.; Viscoli, C.; Gyssens, I.C.; Kern, W.V.; Klyasova, G.; Marchetti, O.; et al. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: Summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica 2013, 98, 1826–1835. [Google Scholar] [CrossRef][Green Version]
- Schlesinger, A.; Paul, M.; Gafter-Gvili, A.; Rubinovitch, B.; Leibovici, L. Infection-control interventions for cancer patients after chemotherapy: A systematic review and meta-analysis. Lancet Infect. Dis. 2009, 9, 97–107. [Google Scholar] [CrossRef]
- Mikulska, M.; Averbuch, D.; Tissot, F.; Cordonnier, C.; Akova, M.; Calandra, T.; Ceppi, M.; Bruzzi, P.; Viscoli, C.; Aljurf, M.; et al. Fluoroquinolone prophylaxis in haematological cancer patients with neutropenia: ECIL critical appraisal of previous guidelines. J. Infect. 2018, 76, 20–37. [Google Scholar] [CrossRef]
- See, I.; Iwamoto, M.; Allen-Bridson, K.; Horan, T.; Magill, S.S.; Thompson, N.D. Mucosal Barrier Injury Laboratory-Confirmed Bloodstream Infection: Results from a Field Test of a New National Healthcare Safety Network Definition. Infect. Control. Hosp. Epidemiol. 2013, 34, 769–776. [Google Scholar] [CrossRef]
- Chaftari, A.-M.; Jordan, M.; Hachem, R.; Al Hamal, Z.; Jiang, Y.; Yousif, A.; Garoge, K.; Deshmukh, P.; Raad, I. A clinical practical approach to the surveillance definition of central line–associated bloodstream infection in cancer patients with mucosal barrier injury. Am. J. Infect. Control 2016, 44, 931–934. [Google Scholar] [CrossRef]
- Kato, Y.; Hagihara, M.; Kurumiya, A.; Takahashi, T.; Sakata, M.; Shibata, Y.; Kato, H.; Shiota, A.; Watanabe, H.; Asai, N.; et al. Impact of mucosal barrier injury laboratory-confirmed bloodstream infection (MBI-LCBI) on central line-associated bloodstream infections (CLABSIs) in department of hematology at single university hospital in Japan. J. Infect. Chemother. 2018, 24, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Metzger, K.E.; Rucker, Y.; Callaghan, M.; Churchill, M.; Jovanovic, B.D.; Zembower, T.R.; Bolon, M.K. The Burden of Mucosal Barrier Injury Laboratory-Confirmed Bloodstream Infection among Hematology, Oncology, and Stem Cell Transplant Patients. Infect. Control. Hosp. Epidemiol. 2015, 36, 119–124. [Google Scholar] [CrossRef]
- Dandoy, C.E.; Kim, S.; Chen, M.; Ahn, K.W.; Ardura, M.I.; Brown, V.; Chhabra, S.; Diaz, M.A.; Dvorak, C.; Farhadfar, N.; et al. Incidence, Risk Factors, and Outcomes of Patients Who Develop Mucosal Barrier Injury–Laboratory Confirmed Bloodstream Infections in the First 100 Days After Allogeneic Hematopoietic Stem Cell Transplant. JAMA Netw. Open 2020, 3, e1918668. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dandoy, C.E.; Haslam, D.; Lane, A.; Jodele, S.; Demmel, K.; El-Bietar, J.; Flesch, L.; Myers, K.C.; Pate, A.; Rotz, S.; et al. Healthcare Burden, Risk Factors, and Outcomes of Mucosal Barrier Injury Laboratory-Confirmed Bloodstream Infections after Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2016, 22, 1671–1677. [Google Scholar] [CrossRef][Green Version]
- de Mooij, C.E.M.; van der Velden, W.J.F.M.; de Haan, A.F.J.; Fazel, S.; van Groningen, L.F.J.; Blijlevens, N.M.A. Grading bloodstream infection risk using citrulline as a biomarker of intestinal mucositis in patients receiving intensive therapy. Bone Marrow Transplant. 2022, 57, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, W.J.; Herbers, A.H.; Feuth, T.; Schaap, N.P.; Donnelly, J.P.; Blijlevens, N.M. Intestinal damage determines the inflammatory response and early complications in patients receiving conditioning for a stem cell transplantation. PLoS ONE 2010, 5, e15156. [Google Scholar] [CrossRef]
- Bachanova, V.; Brunstein, C.G.; Burns, L.J.; Miller, J.S.; Luo, X.; DeFor, T.; Young, J.-A.; Weisdorf, D.J.; Tomblyn, M. Fewer infections and lower infection-related mortality following non-myeloablative versus myeloablative conditioning for allotransplantation of patients with lymphoma. Bone Marrow Transplant. 2009, 43, 237–244. [Google Scholar] [CrossRef]
- Tober, R.; Schnetzke, U.; Fleischmann, M.; Yomade, O.; Schrenk, K.; Hammersen, J.; Glaser, A.; Thiede, C.; Hochhaus, A.; Scholl, S. Impact of treatment intensity on infectious complications in patients with acute myeloid leukemia. J. Cancer Res. Clin. Oncol. 2023, 149, 1569–1583. [Google Scholar] [CrossRef]
- Slavin, M.A.; Grigg, A.P.; Schwarer, A.P.; Szer, J.; Spencer, A.; Sainani, A.; Thursky, K.A.; Roberts, A.W. A randomized comparison of empiric or pre-emptive antibiotic therapy after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007, 40, 157–163. [Google Scholar] [CrossRef][Green Version]
- Vehreschild, J.J.; Böhme, A.; Cornely, O.A.; Kahl, C.; Karthaus, M.; Kreuzer, K.-A.; Maschmeyer, G.; Mousset, S.; Ossendorf, V.; Penack, O.; et al. Prophylaxis of infectious complications with colony-stimulating factors in adult cancer patients undergoing chemotherapy—Evidence-based guidelines from the Infectious Diseases Working Party AGIHO of the German Society for Haematology and Medical Oncology (DGHO). Ann. Oncol. 2014, 25, 1709–1718. [Google Scholar] [CrossRef]
- Richters, A.; van Vliet, M.; Peer, P.G.M.; Verweij, P.E.; Gorkom, B.A.P.L.-V.; Blijlevens, N.M.A.; Donnelly, J.P.; van der Velden, W.J.F.M. Incidence of and risk factors for persistent gram-positive bacteraemia and catheter-related thrombosis in haematopoietic stem cell transplantation. Bone Marrow Transplant. 2014, 49, 264–269. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pagano, L.; Caira, M.; Nosari, A.; Rossi, G.; Viale, P.; Aversa, F.; Tumbarello, M. Italy for the Hema e-Chart Group Etiology of Febrile Episodes in Patients with Acute Myeloid Leukemia: Results from the Hema e-Chart Registry. Arch. Intern. Med. 2011, 171, 1502–1503. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bucaneve, G.; Micozzi, A.; Picardi, M.; Ballanti, S.; Cascavilla, N.; Salutari, P.; Specchia, G.; Fanci, R.; Luppi, M.; Cudillo, L.; et al. Results of a Multicenter, Controlled, Randomized Clinical Trial Evaluating the Combination of Piperacillin/Tazobactam and Tigecycline in High-Risk Hematologic Patients with Cancer with Febrile Neutropenia. J. Clin. Oncol. 2014, 32, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, I.; Lindström, S.; Källgren, M.; Strålin, K.; Mölling, P. 16S rDNA droplet digital PCR for monitoring bacterial DNAemia in bloodstream infections. PLoS ONE 2019, 14, e0224656. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.; Thursky, K.; Spelman, T.; Szer, J.; Bajel, A.; Harrison, S.; Tio, S.Y.; Bupha-Intr, O.; Tew, M.; Worth, L.; et al. [(18)F]FDG-PET-CT compared with CT for persistent or recurrent neutropenic fever in high-risk patients (PIPPIN): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Haematol. 2022, 9, e573–e584. [Google Scholar] [CrossRef]
- Rashidi, A.; Kaiser, T.; Graiziger, C.; Holtan, S.G.; Rehman, T.U.; Weisdorf, D.J.; Khoruts, A.; Staley, C. Specific gut microbiota changes heralding bloodstream infection and neutropenic fever during intensive chemotherapy. Leukemia 2020, 34, 312–316. [Google Scholar] [CrossRef]
- Wang, Y.M.; Abdullah, S.; Luebbering, N.; Langenberg, L.; Duell, A.; Lake, K.; Lane, A.; Hils, B.; Silva, O.V.; Trapp, M.; et al. Intestinal permeability of stem cell transplant patients correlates with systemic acute phase responses and dysbiosis. Blood Adv. 2023, in press. [Google Scholar] [CrossRef]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic Cell Death in Cancer Therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Abreu, M.T. Toll-like receptor signalling in the intestinal epithelium: How bacterial recognition shapes intestinal function. Nat. Rev. Immunol. 2010, 10, 131–144. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Kullberg, B.J.; Van der Meer, J.W.M. Circulating Cytokines as Mediators of Fever. Clin. Infect. Dis. 2000, 31 (Suppl. S5), S178–S184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Spielberger, R.; Stiff, P.; Bensinger, W.; Gentile, T.; Weisdorf, D.; Kewalramani, T.; Shea, T.; Yanovich, S.; Hansen, K.; Noga, S.; et al. Palifermin for Oral Mucositis after Intensive Therapy for Hematologic Cancers. N. Engl. J. Med. 2004, 351, 2590–2598. [Google Scholar] [CrossRef][Green Version]
- Ellis, M.; Zwaan, F.; Hedström, U.; Poynton, C.; Kristensen, J.; Jumaa, P.; Wassell, J.; Al-Ramadi, B. Recombinant human interleukin 11 and bacterial infection in patients with [correction of] haematological malignant disease undergoing chemotherapy: A double-blind placebo-controlled randomised trial. Lancet 2003, 361, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Alsulami, F.J.; Shaheed, S.U. Oral cryotherapy for management of chemotherapy-induced oral mucositis in haematopoietic cell transplantation: A systematic review. BMC Cancer 2022, 22, 442. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, F.; Tendas, A.; Giannarelli, D.; Viggiani, C.; Gumenyuk, S.; Renzi, D.; Franceschini, L.; Caffarella, G.; Rizzo, M.; Palombi, F.; et al. Cryotherapy reduces oral mucositis and febrile episodes in myeloma patients treated with high-dose melphalan and autologous stem cell transplant: A prospective, randomized study. Bone Marrow Transplant. 2016, 52, 154–156. [Google Scholar] [CrossRef] [PubMed]
- de Mooij, C.E.; Netea, M.G.; van der Velden, W.J.; Blijlevens, N.M. Targeting the interleukin-1 pathway in patients with hematological disorders. Blood 2017, 129, 3155–3164. [Google Scholar] [CrossRef][Green Version]
- Kanarek, N.; Grivennikov, S.I.; Leshets, M.; Lasry, A.; Alkalay, I.; Horwitz, E.; Shaul, Y.D.; Stachler, M.; Voronov, E.; Apte, R.N.; et al. Critical role for IL-1beta in DNA damage-induced mucositis. Proc. Natl. Acad. Sci. USA 2014, 111, E702–E711. [Google Scholar] [CrossRef][Green Version]
- Arifa, R.D.; Madeira, M.F.; de Paula, T.P.; Lima, R.L.; Tavares, L.D.; Menezes-Garcia, Z.; Fagundes, C.T.; Rachid, M.A.; Ryffel, B.; Zamboni, D.S.; et al. Inflammasome activation is reactive oxygen species dependent and mediates irinotecan-induced mucositis through IL-1beta and IL-18 in mice. Am. J. Pathol. 2014, 184, 2023–2034. [Google Scholar] [CrossRef]
- Wardill, H.R.; de Mooij, C.E.M.; Ferreira, A.R.D.S.; Havinga, H.; Harmsen, H.J.M.; van der Velden, W.J.F.M.; van Groningen, L.F.J.; Tissing, W.J.E.; Blijlevens, N.M.A. Supporting the gastrointestinal microenvironment during high-dose chemotherapy and stem cell transplantation by inhibiting IL-1 signaling with anakinra. Sci. Rep. 2022, 12, 68. [Google Scholar] [CrossRef]
- Papadia, C.; Sherwood, R.A.; Kalantzis, C.; Wallis, K.; Volta, U.; Fiorini, E.; Forbes, A. Plasma citrulline concentration: A reliable marker of small bowel absorptive capacity independent of intestinal inflammation. Am. J. Gastroenterol. 2007, 102, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Kullenberg, F.; Peters, K.; Sjöblom, M.; Heindryckx, F.; Dahlgren, D.; Lennernäs, H. Anakinra and dexamethasone treatment of idarubicin-induced mucositis and diarrhoea in rats. Basic Clin. Pharmacol. Toxicol. 2023, 132, 507–516. [Google Scholar] [CrossRef]
- de Mooij, C.E.; van Groningen, L.F.; de Haan, A.F.; Biemond, B.J.; Bakker, M.; van der Velden, W.J.; Blijlevens, N. Anakinra: Efficacy in the management of fever during neutropenia and mucositis in autologous stem cell transplantation (AFFECT-2)-study protocol for a multicenter randomized double-blind placebo-controlled trial. Trials 2020, 21, 948. [Google Scholar] [CrossRef]
- Scheinecker, C.; Redlich, K.; Smolen, J.S. Cytokines as Therapeutic Targets: Advances and Limitations. Immunity 2008, 28, 440–444. [Google Scholar] [CrossRef][Green Version]
- Wardill, H.R.; Sonis, S.T.; Blijlevens, N.M.A.; Van Sebille, Y.Z.A.; Ciorba, M.A.; Loeffen, E.A.H.; Cheng, K.K.F.; Bossi, P.; Porcello, L.; Castillo, D.A.; et al. Prediction of mucositis risk secondary to cancer therapy: A systematic review of current evidence and call to action. Support. Care Cancer 2020, 28, 5059–5073. [Google Scholar] [CrossRef] [PubMed]
- Imlay, H.; Laundy, N.C.; Forrest, G.N.; Slavin, M.A. Shorter antibiotic courses in the immunocompromised: The impossible dream? Clin. Microbiol. Infect. 2022, 29, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Guisado, M.; Espigado, I.; Martín-Peña, A.; Gudiol, C.; Royo-Cebrecos, C.; Falantes, J.; Vázquez-López, L.; Montero, M.I.; Rosso-Fernández, C.; Martino, M.D.L.L.; et al. Optimisation of empirical antimicrobial therapy in patients with haematological malignancies and febrile neutropenia (How Long study): An open-label, randomised, controlled phase 4 trial. Lancet Haematol. 2017, 4, e573–e583. [Google Scholar] [CrossRef]
- Paret, R.; Le Bourgeois, A.; Guillerm, G.; Tessoulin, B.; Rezig, S.; Gastinne, T.; Couturier, M.A.; Boutoille, D.; Lecomte, R.; Ader, F.; et al. Safety and risk of febrile recurrence after early antibiotic discontinuation in high-risk neutropenic patients with haematological malignancies: A multicentre observational study. J. Antimicrob. Chemother. 2022, 77, 2546–2556. [Google Scholar] [CrossRef]
- de Jonge, N.A.; Sikkens, J.J.; Zweegman, S.; Beeker, A.; Ypma, P.; Herbers, A.H.; Vasmel, W.; de Kreuk, A.; Coenen, J.L.; Lissenberg-Witte, B.; et al. Short versus extended treatment with a carbapenem in patients with high-risk fever of unknown origin during neutropenia: A non-inferiority, open-label, multicentre, randomised trial. Lancet Haematol. 2022, 9, e563–e572. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, H.; Lu, X.; Xia, L. Viable Bifidobacterium tablets for the prevention of chemotherapy-/radiation-induced mucositis in patients undergoing haematopoietic stem cell transplantation. Support Care Cancer 2023, 31, 282. [Google Scholar] [CrossRef]
- Schwabkey, Z.I.; Wiesnoski, D.H.; Chang, C.-C.; Tsai, W.-B.; Pham, D.; Ahmed, S.S.; Hayase, T.; Turrubiates, M.R.O.; El-Himri, R.K.; Sanchez, C.A.; et al. Diet-derived metabolites and mucus link the gut microbiome to fever after cytotoxic cancer treatment. Sci. Transl. Med. 2022, 14, eabo3445. [Google Scholar] [CrossRef] [PubMed]
- Hayase, E.; Hayase, T.; Jamal, M.A.; Miyama, T.; Chang, C.-C.; Ortega, M.R.; Ahmed, S.S.; Karmouch, J.L.; Sanchez, C.A.; Brown, A.N.; et al. Mucus-degrading Bacteroides link carbapenems to aggravated graft-versus-host disease. Cell 2022, 185, 3705–3719. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Ma, Y.; Qiu, J. Regulation of intestinal immunity by dietary fatty acids. Mucosal Immunol. 2022, 15, 846–856. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, M.; Itano, A.; McInnes, I. Harnessing the small intestinal axis to resolve systemic inflammation. Front. Immunol. 2022, 13, 1060607. [Google Scholar] [CrossRef]
- da Silva Ferreira, A.R.; van der Aa, S.A.J.; Wehkamp, T.; Wardill, H.R.; Klooster, J.P.T.; Garssen, J.; Harthoorn, L.F.; Hartog, A.; Harmsen, H.J.M.; Tissing, W.J.E.; et al. Development of a self-limiting model of methotrexate-induced mucositis reinforces butyrate as a potential therapy. Sci. Rep. 2021, 11, 229. [Google Scholar] [CrossRef]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef]
- Vijayvargiya, P.; Camilleri, M. Update on Bile Acid Malabsorption: Finally Ready for Prime Time? Curr. Gastroenterol. Rep. 2018, 20, 10. [Google Scholar] [CrossRef]
- Saul-McBeth, J.; Dillon, J.; Lee, A.; Launder, D.; Kratch, J.M.; Abutaha, E.; Williamson, A.A.; Schroering, A.G.; Michalski, G.; Biswas, P.; et al. Tissue Damage in Radiation-Induced Oral Mucositis Is Mitigated by IL-17 Receptor Signaling. Front. Immunol. 2021, 12, 687627. [Google Scholar] [CrossRef]
- Tam, J.S.Y.; Crame, E.E.; Elz, A.S.; Coller, J.K.; Wignall, A.; Prestidge, C.A.; Bowen, J.M. Effects of a novel toll-like receptor 4 antagonist IAXO-102 in a murine model of chemotherapy-induced gastrointestinal toxicity. Cancer Chemother. Pharmacol. 2022, 90, 267–278. [Google Scholar] [CrossRef]
- Chang, Y.; Deng, Q.; Zhang, Z.; Zhao, H.; Tang, J.; Chen, X.; Liu, G.; Tian, G.; Cai, J.; Jia, G. Glucagon-like peptide 2 attenuates intestinal mucosal barrier injury through the MLCK/pMLC signaling pathway in a piglet model. J. Cell. Physiol. 2021, 236, 3015–3032. [Google Scholar] [CrossRef]
- Wardill, H.R.; van der Aa, S.A.; Ferreira, A.R.D.S.; Havinga, R.; Tissing, W.J.; Harmsen, H.J. Antibiotic-induced disruption of the microbiome exacerbates chemotherapy-induced diarrhoea and can be mitigated with autologous faecal microbiota transplantation. Eur. J. Cancer 2021, 153, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M.J. Fecal Microbiota Transplantation for Dysbiosis—Predictable Risks. N. Engl. J. Med. 2019, 381, 2064–2066. [Google Scholar] [CrossRef] [PubMed]
- Wardill, H.R.; Sonis, S.T.; Blijlevens, N.M. Using real world data to advance the provision of supportive cancer care: Mucositis as a case study. Curr. Opin. Support. Palliat. Care 2022, 16, 161–167. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blijlevens, N.M.A.; de Mooij, C.E.M. Mucositis and Infection in Hematology Patients. Int. J. Mol. Sci. 2023, 24, 9592. https://doi.org/10.3390/ijms24119592
Blijlevens NMA, de Mooij CEM. Mucositis and Infection in Hematology Patients. International Journal of Molecular Sciences. 2023; 24(11):9592. https://doi.org/10.3390/ijms24119592
Chicago/Turabian StyleBlijlevens, Nicole M. A., and Charlotte E. M. de Mooij. 2023. "Mucositis and Infection in Hematology Patients" International Journal of Molecular Sciences 24, no. 11: 9592. https://doi.org/10.3390/ijms24119592
APA StyleBlijlevens, N. M. A., & de Mooij, C. E. M. (2023). Mucositis and Infection in Hematology Patients. International Journal of Molecular Sciences, 24(11), 9592. https://doi.org/10.3390/ijms24119592





